Abstract
Previous studies have demonstrated that γδ T lymphocytes are important for host resistance to pulmonary infection of the murine lung by log-phase cells of Nocardia asteroides. To study the role of γδ T cells in nocardial interactions in the murine lung, C57BL/6J wild type and C57BL/6J-Tcrd (γδ T-cell knockout mice) were infected intranasally with log-phase cells of N. asteroides GUH-2. At 3, 5, and 7 days after infection, the γδ T cells were quantified by multiparameter flow cytometry. At the same time, Gram and hematoxylin-eosin stains of paraffin sections were performed to monitor the host responses. The data showed that γδ T lymphocytes increased significantly within the lungs after intranasal infection, and the peak of this cellular increase occurred at 5 days. Furthermore, at this time, greater than 50% of the CD3 T-cell receptor (TCR)-positive (CD3+) cells were γδ TCR positive. Histological examination clearly showed divergent inflammatory responses in the lungs of wild-type mice compared to γδ T-cell knockout mice. The C57BL/6J-Tcrd mice were less capable of clearing the organism, and the polymorphonuclear leukocyte response lasted longer than in wild-type C57BL/6J mice. These results showed that γδ T cells were actively involved in modulating the innate host responses to murine pulmonary infection by N. asteroides.
Nocardia asteroides is increasingly recognized as a serious cause of pulmonary disease in both normal and immunocompromised humans (3). A murine model for the investigation of host responses during pulmonary nocardiosis has been developed (6). Cells of N. asteroides GUH-2, during the log phase of growth, adhere to and invade the bronchiolar epithelium of mice following intranasal inoculation (2, 4). At the same time, some of these bacteria reach the alveoli, where they are phagocytized by alveolar macrophages (8, 9). Within hours, the nocardiae initiate growth, which induces an extensive inflammatory response characterized by infiltration with polymorphonuclear phagocytes (PMNs) resulting in bronchopulmonary pneumonia (3, 5, 6). The progressive nature of this pulmonary response is dependent on the relative inoculum size, which dictates the extent of pulmonary damage and host responsiveness (5, 6, 7). For example, with a lethal dose (107 CFU/lung), the mice become acutely ill and die within 3 to 7 days. However, following a lower, nonlethal inoculum, the mice develop symptoms within 24 h that may worsen over the next 24 to 48 h; but at 5 days postinfection, the mice appear improved. Seven days after inoculation, these mice appear to be fully recovered (14, 19).
Histologically, the pneumonia reaches its peak during the first 72 h, but after 5 days the pulmonary infiltrates become stabilized and begin to resolve, so that by 7 days postinfection relatively few inflammatory infiltrates can be found. In general, the structural integrity of the alveoli appears intact and relatively normal (6, 14, 19). These observations suggest that a critical host response occurs within the lungs between 3 and 5 days after intranasal administration of log-phase cells of N. asteroides. During this time period, nocardial growth is terminated, resulting in complete resolution of the inflammatory infiltrates. This process is then followed by repair to the airways, so that after 7 days the lungs appear to be relatively healthy (6, 14, 19).
Previous studies demonstrate that γδ T lymphocytes are important in the initial innate resistance to overwhelming nocardial invasion and growth within the murine lung (18). It is thought that these γδ T cells are responsive to nocardial-induced epithelial damage and that they play a critical role in both PMN recruitment and localized epithelial repair (16, 18). The purpose of the study reported below is to determine whether the number of γδ T cells in the lungs is increased during the termination of nocardial growth and the resolution of lesions 5 to 7 days after pulmonary infection with N. asteroides GUH-2.
MATERIALS AND METHODS
Mice.
Six- to 8-week old C57BL/6J wild-type and C57BL/6J-Tcrd (γδ T-cell deficient) female mice were purchased from Jackson Laboratories (Bar Harbor, Maine) and housed in pathogen-free conditioned animal rooms at the University of California at Davis. The mice were maintained on Purina lab chow and provided water ad libitum. All mice were maintained by the animal resource services (ARS) following standard and approved protocols. The ARS monitored sentinel mice for infectious agents, and none were reported during these studies.
N. asteroides infection.
N. asteroides strain GUH-2 was grown to mid-log phase in brain heart infusion broth (BHI-b; Difco) at 37°C with mild agitation for 16 to 19 h prior to use. The culture was checked for purity by plating a small sample onto BHI agar, and the morphology of the bacteria was monitored microscopically. Then, the culture was centrifuged for 10 min at 50 × g to pellet bacterial aggregates (7). The concentration of bacteria remaining in the supernatant was measured by optical density at 580 nm using a spectrophotometer (Beckman DU 640). The inoculum was then adjusted with fresh BHI-b to approximately 6 × 107 CFU/ml. Serial dilutions were plated on BHI agar to enumerate the actual number of CFU. Either female C57BL/6J or C57BL/6J-Tcrd mice were anesthetized by intraperitoneal injection with Nembutal (50 mg/kg of body weight). Fifty microliters of the nocardial suspension was placed onto the anterior nares, and the mice were permitted to aspirate the inoculum. Previous studies revealed that this procedure reproducibly delivered approximately 1 × 106 to 3 × 106 CFU/lung (6). Control mice from each group were intranasally administered BHI broth without bacteria.
Lung homogenization and determination of CFU.
To assess the actual dosage of GUH-2 in the lung, animals were randomly selected from each group of mice that recovered from the infection process. Three hours after infection, the lungs were removed, placed in 3 ml of Hanks' balanced salt solution (Gibco), and homogenized with a tissue homogenizer (Polytron PT1200). This lung homogenate was serially diluted and plated onto BHI agar. The BHI agar plates were incubated at 37°C for 3 days, and then the colonies were counted. The average number of CFU/mouse was calculated (6, 7).
Pulmonary murine lymphocyte isolation.
At 3, 5, and 7 days postinfection, lungs from groups of mice were intracardially perfused with cold phosphate-buffered saline (PBS) at pH 7.4. The perfused lungs were then removed and minced in RPMI (Difco) to a fine slurry. This slurry was resuspended in 5 ml of digestion medium, which consisted of RPMI, 10% fetal calf serum (FCS), 10 U of DNase I and 20 U of collagenase type VIII per ml, and 50 μM β-mercaptoethanol (1) and incubated for 2 h at 37°C. Undisrupted tissue in the digestion medium was teased with a 20-gauge needle and filtered through a 40-μm nylon cell strainer. The cell filtrate was washed and centrifuged at 200 × g for 10 min. This pellet was then resuspended in RPMI with 10% FCS, carefully overlaid onto a 40 to 70% Percoll gradient, and centrifuged for 30 min at 200 × g. Pulmonary murine lymphocytes were collected from the interface of the 40 to 70% Percoll gradient (1).
Multiparameter flow cytometry.
The red blood cells that were contaminating the lymphocyte preparations were lysed with ammonium chloride. Then, 106 cells were washed and incubated with 10 ng of anti-CD16/32 Fc block (PharMingen, San Diego, Calif.) per ml at 4°C for 20 min to reduce nonspecific binding. These blocked cells were dually stained using anti-CD3 T-cell receptor (TCR) conjugated to fluorescein isothiocyanate (PharMingen) and anti-γδ TCR conjugated to phycoerythrin (PharMingen) for 15 min at 4°C. These stained cells were analyzed with a FACscan cytometer (Becton Dickinson). The resulting dot plot data were analyzed using Cellquest software.
Histology.
To visualize both nocardiae and inflammatory infiltrates, the lungs from wild-type and γδ T-cell-deficient mice were perfused with 10% neutral buffered formalin in 0.1 M PBS and embedded in paraffin. Paraffin sections from these lungs dissected at 3, 5, and 7 days postinfection were stained with the Brown and Brenn modification of the Gram stain as well as with hematoxylin and eosin (H&E).
Statistical analysis.
Statistical significance was performed with the unpaired Student t test. All calculations were computed with Sigmaplot for Apple Macintosh. Statistical significance was indicated at P < 0.05 (n = 3 per experiment, three experiments).
RESULTS
Histological response in murine lung after infection with N. asteroides GUH-2.
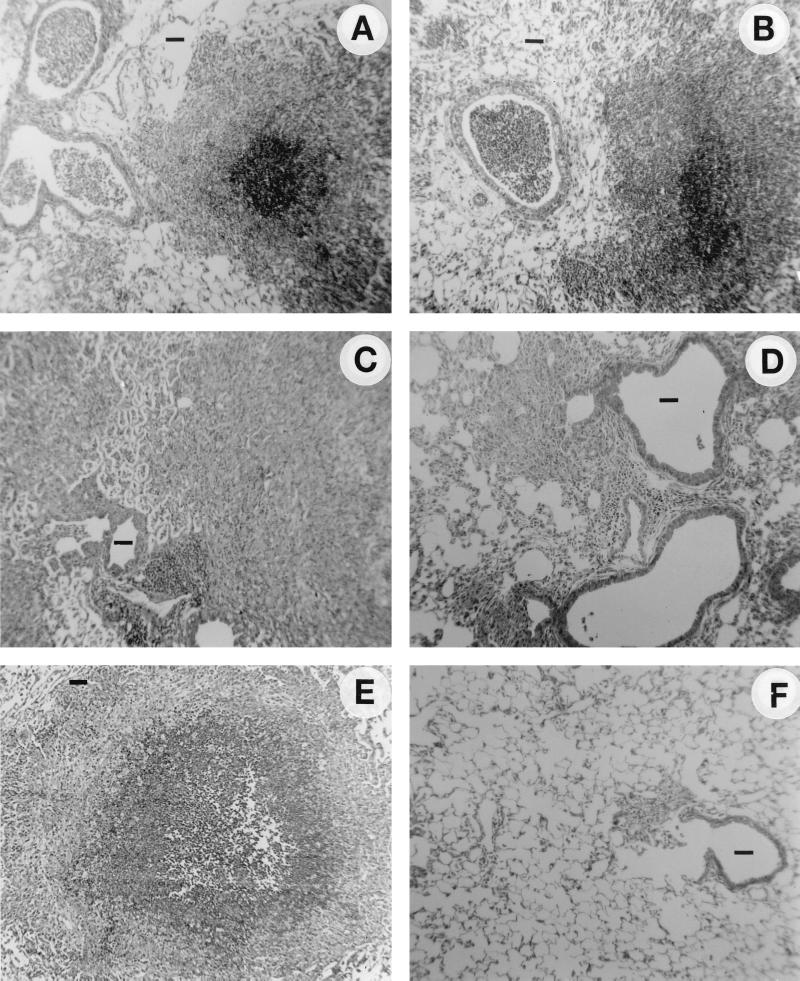
H&E staining of infected lung tissue (Fig. 1) showed that GUH-2 induced an extensive inflammatory response characterized by an infiltration of PMNs in both bronchiolar and alveolar regions of both normal and γδ T-cell-deficient mice. (Fig. 1A and B). At 5 days after infection, histology revealed a divergent inflammatory response between the two groups of animals. The γδ T-lymphocyte-deficient mice continued to show a strong PMN response, whereas the normal (wild type) mice appeared to be improving, with decreased inflammation (Fig. 1C and D). Seven days after infection, the lungs of wild-type mice appeared relatively normal, with only a few small regions of inflammation; in contrast, many centrally located foci of relatively large cellular infiltrates remained in the γδ knockout mice (Fig. 1E and F). Thus, the level of infiltration and resolution of PMNs in the lungs of infected mice, as a function of time, was strongly impaired in the γδ TCR-deficient (knockout) mice (Fig. 1 and 2).
FIG. 1.
Histology of the murine lungs (H&E stain) in normal and γδ T-cell-deficient mice following intranasal infection with a nonlethal dose of log-phase cells of N. asteroides GUH-2. (A) γδ T-cell knockout mice at 3 days after infection; (B) C57BL/6J wild-type mice at 3 days after infection; (C) γδ T-cell knockout mice at 5 days after infection; (D) C57BL/6J wild-type mice at 5 days after infection; (E) γδ T-cell knockout mice at 7 days after infection; (F) C57BL/6J wild-type mice at 7 days after infection. Original magnification, ×100. Bars, approximately 50 μm.
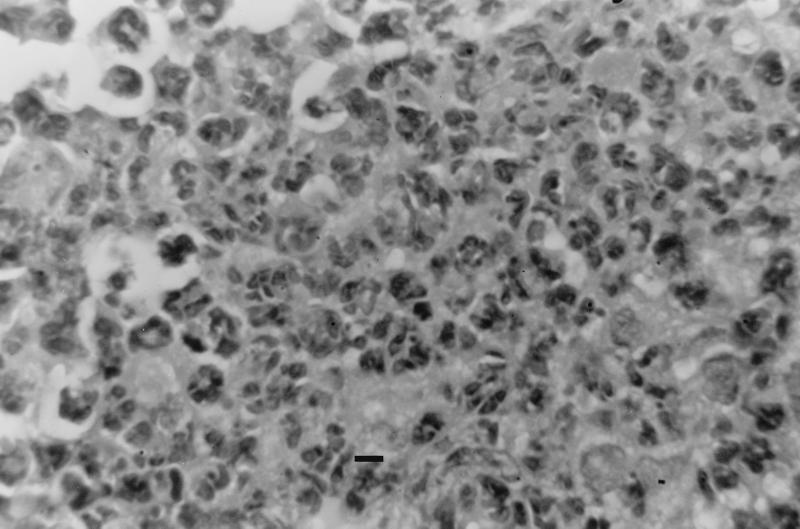
FIG. 2.
High-magnification view of lesion shown in Fig. 1E (bar, 10 μm). Note that lungs from γδ TCR-deficient mice at 7 days postinfection contain foci of cellular infiltration, composed predominantly of PMNs.
Nocardial clearance delayed in γδ TCR-deficient mice.
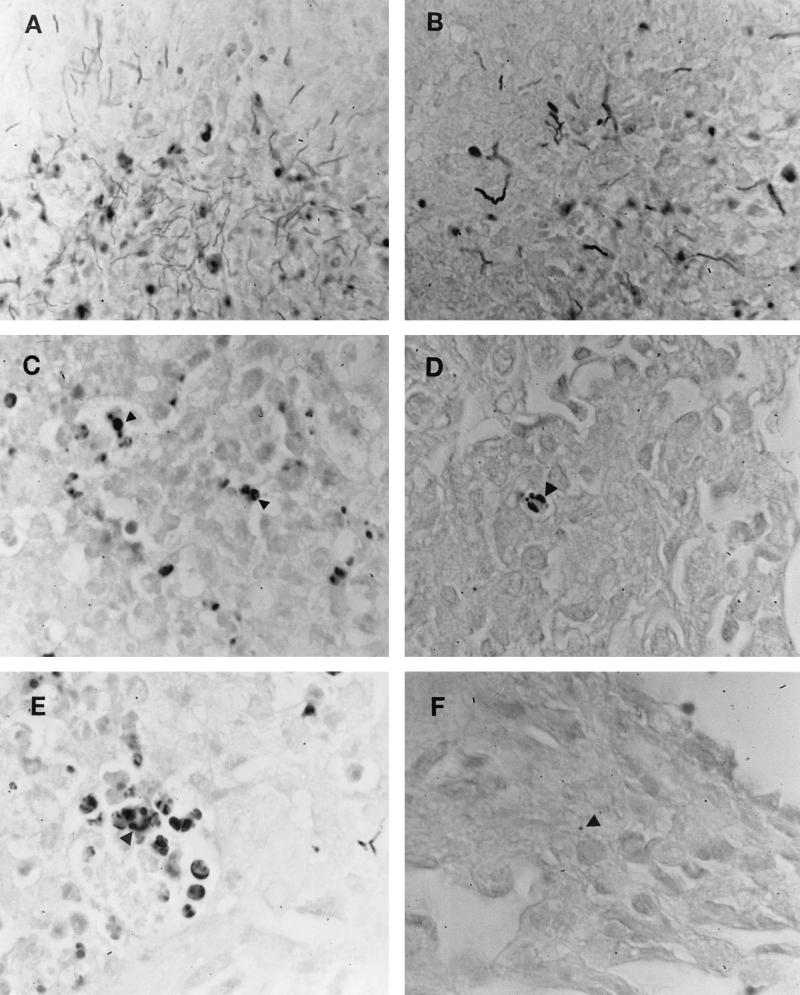
To monitor nocardial clearance, Gram-stained tissues from wild-type and γδ TCR-deficient mice were examined (Fig. 3). The divergent nature observed in histological examination was again paralleled in nocardial clearance. At 3 days, nocardial growth characterized by gram-positive filaments was unchecked in both murine strains (Fig. 3A and B). However, as observed with the PMN stabilization and resolution, the difference in nocardial clearance became apparent at five days. Gram-positive filaments were replaced in wild-type and γδ TCR-deficient mice with gram-positive spherical bodies or L-phase variants of GUH-2 (Fig. 3C and D). These variants were more numerous throughout the lungs of γδ knockout mice than those of their wild-type counterparts. At 7 days, lungs of wild-type mice appeared to be GUH-2 free (Fig. 3E), but gram-positive spherical bodies and nocardial filaments were observed in γδ T-cell-deficient mice (Fig. 3F). These data indicated a delayed nocardial clearance in γδ knockout mice.
FIG. 3.
Gram stain of N. asteroides GUH-2 in the lungs of normal C57BL/6J mice (B, D, and E) at 3, 5, and 7 days after infection compared to lungs from γδ T-cell-deficient mice (A, C, and E) at the same time points. Arrowheads indicate gram-positive nocardial cells.
Increased γδ T lymphocytes in nocardia-infected murine lungs.
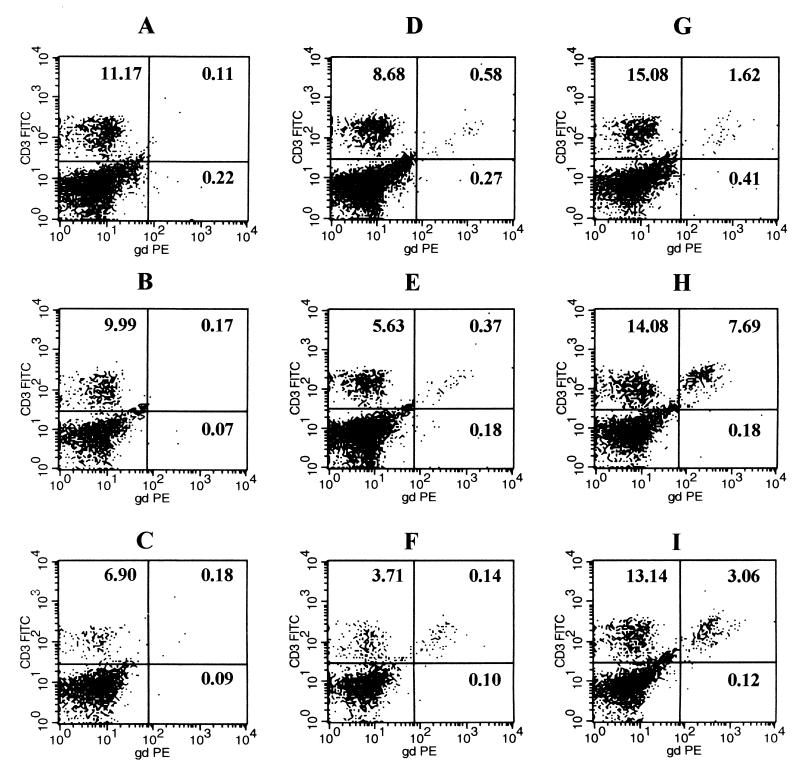
To determine the γδ T-lymphocyte responses in the lungs following a nonlethal infection with N. asteroides GUH-2, multiparameter flow cytometry was used. A dot plot presentation of the data from flow cytometry showed a definite increase in γδ T cells at 5 days after infection in the wild-type C57BL/6J mice compared to the wild-type control mice mock inoculated with BHI broth (Fig. 4H). The mice challenged with GUH-2 had a γδ T-cell response that was first measurable at 3 days, peaked at 5 days, and remained elevated at 7 days after infection (Fig. 4G, H, and I). Also, BHI-b induced a slight but transient response in the lung that was probably due to nonspecific irritation in the lungs (Fig. 4D, E, and F). As expected, all γδ T-cell knockout mice (negative control) failed to show any response in these assays. The use of TCR-deficient mice infected with GUH-2 confirmed the specificity of the γδ TCR antibody and showed that the data were not artifactual, since the γδ T cells detected in the knockout mice were insignificant at all time periods (Fig. 4A, B, and C). Thus, the lungs of mice infected with N. asteroides GUH-2 had increased numbers of γδ T cells that coincided with resolution of the inflammatory response, clearance of bacteria, and repair of damaged lung architecture (compare Fig. 1, 3, and 4).
FIG. 4.
Representative dot plots obtained by FACscan flow cytometry of murine pulmonary γδ T cells after intranasal instillation of log-phase cells of N. asteroides GUH-2 at 3, 5, and 7 days. (A, B, and C) Cells from γδ T-cell knockout mice at 3, 5, and 7 days, respectively; (D, E, and F) cells from sham-inoculated control mice without bacteria at 3, 5, and 7 days, respectively; (G, H, and I) cells from wild-type mice infected with GUH-2 at 3, 5, and 7 days, respectively. Five thousand CD3+ cells per sample were analyzed. The test groups included C57BL/6J-Tcrd (γδ T-cell knockout mice) inoculated with GUH-2 in BHI broth, C57BL/6J wild-type mice inoculated with BHI broth alone, and C57BL/6J wild-type mice inoculated with GUH-2 in BHI broth. Numbers in each panel represent relative number of cells per quadrant compared to total cells. FITC, fluorescein isothiocyanate; PE, phycoerythrin; gd, γδ TCR.
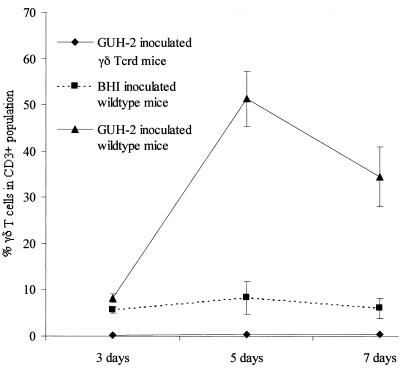
By gating on 5,000 CD3 TCR-positive cells (all T lymphocytes), the distinction was made between lymphocytes and nonlymphocytes that were recovered from the Percoll gradients. The CD3 TCR-positive cells were defined as those cells representing the total T cells in the lungs (αβ plus γδ T cells). By focusing on these CD3 TCR-positive cells, the γδ T-cell response at 3, 5, and 7 days postinfection was expressed as a percentage of the total CD3+ population (Fig. 5). Thus, at 3, 5, and 7 days postinfection, 8.2, 51.3, and 32% of the CD3-positive cells (lymphocytes) were γδ TCR-positive cells, respectively. After 3 days, lungs from the infected γδ TCR-positive wild-type mice showed a minimal response, with a 1.5-fold increase in γδ T cells compared to the control mice. However, at 5 days after infection, the percentage of γδ T cells in the wild-type animals had increased sixfold, and at 7 days the number of γδ T cells was still elevated (Fig. 5). In contrast, the γδ T-cell population remained relatively stable in the BHI broth (sham inoculated) control mice, with the exception of a slight but not significant response at 5 days (Fig. 5).
FIG. 5.
Relative abundance of γδ T cells in murine lungs after intranasal inoculation of N. asteroides GUH-2. A summary of γδ T-cell responses, expressed as percent γδ T cells in the CD3+ population following intranasal infection with GUH-2 at 3, 5, and 7 days. The data points represent the mean ± standard error for each subgroup of three at each time point. Experiments were repeated two more times with similar results. The test groups included C57BL/6J-Tcrd (γδ T-cell knockout mice) inoculated with GUH-2 in BHI broth, C57BL/6J wild-type mice inoculated with BHI broth alone, and C57BL/6J wild-type mice inoculated with GUH-2 in BHI broth.
DISCUSSION
Murine responses to pulmonary infection by N. asteroides appear to depend on the inoculum, the time after infection, the presence of functional T lymphocytes, and an adequate PMN inflammatory response (6, 7, 13–15). With an intranasal inoculum dose slightly less than the 50% lethal dose (LD50) for wild-type mice, γδ T cells are critical to the initial innate resistance to massive and lethal pulmonary invasion by log-phase cells of N. asteroides GUH-2 (18). Thus, when equally challenged with a nearly LD50 dose of log-phase GUH-2, γδ T-cell knockout mice appear to be more deficient at PMN recruitment than their intact, wild-type littermates (16, 18). The lack of PMN recruitment renders γδ T-cell-deficient mice more susceptible to GUH-2 than the controls (16). In the research described here, we did not observe the previously reported PMN deficiency in mice lacking γδ T cells. However, it should be noted that our research utilized an inoculum dose threefold lower than that reported by King et al. (18). In these studies, we showed that recruitment of PMNs was not totally blocked, but delayed. In the studies reported above, we observed no mortality in nocardia-infected γδ T-cell knockout mice using the lower inoculum dose (referred to as a nonlethal dose). We believe that these apparent differences may be related more to the initial severity of epithelial damage in the bronchioles caused by the higher inoculum dose than to a direct, protective role of the γδ T cells per se (16, 18).
Studies have shown that γδ T cells express mRNA for chemokines such as MIP-1α, MIP-1β, RANTES, and lymphotactin (10) and that neutrophil-dependent clearance of nocardiae requires CXC chemokines (19). These observations support the suggestion that γδ T cells are responsible for the recruitment of inflammatory cells after epithelial damage (16). Therefore, even a slight delay in PMN recruitment to the site of nocardial invasion should have a profound influence on the outcome, since many investigators have established a critical role for PMNs in resistance to pulmonary nocardiosis (3, 5, 13–15, 19).
The data presented above demonstrated that the γδ T-cell response in the lung was time dependent. Not only did γδ T cells play an important role in initiating PMN recruitment, they also appeared to be involved in the resolution of the PMN response after infection. Following a nonlethal intranasal infection, there was a time-dependent peak in increased numbers of γδ T cells in the lungs. At 3 days postinfection, the relative percentage of γδ T cells had not increased much above basal levels. However, this ratio was altered dramatically 5 days after infection, when the γδ T cells represented about 50% of the CD3-positive cells in the lungs. In contrast, the numbers of γδ T cells remained at less than 8% of the total CD3-positive cells in sham-inoculated control mice. Histopathology of the pulmonary responses revealed a divergence in PMNs in lesions of mice with γδ T cells compared to knockout mice lacking γδ T cells. This dichotomy was most pronounced at 7 days after infection. At 5 to 7 days, there was a dramatic accumulation of γδ T cells in the lungs of nocardia-infected wild-type mice concomitant with both the appearance and disappearance of neutrophils. These observations implied that the γδ T lymphocytes expedited both the onset of the inflammatory response and its attenuation in order to prevent further tissue damage after the infection had been contained.
Numerous bacterial models that document γδ T-lymphocyte participation exhibit variations on this theme (16). For example, in the Mycobacterium tuberculosis model, γδ T cells regulate cellular trafficking, promote lymphocyte and monocyte infiltration, and limit inflammatory cells that may cause additional tissue damage (12). Furthermore, investigators utilizing the Listeria monocytogenes model concluded that γδ T cells controlled the host inflammatory response to prevent excessive tissue damage (11, 17). The data presented above support the general hypothesis that γδ T cells play a critical role in modulating host PMN responses, repairing epithelial damage and serving as sentinels at mucosal barriers (16, 17).
ACKNOWLEDGMENTS
Public Health Service grant RO1-HL59821 from the National Heart, Lung, and Blood Institute supported this research.
We thank Petar Pujic and LoVelle Beaman and graduate students Terri Ellis, Daniel Barry, and Susanne Kuhlman for helpful comments and discussions during these studies and manuscript preparation.
REFERENCES
- 1.Abraham E, Freitas A A, Coutinho A A. Purification and characterization of intraparenchyma lung lymphocytes. J Immunol. 1990;144:2117–2122. [PubMed] [Google Scholar]
- 2.Beaman B L. Differential binding of Nocardia asteroides in the murine lung and brain suggest multiple ligands on the nocardial surface. Infect Immun. 1996;64:4859–4862. doi: 10.1128/iai.64.11.4859-4862.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaman B L, Beaman L. Nocardia species: host-parasite relationships. Clin Microbiol Rev. 1994;7:213–264. doi: 10.1128/cmr.7.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaman B L, Beaman L. Filament tip-associated antigen involved in adherence to and invasion of murine pulmonary epithelial cells in vivo and HeLa cells in vitro by Nocardia asteroides. Infect Immun. 1998;66:4676–4689. doi: 10.1128/iai.66.10.4676-4689.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaman B L, Beaman L. Nocardia asteroides as an invasive intracellular pathogen of the brain and lungs. Subcell Biochem. 2000;33:167–198. doi: 10.1007/978-1-4757-4580-1_8. [DOI] [PubMed] [Google Scholar]
- 6.Beaman B L, Goldstein E, Gershwin M E, Maslan S, Lippert W. Lung responses of congenitally athymic (nude), heterozygous, and Swiss Webster mice to aerogenic and intranasal infection by Nocardia asteroides. Infect Immun. 1978;22:867–877. doi: 10.1128/iai.22.3.867-877.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaman B L, Maslan S, Scates S, Rosen J. Effect of routes of inoculation on host resistance to Nocardia. Infect Immun. 1980;28:185–189. doi: 10.1128/iai.28.1.185-189.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beaman L, Beaman B L. The timing of exposure of mononuclear phagocytes to recombinant interferon γ and recombinant tumor necrosis factor α alters interactions with Nocardia asteroides. J Leukoc Biol. 1992;51:276–281. doi: 10.1002/jlb.51.3.276. [DOI] [PubMed] [Google Scholar]
- 9.Beaman L, Beaman B L. Differences with interactions of Nocardia asteroides with macrophage, endothelial, and astrocytoma cell lines. Infect Immun. 1994;62:1787–1798. doi: 10.1128/iai.62.5.1787-1798.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boismenu R, Feng L, Xia Y Y, Chang J C, Havran W L. Chemokine expression by intraepithelial γδ T cells: implications for the recruitment of inflammatory cells to damaged epithelia. J Immunol. 1996;157:985–992. [PubMed] [Google Scholar]
- 11.DiTirro J, Rhodes E R, Rhodes A D, Burke J M, Chang J C, Burke J M, Mukasa A, Frank A M, Born W K, Orme I M. Disruption of the cellular inflammatory response to Listeria monocytogenes infection in mice with disruptions in targeted genes. J Immunol. 1998;157:2284–2289. doi: 10.1128/iai.66.5.2284-2289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Souza C D, Cooper A M, Frank A A, Mazzaccaro R J, Bloom B R, Orme I M. An ant-inflammatory role for gamma delta T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217–1221. [PubMed] [Google Scholar]
- 13.Felice G A, Beaman B L, Krick J A, Remington J S. Effects of human neutrophils and monocytes on Nocardia asteroides: failure of killing despite occurrence of the oxidative metabolic burst. J Infect Dis. 1980;142:432–438. doi: 10.1093/infdis/142.3.432. [DOI] [PubMed] [Google Scholar]
- 14.Felice G A, Niewoehner D E. Contribution of neutrophils and cell-mediated immunity to control of Nocardia asteroides in murine lung. J Infect Dis. 1987;156:113–121. doi: 10.1093/infdis/156.1.113. [DOI] [PubMed] [Google Scholar]
- 15.Felice G A. Inhibition of Nocardia asteroides by neutrophils. J Infect Dis. 1985;151:47–56. doi: 10.1093/infdis/151.1.47. [DOI] [PubMed] [Google Scholar]
- 16.Ferrick D A, King D P, Jackson K A, Braun R K, Tam S, Hyde D P, Beaman B L. Intraepithelial γδ T lymphocytes: sentinel cells at mucosal barriers. Springer Semin Immunopathol. 2000;22:283–296. doi: 10.1007/s002810000047. [DOI] [PubMed] [Google Scholar]
- 17.Fu Y X, Roark C E, Kelly K, Drevets D, Campbell P, O'Brien R, Born W. Immune protection and control of inflammatory tissue necrosis by γδ T cells. J Immunol. 1994;153:3101–3115. [PubMed] [Google Scholar]
- 18.King D P, Hyde D M, Jackson K A, Novosad D M, Ellis T N, Putney L, Stovall M Y, Van Winkle L S, Beaman B L, Ferrick D A. Protective response to pulmonary injury requires γδ T lymphocytes. J Immunol. 1999;162:5033–5036. [PubMed] [Google Scholar]
- 19.Moore T A, Newstead M W, Strieter R M, Mehrad B, Beaman B L, Standiford T J. Bacterial clearance and survival are dependent on CXC chemokine receptor-2 ligands in a murine model of pulmonary Nocardia asteroides infection. J Immunol. 2000;164:908–915. doi: 10.4049/jimmunol.164.2.908. [DOI] [PubMed] [Google Scholar]