The SARS‐CoV‐2 coronavirus pandemic led to significant challenges for healthcare institutions. Many institutions implemented preoperative COVID‐19 testing which allowed for safe resumption of elective surgery while maintaining optimum safety of patients and healthcare workers. 1 The American Society of Anesthesiologists and Anesthesia Patient Safety Foundation continue to recommend SARS‐CoV‐2 polymerase chain reaction (PCR) testing for all patients undergoing anesthesia in areas of high COVID‐19 prevalence, but now endorse institutions to adopt a more permissive approach for asymptomatic, vaccinated individuals in areas of low‐moderate community transmission. 2 To date, there are no pediatric‐specific guidelines regarding preoperative COVID‐19 testing. Perioperative testing protocols differ between institutions with respect to the timing of testing, types of tests accepted, length of quarantine prior to surgery, and patient populations tested. Preoperative testing provides reassurance to patients and families that all precautions are being taken to minimize the risk of COVID‐19 exposure as well as protect perioperative staff. Further, active COVID‐19 infection has been associated with adverse outcomes, particularly respiratory complications in the perioperative period. 3 It is recommended to consider testing based on the accuracy, availability, and turnaround time in conjunction with the type of procedure. 4 For example, testing may provide a false reassurance as accuracy can depend on the quality of the swab or the day of incubation as well as the test's sensitivity. 5 Additionally, preoperative testing represents a significant financial burden for institutions in terms of equipment and staffing required for testing. As the pandemic evolves, institutions are evaluating the ongoing need for preoperative testing for pediatric patients.
On February 8, 2022, an anonymous survey was sent to US pediatric institutions to explore COVID‐19 perioperative testing protocols currently in use. This survey was approved by the Pediatric Anesthesia Leadership Council (PALC) section of the Society for Pediatric Anesthesia. Children's National Hospital Institutional Review Board acknowledged the project as meeting criteria for a quality improvement initiative and it did not constitute human subjects research. Sixty‐two pediatric institutions were contacted via a PALC listserv. The same survey was re‐sent to the PALC listserv on June 8, 2022, to assess changes in practice as the pandemic progressed.
In February 2022, 27 out of 62 institutions responded, accounting for a response rate of 44% (Figure 1). 30% of respondents required COVID‐19 testing for all patients prior to anesthesia or sedation, while 44% exempted patients who tested positive within 90 days. One institution did not test patients preoperatively. The remaining respondents (22%) limited testing to certain patients based on the procedure, vaccination status, and presence of symptoms or exposures. Sixty nine percent of respondents accepted only PCR tests. The response rate in June of 2022 was also 44%. Fewer institutions (22%) required testing for all patients prior to anesthesia, and 44% of institutions limited testing to certain patients. Additionally, institutions became more lenient with the type of test accepted.
FIGURE 1.
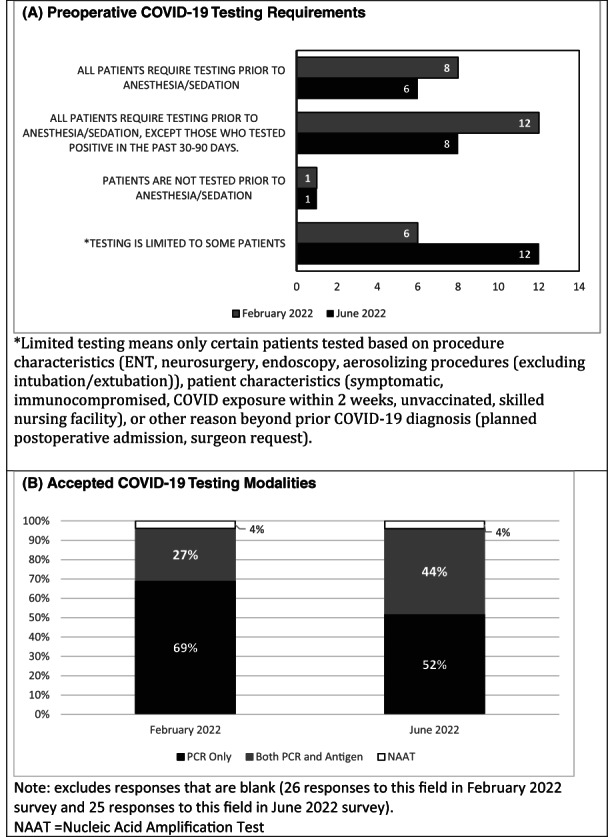
Preoperative COVID‐19 testing survey results. (A) Preoperative COVID‐19 Testing Requirements. (B) Accepted COVID‐19 Testing Modalities.
Extensive preoperative COVID‐19 testing was necessary in the early stages of the pandemic as the long‐term impact of the virus and transmissibility were poorly understood. Knowledge of COVID‐19 diagnosis allows for optimal allocation of perioperative resources such as a negative pressure operating room (OR), personal protective equipment (PPE), and appropriate OR scheduling to ensure staffing while minimizing risks. It also ensures appropriate perioperative risk stratification for the patient. COVID‐19 screening tools are useful at detecting the possibility of disease in symptomatic or exposed individuals, yet imperfect. Universal preoperative COVID‐19 testing may be the only method to identify asymptomatic patients and inform infection prevention decisions to reduce transmission and minimize perioperative risks. Nevertheless, adverse implications should be considered as testing requires dedicated staff and specialized equipment, may reduce operating room efficiency, and be an added burden to patients and families. As significant strides have been made 2 years into the pandemic with the development of infection prevention strategies, symptom, and exposure screening, widespread adoption of COVID‐19 vaccines, greater availability of PPE, and lower disease severity, the need for universal preoperative testing is being reconsidered on an institution‐by‐institution basis. This survey demonstrates testing protocols are changing. While there is a lack of data available regarding outcomes, particularly with new and evolving variants, the data presented is opportune as the pandemic rapidly evolves, and we anticipate preoperative COVID‐19 testing strategies will evolve as well.
FUNDING INFORMATION
The authors have no financial disclosures.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
ACKNOWLEDGEMENT
We are immensely grateful to Pediatric Anesthesia Leadership Council officers Thomas Long, Timothy Martin and Linda Mason for their support, review, and approval of this survey. The authors thank Md Sohel Rana for advice on statistical analysis.
Geng‐Ramos G, Challa C, Pestieau SR, Brennan M, Heitmiller E, Cronin JA. Preoperative COVID‐19 testing at pediatric institutions—Current practice. Pediatr Anesth. 2023;33:86‐88. doi: 10.1111/pan.14575
Section Editor: Britta S von Ungern‐Sternberg
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Geng‐Ramos G, Cronin JA, Heitmiller E, et al. Implementation and expansion of a preoperative COVID‐19 testing process for pediatric surgical patients. Paediatr Anaesth. 2020;30(8):952‐953. doi: 10.1111/pan.13963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. ASA and APSF . ASA/APSF statement on perioperative testing for COVID‐19 virus. 2021. https://www.apsf.org/news‐updates/asa‐and‐apsf‐joint‐statement‐on‐perioperative‐testing‐for‐the‐covid‐19‐virus/. Accessed June 15, 2022.
- 3. Saynhalath R, Alex G, Efune PN, Szmuk P, Zhu H, Sanford EL. Anesthetic complications associated with severe acute respiratory syndrome coronavirus 2 in pediatric patients. Anesth Analg. 2021;133(2):483‐490. [DOI] [PubMed] [Google Scholar]
- 4. American College of Surgeons, American Society of Anesthesiologists, Association of periOperative Registered Nurses, American Hospital Association . Joint statement roadmap for resuming elective surgery after COVID‐19 pandemic. 2020. https://www.asahq.org/about‐asa/newsroom/news‐releases/2020/04/joint‐statement‐on‐elective‐surgery‐after‐covid‐19‐pandemic. Accessed April 17, 2020.
- 5. Schlosser M, Signorelli H, Gregg W, Korwek K, Sands K. COVID‐19 testing processes and patient protections for resumption of elective surgery. Am J Surg. 2021;221(1):49‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


