Abstract
Colloidal contaminants and pathogens are widely distributed in soil, whose tiny sizes and distinct surface properties render unique environmental behaviours. Because of aging, colloids can undergo dramatic changes in their physicochemical properties once in the soil environment, thus leading to diverse or even unpredictable environmental behaviour and fate. Herein, we provide a state‐of‐art review of colloid aging mechanisms and characteristics and implications for risk mitigation. First, we review aging‐induced formation of colloidal contaminants and aging‐associated changes. We place a special focus on emerging nanoplastic (NP) contaminants and associated physical, chemical, and biological aging processes in soil environments. Second, we assess aging and survival features of colloidal pathogens, especially viruses. Viruses in soils may survive from several days to months, or even several years in groundwater, depending on their rates of inactivation and the reversibility of attachment. Furthermore, we identify implications for risk mitigation based on aging mechanisms. Hotspots of (photo)chemical aging of NPs, including plastic gauzes at construction sites and randomly discarded plastic waste in rural areas, are identified as area requiring greater research attention. For COVID‐19, we suggest taking greater care in regions where viruses are persist for long periods, such as cold climate regions. Soil amendment with quicklime (CaO) may act as an effective means for pathogen disinfection. Future risk mitigation of colloidal contaminants and pathogens relies on a better understanding of aging mechanisms and more sophisticated models accurately depicting processes in real soil environments.
Keywords: colloid, inactivation, risk, soil contamination, surface properties, weathering
1. INTRODUCTION
The colloidal phenomenon has been known since the 19th century (Graham, 1861). The rapid oscillation of the plant pollen of Clarkia pulchella in water (i.e., Brownian motion) and the visible route generated when light beams cross smoke (i.e., Tyndall effect) are both because of the colloidal nature of the dispersion system—a dispersing medium with tiny insoluble particles is the distinct feature for both cases. More specifically, the modern definition of colloid is given by the International Union of Pure and Applied Chemistry (IUPAC), which describes a colloid as a system where particles with “at least in one direction a dimension roughly between 1 nm and 1 μm” are dispersed in a medium (Jones et al., 2009). However, the concept is often extrapolated to particles larger than 1 μm (for instance, bacteria) (Steenhuis et al., 2011; Vissers et al., 2018).
Although the colloidal phenomenon was originally used to describe a chemical state of a suspension, this concept has been widely extrapolated, and adopted in soil studies. For instance, colloid‐facilitated transport describes the natural process where contaminants such as heavy metals and organic compounds adsorb to, and co‐migrate with, detached soil particles in porous media (de Jonge et al., 2004; Zhang et al., 2017; Zou & Zheng, 2013). The term “colloidal contaminant” refers to a particulate pollutant (e.g., TiO2, fullerene), including nanoplastic (NP), whose size falls within the nanometre range, which forms a colloidal suspension in the pore water (Khan & Şengül, 2016; Reynaud et al., 2022; Williams et al., 2020). What distinguishes a colloidal contaminant or pathogen is the fact that it has the potential to migrate rapidly in environmental compartments, causing severe human health and ecological risks (Flury & Aramrak, 2017; Liu et al., 2021; Molnar et al., 2015). Their tiny sizes result in high specific surface areas, which enables them to act as vehicles for adsorbed contaminants (Cortés‐Arriagada, 2021; J. Liu et al., 2019) and induces toxicity to organisms (Sasidharan et al., 2018; Sun et al., 2020).
Dynamic surface properties (such as abundant oxygen‐containing functional groups, the presence of eco‐corona, etc.) impacts on their fate (Fadare et al., 2020; Liu et al., 2022; Schultz et al., 2021). Because of their colloidal nature, the stability of these contaminants in aqueous suspension was believed to be the most critical factor controlling their environmental behaviours. Aggregation, therefore, has been extensively investigated and reviewed (Gerba & Betancourt, 2017; Mao et al., 2020). However, aging of colloidal contaminants can change their physiochemical properties with time, leading to more dynamic and unpredictable fates.
For instance, mechanical abrasion increases their surface roughness and alters porous structure, thus changing their interactions with solutes in pore water (Wang, Wu, et al., 2021; Yang et al., 2021); surface oxidation increases their hydrophilicity, thus changing the total interaction energy with the soil matrix (Gao et al., 2022; J. Liu et al., 2019); microbial colonization changes the microbial community structure, altering the mineralization process of these tiny contaminants (Amaral‐Zettler et al., 2020; C. Wang et al., 2022). In particular, when colloidal contaminants and pathogens are released into soil environments (including surface soil, the vadose zone, and groundwater), the heterogeneity and complexity of various physical, chemical, and biological processes that occur in the matrix will significantly alter the colloids (Alimi et al., 2021; Wang et al., 2020), making it much more difficult to predict their fate in soil when compared with freshwater systems.
This review, therefore, investigates the aging phenomenon of colloidal contaminants and pathogens in soil. Herein, unsaturated and saturated zones are collectively referred to as the soil environment (Al‐Kaisi & Lowery, 2017; Briaud, 2013), as an understanding of both zones is needed for risk mitigation. Note that several studies relied on quartz sand as a proxy for soil porous media, whose results are also included and discussed. Based on the IUPAC definition of a colloid, NPs and viruses were selected as representative of colloidal contaminants and pathogens, respectively. We also mention aging mechanisms of other colloids with similar properties, including carbonaceous nanomaterials and natural inorganic nanoparticles to reach a better understanding of their environmental behaviours. Following discussions on the aging processes, special focus is paid to the implications for NP and COVID‐19 risk mitigation. We report that aging can form colloids, change colloids, and damage colloids. It is suggested that aging features may not be a coincidence. Instead, they can be described (semi‐)quantitatively. Implications for risk mitigation and future research directions are also put forward.
2. COLLOIDAL CONTAMINANT (TRANS)FORMATION—THE KEY ROLE OF PHYSICAL, CHEMICAL AND BIOLOGICAL AGING
2.1. Aging leads to colloidal contaminant formation
It has long been acknowledged that in a natural porous media without human disturbance, soil components, such as clay minerals and metal oxides are released from the solid matrix, forming natural colloids in pore water (Figure 1). It can be modelled in a quantitative manner, in which a first‐order kinetic model describes the process (Equation 1) (Grolimund et al., 2001; Grolimund & Borkovec, 2006):
| (1) |
where q refers to the particle concentration on the solid surface and k rel is the release or detachment rate coefficient. When a certain particle is attached to the solid surface then the value of k rel depends on the energy barrier to detachment, E (Equation 2) (Grolimund & Borkovec, 1999):
| (2) |
where k rel,0 represents a “fast release rate coefficient”, RT refers to the thermal energy in molecular units. Interaction energy calculations can be used to estimate the value of E as the sum of the energy barrier height and the depth (magnitude) of the energy well minimum (Bradford & Torkzaban, 2015; Torkzaban & Bradford, 2016).
FIGURE 1.

Various ways in which colloid enter or form in natural porous media. (a) Engineered nanoparticles released to soil directly. (b) Wastewater irrigation, sludge application and droplets result in viruses entering the soil, which will either attach to the soil matrix until inactivation, or transport as a biocolloid in the liquid phase. (c) Secondary NPs formed via the fragmentation of MPs. (d) Minerals and soil organic matter (SOM) released from soil aggregates, thus forming natural colloids that can facilitate contaminant transport in porous media.
However, it was not until recent years that the direct release of colloidal nanoparticles to the soil environment (including the vadose and saturated zones) raised increasing concerns (Figure 1). The emergence of engineered nanoparticles, such as elemental silver nanoparticle [Ag(0)] (Li et al., 2019), gold nanoparticle [Au(0)] (McGivney et al., 2019), titanium dioxide nanoparticle (TiO2) (Pradas Del Real et al., 2018), nanoscale zero‐valent iron (nZVI) (Fajardo et al., 2015), and fullerene (e.g., C60) (Avanasi et al., 2014) have been found in porous media. Notably, primary NPs as intentionally‐manufactured products can be released to the soil environment (Domínguez‐Jaimes et al., 2021). These primary colloids act as toxic contaminants themselves, and carriers for other soil contaminants such as heavy metals and organic contaminants (Figure 1).
Compared with primary colloidal nanoparticles and primary NPs, most NP colloids (i.e., secondary NPs) are formed via the aging of larger plastic pieces (Figure 1). The aging‐induced formation mechanisms of NPs are summarized in the following sections.
2.1.1. Physical fragmentation
Physical fragmentation is an important, yet often overlooked aging mechanism that involves the breaking down of macroplastics and microplastics (MPs) into smaller plastic pieces (Table S1). Evidence from artificial aging approaches in the lab confirms the critical role of mechanical abrasion in NP formation. Experiments involving mixing polystyrene (PS) macroplastics with water in a blender for 5 min resulted in rapid formation of PS–NPs (125 nm diameter). However, in natural conditions, physical aging will not be so intense. Mild mixing is, therefore, needed to simulate fragmentation process. Wet grinding of 106 μm microplastic pieces of polybutyrate adipate‐co‐terephthalate (PBAT) and low‐density polyethylene (LDPE) for 24 h resulted in the formation of NPs (<400 nm) (Astner et al., 2019). It's noteworthy that this fragmentation process did not significantly change the physicochemical properties such as thermal stability and crystallinity, suggesting mechanical abrasion did not result in chemical transformation. Similarly, El Hadri et al. (2020) also used a wet grinding process to produce NPs (below 500 nm) from secondary MPs that were collected from a beach environment.
By modelling the fragmentation process, it was found that the fragmentation phenomenon is controlled by conditional probability (Equation 3) (Wang, Li, et al., 2021):
| (3) |
where X represents the plastic size (mm), a and b are two parameters (a: mm−b−1, b: dimensionless). The equation indicates that the probability of plastics (with original diameter over x) that would be fragmented within x and x + Δx is ax b , which is dependent on the size (Wang, Li, et al., 2021). The size distribution of MPs separated from different land use types, including residential areas, farmlands, roadside soils, forests, and parks followed this rule well, suggesting that historical fragmentation may have contributed to the current presence of plastics in soil. However, to what extent this model can be applied to smaller‐sized NPs remains to be explored.
2.1.2. (Photo)chemical transformation
Photochemical transformation is a potent force leading to plastic downsizing (Table S1). Out‐door exposure to sunlight irradiation of expanded polystyrene (EPS) for 2 years led to the formation of 3.4 × 107–5.7 × 108 NPs cm−2, which was approximately 1 magnitude higher than that for MPs (Song et al., 2020). Soaking different polymers in water with UV irradiation for 112 days also resulted in formation of NPs, whose downsizing ability is highly dependent on the polymer type, i.e., polylactic acid (PLA) > polyethylene terephthalate (PET) (Lambert & Wagner, 2016). Formation of reactive oxygen species (ROS) is the main process in photochemical transformation of polymers. ROS will induce the chain scission process of plastics, leading to downsizing into NPs (Figure 2) (Gewert et al., 2015; Y. Liu et al., 2019). Its evident for polymers with a C‐C backbone [e.g., PS, PE, and polypropylene (PP)] that initiation, propagation and termination downsizes the plastics (Figure 2). At the initiation stage, the main polymer chain is broken by light to generate a free polymer radical. After that oxygen reacts with the as‐formed polymer radical to generate a peroxy radical. Autoxidation process happens apart from the formation of hydroperoxides. During this propagation process, chain scission takes place. Finally, termination occurs when two radicals are combined, thus forming inert products (Gewert et al., 2015). For polymers with heteroatoms in the main chain [e.g., PET, polyurethane (PU)], hydrolysis also played a vital role assisting in photochemical transformation (Gewert et al., 2015). It is of note that a majority of previous works have been done in the aqueous solution. In real soil environments, the exact concentrations of ROS may not be as high as those generated in the lab. The concentration of soil oxidants generated via photosensitization of soil organic matter (SOM) and metal oxides, such as superoxide (O2·−) and hydrogen peroxide (H2O2) are typically extremely low (i.e., within the nmol g−1 range) (Georgiou et al., 2015). To what extent these ROS species in soil break plastics into NPs remains to be explored.
FIGURE 2.

Abiotic degradation pathways for PE (R = H), PP (R = CH3) and PS (R = aromatic ring); after initiation by photolytic cleavage of a C–H bond on the polymer backbone (P = polymer backbone). Reproduced with permission from Gewert et al. (2015) under CC‐BY 3.0 licence.
In comparison, current evidences suggest that chemical oxidation alone may not be able to break MPs into NPs, at least in a shorter term. As a representative of chemical oxidation process, Fenton oxidation even has been extensively used for plastic separation from solid media, although it does generate ROS (Hurley et al., 2018; Vermeiren et al., 2020). In the soil environment, however, the chemical oxidation of macroplastics and MPs are largely unknown.
2.1.3. Biological aging
Soil fauna contribute potently to NP formation. Earthworms turned PE‐MPs into NPs effectively in 21 days via ingestion and cast excretion, while simultaneously suffering from damages to reproductive organs (Kwak & An, 2021). Gut microbiome may have played a vital role, as confirmed by Huerta Lwanga et al. (2018) through extracting microorganisms from the gut, and using them for PE fragmentation in vitro. It was found that Gram‐positive bacteria belonging to Actinobacteria and Firmicutes phyla contributed to biological fragmentation. But biological fragmentation of conventional non‐biodegradable polymers will not directly lead to degradation. For biodegradable polymers such as PBAT and PLA, earthworms assist in their microbial degradation in unique ways, that is, creating a suitable habitat for the proliferation of microorganisms (Figure 3) (Sanchez‐Hernandez et al., 2020). This is accomplished via vermicomposting, during which organic matter is transformed into vermicompost with the help of gut microbiome. Certain species of the genera Streptomyces, Paecilomyces, Trichoderma, and Paenibacillus, which are abundant in vermicompost, were found to facilitate biodegradation of these polymers simultaneously (Sanchez‐Hernandez et al., 2020).
FIGURE 3.

Pictorial representation of the earthworm impact on the environmental fate of mesoplastics and microplastics, and potential microhabitats for polymer biodegradation. Reproduced with permission from Sanchez‐Hernandez et al. (2020). Copyright 2020 American Chemical Society.
In general, earthworms may affect the fate of plastic debris via different ways. Firstly, the middens generated on the surficial soil enhance plastic fragmentation and degradation. Secondly, the burrow walls contain a high content of organic matter, acting as a hotspot for MP degradation. Thirdly, earthworm cast consists of gut microbiome for biodegradation. Furthermore, biodegradable MPs can be directly depolymerized in the gut (Figure 3) (Sanchez‐Hernandez et al., 2020). The role of soil microorganisms in MPs fragmentation into NPs has been reported and reviewed thoroughly. For detailed discussion on the microorganism‐induced fragmentation of plastics, readers are referred to Matjašič et al. (2021) and K. Zhang et al. (2021).
2.2. Aging‐induced transformation in as‐formed colloids
Once formed in porous media, colloidal contaminants suffer from progressive aging. On the one hand, abiotic aging forces, such as freeze–thaw cycling and UV irradiation attack colloids, leading to physicochemical changes (Figure 4, Table S2). On the other hand, coating with soil humic substances and microbial extracellular polymeric substances in turn protect the colloids via the formation of eco‐corona. The following sections will critically assess aging‐induced changes in colloids, including NPs and other colloidal contaminants.
FIGURE 4.

Aging‐induced changes in hydrodynamic diameter and ζ‐potential of NPs. (a) Freeze–thaw aging leads to aggregation because of the increased concentration of NPs and soluble salts in still‐unfrozen water. Sometimes formation of eco‐corona reversed the ζ‐potential of NPs, but in most cases, natural organic matter was not that powerful in turning the ζ‐potential. In comparison, both UV irradiation and ozone oxidation enhanced the stability of NP colloids, while simultaneously decreasing the hydrodynamic diameter via the peeling effect. Data sources: freeze–thaw (Alimi et al., 2021), ozone oxidation (J. Liu et al., 2019), UV irradiation (J. Liu et al., 2019; Y. Liu et al., 2019; Y. Xu et al., 2021), eco‐corona formation (Fadare et al., 2020; Giri & Mukherjee, 2021; Natarajan et al., 2020; Saavedra et al., 2019; Song et al., 2019). (b) The key role of solution ionic strength. The higher the ionic strength, the lower the absolute value of ζ‐potential (because of double layer compression), therefore, the higher the hydrodynamic diameter. Data retrieved from J. Liu et al. (2019). Detailed information regarding aging treatments, and aging‐induced changes are provided in Table S2.
2.2.1. Weathering
Recently, evidence has emerged showing that colloidal NPs are susceptible to physical aging in porous media. Freeze–thaw aging of PS‐NPs decreased the stability of the colloidal suspension, leading to colloid aggregation (Figure 4). This phenomenon was attributed to the solute rejection mechanism, where ice rejects incorporation of insoluble nanoparticles during freezing, resulting in elevated concentrations of inorganic ions and NPs in still‐unfrozen water (Alimi et al., 2021). Enhanced aggregation because of freeze–thaw aging caused greater retention of NPs during migration in the quartz sand media.
Although our understanding on physical aging process of NPs in the terrestrial environment are still lacking, wet‐dry and freeze–thaw‐induced changes from other nanoparticle colloids, including natural or artificial ones, may provide us with fresh insights. Historical wet‐dry cycling events in the Chinese Loess Plateau prevented nanomagnetite (a mixed Fe2+/Fe3+ ferrimagnet) from oxidation, thus leading to enrichment of this magnetic soil mineral in the interglacial/interstadial‐stage fossil soil layer formed 3 million years ago (namely, paleosols) (Ahmed & Maher, 2018). Historical monsoon climate in this region was believed to have caused this phenomenon. In wet summer, a sharp depletion of dissolved oxygen content prevented it from oxidation, whereas in dry winter, an elevation in soil pH also retarded magnetite oxidation (Figure S1). It is interesting that wet‐dry cycle as a physical force would induce redox and pH changes, thus slowing down colloid oxidation in soil. Another study by Ermolin et al. (2019) found that wet‐dry cycling favoured the formation of water‐stable soil aggregates that can immobilize colloids, thus decreasing the mobility of CeO2 and ZnO nanoparticles. As for freeze–thaw cycling, similar observations that this aging force enhanced aggregation and slowed down the transport of colloids in quartz sand have been made for TiO2 nanoparticles because of the same reason mentioned for NPs (Farner et al., 2020). When it comes to the natural soil, however, release of clay colloids will be significant when compared with the modelled quartz sand. In this case, clay colloids released during freeze–thaw cycling would in turn facilitate the transport of metal oxide nanoparticles (G. Xu et al., 2021).
Photochemical transformation causes intense oxidation and morphological changes in as‐formed NPs. Higher surface oxygen content (49.4% vs. 4.2% as confirmed by XPS analysis) together with much rougher morphology were observed for PS‐NPs suffering from mercury lamp (500 W) irradiation for 12 h, suggesting that UV is a powerful aging force for this kind of NP (J. Liu et al., 2019). Oxygen‐containing functional groups (e.g., carboxyl) formed during photochemical transformation may have played a vital role in aggregation performances. For example, aggregation in monovalent NaCl environment was inhibited (indicating higher mobility) because of enhanced electrostatic repulsion, whereas in divalent CaCl2 environment it was promoted (indicating lower mobility) because of the bridging effect between Ca2+ and carboxyl (Y. Liu et al., 2019).
It appears that UV‐induced aging is limited to the surface of NPs. Tian et al. (2019) also noticed a similar phenomenon, that XPS (provided information within the surface layer, several nanometres) suggested the occurrence of surface oxidation, whereas FTIR spectra did not show any significant oxidation in bulk moieties. Simultaneous analysis of water‐soluble products during UV irradiation suggested that small molecules possessing condensed aromatic moieties, with side‐chains containing carbonyl or hydroxyl, could be continuously released from the PS‐NP to the aqueous phase. In other words, UV irradiation “peeled out” small molecules from the surface of NPs (also leading to smaller hydrodynamic diameters as shown in Figure 4) (Tian et al., 2019). Evidence has also shown that there exists a linear relationship between second‐order reaction kinetic constant of UV‐induced photodegradation (k deg) and the square of the PS‐NP diameter (d 2) (Bianco et al., 2020). It can be explained by the fact that hydroxyl radical (·OH) generated during UV irradiation reacts with the surface sites of PS‐NPs (more specifically, the aromatic moieties), rather than the inner polymer bulk (Bianco et al., 2020).
UV‐induced changes are much more complicated for other colloidal contaminants, though. Several types of engineered nanoparticles are even designed to be UV‐sensitive photocatalysts (Ahluwalia et al., 2016; Xu et al., 2019). For those colloids which are out designed for photocatalysis, similar results have been observed. For instance, C60 stability after UV irradiation was also higher in NaCl but lower in CaCl2 for the same mechanisms discussed above (Qu et al., 2010).
2.2.2. Eco‐corona formation
The interactions between colloidal contaminant and organic matter are critical in determining the fate and toxicity of nanoparticles. Sorption of dissolved organic matter to nanoparticle surface results in formation of a coating, namely, eco‐corona (Lynch et al., 2014). In the soil environment, this process occurs in the pore water, which is composed of a mixture of dissolve organic carbon species, including humic substances, lipid components, amino acids and polysaccharides (Schultz et al., 2021). Although reasons why organic matter tends to accumulate on the surface of colloids may vary, there is one thing in common, that is, in order to form an corona, the overall free energy must decrease (according to the second law of thermodynamics) (Equation 4) (Lynch et al., 2014; Norde, 2011):
| (4) |
where Δ ads G is the net changes in Gibbs energy of adsorption, Δ ads H refers to the net changes in enthalpy, Δ ads S represents the net changes in entropy, and T is the absolute temperature. More specifically, four processes account for this change in Gibbs energy (Norde, 2011), which are as follows:
Charge redistribution: Typically, both natural organic matter and the nanoparticle possess a colloidal nature, suggesting that they are electrostatically charged with double layer. During the sorption process, interactions between electrical double layers are therefore inevitable, leading to charge redistribution.
Dispersion interaction (London‐van der Waals interaction): This kind of interaction is always attractive. When atoms approach, their electron obits would influence each other, thereby inducing a subtle dipole moment that causes electromagnetic attraction.
Dehydration: Many low free energy surfaces are hydrophobic, therefore, dehydration promotes adsorption of components from the aqueous solution. The dehydration process of apolar surfaces acts as a driving force for adsorption, increasing the entropy by water molecules released from interactions with hydrophobic side groups.
Conformational changes: Rearrangement of the structure decreases the ordered secondary structure and may either enhance or break intramolecular hydrogen bonding. For instance, soft protein that underwent this process would result in a conformational entropy gain, which may be large enough to make it sorb onto a polar, electrostatically repelling surface.
It should be noted that these criteria were originally proposed for protein corona formation on colloids. Considering the similarity between protein corona and eco‐corona formation (both of which describes a process where organic matter adsorbs onto the nanoparticle colloid), it is proposed that for eco‐corona formation on NPs, electrostatic and van der Waals interactions, and hydrophobic interactions also dominate this process, whose goal is to decrease the overall free energy of the NP‐organic matter system. However, there is currently no evidence whether NPs can interact with all types of natural organic matter and organic contaminants in the soil environment, which is an interesting topic that deserves further investigation.
Eco‐corona protects NPs from other aging forces, such as UV irradiation, by acting as a “filter” that can absorb photons in the UV range, thus diminishing the peeling effect as mentioned above (Natarajan et al., 2021). Corona formation will either enhance or reduce their NPs mobility depending on their susceptibility to several factors. The type of organic macromolecule determines whether NPs will be mobilized or settled after eco‐corona formation. Dissolved black carbon acted as a bridge between PS‐NPs, promoting their settlement via aggregation, whereas humic acid addition increased their mobility by inhibiting aggregation, whose causes remain to be explored (Y. Xu et al., 2021). The surface charge of NPs also matters (Figure 4). Much more significant changes in aggregation behaviour have been found in positively charged PS‐NPs after humic acid coating, when compared with negatively charged ones (Saavedra et al., 2019). It was attributed to the fact that negatively charged humic acid reversed the ζ‐potential of positively charged aminated PS‐NPs from +50 to −47 mV, thus leading to heteroaggregation and settlement (Figure 4). The as‐formed eco‐corona has strong implications for the risk mitigation of NPs (Table S3, Section 4.1).
3. COLLOIDAL PATHOGENS' RELEASE AND INACTIVATION IN SOIL
Nano‐sized viruses are abundant in soil, with reported numbers ranging from 2.2 × 103 virus g−1 in hot deserts to 5.8 × 109 virus g−1 in humid forest soils (Williamson et al., 2017). “Native” viruses, such as bacteriophages, infect soil bacteria and act as drivers of microbial community regulation and nutrient cycling (Kuzyakov & Mason‐Jones, 2018). Human pathogenic viruses mainly pollute soil as a result of application of contaminated organic matter (e.g., sewage sludge, animal waste) (Gessel et al., 2004; Horswell et al., 2010) or surface water (Parashar et al., 2011) (Figure 1). During the COVID‐19 pandemic, other potential contamination pathways of viruses have also drawn much attention. For instance, droplets from infected individuals may bring virus to the soil when infected individual coughs or sneezes (Figure 1) (Klompas et al., 2020; Li et al., 2020). Aerosols containing COVID‐19 may also result in virus deposition to the soil (D. Zhang et al., 2021). There is currently a heated debate whether human infection can occur from exposure to soil with pathogens (namely, indirect transmission) (Anand et al., 2021; WHO, 2020a).
Viruses are attached to the soil matrix through adsorption after entering the soil, suffering from inactivation in the long‐term (Table 1) (Armanious et al., 2016; Kuzyakov & Mason‐Jones, 2018). Inactivation occurs when the virus losses its ability to infect the host because of degradation of the viral genome or disruption of the capsid (protein coat surrounding the nucleic acid) (Gerba, 1984). An understanding of factors that influence inactivation of human pathogenic viruses is crucial for risk management in soils.
TABLE 1.
Survival of pathogenic viruses in the soil environment
| Virus | Environment | Longest survival time | Key factors affecting the survival | Reference |
|---|---|---|---|---|
| Somatic coliphages (as indicators of enteric viruses) | Manure‐applied soil | 130 days | The higher the manure application rate, the higher abundance the viruses would be introduced to the soil, rendering longer survival time till full inactivation | Gessel et al. (2004) |
| Adenovirus | Sewage sludge‐applied soil | Not mentioned | The amount of virus that can be leached out by artificial rain was much lower than that of bacteria, suggesting the strong adsorption (retention) of adenovirus by soil | Horswell et al. (2010) |
| Poliovirus 1 | Different natural soils | 75 days | Temperature: a low temperature of 4°C favoured virus survival. Oxygen content: under aerobic conditions soil bacteria may have contributed to virus inactivation. In contrast, anaerobic soil microorganisms did not affect virus survival | Hurst et al. (1980) |
| Hepatitis A virus (HAV) and hepatitis E virus (HEV) | Natural soil | 13 weeks for HAV at 37°C, 10 weeks for HEV at 37°C | Fluctuating temperature in the real environment shortened the survival time when compared with constant temperature incubation | Parashar et al. (2011) |
| Enteroviruses | Sewage sludge‐applied soil | 2 weeks | High activities of other microorganisms may have contributed to rapid inactivation of enteroviruses | Pourcher et al. (2007) |
| Model enterovirus BE‐1 | Septic tank‐affected groundwater | 2 months | Lower temperature rendered lower inactivation rate | Scandura and Sobsey (1997) |
| Adenovirus | Biosolids‐amended soil with wheat cultivation | Over 180 days | The higher survival time of adenovirus when compared with bacteria was possibly because of the protection effect induced by adsorption to soil solid matrix | Schwarz et al. (2014) |
| Norwalk virus | Groundwater | Over 1266 days | The long‐term persistence of virus RNA in groundwater, as well as virus aggregation may have contributed to long‐term survival and detection by RT‐qPCR | Seitz et al. (2011) |
| Highly Pathogenic Asian Avian Influenza A (subtype H5N1) | Topsoil | 13 days | Low temperature plus high humidity favoured virus survival | Wood et al. (2010) |
| Poliovirus 1 | Soil | Over 180 days | A moderate soil moisture (neither too high nor to low) favoured virus survival | Yeager and O'Brien (1979a) |
Existing literature frequently assumes that inactivation of pathogenic viruses in soil follows a first‐order process, which predicts an exponential decline in virus concentrations with time (Figure 5). Equation (5) describes the first‐order inactivation kinetics as:
| (5) |
where k (d −1) is the inactivation rate constant and N is the virus concentration per volume or gram (whose unit may vary, such as RNA/DNA copies per unit volume or gram, PFU per unit volume or gram, or TCID50 depending on the analysis methods used). Integration of Equation (5) yields the relationship between the initial number of viruses (N 0) and the number of viruses at a given time (N t ) as (Equation 6):
| (6) |
where t (d) is the time. This exponential expression is quite similar to Chick's law which has been extensively used to describe the inactivation of microorganisms during disinfection (Chick, 1908; Dalrymple et al., 2010; Yao et al., 2020). The differences between Chick's law and Equation (6) lie in the fact that virus inactivation in soil is a natural process, in which temperature, humidity, sunlight, and adsorption act as “disinfectants”. A higher rate constant k indicates that environmental conditions are harsher for virus survival.
FIGURE 5.

First‐order inactivation kinetics‐controlled survival features of different viruses in soil. (a) Effects of temperature on the survival of poliovirus 1, showing that a relatively low temperature favoured its survival with lower reaction rate constant (Hurst et al., 1980). (b) Effects of soil moisture on poliovirus 1 survival, suggesting a moderate water content being neither too low nor too high was favourable for virus survival in soil (Yeager & O'Brien, 1979a). (c) Manure application in different rates, namely, 50%, 100%, and 200% of the normal agronomic application rate of 37,000 L ha−1 resulted in different survival rates of the enteric virus indicator somatic coliphages (Gessel et al., 2004). (d) Low temperature plus high humidity favoured the survival of Highly Pathogenic Asian Avian Influenza A (subtype H5N1), whereas UV irradiation and temperature increase contributed to fact inactivation in soil (Wood et al., 2010). (e) Rate constants for different viruses in soil. Original data source: Somatic coliphage (Gessel et al., 2004), poliovirus 1 (Hurst et al., 1980; Yeager & O'Brien, 1979a), hepatitis A virus & hepatitis E virus (Parashar et al., 2011), enteroviruses (*total genome copies of all enteroviruses) (Pourcher et al., 2007), adenovirus (Schwarz et al., 2014), and H5N1 (**Highly Pathogenic Asian Avian Influenza A subtype) (Wood et al., 2010).
Under ideal conditions, pathogenic viruses may stay in the soil‐groundwater system for a long‐term (e.g., over 3 years) (Table 1), threatening human health via contaminating the water source. The rate constant for viruses in soil vary greatly from 0.03 d−1 for poliovirus 1 (in a natural sandy soil) (Hurst et al., 1980) to 6.8 d−1 (for Highly Pathogenic Asian Avian Influenza A subtype H5N1 in room temperature with low humidity) (Wood et al., 2010) (Figure 5). Virus inactivation can occur both in water and when attached to solid surfaces. However, different rates of inactivation have commonly been reported (John & Rose, 2005; Schijven & Hassanizadeh, 2000; Zhao et al., 2008). The inactivation rate has been reported to be higher for attached viruses (Ryan et al., 2002; Sasidharan et al., 2018), but the opposite result has also been observed (Liew & Gerba, 1980; Straub et al., 1993). These differences can be explained by the complex dependency of inactivation on a wide variety of physical, chemical, and biological conditions (Bradford et al., 2013). The long‐term aging features of viruses in soil are highly dependent on environmental factors and soil properties.
Temperature is one key factor determining the inactivation of soil viruses (John & Rose, 2005; Schijven & Hassanizadeh, 2000). Current findings suggest that a low temperature contributed to longer survival of viruses (Figure 5a,d). For instance, poliovirus 1 and coxsackievirus B1 cannot survive in soil during 12 days' incubation at 37°C; however, when temperature was 4°C both viruses were still detectable even after 180 days (Yeager & O'Brien, 1979a). Viral components that are required for host recognition and infection may experience more damaged at higher temperatures because of viral genome degradation, conformational changes in proteins (Harvey & Ryan, 2004), or strong activities of soil enzymes (e.g., proteinase) (Kimura et al., 2008). In addition, a fluctuating temperature was not favourable for the survival of viruses in soil, which was possibly because of lower adaptability of viruses in a changing environment (Parashar et al., 2011). The temperature dependence of inactivation can be accounted for by making k a function of temperature using the Arrhenius equation (Stumm & Morgan, 2012).
Harvey and Ryan (2004) proposed that virus inactivation may occur when they attach in an interaction energy minimum. This implies that virus inactivation increases with the strength of adhesion. This can explain the high rates of solid phase inactivation when negatively charged viruses strongly attach on the surfaces of positively charged metal oxides (Bradford et al., 2006; Ryan et al., 2002) or when solution chemistry conditions produce attachment in a strong primary minimum (e.g., higher ionic strength and lower pH) (Harvey & Ryan, 2004). Nanoscale roughness and charge heterogeneity are known to locally reduce or eliminate the energy barrier between like charged surfaces, and to alter the depth of the interaction minimum (Bradford et al., 2017). Furthermore, the adhesion strength can increase over time because of changes in the conformation of surface structures and creation of chemical bonds, and/or to change with the solution chemistry (Bradford et al., 2021; Sasidharan et al., 2017). This is because the total interaction energy between a colloid and a surface is dominated by joint effects of electric double layer, van der Waals, Born, and steric interaction energies (Bradford et al., 2021). Chemical binding and solution changes following aging will decrease the total interaction energies (Norde, 2011). Similar to the release rate coefficient, these factors are expected to create spatial and temporal variability in the solid phase inactivation rate coefficient. This may explain the release of viable viruses from metal oxides with rough surfaces (Murray & Laband, 1979; Ryan et al., 1999) and diminished inactivation rates in geologic media with high clay content and organic matter (Liew & Gerba, 1980; Straub et al., 1993). Note that adsorbed clays and organic matter alter the surface roughness properties and/or mask charge heterogeneity from metal oxides and may thereby decrease the strength of adhesion and solid phase inactivation (Liang et al., 2021; Ryan et al., 2002). Clay minerals and organic matter in soil also may protect viruses physically from aging forces such as enzyme attack or sunlight (Jin & Flury, 2002).
Soil water content also affects virus survival (Figure 5b). The rate constant for poliovirus in dry soils (moisture content 0.6%) was the highest (i.e., 1.8 d−1), while increasing the soil moisture decreased this constant. It is noteworthy that a moderate moisture content (i.e., 4.7%) was more favourable for virus survival than a high moisture content (i.e., 18%) (Figure 5b). During the drying process, irreversible binding between the virus capsid with the soil matrix occurred, leading to conformational changes. In comparison, in a very humid environment, the high activities of the RNAse accelerated the degradation of the viral RNA (Yeager & O'Brien, 1979b).
Biological factors that influence virus inactivation include the virus type (John & Rose, 2005; Schijven & Hassanizadeh, 2000), viral subpopulations that are more resistant to disinfectants (Chrysikopoulos & Vogler, 2004; Molin & Cvetkovic, 2010), the microbial community (Deng & Cliver, 1995), and the presence of biofilms (Von Borowski & Trentin, 2021). For example, a diverse microbial community thrives in nutrient‐rich environments with high organic matter contents (Bamdad et al., 2022; Nelson & Wear, 2014; Sofo et al., 2022; Z. Zhang et al., 2022). The presence of organic matter can therefore have an antagonistic effect on viruses because of the production of proteolytic enzymes, which degrade the viral genome (Deng & Cliver, 1995). This may explain why Schijven et al. (1999) found that inactivation of MS2 and PRD1 (bacteriophages) were increased by 34‐fold when native groundwater was used as the liquid medium compared with a saline solution containing peptone. Another contributing factor can be the presence of presence of dissolved metal ions that can greatly enhance virus inactivation (Sagripanti et al., 1993).
In these complex cases, separate virus inactivation rates are needed for liquid and solid phases, and virus inactivation is expected to exhibit spatial and temporal variability. Some of these challenges can be overcome using a distribution or multiple first‐order inactivation rates for different phases and environmental conditions (Schijven & Hassanizadeh, 2000) and/or time dependent inactivation coefficients (Chrysikopoulos & Vogler, 2004; Molin & Cvetkovic, 2010; Sim & Chrysikopoulos, 1996). However, these inactivation rate coefficients are expected to be functions of temperature, solution and solid chemistry, water content, nanoscale heterogeneities, clay content, organic matter, and microbial community dynamics. Many of these functional relationships still have not yet be experimentally determined or mathematically described.
4. IMPLICATIONS FOR RISK MITIGATION
Soil contamination is a “hidden reality” when compared with air and water pollution (FAO, 2018). Therefore, identification of hotspots for NP and COVID‐19 contamination is crucial for establishing risk mitigation measures so that accelerated mineralization or inactivation is achieved.
4.1. Nanoplastic
Because of the recalcitrant nature of plastic, it may take centuries for NPs made from conventional polymers to reach full mineralization by native microorganisms in soil (Chamas et al., 2020; SAPEA, 2020). The slow degradation rate, plus continuous input of plastic waste from various sources (including mulching films, dumping, wastewater, etc.) leads to their unidirectional accumulation in the terrestrial environment. Two strategies may slow down or reverse this trend. Firstly, a proper product design for a conventional polymer, along with a high recovery rate of used plastics from the soil environment directly decreases aging‐induced NP generation (Figure 6). For instance, poor selection and retrieval of a plastic mulching film may lead to significant levels of plastic residues left in the field. A recent report released by Food and Agriculture Organization of the United Nations (FAO) suggested that the probability of a PE mulching film to age and generate MPs/NPs (which will be retained in the soil) is directly related to its thickness. When this value increases from 10 to 25 μm, the proportion that can be recovered from the field increases sharply from 32% to 90% (FAO, 2021). Therefore, a thicker mulching film that is more resistant to field aging is recommended to realize a high recovery rate, which may also indicate fewer NPs entering soil. The Chinese government has also set the ambitious goal that the recovery rate of mulching films should reach 85% by 2025 (NDRC and MEE, 2021). A proper plastic product selection is critical to reach that goal.
FIGURE 6.
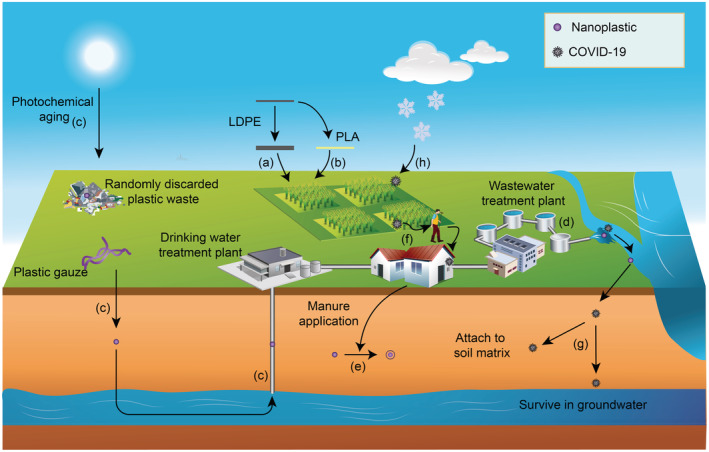
Implications of colloid aging in the soil environment for risk mitigation. (a) A conventional PE mulching film should be thicker to reach a higher retrieval rate, thus decreasing NP release during its soil application. (b) Biodegradable polymer may replace conventional polymer to accelerate mineralization in soil. (c) Photochemical aging of plastic gauze and randomly discarded plastic waste poses elevated risk of colloid migration that threatens both drinking water safety and food security. (d) Tertiary treatment process in a wastewater treatment plant may generate considerable amount of chemically‐aged NPs with high mobility. (e) Application of organic amendments reduces the risks via eco‐corona formation. (f) COVID‐19 in soil may cause indirect transmission. (g) COVID‐19 may survive for a long time in soil and groundwater. (h) A low temperature enables long‐term survival of COVID‐19 in soil.
Secondly, replacing conventional polymers (e.g., PE, PP) by a biodegradable one (e.g., PLA, PBAT) accelerates the microbial degradation process to several months or years in soil (Figure 6). Note that biodegradability is a function of both polymer characteristics and the biochemical nature of the end‐of‐life system (i.e., soil) (Law & Narayan, 2021). The term “biodegradable” also includes those polymers that are “compostable”, but cannot be degraded rapidly in the open environment. The most widely used standards to judge whether a polymer is “biodegradable” are ASTM D6400 and EN 17033. The former uses composting, whereas the latter adopts soil incorporation to assess a polymers' biodegradability (Hayes, 2021). To select a suitable product for soil applications, it is crucial that a proper method be used. Besides, several existing studies have already noticed that a biodegradable polymer can also generate a considerable number of NPs during aging, which may also pose ecological risks (Haider et al., 2019; Qin et al., 2021). It is crucial that the speed of NP generation should not exceed that of biodegradation in soil, so that NPs derived from a biodegradable polymer will not accumulate with time.
(Photo)chemical oxidation is a potent aging force, which will not only result in considerable amount of NP generation but also mobilize as‐formed NPs, whose higher mobility are ascribed to increased hydrophilicity and surface charge negativity (i.e., enhanced electrostatic repulsion with negatively charged soil matrix) (Figure 4). Elevated risks of NPs following UV irradiation or chemical oxidation should not be neglected. For instance, plastic gauzes are extensively used on construction sites or barren lands in China to prevent soil dust, which are usually left in soil for a long time without being retrieved (Mo et al., 2021). Randomly discarded plastic waste in rural areas also face a similar dilemma (Figure 6) (Zeng et al., 2015). Long‐term photochemical aging of these poorly managed plastic debris generates NPs that are highly mobile, which can migrate vertically to groundwater or horizontally to the rhizosphere, posing threat to both drinking water and food safety. Another hotspot for NP oxidation is the tertiary treatment process in a wastewater treatment plant (WWTP) (Figure 6). Advanced oxidation processes (AOPs) (e.g., UV, ozone, Fenton oxidation) are often applied to purify wastewater passing through the biological treatment tank (namely, secondary treatment) (Bixio et al., 2005; Rout et al., 2021). Although some scholars proposed that AOP should theoretically mineralize NPs with the aid of reactive oxygen species (ROS), current evidence suggested that unwanted surface oxidation of plastic particles occurred instead of full mineralization (Ali et al., 2021; Kim et al., 2022). Therefore, elevated risks are expected when those oxidized NPs enter the soil environment via either irrigation or biosolid application. Worse still, chemically weathered NPs exhibit stronger adsorption capacity towards other soil contaminants (such as heavy metals and herbicides) (Davranche et al., 2019; Xiong et al., 2020). In this context, aged NP serves as a vehicle for other contaminants, exhibiting more significant co‐transport in soil when compared with fresh NP.
By contrast, aging with soil organic matter (SOM) tends to decrease the risks of NPs. Eco‐corona can form in soils rich in native organic matter (e.g., humic substances) (Fadare et al., 2020). As‐formed organic coating immobilizes NP in soil pore water, protects NP from progressive UV aging, and reduces the ecotoxicity to organisms (Natarajan et al., 2021; Y. Xu et al., 2021). Application of organic amendments serves as a promising strategy for risk mitigation of NPs with multiple processes and mechanisms. First of all, organic amendments (e.g., compost, manure and biochar) applied to the soil increase the SOM pool (Abagandura et al., 2022; Armolaitis et al., 2022; de Figueiredo et al., 2021; Dong et al., 2022), which may reduce NP mobility via enhanced eco‐corona formation (Figure 6). Secondly, these amendments directly immobilize NP via heteroaggregation or adsorption (Abdoul Magid et al., 2021; Ayaz et al., 2022; Tong et al., 2020; L. Wang et al., 2022). Besides, it is widely accepted that fresh organic amendments will stimulate the activities of soil microorganisms, leading to an elevated mineralization rate of aged SOM (also known as the positive priming effect) (Cordova et al., 2022; Fontaine et al., 2003; Jiang et al., 2022; Kuzyakov et al., 2000; Y. Zhang et al., 2022). A wise use of this priming effect can therefore be proposed, that addition of organic amendments may accelerate the mineralization of biodegradable NPs in soil (in this case, biodegradable NPs can be regarded as the “aged” SOM pool, since they enter the soil environment prior to organic amendments).
4.2. COVID‐19
COVID‐19 may enter the soil environment via different ways, including wastewater, sludge application, droplet or aerosol deposition (Núñez‐Delgado, 2020; WHO, 2020b; D. Zhang et al., 2021). The risks of COVID‐19 in soil is associated with its possible transmission route from soil to human beings, that is, indirect transmission through contact with a fomite (i.e., the contaminated soil) (Figure 6) (WHO, 2020a). It is still unclear whether fomite transmission from soil to humans is viable for COVID‐19, but solid evidence shows that fomite transmission does occur for COVID‐19 on other surfaces (Xie et al., 2020; Yuan et al., 2020). Our current knowledge on the aging and survival of COVID‐19 and other human and animal coronaviruses suggest that non‐porous surfaces lead to quick inactivation, whereas surfaces with well‐developed porous structures favour their survival (Aboubakr et al., 2020).
Typically, the survival time of COVID‐19 on different surfaces range from several hours to days (Marquès & Domingo, 2021; Van Doremalen et al., 2020). Given the high porosity of the soil particle matrix, it is hypothesized that COVID‐19 may also survive for a long time in soil when compared with smooth surfaces. However, there is currently no direct evidence on how long COVID‐19 survives in the soil environment. Based on the current knowledge, it is speculated that as an envelope virus, COVID‐19 may be more susceptible to death in the soil environment when compared with non‐envelope viruses, such as the Norwalk virus (Mancuso et al., 2021; Vasickova et al., 2010).
In particular, extra care must be taken for certain circumstances that favour the survival of COVID‐19 in soil. For instance, a lower inactivation rate occurs at low temperatures (Section 3) (Figure 6). In this context, high latitude regions may serve as long‐term reservoirs of COVID‐19. When thawing occurs it is likely that release of still‐active viruses will pose a threat to humans. A recent study reported that temperature rise in polar regions may have contributed to accelerated COVID‐19 spreading over the world (Hofmeister et al., 2021). It was hypothesized by Hofmeister et al. (2021) that hot Arctic summer may release large amount of both ancient viruses and COVID‐19 during extensive permafrost melting, which would be incorporated in polar air circulation in autumn, during which low temperature and limit sunlight enables viral survival (Hofmeister et al., 2021). Wet deposition occurs when the North Polar Jet stream meets warmer air and this releases infectious viruses to the terrestrial environment (Hofmeister et al., 2021).
More stringent monitoring of sludge quality for soil amendment purposes in winter is recommended – evidence is mounting that COVID‐19 RNA is present in sewage sludge from WWTPs (Balboa et al., 2021; Bogler et al., 2020). Another possible route of COVID‐19 soil contamination should also be assessed, that is, irrigation with wastewater. Risks associated with the use of untreated or partially treated wastewater for irrigation purposes ought to be carefully evaluated, especially in developing countries where this is a common practice (e.g., countries in Southeast Asia and Middle East) (Siddiqui et al., 2020).
For soil disinfection purposes a “typical” disinfectant for surfaces, such as quaternary ammonium, phenolic, and tetraacetyl ethylenediamine in List N of United States Environmental Protection Agency (US EPA) (2021) may not be a suitable candidate because most disinfectants themselves are soil contaminants that can irreversibly damage soil health (Baveye, 2021; Lonigro et al., 2017; Pateiro‐Moure et al., 2013). In this context, selecting a suitable soil amendment which can successfully kill viruses while maintaining soil functions is crucial (Wang, Rinklebe, et al., 2021). Elevating soil temperature, increasing soil pH, and decreasing water content are all feasible means to inactivate soil viruses. Traditional soil amendments (e.g., red mud, zeolite, phosphate rock, lime) that are originally applied to improve nutrient availability or immobilize heavy metals (Arrobas et al., 2022; Arwenyo et al., 2022; Battisti et al., 2022; Christensen et al., 2022; Doni et al., 2021; Yang et al., 2022) may disinfect COVID‐19 successfully, since all of them elevate soil pH so that capsid (protein) can be destroyed. Among these amendments, liming with CaO may serve as a promising strategy for soil disinfection, since the aforementioned three disinfection mechanisms can be achieved simultaneously. Results indicate that applying CaO to compost can effectively kill MS2 coliphage by capsid damage plus RNA exteriorization (Hijikata et al., 2016).
The European Lime Association (EuLA) suggests that using CaO can effectively prevent or disinfect avian influenza in litter or manure in animal houses, with suggested application rate being 10 and 100 kg m−3 for prevention and treatment purposes, respectively (EuLA, 2009). For soil disinfection purposes, the optimum application rate is 0.5 kg m2, with water spraying following CaO application to soil (EuLA, 2009). The US EPA also recommends the application of CaO to disinfect sewage sludge, which requires an elevation of pH to 12, and maintenance for 2 h (US EPA, 2003). Possible application of liming materials for the large‐scale application of COVID‐19‐containing soils to reduce the risks of indirect transmission deserves further investigations.
5. FUTURE RESEARCH DIRECTIONS
Mechanisms of colloid aging in soil deserve further investigation. Our current understanding of colloid aging was mainly extrapolated from the aquatic environment. For instance, most studies that explored NP aging were conducted by exposing NP suspension to UV irradiation. Existing studies on eco‐corona formation were also mainly conducted to test whether organic matter in water bodies change the aggregation behaviour and NP toxicity to aquatic organisms. In the soil environment, however, the crucial role of soil minerals and organic matter compositions cannot be neglected. Different texture and taxonomy, distinct soil pore water compositions, and unique microbial communities for different soils will also make aging processes much more complicated. It is suggested that different soil types be selected (for instance, clay vs. sand in texture, Oxisol vs. Aridisol in taxonomy) to explore how soil properties change the aging patterns of colloids. Besides, characterization methods of colloids should go deeper to the molecular level so that an in‐depth aging mechanism can be explored. In this way a more precise assessment of risks during aging can be made in a compound‐specific manner.
Risks associated with colloid aging can, therefore, be modelled after reaching a comprehensive understanding of aging mechanisms in soil. Parameters directly associated with aging, such as hydrodynamic diameter/zeta potential change, and inactivation rate constant, should be obtained with reliable experimental data. Again, parameters depicting soil properties must also be taken into account, such as soil pH, Eh, total organic matter content, dissolved organic matter content, ionic strength of the pore water, etc. Established theory describing the environmental behaviour of a colloid (e.g., DLVO or XDLVO theory) may be extrapolated with care for aging related parameters. For instance, in a DLVO calculation of interaction energy between colloid and soil using sphere‐plate configuration, interfacial tension values should change after aging, resulting in a different Hamaker constant. Surface potential and radii parameters may also change during aging, both of which cause a different interaction energy between colloid and soil particle. Existing colloid transport models may also be extrapolated with care while assessing the risks of an aged colloid. Parameters related to contaminant retention (adsorption to solid matrix) change with aging, while enhanced or suppressed aggregation after aging also affect the results. Besides, more sophisticated models can be established that may better depict aging‐induced changes in the real soil environment.
Supporting information
TABLE S1 Literature reporting the role of aging in nanoplastic (NP) formation
TABLE S2 Aging‐induced changes in as‐formed nanoplastics
TABLE S3 How aging‐induced formation and aging‐induced changes in as‐formed NPs shed light on its risk mitigation
FIGURE S1 Shrinking‐core model simulation of the soil magnetite nanoparticle oxidation reaction.
ACKNOWLEDGEMENT
This work was supported by the Guangxi Key Research and Development Program (Grant No. GUIKE AB22035036).
Wang, L. , Hu, Z. , Yin, H. , Bradford, S. A. , Luo, J. , & Hou, D. (2022). Aging of colloidal contaminants and pathogens in the soil environment: Implications for nanoplastic and COVID‐19 risk mitigation. Soil Use and Management, 00, 1–22. 10.1111/sum.12849
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abagandura, G. O. , Bansal, S. , Karsteter, A. , & Kumar, S. (2022). Soil greenhouse gas emissions, organic carbon and crop yield following pinewood biochar and biochar‐manure applications at eroded and depositional landscape positions: A field trial in South Dakota, USA. Soil Use and Management, 38, 487–502. 10.1111/sum.12760 [DOI] [Google Scholar]
- Abdoul Magid, A. S. I. , Islam, M. S. , Chen, Y. , Weng, L. , Li, J. , Ma, J. , & Li, Y. (2021). Enhanced adsorption of polystyrene nanoplastics (PSNPs) onto oxidized corncob biochar with high pyrolysis temperature. Science of the Total Environment, 784, 147115. 10.1016/j.scitotenv.2021.147115 [DOI] [PubMed] [Google Scholar]
- Aboubakr, H. A. , Sharafeldin, T. A. , & Goyal, S. M. (2020). Stability of SARS‐CoV‐2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: A review. Transboundary and Emerging Diseases, 68, 296–312. 10.1111/tbed.13707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia, S. , Prakash, N. T. , Prakash, R. , & Pal, B. (2016). Improved degradation of methyl orange dye using bio‐co‐catalyst Se nanoparticles impregnated ZnS photocatalyst under UV irradiation. Chemical Engineering Journal, 306, 1041–1048. 10.1016/j.cej.2016.08.028 [DOI] [Google Scholar]
- Ahmed, I. A. M. , & Maher, B. A. (2018). Identification and paleoclimatic significance of magnetite nanoparticles in soils. Proceedings of the National Academy of Sciences of the United States of America, 115, 1736–1741. 10.1073/pnas.1719186115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, I. , Ding, T. , Peng, C. , Naz, I. , Sun, H. , Li, J. , & Liu, J. (2021). Micro‐ and nanoplastics in wastewater treatment plants: Occurrence, removal, fate, impacts and remediation technologies—A critical review. Chemical Engineering Journal, 423, 130205. 10.1016/j.cej.2021.130205 [DOI] [Google Scholar]
- Alimi, O. S. , Farner, J. M. , & Tufenkji, N. (2021). Exposure of nanoplastics to freeze‐thaw leads to aggregation and reduced transport in model groundwater environments. Water Research, 189, 116533. 10.1016/j.watres.2020.116533 [DOI] [PubMed] [Google Scholar]
- Al‐Kaisi, M. M. , & Lowery, B. (2017). Soil health and intensification of agroecosystems. Academic Press. [Google Scholar]
- Amaral‐Zettler, L. A. , Zettler, E. R. , & Mincer, T. J. (2020). Ecology of the plastisphere. Nature Reviews Microbiology, 18, 139–151. 10.1038/s41579-019-0308-0 [DOI] [PubMed] [Google Scholar]
- Anand, U. , Bianco, F. , Suresh, S. , Tripathi, V. , Núñez‐Delgado, A. , & Race, M. (2021). SARS‐CoV‐2 and other viruses in soil: An environmental outlook. Environmental Research, 198, 111297. 10.1016/j.envres.2021.111297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanious, A. , Aeppli, M. , Jacak, R. , Refardt, D. , Sigstam, T. , Kohn, T. , & Sander, M. (2016). Viruses at solid‐water interfaces: A systematic assessment of interactions driving adsorption. Environmental Science and Technology, 50, 732–743. 10.1021/acs.est.5b04644 [DOI] [PubMed] [Google Scholar]
- Armolaitis, K. , Varnagiryte‐Kabasinskiene, I. , Zemaitis, P. , Stakenas, V. , Beniusis, R. , Kulbokas, G. , & Urbaitis, G. (2022). Evaluation of organic carbon stocks in mineral and organic soils in Lithuania. Soil Use and Management, 38, 355–368. 10.1111/sum.12734 [DOI] [Google Scholar]
- Arrobas, M. , Decker, J. V. , Feix, B. L. , Godoy, W. I. , Casali, C. A. , Correia, C. M. , & Rodrigues, M. A. (2022). Biochar and zeolites did not improve phosphorus uptake or crop productivity in a field trial performed in an irrigated intensive farming system. Soil Use and Management, 38, 564–575. 10.1111/sum.12704 [DOI] [Google Scholar]
- Arwenyo, B. , Varco, J. J. , Dygert, A. , & Mlsna, T. (2022). Phosphorus availability from magnesium‐modified P‐enriched Douglas fir biochar as a controlled release fertilizer. Soil Use and Management, 38, 691–702. 10.1111/sum.12751 [DOI] [Google Scholar]
- Astner, A. F. , Hayes, D. G. , O'Neill, H. , Evans, B. R. , Pingali, S. V. , Urban, V. S. , & Young, T. M. (2019). Mechanical formation of micro‐ and nano‐plastic materials for environmental studies in agricultural ecosystems. Science of the Total Environment, 685, 1097–1106. 10.1016/j.scitotenv.2019.06.241 [DOI] [PubMed] [Google Scholar]
- Avanasi, R. , Jackson, W. A. , Sherwin, B. , Mudge, J. F. , & Anderson, T. A. (2014). C60 fullerene soil sorption, biodegradation, and plant uptake. Environmental Science and Technology, 48, 2792–2797. 10.1021/es405306w [DOI] [PubMed] [Google Scholar]
- Ayaz, M. , Stulpinaite, U. , Feiziene, D. , Tilvikiene, V. , Akthar, K. , Baltenaite‐Gedien, E. , Striugas, N. , Rehmani, U. , Alam, S. , Iqbal, R. , Toleikiene, M. , & Doyeni, M. (2022). Pig manure digestate‐derived biochar for soil management and crop cultivation in heavy metals contaminated soil. Soil Use and Management, 38, 1307–1321. 10.1111/sum.12773 [DOI] [Google Scholar]
- Balboa, S. , Mauricio‐Iglesias, M. , Rodriguez, S. , Martínez‐Lamas, L. , Vasallo, F. J. , Regueiro, B. , & Lema, J. M. (2021). The fate of SARS‐COV‐2 in WWTPS points out the sludge line as a suitable spot for detection of COVID‐19. Science of the Total Environment, 772, 145268. 10.1016/j.scitotenv.2021.145268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamdad, H. , Papari, S. , Lazarovits, G. , & Berruti, F. (2022). Soil amendments for sustainable agriculture: Microbial organic fertilizers. Soil Use and Management, 38, 94–120. 10.1111/sum.12762 [DOI] [Google Scholar]
- Battisti, M. , Moretti, B. , Sacco, D. , Grignani, C. , & Zavattaro, L. (2022). Soil Olsen P response to different phosphorus fertilization strategies in long‐term experiments in NW Italy. Soil Use and Management, 38, 549–563. 10.1111/sum.12701 [DOI] [Google Scholar]
- Baveye, P. C. (2021). Soil health at a crossroad. Soil Use and Management, 37, 215–219. 10.1111/sum.12703 [DOI] [Google Scholar]
- Bianco, A. , Sordello, F. , Ehn, M. , Vione, D. , & Passananti, M. (2020). Degradation of nanoplastics in the environment: Reactivity and impact on atmospheric and surface waters. Science of the Total Environment, 742, 140413. 10.1016/j.scitotenv.2020.140413 [DOI] [PubMed] [Google Scholar]
- Bixio, D. , De Heyder, B. , Cikurel, H. , Muston, M. , Miska, V. , Joksimovic, D. , Schäfer, A. I. , Ravazzini, A. , Aharoni, A. , Savic, D. , & Thoeye, C. (2005). Municipal wastewater reclamation: Where do we stand? An overview of treatment technology and management practice. Water Science and Technology: Water Supply, 5, 77–85. 10.2166/ws.2005.0010 [DOI] [Google Scholar]
- Bogler, A. , Packman, A. , Furman, A. , Gross, A. , Kushmaro, A. , Ronen, A. , Dagot, C. , Hill, C. , Vaizel‐Ohayon, D. , Morgenroth, E. , Bertuzzo, E. , Wells, G. , Kiperwas, H. R. , Horn, H. , Negev, I. , Zucker, I. , Bar‐Or, I. , Moran‐Gilad, J. , Balcazar, J. L. , … Bar‐Zeev, E. (2020). Rethinking wastewater risks and monitoring in light of the COVID‐19 pandemic. Nature Sustainability, 3, 981–990. 10.1038/s41893-020-00605-2 [DOI] [Google Scholar]
- Bradford, S. A. , Kim, H. , Shen, C. , Sasidharan, S. , & Shang, J. (2017). Contributions of nanoscale roughness to anomalous colloid retention and stability behavior. Langmuir, 33, 10094–10105. 10.1021/acs.langmuir.7b02445 [DOI] [PubMed] [Google Scholar]
- Bradford, S. A. , Morales, V. L. , Zhang, W. , Harvey, R. W. , Packman, A. I. , Mohanram, A. , & Welty, C. (2013). Transport and fate of microbial pathogens in agricultural settings. Critical Reviews in Environmental Science and Technology, 43, 775–893. [Google Scholar]
- Bradford, S. A. , Sasidharan, S. , Kim, H. , Gomez‐Flores, A. , Li, T. , & Shen, C. (2021). Colloid interaction energies for surfaces with steric effects and incompressible and/or compressible roughness. Langmuir, 37, 1501–1510. 10.1021/acs.langmuir.0c03029 [DOI] [PubMed] [Google Scholar]
- Bradford, S. A. , Tadassa, Y. F. , & Jin, Y. (2006). Transport of coliphage in the presence and absence of manure suspension. Journal of Environmental Quality, 35, 1692–1701. 10.2134/jeq2006.0036 [DOI] [PubMed] [Google Scholar]
- Bradford, S. A. , & Torkzaban, S. (2015). Determining parameters and mechanisms of colloid retention and release in porous media. Langmuir, 31, 12096–12105. 10.1021/acs.langmuir.5b03080 [DOI] [PubMed] [Google Scholar]
- Briaud, J.‐L. (2013). Geotechnical engineering: Unsaturated and saturated soils. John Wiley & Sons. [Google Scholar]
- Chamas, A. , Moon, H. , Zheng, J. , Qiu, Y. , Tabassum, T. , Jang, J. H. , Abu‐Omar, M. , Scott, S. L. , & Suh, S. (2020). Degradation rates of plastics in the environment. ACS Sustainable Chemistry and Engineering, 8, 3494–3511. 10.1021/acssuschemeng.9b06635 [DOI] [Google Scholar]
- Chick, H. (1908). An investigation of the laws of disinfection. Journal of Hygiene, 8, 92–158. 10.1017/S0022172400006987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, J. T. , Azeez, M. O. , Labouriau, R. , Ravnskov, S. , Kristensen, H. L. , Munkholm, L. J. , & Rubaek, G. H. (2022). Effects of long‐term contrasting lime and phosphorus applications on barley grain yield, root growth and abundance of mycorrhiza. Soil Use and Management, 38, 991–1003. 10.1111/sum.12750 [DOI] [Google Scholar]
- Chrysikopoulos, C. , & Vogler, E. (2004). Estimation of time dependent virus inactivation rates by geostatistical and resampling techniques: Application to virus transport in porous media. Stochastic Environmental Research and Risk Assessment, 18, 67–78. [Google Scholar]
- Cordova, C. , Garrido‐Ruiz, C. , Machuca, A. , Zagal, E. , Orrego, R. , & Finot, V. (2022). Carbon dioxide emissions at local scale linked to soil heterotrophic activity from an experimentally simulated drained peatland in Western Patagonia (Tierra del Fuego, Chile). Soil Use and Management, 38, 304–317. 10.1111/sum.12708 [DOI] [Google Scholar]
- Cortés‐Arriagada, D. (2021). Elucidating the co‐transport of bisphenol A with polyethylene terephthalate (PET) nanoplastics: A theoretical study of the adsorption mechanism. Environmental Pollution, 270, 116192. 10.1016/j.envpol.2020.116192 [DOI] [PubMed] [Google Scholar]
- Dalrymple, O. K. , Stefanakos, E. , Trotz, M. A. , & Goswami, D. Y. (2010). A review of the mechanisms and modeling of photocatalytic disinfection. Applied Catalysis B: Environmental, 98, 27–38. 10.1016/j.apcatb.2010.05.001 [DOI] [Google Scholar]
- Davranche, M. , Veclin, C. , Pierson‐Wickmann, A. C. , El Hadri, H. , Grassl, B. , Rowenczyk, L. , Dia, A. , Ter Halle, A. , Blancho, F. , Reynaud, S. , & Gigault, J. (2019). Are nanoplastics able to bind significant amount of metals? The lead example. Environmental Pollution, 249, 940–948. 10.1016/j.envpol.2019.03.087 [DOI] [PubMed] [Google Scholar]
- de Figueiredo, C. C. , Wickert, E. G. , Vieira Neves, H. C. , Coser, T. R. , & Paz‐Ferreiro, J. (2021). Sewage sludge biochar increases nitrogen fertilizer recovery: Evidence from a N‐15 tracer field study. Soil Use and Management, 37, 689–697. 10.1111/sum.12672 [DOI] [Google Scholar]
- de Jonge, L. W. , Kjærgaard, C. , & Moldrup, P. (2004). Colloids and colloid‐facilitated transport of contaminants in soils: An introduction. Vadose Zone Journal, 3, 321–325. [Google Scholar]
- Deng, M. , & Cliver, D. (1995). Antiviral effects of bacteria isolated from manure. Microbial Ecology, 30, 43–54. [DOI] [PubMed] [Google Scholar]
- Domínguez‐Jaimes, L. P. , Cedillo‐González, E. I. , Luévano‐Hipólito, E. , Acuña‐Bedoya, J. D. , & Hernández‐López, J. M. (2021). Degradation of primary nanoplastics by photocatalysis using different anodized TiO2 structures. Journal of Hazardous Materials, 413, 125452. 10.1016/j.jhazmat.2021.125452 [DOI] [PubMed] [Google Scholar]
- Dong, L. , Wang, J. , Shen, M. , Zhang, H. , Wang, L. , Li, C. , & Lu, C. (2022). Biochar combined with nitrogen fertilizer affects soil properties and wheat yield in medium‐low‐yield farmland. Soil Use and Management, 38, 584–595. 10.1111/sum.12712 [DOI] [Google Scholar]
- Doni, S. , Gispert, M. , Peruzzi, E. , Macci, C. , Mattii, G. B. , Manzi, D. , Masini, C. M. , & Grazia, M. (2021). Impact of natural zeolite on chemical and biochemical properties of vineyard soils. Soil Use and Management, 37, 832–842. 10.1111/sum.12665 [DOI] [Google Scholar]
- El Hadri, H. , Gigault, J. , Maxit, B. , Grassl, B. , & Reynaud, S. (2020). Nanoplastic from mechanically degraded primary and secondary microplastics for environmental assessments. NanoImpact, 17, 100206. 10.1016/j.impact.2019.100206 [DOI] [Google Scholar]
- Ermolin, M. , Fedyunina, N. , & Katasonova, O. (2019). Mobility and fate of cerium dioxide, zinc oxide, and copper nanoparticles in agricultural soil at sequential wetting‐drying cycles. Materials, 12, 1270. 10.3390/ma12081270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EuLA . (2009). Practical guidelines on the use of lime for the prevention and control of avian influenza, foot and mouth disease and other infectious diseases. [Google Scholar]
- Fadare, O. O. , Wan, B. , Liu, K. , Yang, Y. , Zhao, L. , & Guo, L. H. (2020). Eco‐corona vs protein corona: Effects of humic substances on corona formation and nanoplastic particle toxicity in Daphnia magna . Environmental Science and Technology, 54, 8001–8009. 10.1021/acs.est.0c00615 [DOI] [PubMed] [Google Scholar]
- Fajardo, C. , Gil‐Díaz, M. , Costa, G. , Alonso, J. , Guerrero, A. M. , Nande, M. , Lobo, M. C. , & Martín, M. (2015). Residual impact of aged nZVI on heavy metal‐polluted soils. Science of the Total Environment, 535, 79–84. 10.1016/j.scitotenv.2015.03.067 [DOI] [PubMed] [Google Scholar]
- FAO . (2018). Soil pollution: A hidden reality. [Google Scholar]
- FAO . (2021). Assessment of agricultural plastics and their sustainability: A call for action. [Google Scholar]
- Farner, J. M. , De Tommaso, J. , Mantel, H. , Cheong, R. S. , & Tufenkji, N. (2020). Effect of freeze/thaw on aggregation and transport of nano‐TiO2 in saturated porous media. Environmental Science: Nano, 7, 1781–1793. 10.1039/d0en00008f [DOI] [Google Scholar]
- Flury, M. , & Aramrak, S. (2017). Role of air‐water interfaces in colloid transport in porous media: A review. Water Resources Research, 53, 5247–5275. 10.1002/2017WR020597 [DOI] [Google Scholar]
- Fontaine, S. , Mariotti, A. , & Abbadie, L. (2003). The priming effect of organic matter: A question of microbial competition? Soil Biology and Biochemistry, 35, 837–843. 10.1016/S0038-0717(03)00123-8 [DOI] [Google Scholar]
- Gao, J. , Wang, L. , Ok, Y. S. , Bank, M. S. , Luo, J. , Wu, W.‐M. , & Hou, D. (2022). Nanoplastic stimulates metalloid leaching from historically contaminated soil via indirect displacement. Water Research, 218, 118468. [DOI] [PubMed] [Google Scholar]
- Georgiou, C. D. , Sun, H. J. , McKay, C. P. , Grintzalis, K. , Papapostolou, I. , Zisimopoulos, D. , Panagiotidis, K. , Zhang, G. , Koutsopoulou, E. , Christidis, G. E. , & Margiolaki, I. (2015). Evidence for photochemical production of reactive oxygen species in desert soils. Nature Communications, 6, 7100. 10.1038/ncomms8100 [DOI] [PubMed] [Google Scholar]
- Gerba, C. P. (1984). Applied and theoretical aspects of virus adsorption to surfaces. Advances in Applied Microbiology, 30, 133–168. [DOI] [PubMed] [Google Scholar]
- Gerba, C. P. , & Betancourt, W. Q. (2017). Viral aggregation: Impact on virus behavior in the environment. Environmental Science and Technology, 51, 7318–7325. 10.1021/acs.est.6b05835 [DOI] [PubMed] [Google Scholar]
- Gessel, P. D. , Hansen, N. C. , Goyal, S. M. , Johnston, L. J. , & Webb, J. (2004). Persistence of zoonotic pathogens in surface soil treated with different rates of liquid pig manure. Applied Soil Ecology, 25, 237–243. 10.1016/j.apsoil.2003.09.008 [DOI] [Google Scholar]
- Gewert, B. , Plassmann, M. M. , & Macleod, M. (2015). Pathways for degradation of plastic polymers floating in the marine environment. Environmental Sciences: Processes and Impacts, 17, 1513–1521. 10.1039/c5em00207a [DOI] [PubMed] [Google Scholar]
- Giri, S. , & Mukherjee, A. (2021). Ageing with algal EPS reduces the toxic effects of polystyrene nanoplastics in freshwater microalgae Scenedesmus obliquus . Journal of Environmental Chemical Engineering, 9, 105978. 10.1016/j.jece.2021.105978 [DOI] [Google Scholar]
- Graham, T. (1861). Liquid diffusion applied to analysis. Philosophical Transactions of the Royal Society of London, 151, 183–224. [Google Scholar]
- Grolimund, D. , Barmettler, K. , & Borkovec, M. (2001). Release and transport of colloidal particles in natural porous media 2. Experimental results and effects of ligands. Water Resources Research, 37, 571–582. 10.1029/2000WR900286 [DOI] [Google Scholar]
- Grolimund, D. , & Borkovec, M. (1999). Long‐term release kinetics of colloidal particles from natural porous media. Environmental Science and Technology, 33, 4054–4060. 10.1021/es990194m [DOI] [Google Scholar]
- Grolimund, D. , & Borkovec, M. (2006). Release of colloidal particles in natural porous media by monovalent and divalent cations. Journal of Contaminant Hydrology, 87, 155–175. 10.1016/j.jconhyd.2006.05.002 [DOI] [PubMed] [Google Scholar]
- Haider, T. P. , Völker, C. , Kramm, J. , Landfester, K. , & Wurm, F. R. (2019). Plastics of the future? The impact of biodegradable polymers on the environment and on society. Angewandte Chemie ‐ International Edition, 58, 50–62. 10.1002/anie.201805766 [DOI] [PubMed] [Google Scholar]
- Harvey, R. W. , & Ryan, J. N. (2004). Use of PRD1 bacteriophage in groundwater viral transport, inactivation, and attachment studies. FEMS Microbiology Ecology, 49, 3–16. 10.1016/j.femsec.2003.09.015 [DOI] [PubMed] [Google Scholar]
- Hayes, D. G. (2021). Enhanced end‐of‐life performance for biodegradable plastic mulch films through improving standards and addressing research gaps. Current Opinion in Chemical Engineering, 33, 100695. 10.1016/j.coche.2021.100695 [DOI] [Google Scholar]
- Hijikata, N. , Tezuka, R. , Kazama, S. , Otaki, M. , Ushijima, K. , Ito, R. , Okabe, S. , Sano, D. , & Funamizu, N. (2016). Bactericidal and virucidal mechanisms in the alkaline disinfection of compost using calcium lime and ash. Journal of Environmental Management, 181, 721–727. 10.1016/j.jenvman.2016.08.026 [DOI] [PubMed] [Google Scholar]
- Hofmeister, A. M. , Seckler, J. M. , & Criss, G. M. (2021). Possible roles of permafrost melting, atmospheric transport, and solar irradiance in the development of major coronavirus and influenza pandemics. International Journal of Environmental Research and Public Health, 18, 1–24. 10.3390/ijerph18063055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horswell, J. , Hewitt, J. , Prosser, J. , Van Schaik, A. , Croucher, D. , MacDonald, C. , Burford, P. , Susarla, P. , Bickers, P. , & Speir, T. (2010). Mobility and survival of Salmonella typhimurium and human adenovirus from spiked sewage sludge applied to soil columns. Journal of Applied Microbiology, 108, 104–114. 10.1111/j.1365-2672.2009.04416.x [DOI] [PubMed] [Google Scholar]
- Huerta Lwanga, E. , Thapa, B. , Yang, X. , Gertsen, H. , Salánki, T. , Geissen, V. , & Garbeva, P. (2018). Decay of low‐density polyethylene by bacteria extracted from earthworm's guts: A potential for soil restoration. Science of the Total Environment, 624, 753–757. 10.1016/j.scitotenv.2017.12.144 [DOI] [PubMed] [Google Scholar]
- Hurley, R. R. , Lusher, A. L. , Olsen, M. , & Nizzetto, L. (2018). Validation of a method for extracting microplastics from complex, organic‐rich, environmental matrices. Environmental Science and Technology, 52, 7409–7417. 10.1021/acs.est.8b01517 [DOI] [PubMed] [Google Scholar]
- Hurst, C. J. , Gerba, C. P. , & Cech, I. (1980). Effects of environmental variables and soil characteristics on virus survival in soil. Applied and Environmental Microbiology, 40, 1067–1079. 10.1128/aem.40.6.1067-1079.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, N.‐J. , Wang, Y.‐J. , Chu, J. , Kawasaki, S. , Tang, C.‐S. , Cheng, L. , Du, Y.‐J. , Shashank, B. S. , Singh, D. N. , Han, X.‐L. , & Wang, Y.‐Z. (2022). Bio‐mediated soil improvement: An introspection into processes, materials, characterization and applications. Soil Use and Management, 38, 68–93. 10.1111/sum.12736 [DOI] [Google Scholar]
- Jin, Y. , & Flury, M. (2002). Fate and transport of viruses in porous media. Advances in Agronomy, 77, 39–102. [Google Scholar]
- John, D. E. , & Rose, J. B. (2005). Review of factors affecting microbial survival in groundwater. Environmental Science and Technology, 39, 7345–7356. 10.1021/es047995w [DOI] [PubMed] [Google Scholar]
- Jones, R. G. , Pure, I. U. O. , Division, A. C. P. , & Wilks, E. S. (2009). Compendium of polymer terminology and nomenclature: IUPAC recommendations, 2008. RSC Pub. [Google Scholar]
- Khan, S. , & Şengül, H. (2016). Experimental investigation of stability and transport of TiO2 nanoparticles in real soil columns. Desalination and Water Treatment, 57, 26196–26203. [Google Scholar]
- Kim, S. , Sin, A. , Nam, H. , Park, Y. , Lee, H. , & Han, C. (2022). Advanced oxidation processes for microplastics degradation: A recent trend. Chemical Engineering Journal Advances, 9, 100213. 10.1016/j.ceja.2021.100213 [DOI] [Google Scholar]
- Kimura, M. , Jia, Z. J. , Nakayama, N. , & Asakawa, S. (2008). Ecology of viruses in soils: Past, present and future perspectives. Soil Science and Plant Nutrition, 54, 1–32. 10.1111/j.1747-0765.2007.00197.x [DOI] [Google Scholar]
- Klompas, M. , Baker, M. A. , & Rhee, C. (2020). Airborne transmission of SARS‐CoV‐2: Theoretical considerations and available evidence. JAMA, 324, 441–442. 10.1001/jama.2020.12458 [DOI] [PubMed] [Google Scholar]
- Kuzyakov, Y. , Friedel, J. K. , & Stahr, K. (2000). Review of mechanisms and quantification of priming effects. Soil Biology and Biochemistry, 32, 1485–1498. 10.1016/S0038-0717(00)00084-5 [DOI] [Google Scholar]
- Kuzyakov, Y. , & Mason‐Jones, K. (2018). Viruses in soil: Nano‐scale undead drivers of microbial life, biogeochemical turnover and ecosystem functions. Soil Biology and Biochemistry, 127, 305–317. 10.1016/j.soilbio.2018.09.032 [DOI] [Google Scholar]
- Kwak, J. I. , & An, Y. J. (2021). Microplastic digestion generates fragmented nanoplastics in soils and damages earthworm spermatogenesis and coelomocyte viability. Journal of Hazardous Materials, 402, 124034. 10.1016/j.jhazmat.2020.124034 [DOI] [PubMed] [Google Scholar]
- Lambert, S. , & Wagner, M. (2016). Formation of microscopic particles during the degradation of different polymers. Chemosphere, 161, 510–517. 10.1016/j.chemosphere.2016.07.042 [DOI] [PubMed] [Google Scholar]
- Law, K. L. , & Narayan, R. (2021). Reducing environmental plastic pollution by designing polymer materials for managed end‐of‐life. Nature Reviews Materials, 7, 104–116. 10.1038/s41578-021-00382-0 [DOI] [Google Scholar]
- Li, M. , Greenfield, B. K. , Nunes, L. M. , Dang, F. , Liu, H. L. , Zhou, D. M. , & Yin, B. (2019). High retention of silver sulfide nanoparticles in natural soils. Journal of Hazardous Materials, 378, 120735. 10.1016/j.jhazmat.2019.06.012 [DOI] [PubMed] [Google Scholar]
- Li, M. , Yang, Y. , Lu, Y. , Zhang, D. , Liu, Y. , Cui, X. , Yang, L. , Liu, R. , Liu, J. , Li, G. , & Qu, J. (2020). Natural host–environmental media–human: A new potential pathway of COVID‐19 outbreak. Engineering, 6, 1085–1098. 10.1016/j.eng.2020.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, Y. , Luo, Y. , Lu, Z. , Klumpp, E. , Shen, C. , & Bradford, S. A. (2021). Evidence on enhanced transport and release of silver nanoparticles by colloids in soil due to modification of grain surface morphology and co‐transport. Environmental Pollution, 276, 116661. 10.1016/j.envpol.2021.116661 [DOI] [PubMed] [Google Scholar]
- Liew, P. F. , & Gerba, C. P. (1980). Thermostabilization of enteroviruses by estuarine sediment. Applied and Environmental Microbiology, 40, 305–308. 10.1128/aem.40.2.305-308.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Zhang, T. , Tian, L. , Liu, X. , Qi, Z. , Ma, Y. , Ji, R. , & Chen, W. (2019). Aging significantly affects mobility and contaminant‐mobilizing ability of nanoplastics in saturated loamy sand. Environmental Science and Technology, 53, 5805–5815. 10.1021/acs.est.9b00787 [DOI] [PubMed] [Google Scholar]
- Liu, S. , Junaid, M. , Liao, H. , Liu, X. , Wu, Y. , & Wang, J. (2022). Eco‐corona formation and associated ecotoxicological impacts of nanoplastics in the environment. Science of the Total Environment, 836, 155703. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Hu, Y. , Yang, C. , Chen, C. , Huang, W. , & Dang, Z. (2019). Aggregation kinetics of UV irradiated nanoplastics in aquatic environments. Water Research, 163, 114870. 10.1016/j.watres.2019.114870 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Shao, H. , Liu, J. , Cao, R. , Shang, E. , Liu, S. , & Li, Y. (2021). Transport and transformation of microplastics and nanoplastics in the soil environment: A critical review. Soil Use and Management, 37, 224–242. 10.1111/sum.12709 [DOI] [Google Scholar]
- Lonigro, A. , Montemurro, N. , & Laera, G. (2017). Effects of residual disinfectant on soil and lettuce crop irrigated with chlorinated water. Science of the Total Environment, 584–585, 595–602. 10.1016/j.scitotenv.2017.01.083 [DOI] [PubMed] [Google Scholar]
- Lynch, I. , Dawson, K. A. , Lead, J. R. , & Valsami‐Jones, E. (2014). Macromolecular coronas and their importance in nanotoxicology and nanoecotoxicology. Frontiers of Nanoscience, 7, 127–156. [Google Scholar]
- Mancuso, G. , Perulli, G. D. , Lavrnić, S. , Morandi, B. , & Toscano, A. (2021). SARS‐CoV‐2 from urban to rural water environment: Occurrence, persistence, fate, and influence on agriculture irrigation. A review. Water, 13, 764. [Google Scholar]
- Mao, Y. , Li, H. , Huangfu, X. , Liu, Y. , & He, Q. (2020). Nanoplastics display strong stability in aqueous environments: Insights from aggregation behaviour and theoretical calculations. Environmental Pollution, 258, 113760. 10.1016/j.envpol.2019.113760 [DOI] [PubMed] [Google Scholar]
- Marquès, M. , & Domingo, J. L. (2021). Contamination of inert surfaces by SARS‐CoV‐2: Persistence, stability and infectivity. A review. Environmental Research, 193, 110559. 10.1016/j.envres.2020.110559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matjašič, T. , Simčič, T. , Medvešček, N. , Bajt, O. , Dreo, T. , & Mori, N. (2021). Critical evaluation of biodegradation studies on synthetic plastics through a systematic literature review. Science of the Total Environment, 752, 141959. 10.1016/j.scitotenv.2020.141959 [DOI] [PubMed] [Google Scholar]
- McGivney, E. , Gao, X. , Liu, Y. , Lowry, G. V. , Casman, E. , Gregory, K. B. , VanBriesen, J. M. , & Avellan, A. (2019). Biogenic cyanide production promotes dissolution of gold nanoparticles in soil. Environmental Science and Technology, 53, 1287–1295. 10.1021/acs.est.8b05884 [DOI] [PubMed] [Google Scholar]
- Mo, X. , Li, H. , Lian, Y. , Zheng, B. , Dong, J. , & Lu, X. (2021). Estimation of soil microplastic input derived from plastic gauze using a simplified model. Science of the Total Environment, 793, 148577. 10.1016/j.scitotenv.2021.148577 [DOI] [PubMed] [Google Scholar]
- Molin, S. , & Cvetkovic, V. (2010). Microbial risk assessment in heterogeneous aquifers: 1. Pathogen transport (p. 46). Water Resources Research. [Google Scholar]
- Molnar, I. L. , Johnson, W. P. , Gerhard, J. I. , Willson, C. S. , & O'Carroll, D. M. (2015). Predicting colloid transport through saturated porous media: A critical review. Water Resources Research, 51, 6804–6845. 10.1002/2015WR017318 [DOI] [Google Scholar]
- Murray, J. P. , & Laband, S. J. (1979). Degradation of poliovirus by adsorption on inorganic surfaces. Applied and Environmental Microbiology, 37, 480–486. 10.1128/aem.37.3.480-486.1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan, L. , Jenifer, M. A. , & Mukherjee, A. (2021). Eco‐corona formation on the nanomaterials in the aquatic systems lessens their toxic impact: A comprehensive review. Environmental Research, 194, 110669. 10.1016/j.envres.2020.110669 [DOI] [PubMed] [Google Scholar]
- Natarajan, L. , Omer, S. , Jetly, N. , Jenifer, M. A. , Chandrasekaran, N. , Suraishkumar, G. K. , & Mukherjee, A. (2020). Eco‐corona formation lessens the toxic effects of polystyrene nanoplastics towards marine microalgae Chlorella sp. Environmental Research, 188, 109842. 10.1016/j.envres.2020.109842 [DOI] [PubMed] [Google Scholar]
- NDRC and MEE . (2021). China unveils 5‐year plan to control plastic pollution. [Google Scholar]
- Nelson, C. E. , & Wear, E. K. (2014). Microbial diversity and the lability of dissolved organic carbon. Proceedings of the National Academy of Sciences of the United States of America, 111, 7166–7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norde, W. (2011). Colloids and interfaces in life sciences and bionanotechnology. CRC Press. [Google Scholar]
- Núñez‐Delgado, A. (2020). SARS‐CoV‐2 in soils. Environmental Research, 190, 110045. 10.1016/j.envres.2020.110045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parashar, D. , Khalkar, P. , & Arankalle, V. A. (2011). Survival of hepatitis A and E viruses in soil samples. Clinical Microbiology and Infection, 17, E1–E4. 10.1111/j.1469-0691.2011.03652.x [DOI] [PubMed] [Google Scholar]
- Pateiro‐Moure, M. , Arias‐Estévez, M. , & Simal‐Gándara, J. (2013). Critical review on the environmental fate of quaternary ammonium herbicides in soils devoted to vineyards. Environmental Science and Technology, 47, 4984–4998. 10.1021/es400755h [DOI] [PubMed] [Google Scholar]
- Pourcher, A. M. , Françoise, P. B. , Virginie, F. , Agnieszka, G. , Vasilica, S. , & Gérard, M. (2007). Survival of faecal indicators and enteroviruses in soil after land‐spreading of municipal sewage sludge. Applied Soil Ecology, 35, 473–479. 10.1016/j.apsoil.2006.10.005 [DOI] [Google Scholar]
- Pradas Del Real, A. E. , Castillo‐Michel, H. , Kaegi, R. , Larue, C. , De Nolf, W. , Reyes‐Herrera, J. , Tucoulou, R. , Findling, N. , Salas‐Colera, E. , & Sarret, G. (2018). Searching for relevant criteria to distinguish natural vs. anthropogenic TiO2 nanoparticles in soils. Environmental Science: Nano, 5, 2853–2863. 10.1039/c8en00386f [DOI] [Google Scholar]
- Qin, M. , Chen, C. , Song, B. , Shen, M. , Cao, W. , Yang, H. , Zeng, G. , & Gong, J. (2021). A review of biodegradable plastics to biodegradable microplastics: Another ecological threat to soil environments? Journal of Cleaner Production, 312, 127816. 10.1016/j.jclepro.2021.127816 [DOI] [Google Scholar]
- Qu, X. , Hwang, Y. S. , Alvarez, P. J. J. , Bouchard, D. , & Li, Q. (2010). UV irradiation and humic acid mediate aggregation of aqueous fullerene (nC60) nanoparticles. Environmental Science and Technology, 44, 7821–7826. 10.1021/es101947f [DOI] [PubMed] [Google Scholar]
- Reynaud, S. , Aynard, A. , Grassl, B. , & Gigault, J. (2022). Nanoplastics: From model materials to colloidal fate. Current Opinion in Colloid & Interface Science, 57, 101528. [Google Scholar]
- Rout, P. R. , Zhang, T. C. , Bhunia, P. , & Surampalli, R. Y. (2021). Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Science of the Total Environment, 753, 141990. 10.1016/j.scitotenv.2020.141990 [DOI] [PubMed] [Google Scholar]
- Ryan, J. N. , Elimelech, M. , Ard, R. A. , Harvey, R. W. , & Johnson, P. R. (1999). Bacteriophage PRD1 and silica colloid transport and recovery in an iron oxide‐coated sand aquifer. Environmental Science and Technology, 33, 63–73. 10.1021/es980350+ [DOI] [Google Scholar]
- Ryan, J. N. , Harvey, R. W. , Metge, D. , Elimelech, M. , Navigato, T. , & Pieper, A. P. (2002). Field and laboratory investigations of inactivation of viruses (PRD1 and MS2) attached to iron oxide‐coated quartz sand. Environmental Science and Technology, 36, 2403–2413. 10.1021/es011285y [DOI] [PubMed] [Google Scholar]
- Saavedra, J. , Stoll, S. , & Slaveykova, V. I. (2019). Influence of nanoplastic surface charge on eco‐corona formation, aggregation and toxicity to freshwater zooplankton. Environmental Pollution, 252, 715–722. 10.1016/j.envpol.2019.05.135 [DOI] [PubMed] [Google Scholar]
- Sagripanti, J.‐L. , Routson, L. B. , & Lytle, C. D. (1993). Virus inactivation by copper or iron ions alone and in the presence of peroxide. Applied and Environmental Microbiology, 59, 4374–4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐Hernandez, J. C. , Capowiez, Y. , & Ro, K. S. (2020). Potential use of earthworms to enhance decaying of biodegradable plastics. ACS Sustainable Chemistry and Engineering, 8, 4292–4316. 10.1021/acssuschemeng.9b05450 [DOI] [Google Scholar]
- SAPEA . (2020). Biodegradability of plastics in the open environment. [Google Scholar]
- Sasidharan, S. , Bradford, S. A. , Šimunek, J. , & Torkzaban, S. (2018). Minimizing virus transport in porous media by optimizing solid phase inactivation. Journal of Environmental Quality, 47, 1058–1067. 10.2134/jeq2018.01.0027 [DOI] [PubMed] [Google Scholar]
- Sasidharan, S. , Bradford, S. A. , Torkzaban, S. , Ye, X. , Vanderzalm, J. , Du, X. , & Page, D. (2017). Unraveling the complexities of the velocity dependency of E. coli retention and release parameters in saturated porous media. Science of the Total Environment, 603–604, 406–415. 10.1016/j.scitotenv.2017.06.091 [DOI] [PubMed] [Google Scholar]
- Scandura, J. E. , & Sobsey, M. D. (1997). Viral and bacterial contamination of groundwater from on‐site sewage treatment systems. Water Science and Technology, 35, 141–146. [Google Scholar]
- Schijven, J. F. , & Hassanizadeh, S. M. (2000). Removal of viruses by soil passage: Overview of modeling, processes, and parameters. Critical Reviews in Environmental Science and Technology, 30, 49–127. 10.1080/10643380091184174 [DOI] [Google Scholar]
- Schijven, J. F. , Hoogenboezem, W. , Hassanizadeh, M. , & Peters, J. H. (1999). Modeling removal of bacteriophages MS2 and PRD1 by dune recharge at Castricum, Netherlands. Water Resources Research, 35, 1101–1111. [Google Scholar]
- Schultz, C. L. , Bart, S. , Lahive, E. , & Spurgeon, D. J. (2021). What is on the outside matters—Surface charge and dissolve organic matter association affect the toxicity and physiological mode of action of polystyrene nanoplastics to C. elegans . Environmental Science and Technology, 55(9), 6065–6075. 10.1021/acs.est.0c07121 [DOI] [PubMed] [Google Scholar]
- Schwarz, K. R. , Sidhu, J. P. S. , Pritchard, D. L. , Li, Y. , & Toze, S. (2014). Decay of enteric microorganisms in biosolids‐amended soil under wheat (Triticum aestivum) cultivation. Water Research, 59, 185–197. 10.1016/j.watres.2014.03.037 [DOI] [PubMed] [Google Scholar]
- Seitz, S. R. , Leon, J. S. , Schwab, K. J. , Lyon, G. M. , Dowd, M. , McDaniels, M. , Abdulhafid, G. , Fernandez, M. L. , Lindesmith, L. C. , Baric, R. S. , & Moe, C. L. (2011). Norovirus infectivity in humans and persistence in water. Applied and Environmental Microbiology, 77, 6884–6888. 10.1128/AEM.05806-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui, R. , Khamis, M. , Ibrahim, T. , & Khan, N. A. (2020). Irrigation system and COVID‐19 recurrence: A potential risk factor in the transmission of SARS‐CoV‐2. ACS Chemical Neuroscience, 11, 2903–2905. 10.1021/acschemneuro.0c00570 [DOI] [PubMed] [Google Scholar]
- Sim, Y. , & Chrysikopoulos, C. V. (1996). One‐dimensional virus transport in porous media with time‐ dependent inactivation rate coefficients. Water Resources Research, 32, 2607–2611. 10.1029/96WR01496 [DOI] [Google Scholar]
- Sofo, A. , Zanella, A. , & Ponge, J.‐F. (2022). Soil quality and fertility in sustainable agriculture, with a contribution to the biological classification of agricultural soils. Soil Use and Management, 38, 1085–1112. 10.1111/sum.12702 [DOI] [Google Scholar]
- Song, Y. K. , Hong, S. H. , Eo, S. , Han, G. M. , & Shim, W. J. (2020). Rapid production of micro‐ and nanoplastics by fragmentation of expanded polystyrene exposed to sunlight. Environmental Science and Technology, 54, 11191–11200. 10.1021/acs.est.0c02288 [DOI] [PubMed] [Google Scholar]
- Song, Z. , Yang, X. , Chen, F. , Zhao, F. , Zhao, Y. , Ruan, L. , Wang, Y. , & Yang, Y. (2019). Fate and transport of nanoplastics in complex natural aquifer media: Effect of particle size and surface functionalization. Science of the Total Environment, 669, 120–128. 10.1016/j.scitotenv.2019.03.102 [DOI] [PubMed] [Google Scholar]
- Steenhuis, T. S. , Morales, V. L. , Cakmak, M. E. , Salvucci, A. E. , & Zhang, W. (2011). Biocolloids: Transport and retention in soils. Encyclopedia of Earth Sciences Series, 4, 66–70. [Google Scholar]
- Straub, T. M. , Pepper, I. L. , & Gerba, C. P. (1993). Virus survival in sewage sludge amended desert soil. Water Science and Technology, 27, 421–424. 10.2166/wst.1993.0384 [DOI] [Google Scholar]
- Stumm, W. , & Morgan, J. J. (2012). Aquatic chemistry: Chemical equilibria and rates in natural waters (Vol. 126). John Wiley & Sons. [Google Scholar]
- Sun, X. D. , Yuan, X. Z. , Jia, Y. , Feng, L. J. , Zhu, F. P. , Dong, S. S. , Liu, J. , Kong, X. , Tian, H. , Duan, J. L. , Ding, Z. , Wang, S. G. , & Xing, B. (2020). Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana . Nature Nanotechnology, 15, 755–760. 10.1038/s41565-020-0707-4 [DOI] [PubMed] [Google Scholar]
- Tian, L. , Chen, Q. , Jiang, W. , Wang, L. , Xie, H. , Kalogerakis, N. , Ma, Y. , & Ji, R. (2019). A carbon‐14 radiotracer‐based study on the phototransformation of polystyrene nanoplastics in water: Versus in air. Environmental Science: Nano, 6, 2907–2917. 10.1039/c9en00662a [DOI] [Google Scholar]
- Tong, M. , Li, T. , Li, M. , He, L. , & Ma, Z. (2020). Cotransport and deposition of biochar with different sized‐plastic particles in saturated porous media. Science of the Total Environment, 713, 136387. 10.1016/j.scitotenv.2019.136387 [DOI] [PubMed] [Google Scholar]
- Torkzaban, S. , & Bradford, S. A. (2016). Critical role of surface roughness on colloid retention and release in porous media. Water Research, 88, 274–284. 10.1016/j.watres.2015.10.022 [DOI] [PubMed] [Google Scholar]
- US EPA . (2003). Environmental regulations and technology: Control of pathogens and vector attraction in sewage sludge. [Google Scholar]
- US EPA . (2021). List N tool: COVID‐19 disinfectants. https://cfpub.epa.gov/wizards/disinfectants/
- Van Doremalen, N. , Bushmaker, T. , Morris, D. H. , Holbrook, M. G. , Gamble, A. , Williamson, B. N. , Tamin, A. , Harcourt, J. L. , Thornburg, N. J. , Gerber, S. I. , Lloyd‐Smith, J. O. , De Wit, E. , & Munster, V. J. (2020). Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. New England Journal of Medicine, 382, 1564–1567. 10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasickova, P. , Pavlik, I. , Verani, M. , & Carducci, A. (2010). Issues concerning survival of viruses on surfaces. Food and Environmental Virology, 2, 24–34. [Google Scholar]
- Vermeiren, P. , Muñoz, C. , & Ikejima, K. (2020). Microplastic identification and quantification from organic rich sediments: A validated laboratory protocol. Environmental Pollution, 262, 114298. 10.1016/j.envpol.2020.114298 [DOI] [PubMed] [Google Scholar]
- Vissers, T. , Brown, A. T. , Koumakis, N. , Dawson, A. , Hermes, M. , Schwarz‐Linek, J. , Schofield, A. B. , French, J. M. , Koutsos, V. , Arlt, J. , Martinez, V. A. , & Poon, W. C. K. (2018). Bacteria as living patchy colloids: Phenotypic heterogeneity in surface adhesion. Science Advances, 4, eaao1170. 10.1126/sciadv.aao1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Borowski, R. G. , & Trentin, D. S. (2021). Biofilms and coronavirus reservoirs: A perspective review. Applied and Environmental Microbiology, 87, e00859‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Wang, L. , Ok, Y. S. , Tsang, D. C. , & Hou, D. (2022). Soil plastisphere: Exploration methods, influencing factors, and ecological insights. Journal of Hazardous Materials, 430, 128503. [DOI] [PubMed] [Google Scholar]
- Wang, L. , Li, P. , Zhang, Q. , Wu, W. M. , Luo, J. , & Hou, D. (2021). Modeling the conditional fragmentation‐induced microplastic distribution. Environmental Science and Technology, 55, 6012–6021. 10.1021/acs.est.1c01042 [DOI] [PubMed] [Google Scholar]
- Wang, L. , O'Connor, D. , Rinklebe, J. , Ok, Y. S. , Tsang, D. C. W. , Shen, Z. , & Hou, D. (2020). Biochar aging: Mechanisms, physicochemical changes, assessment, and implications for field applications. Environmental Science and Technology, 54, 14797–14814. 10.1021/acs.est.0c04033 [DOI] [PubMed] [Google Scholar]
- Wang, L. , Ok, Y. S. , Tsang, D. C. , Alessi, D. S. , Rinklebe, J. , Mašek, O. , Bolan, N. S. , & Hou, D. (2022). Biochar composites: Emerging trends, field successes and sustainability implications. Soil Use and Management, 38, 14–38. [Google Scholar]
- Wang, L. , Rinklebe, J. , Tack, F. M. G. , & Hou, D. (2021). A review of green remediation strategies for heavy metal contaminated soil. Soil Use and Management, 37, 936–963. 10.1111/sum.12717 [DOI] [Google Scholar]
- Wang, L. , Wu, W. M. , Bolan, N. S. , Tsang, D. C. W. , Li, Y. , Qin, M. , & Hou, D. (2021). Environmental fate, toxicity and risk management strategies of nanoplastics in the environment: Current status and future perspectives. Journal of Hazardous Materials, 401, 123415. 10.1016/j.jhazmat.2020.123415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . (2020a). Modes of transmission of virus causing COVID‐19: Implications for IPC precaution recommendations. [Google Scholar]
- WHO . (2020b). Transmission of SARS‐CoV‐2: Implications for infection prevention precautions. [Google Scholar]
- Williams, T. , Walsh, C. , Murray, K. , & Subir, M. (2020). Interactions of emerging contaminants with model colloidal microplastics, C60 fullerene, and natural organic matter–effect of surface functional group and adsorbate properties. Environmental Science: Processes & Impacts, 22, 1190–1200. [DOI] [PubMed] [Google Scholar]
- Williamson, K. E. , Fuhrmann, J. J. , Wommack, K. E. , & Radosevich, M. (2017). Viruses in soil ecosystems: An unknown quantity within an unexplored territory. Annual Review of Virology, 4, 201–219. 10.1146/annurev-virology-101416-041639 [DOI] [PubMed] [Google Scholar]
- Wood, J. P. , Choi, Y. W. , Chappie, D. J. , Rogers, J. V. , & Kaye, J. Z. (2010). Environmental persistence of a highly pathogenic Avian Influenza (H5N1) virus. Environmental Science and Technology, 44, 7515–7520. 10.1021/es1016153 [DOI] [PubMed] [Google Scholar]
- Xie, C. , Zhao, H. , Li, K. , Zhang, Z. , Lu, X. , Peng, H. , Wang, D. , Chen, J. , Zhang, X. , Wu, D. , Gu, Y. , Yuan, J. , Zhang, L. , & Lu, J. (2020). The evidence of indirect transmission of SARS‐CoV‐2 reported in Guangzhou, China. BMC Public Health, 20, 1–9. 10.1186/s12889-020-09296-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, Y. , Zhao, J. , Li, L. , Wang, Y. , Dai, X. , Yu, F. , & Ma, J. (2020). Interfacial interaction between micro/nanoplastics and typical PPCPs and nanoplastics removal via electrosorption from an aqueous solution. Water Research, 184, 116100. 10.1016/j.watres.2020.116100 [DOI] [PubMed] [Google Scholar]
- Xu, C. , Ravi Anusuyadevi, P. , Aymonier, C. , Luque, R. , & Marre, S. (2019). Nanostructured materials for photocatalysis. Chemical Society Reviews, 48, 3868–3902. 10.1039/c9cs00102f [DOI] [PubMed] [Google Scholar]
- Xu, G. , Zheng, Q. , Yang, X. , Yu, R. , & Yu, Y. (2021). Freeze‐thaw cycles promote vertical migration of metal oxide nanoparticles in soils. Science of the Total Environment, 795, 148894. 10.1016/j.scitotenv.2021.148894 [DOI] [PubMed] [Google Scholar]
- Xu, Y. , Ou, Q. , He, Q. , Wu, Z. , Ma, J. , & Huangfu, X. (2021). Influence of dissolved black carbon on the aggregation and deposition of polystyrene nanoplastics: Comparison with dissolved humic acid. Water Research, 196, 117054. 10.1016/j.watres.2021.117054 [DOI] [PubMed] [Google Scholar]
- Yang, T. , Luo, J. , & Nowack, B. (2021). Characterization of nanoplastics, fibrils, and microplastics released during washing and abrasion of polyester textiles. Environmental Science and Technology, 55, 15873–15881. [DOI] [PubMed] [Google Scholar]
- Yang, X. , Kong, Y. , Guo, E. , Chen, X. , & Li, L. (2022). Organic acid regulation of inorganic phosphorus release from mollisols with different organic matter contents. Soil Use and Management, 38, 576–583. 10.1111/sum.12710 [DOI] [Google Scholar]
- Yao, B. , Luo, Z. , Xiong, W. , Song, B. , Zeng, Z. , & Zhou, Y. (2020). Disinfection techniques of human norovirus in municipal wastewater: Challenges and future perspectives. Current Opinion in Environmental Science and Health, 17, 29–34. 10.1016/j.coesh.2020.08.003 [DOI] [Google Scholar]
- Yeager, J. G. , & O'Brien, R. T. (1979a). Enterovirus inactivation in soil. Applied and Environmental Microbiology, 38, 694–701. 10.1128/aem.38.4.694-701.1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager, J. G. , & O'Brien, R. T. (1979b). Structural changes associated with poliovirus inactivation in soil. Applied and Environmental Microbiology, 38, 702–709. 10.1128/aem.38.4.702-709.1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, J. , Chen, Z. , Gong, C. , Liu, H. , Li, B. , Li, K. , Chen, X. , Xu, C. , Jing, Q. , Liu, G. , Qin, P. , Liu, Y. , Zhong, Y. , Huang, L. , Zhu, B.‐P. , & Yang, Z. (2020). Sewage as a possible transmission vehicle during a coronavirus disease 2019 outbreak in a densely populated community: Guangzhou, China, April 2020. Clinical Infectious Diseases, 73, e1795–e1802. 10.1093/cid/ciaa1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, C. , Niu, D. , & Zhao, Y. (2015). A comprehensive overview of rural solid waste management in China. Frontiers of Environmental Science and Engineering, 9, 949–961. 10.1007/s11783-015-0816-8 [DOI] [Google Scholar]
- Zhang, D. , Zhang, X. , Yang, Y. , Huang, X. , Jiang, J. , Li, M. , Ling, H. , Li, J. , Liu, Y. , Li, G. , Li, W. , Yi, C. , Zhang, T. , Jiang, Y. , Xiong, Y. , He, Z. , Wang, X. , Deng, S. , Zhao, P. , & Qu, J. (2021). SARS‐CoV‐2 spillover into hospital outdoor environments. Journal of Hazardous Materials Letters, 2, 100027. 10.1016/j.hazl.2021.100027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, K. , Hamidian, A. H. , Tubić, A. , Zhang, Y. , Fang, J. K. H. , Wu, C. , & Lam, P. K. S. (2021). Understanding plastic degradation and microplastic formation in the environment: A review. Environmental Pollution, 274, 116554. 10.1016/j.envpol.2021.116554 [DOI] [PubMed] [Google Scholar]
- Zhang, M. , Bradford, S. A. , Šimůnek, J. , Vereecken, H. , & Klumpp, E. (2017). Roles of cation valance and exchange on the retention and colloid‐facilitated transport of functionalized multi‐walled carbon nanotubes in a natural soil. Water Research, 109, 358–366. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Xie, H. , Wang, F. , Sun, C. , & Zhang, X. (2022). Effects of biochar incorporation on soil viable and necromass carbon in the luvisol soil. Soil Use and Management, 38, 318–330. 10.1111/sum.12720 [DOI] [Google Scholar]
- Zhang, Z. , Liu, H. , Liu, X. , Chen, Y. , Lu, Y. , Shen, M. , Dang, K. , Zhao, Y. , Dong, Y. , Li, Q. , & Li, J. (2022). Organic fertilizer enhances rice growth in severe saline‐alkali soil by increasing soil bacterial diversity. Soil Use and Management, 38, 964–977. 10.1111/sum.12711 [DOI] [Google Scholar]
- Zhao, B. , Zhang, H. , Zhang, J. , & Jin, Y. (2008). Virus adsorption and inactivation in soil as influenced by autochthonous microorganisms and water content. Soil Biology and Biochemistry, 40, 649–659. 10.1016/j.soilbio.2007.09.020 [DOI] [Google Scholar]
- Zou, Y. , & Zheng, W. (2013). Modeling manure colloid‐facilitated transport of the weakly hydrophobic antibiotic florfenicol in saturated soil columns. Environmental Science and Technology, 47, 5185–5192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Literature reporting the role of aging in nanoplastic (NP) formation
TABLE S2 Aging‐induced changes in as‐formed nanoplastics
TABLE S3 How aging‐induced formation and aging‐induced changes in as‐formed NPs shed light on its risk mitigation
FIGURE S1 Shrinking‐core model simulation of the soil magnetite nanoparticle oxidation reaction.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


