Abstract
COVID‐19 remains a life‐threatening infectious disease worldwide. Several bio‐active agents have been tested and evaluated in an effort to contain this disease. Unfortunately, none of the therapies have been successful, owing to their safety concerns and the presence of various adverse effects. Various countries have developed vaccines as a preventive measure; however, they have not been widely accepted as effective strategies. The virus has proven to be exceedingly contagious and lethal, so finding an effective treatment strategy has been a top priority in medical research. The significance of vitamin D in influencing many components of the innate and adaptive immune systems is examined in this study. This review aims to summarize the research on the use of vitamin D for COVID‐19 treatment and prevention. Vitamin D supplementation has now become an efficient option to boost the immune response for all ages in preventing the spread of infection. Vitamin D is an immunomodulator that treats infected lung tissue by improving innate and adaptive immune responses and downregulating the inflammatory cascades. The preventive action exerted by vitamin D supplementation (at a specific dose) has been accepted by several observational research investigations and clinical trials on the avoidance of viral and acute respiratory dysfunctions. To assess the existing consensus about vitamin D supplementation as a strategy to treat and prevent the development and progression of COVID‐19 disease, this review intends to synthesize the evidence around vitamin D in relation to COVID‐19 infection.
Keywords: doses, immune system, mechanism, severe acute respiratory syndrome coronavirus 2, vitamin D
1. INTRODUCTION
Genome homology of reports that severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) uses the cell receptors of dipeptidyl peptidase and angiotensin‐converting enzyme 2 (ACE2) to gain entry into the host cell. 1 Similar to severe acute respiratory syndrome coronavirus (SARS‐CoV) and Middle‐East respiratory syndrome coronavirus (MERS‐CoV), the novel virus SARS‐CoV‐2 also causes inflammation and releases pro‐inflammatory mediators, such as tumor necrosis factor‐α (TNF‐α) and interleukin‐1β (IL‐1 β) and IL‐6. 2 Both innate and adaptive immunity play a significant role in eliciting immediate protective immune responses. Several studies have revealed the significant role of vitamin D in controlling these innate as well as adaptive immune responses concurrently via vitamin D receptor (VDR) localized in immune cells, including T cells (CD4+ and CD8+), B cells, monocytes, neutrophils, macrophages, and dendritic cells. 3 Individuals who lacked vitamin D had upregulated expression of IL‐6, where the TNF‐α was found to activate phenotypes of monocytes. 4 Host responses are occasionally altered as the result of overexposure to inflammatory mediators, and the apparent “cytokine storm” causes critical complications in SARS‐CoV‐2‐infected patients like those in acute respiratory distress syndrome (ARDS). 5 Immune defenses can be boosted by vitamin D supplementation, which balances inflammation versus anti‐inflammation. Persistent lack of vitamin D is one of the increasing pathological states worldwide, affecting more than 1 billion of individuals. 6 Research has revealed a prospective relationship between insufficient vitamin D and the occurrence of systemic infection along with several other diseases. 7 , 8 , 9 Vitamin D deficiency alters the body's immunity because vitamin D is a key player in immune modulation, as it affects secretion of antiviral peptides and hence improves innate immunity, which is responsible for maintaining mucosal integrity and defense mechanisms. 10 , 11 , 12 The current hypothesis states that a lack of vitamin D could reduce respiratory immune responses and enhance the chances of SARS‐CoV‐2 infection severity and mortality rate. 13 , 14 Along with this hypothesis, numerous retrospective studies have represented the interrelationship between vitamin D content and the severity of SARS‐CoV‐2 infection. 15 , 16 , 17 , 18 , 19 , 20 As a result, enhancing the immune system by administering vitamin D has become a significant factor. However, there are limited data concerning vitamin D and its ability to successfully prevent the infection; there is a need for more research about vitamin D supplementation and how it can be useful to enhance the immune system and prevent further spread of SARS‐CoV‐2. Evidence from past literature has illustrated the importance of vitamin D in the chronic phase of diseases leading to high mortality rates in patients with coronavirus disease 2019 (COVID‐19). Immune responses are well‐documented as being modulated by vitamin D. There has been a much interest in vitamin D's potential to reduce or avoid negative immunological reactions caused by the severe effects of COVID‐19 on the immune system. A narrative synthesis is necessary to summarize the current level of knowledge in this topic because several new studies have just been released. 21 In order to assess the existing consensus about vitamin D supplementation as a strategy to treat and/or prevent the development or progression of COVID‐19, this review intends to synthesize the evidence around vitamin D in relation to COVID‐19. With reference to significant research and systematic reviews that have been published, we provide an overview of the current level of knowledge in this field. This review was prepared by collecting the relevant scientific information from PubMed, Web of Science/Scopus, and Google Scholar using the following phrases in all possible combinations: “role of vitamin D in respiratory diseases” (292 results), “role of vitamin D in covid‐19” (9 results), “molecular mechanisms of vitamin D" (12 results), “role of vitamin D in viral disease” (5 results). This survey identified a total of 318 published articles, but after scrutiny only 42 included original research evidence focused on the involvement of vitamin D in COVID‐19, published between 2019 and 2022. The selected studies tested the role of vitamin D in diverse respiratory dysfunctions including in vitro and ex vivo findings. Evidence pertaining to the impact of vitamin D in comorbidities, other organ injuries, and other viral manifestations were excluded from the present review.
2. POTENTIAL MOLECULAR MECHANISM OF VITAMIN D
The three main mechanisms involved in reducing microbial infection are physically oriented barriers, cellular immunity, and humoral immunity. 22 After administration, the binding of vitamin D to its receptors in the cell causes the activation of retinoid X receptors, resulting in the relocation of the vitamin D receptor element. The VDR controls the presence of several host genes, such as those for β‐defensin and cathelicidin. 23 Innate immunity of the cell is also enhanced through vitamin D by upregulation of human cathelicidin LL‐37, 1,25‐di‐hydroxy vitamin D, and defensins, and continuing tight junctions, adherens junctions, and gap junctions. 24 , 25 , 26 , 27 Cathelicidins have direct antipathogenic activity in response to a broad range of microorganisms, particularly bacteria (Gram‐positive/Gram‐negative), viruses (enveloped/non‐enveloped), and fungi. 28 The several functions performed by cathelicidin include promoting the triggering of inflammatory cascades and prompting chemotaxis of leukocytes such as monocytes, neutrophils, macrophages, and T lymphocytes. This further helps to clear pathogens from the respiratory tract by persuading apoptotic and autophagic events in epithelial cells exposed to the virus. 10 , 29 Vitamin D also impacts Toll‐like receptors, which play a significant role in the body's innate immune response because they recognize harmful proteins. 30 Vitamin D regulates several genes, such as defensins, which directly destroy virus membrane. When activated, VDR attaches to the VDR element of the cathelicidin promoter region, triggering host defense against viral infections. The release of nitric oxide is also enhanced by vitamin D, which helps in mediating innate immunity. This is exhibited primarily by dampening T‐cell proliferation and the resulting conversion of T helper type 1 (Th1) cells into Th2 cells. 31 , 32 Vitamin D also impacts the maturation of T cells and can further shift Th17 cells toward regulatory T cells, which are anti‐inflammatory. By following this mechanism, vitamin D downregulates pro‐inflammatory cytokines such as IL‐1, IL‐6, IL‐12, and TNF‐α. 33 , 34 , 35 Gene expression associated with anti‐oxidation is improved through vitamin D intake; as a result, glutathione production is enhanced, which shows antimicrobial activities, 36 , 37 , 38 regulatory T cell‐improving features of vitamin D are resolved either by direct or indirect signaling pathways that also influence tolerogenic dendritic cells. 39 Dendritic cells play a key role in vitamin D‐moderated immunoregulation. 40 In addition, vitamin D directly impedes the nuclear factor‐κB pathway, therefore lowering the generation of pro‐inflammatory mediators. Apparently, the control of cytokine regulation and differentiation of T cells shows the dual role of vitamin D in immunopathological conditions. 41 VDR and CYP27B1 may be significant target tissues for vitamin D in the endocrine system. Several studies have reported the downregulation of ACE2 receptors by vitamin D, hence preventing the manifestations of COVID‐19. 42 Figure 1 illustrates how vitamin D can boost the immune response.
FIGURE 1.
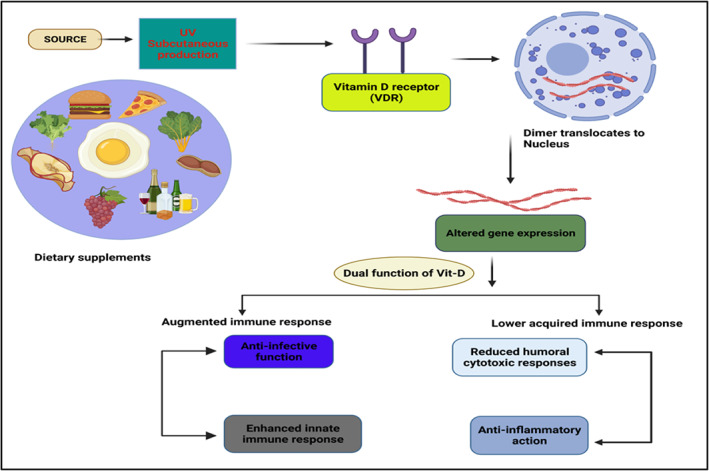
Representation of dual role of vitamin D on the immune system and inflammation. Vitamin D has the potential to alter expression of several genes, showing increased innate immune response and reduced acquired immune response. UV, ultraviolet rays; VDR, vitamin D receptor
3. INTERPLAY BETWEEN VITAMIN D AND ACE2 RECEPTOR
Acute respiratory distress syndrome is mainly due to endothelial defects and enhanced barrier permeability, resulting in increased concurrent diseases and death rates in SARS‐CoV‐2‐infected patients. The nutrition D‐mediated pathway might also keep away the risk of mild respiratory dysfunction. In addition, 1,25(OH)2 D3 is also responsible for suppressing renin, ACE, and angiotensin II (Ang II) expression and for increasing ACE‐2 in lipopolysaccharide‐mediated acute respiratory dysfunction. Nutrition D decreased lipopolysaccharide‐triggered lung injury by various means, including by partially prompting ACE2 and Ang (1‐7) axis action, resulting in the suppression of renin and the ACE and Ang II or angiotensin II receptor type 1 events and drastic exposure of VDR in the lungs. It was concluded that the deactivation of VDR led to dyshomeostasis in the renin‐angiotensin system. VDR and 1,25(OH)2 D are also found to attenuate fibrotic cascades in kidneys, lungs, and liver through negative feedback of the renin‐angiotensin system and the hindering of nuclear factor‐κB. 43 Besides mediating these protective functions, VDR also evades sepsis‐associated lung damage by interfering with the angiopoietin‐2‐TEK receptor triggering tyrosine kinase‐mediated myosin light‐chain kinase events. 44 Vitamin D administration diminishes the acute lung injury by abolishing various pro‐inflammatory actions, inhibiting lung epithelium‐endothelium barrier transfer, and adjusting the renin‐angiotensin system. The analog of vitamin D (Paricalcitol) prevents alveolar barrier function, alleviating lipopolysaccharide‐linked acute lung injury. Figure 2 depicts the entry mechanism of SARS‐CoV‐2 through host ACE2, and Figure 3 shows the impact of vitamin D on ACE2 inhibitors.
FIGURE 2.
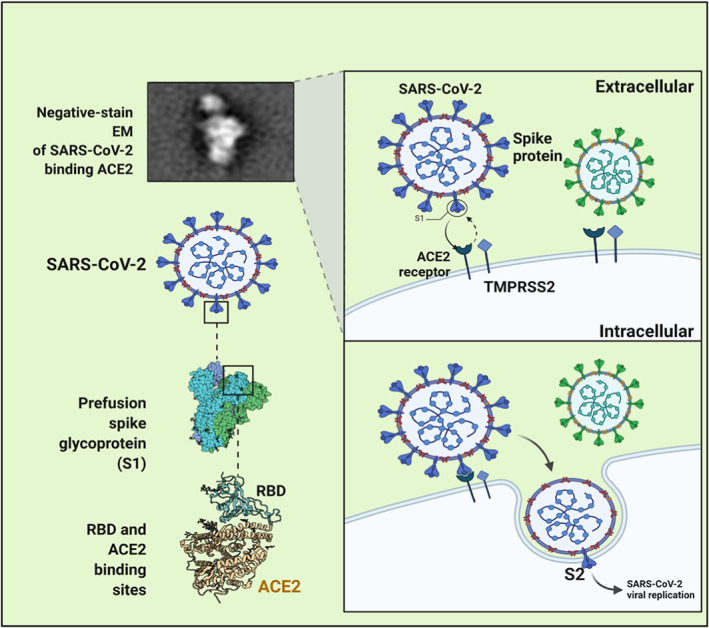
Entry mechanism of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) through host angiotensin‐converting enzyme 2 (ACE2). SARS‐CoV‐2 binds to the surface of host cells via several receptors (ACE2, neuropilin‐1, AXL, and antibody‐Fcγ receptor complexes). The spike (S) protein goes through conformational transition from prefusion to postfusion by using proteases (furin, TMPRSS2, and cathepsins). SARS‐CoV‐2 attaches with the cell receptor ACE2, which comprises integral membrane protein, and moves to the surface of cells after transcription with its N‐terminal signal peptide and binds by C‐terminal transmembrane domain. When receptor‐binding domain starts binding with the tips of one lobe of ACE2 the viral entry initiates
FIGURE 3.
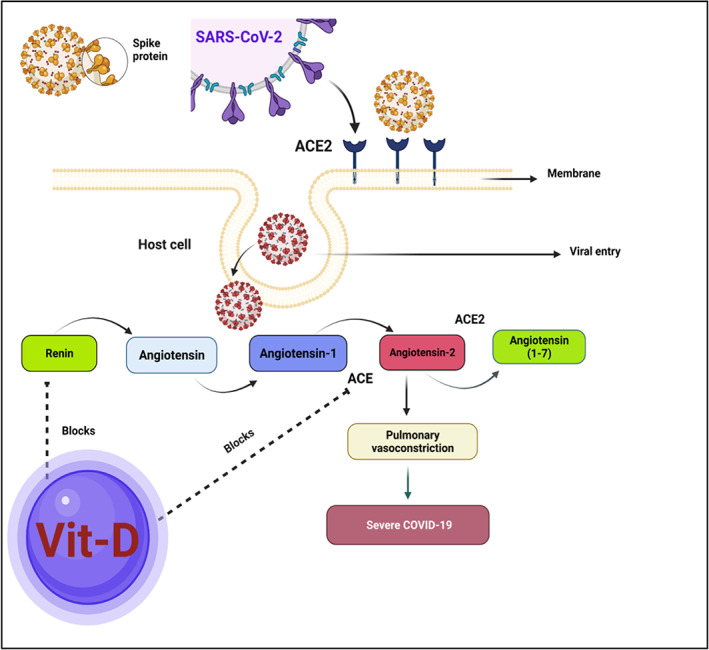
Vitamin D functions in the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) response after angiotensin‐converting enzyme (ACE). The ACE converts Ang I into Ang II. Vasoconstriction, inflammation, and apoptosis are caused by Ang II binding to the Ang II type 1 receptor. Ang (1‐7) counteracts Ang II's effects. As a result, the equilibrium between ACE and ACE2 levels affects the endogenous content of Ang II and Ang (1‐7) 8. Additionally, vitamin D also reduces RAS activity by diminishing renin
4. DOES VITAMIN D EXERT ANTI‐VIRAL POTENTIAL?
Several research and review studies have investigated if vitamin D can lower the chances of microbial infections. 45 A current review also described the active contribution of vitamin D supplementation in patients with COVID‐19, thereby reducing the mortality rate. 46 As observed in several rodent and human cell lines, lymphopenia is a common and significant complication in critically affected SARS‐CoV‐2 patients. Vitamin D can provide a protective mechanism for experimental interstitial pneumonitis. 47 Vitamin D therapy has been proven in a number of in vitro experiments to play a crucial role in maintaining local respiratory homeostasis, mainly via dual pathways: stimulation of the expression of antimicrobial peptides or by direct proliferation of the respiratory infection causing virus. 48 Several investigations have found that vitamin D has antiviral properties, mostly against enveloped viruses. In respiratory viral infections, vitamin D regulates the immune system by raising the number of CD8+ T cells that are virus‐specific (such as against influenza and Epstein‐Barr viruses). Vitamin D also enhances macrophage development and oxidative burst capability while decreasing autophagy (linked to viral replication).
5. THE LACK OF VITAMIN D IN COVID‐19 PATIENTS
Lips and co‐workers report that vitamin D levels are continuing to rise in the populations of around 40 nations. However, more than 50% deficiency is reported among elderly patients. Vitamin D deficiency is found worldwide and is a major undiagnosed and untreated dietary deficiency (with more than 1 billion people being deficient). 49 It was reported that human dipeptidyl peptidase‐4/CD26 has a link between the S1 domains of the SARS‐CoV‐2 spike glycoprotein, which may act as an ideal virulence feature in this infection. 50 This appearance of the dipeptidyl peptidase‐4/CD26 receptor can be minimized by administering careful vitamin D supplementation. It was also stated that adjusting vitamin D supplementation could decrease the adverse downstream immunological sequelae, such as an increase in IL‐6, and late interferon‐γ response in the infected victims. A recent study demonstrated the direct association between vitamin D scarcity and the SARS‐CoV‐2‐infected population. 51 It showed that those who were given the most vitamin D were at lower risk for SARS‐CoV‐2 infection. An additional study found that 84.6% of critically ill COVID‐19 patients in intensive care units (ICUs) were vitamin D deficient (12 ng/mL), compared with only 57.1% of patients in medical wards. 52 Also, COVID‐19 patients in ICUs had lower 25(OH)D serum concentrations than non‐ICU patients, verified in a study of 34 SARS‐CoV‐2‐infected patients. 53 In addition, another study found a link between noninvasive breathing assistance and dependence unit admission (P = 0.042), with older affected individuals having worse consequences. 54 Several studies have found that low vitamin D levels are linked to hospitalization of SARS‐CoV‐2‐infected patients. Moreover, serum vitamin D levels lower than 16 ng/mL have been associated with an enhanced threat of sepsis among critically ill patients. 55 The deficiency of vitamin D concentrations in serum concentrations quadrupled the likelihood of hospitalization owing to SARS‐CoV‐2 infection according to a large Israeli study cohort. A retrospective case study indicated that the serum concentrations of vitamin D among COVID‐19 patients were lower in critical cases compared with acute cases. 56 Not only deficiency factors but also several key parameters are responsible for the severity of infections in the elderly population, including ethnicity, male sex, socioeconomic factors, and concurrent diseases such as obesity, diabetes mellitus, and hypertensive states. 57 A pilot investigation (randomized clinical trial) stated in October 2020 that higher doses of Calcifediol (25(OH)D) can considerably decrease the viral load of ICU patients 58 and a clinical case series concluded that those who took high doses of vitamin D showed enhanced clinical recovery, lower oxygen requirement, and fewer days in hospital. 52 In addition, a study with realistic trial design analyzed in parallel the vitamin D3 states in the early stages of infection. The main motive of vitamin D (1,25(OH)2D3) supplementation is to boost the immune defense mechanism by decreasing pro‐inflammatory cytokine production. 59 Approximately 24% of US citizens and 37% of Canadians were found to be suffering from vitamin D deficiency. 60 Systematic reviews and meta‐analyses have illustrated that vitamin D can prevent respiratory infections. A retrospective multicenter study has reported that patients with vitamin D deficiency usually had worse results than patients with high vitamin D concentrations. 61
6. REGULATION OF AUTOPHAGY BY VITAMIN D
Autophagy is an essential role of the immune system because it safeguards against viral infections. 62 1,25(OH)2D3 influences autophagy by modulating various approaches, including the generation of apoptogenic molecules such as Bcl‐2 along with cell regulatory mechanisms including the mammalian target of rapamycin pathway, class III phosphatidylinositol 3‐kinase complex (an enzyme involved in cellular growth proliferation and differentiation), and cathelicidin biosynthesis. As a result, it accelerates virus clearance and diminishes the viral burden. Vitamin D suppresses SARS‐CoV‐2 replication by increasing autophagy in macrophages. 63 Resistance to SARS‐CoV‐2 may indeed be established with VDR and enough vitamin D. 29
7. IMMUNOMODULATORY FUNCTION OF VITAMIN D: IMMIGRATION OF NEUTROPHILS
Patients infected with SARS‐CoV2 frequently display a change in neutrophil percentage. Neutrophils are specialized cells that cause phagocytosis to protect early responses towards invading pathogens. 64 Microbicidal processes, phagocytic pathways, degranulation, and transfer of extracellular traps in neutrophils contribute to strengthening innate immune responses against infectious illnesses. Because neutrophil extracellular traps include specialized proteolytic enzymes that can degrade bacterial virulence factors, they have antibacterial activity. Neutrophil extracellular traps defend the host by increasing IL‐1 and interferon‐γ in activated neutrophils. 65
8. VITAMIN D DEFICIENCY: EFFECTS ON OXIDATIVE AND NITRATIVE STRESS
The inhibition of oxidative stress provides a promising effect against antiviral infection. Moreover, excessive induction of the oxidative mediators is linked to pathophysiology and tissue damage mechanisms. 66 The phosphatidyl inositol 3‐kinase signaling cascade decreases reactive oxygen species (ROS) and inducible nitric oxide synthase (iNOS) in immune cells, including monocytes and macrophages. The elegance III phosphatidylinositol 3‐kinase complex is an important signaling pathway regulated by 1,25(OH)2D3. As a result, 1,25(OH)2D3 plays a function in redox homeostasis in ROS and iNOS induction to improve antiviral activity and anti‐oxidative hindrance of iNOS, and induction of ROS scavenging pathways to block immunopathology. 32
9. THERAPEUTIC EFFECTS OF VITAMIN D IN COVID‐19 PATIENTS
Age, genetic predispositions, and pre‐existing chronic diseases are also responsible for the respiratory diseases associated with COVID‐19. 13 Vitamin D deficiency is also more common among the elderly and in communities receiving a low amount of vitamin D because of reduced vitamin D uptake and metabolism, mainly in African Americans. 67 Similarly, inadequate vitamin D levels are also prevalent in prolonged incurable diseases such as cardiovascular disease and hyperglycemia, leading to a possible indirect connection between vitamin D inadequacy and high risk of COVID‐19. 68 The previous study has also revealed that SARS‐CoV‐2‐infected patients living in South Asia and Switzerland have respiratory complications due to their vitamin D‐deprived state. 69 Another association was observed between vitamin D and SARS‐CoV‐2, where enhanced mortality rates were observed in northern US states that received less UVB compared with southern regions. Similarly, global data have shown greater rates of mortality where only an average vitamin D supplement is taken. 70 Vitamin D insufficiency is linked to several infections, including human immunodeficiency virus, tuberculosis, dengue, malaria, leprosy, as well as multiple sclerosis, inflammatory bowel disease, and cancer. 71 Low levels of vitamin D are also linked to hepatitis C and liver fibrosis. 72 Two randomized controlled trials (RCTs) report that proper supplementation of vitamin D can provide beneficial results against seasonal influenza. 73 A few limitations were seen in the RCTs, which included various individuals vaccinated against influenza who were given vitamins but showed no beneficial effect. 74 Levels of vitamin D above 50 ng/mL (125 nmoL/L) might be promising in decreasing the severity of COVID‐19. 75 Malek et al describe the role of vitamin D in the modulation of the detrimental endocrine renin‐angiotensin system and downregulated renin synthesis. It seems to restrict renin and the ACE axis, thus improving the expression of ACE2, Mas receptor (MasR), and Ang (1‐7) concentration, that play an important role in the amelioration of acute lung injury. Therefore, vitamin D could be a promising advance in the treatment of COVID‐19 and ARDS. 76 It can influence the activity of the ACE2/Ang (1‐7)/MasR and ACE2/Ang (1‐7) axes. It appears that vitamin D prophylaxis (avoiding overdosing) could help to lessen severity of COVID‐19, particularly in cases of vitamin D insufficiency. 77 Tsujino et al demonstrated that in lung tissue, activation of vitamin D3 results in a protective action against interstitial lung disease. 78 Martineau et al 47 also showed that a regular dose of approximately 2000 IU/day with no additional bolus requirement showed safe and protective action against the virus.
9.1. Function and outcomes of vitamin D in SARS‐CoV‐2 infections in other studies
Several clinical trials and cohort studies indicate the beneficial contribution made by vitamin D in SARS‐CoV‐2 infection. However, a few retrospective observational studies suggest an inter‐relationship between vitamin D and the associated SARS‐CoV‐2 infection. For example, a retrospective study conducted on SARS‐CoV‐2 among 212 patients from three different hospitals in South Asia that evaluated the vitamin D levels in collected serum samples concluded that the level of vitamin D was significantly different in four conditions; mild (78 nmol/L), ordinary cases (68.5 nmol/L), severe cases (53 nmol/L), and critically affected COVID‐19 patients, where the level was too low. The authors of this study indicated the significant correlation among clinical outcomes (P < 0.001) underlying vitamin D status. In Singapore, a small cohort study was conducted 79 among 43 SARS‐CoV‐2‐positive patients who were given a combination therapy of oral vitamin D dose of 1000 IU, magnesium (150 mg), and vitamin B12 (500 μg) and essential oxygen compared with a control group (3 out of 17 vs 16 out of 26; P = 0.006). The findings concluded that the combination treatment of vitamin D, magnesium, and vitamin B12 exerted a considerable defense against clinical pathophysiology due to infection (P = 0.041). Decrease in vitamin D concentration was also observed in critically ill COVID‐19 patients with a pre‐existing medical history. 80 In one of the retrospective observational studies conducted among 186 SARS‐CoV‐2‐positive survivors of COVID‐19, vitamin D levels were found to be significantly lower (P = 0.0016) when compared with the control group. Another study collected data sets from various regions of the world and reported a 15% decrease in disease rate after taking vitamin D supplementation. 81 In Indonesia, a retrospective cohort study was performed among 780 patients and found a higher death rate in patients with low levels of vitamin D. 82 Another retrospective study from the USA concluded that sunlight and vitamin D might reduce the risks of SARS‐CoV‐2 infection. 83
10. BENEFICIAL EFFECTS OF VITAMIN D UPTAKE
According to previous evidence, no data indicates that supplementing vitamin D can minimize the relative severity and high mortality rate of COVID‐19. Very few randomized clinical trials have been registered on the SARS‐CoV‐2‐infected population that compare their vitamin D levels. A small cohort study was conducted that validated the promising results of vitamin D in combination with vitamin B12 and magnesium among the SARS‐CoV‐2 infected patients. Regarding respiratory tract infection prevention, a meta‐analysis demonstrated that dietary vitamin D administration is a safe and robust agent against SARS‐CoV‐2 infections. 84 In addition, the study concluded that those with lower levels of vitamin D took the most advantage from this supplementation. In another identical study, subgroup evaluation specified that each daily or weekly consumption of dietary vitamin D (not more than extra bolus doses) could protect from acute upper respiratory infection, specifically in patients with a lack of dietary vitamin D. Vitamin D administration enhanced gene expression associated with anti‐oxidation (glutathione reductase modifier subunit). 85 Indeed, treatment of adults with vitamin D (100 μg/day) is safe. 36 , 86 The heterogeneity of the population and the dose regimens of vitamin D must be considered in the prevention of SARS‐CoV‐2 infections. Checking the influence of nutrition D administration in RCTs, and a serious serum‐based study of 25(OH)D instead of administered vitamin D dose are suggested. 87
11. VITAMIN D AND COVID‐19: SUMMARY OF THE EVIDENCE
11.1. Overview
The alleged function of vitamin D in the management or therapy of COVID‐19 is dynamic and complex. As previously mentioned, vitamin D has the ability to modulate various components of immunity, potentially affecting the severity and consequences of COVID‐19. Downregulation of ACE2 brought on by SARS‐CoV‐2 infections results in toxic Ang II build‐up, which in turn causes ARDS. It has been discovered that vitamin D reduces the consequences of these interactions between SARS‐CoV‐2 and the renin‐angiotensin‐aldosterone system (RAAS). 88 Vitamin D can activate the vasorelaxant ACE2/Ang (1‐7)/Mas receptor axis, a negative endocrine regulator on the RAAS that guards against acute lung injury and ARDS (Figure 4). By attaching to the VDR and suppressing the expression of renin‐producing enzymes and proteins, vitamin D inhibits the manufacture of renin. 89 It has also been demonstrated that vitamin D promotes ACE2 expression. Data suggest that differing expressions of ACE2, which generate a heightened and more robust immunological response in females, may be the reason for the differential presentation of COVID‐19 between male and female patients (higher likelihood of ICU admissions and death in males). 90 Because males express ACE2 at higher levels than females, its impact on the severity of COVID‐19 has been assessed. 91 A study employing an animal model discovered that females following oophorectomy showed higher ACE2 activity compared with before, demonstrating that many of these alterations can be linked to the presence of estrogen. 92 In essence, COVID‐19 is more severe in men because they express ACE2 more and do not benefit from estrogen's cardioprotective effects. Although intricate mechanisms for the cardiovascular consequences of COVID‐19 have been put forward, 93 it is apparent that damage has been done. 94 ACE2 and the RAAS have been linked in the process by which COVID‐19 substantially increases the strain on the cardiovascular system, and individuals who already have cardiovascular morbidities are more susceptible to problems and mortality. In a case study of 43 patients in China, it was discovered that men were more likely than women to have more severe COVID‐19. 95 This was also found in the USA and Europe. 96 Different amounts of 1,25(OH)2D3 and VDR expression may have a role in the disparities between males and females, according to other observational research. 97 Beyond the mechanistic explanation of how 1,25(OH)2D3 signaling modifies the immune response, women also benefit from vitamin D's synergistic action with estrogen in CD4+ T cells, which inhibits an autoimmune response. 98 However, it might be an oversimplification to attribute gender variations in COVID‐19 to ACE2 or vitamin D. This could be explained by fewer morbidities, in a manner that may be very varied because of individual variations, as estrogen confers a protective effect on cardiovascular and immune health. 99 It is likely that low vitamin D levels cause cytokine storms, poor defense against epithelial cell death, and inadequate epithelial cell repair, ultimately leaving the lungs susceptible to catastrophic immune system dysregulation. It is expected that vitamin D is a more effective immune system modulator than antibody treatment for halting the function of cytokines like IL‐6. 100 The action of the TMPRSS2 is likely to be inhibited by vitamin D and its physiologically active hydroxy derivatives. 101 As a result, the fusion of the viral spike protein and ACE2, which is necessary for viral entry into the host cell, is blocked. The physiologically active compounds that stop the serine proteases from interacting with each other are involved in a variety of biological processes. The involvement of vitamin D in COVID‐19 has also been verified by research employing genomics guided tracing to identify the targets of SARS‐CoV‐2. Glinsky 102 investigated vitamin D as a putative inhibitor of ACE2 expression and discovered that vitamin D appears to decrease ACE2 expression by way of the VDR and other transcription factors in human bronchial smooth muscle cells. Vitamin D influences expression levels of 84 of the 332 SARS‐CoV‐2 prey protein‐coding genes (25%). These prey proteins perform a variety of cellular processes that are interfered with by infection. This shows that vitamin D has the ability to interfere with the function of 19 out of 27 (or 70%) of the SARS‐CoV‐2 proteins, in addition to blocking the production of ACE2. Mechanistic investigations using transcriptome or metabolomic analysis are eagerly awaited to validate the involvement of vitamin D in SARS‐CoV‐2 infections. However, numerous observational studies and open‐label, RCTs have demonstrated a substantial relationship between vitamin D and COVID‐19 (Table 1). 52 , 59 , 62 , 67 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143
FIGURE 4.
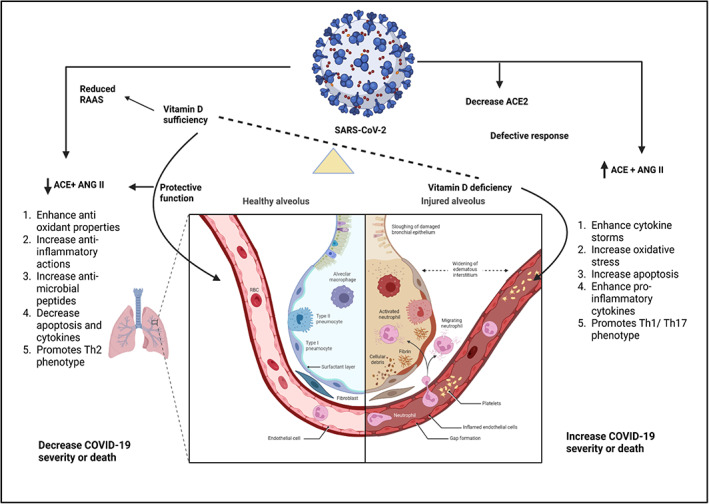
Possible methods by which adequate serum vitamin D levels could protect against COVID‐19 and acute lung injury, whereas vitamin D deficiency might cause a faulty immunological response to COVID‐19, leading to increased severity and/or fatality. Arrows pointing up or down denote a rise or reduction, respectively
TABLE 1.
Characteristics of studies examining the links between vitamin D and COVID‐19
| Study design | Sample size | Main findings | References |
|---|---|---|---|
| Correlation analysis of data | 88 countries | Countries lying near to the equator had fewer COVID‐19 fatalities than those lying farther away; latitude accounted for 16% of this difference | 103 |
| Cohort study | 105 | Lack of vitamin D was linked to increased care requirements and the development of cytokine storms | 104 |
| Retrospective cohort study | 107 | SARS‐CoV‐2‐positive individuals showed lower levels of 25(OH)D | 105 |
| Retrospective cohort study | 4314 | A higher risk of COVID‐19 was associated with inadequate vitamin D levels | 106 |
| Retrospective study | 185 | Lack of vitamin D was linked to an increased risk of invasive mechanical ventilation or death | 107 |
| Randomized prospective open‐label study | 87 | In people with hypovitaminosis D, vitamin D supplementation reduced inflammatory markers | 108 |
| Retrospective cohort study | 56 | A lack of vitamin D in patients with COVID‐19 reported greater levels of inflammatory markers along with considerably reduced level of hemoglobin and lymphocyte counts | 52 |
| Parallel pilot randomized open‐label trial | 76 | In patients who needed to be admitted to the ICU, high dosage calcifediol lessened the severity of COVID‐19 | 109 |
| Multi‐center parallel double‐blind RCT | 240 | When compared with placebo, a single high dose of cholecalciferol had no effect on hospital stay, death, ICU admission, or the need for ventilation | 110 |
| RCT | 40 | A higher percentage of asymptomatic or slightly symptomatic patients on high dosage vitamin D—25(OH)D >50 ng/mL achieved a negative SARS‐CoV‐2 RNA at less than 21 days—than those with vitamin D deficiency | 111 |
| Quasi‐randomized trial | 66 | In an elderly population, vitamin D administration before or after COVID‐19 decreased disease severity and fatality rates | 112 |
| Systematic review and meta‐analysis | 6 retrospective articles | A helpful prognostic indicator of COVID‐19 results could be vitamin D levels | 113 |
| Living Cochrane systematic review | 3 RCTs | The available information is insufficient to definitively determine the advantages or disadvantages of vitamin D supplementation as a COVID‐19 treatment | 114 |
| Open‐label, multicenter, superiority RCT | NA | In SARS‐CoV‐2‐positive patients exhibiting at least one symptom of elevated risk, a single dose of 50 000 IU of vitamin D was compared with a single dose of 200 000 IU of vitamin D. Incomplete results | 115 |
| Quasi‐experimental study | 77 | Before COVID‐19, regular vitamin D administration decreased mortality in elderly individuals at the 3‐month follow up | 116 |
| Systematic review and meta‐analysis | 21 studies | Low vitamin D levels are correlated with severe COVID‐19 illness, which may be explained by the fact that 25(OH)D is inversely correlated with pro‐inflammatory cytokines like interleukin‐6, an increase in C‐reactive protein, and cardiac insufficiency, which are related to the severity of COVID‐19 and its unfavorable outcomes. A 25(OH)D shortage and sensitivity to infection by COVID‐19 have not been linked causally, despite a linkage between higher vitamin D levels, immunological responses, and a better prognosis in other viral infections, according to testing and blood vitamin D tests in SARS‐CoV‐2 patients | 62, 117, 118 |
| Cohort study | 43 | If they did not need oxygen therapy, patients were given 500 μg/day of vitamin B12, 150 mg/day of magnesium, and 1000 IU/day of vitamin D3 (DMB) when they were admitted. Patients who received DMB had much less deterioration to the point of needing oxygen therapy or intensive care support, even after adjusting for demographics and hypertension | 119 |
| Retrospective cohort study | 499 | The incidence of COVID‐19 was associated with vitamin D deficiency status in addition to age and non‐white race. The likelihood of testing positive for COVID‐19 was not substantially associated with the amount of vitamin D supplementation | 120 |
| Retrospective observational analysis | 191 779 | Patients with inadequate 25(OH)D scores had a greater incidence of SARS‐CoV‐2 than patients with sufficient values and those with levels under 55 ng/mL | 121 |
| Observational study | 154 | Patients who were asymptomatic had mean vitamin D levels that were noticeably higher than patients who were gravely unwell. In people who were extremely unwell, vitamin D deficiency was more common. 90 out of 154 patients had vitamin D deficiency (29 asymptomatic; 61 severely ill). Patients with vitamin D deficiency had greater levels of inflammatory markers, an inflammatory response, and a higher mortality rate in their serum (21% vs 3.1%). Patients with severe COVID‐19 had noticeably low levels of vitamin D | 122 |
| Retrospective observational study | 42 | Patients with severe vitamin D deficiency had a 50% chance of dying after 10 days in the hospital, whereas those with insufficiency or moderate deficiency had a 5% chance. Hypovitaminosis D was found to be highly prevalent in 19 patients with acute respiratory failure who were receiving treatment in the ICU, which was associated with a significant mortality risk | 123 |
| Observational study | NA | Government statistics data on mortality were inversely associated with the mean vitamin D levels reported in various states and territories | 124 |
| Retrospective observational study | 444 | A lower incidence of COVID‐19 mortality was associated with the administration of cholecalciferol booster | 125 |
| Retrospective observational trial | 186 | Independent of age, chronic lung illness, and the severity score from the chest computed tomography, vitamin D deficiency at admission was linked to mortality | 126 |
| Retrospective observational study | 149 | A lower level of serum 25(OH)D was linked to higher mortality in COVID‐19 patients | 127 |
| Retrospective observational study | 464 | After sex was ruled out as a factor in COVID‐19 severity or fatality, levels of 25(OH)D <12 ng/mL were found to be strongly associated with an elevated risk of fatal disease and severe sickness | 128 |
| Cohort study | 646 | There is no proven link between low vitamin D levels and COVID‐19 severity or fatality | 129 |
| Observational cohort study | 445 | There was a link between hypocalcemia and the severity of COVID‐19, but there was no linkage between vitamin D and the severity or advancement of the disease. It is also accepted that calcium and vitamin D interact | 130 |
| Retrospective observational study | 1549 | But not mortality, hospital admission and critical care were associated with low vitamin D levels | 131 |
| Retrospective cohort study | 144 | The level of serum vitamin D was negatively linked with hospital mortality and the requirement for mechanical ventilation | 132 |
| Prospective observational study | 410 | There is no link between a vitamin D deficiency and the prevalence of severe COVID‐19, a rise in oxygen use, hospital admissions, or death. There was no appreciable change in the outcome in vitamin D‐deficient patients treated with cholecalciferol | 133 |
| Retrospective, multicenter, non‐randomized cohort study | 537 | After COVID‐19 diagnosis, calcifediol medication was significantly linked to a lower 30‐day mortality rate | 134 |
| Observational study | 157 | It was discovered that vitamin D supplementation had an adverse relationship with mortality in COVID‐19 patients | 135 |
| Cohort study | 1486 | The incidence of COVID‐19 was higher in patients with Parkinson's disease who were younger, heavier, and had chronic obstructive pulmonary disease. Patients who took vitamin D supplements had a lower likelihood of developing it | 136 |
| Retrospective study | 91 | In patients with comorbidities, two doses of 200 000 IU of vitamin D given on consecutive days can enhance outcomes (ICU admission, mortality) | 137 |
| Case‐control study | 60 039 | Statins that raised levels of 25(OH)D, such as rosuvastatin, were the only ones to have a protective effect in COVID‐19 | 138 |
| Retrospective survival study | 16 401 | Following the administration of vitamin D (calcifediol) 15‐30 days before hospitalization, there was a significant decrease in mortality | 139 |
| RCT | 69 | For 2 weeks, patients with mild to severe symptoms of COVID‐19 required less time to recover when given 5000 IU of vitamin D daily rather than 1000 IU. Over time, the interleukin‐6 and body mass index levels in both groups decreased | 140 |
| Population‐based cohort study | 108 343 | Infection rates were more likely to increase with lower serum 25(OH)D levels than disease severity or mortality. Patients with COVID‐19 who received vitamin D supplements compared with those who did not receive any supplements | 59 |
| Clinical case series | 4 | Treatment with calcifediol significantly decreased mortality and ICU admission in hospitalized COVID‐19 patients | 141 |
| Observational study | 838 | Treatment with calcifediol significantly decreased mortality and ICU admission in COVID‐19 patients who were hospitalized | 142 |
| Prospective study | 8297 | A decrease in COVID‐19 incidence was associated with routine vitamin D intake | 67 |
Abbreviations: COVID‐19, coronavirus disease 2019; RCT, randomized controlled trial; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
11.2. Observational studies
There have been various observational studies looking into the role that vitamin D plays in SARS‐CoV‐2 infections. A significant cross‐sectional study that looked at the geographic distribution of COVID‐19 in the USA discovered associations between outcomes including illness severity and COVID‐19 deaths and sunlight exposure as a proxy for vitamin D. 103 In a cohort study of an elderly population, it was found that patients with COVID‐19 who were admitted to a hospital in the UK had lower serum levels of vitamin D compared with healthy controls, and that there was a pronounced correlation between vitamin D deficiency and needing more care or ventilation. Additionally, Baktash et al, 104 reported that a lack of vitamin D is linked to a higher frequency of cytokine storms. Similarly, those who tested positive for SARS‐CoV‐2 were more likely to have lower circulating 25(OH)D concentrations in two trials involving 107 and 4314 patients in Switzerland and Chicago, respectively. 107 Others have discovered links between having enough vitamin D and a lower incidence of COVID‐19, but not in terms of what happens following infection. Some research, however, has presented contradictory, unfavorable findings. Overall, the data point to the significance of the association between vitamin D and COVID‐19, an association that calls for further research through large‐scale, nationally representative investigations. There are obviously many confounding variables in observational data, and up to this point, there have not been any randomized controlled studies to determine whether vitamin D specifically has a role in COVID‐19 susceptibility and consequences. Notably, this narrative review presents some recent associative evidence on the relationship between vitamin D and COVID‐19. One questionnaire‐based study of 1486 Parkinson's patients from Italy found that those who used vitamin D supplements had a lower risk of having COVID‐19. 144 Patients who tested positive for SARS‐CoV‐2 by polymerase chain reaction had decreased serum levels of vitamin D, according to a second small Italian investigation (n = 107). 143 The chance of testing positive for COVID‐19 rose when vitamin D levels were low, according to a third study from the USA (n = 4314). 119
11.3. Clinical trials
In addition to receiving normal therapy, supplementing with vitamin D significantly reduced the inflammatory markers in 87 patients with COVID‐19 and hypovitaminosis D, according to a randomized prospective open‐label study conducted in India. 108 In comparison to individuals receiving no supplements, levels of C‐reactive protein, lactate dehydrogenase, IL‐6, ferritin, and neutrophil to lymphocyte ratios significantly decreased in the patients receiving 60 000 IU of vitamin D supplementation daily for 8 days. A retrospective cohort research that included COVID‐19 patients who were vitamin D deficient found that they had considerably lower lymphocyte and hemoglobin counts and higher levels of inflammatory markers, such as C‐reactive protein. 52 In addition, patients with vitamin D deficiencies were more likely to need oxygen therapy and were more likely to develop pneumonia if their vitamin D levels had not been raised 6 months before being infected by SARS‐CoV‐2. The effectiveness of oral calcifediol, azithromycin, and hydroxychloroquine in treating COVID‐19 was examined in an RCT in Spain. They reported that adequate vitamin D levels brought on by calcifediol supplementation were the most obvious determinant in determining disease prognosis, even when hydroxychloroquine and azithromycin were given in accordance with standard of care treatment. In an RCT conducted in India by Rastogi et al, 110 it was discovered that asymptomatic or mildly symptomatic patients could achieve a negative SARS‐CoV‐2 RNA by day 21 when given high‐dose vitamin D (60 000 IU of daily cholecalciferol for 7 days), achieving a therapeutic target of 25(OH)D greater than 50 ng/mL. In contrast, a different RCT in Brazil found that among 240 hospitalized COVID‐19 patients, a single high dose of 200 000 IU of cholecalciferol had no difference from placebo in terms of reducing hospital length of stay, in‐hospital mortality, admission to the intensive care unit, or need for mechanical ventilation. It should be emphasized that individuals in this trial were given a variety of concurrent drugs and vitamin D after a considerable amount of time had passed since the onset of symptoms (mean 10.3 days). Therefore, it is uncertain if the delayed results may be to blame and whether early or preventive vitamin D treatment may be helpful in treating mild or moderate COVID‐19. In fact, it was found that vitamin D administration during or immediately before COVID‐19 was related to less severe disease and reduced mortality rates in 66 elderly residents of a French nursing home participating in quasi‐randomized research looking into possible infection prevention. 111 The National Institute for Health and Care Excellence assessment includes 12 observational studies examining relationships between serum vitamin D concentrations and the occurrence or treatment of COVID‐19, one small RCT of vitamin D as a therapy, and no trials of vitamin D as a preventive measure. 145
12. RECOMMENDED DOSE
Several recommended doses have been approved depending on various circumstances. Table 2 describes the probable doses. 13 , 146 , 147 , 148 , 149 , 150 , 151
TABLE 2.
Recommended vitamin D dose
| S. No | Suggested doses |
|---|---|
| 1. | With the slight increase in serum concentration of 25(OH)D, the degree of protection also increases; however, the optimal range lies between 40‐60 ng/mL (100‐150 nmol/L). To gain these levels, approximately 2000‐5000 IU/day of vitamin D intake is required in 50% of the population 145 |
| 2. | To sustain 30 ng/mL of the serum concentration of 25(OH)D, administration of several loading doses is needed. One study employed a dose of 100 000‐200 000 IU weekly for 8 consecutive weeks (1800 or 3600 IU/day) 146 |
| 3. | Clinical evidence suggests that either weekly or daily consumption of vitamin D is more efficacious than bolus doses in patients experiencing lung infections. Still, a substantial intake of vitamin D can indeed be deleterious and toxic 147 , 148 |
| 4. | To avoid infection, some studies suggest taking a high amount of vitamin D once a day 149 |
| 5. | High doses (600 000 IU administered orally once) should therefore be avoided because they significantly increase the chance of toxicity |
| 6. | According to the previous research, taking 10 000 IU every day for 1 month will enhance serum 25(OH)D concentrations into the ideal range of 40‐60 ng/mL. 150 To maintain that level, the dose is lowered to 2000‐3000 IU/day after the first month |
| 7. | Patients hospitalized with COVID‐19 should have initial basal serum 25(OH)D amounts evaluated and supplemented to a level of approximately 30 ng/mL (ideally 40‐60 ng/mL), specifically if the baseline level is far less than 10 ng/mL, as this deficit is substantially more prevalent in male patients 151 |
| 8. | In SARS‐CoV‐2‐infected patients with 25(OH)D serum concentrations <20 ng/mL, the suggested dose is 6000‐7000 oral IU/day for 6‐8 weeks. For protection, the dose may vary from 2000‐3000 oral IU/day depending on the age and clinical situation |
| 9. | Vitamin D administration is required when the baseline plasma concentrations of SARS‐CoV‐2‐infected hospitalized patients are <30 ng/mL (optimal 40‐60 ng/mL), especially when the baseline level is 10 ng/mL |
| 10. | While baseline 25(OH)D concentrations in infected patients cannot be determined, 2000‐3000 oral IU per day is suggested |
13. LIMITATIONS AND FUTURE DIRECTIONS
The rapid evolution of SARS‐CoV‐2 and its newly appearing, little known impacts on the immune system severely restrict the field's ability to advance. Re‐examining what little is known is necessary in the light of this phenomenon where more infectious or virulent virus strains result in negative effects even in younger populations that are considered to be more immune‐resilient. Even though the evidence now available strongly supports the concept that vitamin D plays a role, it is not conclusive; and some studies have found no connection between vitamin D and COVID‐19 when other confounding variables are taken into account. 152 Furthermore, the underlying etiology of the vitamin D deficiency, and consequently, the immunological dysfunction identified in COVID‐19, are still up for debate. It has also been suggested that, rather than a shortage of vitamin D itself, diabetes and obesity (both of which are accompanied by vitamin D‐deficient states) are to blame for the rise in COVID‐19 mortality. 153 Others make the case for the “healthy user effect,” which states that people with healthy diets and regular exercise spend more time outside and have better vitamin D levels as a result. This demonstrates associations, and not causalities, between vitamin D insufficiency and poor COVID‐19 outcomes. Taken together, these differences make it impossible to draw firm conclusions and emphasize the necessity of well thought out, appropriately powered experiments to ascertain the function of vitamin D in COVID‐19. There are currently 21 active trials that could provide some insight into this subject in the near future, according to a recent Cochrane review. Vitamin D deficiency has also been advised to be treated preventatively as it lessens the severity of disease following infection. This needs to be taken into account and needs further examination in light of other risk factors like aging and age‐related health degradation. 19 Overall, there is still much left to be done to assess the immunological implications of the mutant SARS‐CoV‐2 strains. Additionally, more research has to be done on the mechanistic processes underpinning the cellular and molecular activities of vitamin D concentrations in the context of COVID‐19.
14. SIGNIFICANCE OF THE VITAMIN D AND SARS‐COV‐2 STUDIES
There has been plenty of discussion on the effect of vitamin D on SARS‐CoV‐2 infection, hospitalization, and mortality rates. Vitamin D deficiency is likely to be a significant factor in transmission and complications, according to a large body of evidence, including known COVID‐19 immunity pathways, vitamin D physiology and its effects on the immune system, and population‐based studies linking vitamin D levels to respiratory infections. 154 Observational studies comparing outcomes across nations show an inverse relationship between vitamin D status and the severity of COVID‐19 disease and its associated mortality, suggesting that vitamin D may influence the immune system's response to infection. 155 In particular, Spain and Italy have recorded the highest rates of COVID‐19 infection and deaths worldwide, as well as high rates of vitamin D insufficiency. In contrast, the Nordic nations have a lower incidence of COVID‐19 infection and death as well as higher levels of vitamin D due to systematic dietary fortification. Other statistics, however, cast doubt on such a connection. For instance, Brazil, a country on the equator, has high rates of both cases and mortality from COVID‐19, whereas Greece, a country with a prevalence of vitamin D deficiency (25(OH)D 20 ng/mL) of at least 50% over a wide age range, 156 is among the nations with the lowest numbers of confirmed COVID‐19 cases and deaths. The results of well‐conducted RCTs demonstrating the impact of vitamin D on COVID‐19 clinical outcomes should be awaited by the entire world. It is only reasonable to wonder if vitamin D could prevent COVID‐19 given that the earlier research studies have shown that it could prevent acute respiratory infection. This topic was covered by Rhodes et al in a narrative review that was published in the Journal of Internal Medicine. 157 The authors compared the mortality of COVID‐19 in relation to the latitude of various nations in order to first establish a definitive association between vitamin D levels and COVID‐19. After adjusting for age, they discovered a 4.4% rise in mortality for every degree of latitude north of 28°. This finding suggests that indirect vitamin D from UV light may have a role in COVID‐19 protection. Furthermore, they draw attention to the fact that risk variables for vitamin D insufficiency overlap with those linked to COVID‐19 death (old age, ethnicity, male sex, obesity, diabetes, and hypertension). The “healthy user effect,” which states that healthier people just spend more time outside and eat more than people who are less healthy, may entirely explain this overlap, despite the fact that it is striking and merits further study. Although there is no direct evidence linking vitamin D levels to the occurrence or results of COVID‐19, there is indirect evidence that vitamin D has an immunomodulatory role in respiratory infections. The similarity of the risk variables for severe COVID‐19 and vitamin D deficiency, including older age, obesity, and minority ethnicity, constitutes additional indirect evidence. In high‐latitude nations, there is a link between the seasonal drop in vitamin D serum concentrations and a larger burden of COVID‐19. 158 Together, the available data make a strong justification for additional studies.
15. CONCLUSION
It has been observed that supplementation of vitamin D plays a major role in the prevention of infections. The defensive role of vitamin D against infections related to the respiratory system has been proven in various randomized clinical trials and meta‐analyses. It has been suggested that vitamin D supplementation be administered in the current pandemic situation to sustain the optimal levels of 25(OH)D circulating in the body (75‐125 nmol/L). Several studies indicate the association of COVID‐19 cases with abnormal vitamin D levels. Maintaining vitamin D serum concentrations (40 and 60 ng/mL) over the year may decrease the risk of respiratory tract infections and SARS‐CoV‐2 infections. Depending on the pandemic wave, large‐scale clinical trials may provide a more accurate picture of the relation between vitamin D deficiency and SARS‐CoV‐2. More studies are needed to study the role of vitamin D on the replication of SARS‐CoV‐2 and the inflection of ACE2. Immune responses against the virus can be achieved by regulating the vitamin D‐dependent mucosal microbiota, particularly in cystic fibrosis cases. Vitamin D is proven to possess multiple immune‐modulating actions, so it seems crucial to consider the prophylactic supplementation and co‐administration of vitamin D to invoke an effective therapeutic benefit in the modulation of the immune system and reduce the severity of SARS‐CoV‐2 infection, especially in elderly patients. Yet, more clinical research, including randomized and controlled clinical trials and large‐scale cohort investigations, are essential to further understand the therapeutic mechanisms of vitamin D in COVID‐19.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
ACKNOWLEDGEMENT
The authors are grateful to the Graduate School of Health, University of Technology Sydney, Australia for its support. Keshav Raj Paudel is supported by a fellowship from Prevent Cancer Foundation and the International Association for the Study of Lung Cancer. Kamal Dua is supported by project grants from the Rebecca L Cooper Medical Research Foundation. Open access publishing facilitated by University of Technology Sydney, as part of the Wiley ‐ University of Technology Sydney agreement via the Council of Australian University Librarians.
Ashique S, Gupta K, Gupta G, et al. Vitamin D—A prominent immunomodulator to prevent COVID‐19 infection. Int J Rheum Dis. 2023;26:13‐30. doi: 10.1111/1756-185X.14477
Contributor Information
Dinesh Kumar Chellappan, Email: dinesh_kumar@imu.edu.my.
Kamal Dua, Email: kamalpharmacist02@gmail.com.
REFERENCES
- 1. Magrone T, Magrone M, Jirillo E. Focus on receptors for coronaviruses with special reference to angiotensin‐converting enzyme 2 as a potential drug target‐a perspective. Endocr Metab Immune Disord. 2020;20(6):807‐811. [DOI] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L. Vitamin D3: a helpful immuno‐modulator. Immunology. 2011;134(2):123‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Manion M, Hullsiek KH, Wilson EM, et al. Vitamin D deficiency is associated with IL‐6 levels and monocyte activation in HIV‐infected persons. PLoS One. 2017;12(5):e0175517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jose RJ, Manuel A. COVID‐19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8(6):e46‐e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment, and prevention. Rev Endocrine Metabolic Dis. 2017;18(2):153‐165. [DOI] [PubMed] [Google Scholar]
- 7. Dankers W, Colin EM, van Hamburg JP, Lubberts E. Vitamin D in autoimmunity: molecular mechanisms and therapeutic potential. Front Immunol. 2017;7:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Infante M, Ricordi C, Sanchez J, et al. Influence of vitamin D on islet autoimmunity and beta‐cell function in type 1 diabetes. Nutrients. 2019;11(9):2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bouillon R, Marcocci C, Carmeliet G, et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr Rev. 2019;40(4):1109‐1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greiller CL, Martineau AR. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7(6):4240‐4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up‐regulated in myeloid cells by 1, 25‐dihydroxyvitamin D3. FASEB J. 2005;19(9):1067‐1077. [DOI] [PubMed] [Google Scholar]
- 12. Wang TT, Dabbas B, Laperriere D, et al. Direct and indirect induction by 1, 25‐dihydroxyvitamin D3 of the NOD2/CARD15‐defensin β2 innate immune pathway defective in Crohn disease. J Biol Chem. 2010;285(4):2227‐2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grant WB, Lahore H, McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID‐19 infections and deaths. Nutrients. 2020;12(4):988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Watkins J. Preventing a covid‐19 pandemic. BMJ. 2020;368:m810. [DOI] [PubMed] [Google Scholar]
- 15. Daneshkhah A, Agrawal V, Eshein A, Subramanian H, Roy HK, Backman V. The possible role of vitamin D in suppressing cytokine storm and associated mortality in COVID‐19 patients. Med Rxiv. 2020. [Google Scholar]
- 16. Darling AL, Ahmadi KR, Ward KA, et al. Vitamin D status, body mass index, ethnicity and COVID‐19: initial analysis of the first‐reported UK biobank COVID‐19 positive cases (n 580) compared with negative controls (n 723). Med Rxiv. 2020. [Google Scholar]
- 17. De Smet D, De Smet K, Herroelen P, Gryspeerdt S, Martens GA. Vitamin D deficiency as risk factor for severe COVID‐19: a convergence of two pandemics. Med Rxiv. 2020. [Google Scholar]
- 18. Hasti CE, Mackay DF, Ho F, et al. Vitamin D concentrations and COVID‐19 infection in UK biobank. Diabetes Metab Syndr. 2020;14(4):561‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020;32(7):1195‐1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lau FH, Majumder R, Torabi R, et al. Vitamin D insufficiency is prevalent in severe COVID‐19. Med Rxiv. 2020. [Google Scholar]
- 21. Zemb P, Bergman P, Camargo CA Jr, et al. Vitamin D deficiency and the COVID‐19 pandemic. J Glob Antimicrob Resist. 2020;22:133–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rondanelli M, Miccono A, Lamburghini S, et al. Self‐care for common colds: the pivotal role of vitamin D, vitamin C, zinc, and echinacea in three main immune interactive clusters (physical barriers, innate and adaptive immunity) involved during an episode of common colds—practical advice on dosages and on the time to take these nutrients/botanicals in order to prevent or treat common colds. Evid Based Complement Alternat Med. 2018;2018:5813095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21(3):319‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schwalfenberg GK. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol Nutr Food Res. 2011;55(1):96‐108. [DOI] [PubMed] [Google Scholar]
- 25. Murdaca G, Pioggia G, Negrini S. Vitamin D and Covid‐19: an update on evidence and potential therapeutic implications. Clinic Mol Allergy. 2020;18(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adams JS, Ren S, Liu PT, et al. Vitamin d‐directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182(7):4289‐4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laaksi I. Vitamin D and respiratory infection in adults. Proc Nutr Soc. 2012;71(1):90‐97. [DOI] [PubMed] [Google Scholar]
- 28. Herr C, Shaykhiev R, Bals R. The role of cathelicidin and defensins in pulmonary inflammatory diseases. Exp Opinion Biol Therapy. 2007;7(9):1449‐1461. [DOI] [PubMed] [Google Scholar]
- 29. Yuk JM, Shin DM, Lee HM, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6(3):231‐243. [DOI] [PubMed] [Google Scholar]
- 30. Liu PT, Stenger S, Li H, et al. Toll‐like receptor triggering of a vitamin D‐mediated human antimicrobial response. Science. 2006;311(5768):1770‐1773. [DOI] [PubMed] [Google Scholar]
- 31. Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Therap. 2008;324(1):23‐33. [DOI] [PubMed] [Google Scholar]
- 32. Sly LM, Lopez M, Nauseef WM, Reiner NE. 1α, 25‐Dihydroxyvitamin D3‐induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3‐kinase and mediated by the NADPH‐dependent phagocyte oxidase. J Biol Chem. 2001;276(38):35482‐35493. [DOI] [PubMed] [Google Scholar]
- 33. Cantorna MT, Snyder L, Lin YD, Yang L. Vitamin D and 1, 25 (OH) 2D regulation of T cells. Nutrients. 2015;7(4):3011‐3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jeffery LE, Burke F, Mura M, et al. 1, 25‐Dihydroxyvitamin D3 and IL‐2 combine to inhibit T cell production of inflammatory cytokines and promote the development of regulatory T cells expressing CTLA‐4 and FoxP3. J Immunol. 2009;183(9):5458‐5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aranow C. Vitamin D and the immune system. J Invest Med. 2011;59(6):881‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lei GS, Zhang C, Cheng BH, Lee CH. Mechanisms of action of vitamin D as supplemental therapy for pneumocystis pneumonia. Antimicrob Agents Chemother. 2017;61(10):e01226‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mousavi S, Bereswill S, Heimesaat MM. Immunomodulatory and antimicrobial effects of vitamin C. Eur J Microbio Immunol. 2019;9(3):73‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Colunga Biancatelli RM, Berrill M, Marik PE. The antiviral properties of vitamin C. Exp Rev Anti‐Infective Therapy. 2020;18(2):99‐101. [DOI] [PubMed] [Google Scholar]
- 39. Penna G, Amuchastegui S, Giarratana N, et al. 1, 25‐Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J Immunol. 2007;178(1):145‐153. [DOI] [PubMed] [Google Scholar]
- 40. Széles L, Keresztes G, Töröcsik D, et al. 1, 25‐dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J Immunol. 2009;182(4):2074‐2083. [DOI] [PubMed] [Google Scholar]
- 41. Chen Y, Zhang J, Ge X, Du J, Deb DK, Li YC. Vitamin D receptor inhibits nuclear factor κB activation by interacting with IκB kinase β protein. J Biol Chem. 2013;288(27):19450‐19458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arboleda JF, Urcuqui‐Inchima S. Vitamin D supplementation: a potential approach for coronavirus/covid‐19 therapeutics. Front Immunol. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beilfuss A, Sowa JP, Sydor S, et al. Vitamin D counteracts fibrogenic TGF‐β signalling in human hepatic stellate cells both receptor‐dependently and independently. Gut. 2015;64(5):791‐799. [DOI] [PubMed] [Google Scholar]
- 44. Kong J, Zhu X, Shi Y, et al. VDR attenuates acute lung injury by blocking ang‐2‐Tie‐2 pathway and renin‐angiotensin system. Mol Endocrinol. 2013;27(12):2116‐2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gruber‐Bzura BM. Vitamin D and influenza—prevention or therapy. Int J Mol Sci. 2018;19(8):2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Singh S, Kola P, Kaur D, et al. Therapeutic Potential of Nutraceuticals and Dietary Supplements in the Prevention of Viral Diseases: A Review. Front Nutr. 2021;8:679312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Martineau AR, MacLaughlin BD, Hooper RL, et al. Double‐blind randomised placebo‐controlled trial of bolus‐dose vitamin D3 supplementation in adults with asthma (ViDiAs). Thorax. 2015;70:451‐457. [DOI] [PubMed] [Google Scholar]
- 48. Zdrenghea MT, Makrinioti H, Bagacean C, Bush A, Johnston SL, Stanciu LA. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev Med Virol. 2017;27(1):e1909. [DOI] [PubMed] [Google Scholar]
- 49. Lips P, Cashman KD, Lamberg‐Allardt C, et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. European Journal of Endocrinology. 2019;180(4):P23–P54. [DOI] [PubMed] [Google Scholar]
- 50. Vankadari N, Wilce JA. Emerging COVID‐19 coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect. 2020;9(1):601‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of vitamin D deficiency and treatment with COVID‐19 incidence. Med Rxiv. 2020. [Google Scholar]
- 52. Castillo ME, Costa LM, Barrios JM, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID‐19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Allegra A, Tonacci A, Pioggia G, Musolino C, Gangemi S. Vitamin deficiency as risk factor for SARS‐CoV‐2 infection: correlation with susceptibility and prognosis. Eur Rev Med Pharmacol Sci. 2020;24(18):9721‐9738. [DOI] [PubMed] [Google Scholar]
- 54. Panagiotou G, Tee SA, Ihsan Y, et al. Low serum 25‐hydroxyvitamin D (25 [OH] D) levels in patients hospitalized with COVID‐19 are associated with greater disease severity. Clin Endocrinol (Oxf). 2020;93(4):508‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Baktash V, Hosack T, Patel N. Vitamin D status and outcomes for hospitalized older patients with COVID‐19. Postgrad Med J. 2020;97(1149):442‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mardani R, Alamdary A, Nasab SM, Gholami R, Ahmadi N, Gholami A. Association of vitamin D with the modulation of the disease severity in COVID‐19. Virus Res. 2020;289:198148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Merzon E, Tworowski D, Gorohovski A, et al. Low plasma 25 (OH) vitamin D level is associated with increased risk of COVID‐19 infection: an Israeli population‐based study. FEBS J. 2020;287(17):3693‐3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rhodes JM, Subramanian S, Laird E, Griffin G, Kenny RA. Perspective: vitamin D deficiency and COVID‐19 severity–plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. J Intern Med. 2021;289(1):97‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ohaegbulam KC, Swalih M, Patel P, Smith MA, Perrin R. Vitamin D supplementation in COVID‐19 patients: a clinical case series. Amer J Therapeutics. 2020;e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Quesada‐Gomez JM, Entrenas‐Castillo M, Bouillon R. Vitamin D receptor stimulation to reduce acute respiratory distress syndrome (ARDS) in patients with coronavirus SARS‐CoV‐2 infections: revised Ms SBMB 2020_166. J Steroid Biochem Mol Biol. 2020;202:105719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Euro J Clinic Nutr. 2020;74(11):1498‐1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Alipio, M. Vitamin D supplementation could possibly improve clinical outcomes of patients infected with Coronavirus‐2019 (COVID‐19). 2020.
- 63. Wu S, Sun J. Vitamin D, vitamin D receptor, and macroautophagy in inflammation and infection. Discov Med. 2011;11(59):325. [PMC free article] [PubMed] [Google Scholar]
- 64. Høyer‐Hansen M, Nordbrandt SP, Jäättelä M. Autophagy as a basis for the health‐promoting effects of vitamin D. Trends Mol Med. 2010;16(7):295‐302. [DOI] [PubMed] [Google Scholar]
- 65. Hong CW. Current understanding in neutrophil differentiation and heterogeneity. Immune Net. 2017;17(5):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lescure FX, Bouadma L, Nguyen D, et al. Clinical and virological data of the first cases of COVID‐19 in Europe: a case series. Lancet Infect Dis. 2020;20(6):697‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Marik PE, Kory P, Varon J. Does vitamin D status impact mortality from SARS‐CoV‐2 infection. Med Drug Discov. 2020;6:100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Martens PJ, Gysemans C, Verstuyf A, Mathieu C. Vitamin D's effect on immune function. Nutrients. 2020;12(5):1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Christakos S, Hewison M, Gardner DG, et al. Vitamin D: beyond bone. Ann N Y Acad Sci. 2013;1287(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Khalil I, Barma P. Sub‐continental atmosphere and inherent immune system may have impact on novel Corona Virus' 2019 (nCovid‐19) prevalence in South East Asia. Mymensingh Med J. 2020;29(2):473‐480. [PubMed] [Google Scholar]
- 71. Benskin LL. A basic review of the preliminary evidence that COVID‐19 risk and severity is increased in vitamin D deficiency. Front Public Health. 2020;8:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Aloia JF, Li‐Ng M. RE: epidemic Influenza and Vitamin D. Epidemiol Infect. 2007;135(7):1095‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hoan NX, Van Tong H, Le Huu Song CG, Velavan TP. Vitamin D deficiency and hepatitis viruses‐associated liver diseases: a literature review. World J Gastroenterol. 2018;24(4):445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhou J, Du J, Huang L, Wang Y, Shi Y, Lin H. Preventive effects of vitamin D on seasonal influenza a in infants: a multicenter, randomized, open, controlled clinical trial. Pediatr Infect Dis J. 2018;37(8):749‐754. [DOI] [PubMed] [Google Scholar]
- 75. Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza a in schoolchildren. Am J Clin Nutr. 2010;91(5):1255‐1260. [DOI] [PubMed] [Google Scholar]
- 76. Malek Mahdavi A. A brief review of interplay between vitamin D and angiotensin‐converting enzyme 2: Implications for a potential treatment for COVID‐19. Rev Med Virol. 2020;30(5):e2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Iddir M, Brito A, Dingeo G, et al. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID‐19 crisis. Nutrients. 2020;12(6):1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tsujino I, Ushikoshi‐Nakayama R, Yamazaki T, Matsumoto N, Saito I. Pulmonary activation of vitamin D3 and preventive effect against interstitial pneumonia. J Clinic Biochem Nut. 2019;65(3):245‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Martineau AR. Vitamin D supplementation and musculoskeletal health. Lancet Diabetes Endocrinol. 2019;7(2):86‐87. [DOI] [PubMed] [Google Scholar]
- 80. Tan CW, Ho LP, Kalimuddin S, et al. A cohort study to evaluate the effect of combination vitamin D, magnesium and vitamin B12 (DMB) on progression to severe outcome in older COVID‐19 patients. Med Rxiv. 2020. [Google Scholar]
- 81. Glicio, EJ . Vitamin D level of mild and severe elderly cases of COVID‐19: a preliminary report. 2020.
- 82. Daneshkhah A, Agrawal V, Eshein A, et al. The possible role of vitamin D in suppressing cytokine storm and associated mortality in COVID‐19 patients [preprint]. Infect Dis. 2020. [Google Scholar]
- 83. Raharusun, P. ; Priambada, S. ; Budiarti, C. ; Agung, E. ; Budi, C. Patterns of COVID‐19 mortality and vitamin D: an Indonesian study. 2020.
- 84. Li Y, Li Q, Zhang N, Liu Z. Sunlight and vitamin D in the prevention of coronavirus disease (COVID‐19) infection and mortality in the United States. Sci Rep. 2020. [Google Scholar]
- 85. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta‐analysis of individual participant data. BMJ. 2017;356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Vieth R, Kimball S, Hu A, Walfish PG. Randomized comparison of the effects of the vitamin D 3 adequate intake versus 100 mcg (4000 IU) per day on biochemical responses and the wellbeing of patients. Nutr J. 2004;3(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bischoff‐Ferrari HA, Shao A, Dawson‐Hughes B, Hathcock J, Giovannucci E, Willett WC. Benefit–risk assessment of vitamin D supplementation. Osteopor Int. 2010;21(7):1121‐1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Giménez VMM, Sanz RL, Marón FJM, Ferder L, Manucha W. Vitamin D‐RAAS connection: an integrative standpoint into cardiovascular and neuroinflammatory disorders. Curr Protein Pept Sci. 2020;21:948‐954. [DOI] [PubMed] [Google Scholar]
- 89. Xu J, Yang J, Chen J, Luo Q, Zhang Q, Zhang H. Vitamin D alleviates lipopolysaccharide‐induced acute lung injury via regulation of the renin‐angiotensin system. Mol Med Rep. 2017;16:7432‐7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ambrosino I, Barbagelata E, Ortona E, et al. Gender differences in patients with COVID‐19: a narrative review. Monaldi Arch Chest Dis. 2020;90. [DOI] [PubMed] [Google Scholar]
- 91. Cai H. Sex difference and smoking predisposition in patients with COVID‐19. Lancet Respir Med. 2020;8:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Dalpiaz PL, Lamas AZ, Caliman IF, et al. Sex hormones promote opposite effects on ACE and ACE2 activity, hypertrophy and cardiac contractility in spontaneously hypertensive rats. PLoS ONE. 2015;10:e0127515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Samidurai A, Das A. Cardiovascular complications associated with COVID‐19 and potential therapeutic strategies. Int J Mol Sci. 2020;21:6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323:2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Jin JM, Bai P, He W, et al. Gender differences in patients with COVID‐19: focus on severity and mortality. Front Public Health. 2020;8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance–United States, January 22–may 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(24):759‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Muscogiuri G, Barrea L, Somma CD, et al. Sex differences of vitamin D status across BMI classes: an observational prospective cohort study. Nutrients. 2019;11:3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Spanier JA, Nashold FE, Mayne CG, Nelson CD, Hayes CE. Vitamin D and estrogen synergy in Vdr‐expressing CD4(+) T cells is essential to induce Helios(+)FoxP3(+) T cells and prevent autoimmune demyelinating disease. J Neuroimmunol. 2015;286:48‐58. [DOI] [PubMed] [Google Scholar]
- 99. Mukherjee S, Pahan K. Is COVID‐19 gender‐sensitive? J Neuroimmune Pharmacol. 2021;16:38‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Silberstein M. COVID‐19 and IL‐6: why vitamin D (probably) helps but tocilizumab might not. Eur J Pharmacol. 2021;899:174031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Song Y, Qayyum S, Greer RA, et al. Vitamin D3 and its hydroxyderivatives as promising drugs against covid‐19: a computational study. J Biomol Struct Dyn. 2021;1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Glinsky GV. Tripartite combination of candidate pandemic mitigation agents: vitamin D, quercetin, and estradiol manifest properties of medicinal agents for targeted mitigation of the COVID‐19 pandemic defined by genomics‐guided tracing of SARS‐CoV‐2 targets in human cells. Biomedicine. 2020;8:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Whittemore PB. COVID‐19 fatalities, latitude, sunlight, and vitamin D. Am J Infect Control. 2020;48:1042‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Baktash V, Hosack T, Patel N, et al. Vitamin D status and outcomes for hospitalised older patients with COVID‐19. Postgrad Med J. 2021;97:442‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of vitamin D status and other clinical characteristics with COVID‐19 test results. JAMA Netw Open. 2020;3:e2019722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Radujkovic A, Hippchen T, Tiwari‐Heckler S, Dreher S, Boxberger M, Merle U. Vitamin D deficiency and outcome of COVID‐19 patients. Nutrients. 2020;12:2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lakkireddy M, Gadiga SG, Malathi RD, et al. Impact of daily high dose oral vitamin D therapy on the inflammatory markers in patients with COVID 19 disease. Sci Rep. 2021;11:10641. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 108. Ünsal YA, Gül ÖÖ, Cander S, et al. Retrospective analysis of vitamin D status on ınflammatory markers and course of the disease in patients with COVID‐19 infection. J Endocrinol Invest. 2021;44(12):2601‐2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Murai IH, Fernandes AL, Sales LP, et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID‐19: a randomized clinical trial. JAMA. 2021;325:1053‐1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rastogi A, Bhansali A, Khare N, et al. Short term, high‐dose vitamin D supplementation for COVID‐19 disease: a randomised, placebo‐controlled, study (SHADE study). Postgrad Med J. 2020;98:87‐90. [DOI] [PubMed] [Google Scholar]
- 111. Annweiler C, Hanotte B, l'Eprevier CGD, Sabatier JM, Lafaie L, Célarier T. Vitamin D and survival in COVID‐19 patients: a quasi‐experimental study. J Steroid Biochem Mol Biol. 2020;204:105771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Munshi R, Hussein MH, Toraih EA, et al. Vitamin D insufficiency as a potential culprit in critical COVID‐19 patients. J Med Virol. 2021;93:733‐740. [DOI] [PubMed] [Google Scholar]
- 113. Stroehlein JK, Wallqvist J, Iannizzi C, et al. Vitamin D supplementation for the treatment of COVID‐19: a living systematic review. Cochrane Database Syst Rev. 2021;5:Cd015043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Annweiler C, Beaudenon M, Gautier J, et al. COvid‐19 and high‐dose VITamin D supplementation TRIAL in high‐risk older patients (COVIT‐TRIAL): study protocol for a randomized controlled trial. Trials. 2020;21:1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Annweiler G, Corvaisier M, Gautier J, et al. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID‐19 patients: the GERIA‐COVID quasi‐experimental study. Nutrients. 2020;12:3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Pereira M, Damascena AD, Azevedo LMG, Oliveira TDA, Santana JDM. Vitamin D deficiency aggravates COVID‐19: systematic review and meta‐analysis. Crit Rev Food Sci Nutr. 2020;62(5):1‐9. [DOI] [PubMed] [Google Scholar]
- 117. Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID‐19. Lancet Infect Dis. 2020;20:656‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tan CW, Ho LP, Kalimuddin S, et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B(12) in combination on progression to severe outcomes in older patients with coronavirus (COVID‐19). Nutrition. 2020;79:111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of Vitamin D Deficiency and Treatment with COVID‐19 incidence. MedRxiv. 2020. [Google Scholar]
- 120. Kaufman HW, Niles JK, Kroll MH, Bi C, Holick MF. SARS‐CoV‐2 positivity rates associated with circulating 25‐ hydroxyvitamin D levels. PLoS One. 2020;15:e0239252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Jain A, Chaurasia R, Sengar NS, Singh M, Mahor S, Narain S. Analysis of vitamin D level among asymptomatic and critically ill COVID‐19 patients and its correlation with inflammatory markers. Sci Rep. 2020;10:20191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Carpagnano GE, Lecce VD, Quaranta VN, et al. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID‐19. J Endocrinol Invest. 2021;44:765‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Padhi S, Suvankar S, Panda VK, Pati A, Panda AK. Lower levels of vitamin D are associated with SARS‐CoV‐2 infection and mortality in the Indian population: an observational study. Int Immunopharmacol. 2020;88:107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ling SF, Broad E, Murphy R, et al. High‐dose cholecalciferol booster therapy is associated with a reduced risk of mortality in patients with COVID‐19: a cross‐sectional multi‐Centre observational study. Nutrients. 2020;12:3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Smet DD, Smet KD, Herroelen P, Gryspeerdt S, Martens GA. Serum 25(OH)D level on hospital admission associated with COVID‐19 stage and mortality. Am J Clin Pathol. 2021;155:381‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Karahan S, Katkat F. Impact of serum 25(OH) vitamin D level on mortality in patients with COVID‐19 in Turkey. J Nutr Health Aging. 2021;25:189‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. AlSafar H, Grant WB, Hijazi R, et al. COVID‐19 disease severity and death in relation to vitamin D status among SARS‐CoV‐2‐positive UAE residents. Nutrients. 1714;2021:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Orchard L, Baldry M, Nasim‐Mohi M, et al. Vitamin‐D levels and intensive care unit outcomes of a cohort of critically ill COVID‐19 patients. Clin Chem Lab Med. 2021;59:1155‐1163. [DOI] [PubMed] [Google Scholar]
- 129. Osman W, Fahdi FA, Salmi IA, Khalili HA, Gokhale A, Khamis F. Serum calcium and vitamin D levels: correlation with severity of COVID‐19 in hospitalized patients in Royal Hospital. Oman Int J Infect Dis. 2021;107:153‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Diaz‐Curiel M, Cabello A, Arboiro‐Pinel R, et al. The relationship between 25(OH) vitamin D levels and COVID‐19 onset and disease course in Spanish patients. J Steroid Biochem Mol Biol. 2021;212:105928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Angelidi AM, Belanger MJ, Lorinsky MK, et al. Vitamin D status is associated with In‐hospital mortality and mechanical ventilation: a cohort of COVID‐19 hospitalized patients. Mayo Clin Proc. 2021;96:875‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jevalikar G, Mithal A, Singh A, et al. Lack of association of baseline 25‐hydroxyvitamin D levels with disease severity and mortality in Indian patients hospitalized for COVID‐19. Sci Rep. 2021;11:6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Alcala‐Diaz JF, Limia‐Perez L, Gomez‐Huelgas R, et al. Calcifediol treatment and hospital mortality due to COVID‐19: a cohort study. Nutrients. 1760;2021:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Cangiano B, Fatti LM, Danesi L, et al. Mortality in an Italian nursing home during COVID‐19 pandemic: correlation with gender, age, ADL, vitamin D supplementation, and limitations of the diagnostic tests. Aging. 2020;12:24522‐24534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Fasano A, Cereda E, Barichella M, et al. COVID‐19 in Parkinson's disease patients living in Lombardy. Italy Mov Disord. 2020;35:1089‐1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Giannini S, Passeri G, Tripepi G, et al. Effectiveness of In‐hospital cholecalciferol use on clinical outcomes in comorbid COVID‐19 patients: a hypothesis‐generating study. Nutrients. 2021;13:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Israel A, Schäffer AA, Cicurel A, et al. Identification of drugs associated with reduced severity of COVID‐19–a case‐control study in a large population. Elife. 2021;10:e68165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Loucera C, Peña‐Chilet M, Esteban‐Medina M, et al. Real world evidence of calcifediol use and mortality rate of COVID‐19 hospitalized in a large cohort of 16,401 Andalusian patients. MedRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Sabico S, Enani MA, Sheshah E, et al. Effects of a 2‐week 5000 IU versus 1000 IU vitamin D3 supplementation on recovery of symptoms in patients with mild to moderate Covid‐19: a randomized clinical trial. Nutrients. 2021;13:2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Oristrell J, Oliva JC, Casado E, et al. Vitamin D supplementation and COVID‐19 risk: a population‐based, cohort study. J Endocrinol Invest. 2021;45(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Nogues X, Ovejero D, Pineda‐Moncusí M, et al. Calcifediol treatment and COVID‐19‐related outcomes. J Clin Endocrinol Metab. 2021;106(10):e4017‐e4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ma H, Zhou T, Heianza Y, Qi L. Habitual use of vitamin D supplements and risk of coronavirus disease 2019 (COVID‐19) infection: a prospective study in UK biobank. Am J Clin Nutr. 2021;113:1275‐1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. D'Avolio A, Avataneo V, Manca A, et al. 25‐hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS‐CoV‐2. Nutrients. 2020;12:1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Fasano A, Cereda E, Barichella M, et al. COVID‐19 in Parkinson's disease patients living in Lombardy, Italy. Mov Disord. 2020;35:1089‐1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. NICE . Vitamin D for covid‐19: evidence reviews for the use of vitamin D supplementation as prevention and treatment of covid‐19. 2020. [PubMed]
- 146. Fabbri A, Infante M, Ricordi C. Editorial‐vitamin D status: a key modulator of innate immunity and natural defense from acute viral respiratory infections. Eur Rev MedPharmacol Sci. 2020;24(7):4048‐4052. [DOI] [PubMed] [Google Scholar]
- 147. Santos RN, Maeda SS, Jardim JR, Lazaretti‐Castro M. Reasons to avoid vitamin D deficiency during COVID‐19 pandemic. Archives Endocrinol Metabol. 2020;64(5):498‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Cipriani C, Romagnoli E, Pepe J, et al. Long‐term bioavailability after a single oral or intramuscular administration of 600,000 IU of ergocalciferol or cholecalciferol: implications for treatment and prophylaxis. J Clin Endocrinol Metab. 2013;98(7):2709‐2715. [DOI] [PubMed] [Google Scholar]
- 149. Martineau AR, Jolliffe DA, Greenberg L, et al. Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta‐analysis. Health Tech Assessment. 2019;23(2):1‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Marcinowska‐Suchowierska E, Kupisz‐Urbańska M, Łukaszkiewicz J, Płudowski P, Jones G. Vitamin D toxicity–a clinical perspective. Front Endocrinol. 2018;9:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Liu G, Hong T, Yang J. A single large dose of vitamin D could be used as a means of coronavirus disease 2019 prevention and treatment. Drug Des Devel Ther. 2020;14:3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Hastie CE, Mackay DF, Ho F, et al. Vitamin D concentrations and COVID‐19 infection in UK biobank. Diabetes Metab Syndr Clin Res Rev. 2020;14:561‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Weir EK, Thenappan T, Bhargava M, Chen Y. Does vitamin D deficiency increase the severity of COVID‐19? Clin Med. 2020;20:e107‐e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta‐analysis of individual participant data. BMJ. 2019;356:2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Daneshkhah A, Agrawal V, Eshein A et al. The possible role of vitamin D in suppressing cytokine storm and associated mortality in COVID‐19 patients, 2020, medRxiv preprint.
- 156. Grigoriou EV, Trovas G, Papaioannou N. Serum 25‐ hydroxyvitamin D status, quantitative ultrasound parameters, and their determinants in Greek population. Arch Osteoporos. 2018;13(1):111. [DOI] [PubMed] [Google Scholar]
- 157. Rhodes JS, Laird E, Griffin G, Kenny RA. Perspective: vitamin D deficiency and COVID‐19 severity ‐ plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2, and thrombosis (R1). J Intern Med. 2020;2:97‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kohlmeier M. Avoidance of vitamin D deficiency to slow the COVID‐19 pandemic. BMJ Nutr Prev Health. 2020;3:67‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]


