Abstract
Ocrelizumab, an anti‐CD20 monoclonal antibody, counteracts induction of humoral immune responses after severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) vaccinations in patients with multiple sclerosis (MS). We aimed to assess if serum ocrelizumab concentration measured at the time of vaccination could predict the humoral response after SARS‐CoV‐2 vaccination. In 52 patients with MS, we found ocrelizumab concentration at the time of vaccination to be a good predictor for SARS‐CoV‐2 IgG anti‐RBD titers after vaccination (comparable to B‐cell count). As the course of ocrelizumab concentration may be predicted using pharmacokinetic models, this may be a superior biomarker to guide optimal timing for vaccinations in B‐cell depleted patients with MS. ANN NEUROL 2023;93:103–108
Ocrelizumab, an anti‐CD20 monoclonal antibody approved for treatment of multiple sclerosis (MS), is associated with an increased risk of a severe course of coronavirus disease 2019 (COVID‐19). 1 Many data show that ocrelizumab inhibits the humoral severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) response after vaccination or infection in patients with MS, whereas T‐cell responses are largely preserved. 2 , 3 , 4 , 5 As absent or decreased humoral responses in patients with MS are associated with breakthrough COVID‐19, 6 it is of great importance to identify modifiable determinants predicting humoral responses in ocrelizumab‐treated patients with MS.
In previous work, 2 markers for predicting humoral responses in ocrelizumab‐treated patients with MS were identified; higher B‐cell counts at the time of vaccination and longer time between last ocrelizumab infusion and vaccination. 2 , 7 However, as peripheral B‐cells are still fully depleted in the majority of patients with MS on ocrelizumab at the moment of redosing, 8 this is an unsuitable biomarker for timing vaccinations. In addition, as seroconversion rates are still low (<25%) in patients receiving vaccination in the weeks prior to ocrelizumab infusion, 2 it is not likely that postponing vaccination until the recommended 12 to 26 weeks after the last ocrelizumab infusion will lead to a clinically significant increase in humoral responses.
Therefore, a better biomarker is needed to establish the optimal timing for booster vaccinations. Drug concentrations of monoclonal antibodies are often used for establishing and predicting the course of pharmacodynamic effects. 9 , 10 Following this reasoning, ocrelizumab concentration could serve as a biomarker for prediction of humoral vaccination responses and might help in timing vaccinations.
In this study, we aimed to evaluate if ocrelizumab concentration could be of added value for predicting humoral responses after SARS‐CoV‐2 vaccination compared to the B‐cell count and the time of vaccination after the last infusion.
Methods
This was a substudy of a prospective multicenter cohort study on SARS‐CoV‐2 vaccination in patients with various immune mediated inflammatory diseases (T2B!; Trial NL8900; Dutch Trial register). 11 The medical ethical committee of the Amsterdam UMC, location AMC (2020.194) approved the study and all participants provided written informed consent.
Participants
For this substudy, participants with a current diagnosis of MS using ocrelizumab were included. Patients previously infected with SARS‐CoV‐2, the SARS‐CoV‐2 antibodies at baseline, or missing data of SARS‐CoV‐2 titers (at T = 70) or ocrelizumab concentrations (at T = 0) were excluded. Ocrelizumab treatment was given at 6‐month intervals.
Study Procedures
All patients were vaccinated by the study team with mRNA‐1,273 (Moderna) at baseline (T = 0). The second vaccination was administered 42 days after the first vaccination following national protocols (T = 42).
At T = 0 and T = 42, serum samples were collected and stored to measure ocrelizumab concentration using an enzyme‐linked immunosorbent assay (ELISA) developed by Sanquin Diagnostic Services, Amsterdam, The Netherlands. This assay captures ocrelizumab from a serum sample using polyclonal anti‐idiotype antibodies specific for ocrelizumab and biotinylated polyclonal anti‐ocrelizumab conjugate to subsequently detect bound ocrelizumab (sandwich ELISA). The anti‐idiotype antibodies were produced by rabbit immunization, as described before. 12 Concentrations were determined by comparing absorbance to a serially diluted calibrator in each plate. The lower limit of quantitation was 0.0025 μg/ml.
Subsequently, at T = 0 and T = 42, fresh whole blood was collected to assess the number of CD19+ B‐cells with a highly sensitive assay. 13
SARS‐CoV‐2 IgG antibodies against RBD (Wuhan strain) were measured as published before using an IgG specific ELISA at T = 0 (prior to the first vaccination), T = 42 (prior to the second vaccination) and T = 70 (28 days after the second vaccination). 14 The cutoff for seroconversion was an IgG anti‐RDB response >4.0 arbitrary units per ml (AU/ml, Sanquin units).
Statistical Analyses
Correlations between ocrelizumab concentration and B‐cell count at baseline were calculated using Spearman's correlation coefficient.
The association between (1) SARS‐CoV‐2 antibody titers (at T = 70) and ocrelizumab concentration (at T = 0), (2) SARS‐CoV‐2 titers (at T = 70) and B‐cell counts (at T = 0), and (3) SARS‐CoV‐2 titers (at T = 70) and the time between the last infusion and the first vaccination were analyzed using a negative binomial regression model correcting for the potential confounding effects of sex. Model quality and differences between models were assessed by the log likelihood and −2 log likelihood ratio with a Chi‐square distribution with 1 degree of freedom for between‐model differences. We repeated the models with ocrelizumab concentration and B‐cell count at the time of the second vaccination (T = 42).
Next, receiver operating characteristic (ROC) curves were constructed using logistic regression models in which the presence of SARS‐CoV‐2 IgG anti‐RDB response (based on the threshold of 4.0 AU/ml) was the dependent variable, and ocrelizumab concentration at T = 0, B‐cell count at T = 0, or both (all models corrected for sex) were entered as independent variables. We compared the area under the curve (AUC) of the 3 different models.
All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) version 26.0 (SPSS, Chicago, IL, USA) and R‐studio version 4.0.3.
Results
Fifty‐three patients were included. Mean age was 43.1 years (SD = 10.1), 40 patients (75.5%) were women, 46 (86.8%) patients were diagnosed with relapsing remitting MS, and 7 patients (13.2%) were diagnosed with primary progressive MS. Patients received a median of 4 ocrelizumab infusions (range = 2–7, the first two 300 mg infusions were counted as one infusion).
Eight patients (15.1%) showed seroconversion at T = 42, and 21 patients (39.6%) showed seroconversion at T = 70. Patients received their first vaccination after a median of 13.3 weeks (range = 2.0–27.4 weeks) after their last ocrelizumab infusion. Nine patients received an ocrelizumab infusion between the first and second vaccinations of whom 4 had seroconverted at T = 70.
At T = 0, there were 35 patients (66%) who had peripheral B‐cell counts under 1 CD19+ cell per μl (range = 0–25 μl). Ocrelizumab concentration in serum at T = 0 ranged from 0.017 μg/ml to 63.8 μg/ml (depending on the time after infusion) and was negatively correlated with B‐cell count (ρ = −0.409, p = 0.002). In only 2 patients, ocrelizumab concentration was below the lower limit of quantification. These samples were taken 17 and 26 weeks after the last ocrelizumab infusion (with B‐cell counts of 25 and 8 CD19+ cells/μl, respectively).
SARS‐CoV‐2 seroconversion at T = 70 was associated with ocrelizumab concentration at T = 0 (Table 1, Fig 1A). Seroconversion was seen in 87.5% (7/8) of participants with concentration <0.1 μg/ml, 45.5% (5/11) of participants with concentration 0.1 to 1.0 μg/ml, 47.1% (8/17) of participants with concentration 1.0 to 10 μg/ml, and 17.6% (3/17) of participants with concentration >10 μg/ml.
TABLE 1.
Regression Models
| SARS‐CoV‐2 IgG anti‐RBD titers at T = 70 | |||||
|---|---|---|---|---|---|
| Model | Risk ratio | 95% CI | p | Log likelihood overall model a | |
| 1 | B‐cell count (T = 0) | 1.467 | 1.232–1.746 | <0.001 | −207.733 |
| 2 | OCR concentration (T = 0) | 0.893 | 0.866–0.920 | <0.001 | −204.768 |
| 3 | Days between last infusion at first vaccination (T = 0) | 1.018 | 1.012–1.025 | <0.001 | −213.008 |
| 4 | B‐cell count + OCR concentration (T = 0) |
1.293 0.925 |
1.118–1.496 0.898–0.954 |
<0.001 <0.001 |
−195.303 |
| 5 | B‐cell count + OCR concentration + days after last infusion (T = 0) | 1.244 | 1.049–1.474 | <0.001 | −195.067 |
| 0.932 | 0.899–0.965 | <0.012 | |||
| 1.003 | 0.995–1.011 | 0.488 | |||
| Model | |||||
| 1 | B‐cell count (T = 42) | 1.101 | 1.072–1‐130 | <0.001 | −181.750 |
| 2 | OCR concentration (T = 42) | 0.995 | 0.989–1.00 | 0.040 | −213.162 |
| 3 | Days after last infusion at second vaccination | 1.015 | 1.011–1.019 | <0.001 | −199.048 |
| 4 | B‐cell count + OCR concentration (T = 42) | 1.107 | 1.074–1.141 | <0.001 | −169.457 |
| 1.005 | 0.999–1.012 | 0.124 | |||
| 5 | B‐cell count + OCR concentration + days after last infusion (T = 42) | 1.100 | 1.063–1.138 | <0.001 | −169.211 |
| 1.004 | 0.997–1.011 | 0.251 | |||
| 1.003 | 0.995–1.010 | 0.484 | |||
The association among B‐cell count, ocrelizumab concentration, days between infusion and vaccination with SARS‐CoV‐2 IgG anti‐RBD titers were analyzed using a negative binomial regression model. Model quality and differences between models were assessed by the log likelihood and 2 log likelihood ratio with a chi‐square distribution with 1 degree of freedom for between model differences. T = 0 is baseline at the first vaccination, T = 42 is 42 days after the first vaccination at the day of the second vaccination, T = 70 is 70 days after the first vaccination (28 days after the second vaccination).
Abbreviations: CI = confidence interval; OCR = ocrelizumab; SARS‐CoV‐2 = severe acute respiratory syndrome‐coronavirus 2.
Corrected for sex.
FIGURE 1.
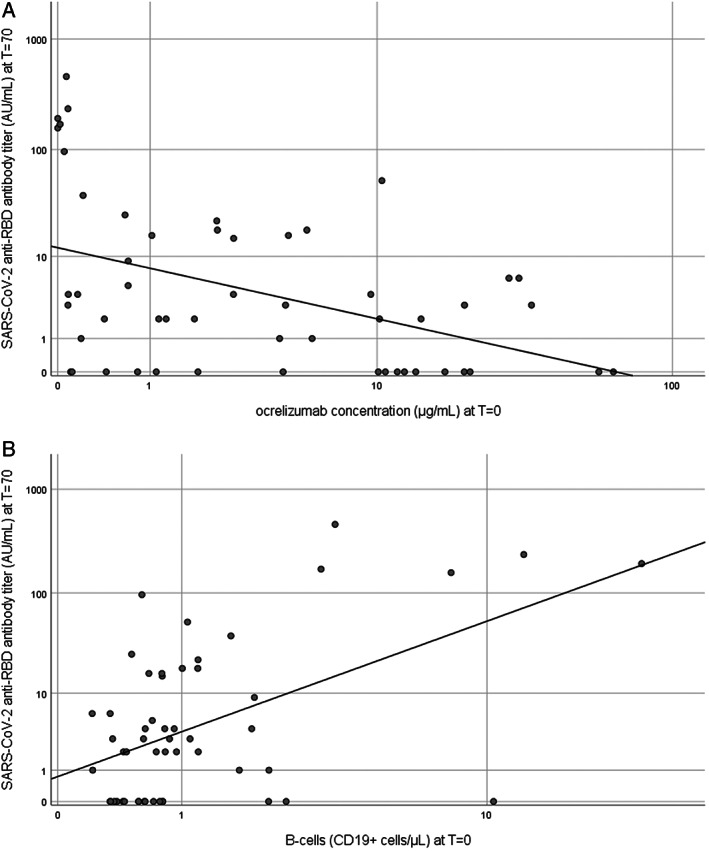
Ocrelizumab concentration and B‐cells in relation to SARS‐CoV‐2 anti‐RBD antibody titer. These scatterplots show ocrelizumab concentration (μg/ml) (A) and B‐cells (CD19+ cells/μl) (B) at T = 0 in relation to SARS‐CoV‐2 anti‐RBD antibody titer (AU/ml) at T = 70. The T = 0 is baseline at the first vaccination, T = 70 is 70 days after the first vaccination (28 days after the second vaccination). SARS‐CoV‐2 = severe acute respiratory syndrome‐coronavirus 2.
Regression models for the different predictors are shown in the Table 1. Both lower ocrelizumab concentration (see the Table 1, and model 1, Fig 1A) and higher B‐cell counts (model 2, Fig 1B) at T = 0 were significantly associated (p < 0.001) with higher SARS‐CoV‐2 titers at T = 70. When combined in one model (model 4), the model performance improved significantly. Figure 2 shows the ROC curves for these models separately. The AUC was 0.705 (95% confidence interval [CI] = 0.560–0.851) for the ocrelizumab concentration at baseline. The optimal cutoff was 5.62 μg/ml (sensitivity 0.857 and 1‐specificity 0.5). The AUC was 0.679 (95% CI = 0.528–0.832) for the B‐cell count. The optimal cutoff was 0.973 cells/μl (sensitivity 0.571 and 1‐specificity 0.291).
FIGURE 2.
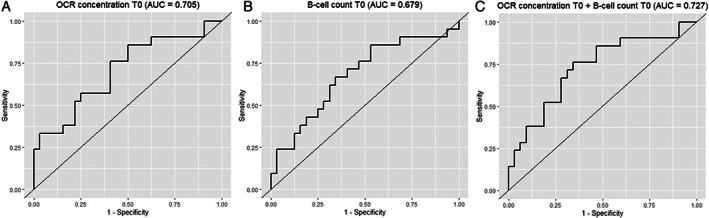
ROC curves for ocrelizumab concentration, B‐cell count, and the combined model at T = 0. Shows the ROC curves for ocrelizumab concentration (μg/ml) at T = 0 (A), B‐cells (CD19+ cells/μl) at T = 0 (B), and the combined model of A en B (C) as independent variables in which the presence of SARS‐CoV‐2 IgG anti‐RDB response at T = 70 (based on the threshold of 4.0 AU/ml) was the dependent variable. ROC curves were constructed using logistic regression models. T = 0 is baseline at the first vaccination, and T = 70 is 70 days after the first vaccination (28 days after the second vaccination). AUC = area under the curve; OCR = ocrelizumab; ROC = receiver operating characteristic; SARS‐CoV‐2 = severe acute respiratory syndrome‐coronavirus 2.
Even though the time between the last infusion and the first vaccination was significantly associated with a higher titer at T = 70 (model 3), there was no extra predictive effect of time between infusion and vaccination when added to the model with ocrelizumab concentration and B‐cell counts (model 5). The effect of baseline determinants on SARS‐CoV‐2 antibody titers at T = 42 could not be modeled due to the limited amount of events (n = 8 with antibody response).
Discussion
To our knowledge, we are the first to identify ocrelizumab concentration as a predicting biomarker for developing a humoral response after vaccination. Ocrelizumab concentration proved to be a comparable predictor for SARS‐CoV‐2 humoral in comparison to B‐cell count, but combined the model improved in comparison to both single parameters. Recently, rituximab concentration was also studied in relation to SARS‐CoV‐2 immunity. 15 In a cohort of patients with MS with variable intervals from the last infusion to vaccination (0.2–2.8 years) and consequently rituximab levels below the detection limit in 55% (33/60), rituximab concentrations were associated with humoral responses after vaccination. We show that also in the approved interval of 6 months, ocrelizumab drug concentration is a good predictor of the humoral response.
Drug concentrations are increasingly used as biomarkers in treatments of monoclonal antibodies for monitoring therapeutic effect and personalizing dosing. 10 , 16 Ocrelizumab concentration can easily be measured by an ELISA assay, which is a relatively straightforward assay to set up, and service testing is available from Sanquin Diagnostics, in the Netherlands. Furthermore, only serum is needed (which could even be retrieved by patients performing a fingerprick at home) 11 and the costs of assessment of ocrelizumab concentration are substantially lower than a quantitative B‐cell measurement by flow cytometry.
In another cohort using the same assay for longitudinal testing of ocrelizumab pharmacokinetics, the median ocrelizumab concentration after 6 months after the last infusion was 0.08 μg/ml (interquartile range [IQR] = 0.03–0.19). 17 As we calculated the optimal cutoff for ocrelizumab concentration predicting seroconversion after SARS‐CoV‐2 vaccination to be at 5.62 μg/ml, we expect that this cutoff can be used within the normal treatment interval of ocrelizumab. Furthermore, as ocrelizumab clearance is time‐dependent and typical for an IgG1 immunoglobulin, the course of the concentration for an individual patient could be predicted by pharmacokinetic models (in contrast to the repopulation of B‐cells). 18 Consequently, with one measurement of ocrelizumab concentration and using the body mass index (BMI) of the patient (which influences ocrelizumab pharmacokinetics), 18 an optimal timing for vaccination could be established.
Ocrelizumab also decreases the humoral responses of other vaccinations (eg, tetanus and influenza). 19 In addition to the use of ocrelizumab concentration for timing SARS‐CoV‐2 vaccinations, this method could also be applied to establish an optimal timing for other vaccination regimes.
The relatively small sample size was a limitation in this study. Therefore, our results need to be validated in a larger independent cohort. Furthermore, it is important to realize that seroconversion does not necessarily induce adequate protection as the titer and antibody functionality (at current corona strains) also influence the level of immunity.
In conclusion, ocrelizumab concentration is a biomarker that, although equal to the B‐cell count in diagnostic accuracy, might be preferred for the prediction of a humoral response after SARS‐CoV‐2 vaccination in ocrelizumab‐treated patients with MS as it could lead to a more reliable prediction of the optimal timing of vaccination.
Author Contributions
Z.K., K.B., A.B., S.H., T.K., G.W., F.L., L.W., F.E., T.R., and J.K. contributed to the conception and design of the study. Z.K., L.H., A.T., M.S., E.S., L.K., P.D., K.B., F.L., L.W., and E.S. contributed to the acquisition and analysis of data. Z.K., L.H., A.T., L.W., E.S., and J.K. contributed to drafting the text or preparing the figures.
Potential Conflicts of Interest
J.K. reported speaking and consulting relationships with Roche, this company manufactures ocrelizumab. Amsterdam UMC, location VUmc, MS Center Amsterdam has received financial support for research activities from Roche.
Acknowledgments
The authors are grateful for ZonMw (The Netherlands Organization for Health Research and Development) for funding this study (grant number 10,430,072,010,007). The sponsor had no role in the design, analyses, or reporting of the study. We are also grateful for the Dutch MS Research Foundation for additional funding of this study (grant number 21‐1135 MS).
Laura Hogenboom and Alyssa A. Toorop authors are contributed equally to this work.
Data Availability
Anonymized data will be shared upon reasonable request from any qualified investigator.
References
- 1. Simpson‐Yap S, De Brouwer E, Kalincik T, et al. Associations of disease‐modifying therapies with COVID‐19 severity in multiple sclerosis. Neurology 2021;97:e1870–e1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Etemadifar M, Nouri H, Pitzalis M, et al. Multiple sclerosis disease‐modifying therapies and COVID‐19 vaccines: a practical review and meta‐analysis. J Neurol Neurosurg Psychiatry 2022;93:986–994. [DOI] [PubMed] [Google Scholar]
- 3. Palomares Cabeza V, Kummer LYL, Wieske L, et al. Longitudinal T‐cell responses after a third SARS‐CoV‐2 vaccination in patients with multiple sclerosis on Ocrelizumab or Fingolimod. Neurol: Neuroimmunol NeuroInflammation 2022;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Kempen ZLE, Strijbis EMM, Al M, et al. SARS‐CoV‐2 antibodies in adult patients with multiple sclerosis in the Amsterdam MS cohort. JAMA Neurol 2021;78:880–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verstegen NJM, Hagen RR, van den Dijssel J, et al. Immune dynamics in SARS‐CoV‐2 experienced immunosuppressed rheumatoid arthritis or multiple sclerosis patients vaccinated with mRNA‐1273. eLife 2022;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sormani MP, Schiavetti I, Inglese M, et al. Breakthrough SARS‐CoV‐2 infections after COVID‐19 mRNA vaccination in MS patients on disease modifying therapies during the Delta and the omicron waves in Italy. EBioMedicine 2022;80:104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Kempen ZLE, Wieske L, Stalman EW, et al. Longitudinal humoral response after SARS‐CoV‐2 vaccination in ocrelizumab treated MS patients: to wait and repopulate? Mult Scler Relat Disord 2022;57:103416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hauser SL, Bar‐Or A, Comi G, et al. Ocrelizumab versus interferon Beta‐1a in relapsing multiple sclerosis. N Engl J Med 2017;376:221–234. [DOI] [PubMed] [Google Scholar]
- 9. van Kempen ZL, Toorop AA, Sellebjerg F, et al. Extended dosing of monoclonal antibodies in multiple sclerosis. Mult Scler 2022;28:2001–2009. [DOI] [PubMed] [Google Scholar]
- 10. van Kempen ZLE, Hoogervorst ELJ, Wattjes MP, et al. Personalized extended interval dosing of natalizumab in MS: a prospective multicenter trial. Neurology 2020;95:e745–e754. [DOI] [PubMed] [Google Scholar]
- 11. Wieske L, van Dam KPJ, Steenhuis M, et al. Humoral responses after second and third SARS‐CoV‐2 vaccination in patients with immune‐mediated inflammatory disorders on immunosuppressants: a cohort study. Lancet Rheumatol 2022;4:e338–e350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rispens T, Leeuwen A, Vennegoor A, et al. Measurement of serum levels of natalizumab, an immunoglobulin G4 therapeutic monoclonal antibody. Anal Biochem 2011;411:271–276. [DOI] [PubMed] [Google Scholar]
- 13. Koutsakos M, Rowntree LC, Hensen L, et al. Integrated immune dynamics define correlates of COVID‐19 severity and antibody responses. Cell Rep Med 2021;2:100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Steenhuis M, van Mierlo G, Derksen NI, et al. Dynamics of antibodies to SARS‐CoV‐2 in convalescent plasma donors. Clin Transl Immunol 2021;10:e1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Asplund Hogelin K, Ruffin N, Pin E, et al. B‐cell repopulation dynamics and drug pharmacokinetics impact SARS‐CoV‐2 vaccine efficacy in anti‐CD20‐treated multiple sclerosis patients. Eur J Neurol 2022;29:3317–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mould DR. Why therapeutic drug monitoring is needed for monoclonal antibodies and how do we implement this? Clin Pharmacol Ther 2016;99:351–354. [DOI] [PubMed] [Google Scholar]
- 17. Toorop AA, Hogenboom L, Bloem K, et al. Ocrelizumab Concentration and Anti‐Drug Antibodies are Associated with B‐cell Course in Multiple Sclerosis. 2022. [DOI] [PubMed] [Google Scholar]
- 18. Gibiansky E, Petry C, Mercier F, et al. Ocrelizumab in relapsing and primary progressive multiple sclerosis: pharmacokinetic and pharmacodynamic analyses of OPERA I, OPERA II and ORATORIO. Br J Clin Pharmacol 2021;87:2511–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bar‐Or A, Calkwood JC, Chognot C, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology 2020;95:e1999–e2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared upon reasonable request from any qualified investigator.


