Abstract
Background and purpose
Population‐based studies suggest severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vaccines may trigger neurological autoimmunity including immune‐mediated thrombotic thrombocytopenia. Long‐term characterization of cases is warranted to facilitate patient care and inform vaccine‐hesitant individuals.
Methods
In this single‐center prospective case study with a median follow‐up of 387 days long‐term clinical, laboratory and imaging characteristics of patients with neurological autoimmunity diagnosed in temporal association (≤6 weeks) with SARS‐CoV‐2 vaccinations are reported.
Results
Follow‐up data were available for 20 cases (central nervous system demyelinating diseases n = 8, inflammatory peripheral neuropathies n = 4, vaccine‐induced immune thrombotic thrombocytopenia n = 3, myositis n = 2, myasthenia n = 1, limbic encephalitis n = 1, giant cell arteritis n = 1). Following therapy, the overall disability level improved (median modified Rankin Scale at diagnosis 3 vs. 1 at follow‐up). The condition of two patients worsened despite immunosuppressants possibly related to their autoimmune diagnoses (limbic encephalitis n = 1, giant cell arteritis n = 1). At 12 months’ follow‐up, 12 patients achieved complete clinical remissions with partial responses in five and stable disease in one case. Correspondingly, autoimmune antibodies were non‐detectable or titers had significantly lowered in all, and repeat imaging revealed radiological responses in most cases. Under vigilant monitoring 15 patients from our cohort underwent additional SARS‐CoV‐2 vaccinations (BNT162b2 n = 12, mRNA‐1273 n = 3). Most patients (n = 11) received different vaccines than prior to diagnosis of neurological autoimmunity. Except for one short‐lasting relapse, which responded well to steroids, re‐vaccinations were well tolerated.
Conclusions
In this study long‐term characteristics of neurological autoimmunity encountered after SARS‐CoV‐2 vaccinations are defined. Outcome was favorable in most cases. Re‐vaccinations were well tolerated and should be considered on an individual risk/benefit analysis.
Keywords: autoimmune, cerebral venous sinus thrombosis, COVID‐19, Guillain–Barré syndrome, multiple sclerosis, myelitis, myositis
In this study we define long‐term characteristics of various neurological autoimmune disorders encountered after SARS‐CoV‐2 vaccinations. Long‐term outcome was favorable in most cases. Re‐vaccinations were well‐tolerated and should be considered on an individual risk/benefit analysis.

INTRODUCTION
In the coronavirus 2019 (COVID‐19) pandemic with >450 million cases and >6 million deaths worldwide, vaccines against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) remain the principal countermeasure [1, 2]. Five vaccines (Pfizer‐BioNTech BNT162b2, Moderna mRNA‐1273, AstraZeneca ChAdOx1 nCov‐19, Janssen/Johnson&Johnson Ad26.COV2.S, Novavax NVX‐CoV2373) have been approved by the European Medicines Agency based on randomized clinical trials [3, 4, 5, 6, 7]. In August 2022, based on the Johns Hopkins University COVID‐19 vaccination resource center data an average of 20% of the overall European population remain unvaccinated [8]. Concerns regarding safety, particularly blood‐clotting risks including vaccine‐induced immune thrombotic thrombocytopenia (VITT) and long‐term side effects are stated as the most prominent reasons [9, 10, 11].
Various neurological autoimmune conditions diagnosed in temporal association with SARS‐CoV‐2 vaccinations have been previously reported by ourselves and others [9, 10, 12, 13, 14, 15, 16, 17, 18]. Since those publications the long‐term outcome and re‐vaccination of our patients has frequently been enquired about. Whilst such data are available for VITT to a limited extent, longitudinal characterization of other potential neurological autoimmune sequelae of SARS‐CoV‐2 vaccinations is warranted [19, 20]. The long‐term characteristics of patients diagnosed with various neurological autoimmune conditions following SARS‐CoV‐2 vaccinations are therefore defined here.
METHODS
A short‐term follow‐up of this cohort and detailed methods have been described previously [13]. Briefly, 21 patients with neurological autoimmunity diagnosed within 6 weeks of SARS‐CoV‐2 vaccination between 1 March and 1 June 2021 at the Department of Neurology or Division of Rheumatology at Heidelberg University or affiliated teaching hospitals were included. Previous studies assumed a potential causative link between vaccination and autoimmunity if onset occurred within 4–8 weeks after administration [21]. Our study protocol like others included patients within a 6‐week interval [22]. However, all included cases occurred within 4 weeks. Chart review allowed collection of clinical, laboratory and radiological data from follow‐up visits. Physicians involved in outpatient care of study participants were consulted. Structured telephone interviews with patients or their legal guardians provided additional information. The modified Rankin Scale (mRS) was selected for disability assessment of the entire cohort allowing an overview of functional outcome across various neurological autoimmune conditions. Additionally, where available disease‐specific disability scales are reported within the Results section (e.g., Expanded Disability Status Scale [EDSS] for central nervous system [CNS] demyelinating conditions). Antibody panels assessed for our patients were previously described in detail [13]. The last follow‐up was conducted on 5 August 2022. SPSS version 27 (IBM) was used for descriptive statistics. Consensus‐based clinical case reporting (CARE) guidelines were followed [23]. The Heidelberg University institutional review board approved this study (S‐373/2021). Participants or their legal guardians provided written informed consent prior to study entry.
RESULTS
Patients with neurological autoimmunity a median of 11 days (range 3–23) after SARS‐CoV‐2 vaccination were monitored with a median follow‐up of 387 days (range 120–500) after inhouse diagnosis. Diagnoses included CNS demyelinating diseases (n = 8: multiple sclerosis [MS] n = 2; myelitis n = 2; optic neuritis n = 4), inflammatory peripheral neuropathies (n = 4: Guillain–Barré syndrome [GBS] n = 2; L5 radiculitis n = 1; facial palsy n = 1), VITT (n = 3), inflammatory myopathies (n = 3), limbic encephalitis (n = 1), myasthenia (n = 1) and giant cell arteritis (GCA) (n = 1). One individual with myositis declined further participation and was lost to follow‐up. Baseline characteristics of our follow‐up cohort (n = 20) including neurological autoimmune diagnosis, previously known autoimmunity, age, gender, vaccine type and dose administered prior to onset, intervals from vaccination to symptom onset and subsequent treatment are summarized on a single case level in Table 1.
TABLE 1.
Baseline characteristics
| ID | Autoimmune diagnosis | Known AID | Age | Gender | Vaccination type | Vaccination dose | Interval onset (days) | Treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | VITT + CVST | None | 43 | F | ChAdOx1 | 1 | 11 | UFH, LMWH, IVIGs, dexa, apixaban |
| 2 | VITT + CVST | Hashimoto thyreoditis | 27 | F | ChAdOx1 | 1 | 12 | Argatroban, IVIGs, dexa, dabigatran |
| 3 | VITT + CVST | Atopic dermatitis | 25 | F | ChAdOx1 | 1 | 14 | Argatroban, dabigatran |
| 4 |
Optic neuritis |
Uveitis (Dx 2019) | 57 | F | BNT162b2 | 2 | 15 | MP pulse, 5× PP |
| 5 |
Optic neuritis (MS conversion) |
None | 31 | F | BNT162b2 | 2 | 21 | MP pulse, dimethyl fumarate |
| 6 |
Optic neuritis (MS conversion) |
None | 50 | F | BNT162b2 | 1 | 8 | MP pulse |
| 7 | Optic neuritis | None | 57 | F | BNT162b2 | 1 | 23 | MP pulse |
| 8 | Myelitis | None | 34 | F | ChAdOx1 | 1 | 14 | MP pulse |
| 9 | Myelitis | None | 55 | F | BNT162b2 | 2 | 3 | MP pulse |
| 10 | MS relapse | RR‐MS (Dx 2019) | 29 | F | mRNA‐1273 | 1 | 21 | MP pulse, teriflunomid |
| 11 | MS relapse | rSP‐MS (Dx 1999) | 61 | M | ChAdOx1 | 1 | 10 | MP pulse, siponimod |
| 12 | GBS | None | 34 | M | ChAdOx1 | 1 | 12 | IVIGs |
| 13 | GBS | None | 66 | M | ChAdOx1 | 1 | 8 | IVIGs |
| 14 | L5 radiculitis | None | 54 | F | BNT162b2 | 1 | 3 | MP pulse |
| 15 | Facial palsy | None | 39 | F | BNT162b2 | 1 | 5 | MP taper |
| 16 | Myositis | None | 22 | F | BNT162b2 | 2 | 6 | MP taper, IVIGs, azathioprine |
| 17 | Myositis relapse | Myositis (Dx 2014), Crohn's (Dx 2002) | 36 | F | BNT162b2 | 1 | 6 | MP taper |
| 18 | Limbic encephalitis | None | 80 | F | BNT162b2 | 2 | 15 | MP pulse, 5× PP |
| 19 | GCA | None | 79 | M | BNT162b2 | 1 | 17 | MP taper |
| 20 | MG relapse | MG (Dx 3 months prior) | 79 | F | ChAdOx1 | 1 | 6 | MP, 5× PP, MMF |
Abbreviations: AID, autoimmune disease; CVST, cerebral venous sinus thrombosis; dexa, dexamethasone; Dx, diagnosis; F, female; GBS, Guillain–Barré syndrome, GCA, giant cell arteritis; ID, identifier; IVIGs, intravenous immunoglobulins; LMWH, low molecular weight heparin; M, male; MG, myasthenia gravis; MMF, mycophenolate mofetil; MP, methylprednisolone; MS, multiple sclerosis; PP, plasmapheresis; RR‐MS, relapsing–remitting MS; rSP‐MS, relapsing secondary‐progressive MS; UFH, unfractionated heparin; VITT, vaccine‐induced thrombotic thrombocytopenia.
Follow‐up investigations were tailored to the respective autoimmune conditions. Baseline and follow‐up serological, radiological and cerebrospinal fluid findings are summarized on a single case level in Table 2. Representative magnetic resonance imaging (MRI) findings at initial diagnosis and follow‐up are shown in Figure 1.
TABLE 2.
Serological, radiological and CSF findings
| At diagnosis | At follow‐up | |||||
|---|---|---|---|---|---|---|
| ID | Serology | Imaging | CSF | Serology | Imaging | CSF |
| 1 | PF4‐Ab positive | MRI: CVST, S. trans b/l, S. sigm R, congestive ICB temporo‐occipital L | NP | PF4‐Ab negative | MRI: no CVST, resorption ICB, parenchymal defect temporo‐occipital L | NP |
| 2 | PF4‐Ab positive | MRI: CVST, S. trans + sigm L, congestive ICB parieto‐occipital L | NP | PF4‐Ab negative | MRI: no CVST, resorption ICB, parenchymal defect parieto‐occipital L | NP |
| 3 | N/A | MRI: CVST, S. sphen R, vena jugularis R, no parenchymal defect | NP | N/A | MRI: no CVST, no parenchymal defect | NP |
| 4 | Negative (NMO/M) |
MRI: T2 hyperint. Optic nerve + C/E |
3c, Alb↑, OCB II | NP |
MRI: no C/E or T2 hyperint. Optic nerve, no new lesions |
Unremarkable |
| 5 | Negative (NMO/M) |
MRI: T2‐hyperint. Optic nerve + C/E |
13c, Alb norm, OCB II | NP |
MRI: no C/E or T2‐hyperint. Optic nerve, no new lesions sMRI: T2‐hyperint. lesions Th6‐8, no C/E |
N/A |
| 6 | Negative (NMO/M) |
MRI: T2‐hyperint. Optic nerve + C/E |
12c, Alb↑, OCB II | NP |
MRI: no C/E or T2 hyperint. Optic nerve, new lesions: jc, no C/E |
6c, Alb↑, OCB II |
| 7 | Negative (NMO/M) |
MRI: T2‐hyperint. Optic nerve + C/E |
7c, Alb norm, OCB III | NP | N/A | N/A |
| 8 | Negative (NMO/M) |
sMRI: T2‐hyperint. Lesion Th4‐9 + C/E |
5c, Alb↑, OCB II | NP |
sMRI: mild residual T2‐hyperint. Th7, no C/E MRI: no lesions |
Unremarkable |
| 9 | Negative (NMO/M) |
sMRI: T2 hyperint. Lesion Th1 + C/E |
8c, Alb norm, OCB II | NP |
sMRI: regression T2 hyperint. Th1 MRI: no lesions |
N/A |
| 10 | Negative (NMO/M) | MRI: new pv + jc C/E lesions | 3c, Alb norm, OCB II | NP | MRI: new jc + pv lesions, no C/E | 5c, Alb norm, OCB II |
| 11 | NP | MRI: new jc C/E lesions | NP | NP | NP | NP |
| 12 | Negative (ANP) | NP | 2c, Alb↑↑, no OCB | NP | NP | Unremarkable |
| 13 | Negative (ANP) | NP | N/A | NP | NP | NP |
| 14 | Negative (ANP) | MRI: T2‐hyperint. L5 N root R + common peroneal/tibial N R, edema R LE M | 1c, Alb↑, no OCB | NP | MRI: regression T2‐hyperint. L5 N root + N R LE, regression edema R LE M | N/A |
| 15 | Negative (ANP) |
MRI: T2‐hyperint. Facial nerve R |
6 and 7c, Alb↑, OCB II | NP | NP | Unremarkable |
| 16 | Negative (Myositis) | MRI LE: T2‐hyperint. L LE M + C/E | NP | Negative (Myositis) | MRI LE: complete remission | NP |
| 17 | PM/Scl‐75‐Ab + | MRI LE: progressive T2‐hyperint. b/l LE M + C/E | NP | PM/Scl‐75‐Ab Negative | MRI LE: milder b/l LE T2‐hyperint., no C/E | NP |
| 18 | Negative (Enceph.) | b/l temporal FLAIR hyperint. | 13c, Alb↑, OCB II | N/A | ||
| 19 | NP |
US: halo sign temporal artery CT: old stroke putamen L |
NP | NP | CT: MCA stroke R | NP |
| 20 |
AchR‐Ab (8 nmol/l) |
MRI: unremarkable | Unremarkable | AchR‐Ab (1 nmol/l) | NP | NP |
Abbreviations: Ab, antibodies; AchR, acetylcholine receptor; Alb, albumin; ANP, autoimmune neuropathy panel; b/l, bilateral; c, cells; C/E, contrast enhancement; CSF, cerebrospinal fluid; CT, computed tomography; CVST, cerebral venous sinus thrombosis; Enceph., encephalitis panel; FLAIR, fluid‐attenuated inversion recovery; hyperint, hyperintensities; ICB, intracranial bleeding; ID, identifier; jc, juxtacortical; L, left; M, muscle, LE, lower extremity; MCA, middle cerebral artery; MRI, magnetic resonance imaging; N, nerve; N/A, not available; NMO/M, neuromyelitis optica/myelitis panel; NP, not performed; OCB, oligoclonal bands; PF4, platelet‐factor 4, PM/Scl, polymyositis/scleroderma; pv, periventricular; R, right; S., sinus; sigm, sigmoideus; sMRI, spinal MRI; sphen, sphenoidalis; Th, thoracal segment; trans, transversus; US, ultrasound.
FIGURE 1.
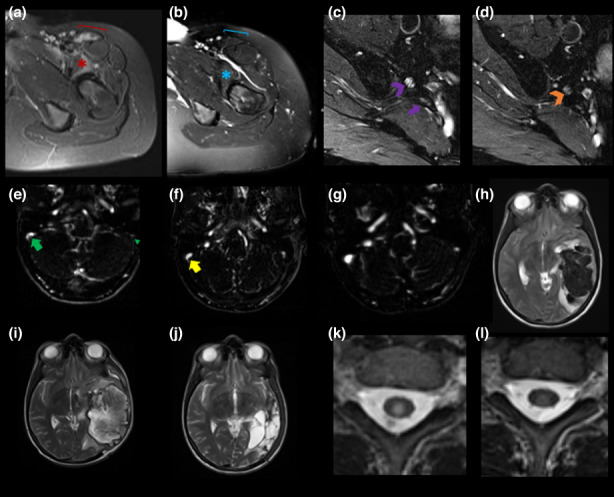
Magnetic resonance imaging (MRI) findings during follow‐up. MRI obtained at diagnosis of neurological autoimmunity (a), (c), (e), (h), (k) after SARS‐CoV‐2 vaccination and at follow‐up (b), (d), (f), (g), (i), (j), (l) are shown including cases of inflammatory myopathy (a) and (b), L5 radiculitis (c) and (d), vaccine‐induced thrombotic thrombocytopenia (VITT) (e)–(j) and acute myelitis (k), (l). (a), (b) Axial T2‐weighted (T2w) and fat suppressed (fs) MRI of left pelvic and proximal thigh musculature are shown. (a) T2w hyperintensities of left iliopsoas (red *) and sartorius (red bracket) muscles consistent with an inflammatory myopathy (b) completely resolved (blue * and bracket) under steroid therapy over 11 months. (c), (d) Axial T2w fs MR neurography of the lumbosacral plexus at the level of the pelvis revealed T2w hyperintense L5 nerve root enlargement ((c) violet arrowhead) in line with an L5 radiculitis with subtotal remission 9 months later ((d) orange arrowhead) following immunosuppressive therapy. Normal appearance of right S1 nerve root ((c) violet arrow). (e)–(j) Axial reconstructions of contrast‐enhanced MR venography (e)–(g) and T2w axial MRI (h)–(j) are shown. (e) At diagnosis of VITT, complete thrombotic occlusion of the left hypoplastic sigmoid sinus (green arrowhead) and a non‐occlusive thrombus in the right sigmoid sinus (green arrow) were noted. Following therapy, marked regression ((f) 3 weeks after diagnosis) and complete resolution of thrombotic occlusions ((g) 9 months after diagnosis) were observed. CVST resulted in congestive intraparenchymal bleeding in the left temporal lobe at diagnosis (h) with associated midline shift and small intraventricular hemorrhage. Following decompressive craniotomy brain parenchyma protrusion and unchanged midline shift was noted ((i) 3 weeks after diagnosis). Nine months after onset, complete resorption of hemorrhage and a residual left temporal parenchymal defect with ex vacuo hydrocephalus was found (j). (k), (l) Axial T2w MRI of the spinal cord revealed a delineated central T2w hyperintensity with concomitant mild swelling at the level of the first thoracal vertebral body in line with acute myelitis (k). One year after therapy, marked regression of the spinal cord lesion and complete remission of spinal cord swelling were evident (l).
Amongst patients with CNS demyelinating diseases, four individuals with previous sensorimotor deficits (EDSS at diagnosis: myelitis 4.5 for both cases; MS 3.5 and 3) achieved complete clinical remissions. In optic neuritis cases (EDSS at diagnosis: 5, n = 1; 4, n = 1; 3, n = 2), visual acuity normalized in two (EDSS 0) and improved in one individual (EDSS 1) with stable vision in the remaining patient (EDSS 5). Follow‐up MRI was obtained in six cases (transverse myelitis n = 2, optic neuritis n = 3, MS n = 1). At follow‐up, optic nerve contrast enhancement and T2‐weighted hyperintensities resolved in optic neuritis cases. Conversion to MS was found in two patients with new lesions detected on spinal and cerebral MRI, respectively. Novel but clinically silent T2/fluid‐attenuated inversion recovery hyperintensities were found in the MS case. Re‐imaging of myelitis cases revealed regressive, mild residual T2 hyperintensities. Data from repeat lumbar punctures were available for review in four patients (optic neuritis n = 2, transverse myelitis n = 1, MS n = 1). Findings including cell counts and oligoclonal bands normalized in two individuals (optic neuritis n = 1, transverse myelitis n = 1) whilst two patients had persistent mild lymphocytic pleocytosis and type II oligoclonal bands (optic neuritis with conversion to MS n = 1, MS n = 1).
Participants diagnosed with inflammatory neuropathies (n = 4) were symptom‐free (GBS n = 1, facial palsy n = 1), had mild residual lower extremity sensory deficits (GBS n = 1) and improved sensorimotor weakness of the lower extremities, yet intermittently still required a walking aid for ambulation (L5 radiculitis n = 1). In the latter case follow‐up MRI (Figure 1c,d) was obtained and revealed subtotal remission of T2 hyperintense nerve root enlargement and marked regression of subsequent muscle edema. Repeat lumbar punctures were available in two cases (GBS n = 1, facial palsy n = 1) with normal findings at follow‐up.
Patients with VITT and cerebral venous sinus thrombosis (CVST) resulting in congestive bleedings (n = 2), who at diagnosis presented with left hemispheric syndromes (National Institutes of Health Stroke Scale [NIHSS] ≥12), had mild residual deficits (NIHSS ≤2). The patient with CVST and no congestive bleeding, who initially presented with severe headache, had no residual deficits (NIHSS 0). Anti‐platelet factor 4 (PF4) antibodies were no longer detectable 3 months after diagnosis in both individuals, where they had previously been assessed. Follow‐up MRI revealed resolution of thrombotic occlusions in all individuals with complete resorption of intraparenchymal hemorrhages but residual parenchymal defects in both cases of congestive bleedings following anticoagulation and intravenous immunoglobulin (IVIG) therapy. Anticoagulation included heparin in one case, which is contraindicated in VITT. Later insights into the pathomechanism of VITT led to guidelines recommending anticoagulants used in the setting of heparin‐induced thrombotic thrombocytopenia (e.g., argatroban, apixaban). Representative radiological findings are presented in Figure 1(e–j).
Myositis cases (n = 2) had presented with lower extremity muscle weakness, swelling and pain rendering them unable to ambulate at diagnosis. Another clinical relapse, unrelated to re‐vaccinations, occurred in one case 9 months after diagnosis prompting IVIG treatment and the initiation of azathioprine, which achieved clinical remission. The second patient had only mild pre‐existing muscle weakness, which again allowed ambulation following immunosuppressive therapy. Correspondingly, creatine kinase level normalized (868 U/l at diagnosis) in one case and was significantly lower in the other case (215 U/l vs. 11,105 U/l at diagnosis). Myositis antibodies, found at diagnosis (PM/Scl‐75), were no longer detectable. Repeat MRI was performed in both cases and revealed complete resolution of muscle edema in the first case (Figure 1a,b) and reduced but persisting mild edema in the second case.
Myasthenic crisis requiring intubation and mechanical ventilation at diagnosis (Besinger score 2.8) responded well to intensive care, intravenous and oral pyridostigmine and immunosuppressive therapy (steroids, mycophenolate mofetil). Mild generalized weakness (Besinger score 0.6), that had pre‐existed before vaccination, and a significantly lowered acetylcholine receptor antibody titer (1.07 vs. 8.26 nmol/l at diagnosis) were found at follow‐up. The condition of two patients (limbic encephalitis n = 1, GCA n = 1) worsened despite immunosuppressive therapy, possibly related to their neurological autoimmune diagnoses. An 80‐year‐old lady, who presented with severe impairment of short‐term memory and confusion at diagnosis, died 4 months after newly diagnosed limbic encephalitis. A 79‐year‐old gentleman with GCA (NIHSS at GCA diagnosis 1), who initially consulted our institution for severe left temporal headache, jaw claudication, mild dysarthria and fatigue, had additional cardiovascular risk factors (hypertonia, diabetes type II, smoke abuse). He suffered from right middle cerebral artery territory stroke 8 months after diagnosis, which resulted in persisting neglect, dysarthria and left‐sided hemiparesis (NIHSS 7).
Following immunosuppressive treatment including oral (n = 7: myositis n = 2, multiple sclerosis n = 2, GCA n = 1, myasthenia n = 1, facial palsy n = 1) and/or systemic (n = 12: CNS demyelinating diseases n = 8, limbic encephalitis n = 1, L5 radiculitis n = 1, VITT n = 2) steroids, IVIGs (n = 5: VITT n = 2, GBS n = 2, myositis n = 1), apheresis (n = 3: limbic encephalitis n = 1, optic neuritis n = 1, myasthenia n = 1) and the introduction/change of long‐term immunosuppressive treatment (n = 5: optic neuritis with MS conversion n = 1, MS n = 2, myositis n = 1, myasthenia n = 1) the disability level decreased (median mRS score at diagnosis 3 vs. 1 at follow‐up). mRS distributions before, at diagnosis of neurological autoimmunity and at 12 months’ follow‐up are summarized in Figure 2 for the entire cohort (a) and its largest subgroups (b)–(d).
FIGURE 2.
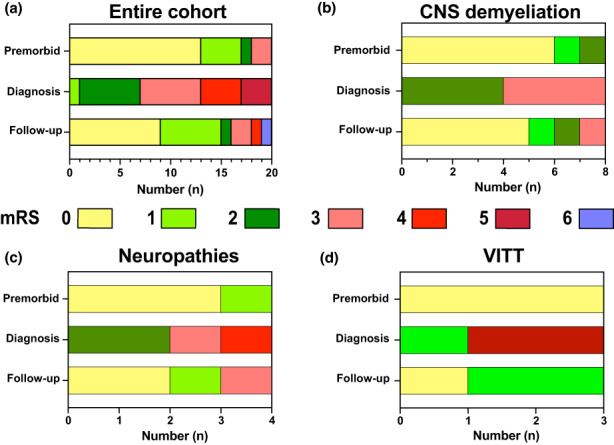
Modified Rankin Scale (mRS) during follow‐up. mRS values are shown for the entire cohort (a) and its largest subgroups (b)–(d): (b) CNS demyelinating disorders, (c) inflammatory neuropathies, (d) VITT before, at diagnosis and at 12 months’ follow‐up. Median mRS of the entire cohort and its subgroups improved after immunosuppressive therapy.
Re‐vaccination data are summarized in Figure 3 for the entire cohort (a) and its largest subgroups (b)–(d). During follow‐up, 15 individuals (VITT n = 2, optic neuritis n = 2, MS n = 2, myelitis n = 2, neuropathies n = 3, myositis n = 2, myasthenia n = 1, GCA n = 1) underwent additional SARS‐CoV‐2 vaccinations under vigilant monitoring. At re‐vaccination four patients (GCA n = 1, myositis n = 2, optic neuritis n = 1) received low‐dose oral steroids whilst long‐term immunosuppressive regimens were administered in five cases (myasthenia n = 1, MS n = 2, myositis n = 1, optic neuritis n = 1). Following ChAdOx1 vaccinations (n = 7: VITT n = 2, MS n = 1, myelitis n = 1, myasthenia n = 1, GBS n = 2), BNT162b2 was administered without any relapses. After BNT162b2 with subsequent diagnosis of autoimmunity (n = 7: myositis n = 2, optic neuritis n = 2, myelitis n = 1, GCA n = 1, facial palsy n = 1), individuals again received BNT162b2 (n = 4: myopathies n = 2, optic neuritis n = 1, myelitis n = 1) or mRNA‐1273 (n = 3: optic neuritis n = 1, GCA n = 1, facial palsy n = 1). Relapse was found in a myositis case 10 days after BNT162b2 re‐vaccination, which, however, responded well to intensified oral steroid therapy and lasted only 4 weeks. Following mRNA‐1273 vaccination (MS n = 1) BNT162b2 was administered without a relapse. Most patients (n = 3 of 4, excluding the deceased patient) without further SARS‐CoV‐2 vaccinations had the intention to receive their second and/or third dose with protein‐based vaccines (n = 2: VITT n = 1, optic neuritis n = 1) or after full clinical remission (optic neuritis n = 1). Vaccination titers were not routinely determined and were available in five individuals only. They were assessed 6–10 months after neuro‐autoimmunity diagnosis with subsequent immunosuppressive therapy (myelitis n = 1, facial palsy n = 1, myasthenia n = 1, GBS n = 1, L5 radiculitis n = 1) and at least 4 weeks after last vaccination. Quantifiable titers (Elecsys Anti‐SARS‐CoV‐2S assay, Roche, Switzerland) were achieved in four individuals, who had received two additional mRNA‐based vaccinations after onset of neurological autoimmunity (BNT162b2: myasthenia 2142 U/ml 4 weeks after third dose, myelitis 1948 U/ml 8 weeks after third dose, GBS 1683 U/ml 5 weeks after third dose; mRNA‐1273: facial palsy 1239 U/ml 6 weeks after third dose). No titer was detected in the fifth individual without further SARS‐CoV‐2 vaccinations after diagnosis of neurological autoimmunity (L5 radiculitis following BNT162b2 vaccination, 6 and 10 months after last vaccination). During the follow‐up period four patients (optic neuritis n = 1, VITT n = 1, GBS n = 1, myositis n = 1), who had all undergone at least one additional vaccination, got infected with SARS‐CoV‐2. All four were oligosymptomatic and did not require inpatient care.
FIGURE 3.

Re‐vaccination of patients with neurological autoimmunity after COVID‐19 vaccinations. Re‐vaccination data are summarized for the entire cohort (a) and its largest subgroups (b)–(d): (b) CNS demyelinating disorders; (c) inflammatory neuropathies; (d) VITT. Vaccines administered before diagnosis of neurological autoimmunity are shown along the x‐axis whereas different colors indicate vaccine types selected for re‐vaccinations. Following ChAdOx1 vaccinations most patients received mRNA‐based vaccines whereas re‐vaccination hesitancy was high after BNT162b2 vaccinations (a)–(d).
DISCUSSION
Previous population‐ and registry‐based studies suggest that SARS‐CoV‐2 vaccinations may be associated with several autoimmune sequelae most prominently including VITT in ChAdOx1 or Ad26.COV2.S recipients [12, 14]. A Scottish national cohort study identified an incidence of 1.13/100,000 for immune thrombocytopenia (adjusted rate ratio [aRR] 5.77) within 27 days after ChAdOx1 vaccinations, which equaled an estimated excess of 0.46 cases/100,000 in comparison to expected events [14]. A German national cohort study estimated a CVST incidence of 1.52/100,000 in ChAdOx1 recipients within 31 days after administration. Compared to mRNA‐based vaccines the aRR was 9.68 with an increased risk for female recipients (aRR 3.14) [12]. Based on the US Vaccine Adverse Event Reporting System (VAERS) the incidence of thrombosis with thrombocytopenia syndrome after Ad26.COV2.S vaccination was estimated at 1/263,000 [24]. Similarly using the VAERS dataset Ad26.COV2.S was associated with an excess of 6.36/100,000 GBS cases [25]. Another American study also found an increased GBS incidence within 3 weeks of Ad26.COV2.S administration (32.4/100,000) with an aRR of 20.56 compared to mRNA‐based vaccines [26]. Regarding ChAdOx1 vaccinations, an analysis of the UK National Immunoglobulin Database suggested an excess of 0.57 GBS cases/100,000 within the first 6 weeks after administration [22]. Although controversial, following aggregation and re‐analysis of BNT162b2 and mRNA‐1273 vaccine trial data, aRR for facial palsy was 7.0 after administration of mRNA‐based COVID‐19 vaccines compared to the placebo arm [17]. Another study suggested an excess of 4.8 and 2.0 facial palsy cases/100,000 after administration of mRNA‐based CoronaVac and BNT162b2 vaccines, respectively. In the nested control analysis a significant increase was only identified for CoronaVac recipients, however [27].
The design of these studies, however, makes long‐term follow‐up of individuals with autoimmunity in temporal association with COVID‐19 vaccines difficult. Long‐term clinical characterization including data on re‐vaccinations is warranted to facilitate care of patients with neurological autoimmunity following SARS‐CoV‐2 vaccinations and to inform vaccine‐hesitant individuals. In this study, follow‐up data on a previously reported cohort of neurological autoimmunity after various SARS‐CoV‐2 vaccines are therefore provided.
Following therapy, the long‐term outcome of most patients a year after diagnosis was favorable and the degree of disability overall improved. This is in line with previous short‐term assessments and included patients with VITT and subsequent CVST, which was previously linked to a rather poor prognosis, suggesting early recognition and guideline‐based therapies allow improved outcomes [9, 13, 19, 28]. Whilst it cannot be excluded that the worsened condition in two cases resulted from autoimmunity despite immunosuppressive therapy, both were elderly patients with significant other comorbidities and cardiovascular risk factors which may have influenced outcome.
Corresponding to clinical improvements, antibodies detected at diagnosis of various neurological autoimmune conditions were negative or titers had lowered following therapy. Similarly, in previous VITT case series PF4‐dependent platelet activation assays became negative a median of 12 weeks after diagnosis, suggesting that PF4 antibodies are transient after diagnosis of VITT [19]. Whether such serological observations may guide treatment, for example the duration of anticoagulation following VITT, or predict outcome warrants further investigation. Imaging findings in our cohort correlated well with clinical improvements. Follow‐up imaging is hence recommended in individuals with relevant findings at diagnosis and could similarly help to guide therapy.
In comparison to published longitudinal cohorts of various neurological conditions unrelated to vaccinations, VITT with subsequent CVST was associated with distinct serological and clinical characteristics that may imply a causative link as previously suggested. PF4 antibodies, detected in VITT, were not found in 93 CVST samples obtained prior to the COVID‐19 pandemic [29]. Other than in VITT, thrombocytopenia (8.4%) was found in the minority of CVST patients and heparin‐induced thrombocytopenia occurred (0.1%) at an incidence that is expected based on data from the general population (0.1%–0.5%) [30]. Similarly, the successful treatment of CVST with IVIGs is unusual given their known coagulatory side effects and reflects suppression of PF4‐antibody‐mediated platelet activation [31]. Like heparin‐induced thrombocytopenia with subsequent thrombosis (28% [32]), mortality in an international VITT registry study was high [28]. The outcome of CVST patients in this study was more favorable and may reflect increasing knowledge of VITT, but also young age, no altered consciousness at diagnosis and no involvement of the sinus rectus, which were previously identified as positive prognostic factors [33]. Longitudinal assessment of CVST unrelated to vaccination suggests that the majority of patients (79%) with congestive bleeding and those without (91%) achieve an mRS ≤2 at 6 months’ follow‐up [34]. Characteristics of other larger subgroups of neurological autoimmune conditions from this study (N ≥ 3: CNS demyelinating disorders, peripheral neuropathies) lacked clearly distinct serological, clinical and outcome characteristics separating them from respective diseases unrelated to COVID‐19 vaccinations. Whilst the size of these sub‐cohorts may have hindered the identification of distinct characteristics, this observation parallels reports of immune‐checkpoint‐inhibitor‐induced autoimmunity [35]. This may suggest that inflammatory reactions following vaccinations may have stimulated already existing pathways of autoimmunity rather than having induced them resulting from cross‐reactivity with the SARS‐CoV‐2 spike protein.
The clinical follow‐up of CNS demyelinating disorders from the study match expected courses of respective conditions. Irrespective of treatment vision improves in most patients with optic neuritis with visual acuity ≥20/40 in 91%–95% of patients at 1‐year follow‐up [36]. Correspondingly, vision improved in three out of four cases from this study. However, vision remains poor in a small percentage (3%–5%) of patients with the severity of initial vision loss being the main negative prognosticator [37]. Accordingly, unchanged vision was noted in an individual with severe vision loss (20/400) at diagnosis. During follow‐up, the diagnosis of MS was established in two cases of optic neuritis in line with 25% of patients with no brain lesions developing MS during extended follow‐up [38]. Previous reports suggest myelitis outcome can be favorable resembling cases included in this study. In a retrospective study including 87 patients most had no significant disability after a mean follow‐up of 2.9 years with higher disability scores at diagnosis identified as a negative prognostic factor. Disease course was monophasic in all but 13% of patients with later conversion to MS [39]. However, the conversion rate increases with longer follow‐up (79% after a median 6.2 years [40]) and conversion/relapse may still occur in our cases. Relapsing MS (relapsing–remitting MS, relapsing secondary‐progressive MS), diagnosed in two cases included in this paper, can be characterized by full or at least partial recovery between relapses as in our cases. Yet despite disease‐modifying treatments no disease evidence—defined as absence of new lesions on MRI, no relapses or EDSS progression—is seen in the minority of patients (46% at 1‐year and 7.9% at 7‐year follow‐up) [41]. The detection of new, clinically silent lesions in one case matches the natural disease course of relapsing–remitting MS. Serological investigations were negative in all CNS demyelinating disorder cases from this series. Only transient oligoclonal band detection in two cases from this study is unusual but has been reported in the context of CNS neuroinflammatory conditions such as acute disseminated encephalomyelitis, myelin oligodendrocyte glycoprotein immunoglobulin G associated encephalomyelitis, distinct MS cases or following successful MS treatment [42, 43, 44].
Clinical courses of inflammatory neuropathies also resembled follow‐up characteristics of cases unrelated to vaccination. In previous studies, the nadir of symptoms was typically reached within 2 weeks and 83%–87% of GBS patients with IVIGs and/or apheresis treatment were able to ambulate 48 weeks after onset with a mean disability scale improvement of 2.5 [45]. In agreement with these numbers, after IVIG treatment both GBS cases recovered with no or only minimal persisting deficits at 12 months’ follow‐up. As in our facial palsy case, complete clinical remission at 1‐year follow‐up is achieved in most (72%) patients treated with steroids [46]. Some patients with inflammatory neuropathies including the L5 radiculitis case from this series, however, may only experience partial clinical remission and do not regain the ability to walk independently 1 year after onset. Unlike in two previous studies reporting GBS cases after ChAdOx1 vaccinations, bilateral facial palsy was not found in our patients, who overall displayed less severe symptoms in contrast to the described areflexic quadriplegia requiring mechanical ventilation [15, 16]. In agreement with both studies, serological investigations including antiganglioside antibodies were negative in all patients from this study. Similarly, no distinct cerebrospinal fluid findings were noted in the study and remission of albuminocytological dissociation in patients with full recovery is in line with the literature [47].
Several studies have previously shown the safety and benefits of SARS‐CoV‐2 vaccines in individuals with known neurological autoimmunity including MS and GBS amongst others [48, 49, 50, 51]. Yet to our knowledge, re‐vaccination of patients with neurological autoimmunity diagnosed following SARS‐CoV‐2 vaccines so far has only been addressed in VITT [19, 20]. In a series of 40 ChAdOx1 recipients additional vaccinations (ChAdOx1 n = 5, mRNA‐1273 n = 2, BNT162b2 n = 33) were safe and well tolerated [13]. As VITT is typically diagnosed after administration of adenoviral vector vaccines and has a distinct pathomechanism, this observation may not be transferable, however, to other cases of neurological autoimmunity after vaccination, which also occur after mRNA‐based vaccinations [9, 10]. Under vigilant monitoring and low‐dose immunosuppression in some cases, except for one short‐lasting relapse additional SARS‐CoV‐2 vaccinations were well tolerated in our cohort. Different vaccines were selected in most cases (e.g., BNT162b2 after ChAdOx1, mRNA‐1273 after BNT162b2, although the latter is also mRNA‐based). Re‐vaccination hesitancy was higher amongst mRNA‐based SARS‐CoV‐2 vaccine recipients in our cohort probably because of the paucity of re‐vaccination data for individuals with new onset of neurological autoimmunity after administration of these vaccines.
Vaccination titers were only available in five patients from this study. Interestingly, four individuals with two additional COVID‐19 vaccinations after onset of autoimmunity had quantifiable titers whereas the titer was negative in the fifth patient without further administrations. Although the numbers do not allow firm conclusions and titers yet need to be correlated with antiviral protection, this appears in line with lower titers and seroconversion rates in immunosuppressed patients [50, 52, 53]. A recent meta‐analysis found seroconversion occurred in 53% and 75% of patients with immune‐mediated inflammatory disorders compared with immunocompetent controls after the first and second vaccination dose, respectively [52]. An additional third booster vaccination achieved successful seroconversion in most non‐responders [52]. This underlines the importance of completing a full vaccination schedule, ideally including a booster dose, in immunosuppressed patients as in our cohort following an individual risk/benefit analysis.
The size and heterogeneity of our cohort, the latter resulting from the important summary of a broad range of neurological autoimmune conditions encountered after vaccinations, remain limitations although several impactful previous studies covering neurological autoimmunity after SARS‐CoV‐2 vaccinations included fewer patients [9, 10, 15]. Whilst it permits descriptive juxtaposition with published cohorts of neurological autoimmunity unrelated to vaccinations, it does not allow statistical comparison of clinical characteristics. The single‐center design and subsequent prospective follow‐up could also have caused a selection bias, which may have influenced outcomes. Given the unchanged study design, it still cannot be ruled out that cases of neurological autoimmunity merely coincided with COVID‐19 vaccinations in our follow‐up study. Lastly, whilst our clinical experience is described, clear recommendations for vaccine selection after autoimmune events in temporal association with previous SARS‐CoV‐2 vaccinations cannot be established. The strengths of this study include the long follow‐up, the inclusion of patients with neurological autoimmunity other than VITT with subsequent CVST, the description of our re‐vaccination experience, and the important research topic given the continuous global rollout of SARS‐CoV‐2 vaccines.
CONCLUSIONS
In this follow‐up study the longitudinal characteristics of patients with various neurological autoimmune diseases diagnosed in temporal association with various SARS‐CoV‐2 vaccines are summarized. Overall, neurological autoimmunity responded well to therapy and most patients had a favorable long‐term outcome. Based on our experience, under vigilant outpatient monitoring additional SARS‐CoV‐2 vaccinations may be considered and were well tolerated. An individual risk/benefit analysis should also evaluate the mortality and potential complications associated with COVID‐19 itself.
FINANCIAL DISCLOSURES
Dr Doubrovinskaia, Dr Mooshage, Dr Seliger, Dr Lorenz reports no disclosures. Dr Nagel reports consultation fees from Brainomix and payment for lectures including service on speakers' bureaus from Boehringer Ingelheim and Pfizer (all unrelated to the present work). Mr Lehnert reports no disclosures. Dr Purrucker reports consultation fees and travel expenses from Abbott, Akcea, Bayer, Boehringer Ingelheim, Daiichi Sanyo and Pfizer (all unrelated to the present work). Dr Wildemann, Dr Bendszus, Dr Wick, Dr Schönenberger, Dr Kaulen report no disclosures.
AUTHOR CONTRIBUTIONS
SD, CMM and LDK drafted the manuscript. SD, CMM, CS and LDK performed chart review and conducted structured interviews. CMM and MB provided radiological evaluation. All authors analyzed and interpreted data, critically revised, read and approved the final manuscript. SS and LDK designed and supervised the study.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGEMENTS
Dr Leon D. Kaulen has been funded by a fellowship of the DKFZ Clinician Scientist Program, supported by the Dieter Morszeck Foundation. Open Access funding enabled and organized by Projekt DEAL.
Doubrovinskaia S, Mooshage CM, Seliger C, et al. Neurological autoimmune diseases following vaccinations against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2): A follow‐up study. Eur J Neurol. 2023;30:463‐473. doi: 10.1111/ene.15602
Sofia Doubrovinskaia and Christoph Mooshage contributed equally.
See commentary by P. Berlit on page 303.
Contributor Information
Silvia Schönenberger, Email: silvia.schoenenberger@med.uni-heidelberg.de.
Leon D. Kaulen, Email: leon.kaulen@med.uni-heidelberg.de.
DATA AVAILABILITY STATEMENT
Data will be shared upon request by qualified researchers.
REFERENCES
- 1. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20(5):533‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. COVID‐19 Data Repository by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University Available from: https://github.com/CSSEGISandData/COVID‐19.
- 3. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2020;384(5):403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV‐19 vaccine against SARS‐CoV‐2: a preliminary report of a phase 1/2, single‐blind, randomised controlled trial. Lancet. 2020;396(10249):467‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single‐dose Ad26.COV2.S vaccine against COVID‐19. N Engl J Med. 2021;384(23):2187‐2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heath PT, Galiza EP, Baxter DN, et al. Safety and efficacy of NVX‐CoV2373 COVID‐19 vaccine. N Engl J Med. 2021;385(13):1172‐1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. COVID‐19 Data Repository by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University . Vaccine tracker. International vaccination efforts. Available from: https://coronavirus.jhu.edu/vaccines/international.
- 9. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov‐19 vaccination. N Engl J Med. 2021;384(22):2092‐2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384(22):2124‐2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pickles K, Copp T, Dodd RH, et al. COVID‐19 vaccine intentions in Australia. Lancet Infect Dis. 2021;21(12):1627‐1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schulz JB, Berlit P, Diener HC, et al. COVID‐19 vaccine‐associated cerebral venous thrombosis in Germany. Ann Neurol. 2021;90(4):627‐639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaulen LD, Doubrovinskaia S, Mooshage C, et al. Neurological autoimmune diseases following vaccinations against SARS‐CoV‐2: a case series. Eur J Neurol. 2022;29(2):555‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simpson CR, Shi T, Vasileiou E, et al. First‐dose ChAdOx1 and BNT162b2 COVID‐19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 2021;27(7):1290‐1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maramattom BV, Krishnan P, Paul R, et al. Guillain–Barré syndrome following ChAdOx1‐S/nCoV‐19 vaccine. Ann Neurol. 2021;90(2):312‐314. [DOI] [PubMed] [Google Scholar]
- 16. Allen CM, Ramsamy S, Tarr AW, et al. Guillain–Barré syndrome variant occurring after SARS‐CoV‐2 vaccination. Ann Neurol. 2021;90(2):315‐318. [DOI] [PubMed] [Google Scholar]
- 17. Ozonoff A, Nanishi E, Levy O. Bell's palsy and SARS‐CoV‐2 vaccines. Lancet Infect Dis. 2021;21(4):450‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Muir K‐L, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021;384:1964‐1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schönborn L, Thiele T, Kaderali L, Greinacher A. Decline in pathogenic antibodies over time in VITT. N Engl J Med. 2021;385(19):1815‐1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lacy J, Pavord S, Brown KE. VITT and second doses of COVID‐19 vaccine. N Engl J Med. 2021;386(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wraith DC, Goldman M, Lambert PH. Vaccination and autoimmune disease: what is the evidence? Lancet. 2003;362(9396):1659‐1666. [DOI] [PubMed] [Google Scholar]
- 22. Keh RYS, Scanlon S, Datta‐Nemdharry P, et al. COVID‐19 vaccination and Guillain–Barré syndrome: analyses using the national immunoglobulin database. Brain. 2022;awac067. doi: 10.1093/brain/awac067. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus‐based clinical case reporting guideline development. J Med Case Reports. 2013;7(1):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. See I, Lale A, Marquez P, et al. Case series of thrombosis with thrombocytopenia syndrome after COVID‐19 vaccination—United States, December 2020 to August 2021. Ann Intern Med. 2022;175(4):513‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woo EJ, Mba‐Jonas A, Dimova RB, Alimchandani M, Zinderman CE, Nair N. Association of Receipt of the Ad26.COV2.S COVID‐19 vaccine with presumptive Guillain–Barré syndrome, February–July 2021. JAMA. 2021;326(16):1606‐1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hanson KE, Goddard K, Lewis N, et al. Incidence of Guillain–Barré syndrome after COVID‐19 vaccination in the vaccine safety datalink. JAMA Netw Open. 2022;5(4):e228879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wan EYF, Chui CSL, Lai FTT, et al. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS‐CoV‐2 vaccines: a case series and nested case–control study. Lancet Infect Dis. 2022;22(1):64‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sánchez van Kammen M, Aguiar de Sousa D, Poli S, et al. Characteristics and outcomes of patients with cerebral venous sinus thrombosis in SARS‐CoV‐2 vaccine‐induced immune thrombotic thrombocytopenia. JAMA Neurol. 2021;78(11):1314‐1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sánchez van Kammen M, Heldner MR, Brodard J, et al. Frequency of thrombocytopenia and platelet factor 4/heparin antibodies in patients with cerebral venous sinus thrombosis prior to the COVID‐19 pandemic. JAMA. 2021;326(4):332‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dhakal B, Kreuziger LB, Rein L, et al. Disease burden, complication rates, and health‐care costs of heparin‐induced thrombocytopenia in the USA: a population‐based study. Lancet Haematol. 2018;5(5):e220‐e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bourguignon A, Arnold DM, Warkentin TE, et al. Adjunct immune globulin for vaccine‐induced thrombotic thrombocytopenia. N Engl J Med. 2021;385:720‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuter DJ, Konkle BA, Hamza TH, et al. Clinical outcomes in a cohort of patients with heparin‐induced thrombocytopenia. Am J Hematol. 2017;92(8):730‐738. [DOI] [PubMed] [Google Scholar]
- 33. de Bruijn SF, de Haan RJ, Stam J. Clinical features and prognostic factors of cerebral venous sinus thrombosis in a prospective series of 59 patients. For the Cerebral Venous Sinus Thrombosis Study Group. J Neurol Neurosurg Psychiatry. 2001;70(1):105‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Girot M, Ferro JM, Canhão P, et al. Predictors of outcome in patients with cerebral venous thrombosis and intracerebral hemorrhage. Stroke. 2007;38(2):337‐342. [DOI] [PubMed] [Google Scholar]
- 35. Sechi E, Markovic SN, McKeon A, et al. Neurologic autoimmunity and immune checkpoint inhibitors: autoantibody profiles and outcomes. Neurology. 2020;95(17):e2442‐e2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beck RW, Cleary PA. Optic neuritis treatment trial. One‐year follow‐up results. Arch Ophthalmol. 1993;111(6):773‐775. [DOI] [PubMed] [Google Scholar]
- 37. Optic Neuritis Study Group . Visual function 5 years after optic neuritis: experience of the Optic Neuritis Treatment Trial. Arch Ophthalmol. 1997;115(12):1545‐1552. [PubMed] [Google Scholar]
- 38. Optic Neuritis Study Group . Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow‐up. Arch Neurol. 2008;65(6):727‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cobo Calvo Á, Mañé Martínez MA, Alentorn‐Palau A, Bruna Escuer J, Romero Pinel L, Martínez‐Yélamos S. Idiopathic acute transverse myelitis: outcome and conversion to multiple sclerosis in a large series. BMC Neurol. 2013;13(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gajofatto A, Monaco S, Fiorini M, et al. Assessment of outcome predictors in first‐episode acute myelitis: a retrospective study of 53 cases. Arch Neurol. 2010;67(6):724‐730. [DOI] [PubMed] [Google Scholar]
- 41. Rotstein DL, Healy BC, Malik MT, Chitnis T, Weiner HL. Evaluation of No evidence of disease activity in a 7‐year longitudinal multiple sclerosis cohort. JAMA Neurol. 2015;72(2):152‐158. [DOI] [PubMed] [Google Scholar]
- 42. Jarius S, Lechner C, Wendel EM, et al. Cerebrospinal fluid findings in patients with myelin oligodendrocyte glycoprotein (MOG) antibodies. Part 2: Results from 108 lumbar punctures in 80 pediatric patients. J Neuroinflammation. 2020;17(1):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dale RC, de Sousa C, Chong WK, Cox TC, Harding B, Neville BG. Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain. 2000;123(Pt 12):2407‐2422. [DOI] [PubMed] [Google Scholar]
- 44. von Glehn F, Farias AS, de Oliveira ACP, et al. Disappearance of cerebrospinal fluid oligoclonal bands after natalizumab treatment of multiple sclerosis patients. Mult Scler J. 2011;18(7):1038‐1041. [DOI] [PubMed] [Google Scholar]
- 45. Plasma Exchange/Sandoglobulin Guillain‐Barré Syndrome Trial Group . Randomised trial of plasma exchange, Intravenous immunoglobulin, and combined treatments in Guillain–Barré syndrome. Lancet. 1997;349(9047):225‐230. [PubMed] [Google Scholar]
- 46. Engström M, Berg T, Stjernquist‐Desatnik A, et al. Prednisolone and valaciclovir in Bell's palsy: a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet Neurol. 2008;7(11):993‐1000. [DOI] [PubMed] [Google Scholar]
- 47. Segurado OG, Krüger H, Mertens HG. Clinical significance of serum and CSF findings in the Guillain–Barré syndrome and related disorders. J Neurol. 1986;233(4):202‐208. [DOI] [PubMed] [Google Scholar]
- 48. Di Filippo M, Cordioli C, Malucchi S, et al. mRNA COVID‐19 vaccines do not increase the short‐term risk of clinical relapses in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2022;93(4):448‐450. [DOI] [PubMed] [Google Scholar]
- 49. Shapiro Ben David S, Potasman I, Rahamim‐Cohen D. Rate of recurrent Guillain–Barré syndrome after mRNA COVID‐19 vaccine BNT162b2. JAMA. Neurology. 2021;78(11):1409‐1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. König M, Torgauten HM, Tran TT, et al. Immunogenicity and safety of a third SARS‐CoV‐2 vaccine dose in patients with multiple sclerosis and weak immune response after COVID‐19 vaccination. JAMA Neurol. 2022;79(3):307‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Farina A, Falso S, Cornacchini S, et al. Safety and tolerability of SARS‐Cov‐2 vaccination in patients with myasthenia gravis: a multicenter experience. Eur J Neurol. 2022;29(8):2505‐2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee A, Wong SY, Chai LYA, et al. Efficacy of COVID‐19 vaccines in immunocompromised patients: systematic review and meta‐analysis. BMJ. 2022;376:e068632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tortorella C, Aiello A, Gasperini C, et al. Humoral‐ and T‐cell‐specific immune responses to SARS‐CoV‐2 mRNA vaccination in patients with MS using different disease‐modifying therapies. Neurology. 2022;98(5):e541‐e554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be shared upon request by qualified researchers.


