Abstract
Objective
To describe the epidemiology of COVID‐19 in one region of New Zealand in the context of the national lockdown and provide a reference for comparing infection dynamics and control measures between SARS‐Cov‐2 strains.
Methods
Epidemiological linking and analysis of COVID‐19 cases and their close contacts residing in the geographical area served by the Southern District Health Board (SDHB).
Results
From 13 March to 5 April 5 2020, 186 cases were laboratory‐confirmed with wild‐type Sars‐Cov‐2 in SDHB. Overall, 35·1% of cases were attributable to household transmission, 27·0% to non‐household, 25·4% to overseas travel and 12·4% had no known epidemiological links. The highest secondary attack rate was observed in households during lockdown (15·3%, 95%CI 10·4–21·5). The mean serial interval in 50 exclusive infector‐infectee pairs was 4·0 days (95%CI 3·2–4·7days), and the mean incubation period was 3.4 days (95%CI 2·7–4·2).
Conclusions
The SARS‐CoV‐2 incubation period may be shorter than early estimates that were limited by uncertainties in exposure history or small sample sizes.
Implications for public health
The continuation of household transmission during lockdown highlights the need for effective home‐based quarantine guidance. Our findings of a short incubation period highlight the need to contact trace and isolate as rapidly as possible.
Key words: COVID‐19, epidemiology, New Zealand
Coronavirus disease 2019 (COVID‐19) is caused by severe acute respiratory coronavirus 2 (SARS‐CoV‐2), a novel respiratory virus that is causing worldwide morbidity, mortality, and social and financial disruption. Preventing illness and death associated with COVID‐19 requires every affected country to take measures to reduce transmission.1
When the first case of COVID‐19 was identified in New Zealand in late February 2020, efforts to trace infection sources, identify close contacts and manage cases were challenged by a nascent understanding of the incubation period, infectiousness, and serial interval. The reporting of these parameters from a range of contexts and across Sars‐Cov‐2 strains will help establish a firm evidence base on which public health decisions can be made.
Faced with considerable uncertainty about the epidemiological characteristics of SARS‐CoV‐2 transmission, and the witnessing of devastating impact overseas, the New Zealand Government introduced travel restrictions, contact tracing and a four‐level COVID‐19 Alert system of population‐wide response measures2 (for a detailed outline of New Zealand's COVID‐19 response, see Jefferies et al.3 and Summers et al.4). New Zealand moved to Alert level 3 on 23 March 2020, 26 days after the first recorded case in New Zealand, and at a total national case number of 102. From 26 March to 28 April 2020, New Zealand was in Alert level 4—everyone except essential workers was required to go into ‘lockdown’. People were instructed to stay at home other than for essential personal movement. Educational facilities, public venues and non‐essential businesses were closed. A COVID‐19 Protection Framework5 replaced the COVID‐19 Alert system in December 2021. The framework was developed in response to the emergence of the Omicron strain and one of its primary aims is to reduce the need for lockdowns.
Households were the core COVID‐19 management unit in New Zealand during the early epidemic period (March through July 2020), with cases and their close contacts being isolated within their homes. The effects of home‐based lockdown and case management need to be understood to refine future COVID‐19 public health response strategies. Using data collected during our management of COVID‐19 cases and their contacts residing in our district, we describe the early epidemiology of COVID‐19 in this region in the context of the national public health response.
Methods
Study population
All COVID‐19 cases living in the geographical area served by the Southern District Health Board (hereafter, SDHB) and their close contacts were included in this study. Cases were confirmed via reverse transcription‐polymerase chain reaction (PCR) from a nasopharyngeal with oropharyngeal swab. SDHB encompasses the two southernmost regions of New Zealand, Otago and Southland, with a combined population of approximately 330,000.6 Compared with the New Zealand average, the SDHB population has a similar age structure, a lower proportion of Māori and Pacific peoples, a higher proportion of people living in the least deprived section of the population, and a lower proportion in the most deprived section.6
Data sources
COVID‐19 became notifiable in New Zealand from 30 January 2020. Local public health units collected case and close contact information via direct interview using a standardised form and recorded it in EpiSurv, the national notifiable diseases database. Contact information was similarly collected and entered in a Research Electronic Data Capture (REDCap) secure online database.
We extracted the records of confirmed COVID‐19 cases identified between 13 March and 5 April 2020 and their close contacts, who were followed up until 14 days after their last exposure to the COVID‐19 case. Since 6 April 2020, no further confirmed cases were identified in SDHB for the remainder of 2020. The last case follow up was 20 May 2020, when the last case was deemed fully recovered. The last close contact follow‐up date was 30 April 2020.
We used information on case age, sex, exposure history, dates of symptom onset and recovery to describe case demographics and estimate time‐to‐recovery, incubation period and serial interval. Recovery was defined as full recovery from all new‐onset COVID‐19 symptoms (i.e. not baseline symptoms from pre‐existing conditions) or at least 28 days since onset for those with lingering symptoms. Information on close contact age, sex, address, contact setting and infection status was used to identify close contact characteristics, define contact type and estimate secondary attack rates.
The University of Otago Human Ethics Committee (HD20/067), Dunedin, New Zealand, approved this study.
Epidemiological parameters
We defined the source of SARS‐CoV‐2 infection as being one of four categories: recent overseas travel; close contact household; close contact non‐household (including cluster); or unknown (presumed community) if no epidemiological links were found. Travel was defined as having returned from overseas in the 14 days before symptom onset (or positive test result for asymptomatic cases). Close contact household had an epidemiological link to a case living at the same address. Close contact non‐household had either 1) an epidemiological link to a case who did not live at the same address, or 2) exposure to a known cluster. Community was a definition by exclusion from the other categories. Close contact status required being within two metres of a case for more than 15 minutes or being linked to a known cluster.
The incubation period and serial interval were estimated using data of confirmed case pairs with clear, exclusive epidemiological links and no overseas travel history (see Supplementary Materials for pair contact type and exposure dates). For the incubation period analysis, we considered the interval between the first and last recorded dates of exposure (i.e. interval censoring) with an equal probability of exposure for each date. For 32/50 pairs, exposure to the infector was in the household and therefore continuous. In these instances, first exposure date was set at a maximum of 48 hours before symptom onset in the infector (i.e. we limited the pre‐symptomatic infectious period to 48 hours) unless an exact first exposure date within the 48‐hour cut‐off was ascertained via case interview. Last exposure date was set as the day of symptom onset in the infectee unless an exact date before symptom onset was established. Sensitivity analyses for different censoring rules (last exposure date set as day before symptom onset in infectee when unknown, known last exposure date only, first exposure date only) are presented in the Supplementary Materials.
Onward transmission rates were calculated as the percentage of cases who went on to infect at least one contact. Logistic regression was used to identify onward transmission risk by case age, sex and infection source. The Wilcoxin rank‐sum test was used to test for a difference in the median number of contacts between cases who transmitted onwards and those that did not. Secondary attack rates were calculated as the percentage of close contacts who were later confirmed to have COVID‐19. Rates were calculated by contact sex, age, and exposure setting (household versus non‐household). Poisson regression was used to test for differences in attack rates by age, sex, exposure setting and lockdown status.
Parametric distributions (Weibull, gamma and lognormal) were fitted to case isolation and recovery times, serial intervals, and incubation periods. The best fit was selected based on the minimum Akaike information criterion.7
Results
In total, 186 COVID‐19 cases were PCR positive laboratory‐confirmed in SDHB between 13 March and 5 April 2020. Of the 186 cases, seven were asymptomatic. Test seeking among the seven asymptomatic cases was primarily due to being a close contact (6/7). One asymptomatic case was tested due to having a recent travel history and wanting to confirm their COVID‐19 status before attending a family event.
From the first confirmed case to the onset of population lockdown (11 March to 25 March 2020; hereafter pre‐lockdown) COVID‐19 case numbers grew to a peak of 18 new cases per day (Figure 1 ). During the period from lockdown onset to the last confirmed COVID‐19 case in SDHB (26 March to 5 April 5 2020; hereafter during lockdown), a steady decline in new cases was observed. Alert level 4 lockdown restrictions remained in place in New Zealand until 26 April 2020.
Figure 1.
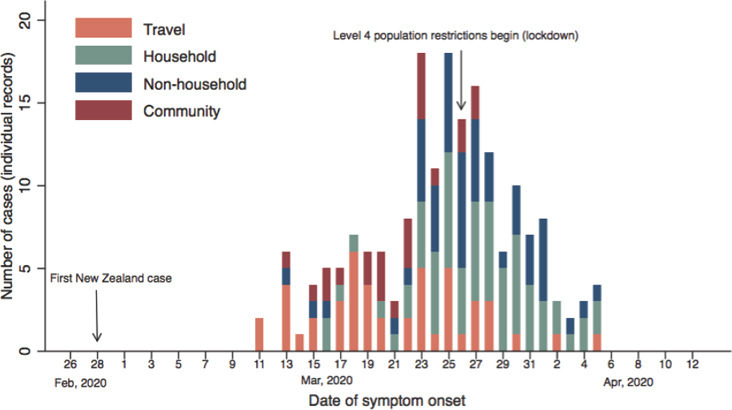
Temporal distribution of COVID‐19 cases in the Southern district, by source of infection. If no travel history or known exposure to a confirmed case or known outbreak, we assumed community transmission. COVID‐19: Coronavirus disease 2019.
The median case age was 36 years (Interquartile range [IQR] 25–55, Table 1 ). The proportion of cases among individuals younger than 15 years was low (4/186, 2%). There were approximately equal numbers of female and male cases (99 versus 87, respectively). Two cases were pregnant. Twenty‐four cases (24/186, 13%) reported underlying conditions, most commonly hypertension (8/24), asthma (7/24), cardiovascular disease (4/24) and diabetes (3/24).
Table 1.
Characteristics of laboratory‐confirmed COVID‐19 cases in SDHB by lockdown status.
| All (N=186) |
Pre‐lockdown (Mar 11–25; N=101) |
During lockdown (Mar 26–Apr 14; N=85) |
|
|---|---|---|---|
| Exposure history | |||
| Overseas travel within 14‐days before symptom onset | 47/185 (25%) | 37/100 (37%) | 10/85 (12%) |
| Close contact with another COVID‐19 case: household | 65/185 (35%) | 24/100 (24%) | 41/85 (48%) |
| Close contact with another COVID‐19 case: non‐household | 50/185 (27%) | 20/100 (20%) | 23/85 (35%) |
| Community: no travel or close contact with another COVID‐19 case | 23/185 (12%) | 19/100 (19%) | 4/85 (5%) |
| Age, years | 36 (25–55) | 39 (25–56) | 35 (23–55) |
| Age group, years | |||
| <1 | 0/186 (0%) | 0/101 (0%) | 0/85 (0%) |
| 1–4 | 0/186 (0%) | 0/101 (0%) | 0/85 (0%) |
| 5–14 | 4/186 (2%) | 1/101 (1%) | 3/85 (4%) |
| 15–24 | 38/186 (20%) | 18/101 (18%) | 20/85 (24%) |
| 25–44 | 62/186 (33%) | 36/101 (36%) | 26/85 (31%) |
| 45–64 | 70/186 (38%) | 39/101 (39%) | 31/85 (37%) |
| >64 | 12/186 (6%) | 7/101 (7%) | 5/85 (6%) |
| Sex | |||
| Female | 99/186 (53%) | 52/101 (51%) | 47/85 (55%) |
| Male | 87/186 (47%) | 49/101 (49%) | 38/85 (45%) |
| Ethnicity | |||
| Māori | 12/185 (7%) | 1/101 (1%) | 11/84 (13%) |
| Pacific Peoples | 2/185 (1%) | 0/101 (0%) | 2/84 (2%) |
| Asian | 5/185 (3%) | 2/101 (2%) | 3/84 (4%) |
| Middle Eastern / Latin American / African | 6/185 (3%) | 1/101 (1%) | 1/84 (1%) |
| European or Other | 160/185 (87%) | 67/101 (80%) | 67/84 (80%) |
Notes:
Data are median (IQR), or n/N (%). Lower denominations indicate missing data, excluded from the analysis. Percentages might not total 100% because of rounding. Table includes all cases (primary and secondary) residing in SDHB at time of infection. We allocated cases to the two time periods according to their symptom onset date. COVID‐19: Coronavirus disease 2019
Source of infection was ascertained for 185/186 (99%) cases. Overall, 65/185 (35%) confirmed cases were attributable to household transmission, 47/185 (25%) to recent overseas travel, 50/185 (27%) to non‐household close contact, and 23/185 (12%) to community transmission. No new cases of community transmission were recorded after two days of population lockdown.
The mean time from symptom onset to official reporting shortened as the outbreak progressed, decreasing from 6·8 days (95% Confidence interval [CI] 6·1–7·5) pre‐lockdown to 4·7 days (95%CI 4·1–5·4) during lockdown; difference: 2·0 days (95%CI 1·0–3·0, p<0·001). Eighty per cent of cases were isolated within four days of symptom onset. The median time from symptom onset to isolation reduced from 2·6 days (95%CI 1·7–3·5) pre‐lockdown to ‐1·6 days (95%CI ‐2·5–0·7) during lockdown, where negative days represent isolation before symptom onset.
As of 20 May 2020, two cases had died and the remaining 184 cases had recovered. We estimated that the median time to recovery was 19·9 days (95%CI 18·6–21·3 days). Five per cent of cases had recovered 10 days after symptom onset, and 95% after 39 days. Age, but not sex, was associated with time to recovery in parametric survival models, with recovery significantly shorter in younger adults. We estimated a median time to recovery of 16·7 days (95%CI 15·3–18·0) in individuals aged 25 years, and 23·8 days (95%CI 21·8–25·8) among those aged 54 years (representing the 25th and 75th percentiles of the observed age distribution, respectively).
We analysed the time between symptom onset (the serial interval) in 50 exclusive infector‐infectee pairs. The serial interval followed a gamma distribution with an estimated mean of 4·0 days (95%CI 3·2–4·7). When pairs were stratified by infector symptom onset in relation to lockdown, we found a serial interval of 5·0 days (95%CI 4·1–5·8) pre‐lockdown and 3·9 days (95%CI 2·6–5·3) during lockdown. Using interval‐censored lognormal regression on the same 50 case pairs used in the serial interval analysis we estimated the mean incubation period for COVID‐19 to be 3.4 days (95%CI 2·7–4·2) and that 95% of those who develop symptoms will do so within nine days of exposure. The median length of exposure to a case during their infectious period was 5 days (IQR 3–7 days, max 13 days). Setting the last possible exposure date as the day before symptom onset made little difference to the incubation period estimate (see Supplementary Materials).
Overall, 1,060 close contacts were identified for 183 COVID‐19 cases (three cases had no close contacts) in SDHB between 11 March and 5 April 2020. Among the 1,060 close contacts, 55 tested PCR‐positive for SARS‐CoV‐2 infection, with an overall secondary attack rate of 5·2% (95%CI 3·9–6·7, Table 2 ). Most cases (150/183, 82·0%) did not infect any of their close contacts with SARS‐CoV‐2 (Table 3 ); 19/183 (10·4%) infected one close contact, 10/183 (5·5%) infected two and 2/183 (1·1%) infected three. Two cases infected five of their close contacts. There was no significant difference in the median number of contacts between cases who transmitted onwards and those who did not (Wilcoxon rank‐sum test, z=‐1.235, p=0.430). Onward transmission was not predicated by case age or sex in logistic regression models. However, cases with community‐acquired infection were more likely to transmit SARS‐CoV‐2 to others than cases acquiring infection in other settings (Odds ratio [OR] 3.6, 95%CI 1.4–9.3, p=0.008). Cases with household‐acquired infection were less likely to transmit to others (OR 0·36, 95%CI 0.1–0.9, p=0.033).
Table 2.
Group‐specific attack rates and risk factors for confirmed SARS‐CoV‐2 infection among close contacts of SDHB cases.
| All |
Last contact with infector pre‐lockdown (before March 26) |
Last contact with infector during lockdown (after March 25) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of contacts | Number of secondary cases | Secondary attack rate, 95%CI | Number of contacts | Number of secondary cases | Secondary attack rate, 95%CI | Number of contacts | Number of secondary cases | Secondary attack rate, 95%CI | |
| Total | 1,060 | 55 | 5·2% (3·9–6·7) | 768/1060 | 24 | 3·1% (2·0–4·6) | 292/1060 | 31 | 10·6% (7·3–14·7) |
| Exposure setting | |||||||||
| Household | 277/1049 | 36 | 13·0% (9·3–17·5) | 101/766 | 9 | 8·9% (4·2–16·2) | 176/283 | 27 | 15·3% (10·4–21·5) |
| Non‐household | 771/1049 | 19 | 2·5% (1·5–3·8) | 665/766 | 15 | 2·3% (1·3–3·7) | 107/283 | 4 | 3·7% (1·0–9·3) |
| Age group, years | |||||||||
| <1 | 9/987 | 0 | 0·0% (0·0–33·6*) | 6/715 | 0 | 0·0% (0·0–45·9*) | 3/272 | 0 | 0·0% (0·0–70·8*) |
| 1–4 | 4/987 | 0 | 0·0% (0·0–60·2*) | 3/715 | 0 | 0·0% (0·0–70·8*) | 1/272 | 0 | 0·0% (0·0–97·5*) |
| 5–14 | 60/987 | 1 | 1·7% (0·0–8·9) | 38/715 | 1 | 2·6% (0·1–13·8) | 22/272 | 0 | 0·0% (0·0–15·4*) |
| 15–24 | 222/987 | 11 | 5·0% (2·5–8·7) | 156/715 | 2 | 1·3% (0·2–4·6) | 66/272 | 9 | 13·6% (6·4–24·3) |
| 25–44 | 317/987 | 22 | 6·9% (4·4–10·3) | 227/715 | 8 | 3·5% (1·5–6·8) | 90/272 | 14 | 15·6% (8·8–24·7) |
| 45–64 | 310/987 | 18 | 5·8% (3·5–9·0) | 254/715 | 11 | 4·3% (2·2–7·6) | 56/272 | 7 | 12·5% (5·2–24·1) |
| >64 | 65/987 | 2 | 3.1% (0·3–10·7) | 31/715 | 2 | 6·5% (0·8–21·4) | 34/272 | 0 | 0·0% (0·0–10·3*) |
| Sex | |||||||||
| Female | 522/1033 | 33 | 6·3% (4·4–8·8) | 378/754 | 14 | 3·7% (2·0–6·1) | 144/279 | 19 | 13·2% (8·1–19·8) |
| Male | 511/1033 | 21 | 4·1% (2·6–6·2) | 376/754 | 10 | 2·7% (1·3–4·8) | 135/279 | 11 | 8·1% (4·1–14·1) |
Note:
One‐sided, 97.5% confidence interval. Lower denominations indicate missing data, excluded from the analysis. Percentages might not total 100% because of rounding. Secondary cases include close contacts of SDHB cases who reside in other geographical areas. COVID‐19: Coronavirus disease 2019.
Table 3.
Characteristics of laboratory‐confirmed confirmed COVID‐19 cases in SDHB by onward transmission status.
| Transmitted onwards | No onward transmission | |
|---|---|---|
| Exposure history | ||
| Overseas travel within 14‐days before symptom onset | 6/46 (13.0) | 40/46 (87.0) |
| Close contact with another COVID‐19 case: household | 6/63 (9.5) | 57/63 (90.5) |
| Close contact with another COVID‐19 case: non‐household | 12/50 (24.0) | 38/50 (76.0) |
| Community: no travel or close contact with another COVID‐19 case | 9/23 (39.1) | 14/23 (60.9) |
| Age, years | ||
| Age group, years | ||
| <1 | 0/0 (0.0) | 0/0 (0.0) |
| 1–4 | 0/0 (0.0) | 0/0 (0.0) |
| 5–14 | 0/3 (0.0) | 3/3 (100.0) |
| 15–24 | 3/38 (7.9) | 35/38 (92.1) |
| 25–44 | 13/60 (21.7) | 47/60 (78.3) |
| 45–64 | 14/70 (20.0) | 56/70 (80.0) |
| >64 | 3/12 (25.0) | 9/12 (75.0) |
| Sex | ||
| Female | 17/33 (51.5) | 80/150 (53.3) |
| Male | 16/33 (48.5) | 70/150 (46.7) |
| Number of contacts | 6 (2–12) | 4 (2–11) |
Notes:
Data are median (IQR), or n/N (%). Lower denominations indicate missing data, excluded from the analysis. Percentages might not total 100% because of rounding. Table includes all cases (primary and secondary) residing in SDHB at time of infection. COVID‐19: Coronavirus disease 2019.
Most secondary infection (36/55, 65%) was related to household contact. The secondary attack rate among household contacts increased from 8·9% (95%CI 4·2–16·2) pre‐lockdown to 15·3% (95%CI 10·4–21·5) during lockdown. In univariate Poisson regression, lockdown and household exposure were each associated with a higher risk of secondary infection among close contacts (Relative Risk [RR] 3·4, 95%CI 2·0–5·8, p<0.005; and RR 5·3, 95%CI 3·0–9·2, p<0.005, respectively). When modelled together, only household contact remained a significant predictor of secondary infection (household RR 4·0, 95%CI 2·1–8.7, p<0.005; lockdown RR 1·7, 95%CI 0·9–3·2, p=0·09). There were no differences in infection risk by close contact age group or sex.
Discussion
COVID‐19 cases were predominantly spread across adult age brackets and a high proportion of transmission was locally acquired. In contrast, nationally, the highest proportion of cases was aged 20–34 years, and the main infection source was overseas acquisition.4 SDHB experienced the highest cumulative incidence of COVID‐19 cases in New Zealand, at 64·6 per 100,000 population (the national cumulative incidence being 30.3 per 100,000) during the study period.4 For the week ending 22 March 2020 (three days before lockdown onset), low levels of influenza‐like activity were observed in New Zealand.8
We estimated an incubation period of 3.4 days, based on case interview data from established, exclusive pairs with well‐defined, short exposure periods in a low‐prevalence population. Results from 14 meta‐analyses suggest an incubation period of five to six days.9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 Most published incubation period estimates, the basis of the meta‐analyses, are derived from studies of one of two exposure types – exposure to Wuhan23, 24, 25, 26, 27, 28 or to a confirmed COVID‐19 case.29, 30, 31, 32, 33, 34, 35, 36, 37, 38 Studies based on travel to Wuhan tend to report longer incubation periods than those based on close contact (with one exception, based on very early data39). For example, the Wuhan exposure studies of Backer et al.25 and Linton et al.26 report estimates of 6·4 and 5·6 days, respectively. Some, but not all,27 travel‐based studies are affected by wide exposure windows, which may increase their incubation period estimates. They also assume that infection was acquired in Wuhan. Where this assumption is violated, the incubation period will be overestimated.
Although potentially more accurate in exposure window determination, close contact studies based on low numbers may lack precision in their incubation period estimates. They may also be biased towards close and prolonged contact situations34 or the reporting of unusually virulent clusters. Huang et al.33 found a median incubation period of just two days among seven infected contacts with two‐hour exposures to a COVID‐19 case. Pung et al.34 reported a four‐day incubation period among 19 pairs linked to the first three clusters in Singapore. Ki et al.37 also report a four‐day incubation period estimate based on a 10‐case series in Korea. Our estimate, based on a greater number of direct transmission pairs than most published studies, also demonstrates a short incubation period. However, it may also be biased towards close and prolonged contact situations due to the high proportion of household pairs in our analysis. One study32 used a similar number of case pairs (n=49) to ours and estimated an incubation period of 5·2 days. Like us, they excluded cases with a travel history and used only pairs with confirmed epidemiological links. However, it is unclear whether the infector's infectious period was accounted for in their exposure window determination. If the start date of exposure occurred considerably before the onset of infectiousness in the infector, the incubation period estimate will be biased upward.
Cases with community‐acquired infection were the highest risk group for onward transmission. Household contacts were the highest risk group for secondary infection. Confining people in their home environment increases contact among household members. Attack rates were already higher for household contacts than in any other setting before lockdown, and during lockdown almost doubled, from 8% to 15% (although the difference was not statistically significant). Between‐country differences exist in reported household secondary attack rates. In Taiwan, a rate of 4·6% was observed,40 in China 11.2%23 and 12.4%41 and Korea 16.5%.42 Taiwan's low secondary attack rates may be due to their practice of admitting cases to isolation rooms.43 New Zealand adopted this strategy in its second wave response (from August 2020 onwards). Together, our onward transmission and secondary infection data suggest that community cases were infecting their households, but those household members were unlikely to infect others outside the household, likely due to the national lockdown.
Our data were collected during an ongoing outbreak response subject to changing protocols. Notably, COVID‐19 testing criteria expanded considerably during the outbreak. We may have missed early community‐transmission cases due to testing being limited to those with a travel history or contact with another case, although a later serology study in the SDHB suggests few were missed.44 Further, we recognised that community transmission was occurring earlier in SDHB than it was elsewhere in New Zealand due to tourism‐related operators reporting COVID‐19 symptoms and changed our local testing criteria accordingly.
COVID‐19 PCR testing among close contacts at the time of this outbreak was not mandatory. It is possible that some close contacts were infected with SARS‐CoV‐2 but did not seek testing or alert health authorities. Asymptomatic cases may have been missed in both the close contact and general population. Consequently, our secondary transmission rates generally represent only clinical cases and may be underestimated. In addition, not all close contacts of travel‐related cases were captured in our data and therefore secondary transmission rates may be overestimated in our non‐household group.
A further limitation with our close contact data is that close contacts were only recorded against one case. If a contact were exposed to both an infectious household member and an infectious workmate, they were recorded as a contact of the household case. This classification of doubly exposed contacts into household exposure may have inflated secondary attack rates in the household group. However, in multiple‐exposure situations, household transmission is likely to pose a greater risk due to the prolonged nature of contact within the home.
Although our data were gathered during two markedly different population control periods, the lack of lag period between the lockdown onset and peak daily new cases suggests that the SDHB population were already taking steps to prevent transmission. This may have been due to strong media reporting of COVID‐19 spread and impact overseas. The lockdown regulations were implemented early in the outbreak relative to the lockdowns in other countries.4 Our findings may not be generalizable to populations using different population control measures or at different stages of the epidemic. The less deprived nature of the SDHB population, and the higher proportion of non‐Māori and non‐Pacific Peoples may limit the generalizability of our findings to the rest of New Zealand.
SDHB cases were ascertained, managed, and recorded by our public health organisation. Our data are robust to potential biases in case ascertainment and data collection. We did not rely on third party information, but directly communicated with cases and their contacts. Where necessary, follow‐up interviews were conducted to resolve ambiguities or obtain complete information.
Our results give similar estimates of the serial interval to those found in the literature and reinforce the conclusion that most infections are caused by a minority. We show that the mean incubation period can be as short as 3.4 days when evaluated using well‐defined exposure windows and exclusive case pairs. This is a valuable reference for comparing incubation periods between SARS‐Cov‐2 strains. Our results also have important implications for public health measures. Our data support non‐pharmaceutical interventions such as lockdowns, physical distancing, and increased hygiene as preventing transmission between people not living together. The success of household‐based prevention measures depends on how quickly and effectively symptomatic individuals self‐isolate and the stringency of within‐house hygiene in the absence of symptoms. These measures will be particularly challenging in crowded households. Our research highlights the importance of effective home‐based quarantine guidance, especially in the context of lockdowns, during which secondary transmission risk may increase.
Footnotes
The authors have stated they have no conflicts of interest.
Supporting Information
Additional supporting information may be found in the online version of this article:
Supplementary Materials:
Case and contact definitions;
Case and contact management and surveillance;
Non‐pharmaceutical interventions;
Completeness of variables used in the study;
Incubation period;
Incubation period analysis.
References
- 1.World Health Organization . WHO; Geneva (CHE): 2020. Overview of Public Health and Social Measures in the Context of COVID‐19. [Google Scholar]
- 2.New Zealand Department of the Prime Minister and Cabinet . Government of New Zealand; Wellington (NZ): 2020. Unite Against COVID ‐ 19 ‐ Alert System Overview | Unite against COVID‐19 [Internet]https://covid19.govt.nz/alert-system/alert-system-overview/ [cited 2020 Sep 18]. Available from: [Google Scholar]
- 3.Jefferies S, French N, Gilkison C, et al. COVID‐19 in New Zealand and the impact of the national response: A descriptive epidemiological study. Lancet Public Health. 2020;5(11):e612–ee23. doi: 10.1016/S2468-2667(20)30225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Summers DJ, Cheng DH‐Y, Lin PH‐H, et al. Potential lessons from the Taiwan and New Zealand health responses to the COVID‐19 pandemic. Lancet Reg Health West Pac. 2020;4 doi: 10.1016/j.lanwpc.2020.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.New Zealand Department of the Prime Minister and Cabinet . Government of New Zealand; Wellington (NZ): 2020. Unite Against COVID‐19 ‐ Traffic Lights [Internet]https://covid19.govt.nz/traffic-lights/ [cited 2022 Jul 22]. Available from: [Google Scholar]
- 6.New Zealand Ministry of Health . Government of New Zealand; Wellington (NZ): 2019. Population of Southern District Health Board [Internet]https://www.health.govt.nz/new-zealand-health-system/my-dhb/southern-dhb/population-southern-dhb [cited 2020 Sep 21]. Available from: [Google Scholar]
- 7.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–723. [Google Scholar]
- 8.New Zealand Public Health Group . New Zealand Ministry of Health; Wellington (NZ): 2020. Flutracking Reports [Internet]https://info.flutracking.net/reports/new-zealand-reports/ [cited 2022 Jul 22]. Available from: [Google Scholar]
- 9.Xin H, Wong JY, Murphy C, et al. The incubation period distribution of coronavirus disease 2019 (COVID‐19): A systematic review and meta‐analysis. Clin Infect Dis. 2021;73(12):2344–2352. doi: 10.1093/cid/ciab501. [DOI] [PubMed] [Google Scholar]
- 10.Quesada JA, López‐Pineda A, Gil‐Guillén VF, et al. Incubation period of COVID‐19: A systematic review and meta‐analysis. Rev Clin Esp (Barc). 2021;221(2):109–117. doi: 10.1016/j.rceng.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alene M, Yismaw L, Assemie MA, et al. Serial interval and incubation period of COVID‐19: A systematic review and meta‐analysis. BMC Infect Dis. 2021;21(1):257. doi: 10.1186/s12879-021-05950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rai B, Shukla A, Dwivedi LK. Incubation period for COVID‐19: A systematic review and meta‐analysis. Z Gesundh Wiss. 2021:1–8. doi: 10.1007/s10389-021-01478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elias C, Sekri A, Leblanc P, et al. The incubation period of COVID‐19: A meta‐analysis. Int J Infect Dis. 2021;104:708–710. doi: 10.1016/j.ijid.2021.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li B, Zhang S, Zhang R, et al. Epidemiological and clinical characteristics of COVID‐19 in children: A systematic review and meta‐analysis. Front Pediatr. 2020;8 doi: 10.3389/fped.2020.591132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wassie GT, Azene AG, Bantie GM, et al. Incubation period of severe acute respiratory syndrome novel coronavirus 2 that causes coronavirus disease 2019: A systematic review and meta‐analysis. Curr Ther Res Clin Exp. 2020;93 doi: 10.1016/j.curtheres.2020.100607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pormohammad A, Ghorbani S, Khatami A, et al. Comparison of influenza type A and B with COVID‐19: A global systematic review and meta‐analysis on clinical, laboratory and radiographic findings. Rev Med Virol. 2021;31(3) doi: 10.1002/rmv.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie Y, Wang Z, Liao H, et al. Epidemiologic, clinical, and laboratory findings of the COVID‐19 in the current pandemic: Systematic review and meta‐analysis. BMC Infect Dis. 2020;20(1):640. doi: 10.1186/s12879-020-05371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAloon C, Collins Á, Hunt K, et al. Incubation period of COVID‐19: A rapid systematic review and meta‐analysis of observational research. BMJ Open. 2020;10(8) doi: 10.1136/bmjopen-2020-039652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khalili M, Karamouzian M, Nasiri N, et al. Epidemiological characteristics of COVID‐19: A systematic review and meta‐analysis. Epidemiol Infect. 2020;148 doi: 10.1017/S0950268820001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang T, Ding S, Zeng Z, et al. Estimation of incubation period and serial interval for SARS‐CoV‐2 in Jiangxi, China, and an Updated Meta‐Analysis. J Infect Dev Ctries. 2021;15:326–332. doi: 10.3855/jidc.14025. [DOI] [PubMed] [Google Scholar]
- 21.Dhouib W, Maatoug J, Ayouni I, et al. The incubation period during the pandemic of COVID‐19: A systematic review and meta‐analysis. Syst Rev. 2021;10(1):101. doi: 10.1186/s13643-021-01648-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He W, Yi GY, Zhu Y. Estimation of the basic reproduction number, average incubation time, asymptomatic infection rate, and case fatality rate for COVID‐19: Meta‐analysis and sensitivity analysis. J Med Virol. 2020;92:2543–2550. doi: 10.1002/jmv.26041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bi Q, Wu Y, Mei S, et al. Epidemiology and transmission of COVID‐19 in 391 cases and 1286 of their close contacts in Shenzhen, China: A retrospective cohort study. Lancet Infect Dis. 2020;20(8):911–919. doi: 10.1016/S1473-3099(20)30287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (CoVID‐19) from publicly reported confirmed cases: Estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Backer JA, Klinkenberg D, Wallinga J. Incubation period of 2019 novel coronavirus (2019‐ nCoV) infections among travellers from Wuhan, China, 20 28 January 2020. Euro Surveill. 2020;25(5) doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linton N, Kobayashi T, Yang Y, et al. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: A statistical analysis of publicly available case data. J Clin Med. 2020;9:538. doi: 10.3390/jcm9020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong T. Longer incubation period of coronavirus disease 2019 (COVID19) in older adults. Aging Med (Milton). 2020;3(2):102–109. doi: 10.1002/agm2.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viego V, Geri M, Castiglia J, et al. Incubation period and serial interval of Covid‐19 in a chain of infections in Bahia Blanca (Argentina) Cien Saude Colet. 2020;25(9):3503–3510. doi: 10.1590/1413-81232020259.20852020. [DOI] [PubMed] [Google Scholar]
- 30.Tian S, Hu N, Lou J, et al. Characteristics of COVID‐19 infection in Beijing. J Infect. 2020;80:401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Litvinova M, Wang W, et al. Evolving epidemiology and transmission dynamics of coronavirus disease 2019 outside Hubei province, China: A descriptive and modelling study. Lancet Infect Dis. 2020;20(7):793–802. doi: 10.1016/S1473-3099(20)30230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang L, Zhang X, Zhang X, et al. Rapid asymptomatic transmission of COVID‐19 during the incubation period demonstrating strong infectivity in a cluster of youngsters aged 16‐23 years outside Wuhan and characteristics of young patients with COVID‐19: A prospective contact‐tracing study. J Infect. 2020;80:e1–e13. doi: 10.1016/j.jinf.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pung R, Chiew CJ, Young BE, et al. Investigation of three clusters of COVID‐19 in Singapore: Implications for surveillance and response measures. Lancet. 2020;395:1039–1046. doi: 10.1016/S0140-6736(20)30528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capon A, Ousta D, Ferson M, et al. A multiple site community outbreak of COVID‐19 in Sydney, Australia. Aust N Z J Public Health. 2021;45:129–132. doi: 10.1111/1753-6405.13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Böhmer MM, Buchholz U, Corman VM, et al. Investigation of a COVID‐19 outbreak in Germany resulting from a single travel‐associated primary case: A case series. Lancet Infect Dis. 2020;20:920–928. doi: 10.1016/S1473-3099(20)30314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ki M, Task Force for 2019‐nCoV Epidemiologic characteristics of early cases with 2019 novel coronavirus (2019‐nCoV) disease in Korea. Epidemiol Health. 2020;42 doi: 10.4178/epih.e2020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS‐Cov-2) outside of Wuhan, China: Retrospective case series. BMJ. 2020;368 doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leung C. The difference in the incubation period of 2019 novel coronavirus (SARS‐CoV-2) infection between travelers to Hubei and non‐travelers: The need of a longer quarantine period. Infect Control Hosp Epidemiol. 2020;41(5):594–596. doi: 10.1017/ice.2020.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng H‐Y, Jian S‐W, Liu D‐P, et al. Contact tracing assessment of COVID‐19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020;180(9):1156–1163. doi: 10.1001/jamainternmed.2020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jing QL, Liu MJ, Zhang Z‐Bin, et al. Household secondary attack rate of COVID‐19 and associated determinants in Guangzhou, China: A retrospective cohort study. Lancet Infect Dis. 2020;20(10):1141–1150. doi: 10.1016/S1473-3099(20)30471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park SY, Kim YM, Yi S, et al. Coronavirus disease outbreak in call center, South Korea. Emerg Infect Dis. 2020;26:1666–1670. doi: 10.3201/eid2608.201274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin C, Mullen J, Braund WE, et al. Reopening safely – Lessons from Taiwan's COVID‐19 response. J Glob Health. 2020;10(2) doi: 10.7189/jogh.10.020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Craigie A, McGregor R, Whitcombe A, et al. SARS‐CoV‐2 antibodies in the Southern Region of New Zealand, 2020. Pathology. 2021;53(5):645–651. doi: 10.1016/j.pathol.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article:
Supplementary Materials:
Case and contact definitions;
Case and contact management and surveillance;
Non‐pharmaceutical interventions;
Completeness of variables used in the study;
Incubation period;
Incubation period analysis.


