Abstract
Long coronavirus disease (COVID) or postacute sequelae of coronavirus disease of 2019 (COVID‐19) is widely reported but the data of long COVID after infection with the Omicron variant is limited. This study was conducted to estimate the incidence, characteristics of symptoms, and predictors of long COVID among COVID‐19 patients diagnosed during the Omicron wave in Eastern India. The cohort of COVID‐19 patients included were adults (≥18 years) diagnosed as severe acute respiratory syndrome coronavirus 2 positive with Reverse Transcription Polymerase Chain Reaction. After 28 days of diagnosis; participants were followed up with a telephonic interview to capture data on sociodemographic, clinical history, anthropometry, substance use, COVID‐19 vaccination status, acute COVID‐19 symptoms, and long COVID symptoms. The long COVID symptoms were self‐reported by the participants. Logistic regression was used to determine the predictors of long COVID. The median follow‐up of participants was 73 days (Interquartile range; 67–83). The final analysis had 524 participants' data; among them 8.2% (95% Confidence Interval [CI]: 6%–10.9%) self‐reported long COVID symptoms. Fatigue (34.9%) was the most common reported symptom followed by cough (27.9%). In multivariable logistic regression only two predictors were statistically significant—number of acute COVID‐19 symptoms ≥ five (Adjusted odds ratio (aOR) = 2.95, 95% CI: 1.30–6.71) and past history of COVID‐19 (aOR = 2.66, 95% CI: 1.14–6.22). The proportion of self‐reported long COVID is considerably low among COVID‐19 patients diagnosed during the Omicron wave in Eastern India when compared with estimates during Delta wave in the same setting.
Keywords: COVID‐19, long COVID, Omicron, postacute COVID‐19 syndrome
1. INTRODUCTION
Long coronavirus disease (COVID) or postacute sequelae of coronavirus disease of 2019 (COVID‐19) is a debilitating condition affecting millions worldwide. 1 It is defined as an ongoing symptomatic COVID‐19 from 4 weeks up to 12 weeks and post‐COVID‐19 syndrome which is defined as signs and symptoms that develop during or after an infection consistent with COVID‑19, continue for more than 12 weeks and are not explained by an alternative diagnosis. 2 A recent systematic review and meta‐analysis had estimated the global prevalence of long COVID to be 43%. 3 Long COVID affects multiple organ systems and presents with a multitude of symptoms. 4 There is no definite treatment protocol for long COVID, and management is tailored to each patient. 5 Lack of consensus and poor understanding of long COVID has led to misdiagnosis and ignorance of patient symptoms and increased suffering.
Most of the reports on long COVID are following infections with COVID‐19 variants like Delta. Our research team had conducted a hospital‐based retrospective cohort study among Reverse transcription polymerase chain reaction (RT‐PCR) diagnosed COVID‐19 patients, following the Delta wave in Eastern India. 6 We found that 29.2% self‐reported having long COVID symptoms 4 weeks after diagnosis. 6 But data on long COVID after infection with the Omicron variant is not yet available and World Health Organization (WHO) has recognized this as a research priority. 7
In Eastern India, January 2022 witnessed the third wave of COVID‐19 which was predominantly caused by the Omicron variant. 8 The Omicron variant is reported to cause mild COVID‐19 disease, but with increased infectivity. 9 , 10 The severity of Omicron infection is found to be less than that of Delta. 11 This may be due to the innate nature of the new variant or because of the presence of population immunity developed due to previous infections and vaccination. 12 Anecdotal evidence suggests that the incidence of long COVID following Omicron infection may be low. However, since the numbers of people getting infected are high, the burden of long COVID may be more with the Omicron wave. 13 Thus, it is pertinent that we address this study gap for a better understanding of the condition and health system preparedness. In this study, we replicate the methodology used during the Delta wave and study a new cohort of COVID‐19 patients who were diagnosed during the Omicron wave. 6
2. METHODOLOGY
Previously we had studied the prevalence, characteristics, and predictors of long COVID among COVID‐19 patients who were diagnosed during the second wave (Delta variant) in India. The present study uses the same methodology but with a different cohort of patients who were diagnosed with COVID‐19 during the Omicron wave. Briefly, we acquired the data of RT‐PCR diagnosed cases of COVID‐19 patients from COVID‐19 screening Outpatient Department (OPD) of a tertiary care government hospital and research institute in Eastern India (All India Institute of Medical Sciences [AIIMS] Bhubaneswar). The data on patients admitted to COVID‐19 wards, and the Intensive Care Unit (ICU) were also collected. The database was cleaned by removing individuals aged less than 18 years, missing phone numbers, or were reported dead. The patients in our cohort were diagnosed during a period ranging from the first week of January to the middle of February 2022. The diagnosis did not include the type of variant, but based on the Indian severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) Genomics Consortium (INSACOG) data, the variant in circulation was predominately Omicron (more than 92%). 14
The database was divided into three parts and handed over to data collectors who are postgraduate students and authors of this paper. One set contained data of only healthcare workers of the hospital, the second set had outpatient data, and the third data set contained outpatient and inpatient data. They contacted the patients in the list consecutively and with a convenient sampling approach, they continued till the required sample size was achieved.
A data collection form was developed in Epicollect5 (https://five.epicollect.net). The form was pretested using data from the cleaned database and this was not included in the analysis. Each data collector was trained, mentored, and monitored by a senior who had previous experience in conducting a similar study. After completing an online training session on conducting a telephonic interview and extensive pretesting, the forms were finalized. Data collection started in the last week of February 2022. The individuals who completed 28 days after their COVID‐19 diagnosis was contacted for telephonic interview. The response to each call was recorded in a Google sheet, and three attempts were made to contact a person if they were unavailable. The data collection was monitored by mentors in real‐time using Google sheet.
The individuals who were available, who met the all‐eligibility criteria (Age more than 18 years and not pregnant at the time of COVID‐19 diagnosis), and who gave consent for data collection were interviewed telephonically. Data on the demographic, socioeconomic status, medical history, including chronic disease and substance use, acute COVID‐19 symptoms, COVID‐19 vaccination, long COVID symptoms were collected. Body Mass Index (BMI) was calculated from self‐reported height and weight and classified according to WHO criteria. 15 The data collection form was made by adapting the questions from WHO Global COVID‐19 Clinical Platform Case Report Form (CRF) for post‐COVID condition (post‐COVID‐19 CRF). 16
2.1. Sample size and statistical analysis
We estimated the prevalence of long COVID in mild to moderate cases of COVID‐19 as 20% and in severe cases as 50%, based on previous literature and assuming a lower prevalence of long COVID in the Omicron variant compared with Delta. 6 A relative precision of 20% was used in both cases. Thus, the sample size was calculated to be 400 in mild to moderate cases and 100 in severe to critical cases. The data collectors called the patients consecutively from the list and the data from the interview was recorded using the Epicollect5 form on their smartphones. The final data exported to Microsoft Excel and later analyzed using statistical software STATA version 16 (StataCorp).
The baseline characteristics of participants were described using proportions and means with standard deviation. The primary objective of the study was the self‐reported proportion of long COVID among COVID‐19‐positive individuals after 28 days of diagnosis. The characteristics of symptoms were also reported in proportions. To find the predictors of long COVID, logistic regression was used with the presence or absence of self‐reported long COVID as the dependent variable. A p‐value less than 0.05 was considered statistically significant. To adjust for covariates, a multivariable logistic regression model was fitted with the clinically significant independent variables and those variables with a p‐value less than 0.2 in univariable regression. Wherever appropriate, categories of independent variables were collapsed to increase the power of analysis.
2.2. Ethical clearance
The study began only after obtaining the ethics approval from the Institutional Ethics Committee (IEC) of AIIMS Bhubaneswar (IEC Number: T/IM‐NF/CM& FM/21/37). The ethics committee approved the process of taking only verbal consent. The participants who were found to have long COVID at the time of the interview were asked to review in long COVID Outpatient (OPD) of Pulmonary Medicine at AIIMS Bhubaneswar.
3. RESULTS
The COVID‐19 screening OPD data and the data from the admission register of AIIMS Bhubaneswar were retrieved and cleaned, and a list of 2751 potential participants was finalized. We made 789 telephone calls for conducting the interview and 613 calls were received. By dropping the individuals who did not meet our inclusion and exclusion criteria and those who denied consent, we successfully completed 535 interviews. During the final analysis of data, 11 entries were dropped because they were duplicates or had some data errors, and the final list of 524 participants' data was analyzed. The median number of days of follow‐up was 73 with an interquartile range from 67 to 83 days (Figure 1). (Flow chart showing the selection of study participants.).
Figure 1.
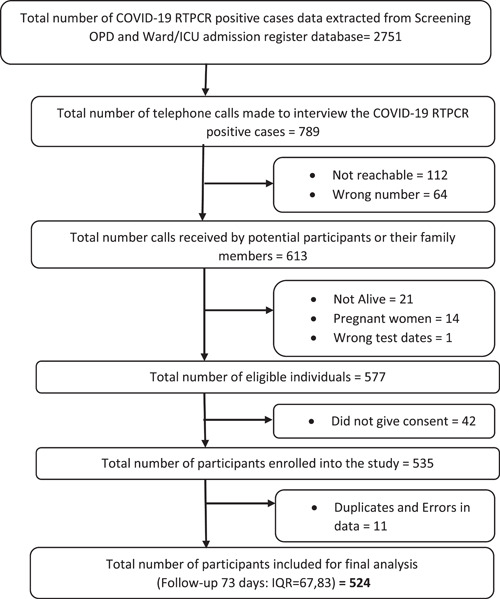
Flow chart showing the selection of study participants. ICU, Intensive Care Unit; OPD, Outpatient Department; RT‐PCR, reverse transcription polymerase chain reaction.
The mean age of the study participants was 36 years (SD = 14) and 40.5% were females. Most of the participants had graduate‐level education and were professionals with 25.2% reporting that their job involved COVID‐19 management. The BMI derived from self‐reported anthropometric values shows that 30.1% of the participants were overweight and 6.5% were obese. Only 10.1% reported having contracted or were diagnosed to have COVID‐19 before the present diagnosis. Diabetes and hypertension were reported to be around 8%–9%, and few participants had other medical conditions. Among the 12.8% of participants who self‐reported substance use, the most used substance was alcohol. Regarding COVID‐19 vaccinations, 88.9% of participants reported to have received two doses, and a small percentage (2.9%) had received the third dose or the precautionary dose (Table 1). (Sociodemographic characteristics, past medical history, and vaccination status of participants [n = 524]). The most common vaccine received by the participants was Covaxin (74.7%).
Table 1.
Sociodemographic characteristics, past medical history, and vaccination status of participants (n = 524)
| Socio‐demographic characteristics | ||||
|---|---|---|---|---|
| Variable | n (%) | |||
| Age (mean (SD); range) | 36 (14); 19–90 | |||
| Females | 212 (40.5) | |||
| Education | Illiterate/no formal education | 20 (3.8) | ||
| Studied up to 10 std or below | 110 (21) | |||
| Higher secondary | 79 (15.1) | |||
| Graduate | 216 (41.2) | |||
| Postgraduate and above | 98 (18.7) | |||
| Did not wish to answer | 1 | |||
| Current occupation | Unemployed/homemaker | 88 (16.8) | ||
| Student | 69 (13.2) | |||
| Professionals/technical/administrators | 192 (36.6) | |||
| Skilled manual laborer | 38 (7.3) | |||
| Unskilled manual laborer | 19 (3.6) | |||
| Retired | 27 (5.2) | |||
| Agriculture | 22 (4.2) | |||
| Other | 69 (13.5) | |||
| Occupation involving COVID‐19 management | 132 (25.2) | |||
| BMI (n = 484) (self‐reported) | Underweight (<18.5) | 23 (4.4) | ||
| Normal (18.5–24.9) | 308 (59) | |||
| Overweight (25.0–29.9) | 157 (30.1) | |||
| Obese (≥30.0) | 34 (6.5) | |||
| Past medical history | ||||
| History of COVID‐19 before the current episode | 53 (10.1) | |||
| Diagnosed to have diabetes | 42 (8) | |||
| Diagnosed to have hypertension | 49 (9.3) | |||
| Diagnosed to have anxiety/depression | 6 (1.1) | |||
| Diagnosed to have asthma | 8 (1.5) | |||
| Diagnosed to have tuberculosis | 7 (1.3) | |||
| Diagnosed to have cancer | 39 (7.4) | |||
| Diagnosed to have other medical conditions | 40 (7.6) | |||
| Participants who gave a history of substance use | 67 (12.8) | |||
| Smoking status | Current smoker | 24 (4.6) | ||
| Former (not smoked more than 1 year) | 6 (1.1) | |||
| Chewable tobacco | 32 (6.1) | |||
| Alcohol use | 48 (9.2) | |||
| COVID‐19 vaccination status | ||||
| Participants who received two doses plus a precautionary dose of COVID‐19 vaccine | 15 (2.9) | |||
| Participants who received two doses of COVID‐19 vaccine | 466 (88.9) | |||
| Participants who received one dose of COVID‐19 vaccine | 17 (3.2) | |||
| Participants who did not receive COVID‐19 vaccine | 26 (5) | |||
Abbreviations: BMI, Body Mass Index; COVID‐19, coronavirus disease of 2019.
The symptoms reported during the acute illness of COVID‐19 were classified into three categories, and 63.9% of the participants had one to four symptoms during the acute phase of the illness. The most common symptom was fever, followed by cough and body ache. The majority of participants had only mild to moderate severity of acute COVID‐19 (96.4%) and were treated at home (94.8%). Only 2.1% of participants reported having been admitted to the ICU (Table 2). (Clinical features and management of acute illness of COVID‐19 among participants [n = 524].)
Table 2.
Clinical features and management of acute illness of COVID‐19 among participants (n = 524)
| Clinical features and management of acute illness of COVID‐19 | n (%) | |
|---|---|---|
| Total number of symptoms reported by each participant | No symptoms | 131 (25.0) |
| 1–4 symptoms | 335 (63.9) | |
| 5 or more symptoms | 58 (11.1) | |
| Most common symptoms reported | Fever | 331 (63.2) |
| Cough | 203 (38.7) | |
| Body ache | 176 (33.6) | |
| Tiredness | 101 (19.3) | |
| Sore throat | 96 (18.3) | |
| Running nose | 64 (12.2) | |
| Loss of smell | 28 (5.3) | |
| Loss of taste | 25 (4.8) | |
| Severity of acute illness | Mild/moderate—did not receive oxygen | 505 (96.4) |
| Severe—required oxygen or was told you required oxygen | 17 (3.2) | |
| Critical—received invasive ventilation t | 2 (0.4) | |
| Highest care received during acute illness | Home isolation | 497 (94.8) |
| Admitted to the hospital (Not in ICU) | 16 (3.1) | |
| Admitted to ICU | 11 (2.1) | |
Abbreviations: COVID‐19, coronavirus disease of 2019; ICU, Intensive Care Unit.
In the total sample, 43 participants self‐reported having long COVID symptoms. Thus, the percentage of participants who self‐reported long COVID symptoms was 8.2% (95% Confidence Interval [CI] 6%–10.9%). Among the long COVID patients, the majority (93%) perceived the symptoms to be not severe in nature. The most common long COVID symptom was fatigue followed by a cough. Most of the patients did not report any limitation of activity due to long COVID and only 15 patients consulted a healthcare practitioner for their symptoms (Table 3). (Self‐reported long COVID symptoms and their features).
Table 3.
Self‐reported long COVID symptoms and their features
| Variable | n (%) | |
|---|---|---|
| Number of participants who self‐reported long COVID symptoms (n = 524) | Long COVID | 43 (8.2) |
| No symptoms of long COVID | 481 (91.8) | |
| Perceived severity of long COVID symptoms (n = 43) | Not severe | 40 (93) |
| Severe | 3 (7) | |
| Most common self‐reported long COVID symptoms (n = 43) | Fatigue | 15 (34.9) |
| Cough | 12 (27.9) | |
| Chest pain | 3 (7) | |
| Breathing difficulty | 2 (4.7) | |
| Anxiety/depression | 2 (4.7) | |
| Fever | 2 (4.7) | |
| Limitation of activity (n = 43) | Activity limited a lot | 2 (4.7) |
| Activity limited a little | 15 (34.9) | |
| No activity limitation | 26 (60.5) | |
| Consulted a healthcare practitioner (n = 43) | 15 (34.9) | |
Abbreviation: COVID, coronavirus disease.
In univariable logistic regression, age and sex were not statistically significant predictors. Education when classified into two categories showed that individuals having a postgraduate degree or higher had increased odds of reporting long COVID symptoms. Participants having a BMI greater than or equal to 25 had an odds ratio (OR) of 1.92 (95% CI 1.03–3.60) compared with BMI less than 25. The participants who had a prior history of infection with COVID‐19 and those who reported five or more symptoms during the acute phase of COVID‐19 at this time of diagnosis had increased odds of developing long COVID. These two predictors remained statistically significant even after adjusting for covariates in multivariable logistic regression: prior infection with COVID‐19 (adjusted OR = 2.66 [95% CI 1.14–6.22]) and five or more symptoms during the acute phase of COVID‐19 (Adjusted odds ratio 2.95 [95% CI 1.30–6.71)]. Pre‐existing medical condition, substance abuse, COVID‐19 vaccination, and severity of the illness were not statistically significant predictors of long COVID (Table 4). (Predictors of self‐reported long COVID symptoms).
Table 4.
Predictors of self‐reported long COVID symptoms
| Variable | Univariable logistic regression | Multivariable logistic regression | |||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | p‐Value | Adjusted odds ratio (95% CI) | p‐Value | ||
| Age categories | 18–45 years | Reference | ‐ | Reference | ‐ |
| 46 years & above | 0.63 (0.27–1.44) | 0.27 | 0.86 (0.30–2.43) | 0.77 | |
| Sex | Male | Reference | ‐ | Reference | ‐ |
| Female | 1.78 (0.95–3.32) | 0.07 | 1.57 (0.77–3.19) | 0.21 | |
| Education | Graduate or below | Reference | ‐ | Reference | ‐ |
| Postgraduate | 2.28 (1.16–4.50) | 0.02 | 1.62 (0.75–3.49) | 0.22 | |
| Occupation involving COVID‐19 management | 1.49 (0.76–2.90) | 0.25 | 0.82 (0.37–1.79) | 0.61 | |
| BMI | Less than 25 | Reference | ‐ | Reference | ‐ |
| BMI > = 25 | 1.92 (1.03–3.60) | 0.04 | 1.83 (0.93–3.61) | 0.08 | |
| History of substance use | 0.89 (0.34–2.35) | 0.81 | 0.75 (0.25–2.22) | 0.61 | |
| Past history of COVID‐19 | 3.09 (1.42–6.69) | 0.00 | 2.66 (1.14–6.22) | 0.02 | |
| Pre‐existing medical condition | 0.93 (0.57–1.51) | 0.76 | 1.19 (0.65–2.17) | 0.58 | |
| COVID‐19 vaccination | Zero to one dose | Reference | ‐ | Reference | ‐ |
| Two or more dose | 1.91 (0.45–8.18) | 0.38 | 1.76 (0.37–8.28) | 0.48 | |
| Number of acute COVID‐19 symptoms | Up to 4 symptoms | Reference | ‐ | Reference | ‐ |
| Five or more | 4.20 (2.04–8.62) | 0.00 | 2.95 (1.30–6.71) | 0.01 | |
| Severity of COVID‐19 disease | Mild/moderate | Reference | ‐ | Reference | |
| Severe/critical | 1.33 (0.30–5.96) | 0.71 | 1.59 (0.29–8.74) | 0.60 | |
| Highest level of care received during COVID‐19 | Home isolation | Reference | ‐ | ‐ | ‐ |
| Admitted to hospital | 0.89 (0.20–3.89) | 0.88 | ‐ | ‐ | |
Abbreviations: BMI, Body Mass Index; COVID, coronavirus disease; COVID‐19, coronavirus disease of 2019.
4. DISCUSSION
Long COVID is extensively researched worldwide, but there is a paucity of evidence from low‐ and middle‐income countries. 17 The emergence of new COVID‐19 variants like Omicron has resulted in a greater number of people getting infected with the virus, and this may translate to an increased burden of long COVID cases. 13 , 18 Although the severity of new COVID‐19 variants is observed to be mild, in certain population like pregnant women, it can cause severe diseases outcomes. 19 It was imperative that the effects of new variants on the development of long COVID is studied extensively to mitigate a public health crisis as well as to devise better management strategies.
Our primary objective was to estimate the proportion of patients who self‐reported long COVID symptoms after getting diagnosed with COVID‐19 during the Omicron wave in Eastern India. We found that only 8.2% (95% CI 6%–10.9%) reported long COVID after a minimum of 28 days of diagnosis. This is a considerably smaller percentage reporting long COVID when compared with the 29.2% reported by the cohort of COVID‐19 patients during the Delta wave in the same setting. 6 Since the number of participants having severe illness was considerably less, we have only reported the proportion in total sample.
This difference is coherent because the Omicron variant of COVID‐19 is widely reported to be causing a mild disease when compared with the Delta variant. 9 , 10 , 20 This may be because the virus is inherently mild, or it is due to pre‐existing immunity because of previous infection and vaccination. 12 , 21 We could not find any other studies from India which reported long COVID symptoms after the Omicron wave, for a direct comparison. However, the findings from self‐reported cases of long COVID in COVID Symptom Study app from UK shows that odds of having long COVID is less (0.24–0.50) with the Omicron variant compared with Delta variant. 22
The most common long COVID symptom reported in our study was fatigue, followed by cough. The symptoms were self‐reported during the telephonic interview and no objective assessment was done. The finding is consistent with the available literature, which reported fatigue as the most common long COVID symptom. 23 , 24 Cough, chest pain, and breathing difficulties were also widely reported long COVID symptoms. 25
Regarding the predictors, two independent variables that came out to be statistically significant on occurrence of long COVID‐history of COVID‐19 and the number of symptoms during the acute phase of COVID‐19. Our results showing increased odds of long COVID in individuals reporting five or more symptoms during the acute phase of illness is consistent with the COVID symptoms app study which reported that patients experiencing more than five symptoms during the first week of COVID‐19 illness had a higher‐odds of developing long COVID. 26 The higher odds of long COVID seen in participants having a history of COVID‐19 in the past may be related to the possibility of a chronic SARS‐CoV‐2 infection. 27
In our study, other characteristics like age and sex were not significant predictors although previous literature has reported these variables to be statistically significant. 28 In univariable regression, the unadjusted odds ratio was statistically significant for BMI greater than 25. Evidence shows that BMI could be a significant risk factor for the development of long COVID. 29 Since the BMI in our study was not based on actual measurements but on self‐reported values, our method of data collection could have affected the result. Similarly, a significant association seen between higher education and long COVID in our study may be related to increased reporting of symptoms. Recent studies have reported that vaccination decreases the odds of having long COVID, but no such association was found in our study. 30 , 31
The major strength of our study is that all participants were confirmed to be positive for SARS‐CoV‐2 infection using the RT‐PCR test thereby minimizing the chances of misclassification. The data collection form was adapted from the standard format used by WHO and underwent thorough pretesting. The data collectors who interviewed the participants were postgraduate students who had training and understanding of COVID‐19 disease and mentored by seniors who were previously involved with similar studies.
There were a few limitations as well. We did purposive sampling, and this sample may not be representative of the population. Since we conducted the follow‐up using a telephonic interview, all the data captured were self‐reported and no clinical examination or investigations were done to confirm the findings. Even though we expect that the majority of cases were of Omicron during the study period, we did not test for the variant.
Our study concluded that after the Omicron wave only around 8.2% reported long COVID which is significantly less in proportion compared with the Delta wave estimates in the same setting. The low percentage still has public health importance when we consider the total number of people getting infected with Omicron. Further studies are warranted to better understand the long COVID by studying the clinical, biochemical, and radiological features of the affected individuals.
AUTHOR CONTRIBUTIONS
M. C. Arjun and Arvind K. Singh contributed to development of concept, literature search, and design of the study. Payel Roy, Mythry Ravichandran, Srijani Mandal, Debkumar Pal, Alekhya Gajjala, Kajal Das, Mahalingam Venkateshan, M. C. Arjun, Arvind K. Singh, Binod K. Patro, Sonu Hangma Subba, Prasanta Raghab Mohapatra, and Baijayantimala Mishra participated in data acquisition and data analysis of the study. M. C. Arjun prepared the manuscript. Payel Roy, Mythry Ravichandran, Srijani Mandal, M. C. Arjun, Arvind Kumar Singh, Binod Kumar Patro, Sonu Hangma Subba, Prasanta Raghab Mohapatra, and Baijayantimala Mishra participated in manuscript editing and Payel Roy and M. C. Arjun in review.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The study began only after obtaining the ethics approval from the Institutional Ethics Committee (IEC) of AIIMS Bhubaneswar (IEC Number: T/IM‐NF/CM&FM/21/37). The ethics committee approved the process of taking only verbal consent.
Arjun MC, Singh AK, Roy P, et al. Long COVID following Omicron wave in Eastern India—A retrospective cohort study. J Med Virol. 2022;95:e28214. 10.1002/jmv.28214
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Coronavirus disease (COVID‐19) 2022. https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease
- 2.Overview | COVID‐19 rapid guideline: managing the long‐term effects of COVID‐19 | Guidance | NICE. Nice.org.uk. Accessed July 28, 2022. https://www.nice.org.uk/guidance/ng188
- 3. Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post‐COVID‐19 condition or long COVID: a meta‐analysis and systematic review. J Infect Dis. 2022;225(8)jiac136. 10.1093/infdis/jiac136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lopez‐Leon S, Wegman‐Ostrosky T, Perelman C, et al. More than 50 long‐term effects of COVID‐19: a systematic review and meta‐analysis. Sci Rep. 2021;11(1):16144. 10.1038/s41598-021-95565-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National comprehensive guidelines for management of postcovid sequelae. Mohfw.gov.in. Accessed July 27, 2022. https://www.mohfw.gov.in/pdf/NationalComprehensiveGuidelinesforManagementofPostCovidSequelae.pdf
- 6. Arjun MC, Singh AK, Pal D, et al. Prevalence, characteristics, and predictors of Long COVID among diagnosed cases of COVID‐19. medRxiv. 2022. 10.1101/2022.01.04.21268536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Can ‘long COVID’ infect you after Omicron? WHO answers. mint. Accessed July 12, 2022. https://www.livemint.com/science/health/can-long-covid-infect-you-after-omicron-who-on-symptoms-vaccination-and-the-variant-11644404550699.html
- 8. Singhal T. The emergence of Omicron: challenging times are here again. Indian J Pediatr. 2022;89(5):490‐496. 10.1007/s12098-022-04077-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raju MK, Vivian Thangaraj JW, Selvavinayagam TS, et al. Clinical profile of patients infected with suspected SARS‐CoV‐2 Omicron variant of concern, Tamil Nadu, India, December 2021–January 2022. Indian J Med Res. 2022;155(1):165‐170. 10.4103/ijmr.ijmr_312_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abdullah F, Myers J, Basu D, et al. Decreased severity of disease during the first global Omicron variant COVID‐19 outbreak in a large hospital in Tshwane, South Africa. Int J Infect Dis. 2022;116:38‐42. 10.1016/j.ijid.2021.12.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wrenn JO, Pakala SB, Vestal G, et al. COVID‐19 severity from Omicron and Delta SARS‐CoV‐2 variants. Influenza Other Respir Viruses. 2022;16(5):832‐836. 10.1111/irv.12982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sigal A. Milder disease with Omicron: is it the virus or the pre‐existing immunity. Nat Rev Immunol. 2022;22(2):69‐71. 10.1038/s41577-022-00678-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harvard experts on Omicron and long COVID. Harvard Gazette. Accessed July 30, 2022. https://news.harvard.edu/gazette/story/2022/02/harvard-experts-expect-new-wave-of-long-covid-cases/
- 14.Indian SARS‐CoV‐2 Genomics Consortium (INSACOG). Accessed September 20, 2022. https://research.nibmg.ac.in/insacog/
- 15.Obesity and overweight. Who.int. Accessed July 4, 2022. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 16.Global COVID‐19 Clinical Platform Case Report Form (CRF) for Post COVID condition (Post COVID‐19 CRF). Who.int. Accessed July 4 2022. https://www.who.int/publications/i/item/global-covid-19-clinical-platform-case-report-form‐(crf)‐for‐post‐covid‐conditions‐(post‐covid‐19‐crf‐).
- 17. Michelen M, Manoharan L, Elkheir N, et al. Characterising long COVID: a living systematic review. BMJ Glob Health. 2021;6(9):e005427. 10.1136/bmjgh-2021-005427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eggink D, Andeweg SP, Vennema H, et al. Increased risk of infection with SARS‐CoV‐2 Omicron BA.1 compared with Delta in vaccinated and previously infected individuals, the Netherlands, 22 November 2021 to 19 January 2022. Euro Surveill. 2022;27(4):2101196. 10.2807/1560-7917.ES.2022.27.4.2101196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sahin D, Tanacan A, Anuk AT, et al. Comparison of clinical features and perinatal outcomes between pre‐variant and post‐variant periods in pregnant women with SARS‐CoV‐2: analysis of 1935 cases. Arch Gynecol Obstet. 2022;305(3):1‐10. 10.1007/s00404-022-06493-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kahn F, Bonander C, Moghaddassi M, et al. Risk of severe COVID‐19 from the Delta and oOmicron variants in relation to vaccination status, sex, age and comorbidities—surveillance results from Southern Sweden, July 2021 to January 2022. Euro Surveill. 2022;27(9):2200121. 10.2807/1560-7917.ES.2022.27.9.2200121/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Madhi SA, Kwatra G, Myers JE, et al. Population immunity and Covid‐19 severity with Omicron variant in South Africa. N Engl J Med. 2022;386(14):1314‐1326. 10.1056/NEJMoa2119658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Antonelli M, Pujol JC, Spector TD, Ourselin S, Steves CJ. Risk of long COVID associated with delta versus omicron variants of SARS‐CoV‐2. Lancet. 2022;399(10343):2263‐2264. 10.1016/S0140-6736(22)00941-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in Post‐COVID‐19 syndrome: a systematic review and meta‐analysis. Brain Behav Immun. 2022;101:93‐135. 10.1016/j.bbi.2021.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iqbal FM, Lam K, Sounderajah V, Clarke JM, Ashrafian H, Darzi A. Characteristics and predictors of acute and chronic post‐COVID syndrome: a systematic review and meta‐analysis. EClinicalMedicine. 2021;36:100899. 10.1016/j.eclinm.2021.100899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cabrera Martimbianco AL, Pacheco RL, Bagattini ÂM, Riera R. Frequency, signs and symptoms, and criteria adopted for long COVID‐19: a systematic review. Int J Clin Pract. 2021;75(10):e14357. 10.1111/ijcp.14357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626‐631. 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O'Donnell JS, Chappell KJ. Chronic SARS‐CoV‐2, a cause of post‐acute COVID‐19 sequelae (Long‐COVID). Front Microbiol. 2021;12:724654. 10.3389/fmicb.2021.724654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co‐occurrence, and evolution of long‐COVID features: a 6‐month retrospective cohort study of 273,618 survivors of COVID‐19. PLoS Med. 2021;18(9):e1003773. 10.1371/journal.pmed.1003773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vimercati L, De Maria L, Quarato M, et al. Association between long COVID and Overweight/Obesity. J Clin Med. 2021;10(18):4143. 10.3390/jcm10184143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Antonelli M, Penfold RS, Merino J, et al. Risk factors and disease profile of post‐vaccination SARS‐CoV‐2 infection in UK users of the COVID symptom study app: a prospective, community‐based, nested, case‐control study. Lancet Infect Dis. 2022;22(1):43‐55. 10.1016/S1473-3099(21)00460-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The effectiveness of vaccination against Long Covid: a rapid evidence briefing (February 2022). Patient Safety Learning—the hub. Accessed July 27, 2022. https://www.pslhub.org/learn/coronavirus-covid19/data-and-statistics/the-effectiveness-of-vaccination-against-long-covid-a-rapid-evidence-briefing-february-2022-r6159/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


