Abstract
Autoimmune hemolytic anemia (AIHA) is caused by the production of autoantibodies against RBCs. COVID‐19 vaccines can reduce the risk of severe disease, however, various adverse effects such as AIHA were observed following vaccination. This review aimed to assess the relationship of AIHA and COVID‐19 vaccination using the PRISMA guidelines. Among 18 cases included in this review, new post‐vaccination AIHA development was reported in 11 patients (7 women and 4 men) with a median age of 67.0 years. In 7 of 11 and 3 of 11 cases, the onset of symptoms occurred after first and second vaccine dose with median times of 7 and 14 days, respectively. In 1 of 11 cases, the AIHA occurred on Day 17 after booster vaccination. Ten of 11 and 1 of 11 AIHA patients received mRNA‐ and vector‐based vaccine, respectively. After vaccination, 9 of 11, 1 of 11, and 1 of 11 AIHA patients developed warm IgG, cold IgM, and mixed autoantibodies against RBCs, respectively. Significant AIHA exacerbation was reported in seven patients (four women and three men) with a median age of 73.0 years. In 4 of 7 and 2 of 7 exacerbated AIHA cases, the onset of symptoms occurred after first and second vaccine dose with median times of 7 and 3 days, respectively. In 1 of 7 exacerbated AIHA cases, the onset of symptoms was observed on Day 2 after booster vaccination. All exacerbated AIHA cases received mRNA‐based vaccines; 3 of 7 and 4 of 7 exacerbated AIHA cases developed IgG and IgM against RBCs, respectively. This review provides a comprehensive explanation regarding the AIHA development and exacerbation after COVID‐19 vaccination.
Keywords: autoimmune hemolytic anemia, COVID‐19, SARS‐CoV‐2, vaccination
1. INTRODUCTION
Since the emergence of the SARS‐CoV‐2 pandemic, more than 548 million infections, and 6.3 million deaths have been documented, as of July 2, 2022. 1 While there is currently no verified treatment for the COVID‐19, vaccination against SARS‐CoV‐2 is the most efficient approach to remarkably limit the COVID‐19 pandemic and decrease the risk of severe COVID‐19. Vaccination can provide protection against serious infection by inducing neutralizing antibodies to SARS‐CoV‐2 spike (S) protein. 2 As of January 12, 2022, World Health Organization (WHO) has confirmed that some inactivated‐based vaccines (Sinovac, Covaxin, and Sinopharm), vector‐based vaccines (AstraZeneca/Oxford and Johnson and Johnson), mRNA‐based vaccines (Moderna and Pfizer/BioNTech), and a subunit protein‐based vaccine (Nuvaxovid) are effective and safe. 3 , 4 As of July 2, 2022, more than 12.0 billion vaccine doses have been administered, globally. 1 The results from some studies indicate that the current available COVID‐19 vaccines are overall efficient and safe. 5 , 6 , 7 However, various adverse events such as thrombotic thrombocytopenia, thyroid dysfunctions, neurologic disorders, and myocarditis have been reported after COVID‐19 vaccination. 8 , 9 , 10 , 11 Development of the new‐onset and exacerbation of autoimmune hemolytic anemia (AIHA) were also reported after COVID‐19 vaccination. Thereby, thorough monitoring of the adverse effects of vaccination is required. The attention to COVID‐19 vaccination‐related AIHA was reported in a few studies. However, a summary review is required to generate a comprehensive view to combine their findings. Therefore, we reviewed the published papers to compile their results and provide a comprehensive view regarding the features of AIHA development and exacerbation after COVID‐19 vaccination.
2. METHODS
2.1. Search strategy
Using the guidelines of the PRISMA, we performed a systematic review of the literature to find all articles concerning AIHA after COVID‐19 vaccination by searching for published papers until July 3, 2022, in major databases including PubMed, Web of Science, PubMed Central, and Scopus. The following keywords were used: COVID‐19, SARS‐CoV‐2, vaccination, vaccine, and anemia. Inclusion criteria were administration of the SARS‐CoV‐2 vaccine, and case reports describing patients with the approved AIHA development and exacerbation after vaccination. Unrelated publications and reviews were excluded from study.
2.2. Study eligibility criteria
Abstracts and titles were screened according to specific inclusion criteria. The search method is depicted in Figure 1. In total, 53 papers were selected for screening. We included 15 reports in the study, 11 of which reported the development of the new‐onset AIHA after COVID‐19 vaccination and 4 of which reported AIHA exacerbation after vaccination. Figure 1 shows the PRISMA flow diagram used in this systematic review. Two experienced reviewers (A.J. and S.M.J.M) independently reviewed all included papers to assess possible bias risk. As all included papers were case reports (not randomized clinical trials), so there was no disagreement between these two reviewers regarding the study bias point of view.
FIGURE 1.
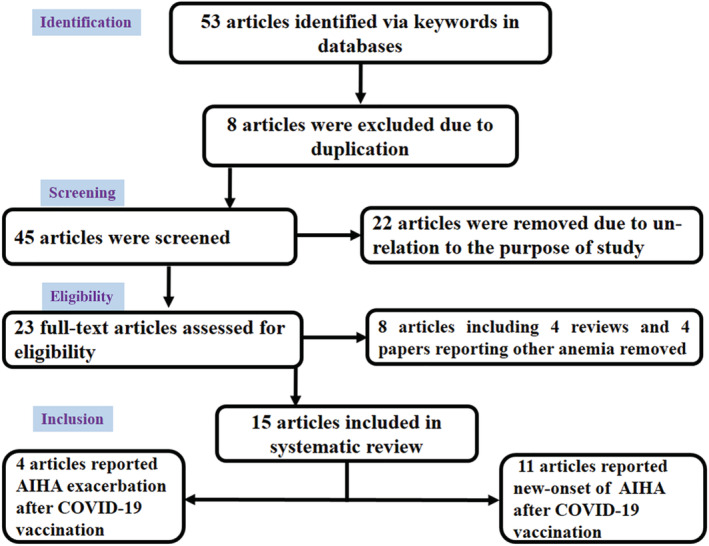
PRISMA flow chart used in this systematic review
2.3. Data extraction
Study outcomes, including patients' data and laboratory data were extracted. Patient data: age, sex, comorbidities, type and brand of administrated vaccines, interval times from COVID‐19 vaccination, and development of the new‐onset AIHA or AIHA exacerbation as well as therapeutic programs for AIHA (Tables 1 and 3). Laboratory data: AIHA diagnosis, hemoglobin levels at presentation, as well as, other related tests such as lactate dehydrogenase (LDH) haptoglobin, and bilirubin levels (Tables 1 and 3).
TABLE 1.
Characteristics of patients with new‐onset development of autoimmune hemolytic anemia (AIHA) following COVID‐19 vaccination
| Country (case no.) | Type of AIHA | Gender‐age, years | Vaccine name (vaccine type) | Onset time of symptoms‐primary clinical symptoms | Hematologic test results | Other tests | Medication and outcome | Reference |
|---|---|---|---|---|---|---|---|---|
| India (1) a | Warm AIHA | M‐71 | Oxford/AstraZeneca |
‐ 30 days after first dose. ‐ Fatigue, Hb: 5 mg/dl |
‐ Normocytic normochromic anemia with RBC agglutination, polychromasia, nucleated RBC, spherocytosis. ‐ High reticulocyte count (6.6%) low haptoglobin level. ‐ MCV 85 fl, MCH 31 pg, MCHC 37 g/dl, platelet count 2.83 lakhs. ‐ DAT: anti‐RBC IgG showed 4+ reaction. |
‐ Unconjugated hyperbilirubinemia with raised LDH. ‐ Transferrin saturation: 108.38%, Ferritin level: 2238.7 ng/ml. Vitamin B12 and folic acid levels were normal. ‐ Bone marrow: hypercellular with erythroid hyperplasia. |
‐ Treatment was done with high dose IVIG. ‐ Blood transfusion was done whenever Hb dropped <5 mg/dl. ‐ Hb improved by 2 weeks and there was no need to blood transfusion after 12 days of IVIG. |
12 |
| USA (2) b | Warm AIHA | F‐66 | Moderna mRNA vaccine |
‐ 4 months after second dose. ‐ Hb: 9.9 mg/dl; Hct: 29.4%. |
‐ High reticulocyte count, moderate number of spherocytes. ‐ DAT: anti‐RBC IgG showed 4+ reaction (without C3). |
‐ Total bilirubin: 2.5 mg/dl; direct bilirubin: 0.7 mg/dl. ‐ High serum LDH, and low serum haptoglobin level. ‐ Physical examination: slight uvular icterus. |
‐ Treatment was done by prednisone. ‐ Hgb/Hct began to be normalized within 2 weeks of treatment. |
13 |
| Portugal (3) | Warm AIHA | F‐88 | COVID‐19 mRNA vaccine |
‐ 2 days after second dose. ‐ Asthenia, jaundice. |
‐ Normocytic normochromic anemia ‐ Hb: 4.5 mg/dl, hematocrit: 13.8%, normal mean globular volume, normal mean globular hemoglobin concentration, normal proportion of reticulocytes, normal platelet counts. ‐ DAT: High titers of anti‐RBC IgG and high anti‐C3d titers. No cold agglutinins were detected. |
‐ High LDH, total bilirubin and indirect bilirubin, AST levels, normal ALT level, low haptoglobin level. ‐ Acute kidney injury with a high creatinine and urea with mild compensated metabolic acidosis. ‐ Urinalysis: hemoglobinuria without bilirubinuria. |
‐ Treatment was done using transfusion with erythrocyte concentrate. ‐ Methylprednisolone therapy results in stabilization of Hb level and decreased bilirubin. ‐ On the fifth day, reticulosis was 9.3% and kidney injury improved. ‐ From the sixth day, normalization of Hb and hematocrit was begun. The corticosteroid therapy was switched to oral. ‐ On the 11th day patient was asymptomatic with a Hb of 7.2 g/dl. |
14 |
| Switzerland (4) | Warm AIHA | M‐77 | Moderna mRNA‐1273 |
‐ 5 days after first dose. ‐ Weakness, fatigue and shortness of breath. |
Normochromic normocytic anemia with increased reticulocytes mild leukocytosis. DAT: Warm AIHA was diagnosed. |
‐ High transaminases, LDH and bilirubin and low haptoglobin. ‐ Abdominal ultrasound: discrete inhomogeneous liver parenchyma. ‐ Anti‐MPO, p‐ANCA, c‐ANCA antibodies were normal. |
‐ Treatment was done using prednisone. ‐ Two weeks later, Hb level increased to 112 g/L, reticulocytes, bilirubin, and LDH levels also decreased. ‐ Prednisone gradually tapered and the Hb level was almost normalized after 10 weeks. |
15 |
| USA (5) c | Warm AIHA | M‐84 | Pfizer‐BioNTech |
‐ 19 days after first dose. ‐ Increased urinary frequency and dizziness. ‐ Urine culture was positive for Enterobacter cloacae. |
‐ Patient's chronic anemia was worsened. ‐ Hb: 8.8 g/dl and more reduced later, high MCV 107, elevated reticulocyte counts. ‐ Leukocytosis, high reticulocyte count, and polychromasia. ‐ Leukoerythroblastic reaction with macrocytic anemia and reticulocytosis, along with moderate neutrophilia and monocytosis. ‐ DAT: Test was positive for anti‐RBC IgG, while was negative for C3. |
LDH was elevated, Haptoglobin was less. |
‐ Treatment was done using Methylprednisolone and prednisone. ‐ Transfusion using packed was also done. ‐ Hb level increased to 7.7 g/dl on the second day of hospitalization and remained stable at discharge on the third hospital day as well as during the follow‐up. ‐ Hb reached to 9.0 g/dl 1 week after the discharge. |
16 |
| USA (6) | Mixed‐AIHA | F‐41 | Moderna mRNA‐1273 |
‐ 7 days after first dose. ‐ Fatigue, dark urine, dyspnea. |
‐ Hb: 7.1 g/dl, low MCV, high reticulocyte count. ‐ Mixed warm/cold AIHA was observed. ‐ Warm autoantibody consisted with the presence of either IgG4 or IgG3. |
‐ H levels of total and direct bilirubin ‐ Bilirubin, low haptoglobin, high LDH. |
‐ Transfusion using packed was also done. ‐ Various types of symptoms and hematological complications was observed. ‐ Treatment was continued using prednisone, mycophenolate mofetil, IVIG and rituximab up to 80 days after vaccination to manage her complications. |
17 |
| Japan (7) d | Warm AIHA | F‐75 | Pfizer‐BioNTech |
‐ 14 days after first dose. ‐Anemia |
Direct and indirect coombs tests: positive hemolytic anemia | Elevation of serum LDH and indirect bilirubin levels. | Oral prednisolone therapy was done. The anemia improved soon after the treatment. | 18 |
| Saudi Arabia (8) | Cold AIHA | F‐45 | Pfizer‐BioNTech |
‐ 3 days after first dose. ‐ Shortness of breath, palpitations, fatigue and dark urine. |
‐ Hb: 57 g/L, 10% reticulocytes. ‐ Peripheral blood film: marked agglutination. ‐ DAT: test was positive for C3. |
‐ Physical examination: Tachycardia, jaundice and pallor. ‐High LDH level, high total bilirubin, and direct bilirubin. |
‐ Transfusion was also done. ‐ The patient was also treated with rituximab. ‐Complete remission was achieved 8 weeks after rituximab therapy. |
19 |
| USA (9) e | Warm AIHA | F‐42 | Moderna |
‐ 7 days after first dose. ‐ Dizziness and light headedness, breath shortness with activity, palpitations, blurred vision, weakness, and fatigue. |
‐ Hb: 4.5 g/dl, MCV: 90 fl, absolute reticulocyte count: 19.9 × 109/L. ‐ A peripheral blood smear: A few schistocytes, polychromasia, and anisocytosis. ‐ DAT: test was positive for IgG and C3 with identification of warm autoantibodies. |
‐ High LDH level and low haptoglobin level. ‐ Mildly elevated indirect bilirubin. Creatinine was normal range ‐ Normal serum iron and iron saturation but mildly elevated ferritin, normal folate and severely low vitamin B12 level. ‐ Anti‐gastric parietal cell IgG and intrinsic factor antibodies were positive. |
‐ Treatment was done using methylprednisolone, and cyanocobalamin. ‐ On 10th day Hb improved and she was discharged. ‐ On the follow‐up hematologic parameters were improved. |
20 |
| Belgium (10) | Warm AIHA | M‐67 | Pfizer‐BioNTech |
‐ 17 days after third dose. ‐Fever, general weakness, fatigue, jaundice, dark urine. |
‐ Hb: 7.5 g/dl, reticulocytopenia, decreased platelet count, ‐ A peripheral blood smear: A few schistocytes, polychromasia, and anisocytosis. ‐ DAT: test was positive for IgG and C3 ‐ DAT: test was positive for IgG and C3 with identification of warm autoantibodies. |
‐ Undetectable level of haptoglobin, high level of LDH and ferritin, total and indirect bilirubin was raised. |
‐ Methylprednisolone was initiated, but in the next morning the Hb level was critically reduced. ‐ Transfusion was delayed as compatible RBC was unavailable. With a delay, compatible RBC was administered. ‐ Methylprednisolone dose was raised due to poor response. On Day 12, a daily plasma exchange was done, as well as weekly rituximab injections. The patient was finally discharged on Day 21, with a steady Hb level of about 7.0 g/dl. |
21 |
| Japan (11) f | Warm AIHA | F‐53 | Pfizer‐BioNTech |
‐ 14 days after second dose. ‐ Yellow skin and conjunctiva and anemic palpebral. |
‐ Hb: 6.9 g/dl. ‐ Low platelet count. ‐ Erythroid hyperplasia with megaloblastic alterations on the smear of bone marrow. ‐ Both direct and indirect coombs tests were positive. ‐ Cold agglutinin level was low. |
‐ High indirect bilirubin, elevated LDH, low haptoglobin, positive lupus coagulant and anti‐nuclear antibody. | ‐ Treatment with prednisolone together with once RBC transfusion gradually improved the symptoms. | 22 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; DAT, direct antiglobulin test; IVIG, intravenous immunoglobulin; LDH, lactate dehydrogenase; MPO, myeloperoxidase.
The patient had a history of COVID‐19 infection around 6 months before vaccination.
The patient had long‐term psoriatic arthritis on adalimumab therapy for >5 years. Her Hgb/Hct, platelet and WBC counts were normal 4 months earlier.
The patient had a history of malignancies, cardiovascular and digestive disorders with mild chronic anemia 4 months before presentation.
The patient had history of chemotherapy for lung cancer and a history of H. pylori eradication for idiopathic thrombocytopenic purpura (ITP).
The patient had a history of hypertension, congenitally mute and deaf, iron deficiency anemia, and provoked venous sinus thrombosis treated with anticoagulation.
The patients displayed coexistence of AIHA and immune thrombocytopenia (Evans syndrome) associated with the systemic lupus erythematosus.
TABLE 3.
Characteristics of cases with exacerbated previous autoimmune hemolytic anemia (AIHA) following COVID‐19 vaccination
| Country (case no.) | Anemia type | Gender‐age, years | Vaccine name (vaccine type) | Onset time of symptoms‐primary clinical symptoms | Hematologic test results | Other tests | Medication and outcome | Reference |
|---|---|---|---|---|---|---|---|---|
| Spain (1) a | Cold AIHA | F‐57 | mRNA‐based vaccine |
‐ 2 days after first dose. ‐ Chills, weakness, shortness of breath upon exertion, lumbar pain, jaundice, and mild hemoglobinuria. |
‐ Hb: 8.6 g/dl, high reticulocyte count, spherocytes on the peripheral blood smear. ‐ DAT: Test was positive for C3b and negative for IgG. A cold agglutinin with anti‐I specificity was identified. The cryoagglutinin titer increased after the first dose of the vaccine. |
‐Bilirubin (2.9 mg/dl), LDH (462 U/L), and ‐ Inflammation parameters: Ferritin or D‐dimer were elevated at 426 and 726 ng/ml, respectively |
‐Treatment with prednisone improved blood factors and Hb level. ‐ Seventh days before the second of the vaccine, prednisone dose was reduced. ‐ 2 days after the second dose of vaccine, same symptoms, and laboratory data observed. ‐ Prednisone dose was then increased causing improvement of symptoms and laboratory parameters, |
23 |
| Italy (2) b | Cold AIHA | M‐59 | Moderna |
‐ 1 days after second dose. ‐ Fever, dark urine and jaundice. |
‐ Hb drop from 10.1 to 6.8 g/dl and LDH elevation (1.8 ×ULN) 5 days after the second dose of moderna vaccine. | Low levels of complement components: C3 level was 55 mg/dl and C4 was undetectable. |
‐Treatment with prednisone and erythropoietin stabilized Hb at about 8.2 g/dl. ‐ 2 weeks later, rituximab was used with progressive and full recovery. |
24 |
| Italy (3) b | Warm AIHA | F‐79 | Pfizer‐BioNTech |
‐ After first dose. |
‐ Hb decrease from 10.4 to 9.1 g/dl (LDH 1.2 to 1.7 × ULN) after the first dose of Pfizer vaccine. | Not reported. | She need a slight increase of steroid dose (to 5 mg day prednisone) and remained stable following the second dose. | 25 |
| Italy (4) b | Warm AIHA | M‐73 | Moderna | ‐ 7 days after first dose. | ‐ Hb decrease from 13.9 to 9.1 g/dl, LDH 1.1 to 1.6 × ULN. | Not reported. | Treatment with prednisone 0.5 mg/kg day cause prompt response. | |
| Italy (5) b | Cold AIHA | M‐77 | Moderna | ‐ 7 days after first dose. | ‐ Hb drop from 9.3 to 7.2 g/dl. | Not reported. | Treatment was done with steroids, rituximab, and erythropoietin. | |
| Italy (6) b | Warm AIHA | M‐73 | Pfizer‐BioNTech | ‐ 5 days after second dose. | ‐ Hb drop from 14 to 7.4 g/dl, LDH 1.1 to 2.3 × ULN. | Not reported. | Treatment with high dose intravenous steroids improved symptoms about 1 week. | |
| Philippines (7) c | Warm AIHA | F‐33 | Moderna |
‐ 2 days after booster dose. ‐ Dizziness, generalized weakness and pallor. |
‐ Hb: 5.2 g/dl, high reticulocyte counts on the peripheral blood smear. ‐ DAT: positive for IgG and C3. |
‐ Elevated LDH and hyperferritinemia. | An intravenous steroid was administered to the patient. To relieve the anemia symptoms, packed red blood cells were transfused. During the hospitalization, hematologic indices steadily increased. | 26 |
Abbreviations: DAT, direct antiglobulin test; LDH, lactate dehydrogenase.
The patient had a history of cold agglutinin disease (CAD) in 2016 that had been improved using corticosteroids.
The patients suffered from mentioned AIHA before COVID‐19 vaccination.
Primary vaccination using an inactivated vaccine had no adverse effects.
3. RESULTS
3.1. Development of AIHA after COVID‐19 vaccination
AIHA is caused by IgG and/or IgM autoantibodies that react with red blood cell (RBC)‐related surface antigens. 27 , 28 Warm antibodies (mostly of the IgG type) account for 65%–70% of AIHA cases. Depending on the IgG subtype (IgG1, IgG3) and concentration, these antibodies can also activate complement on the RBC surface, resulting in both intravascular hemolysis as well as extravascular phagocytosis mediated by C3b receptors. 29 , 30 Warm autoantibodies can be detected by direct antiglobulin test (DAT) positivity for IgG or IgG + C3b. 31 Cold autoantibodies (usually of the IgM class) account for 20%–25% of AIHAs that bind at cold temperatures and rapidly activate complement, causing intravascular RBC lysis. 29 Cold autoantibodies are usually from IgM isotype, however, rare cases of warm IgM‐related AIHAs can also occur. 28 , 32 The RBC‐reactive IgM autoantibodies can be detected by the DAT with a strong positive result for C3d but a negative or weakly positive result for IgG. 28 , 33 AIHA has a wide range of clinical manifestations, from asymptomatic to severe types with deadly outcomes. 29
Tables 1 and 2 summarize the main features of the 11 patients who developed AIHA after COVID‐19 vaccination, as of July 3, 2022. Among 18 cases with post‐vaccination AIHA, development of the new AIHA was reported in 11 patients (7 women [63.6%] and 4 men [36.4%]). Accordingly, the women/men ratio was 1.75. The median of age was 74.0 years (with a range of 67–84 years) for men, and 53.0 years (with a range of 41–88 years) for women. In whole cases with AIHA development after vaccination, the median age was 67 years (with a range of 41–88 years) (Table 2).
TABLE 2.
Age of patients and the onset time of autoimmune hemolytic anemia (AIHA) symptoms following COVID‐19 vaccination according to the gender of individuals
| Observed AIHA | Gender | No. (%) | Age, years mean ± SD median (min − max) | Cases with disease onset after first dose | Onset time after first dose (days) | Cases with disease onset after second dose | Onset time after second dose (days) |
|---|---|---|---|---|---|---|---|
| AIHA development | Men a | 4 (36.4%) |
74.75 ± 7.41 74 (67–84) |
3 (75.0% b ) |
18.0 ± 12.5 c 19.0 (5–30) d |
– | – |
| Women | 7 (64.6%) |
58.57 ± 18.21 53.00 (41–88) |
4 (57.1% b ) |
7.75 ± 4.57 7.0 (3–14) |
3 (42.9% b ) |
45.33 ± 64.94 14.0 (2–120) |
|
| Total | 11 (100.0%) |
64.45 ± 16.79 67.0 (41–88) |
7 (63.6% e ) |
12.14 ± 9.63 7.0 (3–30) |
3 (27.3% e ) |
45.33 ± 64.94 14.0 (2–120) |
|
| AIHA exacerbation | Men | 3 (42.9%) |
69.66 ± 9.45 73.0 (59–77) |
2 (66.7% b ) |
7.0 ± 0.0 7.0 (7.0–7.0) |
1 (33.3% b ) |
1.0 ± 0.0 1 (1.0–1.0) |
| Women f | 4 (57.1%) |
60.50 ± 20.55 65.0 (33–79) |
2 (50.0% b ) |
2.0 ± 0.0 2.0 (2.0–2.0) |
1 (25.0% b ) |
5.0 ± 0.0 5 (5.0–5.0) |
|
| Total | 7 (100.0%) |
64.42 ± 16.27 73.0 (33–79) |
4 (57.1% e ) |
5.33 ± 2.88 7.0 (2.0–7.0) |
2 (28.6% e ) |
3.0 ± 2.82 3 (1.0–5.0) |
In 1 of 4 (25.5%) men, the onset of AIHA symptoms was observed on Day 17 following the booster vaccination.
The percent was calculated within a specified gender.
The onset time expressed as mean ± SD.
The onset time expressed as median (min − max).
The percent was calculated according to the onset time of symptoms.
In 1 of 4 (25.5%) women, the AIHA exacerbation was observed on Day 2 following the booster vaccination.
Four of seven (57.1%) and 3 of 7 (42.9%) women displayed the onset of AIHA symptoms following the first and second vaccine dose, with a median time of 7.0 days (with a range of 3–14 days) and 14.0 days (with a range of 2–120 days) following vaccination, respectively. Three of four (75.0%) men displayed the onset of AIHA symptoms following the first vaccine dose, with a median time of 19.0 days (with a range of 5–30 days). In 1 of 4 (25.5%) men, the onset of AIHA symptoms was observed on Day 17 following the booster vaccination. Overall, in 7 of 11 (63.6%) AIHA cases, the onset of the symptoms occurred following the first vaccine dose with a median time of 7 days (ranged: 3–30 days) after vaccination. Moreover, 3 of 11 (27.3%) AIHA patients developed the symptoms after the second dose of vaccine with a median time of 14.0 days (ranged: 2–120 days) after vaccination (Table 2). In 1 of 11 (9.1%) AIHA, the onset of AIHA symptoms was observed on Day 17 following the booster vaccination.
Ten of 11 (90.9%) AIHA patients received the mRNA‐based vaccines and 1 of 11 (9.1%) received a vector‐based vaccine. According to the vaccine name, 5 of 11 (45.5%) AIHA cases received the Pfizer vaccine, 4 of 11 (36.4%) AIHA cases received the Moderna vaccine, and 1 of 11 (9.1%) cases received the AstraZeneca vaccine. The name of the administrated vaccine was not originally reported for one case who received the mRNA‐based vaccine (Table 1).
Nine of 11 (81.8%) patients developed warm (IgG) anti‐RBC autoantibodies, 1 of 11 (9.1%) patients developed cold (IgM) anti‐RBC autoantibodies, and 1 of 9 (9.1%) patients developed mixed (IgG + IgM) anti‐RBC autoantibodies after COVID‐19 vaccination. In one case, the development of the AIHA was observed 4 months after vaccination with the second dose of the Moderna vaccine, indicating the delayed onset of AIHA. 13 Campos‐Cabrera et al. also reported five patients (without their characteristics) with secondary AIHA to COVID‐19 vaccination, whose Hb levels reduced to <7 mg/dl, that were recovered using a treatment program with dexamethasone. 34
3.2. Exacerbation of AIHA after COVID‐19 vaccination
Patients with idiopathic warm AIHA usually respond to corticosteroid therapy. However, a number of patients display disease relapse after steroid‐mediated remission. The short duration of maintenance and quick tapering of corticosteroids as well as infections are possible risk factors for relapsed/recurrent hemolysis in idiopathic AIHA. 35
Tables 2 and 3 summarize the main features of the seven patients who displayed AIHA exacerbation after COVID‐19 vaccination, as of July 3, 2022. Among 18 cases with post‐vaccination AIHA, significant AIHA exacerbation was reported in seven patients (4 women [57.1%] and 3 men [42.9%]). Accordingly, the women/men ratio with significant exacerbation of AIHA was 1.33. Among exacerbated AIHA cases, the median of age was 73.0 years (with a range of 59–77 years) for men and 65.0 years (with a range of 33–79 years) for women. In whole, exacerbated AIHA cases, the median of age was 73 years (with a range of 33–79 years) (Table 2).
Two of three (66.7%) men and 2 of 4 (50.0%) women displayed AIHA exacerbation following the first vaccine dose, with a median time of 7.0 and 2.0 days, respectively. One of three (33.3%) men and 1 of 4 (25.0%) women displayed the AIHA exacerbation on 1 and 5 days following the second vaccine, respectively. In 1 of 4 (25.5%) women, the AIHA exacerbation was observed on Day 2 following the booster vaccination. Overall, in 4 of 7 (57.1%) exacerbated AIHA cases, the onset of the symptoms occurred following the first vaccine dose with a median time of 7 days (with a range of 2–7 days) after vaccination. Moreover, 2 of 7 (28.6%) exacerbated AIHA cases developed the symptoms after the second dose of vaccine with a median time of 3 days (with a range of 1–5 days) after vaccination. In 1 of 7 (14.3%) exacerbated AIHA cases, the onset of the symptoms was observed on Day 2 following the booster vaccination.
Seven of seven (100.0%) exacerbated AIHA cases received mRNA‐based vaccines. According to the vaccine name, 4 of 7 (57.1%) exacerbated AIHA cases received the Moderna vaccine and 2 of 7 (28.6%) cases received the Pfizer vaccine. The name of the administrated vaccine was not originally reported for one exacerbated case (Table 3).
Three of seven (42.9%) patients with exacerbated AIHA developed warm (IgG) anti‐RBC autoantibodies, while 4 of 7 (57.1%) patients developed cold (IgM) anti‐RBC autoantibodies. The level of Hb in patients with exacerbated AIHA reached 7.62 ± 1.41 mg/dl, with a median level of 7.4 mg/dl (ranged 5.2–9.1 mg/dl). The results from a study from Italy indicated that the SARS‐CoV‐2 vaccination may be associated with a clinically significant decrease in hematologic values in 7.4% of patients suffering from AIHA and a mild decrease was observed in about 10.0% of AIHA patients. All cases have been successfully managed with steroids and transfusion support. 25 Campos‐Cabrera et al. reported seven patients (without their characteristics) with preexisting AIHA who developed hemolysis after vaccination. Five of them with Hb levels >7 mg/dl were recovered without any treatment, while two patients with Hb levels <7 mg/dl were recovered using a treatment program with dexamethasone. 34
4. DISCUSSION
AIHA can emerge as a primary (idiopathic) disorder or secondarily in association with other diseases, including autoimmune disorders (such as lupus erythematosus, rheumatoid arthritis, antiphospholipid syndrome, inflammatory bowel disease, and autoimmune thyroid diseases), immunodeficiency disorders (such as common variable immunodeficiency and IgA deficiency), lymphoproliferative disorders (such as chronic lymphocytic leukemia, non‐Hodgkin lymphoma, Hodgkin lymphoma, and Waldenstrom macroglobulinemia), and infections (such as SARS‐CoV‐2, epstein barr virus, cytomegalovirus, HIV, hepatitis C virus and Mycoplasma pneumonia). 29 , 30 , 36 Treatment with a number of drugs (such as penicillins, cephalosporin, alpha methyl dopa, fludarabine, and oxaliplatinum) was also associated with AIHA development. 29 , 30 COVID‐19 is associated with both warm AIHA (28% of patients) and cold AIHA (38% of patients). 36
The occurrence of AIHA was reported after vaccination with the influenza vaccine. 37 , 38 Other vaccines, such as DTP (diphtheria‐tetanus‐pertussis), also can cause AIHA due to the induction of warm and cold anti‐RBC antibodies. 39 The results of this study indicate the occurrence of AIHA following COVID‐19 vaccination. However, the etiology of AIHA remains to be determined in future studies. Vaccines, like infectious agents, can contribute to the development of autoimmune disorders through a variety of pathways, including molecular mimicry, polyclonal activation of lymphocytes, epitope spreading, bystander activation, and presentation of the cryptic determinants of antigens. 5 If a vaccine's antigen has structural similarities with self‐antigens, then the immune response to that vaccine may extend to host cells expressing the similar autoantigen. The cross‐reactive antibody response against the RBCs can result from an antibody response to SARS‐CoV‐2 proteins. The molecular mimicry between ankyrin‐1, an RBC membrane protein, and the SARS‐CoV‐2 S protein was postulated by Angileri et al. They identified a 100.0% similarity between an ankyrin‐1 putative immunogenic epitope and an S protein‐predicted epitope for B cells using an epitope database. 40 It was proposed that this molecular similarity can be considered as an etiologic basis for AIHA development in COVID‐19 patients. We can speculate that the same cross‐reactivity may contribute to the AIHA development and exacerbation after COVID‐19 vaccination.
Vaccines may contain additional components, such as adjuvants that boost the immune response to the antigen, preservatives and stabilizers, and occasionally traces of antibiotics to prevent bacterial and fungal contamination. 5 The etiologic role of some antibiotics (such as penicillin and cephalosporin) in the induction of AIHA was clearly explained. 41 High titers of anti‐cardiolipin antibodies following influenza vaccination in lupus patients, as well as anti‐ovarian and anti‐thyroid antibodies following HPV vaccination, have been related to vaccine‐related adjuvants (aluminum and thimerosal). 42 The post‐vaccination AIHA developed with a higher rate in females compared with males. Like most autoimmune disorders, these findings can be attributed to the immunological impacts of sex hormones. For instance, testosterone stimulates, while estrogen dampens the regulatory T (Treg) cell activity. 43
The therapeutic approach to warm AIHA is significantly different from patients with cold agglutinin hemolysis. In secondary AIHA, treatment on the associated disorder should be considered. Corticosteroids are the primary therapeutic agent for the treatment of warm AIHA.
Splenectomy was the only effective therapy for warm AIHA before the introduction of corticosteroid therapy. Splenectomy is still an option, with a response rate of about 80.0%. 29 , 30 However, splenectomy may have complications, such as severe infection. Monoclonal anti‐CD20 antibody, rituximab, and cyclophosphamide are effective for the treatment of both warm and cold AIHA. IVIG may be used in combination with other therapies, particularly in severe hemolysis cases. 29 , 30 The inhibitors of complement are in clinical trials for cold AIHA treatment with promising results. 44 Clinical trials are also ongoing to assess the safety and effectiveness of new AIHA therapies such as fostamatinib (a Syk inhibitor) and ibrutinib (a Bruton tyrosine kinase inhibitor). 17
The data presented here indicated that the administration of a corticosteroid drug was used in the treatment program of the majority (9/11) of patients with new‐onset of AIHA development followed by the blood transfusion that was used in 6 of 11 patients. All patients with AIHA exacerbation used a corticosteroid, and 2 of 7 exacerbated cases used a corticosteroid together with erythropoietin plus rituximab in their treatment program.
5. CONCLUSION
AIHA may be considered as a very rare complication of COVID‐19 vaccination that occurs following vaccination. The direct causal relationship between COVID‐19 vaccination and AIHA is still ambiguous. Prospective studies evaluating RBC‐related autoantibodies before and after vaccination, as well as comparisons with an unvaccinated control group, should be done to determine whether there is an increase in AIHA incidence following SARS‐CoV‐2 vaccination. Both cold and warm anti‐RBC autoantibodies contribute to AIHA pathogenesis. Hematologic monitoring, especially in patients with a history of AIHA before and after COVID‐19 vaccination appears appropriate to promptly manage AIHA. It is also important to record AIHA events and follow‐up patients who develop this complication over time. Globally, there is no exact statistically data about the coverage of each COVID‐19 vaccine type. The Pfizer and Modern vaccines are the most administrated vaccines in European countries. 45 In fact, most cases of AIHA have been reported from the European countries and the United States, where most of the vaccines used are mRNA based. Therefore, the increased incidence of AIHA after vaccination with mRNA‐based vaccines may be attributed to the higher coverage of these vaccines. However, the post‐vaccination AIHA is manageable and the advantages of immunization can exceed these risks.
AUTHOR CONTRIBUTIONS
Abdollah Jafarzadeh conceptualized the manuscript; Abdollah Jafarzadeh, Sara Jafarzadeh, Mohammad Pardehshenas, Maryam Nemati, and Seyed Mohammad Javad Mortazavi drafted the manuscript; Abdollah Jafarzadeh and Seyed Mohammad Javad Mortazavi scrutinized the scientific‐content and edited the manuscript; All authors approved the submitted version.
CONFLICT OF INTEREST
The authors have no conflict of interest.
Jafarzadeh A, Jafarzadeh S, Pardehshenas M, Nemati M, Mortazavi SMJ. Development and exacerbation of autoimmune hemolytic anemia following COVID‐19 vaccination: A systematic review. Int J Lab Hematol. 2022;1‐11. doi: 10.1111/ijlh.13978
Contributor Information
Abdollah Jafarzadeh, Email: jafarzadeh14@yahoo.com.
Seyed Mohammad Javad Mortazavi, Email: mortazavismj@gmail.com.
DATA AVAILABILITY STATEMENT
The data will be available upon request from the corresponding authors.
REFERENCES
- 1. World Health Organization . WHO COVID‐19 dashboard. 2021. https://covid19.who.int/
- 2. Kyriakidis NC, López‐Cortés A, González EV, Grimaldos AB, Prado EO. SARS‐CoV‐2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines. 2021;6(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shiravi AA, Ardekani A, Sheikhbahaei E, Heshmat‐Ghahdarijani K. Cardiovascular complications of SARS‐CoV‐2 vaccines: an overview. Cardiol Ther. 2021;11:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Association . COVID‐19 vaccines advice. Accessed December 18, 2021. https://wwwwhoint/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice
- 5. Olivieri B, Betterle C, Zanoni G. Vaccinations and autoimmune diseases. Vaccine. 2021;9(8):815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lim HX, Arip M, Yahaya AAA‐F, Jazayeri SD, Poppema S, Poh CL. Immunogenicity and safety of SARS‐CoV‐2 vaccines in clinical trials. Front Biosci. 2021;26(11):1286‐1304. [DOI] [PubMed] [Google Scholar]
- 8. Rzymski P, Perek B, Flisiak R. Thrombotic thrombocytopenia after COVID‐19 vaccination: in search of the underlying mechanism. Vaccine. 2021;9(6):559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Y, Xu Z, Wang P, et al. New‐onset autoimmune phenomena post‐COVID‐19 vaccination. Immunology. 2021;165:386‐401. [DOI] [PubMed] [Google Scholar]
- 10. Jafarzadeh A, Nemati M, Jafarzadeh S, Nozari P, Mortazavi SMJ. Thyroid dysfunction following vaccination with COVID‐19 vaccines: a basic review of the preliminary evidence. J Endocrinol Investig. 2022;45:1835‐1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen F, Cao P, Liu H, Cai D. The impact of COVID‐19 and vaccine on human nervous system. Neuroendocrinology. 2022;1‐12:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simon S, Paul M, Thomas J, Abraham LK. Autoimmune haemolytic anaemia following ChAd Ox 1 nCoV‐19 in haemodialysis. J Assoc Physicians India. 2022;70(1):11‐12. [PubMed] [Google Scholar]
- 13. Fatima Z, Reece BRA, Moore JS, Means RT Jr. Autoimmune hemolytic anemia after mRNA COVID vaccine. J Investig Med High Impact Case Rep. 2022;10:23247096211073258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brito S, Ferreira N, Mateus S, et al. A case of autoimmune hemolytic anemia following COVID‐19 messenger ribonucleic acid vaccination. Cureus. 2021;13(5):e15035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaignard ME, Lieberherr S, Schoenenberger A, Benz R. Autoimmune hematologic disorders in two patients after mRNA COVID‐19 vaccine. HemaSphere. 2021;5(8):e618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murdych TM. A case of severe autoimmune hemolytic anemia after a receipt of a first dose of SARS‐CoV‐2 vaccine. Int J Lab Hematol. 2022;44(1):e10‐e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gadi SRV, Brunker PAR, Al‐Samkari H, et al. Severe autoimmune hemolytic anemia following receipt of SARS‐CoV‐2 mRNA vaccine. Transfusion. 2021;61(11):3267‐3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okuno S, Hashimoto K, Shimizu R, Takagi E, Kajiguchi T. Development of autoimmune hemolytic anemia after BNT162b2 mRNA COVID‐19 vaccination. Rinsho Ketsueki. 2021;62(10):1510‐1514. [DOI] [PubMed] [Google Scholar]
- 19. Aoun SA, Motabi I. Cold agglutinin disease after COVID‐19 vaccine. Br J Haematol. 2021;195(5):650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jaydev F, Kumar V, Khatri J, Shahani S, Beganovic S. A case of autoimmune hemolytic anemia after the first dose of COVID‐19 mRNA‐1273 vaccine with undetected pernicious anemia. Case Rep Hematol. 2022;2022:2036460‐2036465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Bruyne S, Van Landeghem S, Schauwvlieghe A, Noens L. Life‐threatening autoimmune hemolytic anemia following mRNA COVID‐19 vaccination: don't be too prudent with the red gold. Clin Chem Lab Med. 2022;60(6):e125‐e128. [DOI] [PubMed] [Google Scholar]
- 22. Hidaka D, Ogasawara R, Sugimura S, et al. New‐onset Evans syndrome associated with systemic lupus erythematosus after BNT162b2 mRNA COVID‐19 vaccination. Int J Hematol. 2022;115(3):424‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pérez‐Lamas L, Moreno‐Jiménez G, Tenorio‐Núñez MC, et al. Hemolytic crisis due to Covid‐19 vaccination in a woman with cold agglutinin disease. Am J Hematol. 2021;96(8):E288‐E291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fattizzo B, Pasquale R, Bellani V, Barcellini W, Kulasekararaj AG. Complement mediated hemolytic anemias in the COVID‐19 era: case series and review of the literature. Front Immunol. 2021;12:791429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fattizzo B, Giannotta JA, Cecchi N, Barcellini W. SARS‐CoV‐2 vaccination in patients with autoimmune cytopenias: the experience of a reference center. Am J Hematol. 2021;96(11):E413‐e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mesina FZ. Severe relapsed autoimmune hemolytic anemia after booster with mRNA‐1273 COVID‐19 vaccine. Hematol Transfus Cell Ther. 2022. doi: 10.1016/j.htct.2022.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Berentsen S, Barcellini W. Autoimmune hemolytic anemias. N Engl J Med. 2021;385(15):1407‐1419. [DOI] [PubMed] [Google Scholar]
- 28. Jäger U, Barcellini W, Broome CM, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the first international consensus meeting. Blood Rev. 2020;41:100648. [DOI] [PubMed] [Google Scholar]
- 29. Liebman HA, Weitz IC. Autoimmune hemolytic anemia. Med Clin North Am. 2017;101(2):351‐359. [DOI] [PubMed] [Google Scholar]
- 30. Hill A, Hill QA. Autoimmune hemolytic anemia. Hematology Am Soc Hematol Educ Program. 2018;2018(1):382‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barcellini W, Zaninoni A, Giannotta JA, Fattizzo B. New insights in autoimmune hemolytic anemia: from pathogenesis to therapy stage 1. J Clin Med. 2020;9(12):3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barcellini W, Fattizzo B. How I treat warm autoimmune hemolytic anemia. Blood. 2021;137(10):1283‐1294. [DOI] [PubMed] [Google Scholar]
- 33. Berentsen S. New insights in the pathogenesis and therapy of cold agglutinin‐mediated autoimmune hemolytic anemia. Front Immunol. 2020;11:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Campos‐Cabrera G, Torres‐Salgado F‐G, Campos‐Cabrera S, Campos‐Villagomez J‐L, Campos‐Cabrera V. Autoimmune cytopenias and COVID‐19 vaccination: relapse and suggested treatment. Blood. 2021;138:4141. [Google Scholar]
- 35. Dussadee K, Taka O, Thedsawad A, Wanachiwanawin W. Incidence and risk factors of relapses in idiopathic autoimmune hemolytic anemia. J Med Assoc Thai. 2010;93(suppl 1):S165‐S170. [PubMed] [Google Scholar]
- 36. Jacobs JW, Booth GS. COVID‐19 and immune‐mediated RBC destruction. Am J Clin Pathol. 2022;157(6):844‐851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Montagnani S, Tuccori M, Lombardo G, et al. Autoimmune hemolytic anemia following MF59‐adjuvanted influenza vaccine administration: a report of two cases. Ann Pharmacother. 2011;45(1):e8. [DOI] [PubMed] [Google Scholar]
- 38. Gani I, Hinnant G, Kapoor R, Savage N. Autoimmune hemolytic anemia in a renal transplant patient following seasonal influenza vaccination. Case Rep Hematol. 2019;2019:3537418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Downes KA, Domen RE, McCarron KF, Bringelsen KA. Acute autoimmune hemolytic anemia following DTP vaccination: report of a fatal case and review of the literature. Clin Pediatr. 2001;40(6):355‐358. [DOI] [PubMed] [Google Scholar]
- 40. Angileri F, Légaré S, Marino Gammazza A, Conway de Macario E, Macario AJL, Cappello F. Is molecular mimicry the culprit in the autoimmune haemolytic anaemia affecting patients with COVID‐19? Br J Haematol. 2020;190(2):e92‐e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Garratty G. Drug‐induced immune hemolytic anemia. Hematol Am Soc Hematol Educ Program. 2009;2009:73‐79. [DOI] [PubMed] [Google Scholar]
- 42. Vadalà M, Poddighe D, Laurino C, Palmieri B. Vaccination and autoimmune diseases: is prevention of adverse health effects on the horizon? EPMA J. 2017;8(3):295‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Singh RP, Bischoff DS. Sex hormones and gender influence the expression of markers of regulatory T cells in SLE patients. Front Immunol. 2021;12:619268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Röth A, Barcellini W, D'Sa S, et al. Sutimlimab in cold agglutinin disease. N Engl J Med. 2021;384(14):1323‐1334. [DOI] [PubMed] [Google Scholar]
- 45. Ritchie H, Mathieu E, Rodés‐Guirao L, et al. Coronavirus pandemic (COVID‐19). Published online at OurWorldInData.org. 2020. Accessed March 15, 2022. https://ourworldindataorg/coronavirus
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will be available upon request from the corresponding authors.


