Abstract
Objective
The objective of this systematic review is to summarize in vitro, preclinical, and human data related to omadacycline and Clostridioides difficile infection (CDI).
Data Sources
PubMed and Google Scholar were searched for “omadacycline” AND (“Clostridium difficile” OR “C difficile” OR “Clostridioides difficile”) for any studies published before February 15, 2022. The US Food and Drug Administration (FDA) Adverse Events Reporting System (AERS) was searched for omadacycline (for reports including “C. difficile” or “CDI” or “gastrointestinal infection”). The publications list publicly available at Paratek Pharmaceuticals, Inc. Web site was reviewed.
Study Selection and Data Extraction
Publications presenting primary data on omadacycline and C. difficile published in English were included.
Data Synthesis
Preclinical and clinical evidence was extracted from 14 studies. No case reports in indexed literature and no reports on FDA AERS were found. Omadacycline has potent in vitro activity against many C. difficile clinical strains and diverse ribotypes. In phase 3 studies, there were no reports of CDI in patients who received omadacycline for either community-acquired bacterial pneumonia or acute bacterial skin and skin structure infection.
Relevance to Patient Care and Clinical Practice
Omadacycline should be considered a low-risk antibiotic regarding its propensity to cause CDI.
Conclusions
Reducing the burden of CDI on patients and the health care system should be a priority. Patients with appropriate indications who are at heightened risk of CDI may be suitable candidates for omadacycline therapy. In these patients, omadacycline may be preferable to antibiotics with a high CDI risk.
Keywords: infectious diseases, bacterial infections, hospital-acquired infections, health care–associated infections, antibiotics, antibiotic resistance, respiratory infections, skin infections
Video Abstract
Introduction
Clostridioides difficile infection (CDI) is a major worldwide public health threat with an estimated 460 000 cases annually in the United States, and approximately 124 000 cases or a cumulative incidence of 8 per 100 000 persons annually in Europe.1-3 Although a large spectrum of symptoms are possible, patients with CDI typically exhibit diarrhea ranging from mild to severe, which can be accompanied by abdominal pain. 4 In addition to a significant clinical burden of disease, the health care–related costs are also substantial, estimated at more than $5 billion annually in the United States.5,6 Patient health-related quality of life (HRQoL) also worsens with CDI, resulting in impaired daily activities and reduced work productivity. 7 CDI recurrence, a significant driver of the clinical, economic, and HRQoL outcomes, occurs in approximately 25% of patients after an initial infection and in 50% of patients who have already experienced a recurrence of CDI.8-11
The pathophysiology of CDI involves the disruption of the human gut microbiota, usually from exposure to high-risk antibiotics, which allows the C. difficile spores to germinate, produce 2 endotoxins (toxins A and B), and cause disease. In general, antibiotics that substantially disrupt the normal host gut microbiota are considered to be high-risk antibiotics and the most significant factor for developing CDI.12-16
Emergence of epidemic strains with reduced antibiotic susceptibility, such as the clindamycin-resistant J strain or the fluoroquinolone-resistant ribotype 027, have made CDI increasingly difficult to prevent, manage, and treat.16,17 Resistance to antibiotics commonly used to treat CDI also complicates treatment approaches and heightens the need for new drug development in this area.18,19
Based on these factors, antibiotics should be evaluated for 2 criteria as they relate to novel CDI therapeutics: minimal disruption in the host gut microbiota and potent activity against C. difficile. Historically, tetracycline-class antibiotics (eg, tetracycline, doxycycline, minocycline) have been associated with a low risk of developing CDI.14,20 However, the use of tetracyclines has decreased in recent decades, owing in part to the development of resistance by other pathogens to tetracycline-class agents.21,22 Newer tetracycline analogs (omadacycline, eravacycline, and tigecycline) have been developed to overcome common mechanisms of resistance and, as such, they retain antibacterial activity against many pathogens that are considered nonsusceptible to earlier tetracyclines.23,24
Here we summarize and contextualize the breadth of evidence related to C. difficile and omadacycline, an aminomethylcycline antibiotic in the tetracycline class. Omadacycline overcomes the 2 main mechanisms of tetracycline resistance: efflux and ribosomal protection. 25 Omadacycline is available in oral and intravenous (IV) formulations and is approved in the United States to treat adults with community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI). The purpose of this systematic review is to summarize in vitro, preclinical, and human data as they relate to omadacycline, C. difficile, and CDI.
Methods
PubMed and Google Scholar were searched for “omadacycline” AND (“Clostridium difficile” OR “C. difficile” OR “Clostridioides difficile”) for any studies published before February 15, 2022. The US Food and Drug Administration (FDA) Adverse Events Reporting System (AERS) was searched for reports including omadacycline with mention of “C. difficile” or “CDI” or “gastrointestinal [GI] infection” through February 15, 2022. 26 The bibliography publicly available on the Paratek Pharmaceuticals, Inc. Web site was reviewed. 27 We included all publications presenting primary data published in English on omadacycline in relation to C. difficile, including in vitro, in vivo, and clinical data. Reports on a secondary analysis and review papers were excluded from this analysis. Likewise, data on C. difficile that did not also include omadacycline were excluded.
All authors reviewed the available studies to confirm inclusion in the review. In case of disagreement on study inclusion, authors were to conduct a scientific debate and vote on inclusion. Relevant preclinical and clinical evidence was extracted from the studies following Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. 28
Results
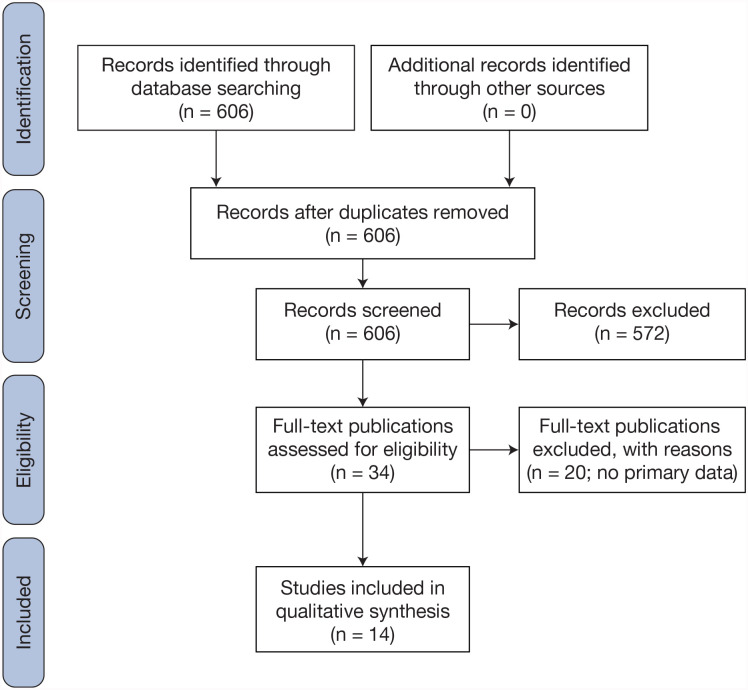
The PubMed search returned 7 results, 5 of which contained primary data on omadacycline and C. difficile; Google Scholar search returned 606 results, 14 of which contained primary data on omadacycline and C. difficile (7 overlapping results with PubMed); there were no reports of omadacycline related to CDI or GI infection on the FDA AERS Web site. A total of 14 studies of omadacycline and C. difficile were unanimously included covering in vitro activity, preclinical models, clinical trials, and health economics and outcomes research (HEOR); no case reports were found (Figure 1).29-43
Figure 1.
PRISMA flow diagram.
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
In Vitro Activity
The in vitro activity of omadacycline and comparators was assessed in 4 studies (noted as Panels 1-4) and the results are summarized in Table 1. In the Panel 1 study, omadacycline had broth dilution MIC50/90 values of 0.25/0.5 mg/L against 21 C. difficile isolates collected between 2006 and 2016 in the United States. 29 Metronidazole had MIC50/90 values of 0.5/1 mg/L. In the Panel 2 study, omadacycline had broth dilution MIC50/90 values of 0.12/0.12 mg/L and MIC values ranged from 0.06 to 0.12 mg/L against 27 C. difficile isolates. 30
Table 1.
Summary of In Vitro Activity of Omadacycline and Select Comparators Against Clostridioides difficile Isolates.
| MIC50/MIC90 (mg/L) | Panel 1 (n = 21)29,a |
Panel 2 (n = 27)30,a |
Panel 3 (n = 65)36,a |
Panel 4 (n = 250)37,b |
|---|---|---|---|---|
| Omadacycline | 0.25/0.5 | 0.12/0.12 | 0.25/1 | 0.031/0.031 |
| Metronidazole | 0.5/1 | 0.12/0.25 | 0.25/1 | 0.5/2 |
| Vancomycin | – | – | 0.5/2 | 2/2 |
| Fidaxomicin | – | – | 0.12/0.5 | 0.016/0.063 |
| Tigecycline | 0.25/0.25 | – | 0.03/0.12 | – |
| Moxifloxacin | 2/>16 | – | 2/4 | – |
| Clindamycin | 8/>32 | 8/>16 | – | – |
| Doxycycline | – | 0.03/1 | – | – |
Source of C. difficile isolates: Panel 1: US, 2006-2016; Panel 2: Not described; Panel 3: Sweden, 2015-2018; Panel 4: Houston, TX, USA 2015-2018.
Abbreviations: MIC, minimum inhibitory concentration; – entries denote an antibiotic that was not tested in a panel of C. difficile isolates.
MIC values determined by agar dilution.
MIC values determined by broth dilution.
In the Panel 3 study, omadacycline had agar dilution MIC50/90 values of 0.25/1.0 mg/L, and an MIC range of 0.25 to 16 mg/L against 65 C. difficile isolates collected between 2015 and 2018 at Karolinska Hospital in Stockholm, Sweden. 36 Against ribotype 027 (n = 1), omadacycline had an MIC value of 0.5 mg/L.
In the Panel 4 study, omadacycline demonstrated potent in vitro activity, with a broth dilution MIC50/90 value of 0.031/0.031 mg/L and MIC values ranging from 0.016 to 0.13 mg/L against 250 C. difficile isolates collected between 2015 and 2018 from 13 hospitals in the Houston, TX, region. 37 Omadacycline displayed equally potent activity among the 7 different ribotypes in this collection and regardless of CDI disease severity or vancomycin susceptibility. 37 In time-kill kinetic studies, bacterial killing was similar to (or more potent than) fidaxomicin, metronidazole, and vancomycin, with omadacycline demonstrating bactericidal effects by 24 hours (>3 log10 colony-forming units [CFU]/mL killing).
Although selection of the comparator antibiotics differed among the 4 studies, the guideline-endorsed treatments for CDI had the following susceptibility ranges: metronidazole MIC50 values were 0.12 to 0.5 mg/L and MIC90 values were 0.25 to 2 mg/L; and vancomycin MIC50 values were 0.5 to 2 mg/L and the MIC90 value was 2 mg/L.
Another in vitro study examined the extent of C. difficile spore eradication when germinants were combined with omadacycline or vancomycin. Antibiotic concentrations chosen for both omadacycline and vancomycin reflected a midpoint between fecal concentration reached during clinical dosing and the MIC values obtained for C. difficile strain used. There was >99% spore and vegetative cell eradication when germinants were combined with omadacycline, and >94% spore eradication when germinants were combined with vancomycin. 38 Among the 4 strains in the study, the MIC range for omadacycline was 0.031 to 0.125 mg/L, while the MIC range for vancomycin was 1 to 4 mg/L when tested via broth microdilution. The combination of germinants with either omadacycline or vancomycin did not result in significant production of toxins A or B.
Preclinical Models
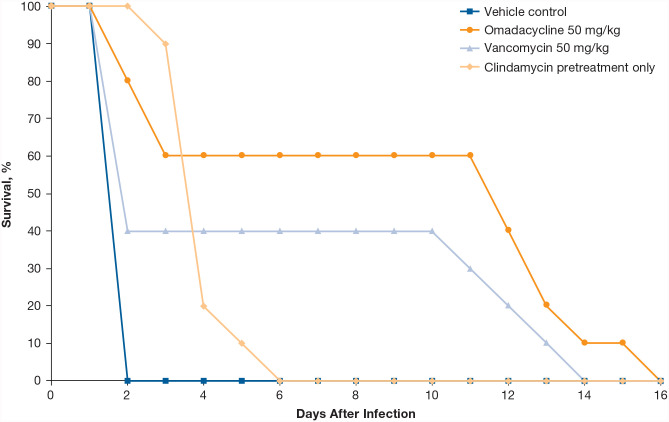
Efficacy of omadacycline was determined in a hamster model of C. difficile–associated diarrhea using the C. difficile American Type Culture Collection 43596 strain (omadacycline, tigecycline, metronidazole, and vancomycin MIC values were all 0.06 mg/L). 30 Hamsters were pretreated with subcutaneous clindamycin 24 hours before oral gavage of C. difficile; 24 hours after inoculation, hamsters received oral omadacycline (50 mg/kg), oral vancomycin (50 mg/kg), or vehicle for 5 days (n = 10 each treatment group). The median survival after inoculation was 12 days for omadacycline-treated animals, compared with 2 days for vancomycin-treated animals, and 4 days for vehicle-treated animals (Figure 2).
Figure 2.
Survival of hamsters infected by oral gavage with C. difficile after treatment with omadacycline or comparators.
All treatment groups received clindamycin pretreatment. N = 10 per group. Figure adapted from Kim et al. 30
A murine model of C. difficile relapse evaluated the in vivo efficacy of spore reservoir eradication. 39 In this study, vancomycin or omadacycline alone was compared with either antibiotic combined with a germinant solution (sodium taurocholate, taurine, sodium docusate, and calcium gluconate) following the establishment of CDI. Both omadacycline and vancomycin alone had 60% survival by Day 15, while omadacycline or vancomycin combined with germinant solution had 100% survival (P = 0.004 vs the respective antibiotic alone). The group receiving omadacycline alone showed less severe clinical disease, less toxin production, and reduction in detectable spores after treatment compared with the group receiving vancomycin alone (60% vs 100%, respectively; P = 0.087). Notably, no mice receiving germinants with either omadacycline or vancomycin were spore or toxin positive.
Using a triple-stage chemostat human gut model, researchers investigated the effects of omadacycline instillation on normal gut microbiome populations and the subsequent potential for induction of CDI compared with moxifloxacin. 40 The model was designed to reproduce the spatial, temporal, nutritional, and physicochemical characteristics of the proximal-to-distal bowel, with 3 vessels of increasing pH that were continuously sparged with nitrogen. 44 The model was inoculated with a pooled human fecal slurry from healthy volunteers that stabilized for 2 weeks, and was then challenged twice with 107 CFU/mL of C. difficile spores (ribotype 027). Omadacycline (430 mg/L) or moxifloxacin (43 mg/L) was instilled once per day for 7 days to achieve desired exposures with oral dosing and model the observed antibacterial concentration in the human gut. Omadacycline concentrations in the gut model peaked at 242 to 48 mg/L in the modeled proximal-to-distal gut sections, respectively. Moxifloxacin concentrations peaked at 55 to 25 mg/L, respectively. Moxifloxacin instillation caused a decline in enterococci and Bacteroides fragilis populations (~4 log10 CFU/mL for both), a decline in bifidobacteria and lactobacilli (~3 log10 CFU/mL), followed by simulated CDI (vegetative cell proliferation and detectable toxin). Omadacycline instillation decreased populations of bifidobacteria (~8 log10 CFU/mL), B. fragilis group populations (7-8 log10 CFU/mL), lactobacilli (2-6 log10 CFU/mL), and enterococci (4-6 log10 CFU/mL). Despite these microbial shifts, there was no evidence of C. difficile germination or toxin production in the omadacycline-instilled model over the 3-week observation. In contrast to moxifloxacin, omadacycline exposure did not facilitate simulated CDI.
Clinical Trials
To date, there have been no reports of CDI in participants enrolled in clinical trials of omadacycline. In the phase 3 OPTIC study in CABP (NCT02531438), 2.1% (8/388) of patients who received moxifloxacin developed CDI versus no patients receiving omadacycline (n = 386). 41 In the IV-to-oral phase 3 OASIS-1 study in ABSSSI (NCT02378480), there were no reports of CDI in either the omadacycline treatment group (n = 323) or the linezolid group (n = 322). 42 Likewise, in the oral-only OASIS-2 phase 3 study in ABSSSI (NCT02877927), there were no reports of CDI associated with omadacycline (n = 368) or linezolid (n = 367). 43 No cases of CDI were reported in the omadacycline treatment groups or in the comparator groups receiving levofloxacin or nitrofurantoin in phase 1b/2 studies in acute pyelonephritis or cystitis (data on file).31-33
Health Economics and Outcomes Research
Taking the aforementioned data from clinical trials of omadacycline and data on the health care burden of CDI, the estimated potential effects of omadacycline use on CDI-related outcomes have been described. A conceptual health care–decision analytic model was created to estimate the incremental costs associated with treating 100 patients who are hospitalized for CABP with an initial 5-day inpatient regimen of omadacycline instead of moxifloxacin. 34 The use of omadacycline had the potential to reduce the CDI-related economic burden if it could avoid approximately 5 to 10 cases of moxifloxacin-associated CDI per 100 hospitalized patients, assuming the attributable CDI cost was approximately $30,000 per episode.
A separate modeling study assessed the economic impact of substituting current guideline-concordant therapy for CABP (fluoroquinolones and third-generation cephalosporins) with omadacycline in hospitalized patients at high risk of CDI, ie, with a Davis risk score ≥6 (calculated based on this scoring system: number of high-risk antibiotics received = 1 point each [maximum 5 points]; receipt of proton-pump inhibitors = 1 point; age 40-55 years = 1 point; age >55 years = 2 points; and Charlson Comorbidity Index score [1 comorbidity = 1 point, >1 comorbidity = 2 points]). 35 In the phase 3 OPTIC CABP study, 14% of patients with a Davis risk score ≥6 who received moxifloxacin developed CDI versus 0% of patients who received omadacycline. 41 As modeled, use of omadacycline in patients at high risk of CDI could result in cost savings to hospitals compared with use of guideline-concordant antibiotics, if omadacycline reduces excess CDI by 5 to 20 cases per 100 treated patients with CABP. 35
Discussion
The relationship between antibiotics and CDI is 2-fold. First, antibiotics that minimally disrupt the human gut microbiota will likely lower the risk of developing CDI relative to antibiotics that cause profound dysbiosis to the gut microbiota and allow C. difficile spores to germinate. This is an important consideration not only when choosing antibiotics to treat infections, but also for antimicrobial stewardship teams that wish to minimize CDI at their institution. Second, antibiotics that cause minimal dysbiosis and have activity against C. difficile may also be investigated as a potential treatment option for CDI. The purpose of this systematic review was to appraise the data on omadacycline, a newer tetracycline analog, as it relates to these 2 CDI concepts.
The compilation of in vitro activity data shows that omadacycline has potent activity against many C. difficile clinical strains and diverse ribotypes. Omadacycline demonstrated similar or more potent in vitro activity than comparators, including current guideline-recommended therapies for CDI, and against hypervirulent strains (eg, ribotype 027). 45 It is important to note that the FDA has not established breakpoint interpretive criteria for omadacycline against C. difficile, and that the clinical relevance of the MIC values presented here is therefore unknown. It is unclear why omadacycline in vitro activity differed between some of the panels; reasons may include the different testing methodologies used, ie, broth dilution compared with agar dilution, or the different strain collections assessed. It has been reported that a negative bias, ie, lower MIC values, for broth microdilution exists when compared with agar dilution when testing antimicrobials against C. difficile. 46 While omadacycline was not tested in that report, the authors note that the 2 methodologies may not be equivalent, and that greater variability in MIC values was found when using broth dilution methods. 46
From preclinical data, it appears that omadacycline achieves high enough concentrations in the gut to prevent C. difficile proliferation; GI concentrations were found to be 2 to 3 times higher than systemic concentrations, and approximately 81% of a single dose of oral omadacycline is excreted in the feces.47,48 For comparison, virtually all oral vancomycin is excreted in the feces, and approximately 14% of oral metronidazole is excreted by the fecal route.49,50 Preclinical models also demonstrated durable survival of omadacycline-treated animals that had CDI, as well as no evidence of simulated CDI development after omadacycline exposure, indicating a favorable preclinical profile. The clinical data suggest that omadacycline may have a low propensity to induce CDI in patients. 40 Results of the gut microbiota exposure to omadacycline were similar to those obtained with tigecycline. 51 Given the lack of association of tigecycline with CDI, this provides further confidence that omadacycline truly has a low CDI risk propensity. 52 Data from other newer tetracycline analogs (ie, tigecycline, eravacycline) show similar in vitro and in vivo results.46,53-56
Relevance to Patient Care and Clinical Practice
Based on available evidence, omadacycline should be considered a low-risk antibiotic regarding its propensity to cause CDI, similar to other tetracyclines.57-59 This should be considered when optimizing the selection of antibiotics in patients with susceptible infections. Omadacycline is currently indicated for adults with CABP or ABSSSI. 60 In clinical trials of CABP, omadacycline was compared with moxifloxacin. Quinolones, including moxifloxacin, are considered high-risk CDI antibiotics, and health care systems have targeted fluoroquinolone use in order to reduce CDI cases.14,15,61 There are no comparative data for ceftriaxone and omadacycline; however, ceftriaxone is the preferred antibiotic in hospitalized patients with CABP. 62 Ceftriaxone is also considered a high-risk antibiotic for developing CDI due to its broad spectrum, high fecal concentrations, and disruption of the gut microbiota. Notably, a systematic review and meta-analysis found no association of CDI risk with aminoglycosides, tetracyclines, or macrolides. 58 For individuals especially prone to CDI (eg, those aged ≥65 years), CDI risk should be considered when choosing between agents for treatment of a non-CDI infection. Current CABP and antibiotic stewardship guidelines recommend considering patient history of CDI (CABP) and reducing use of high-risk CDI antibiotics (stewardship), but neither make specific recommendations on which agents are preferred in these regards.62,63 In patients with preexposure to high-risk antibiotics, treatment with omadacycline for a susceptible indicated infection may minimize further microbiota disruption that could potentiate CDI. Ongoing studies to specifically investigate microbiota disruption between omadacycline and comparators should provide further evidence about its role and the risk of developing CDI.
In vitro susceptibility studies, an ex vivo model mimicking the human GI tract, and an in vivo hamster model also provide support for further development of omadacycline as a therapeutic for CDI. This is especially appealing as an alternative to metronidazole when IV therapy is required. 45 Omadacycline is largely excreted as active drug via the feces regardless of route of administration. 47 Thus, the ability for direct IV-to-oral conversion would also have appeal to stewardship teams. Omadacycline also likely results in less disruption to the human GI tract than oral vancomycin due to the latter’s activity against enterococci, but studies are needed to validate this hypothesis and provide guidance on its preference, if any, over vancomycin. There is evidence that another newer tetracycline analog, tigecycline, is an effective therapeutic option for patients with CDI, especially those with severe disease, but this option is restricted by substantial tolerability issues. 64 The promising characteristics of omadacycline, including IV-to-oral possibility and a more favorable safety profile compared with tigecycline, provide a solid basis to further evaluate this newer tetracycline analog in phase 2/3 studies of CDI to generate comparative clinical data before possible therapeutic use.
Limitations
Limitations of this review include the lack of clinical trials testing omadacycline in CDI, and that there are no data on CDI-related outcomes as endpoints in other clinical trials of omadacycline. Furthermore, in vitro potency of omadacycline against C. difficile may not translate into a clinical response. There are limited data on the HEOR implications of omadacycline and few comparator scenarios that have been examined.
Conclusion
Clinicians should carefully consider first the need for antibiotics in general, and then the choice of specific antibiotic when warranted, especially in patients with risk factors for developing CDI. Based on this review, omadacycline should be considered in patients who have appropriate indications and are at heightened risk of CDI. Omadacycline may be preferable to antibiotics that are known to confer a higher risk of developing CDI (eg, fluoroquinolones, third-generation cephalosporins, carbapenems, clindamycin). Further research should be pursued to investigate omadacycline as a treatment option for CDI.
Acknowledgments
Medical editorial assistance, funded by Paratek Pharmaceuticals, Inc., was provided by Agnella Izzo Matic, PhD, CMPP, of Innovative Strategic Communications (Milford, PA, USA).
Footnotes
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Kevin W. Garey has received research funding from Paratek Pharmaceuticals, Inc. Warren Rose has received research funding and consulting fees from Paratek Pharmaceuticals, Inc. Mark Wilcox has received consulting fees from Paratek Pharmaceuticals, Inc. Alisa W. Serio and Kyle Gunter are employees and stockholders of Paratek Pharmaceuticals, Inc.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This review was supported by Paratek Pharmaceuticals, Inc.
ORCID iD: Kyle Gunter  https://orcid.org/0000-0003-1967-995X
https://orcid.org/0000-0003-1967-995X
References
- 1. Guh AY, Mu Y, Winston LG, et al. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med. 2020;382:1320-1330. doi: 10.1056/NEJMoa1910215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. European Centre for Disease Prevention Control. Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals, 2011-2012. Stockholm: ECDC. Published; 2013. Accessed January 22, 2022. https://www.ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/healthcare-associated-infections-antimicrobial-use-PPS.pdf. [Google Scholar]
- 3. Balsells E, Shi T, Leese C, et al. Global burden of Clostridium difficile infections: a systematic review and meta-analysis. J Glob Health. 2019;9(1):010407. doi: 10.7189/jogh.09.010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fernández-García L, Blasco L, López M, Tomás M. Clostridium difficile infection: pathogenesis, diagnosis and treatment. In: Clostridium Difficile: A Comprehensive Overview. IntechOpen; 2017. doi: 10.5772/67754. [DOI] [Google Scholar]
- 5. Ghantoji SS, Sail K, Lairson DR, DuPont HL, Garey KW. Economic healthcare costs of Clostridium difficile infection: a systematic review. J Hosp Infect. 2010;74(4):309-318. doi: 10.1016/j.jhin.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 6. Desai K, Gupta SB, Dubberke ER, Prabhu VS, Browne C, Mast TC. Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach. BMC Infect Dis. 2016;16:303. doi: 10.1186/s12879-016-1610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heinrich K, Harnett J, Vietri J, Chambers R, Yu H, Zilberberg M. Impaired quality of life, work, and activities among adults with Clostridium difficile infection: a multinational survey. Dig Dis Sci. 2018;63(11):2864-2873. doi: 10.1007/s10620-018-5222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smits WK, Lyras D, Lacy DB, Wilcox MH, Kuijper EJ. Clostridium difficile infection. Nat Rev Dis Prim. 2016;2:16020. doi: 10.1038/nrdp.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825-834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shah DN, Aitken SL, Barragan LF, et al. Economic burden of primary compared with recurrent Clostridium difficile infection in hospitalized patients: a prospective cohort study. J Hosp Infect. 2016;93(3):286-289. doi: 10.1016/j.jhin.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 11. Garey KW, Aitken SL, Gschwind L, et al. Development and validation of a Clostridium difficile health-related quality-of-life questionnaire. J Clin Gastroenterol. 2016;50(8):631-637. doi: 10.1097/MCG.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chalmers JD, Akram AR, Singanayagam A, Wilcox MH, Hill AT. Risk factors for Clostridium difficile infection in hospitalized patients with community-acquired pneumonia. J Infect. 2016;73(1):45-53. doi:10.1016/j.jinf.2016.04.008 [DOI] [PubMed] [Google Scholar]
- 13. Eze P, Balsells E, Kyaw MH, Nair H. Risk factors for Clostridium difficile infections: an overview of the evidence base and challenges in data synthesis. J Glob Heal. 2017;7:010417. doi: 10.7189/jogh.07.010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deshpande A, Pasupuleti V, Thota P, et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36(4):452-460. doi: 10.1017/ice.2014.88. [DOI] [PubMed] [Google Scholar]
- 15. Tabak YP, Srinivasan A, Yu KC, et al. Hospital-level high-risk antibiotic use in relation to hospital-associated Clostridioides difficile infections: retrospective analysis of 2016-2017 data from US hospitals. Infect Control Hosp Epidemiol. 2019;40:1229-1235. doi: 10.1017/ice.2019.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnson S, Samore MH, Farrow KA, et al. Epidemics of diarrhea caused by a clindamycin-resistant strain of Clostridium difficile in four hospitals. N Engl J Med. 1999;341:1645-1651. doi: 10.1056/NEJM199911253412203. [DOI] [PubMed] [Google Scholar]
- 17. Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079-1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 18. Shen W-J, Deshpande A, Hevener KE, et al. Constitutive expression of the cryptic vanGCd operon promotes vancomycin resistance in Clostridioides difficile clinical isolates. J Antimicrob Chemother. 2020;75:859-867. doi: 10.1093/jac/dkz513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deshpande A, Wu X, Huo W, Palmer KL, Hurdle JG. Chromosomal resistance to metronidazole in Clostridioides difficile can be mediated by epistasis between iron homeostasis and oxidoreductases. Antimicrob Agents Chemother. 2020;64:e00415-e00420. doi: 10.1128/AAC.00415-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brown KA, Khanafer N, Daneman N, Fisman DN. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother. 2013;57(5):2326-2332. doi: 10.1128/AAC.02176-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65(2):232-260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention. Outpatient Antibiotic Prescriptions: United States, 2018. Accessed January 22, 2022. https://stacks.cdc.gov/view/cdc/112114.
- 23. Cho JC, Childs-Kean LM, Zmarlicka MT, Crotty MP. Return of the tetracyclines: omadacycline, a novel aminomethylcycline antimicrobial. Drugs Today (Barc). 2018;54(3):209-217. doi: 10.1358/dot.2018.54.3.2800620. [DOI] [PubMed] [Google Scholar]
- 24. Heaney M, Mahoney MV, Gallagher JC. Eravacycline: the tetracyclines strike back. Ann Pharmacother. 2019;53(11):1124-1135. doi: 10.1177/1060028019850173. [DOI] [PubMed] [Google Scholar]
- 25. Tanaka SK, Steenbergen J, Villano S. Discovery, pharmacology, and clinical profile of omadacycline, a novel aminomethylcycline antibiotic. Bioorg Med Chem. 2016;24:6409-6419. doi: 10.1016/j.bmc.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 26. US. Food and Drug Administration. FDA Adverse Events Reporting System (FAERS) Public Dashboard. Accessed January 22, 2022. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard.
- 27. Paratek Pharmaceuticals Inc. Publications and Presentations. Accessed January 22, 2022. https://www.paratekpharma.com/medical-affairs/publications-and-presentations.
- 28. British Medical Journal. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stapert L, Wolfe C, Shinabarger D, Marra A, Pillar C. In vitro activities of omadacycline and comparators against anaerobic bacteria. Antimicrob Agents Chemother. 2018;62:e00047-e00018. doi: 10.1128/AAC.00047-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim O, Leahy RG, Traczewski M, Macone A, Steenbergen J, Tanaka SK. Activity and efficacy of omadacycline against Clostridium difficile. Poster P1325 Presented at the 26th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID); April 9-12, 2016; Amsterdam, The Netherlands. [Google Scholar]
- 31. Overcash JS, Bhiwandi P, Garrity-Ryan L, et al. Pharmacokinetics, safety, and clinical outcomes of omadacycline in women with cystitis: results from a phase 1b study. Antimicrob Agents Chemother. 2019;63(5):e02083-18. doi: 10.1128/AAC.02083-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Overcash JS, Tzanis E, Manley A, et al. Omadacycline in female adults with cystitis: results from a randomized, double-blinded, adaptive phase 2 study. Open Forum Infect Dis. 2020;7:S827-S828. doi: 10.1093/ofid/ofaa439.1866. [DOI] [Google Scholar]
- 33. Overcash JS, Tzanis E, Manley A, et al. Omadacycline in female adults with acute pyelonephritis: results from a randomized, double-blind, adaptive phase 2 study. Open Forum Infect Dis. 2020;7:S827. doi: 10.1093/ofid/ofaa439.1865. [DOI] [Google Scholar]
- 34. Lodise TP, Mistry R, Young K, LaPensee K. Decision analysis: omadacycline relative to moxifloxacin among hospitalized community-acquired bacterial pneumonia patients at risk of Clostridioides difficile infection. Clin Drug Investig. 2021;41(3):269-275. doi: 10.1007/s40261-021-01005-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lodise T, Rodriguez M, Chitra S, Wright K, Patel N. Potential cost savings associated with targeted substitution of omadacycline in current guideline concordant inpatient agents for the treatment of adult hospitalized patients with community-acquired bacterial pneumonia at high risk for Clostridioides difficile infections: results of healthcare-decision analytic model from the United States hospital perspective. Antibiotics (Basel). 2021;10(10):1195. doi: 10.3390/antibiotics10101195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Camporeale A, Tellapragada C, Kornijenko J, Nord CE, Giske CG. In vitro activity of omadacycline and five comparators against contemporary ribotypes of Clostridioides difficile in Stockholm, Sweden. Microbiol Spectr. 2021;9(2):e0144021. doi: 10.1128/Spectrum.01440-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Begum K, Bassères E, Miranda J, et al. In vitro activity of omadacycline, a new tetracycline analog, and comparators against Clostridioides difficile. Antimicrob Agents Chemother. 2020;64:e00522-20. doi: 10.1128/AAC.00522-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Budi N, Godfrey JJ, Safdar N, Shukla SK, Rose WE. Omadacycline compared to vancomycin when combined with germinants to disrupt the life cycle of Clostridioides difficile. Antimicrob Agents Chemother. 2021;65(5):e01431-20. doi: 10.1128/AAC.01431-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Budi N, Godfrey J, Shukla S, Safdar N, Rose W. Efficacy of germinants and omadacycline for preventing Clostridioides difficile relapse in a murine model. Poster 1037 Presented at Idweek, September 29 to October 3, 2021(Virtual). [Google Scholar]
- 40. Moura IB, Buckley AM, Ewin D, et al. Omadacycline gut microbiome exposure does not induce Clostridium difficile proliferation or toxin production in a model that simulates the proximal, medial, and distal human colon. Antimicrob Agents Chemother. 2019;63(2):e01581-18. doi: 10.1128/AAC.01581-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stets R, Popescu M, Gonong JR, et al. Omadacycline for community-acquired bacterial pneumonia. N Engl J Med. 2019;380:517-527. doi: 10.1056/NEJMoa1800201. [DOI] [PubMed] [Google Scholar]
- 42. O’Riordan W, Green S, Overcash JS, et al. Omadacycline for acute bacterial skin and skin-structure infections. N Engl J Med. 2019;380:528-538. doi: 10.1056/NEJMoa1800170. [DOI] [PubMed] [Google Scholar]
- 43. O'Riordan W, Cardenas C, Shin E, et al. Once-daily oral omadacycline versus twice-daily oral linezolid for acute bacterial skin and skin structure infections (OASIS-2): a phase 3, double-blind, multicentre, randomised, controlled, non-inferiority trial. Lancet Infect Dis. 2019;19(10):1080-1090. doi: 10.1016/S1473-3099(19)30275-0. [DOI] [PubMed] [Google Scholar]
- 44. Baines SD, Freeman J, Wilcox MH. Effects of piperacillin/tazobactam on Clostridium difficile growth and toxin production in a human gut model. J Antimicrob Chemother. 2005;55(6):974-982. doi: 10.3390/antibiotics10101195. [DOI] [PubMed] [Google Scholar]
- 45. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:e1-e48. doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hastey CJ, Dale SE, Nary J, et al. Comparison of Clostridium difficile minimum inhibitory concentrations obtained using agar dilution vs broth microdilution methods. Anaerobe. 2017;44:73-77. doi: 10.1016/j.anaerobe.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 47. Rodvold KA, Pai MP. Pharmacokinetics and pharmacodynamics of oral and intravenous omadacycline. Clin Infect Dis. 2019;69:S16-S22. doi: 10.1093/cid/ciz309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Flarakos J, Du Y, Gu H, et al. Clinical disposition, metabolism and in vitro drug–drug interaction properties of omadacycline. Xenobiotica. 2017;47(8):682-696. doi: 10.1080/00498254.2016.1213465. [DOI] [PubMed] [Google Scholar]
- 49. Gonzales M, Pepin J, Frost EH, et al. Faecal pharmacokinetics of orally administered vancomycin in patients with suspected Clostridium difficile infection. BMC Infect Dis. 2010;10:363. doi: 10.1186/1471-2334-10-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lamp KC, Freeman CD, Klutman NE, Lacy MK. Pharmacokinetics and pharmacodynamics of the nitroimidazole antimicrobials. Clin Pharmacokinet. 1999;36(5):353-373. doi: 10.2165/00003088-199936050-00004. [DOI] [PubMed] [Google Scholar]
- 51. Baines SD, Saxton K, Freeman J, Wilcox MH. Tigecycline does not induce proliferation or cytotoxin production by epidemic Clostridium difficile strains in a human gut model. J Antimicrob Chemother. 2006;58:1062-1065. doi: 10.1093/jac/dkl364. [DOI] [PubMed] [Google Scholar]
- 52. Wilcox MH. Evidence for low risk of Clostridium difficile infection associated with tigecycline. Clin Microbiol Infect. 2007;13(10):949-952. doi: 10.1111/j.1469-0691.2007.01792.x. [DOI] [PubMed] [Google Scholar]
- 53. Khanafer N, Daneman N, Greene T, et al. Susceptibilities of clinical Clostridium difficile isolates to antimicrobials: a systematic review and meta-analysis of studies since 1970. Clin Microbiol Infect. 2018;24(2):110-117. doi: 10.1016/j.cmi.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 54. Buckley AM, Altringham J, Clark E, et al. Eravacycline, a novel tetracycline derivative, does not induce Clostridioides difficile infection in an in vitro human gut model. J Antimicrob Chemother. 2020;76:171-178. doi: 10.1093/jac/dkaa386. [DOI] [PubMed] [Google Scholar]
- 55. Bassères E, Begum K, Lancaster C, et al. In vitro activity of eravacycline against common ribotypes of Clostridioides difficile. J Antimicrob Chemother. 2020;75:2879-2884. doi: 10.1093/jac/dkaa289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Norén T, Alriksson I, Åkerlund T, Burman LG, Unemo M. In vitro susceptibility to 17 antimicrobials of clinical Clostridium difficile isolates collected in 1993-2007 in Sweden. Clin Microbiol Infect. 2010;16:1104-1110. doi: 10.1111/j.1469-0691.2009.03048.x. [DOI] [PubMed] [Google Scholar]
- 57. Tariq R, Cho J, Kapoor S, et al. Low risk of primary Clostridium difficile infection with tetracyclines: a systematic review and metaanalysis. Clin Infect Dis. 2018;66:514-522. doi: 10.1093/cid/cix833. [DOI] [PubMed] [Google Scholar]
- 58. Slimings C, Riley TV. Antibiotics and healthcare facility-associated Clostridioides difficile infection: systematic review and meta-analysis 2020 update. J Antimicrob Chemother. 2021;76:1676-1688. doi: 10.1093/jac/dkab091. [DOI] [PubMed] [Google Scholar]
- 59. Turner RB, Smith CB, Martello JL, Slain D. Role of doxycycline in Clostridium difficile infection acquisition. Ann Pharmacother. 2014;48(6):772-776. doi: 10.1177/1060028014528792. [DOI] [PubMed] [Google Scholar]
- 60. NUZYRA [package insert], 2021. King of Prussia, PA: Paratek Pharmaceuticals, Inc. [Google Scholar]
- 61. Tischendorf J, Brunner M, Knobloch MJ, et al. Evaluation of a successful fluoroquinolone restriction intervention among high-risk patients: a mixed-methods study. PLoS One. 2020;15(8):e0237987. doi: 10.1371/journal.pone.0237987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65:1-12. doi: 10.15585/mmwr.rr6506a1. [DOI] [PubMed] [Google Scholar]
- 64. Kechagias KS, Chorepsima S, Triarides NA, Falagas ME. Tigecycline for the treatment of patients with Clostridium difficile infection: an update of the clinical evidence. Eur J Clin Microbiol Infect Dis. 2020;39(6):1053-1058. doi: 10.1007/s10096-019-03756-z. [DOI] [PubMed] [Google Scholar]