Abstract
Background
In the era of antiretroviral therapy, HIV-positive patients have reduced mortality from HIV infection and increased morbidity from end stage heart failure. The number of HIV-positive heart transplantation recipients remains scant. Long-term survival has not been rigorously studied. We compared survival outcomes of heart transplantation in HIV-positive recipients to HIV-negative recipients.
Methods
Clinical data from all adult heart transplantations were extracted from the UNOS Dataset. The impact of recipient HIV status was analyzed with Cox proportional hazards modeling, 1:3 propensity score matching and Kaplan-Meier survival analysis.
Results
Seventy-five HIV-positive recipients and 29,848 HIV-negative recipients were identified. Race distributions differed between the recipient groups, with Blacks comprising a larger proportion of the HIV-positive recipient group (46.7% versus 20.9%, p<0.001). The mean year of transplant was significantly later in the HIV-positive recipient group. The rate of acute rejection in the HIV-positive group was higher than the HIV-negative group (38.7% versus 17.7%, p<0.001), as was rate of antirejection treatment administration such as IVIG or plasmapheresis (26.7% versus 10.4%, p<0.001). There was no difference in 30-day, 1-year, and 5-year survival of HIV-positive recipients versus HIV-negative recipients. Recipient HIV infection was not a significant covariate in predicting survival in a Cox proportional hazards model.
Summary
Short- and moderate-term survival following heart transplantation is similar in HIV-positive and HIV-negative recipients, although data are very limited. This suggests that HIV-positive recipients should not be excluded from transplant candidacy solely based upon HIV serostatus.
Introduction
Due to the major therapeutic revolution of combination antiretroviral therapy (ART), the lethality of human immunodeficiency virus (HIV) has significantly reduced.1,2 The gap in life expectancy in the United States between people with and without HIV has narrowed from 44 years in 1997 to 12 years in 2011.3 Five, 10, and 15 year survival after seroconversion are now 99%, 93%, and 89% respectively.4 As a result, cardiovascular disease and heart failure have become increasingly prevalent. The reason for a rise in heart failure among HIV patients is multifactorial, including direct myocardial effects of the virus itself5; high prevalence of contributory comorbidities such as hypertension, dyslipidemia and heavy alcohol consumption6; antiretroviral therapy7 and immune response.8 It is estimated that approximately 2,300 HIV positive patients are living with advanced heart failure.9
Cardiac transplantation in HIV-positive recipients has been extremely limited, with the first US case report published in 200310 and a few subsequent case series.9, 11 Modest case series have reported survival outcomes comparable to the general cardiac transplantation population).9 Despite this, HIV infection is considered a relative contraindication to transplantation at most centers.12 With the passage of the HIV Organ Policy Equity Act in 2013 legalizing the study of organ donation between HIV-positive donors and recipients, increased attention has been given to this topic within the transplantation field. Given the changing landscape, the purpose of this study was to compare survival outcomes of cardiac transplantation in HIV-positive recipients with HIV negative recipients.
Methods
Patient population and data collection
The United Network for Organ Sharing (UNOS) provided Standard Transplant Analysis and Research files containing de-identified donor and recipient data from October 1987 to March 2019. The database contains prospectively collected donor and recipient data for all organ transplants performed in the United States. The UNOS database was reviewed for all first-time heart transplant recipients between January 2005 and June 2019 and their donors. Multi-organ transplants, age under 18, and patients with missing survival data were excluded.
Patients were identified as HIV-positive if they were noted to have HIV positive serology at the time of transplant. This study was deemed exempt by Duke University’s Institutional Review Board.
Outcomes and statistics
Descriptive analysis of baseline donor and recipient characteristics were performed, stratified by recipient HIV status. Donor/recipient sex mismatch was defined as a female donor with male recipient. Baseline donor and recipient demographic data were described as percent (count) for categorical variables and median (interquartile range) for continuous variables. Unadjusted comparisons between cohorts were performed using the Wilcoxon rank sum test for continuous variables and the Pearson chi-squared test or Fisher’s exact test for categorical variables. The primary outcome was overall survival. Secondary outcomes of interest included length of hospital stay, acute rejection prior to discharge, and the occurrence of the following in the first 5 years following transplant: coronary artery disease diagnosis, end-stage renal disease requiring dialysis during follow-up, and hospitalizations during follow-up.
Unadjusted survival was estimated with the Kaplan-Meier method, and compared with the log-rank test. The adjusted association between HIV status and post-transplant survival was assessed using multivariable Cox proportional hazards modeling. Covariates were selected a priori based upon clinical experience, prior literature, and variable availability within the dataset and in addition to recipient HIV status included recipient age, sex, race/ethnicity, heart failure etiology, history of prior cardiac surgery, diabetes, pre-transplant mechanical circulatory support usage, donor/recipient sex mismatch, donor age, graft ischemic time, transplant era, and annualized heart transplant center volume. Linearity of continuous variables with the hazard of the outcome was confirmed using restricted cubic splines with 4 pre-specified knots based upon each variable’s distribution. Where linearity was violated, continuous variables were modeled using piecewise linear splines for ease of interpretation. At least a 10:1 ratio of events to degrees of freedom was maintained to prevent overfitting.
To further account for imbalances in baseline demographic characteristics between the two cohorts, a propensity score matching sensitivity analysis was undertaken by matching each HIV positive recipients to 3 HIV negative recipients using several covariates, which were selected according to similar studies and clinical expertise. Using HIV status as the exposure, an optimal 1:3 propensity score matching algorithm was performed without replacement using a nearest neighbor algorithm, where the closest match for each patient is chosen one at a time without trying to minimize a global distance measure, with a standard caliper width of 0.1 of the standard deviation of the propensity score (R packages: “matchit”, “optmatch”, R Foundation for Statistical Computing, Vienna, Austria). Variables included in the regression model were donor age, race, cause of death, diabetes, cocaine use, alcohol use, and cigarette use as well as recipient age, sex, race, medical condition, diabetes, IV antibiotic requirement, VAD use, inotropes, heart failure etiology, transplant year, and ischemic time.
Two-sided p-values <0.01 were considered statistically significant. Multivariable modeling was performed as complete case analyses. All statistical analyses were performed using R version 3.5.1.
Results
A total of 75-HIV positive recipients and a corresponding 29,848 HIV-negative recipients met inclusion criteria. There were no HIV-positive donors. Baseline demographic and clinical characteristics of donors and recipients are represented in Tables 1, 2, and Supplemental Table 1, respectively. There were no significant differences among the donors. The median length of follow-up time was 1.99 years (IQR, 0.56–4.75) in the HIV-positive recipients and 3.78 years (IQR, 1.05–7.04) in the HIV-negative recipients (p=0.001). Among the recipients, there was a significant difference in the distribution of race, with more HIV-positive recipients being Black compared to HIV-negative recipients (46.7% versus 20.9%, overall p<0.001). The mean year of transplant was significantly later in the HIV-positive recipient group [2016, interquartile range (IQR) 2014–2018 compared to the HIV-negative recipient group (2013, IQR 2009–2016). There were no other significant differences in demographic data, preoperative clinical acuity, preoperative mechanical support, distance from donor hospital to transplant center, or induction immunosuppression.
Table 1.
Donor Characteristics. Demographic and Clinical Characteristics of Heart Donors, Stratified by HIV Status of Recipient, January 2005 – June 2019
| Variable | HIV - recipient | HIV + recipient | p-value |
|---|---|---|---|
| (n=29,848) | (n=75) | ||
| Male gender | 21,183 (71.0%) | 55 (73.3%) | 0.747 |
| Donor age (median years, IQR) | 30 (22–40) | 33 (23–38) | 0.481 |
| Donor BMI (median kg/m2, IQR) | 26.3 (23.2–30.2) | 25.2 (23.0–29.8) | 0.308 |
| Donor ethnicity | 0.111 | ||
| White | 19,171 (64.2%) | 44 (58.7%) | |
| Black | 4,741 (15.9%) | 12 (16.0%) | |
| Hispanic | 4,997 (16.7%) | 19 (25.3%) | |
| Other | 939 (3.1%) | - | |
| Donor history | |||
| Cigarette use | 4,023 (13.5%) | 10 (13.3%) | 1.000 |
| Cocaine use | 5,468 (18.3%) | 21 (28.0%) | 0.044 |
| Alcohol abuse | 4,775 (16.0%) | 10 (13.3%) | 0.638 |
| Diabetes | 1,008 (3.4%) | 3 (4.0%) | 1.000 |
| LVEF | 60 (55–65) | 60 (59–65) | 0.514 |
| Inotrope use at procurement | 13590 (45.5%) | 24 (32.0%) | 0.025 |
| Donor cause of death | 0.012 | ||
| Anoxia | 7,602 (25.5%) | 28 (37.3%) | |
| Cerebrovascular/stroke | 5,787 (19.4%) | 10 (13.3%) | |
| Head trauma | 15,633 (52.4%) | 35 (46.7%) | |
| CNS tumor | 180 (0.6%) | 2 (2.7%) | |
| Other | 645 (2.2%) | - | |
| ABO blood type | 0.147 | ||
| A | 10,732 (36.0%) | 28 (37.3%) | |
| B | 3,301 (11.1%) | 11 (14.7%) | |
| AB | 646 (2.2%) | 4 (5.3%) | |
| O | 15,169 (50.8%) | 32 (42.7%) | |
| IQR, interquartile range; LVEF, left ventricular ejection fraction; CNS, central nervous system | |||
Table 2.
Recipient Characteristics. Demographic and Clinical Characteristics of Orthotopic Heart Transplant Recipients, Stratified by HIV Status of Recipient, January 2005 – June 2019
| Variable | HIV - recipient | HIV + recipient | p-value |
|---|---|---|---|
| (n=29,848) | (n=75) | ||
| Male sex | 22,114 (74.1%) | 60 (80.0%) | 0.301 |
| Sex mismatch | 4,165 (14.0%) | 13 (17.3%) | 0.499 |
| Age (median years, IQR) | 56 (46–63) | 50 (38–61) | 0.004 |
| BMI (median kg/m2, IQR) | 27.0 (23.7–30.6) | 27.3 (24.4–30.0) | 0.788 |
| Ethnicity | <0.001 | ||
| White | 19,817 (66.4%) | 31 (41.3%) | |
| Black | 6,237 (20.9%) | 35 (46.7%) | |
| Hispanic | 2,456 (8.2%) | 7 (9.3%) | |
| Other | 1,338 (4.5%) | 2 (2.7%) | |
| Recipient history | |||
| Diabetes | 8,277 (27.7%) | 15 (20.0%) | 0.172 |
| Malignancy | 2,339 (7.8%) | 4 (5.3%) | 0.555 |
| Cerebrovascular disease | 1,629 (5.5%) | 3 (4.0%) | 0.764 |
| Heart failure etiology | 0.003 | ||
| Ischemic | 9,505 (31.8%) | 14 (18.7%) | |
| Non-ischemic dilated | 14,512 (48.6%) | 51 (68.0%) | |
| Other | 5,831 (19.5%) | 10 (13.3%) | |
| Recipient creatinine (median mg/dL, IQR) | 1.2 (1.0–1.5) | 1.2 (1.0–1.5) | 0.739 |
| Recipient bilirubin (median mg/dL, IQR) | 0.7 (0.5–1.2) | 0.6 (0.4–1.1) | 0.088 |
| Pre-transplant status | 0.262 | ||
| Intensive care unit | 9,159 (30.7%) | 19 (25.3%) | |
| Hospitalized (non-ICU) | 4,823 (16.2%) | 17 (22.7%) | |
| Not hospitalized | 15,863 (53.2%) | 39 (52.0%) | |
| Medical therapy | |||
| IV antibiotics in two weeks before transplant | 3,087 (10.3%) | 9 (12.0%) | 0.779 |
| IV inotropes prior to transplant | 11,470 (38.4%) | 20 (26.7%) | 0.049 |
| Ventilator support prior to transplant | 440 (1.5%) | 4 (5.3%) | 0.022 |
| Durable LVAD support prior to transplant | 10,425 (34.9%) | 29 (38.7%) | 0.577 |
| Temporary MCS prior to transplant | 3,692 (12.4%) | 17 (22.7%) | 0.011 |
| IABP | 2,985 (10.0%) | 12 (16.0%) | 0.125 |
| ECMO | 421 (1.4%) | 2 (2.7%) | 0.667 |
| Temporary VAD | 520 (1.7%) | 4 (5.3%) | 0.054 |
| ABO blood type | 0.591 | ||
| A | 12,071 (40.4%) | 29 (38.7%) | |
| B | 4,445 (14.9%) | 15 (20.0%) | |
| AB | 1,659 (5.6%) | 5 (6.7%) | |
| O | 11,673 (39.1%) | 26 (34.7%) | |
| Days on waitlist (median days, IQR) | 90 (26–258) | 87 (33–227) | 0.683 |
| Waitlist status at transplant | - | ||
| Old 1A | 16,414 (55.0%) | 42 (56.0%) | |
| Old 1B | 9,418 (31.6%) | 16 (21.3%) | |
| Old 2 | 2,029 (6.8%) | 3 (4.0%) | |
| New 1 | 168 (0.6%) | 1 (1.3%) | |
| New 2 | 887 (3.0%) | 7 (9.3%) | |
| New 3 | 504 (1.7%) | 1 (1.3%) | |
| New 4 | 352 (1.2%) | 3 (4.0%) | |
| New 5 | 8 (0.0%) | - | |
| New 6 | 68 (0.2%) | 2 (2.7%) | |
| Graft ischemic time (median hours, IQR) | 3.2 (2.4–3.8) | 3.4 (2.7–3.9) | 0.224 |
| Distance from donor hospital to transplant center (median miles, IQR) | 91 (13–287) | 164 (18–404) | 0.053 |
| Antibody-based induction immunosuppression | 15,177 (50.8%) | 33 (44.0%) | 0.285 |
| Year of transplant (median, IQR) | 2013 (2009–2016) | 2016 (2014–2018) | <0.001 |
| Length of follow up (median years, IQR) | 3.78 (1.05–7.04) | 1.99 (0.56–4.75) | 0.001 |
| IQR, interquartile range; BMI, body mass index; ICU, intensive care unit; IV, intravenous; LVAD, left ventricular assist device; MCS, mechanical circulatory support; IABP, intra-aortic balloon pump; ECMO, extracorporeal membrane oxygenation; VAD, ventricular assist device | |||
Table 3 displays the unadjusted transplant outcomes by HIV status. HIV-positive recipients had significantly longer lengths of hospital stay compared to their HIV-negative peers (18 days, IQR 12–31; 15 days, IQR 11–23 respectively). The rate of acute rejection during initial hospitalization in the HIV-positive recipient group (38.7%) was higher than the HIV-negative recipient group (17.7%, p<0.001), as was rate of antirejection treatment administration such as IVIG or plasmapheresis (26.7% versus 10.4%, p<0.001). There were no differences in coronary artery disease at follow up, renal failure, postoperative rehospitalization for rejection or infection.
Table 3.
Selected Short-Term Unadjusted Clinical Outcomes of Orthotopic Heart Transplant Recipients January 2005 – June 2019, Stratified by Recipient HIV Status
| Variable | HIV - recipient | HIV + recipient | p-value |
|---|---|---|---|
| (n=29,848) | (n=75) | ||
| Length of hospital stay (days, median [IQR]) | 15 (11–23) | 18 (12–31) | 0.006 |
| Acute rejection episode prior to discharge | 5,286 (17.7%) | 29 (38.7%) | <0.001 |
| Treated with antirejection medication | 3,091 (10.4%) | 20 (26.7%) | <0.001 |
| Coronary artery disease at follow up | 6,824 (22.9%) | 10 (13.3%) | 0.068 |
| End stage renal disease requiring dialysis | 1,874 (6.3%) | 4 (5.3%) | 0.921 |
| Hospitalized during follow-up | 16,077 (53.9%) | 35 (46.7%) | 0.257 |
| For rejection | 3,617 (12.1%) | 6 (8.0%) | 0.360 |
| For infection | 7,534 (25.2%) | 10 (13.3%) | 0.025 |
| IQR, interquartile range | |||
A Cox proportional hazards model was created (Table 4) to adjust for potential independent predictors of survival and confounders. HIV infection was not associated with an increased risk of mortality. Other identified independent predictors of worse survival included higher donor age, higher recipient age, Black recipients, non-ischemic dilated cardiomyopathy etiology, recipient diabetes, recipient ECMO support prior to transplant, recipient prior cardiac surgery, graft ischemic time, transplant era 2005–2009, and lower transplant annualized volume.
Table 4.
Cox Proportional Hazards Model Results of Post Transplant Survival, January 2005 – June 2019
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Predictor | Hazard Ratio | Lower | Upper | p-value |
| HIV positive recipient | 0.94 | 0.53 | 1.65 | 0.820 |
| Donor age (per 5 years) | 1.06 | 1.05 | 1.07 | <0.001 |
| Recipient age | ||||
| <45 (per 5 years) | 0.91 | 0.89 | 0.93 | <0.001 |
| >45 (per 5 years) | 1.20 | 1.16 | 1.24 | <0.001 |
| Recipient male sex | 0.96 | 0.91 | 1.02 | 0.181 |
| Recipient ethnicity (reference: White) | ||||
| Black | 1.32 | 1.24 | 1.40 | <0.001 |
| Hispanic | 1.00 | 0.91 | 1.10 | 0.995 |
| Other | 0.96 | 0.85 | 1.09 | 0.550 |
| Donor/recipient sex mismatch | 1.05 | 0.98 | 1.13 | 0.147 |
| Recipient heart failure etiology (reference: ischemic) | ||||
| Non-ischemic dilated | 0.79 | 0.75 | 0.84 | <0.001 |
| Other | 0.98 | 0.89 | 1.02 | 0.13 |
| Recipient diabetes | 1.27 | 1.20 | 1.33 | <0.001 |
| Recipient durable LVAD support | 1.07 | 1.01 | 1.14 | 0.016 |
| Recipient ECMO support | 1.95 | 1.61 | 2.37 | <0.001 |
| Recipient temporary VAD support | 1.21 | 1.00 | 1.45 | 0.048 |
| Recipient IABP support | 1.06 | 0.98 | 1.16 | 0.161 |
| Recipient prior cardiac surgery | 1.13 | 1.07 | 1.19 | <0.001 |
| Graft ischemic time (per hour) | 1.08 | 1.06 | 1.11 | <0.001 |
| Transplant era (reference: 2005–2009) | ||||
| 2010–2014 | 0.85 | 0.78 | 0.88 | <0.001 |
| 2015–2019 | 0.82 | 0.77 | 0.89 | <0.001 |
| Transplant center annualized volume | ||||
| <20 (per 5 transplants) | 0.93 | 0.90 | 0.96 | <0.001 |
| >20 (per 5 transplants) | 1.07 | 1.04 | 1.11 | <0.001 |
| LVAD, left ventricular assist device; ECMO, extracorporeal membrane oxygenation; VAD, ventricular assist device; IABP, intra-aortic balloon pump | ||||
Thirty-day, 1-year, and 5-year survival of the HIV-positive recipient cohort was 96.0%, 88.1%, and 80.2%, respectively; survival in the HIV-negative recipient cohort survival was 96.1%, 90.1%, and 78.2%, respectively. Kaplan-Meier survival analysis (Figure 1) demonstrated no difference in survival between the HIV-positive and HIV-negative recipient groups.
Figure.1.
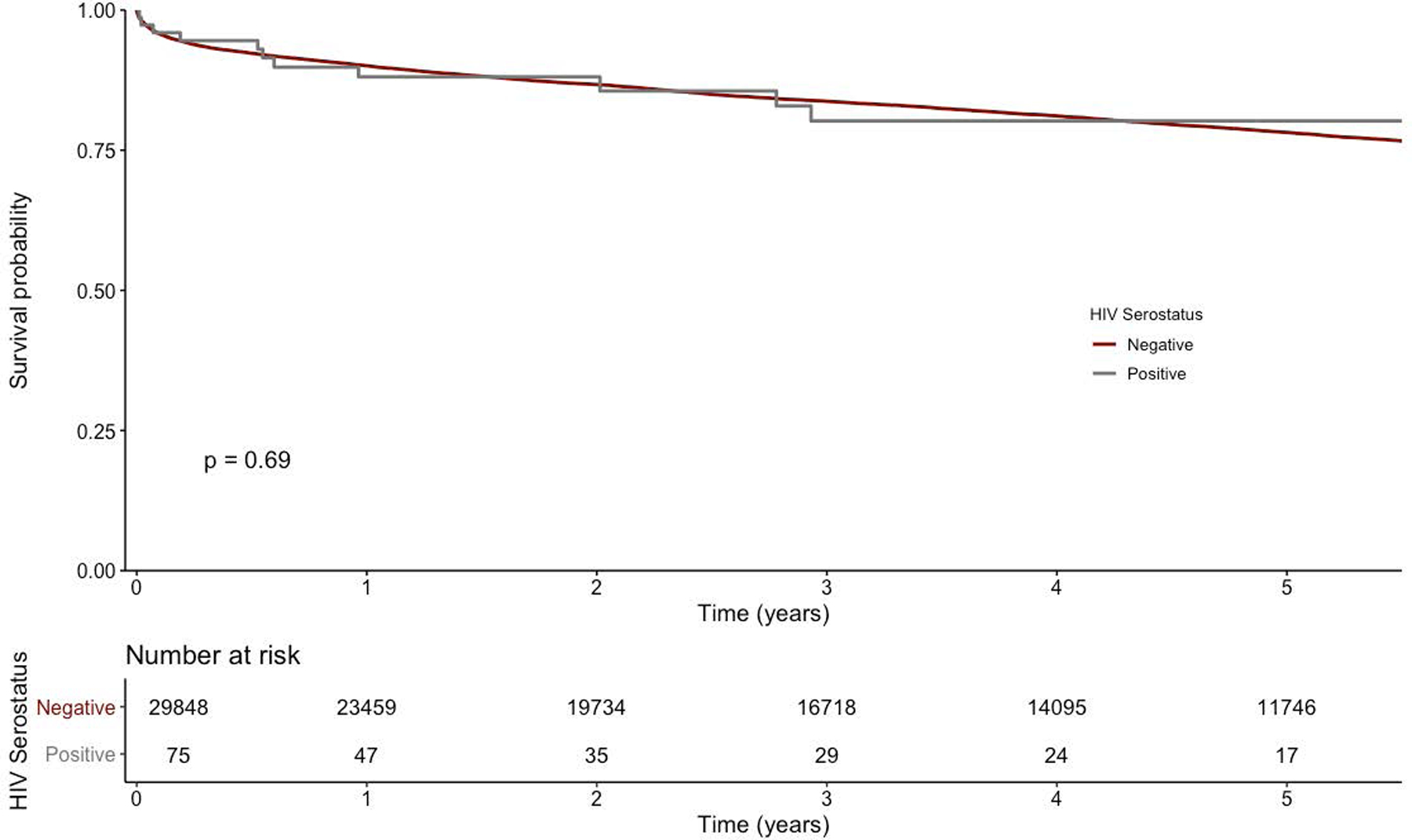
Kaplan-Meier curve for orthotopic heart transplant patients stratified by recipient HIV status. P value represents the two-sided log-rank test. Numbers at risk are provided at the bottom of the graph.
One:three propensity score matching was undertaken to compare HIV-positive recipients with matched HIV-negative controls to assess risk of mortality between the two groups (Supplemental Tables 2, 3, and 4). For the selected group of HIV-negative matched controls, rate of ICU status prior to transplant was higher (45.8%) compared to the HIV-positive recipient group (25.3%) and year of transplant was later (2019, IQR 2008–2019) compared to the HIV-positive recipient group (2016, IQR 2014–2018).
Comment
Heart failure in patients with HIV has become more common.13 In the ART era, the more distinctive HIV-related severe dilated cardiomyopathy has become less common, mirroring decreases in other forms of severe organ function uniquely characteristic of HIV. However, heart failure that is more typical in pathophysiology has become more common overall. 13 In the pre-ART era, 10% of patients with HIV died of cardiac disease, while the incidence in the ART era is closer to 25%. In the ART era, diastolic dysfunction predominates, occurring in 43–50% of cases compared to systolic dysfunction in only 8%.12,14–16
In this retrospective analysis of the Thoracic UNOS database, we analyzed survival outcomes of HIV-positive heart transplant recipients compared with all HIV-negative recipients as well as with a 1:3 propensity matched control group. The 1- and 5-year survival rates were comparable; no significant difference in survival between the groups was appreciated and HIV status was found to not be an independent predictor of mortality risk. Compared to the general heart transplantation population as reported in from the ISHLT Registry, the HIV-positive recipient population had a younger median age, higher proportion of Black patients, and a higher proportion of non-ischemic dilated cardiomyopathy etiology.
Our findings suggest that HIV infection in an otherwise healthy transplant recipient would not significantly impact survival. These findings are supported by previously published case series of HIV-positive heart transplant recipients.9 In a case series of 18 HIV-positive heart recipients, Uriel et al reported a slightly lower pre-transplant VAD rate (38%) with survival comparable to the general heart transplant recipient population (1-, 2-, and 5-year survival 100%, 100%, and 63% respectively). A prior case series of 7 recipients showed similar survival results.11
We report a higher rate of initial acute rejection in the HIV-positive recipient group, which has not been well described elsewhere in the heart transplantation literature. These findings are similar to that described in the kidney transplant literature, in which the HIV-infected cohort of kidney recipients had a higher-than-expected rate of rejection by a factor of 2 to 3.17 This may in part be explained by a high rate of alternative dosing regimens noted in these patients. Other potentially significant factors could include the use of antiretrovirals that affect cytochrome P-450–3A metabolizing enzymes18; transmission of leukocyte antigen molecules of the host to another host, thus inducing allosensitization19; increased responsiveness of T cells in HIV infection and nonspecific enhancement of alloimmunity20; and the role of memory alloreactive T cells.21,22 In our study there was no difference in rate of hospitalization for subsequent rejection episodes, suggesting that the long-term clinical impact of the acute rejection may be modest. Our UNOS data lacks sufficient granularity to indicate the immunologic status of recipients that could support or refute these theories. Close study of post-transplant immunologic function in this population in the future is warranted.
Despite an increase in the number of HIV infected patients comprising the overall heart failure population, HIV infection is still considered a relative contraindication to transplant at most centers.12 A survey of US and Canadian transplant centers conducted by Uriel et al in 2014 found that 57% reported HIV infection to be an absolute contraindication to listing; rationales included 1) perception of HIV-positive patients as high risk recipients to be avoided given scarce organ supply; 2) concern for immunosuppression-triggered progression of HIV to AIDS, and 3) drug interactions which could worsen outcomes. Fifty-nine percent of transplant centers reported that high risk donors should be avoided given organ scarcity. Given that we were able to 1:3 match HIV negative recipients with a risk profile similar to those who were HIV-positive, and that survival outcomes were comparable to the general heart transplantation population, one could argue that organ donation in high risk recipients is already readily occurring with acceptable results. With respect to the concern that immunosuppression required for heart transplantation could trigger progression to AIDS: although our data do not contain this level of detail on post-transplant AIDS or concomitant opportunistic infections, in the kidney transplant experience, HIV remained stable post-transplantation with few HIV-associated complications.17 Lastly, concerning potential drug interactions potentially worsening clinical outcomes: this theory remains valid given the elevated rejection rates seen in the kidney population, which may in part reflect the impact of certain antiretrovirals on cytochrome P-450 metabolism.
There are several limitations to our study. We do not know how many HIV-positive patients were screened prior to arriving at the final group of candidates that ultimately received transplantation; presumably, the HIV-positive recipient cohort is a highly selected subgroup of the HIV positive population overall. Given the nature of how UNOS data are reported, we were unable to obtain any information about the HIV-positive patients that were deemed inappropriate for transplant listing. Each center may also have a unique screening process which is not described in this study. As alluded to prior, we were unable to examine outcomes based on typical markers of HIV disease severity, namely CD4 count, viral load via RT-PCR, degree of immunosuppression, rejection grade, specific rejection therapy received, and allograft dysfunction.
Furthermore the quality of the outcomes data is highly dependent upon the quality of the input data from each reporting center. Given the average year of transplant was later in the HIV-positive recipient group, clinical outcomes such as renal failure and coronary artery disease may be favorably biased toward that group because the reporting time was shorter. Additionally, the quantification of risk that is required to perform propensity score matching may not accurately reflect the complexity of all of the clinically relevant covariates that each transplant recipient represents, thus running the risk of oversimplification. Though the propensity score matching did not result in perfect matching, it is unlikely that this would have significantly impacted the results, given that the Cox proportional hazards model results supported the same conclusions.
Finally, the outcomes from this study are based on subjects transplanted under prior heart transplant allocation guidelines, and there was insufficient data to fully re-categorize all studied recipients into the current allocation scheme. Early studies have demonstrated an increased use of temporary mechanical circulatory support in the current allocation era.23 Due to the sample size of this study, we cannot exclude the possibility of an interaction between use of temporary mechanical support and HIV status in post-transplant outcomes. Temporary mechanical support use was factored into our propensity match and multivariable analysis, and as expected, was a risk factor for mortality, while HIV status did not impact outcomes after heart transplantation.
Conclusion
As HIV survivorship continues to improve, there will be an increasing population of HIV-positive patients with heart failure, and some that progress to end stage heart failure. More research is required to understand the post-transplant outcomes in HIV-positive recipients, and to more closely examine how rejection occurs as it does in renal transplant recipients. Infectious and neoplastic processes need to be examined closely as longer-term survival data are gathered. These results suggest that heart transplantation in HIV-positive recipients confers a higher rate of initial acute rejection, but the same survival as seen in similar patients that are HIV-negative. Preliminary analyses indicate that this is an underrepresented population. We conclude that HIV infection should not, in isolation, be considered an absolute contraindication to transplant.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health [5T32HL069749 to Dr. Jawitz and FT32CA093245 to Dr. Raman].
Footnotes
This manuscript was presented at the 56th Annual Meeting of the Southern Thoracic Surgical Association in Marco Island, FL in November 2019
Disclosures
None declared.
References
- 1.Aguero F, Castel MA, Cocchi S, et al. An update on heart transplantation in HIV-infected patients. Am J Transplantation 2015; 16: 21–18. [DOI] [PubMed] [Google Scholar]
- 2.Kendall CE, Wong J, Taljaard M, et al. A cross‐sectional, population‐based study measuring comorbidity among people living with HIV in Ontario. BMC Public Health 2014; 14: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcus JL, Chao CR, Leyden WA et al. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. JAIDS Sept 2016(73):39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ewings FM, Bhaskaran K, McLean K, et al. Survival following HIV infection of a cohort followed up from seroconversion in the UK. AIDS 2008;22:89–95. [DOI] [PubMed] [Google Scholar]
- 5.Butt AA, Chang CC, Kuller L et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med 2011. Apr 25;171(8):737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laonigro I, Correale M, Di Biase M, Altomare E. Alcohol abuse and heart failure. Eur J Heart Fail 2009;11(5):453–62. [DOI] [PubMed] [Google Scholar]
- 7.Dalakas MC, Illa I, Pezeshkpour GH, et al. Mitochondrial myopathy caused by long-term zidovudine therapy. N Engl J Med 1990;322(16):1098–105. [DOI] [PubMed] [Google Scholar]
- 8.Herskowitz A, Wu TC, Willoughby SB, et al. Myocarditis and cardiotropic viral infection associated with severe left ventricular dysfunction in late-stage infection with human immunodeficiency virus. J Am Coll Cardiol 1994;24:1025–32. [DOI] [PubMed] [Google Scholar]
- 9.Uriel N, Nahumi N, Colombo PC, et al. Advanced heart failure in patients infected with human immunodeficiency virus: Is there equal access to care? J Heart Lung Transplant 2014;33:924–30. [DOI] [PubMed] [Google Scholar]
- 10.Calabrese LH, Albrecht M, Young J et al. Successful Cardiac Transplantation in an HIV-1-Infected patient with advanced disease. New Engl J Med 2003;348:2323–8. [DOI] [PubMed] [Google Scholar]
- 11.Uriel N, Jorde UP, Cotarlan V, et al. Heart transplantation in human immunodeficiency virus–positive patients. J Heart Lung Transplant 2009;28:667–9. [DOI] [PubMed] [Google Scholar]
- 12.Cerrato E, D’Ascenzo F, Biondi‐Zoccai G, et al. Cardiac dysfunction in pauci symptomatic human immunodeficiency virus patients: A meta‐analysis in the highly active antiretroviral therapy era. Eur Heart J 2013;34:1432–6. [DOI] [PubMed] [Google Scholar]
- 13.Remick J, Georgiopoulou V, Marti C et al. Heart failure in patients with human immunodeficiency virus infection: epidemiology, pathophysiology, treatment, and future research. Circulator 2014. Apr;129(17):1781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reinsch N, Neuhaus K, Esser S et al. German Competence Network for Heart Failure; German Competence Network for HIV AIDS, Prevalence of cardiac diastolic dysfunction in HIV-infected patients: results of the HIV-HEART study. HIV Clin Trials 2010;11(3):156–62. [DOI] [PubMed] [Google Scholar]
- 15.Hsue PY, Hunt PW, Ho JE et al. Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail 2010;3(1):132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher SD, Easley KA, Orav EJ et al. Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection (P2C2 HIV) Study Group, Mild dilated cardiomyopathy and increased left ventricular mass predict mortality: the prospective P2C2 HIV Multicenter Study. Am Heart J 2005;150(3):439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stock PG, Barin B, Murphy B et al. Outcomes of kidney transplantation in HIV-infected recipients. N Engl J Med 2010;363:2004–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frassetto L, Baluom M, Jacobsen W, et al. Cyclosporine pharmacokinetics and dosing modifications in human immunodeficiency virus-infected liver and kidney transplant recipients. Transplantation 2005;80:13–17 [DOI] [PubMed] [Google Scholar]
- 19.Langlois AJ, Weinhold KJ, Matthews TJ, Greenberg ML, Bolognesi DP. Detection of anti-human cell antibodies in sera from macaques immunized with whole inactivated virus. AIDS Res Hum Retroviruses 1992;8:1641–52. [DOI] [PubMed] [Google Scholar]
- 20.Wu Z, Bensinger SJ, Zhang J, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med 2004;10:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang HY, Dundon PL, Nahill SR, Welsh RM. Virus-induced polyclonal cytotoxic T lymphocyte stimulation. J Immunol 1989;142:1710–8. [PubMed] [Google Scholar]
- 22.Selin LK, Brehm MA, Naumov YN, et al. Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity. Immunol Rev 2006;211:164–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cogswell R, John R, Estep JD et al. An early investigation of outcomes with the new 2018 donor heart allocation system in the United States. J Heart Lung Transplant 2020. Jan;39(1):1–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


