Dear Editor,
During the epidemic of COVID‐19 disease worldwide, applying COVID‐19 vaccination can effectively protect humans from serious symptoms and help reduce long‐term effects. 1 However, COVID‐19 vaccines have been linked to some potential side effects. COVID‐19 mRNA vaccine‐associated dermatomyositis (DM) with the anti‐TIF‐1y‐positive case was first reported in 2021. 2 Two reports about MDA5‐DM patients after mRNA or adenovirus vector COVID‐19 vaccines were searched out in the PubMed, Science Direct and SCOPUS by June 2022. 3 , 4 We first reported an MDA5‐DM with rapid progressive interstitial lung disease (RP‐ILD) patient after an inactivation COVID‐19 vaccine, exhibiting time course of the disease development.
A 39‐year‐old‐Chinese male received two doses of Sinopharm [Vero cell] Inactivated COVID‐19 Vaccine in July 2021. Ten days after the second dosage, the rash appeared on his index finger, 7 weeks later, local rash progressed over his face, hands, elbows and back (Figure 1a–c). Subsequently, hands became swelling (circle), vasculitis necrosis formed in the fingertips (Figure 1d,e), Gottron's papules were observed overlying the extensor of interphalangeal and metacarpal joints (Figure 1f,g). Skin biopsy showed focal dyskeratosis, red blood cells exuded, perivascular lymphocytes infiltrated in the superficial dermis (Figure 2a) and increased mucin accumulated in the dermis (Figure 2d).
FIGURE 1.
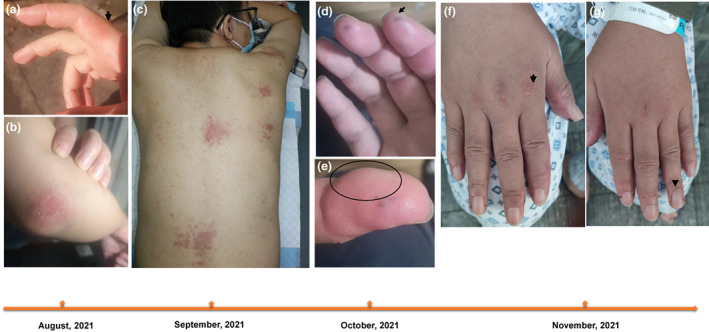
Dermatological rash in the patient. Erythematous rash appeared on the fingers (a), arrows point to the desquamate red patchy rash on the index finger, elbow (b), and back (c) of the patient, necrosis in the fingertips (arrow in d), swelling appeared on the hands (circled in the e), the fingerprint was not available due to swelling, while vasculitis formed on the fingertips (arrows) of the patient. Gottron's papulars on both hands of the patient. Papulars appeared on the knuckles and palmar joints (arrow in f) and vascular damage on the nailfold (arrow in g).
FIGURE 2.
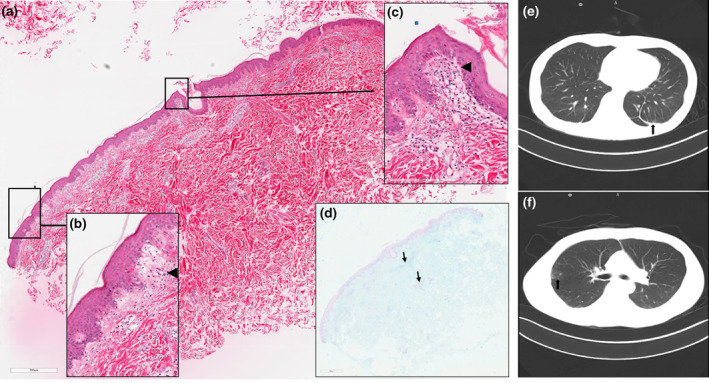
Skin biopsy on the back and high‐resolution computed tomography imaging of the chest. Arrowheads marked vacuolar interface (a), lymphocytes and erythrocytes infiltration in the superficial perivascular (c). Increased amounts of mucin and lymphocytes in the dermis (d). Magnification: (a) 400×; (b) 10×; (c) 400×; (d) 10×. Scattered patchy, flocculent ground glass density shadow on both lungs (arrow in e), nodules (arrow in f).
When he accessed the hospital, lab tests revealed elevated levels of creatine kinase (793 U/L), lactate dehydrogenase (542 U/L), alanine aminotransferase (80 U/L) and aspartate aminotransferase (124 U/L). Both anti‐Ro52 and anti‐MDA5 antibodies were found to be positive.
Computed tomography thorax depicted scattered flocculent ground‐glass density shadow (Figure 2e), multiple nodules (Figure 2f), early fibrous proliferation and 6 days later, another CT showed RP‐ILD.
His presentation met 2017 ACR/EULAR classification criteria 5 (15.1 score). He was diagnosed with MDA5‐DM with RP‐ILD, after successful following treatment with prednisolone, antibiotic, Rituximab and IVIG for 2 months, the condition was under‐controlled.
For our patient, no relevant medication history, absence of any additional triggering factors and negative result for SARS‐CoV‐2, which suggested vaccination may be associated with the MDA5‐DM. The inactivated vaccine is prepared by strain culture in Vero cells. After inactivation, the virus lost its infectivity and pathogenicity, but nucleic acid can be recognized by the immune system to produce corresponding antibodies. A retrospective study in China indicated that COVID‐19‐infected patients with higher MDA5 antibody titers are more likely to develop severe disease. 6 Three immunogenic epitopes in DM patients with high‐sequence identity to SARS‐CoV‐2 proteins were identified. 7 Therefrom we conjectured the virus dsRNA was detected by MDA5 in the cytoplasm and triggered the autoimmunity, mechanism of post‐vaccination DM may be the same as the COVID‐19‐related immune myopathy.
Dermatomyositis case with an early ILD after the COVID‐19 mRNA vaccination was firstly reported in 2021. 8 Anti‐Ro52 antibody is highly prevalent in anti‐MDA5‐positive CADM‐ILD patients, and subgroup of patients with two antibodies presented an increased frequency of RP‐ILD, 9 in our Rheumatology and Immunology ward, totally five MDA5‐DM patients after inactivated COVID‐19 vaccine coexistence of Ro‐52, four patients showed RP‐ILD.
Statistically, the latency period of most MDA5‐DM symptoms onset is a few days after the second dose, 3 , 4 hypersensitivity reactions after the second dose maybe due to the immune response is a delayed type of T‐cell‐mediated hypersensitivity.
Additionally, our patient had been diagnosed with tuberculosis and cured 13 years ago, and his father has type II diabetes. Although he was physically healthy once, his genetic predisposition maybe influenced and triggered autoimmunity by the COVID‐19 vaccine.
Generally, three kinds of dominat COVID‐19 vaccines may trigger MDA5‐DM, involving the Asian, Hispanic and Caucasian races. Further investigation into the pathologic mechanism underlying vaccine‐associated DM is necessary. Furthermore, clinicians may be aware of the unexpected adverse event after COVID‐19 vaccination and give proper treatment.
FUNDING INFORMATION
This work was supported by the National Natural Science Foundation of China (81303139 and 82103726), Shenzhen Sanming Project (no. SZSM201812059), Shenzhen Key Medical Discipline Construction Fund (no. SZXK040) and Project of Shenzhen Municipal Health Commission (no. SZXJ2017046).
CONFLICT OF INTEREST
The first author is the wife of the patient, the other authors have no potential conflicts of interest to disclose.
ETHICS STATEMENT
Ethics committee approval was received for this study from the ethics committee of Peking University Shenzhen Hospital (2017‐325‐[R1]). The patient in this manuscript has given written informed consent to the publication of their case details.
ACKNOWLEDGEMENTS
The authors thank Dr. Gang Ma, Dr. Bo Yu, Dr. Qingwen Wang, Dr. Hua Luo, Dr. Guanxun Chen, Dr. Xia Dou, Dr. Haiyan Huang, N. Yanfen Zou, Dr. JiYang Lv, Dr. Changbin Shen, Dr. Qiufeng Huang, Dr. Zizhuo Li, Dr. Yufang Yao, Dr. DanDan Li and Dr. YuanYuan Zheng in Peking University Shenzhen Hospital. At last, we also thank our patients.
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Cho K, Park S, Kim EY, Koyanagi A, Jacob L, Yon DK, et al. Immunogenicity of COVID‐19 vaccines in patients with diverse health conditions: a comprehensive systematic review. J Med Virol. 2022;94(9):4144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu M, Karim M, Ashinoff R. COVID‐19 vaccine‐associated dermatomyositis. JAAD Case Rep. 2022;23:58–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gonzalez D, Gupta L, Murthy V, Gonzalez EB, Williamson KA, Makol A, et al. Anti‐MDA5 dermatomyositis after COVID‐19 vaccination: a case‐based review. Rheumatol Int. 2022;42(9):1629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carrasco L, Arthur A, Gaitan RJ, Case C. A rapidly progressive and rare illness: autoantibodies against melanoma diferentiation‐associated protein 5 (anti‐MDA5): amyopathic dermatomyositis with progressive interstitial lung disease that developed after covid‐19 vaccine. Chest J. 2021;160(4):A680–1. [Google Scholar]
- 5. Lundberg IE, Tjarnlund A, Bottai M, Werth VP, Pilkington C, de Visser M, et al. 2017 European league against rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis. 2017;76(12):1955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang G, Wang Q, Wang Y, Liu C, Wang L, Chen H, et al. Presence of anti‐MDA5 antibody and its value for the clinical assessment in patients with COVID‐19: a retrospective cohort study. Front Immunol. 2021;12:791348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Megremis S, Walker TDJ, He X, Ollier WER, Chinoy H, Hampson L, et al. Antibodies against immunogenic epitopes with high sequence identity to SARS‐CoV‐2 in patients with autoimmune dermatomyositis. Ann Rheum Dis. 2020;79(10):1383–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gouda W, Albasri A, Alsaqabi F, Al Sabah HY, Alkandari M, Abdelnaby H. Dermatomyositis following BNT162b2 mRNA COVID‐19 vaccination. J Korean Med Sci. 2022;37(5):e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu A, Ye Y, Fu Q, Lian X, Chen S, Guo Q, et al. Prognostic values of anti‐Ro52 antibodies in anti‐MDA5‐positive clinically amyopathic dermatomyositis associated with interstitial lung disease. Rheumatology (Oxford). 2021;60(7):3343–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.


