Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) variants of concern (VOCs) have prolonged coronavirus disease 2019 (COVID‐19) pandemic by escaping pre‐existing immunity acquired by natural infection or vaccination. Elucidation of VOCs' mutation trends and evasion of neutralization is required to update current control measures. Mutations and the prevalence of VOCs were analyzed in the global immunization coverage rate context. Lentivirus‐based pseudovirus neutralization analysis platforms for SARS‐CoV‐2 prototype strain (PS) and VOCs, containing Alpha, Beta, Gamma, Delta, and Omicron, were constructed based on the spike protein of each variant and HEK 293T cell line expressing the human angiotensin‐converting enzyme 2 (hACE2) receptor on the surface, and an enhanced green fluorescent protein reporter. Serum samples from 65 convalescent individuals and 20 WIBP‐CorV vaccine recipients and four therapeutic monoclonal antibodies (mAbs) namely imdevimab, casirivimab, bamlanivimab, and etesevimab were used to evaluate the neutralization potency against the variants. Pseudovirus‐based neutralization assay platforms for PS and VOCs were established, and multiplicity of infection (MOI) was the key factor influencing the assay result. Compared to PS, VOCs may enhance the infectivity of hACE2‐293T cells. Except for Alpha, other VOCs escaped neutralization to varying degrees. Attributed to favorable and emerging mutations, the current pandemic Omicron variant of all VOCs demonstrated the most significant neutralization‐escaping ability to the sera and mAbs. Compared with the PS pseudovirus, Omicron had 15.7‐ and 3.71‐fold decreases in the NT50 value (the highest serum dilution corresponding to a neutralization rate of 50%); and correspondingly, 90% and 43% of immunization or convalescent serum samples lost their neutralizing activity against the Omicron variant, respectively. Therefore, SARS‐CoV‐2 has evolved persistently with a strong ability to escape neutralization and prevailing against the established immune barrier. Our findings provide important clues to controlling the COVID‐19 pandemic caused by new variants.
Keywords: COVID‐19, immune escape, neutralizing antibodies, SARS‐CoV‐2, VOCs
1. INTRODUCTION
The worldwide pandemic of severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) and its disease coronavirus disease 2019 (COVID‐19) has lasted more than 2 years, seriously threatening global public health and economic development. 1 To conquer the pandemic, at least 16 vaccines and 9 monoclonal antibodies (mAbs) have been developed based on the spike protein (S) of the SARS‐CoV‐2 prototype strain (PS) and approved for vaccination or treatment at unprecedented rates. 1 , 2 The impression that the pandemic will end soon is starting to emerge, and public health interventions have been even liberalized in an increasing number of countries and regions. However, since the SARS‐CoV‐2 variant carrying the D614G mutation was found in February 2020, 3 many more mutations in the receptor‐binding domain (RBD) of the S protein have occurred and have significantly changed the viral characteristics. Variants of concern (VOCs) have resulted in subsequent waves of pandemics, 4 , 5 such as B.1.1.7 (Alpha), 6 B.1.617.2 (Delta), 7 B.1.1.529 (Omicron), 8 and the latest emerging Omicron sublineages BA.2.12.1, BA.4, and BA.5. 9 , 10 Evidently, there is an urgent need to update current control measures for pandemics caused by these VOCs and future potential variants.
Neutralizing antibodies (nAbs) play decisive roles in preventing reinfection and underscoring clinical COVID‐19 disease progression. 11 , 12 , 13 Our previous study confirmed that nAbs persist in the sera of convalescent individuals for at least 1 year following natural infection. 13 Immunization with different vaccines could induce nAb response with varying intensity. 14 Furthermore, the quantitative nAb titer has been considered an alternative biomarker for evaluating the efficacy of the COVID‐19 vaccine. 15 Several studies reported that VOCs showed variable abilities to escape pre‐existing immunity acquired after infection or vaccination. 16 , 17 , 18 , 19 , 20 However, the mutation trends and the abilities of VOCs to escape neutralizing activity during the pandemic remain unknown. To this end, lentivirus‐based pseudovirus neutralization assay platforms for VOCs were first constructed respectively. Differential neutralizing efficacies of convalescent and WIBP‐CorV‐vaccinated sera and therapeutic mAbs against different pseudoviruses were then evaluated and the abilities of VOCs to escape neutralization were correlated with their mutations with the pandemic to demonstrate their mutation trends, to provide a scientific basis for developing effective strategies to prevent and control the pandemic of mutant strains.
2. MATERIALS AND METHODS
2.1. Serum samples
Sixty‐five serum samples were collected from convalescent individuals enrolled in Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology between March and April 2021, approximately 1 year after SARS‐CoV‐2 infection. In addition, sera from healthy controls were collected as previously described. 13 , 21 Healthy individuals were defined as those with negative test results for SARS‐CoV‐2 specific nucleic acid and antibodies without any significant clinical symptoms or epidemiological exposure history. Twenty serum samples were obtained from vaccine recipients enrolled 1 month after receiving two doses of an inactivated vaccine (WIBP‐CorV) between June and July 2021. The vaccine recipients had no prior SARS‐CoV‐2 infection or autoimmune diseases and did not take immunosuppressive drugs. Serum samples were stored at −80°C until use. The study protocols were approved respectively by the Ethics Committee of Tongji Hospital (IRB ID: TJ‐IRB20210137) and Southern University of Science and Technology Hospital, Shenzhen, China (IRB ID: NKDYY2022‐001). Written informed consent was obtained from all the participants.
2.2. Mutation and prevalence of SARS‐CoV‐2 VOCs
Based on SARS‐CoV‐2 sequence data in GISAID between July 1, 2020, and May 5, 2022, the frequencies of all mutation positions in the S protein of VOCs were analyzed using the Analyze Align platform. The prevalence of VOCs and mutations was analyzed using CovGlobe (https://covglobe.org/) and CoronaTrend (https://coronatrend.live/), respectively. Vaccination coverage data were obtained from Our World in Date (https://ourworldindata.org/covid-vaccinations).
2.3. Construction of cells and pseudoviruses
Human angiotensin‐converting enzyme 2 (hACE2)‐293T cells were constructed by stably expressing the hACE2 receptor on the surface of HEK293T cell lines (Genomeditech). hACE2‐293T cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and puromycin (60210ES25; YEASEN; 0.75 μg/ml) at 37°C and 5% CO2.
Pseudoviruses were constructed using lentiviral vector packaging systems (Genomeditech). Sequences coding for the S protein of SARS‐CoV‐2 PS and VOCs (Alpha, Beta, Gamma, Delta, and Omicron) were designed, synthesized, and used to replace the lentiviral envelope protein VSV‐G to construct the shuttle plasmids. To package recombinant pseudoviruses expressing the S protein, the shuttle plasmids, lentiviral packaging plasmid (containing structural proteins Gag and Pol), and pGMLV‐CMV‐eGFP reporter plasmid were cotransfected into HEK 293T cells. The resultant pseudoviruses were confirmed to express the S protein of SARS‐CoV‐2 on the surface of infected cells and carry eGFP reporter. Finally, the purified virus with different dilutions was used to infect hACE2‐293T cells and to quantify the content of the viral solution by detecting eGFP‐expressing cells (GECs) and using a FACS‐CytoFLEX flow cytometer (Beckman Coulter). The positive rate of GECs was calculated after collecting 2 × 103 cells and the viral content was expressed as GECs/ml.
2.4. Comparison of the entering efficiency of pseudovirus to hACE2‐293T cells
One hundred microliters of 104 hACE2‐293T cells were seeded into 96‐well plates for 24 h. Cells were infected with purified SARS‐CoV‐2 PS and VOCs pseudoviruses (multiplicity of infection, MOI of virus: cell = 1) in triplicates for 24 h and then refreshed for 48 h. Fluorescence microscopy was used to confirm the entrance of the pseudoviruses. The number of GECs was detected by FACS, and the results were expressed as the positive rate and compared between different pseudovirus‐treated cells.
2.5. Establishment of pseudovirus neutralization assay platforms
After heat inactivation at 56°C for 30 min, the serum samples were serially diluted twofold, ranging from 1:20 to 1:2560. An equal volume of the SARS‐CoV‐2 PS pseudovirus was added to the serum dilutions and mixed for 1 h at 37°C. Serum‐pseudovirus mixture was added into 104 hACE2‐293T cells in triplicates, and serum dilutions ranged from 1:40 to 1:5120. MOI values of 0.5, 1, 2, and 4 were used to optimize the pseudovirus neutralization assay platform. After further incubation for 48 or 72 h, the cells were collected, and the number of eGFP‐expressing cells was detected by FACS. Cells treated with either the culture medium (cell control, CC) or pseudovirus alone (virus control, VC) were used as controls. The positive rate of eGFP‐expressing cells (PRG) was calculated after collecting 2 × 103 cells. The neutralization rate (%) was calculated using the following formula:
2.6. Detection of neutralization activity of sera and mAbs
Pseudovirus neutralization assay platforms for VOCs with respective MOI values were also established, as previously mentioned. The neutralization activity of each serum sample against PS and VOCs pseudoviruses was determined. To replace sera, mAbs such as imdevimab (REGN10987), casirivimab (REGN10933), bamlanivimab (LY‐CoV555), and etesevimab (JS016) (AtaGenix) were used as threefold serial dilutions, ranging from 1 × 10–4 to 10 μg/ml. The results are expressed as the mean ± SD of 50% neutralization titer (NT50). NT50 was the highest serum dilution, corresponding to a neutralization rate of 50%. In this study, an NT50 < 10 was defined as a lack of neutralizing activity. 19
2.7. Statistical analysis
Nonlinear regression SPSS 23.0 was used to calculate the NT50 for each sample. D'Agostino & Pearson test was used to test the normality of the data with the GraphPad Prism 8.0 software. Student's t test was performed for comparisons between two different groups, and one‐way analysis of variance was used for comparisons between three or more groups. Nonparametric tests, the Mann–Whitney U test, and the Friedman test with Dunn's post hoc test were used for nonnormally distributed data. p < 0.05 was defined as statistically different.
3. RESULTS
3.1. SARS‐CoV‐2 continues to mutate based on inheriting key mutations during the pandemic
The SARS‐CoV‐2 PS caused the first wave of the global pandemic. In December 2020, Alpha began to cause a new pandemic wave and was defined as the first VOC. Subsequently, the virus continued to evolve with the emergence of Beta, Gamma, Delta, and, more recently, Omicron. Except for Beta and Gamma, other VOCs have caused pandemics. In particular, the Omicron has now become the major epidemic variant, even though nearly 70% of the world population has been vaccinated with different types of vaccines (Figure 1A). Omicron contained more than 30 mutations in the S protein. Most mutations were not found in previously circulating strains, including deletions, insertions, and substitutions (Figure 1B,C). Among the VOCs, there were some high‐frequency mutation sites, including deletions of 69, 70, 144, 156, and 157, and substitutions of T19R, T95I, G142D, R158G, L452R, T478K, E484K, N501Y, A570D, D614G, P681R/H, T716I, D950N, S982A, and D1118H (Figure 1D). Among these mutations, D614G was present in all the variants. Omicron mainly inherited some mutations from the pandemic variants, such as the three amino acid deletions in Alpha (H69, V70, and Y144) and four mutations in Delta (N501Y, P681H, G142D, and T478K). Two mutations emerging in regional epidemic variants, K417N of Beta and K417T and H655Y of Gamma, were also present in Omicron (Figure 1C,D). Interestingly, although absent in Delta, N501Y and P681H were highly prevalent and regained popularity with the emergence of Omicron. Mutations T478K and G142D have spiked rapidly since the Delta pandemic in April 2021 and currently have a prevalence of approximately 80%. Omicron also retained some mutations (S477N and H655Y) that were prevalent at low frequencies (Figure 1E).
Figure 1.
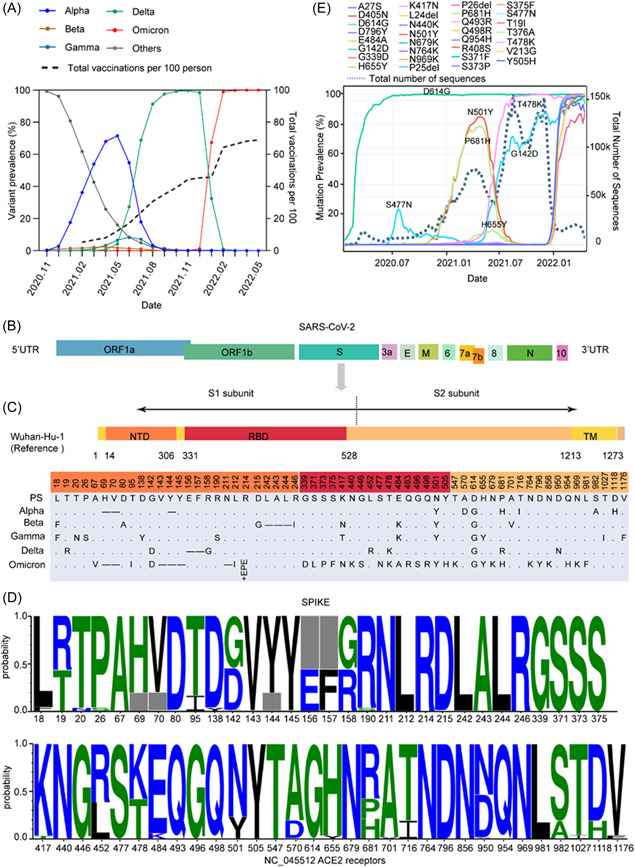
Mutation landscape and prevalence of mutations of the S protein in VOCs under immune stress. (A) The prevalence of VOCs and the number of vaccinations per 100 people over time. (B) Schematic representation of the SARS‐CoV‐2 genomic structure. (C) Schematic representation of the spike protein of the SARS‐CoV‐2 prototype strain Wuhan‐Hu‐1 (NC_045512) and comparison of mutations between the spike protein of different VOCs. NTD, N‐terminal domain; RBD, receptor‐binding domain; TM, transmembrane domain. (.) indicates no amino acid mutations and (−) indicates amino acid deletions. (D) The frequency of different mutations on the spike protein of VOCs. The horizontal axis shows mutation positions in the S protein, and the gray box indicates deletions. (E) The prevalence of major mutations over time. VOCs, variants of concern.
3.2. SARS‐CoV‐2 variant enhances the ability to infect cells during the pandemic
To compare the entry efficiency of VOCs into hACE2‐293T cells, PS and VOCs pseudoviruses were used to infect hACE2‐293T cells at the same MOI value (MOI = 1). As shown in Figure 2A, all these pseudoviruses could enter hACE2‐293T cells with variable numbers of cells expressing fluorescence and changing fluorescence intensities, as demonstrated by fluorescence microscopy. FACS also demonstrated that PS and VOCs pseudoviruses had different efficiencies in entering the cells through the hACE2 receptor (Figure 2B,C). Compared to the PS pseudovirus, VOCs pseudoviruses showed a stronger ability to enter hACE2‐293T cells. Beta pseudovirus had the highest efficiency among all pseudoviruses, followed by Alpha and Delta pseudoviruses (Figure 2B,C). Our results indicate that the infection ability of SARS‐CoV‐2 variants may have been enhanced during the pandemic.
Figure 2.
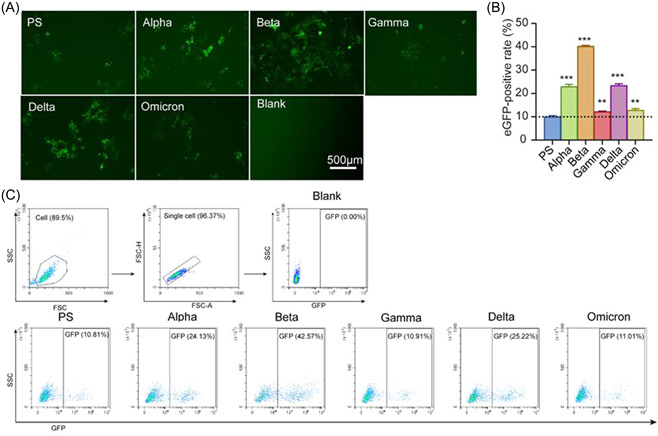
The potency of PS and VOCs pseudoviruses entry into hACE2‐293T. (A) Representative fluorescence photographs observed by inverted fluorescence microscopy at 48 h postinfection. Scale bar = 500 μm. (B) The gating strategies and the representative flow dot plots show the eGFP‐positive rate of VOCs. (C) hACE2‐293T cells (104 cells/well) were infected using SARS‐CoV‐2 PS and VOCs pseudoviruses, and eGFP‐positive cells were detected by FACS at 48 h postinfection, and the experiment was set up in triplicates. The results were expressed as mean ± SD. **p < 0.01 versus PS control, ***p < 0.001 versus PS control. eGFP, enhanced green fluorescent protein; FACS, fluorescence‐activated cell sorting; PS, prototype strain; VOCs, variants of concern.
3.3. MOI is the key factor influencing the results of pseudovirus‐based neutralization assay platforms
Eighteen convalescent serum samples and three healthy controls were used to establish the platforms for pseudovirus‐based neutralization assays. Different MOI values of the PS pseudovirus, ranging from 0.5 to 4, were explored to establish the neutralization curves. Notably, different MOI values produced different curves and NT50 values. The lower the MOI value, the higher the serum dilution revealed by the NT50 (Figure 3A). Furthermore, MOI values of 1 and 2 were then chosen to compare the neutralizing activity of the CB6 antibody against PS pseudovirus with the data from real live PS viral experiments, as previously described. 22 When an MOI of 1 was used, PS pseudovirus‐ and real‐live virus‐based neutralization assays produced similar NT50 results (Figure 3B). In addition, coincubation times of 48 and 72 h had no clear effects on the neutralization curves (Figure 3C). Finally, the reproducibility of the PS pseudovirus‐based neutralization assay platform with an MOI of 1 and a coincubation time of 48 h was confirmed by three independent replicate assays with the same serum sample (Figure 3D).
Figure 3.
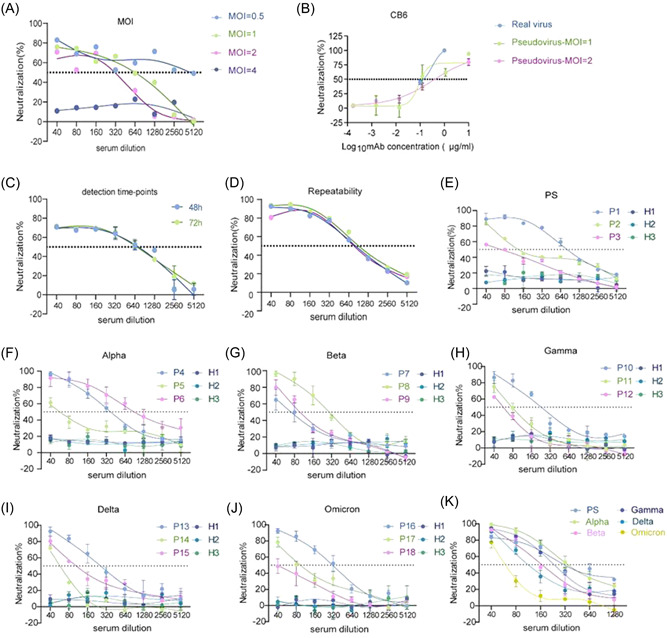
Establishment and optimization of pseudovirus‐based neutralization analysis platform. (A) Neutralization curves of PS pseudovirus infected with hACE2‐293T cells at different MOI values, ranging from 0.5 to 4. (B) Comparison of neutralization curves of CB6 antibody against real virus and PS pseudovirus with MOI values of 1 and 2. (C) The effects of 48 or 74 h coincubation on the neutralization curves against PS pseudovirus. (D) The reproducibility of PS pseudovirus (MOI = 1) based neutralizing curves with the same serum sample was confirmed by three independent replicates. (E–J) Specificity and sensitivity of PS and VOCs pseudovirus‐based neutralization assay platforms. Three healthy control serum samples, H1–H3, were used to assess specificity. Eighteen convalescent serum samples, P1–P18, were used to assess sensitivity. (K) Representative neutralization curves of the same convalescent serum sample against PS and VOCs pseudoviruses. The dotted line indicates the 50% neutralization rate (NT50). MOI, multiplicity of infection; PS, prototype strain; VOCs, variants of concern.
Because the entry efficiency of the PS pseudovirus with an MOI of 1 was approximately 10%, the MOI values for different VOCs pseudoviruses to achieve the same efficiency were chosen to establish VOCs pseudovirus‐based neutralization assay platforms. Specificity was confirmed using 18 convalescent samples and three healthy controls. All platforms also showed good specificity because the neutralization rates of the three controls were less than 20%, regardless of serum dilution (Figure 3E–J). In particular, these platforms plotted good neutralization curves and showed neutralizing activity for each of the three convalescent serum samples. Furthermore, when the same convalescent serum sample was used, the same NT50 value was observed only between the PS and Alpha pseudovirus‐based assays, whereas others detected different NT50 results (Figure 3K). Together, these data demonstrate the successful establishment of neutralization assay platforms for different pseudoviruses.
3.4. SARS‐CoV‐2 variant shows the enhanced ability to escape vaccine‐induced neutralizing activity during the pandemic
To evaluate the ability of VOCs to escape the nAb response from sera after vaccination, serum samples were collected from 20 healthy adults (Supporting Information: Table S1) 1 month after vaccination with two doses of the WIBP‐CorV vaccine. Compared to PS pseudovirus, sera from these vaccine recipients exhibited a decrease in neutralizing activity against VOCs pseudovirus, except for Alpha pseudovirus (Figure 4A,B). Gamma and Delta showed the lowest ability to escape the vaccine‐induced nAb response (Figure 4D,E), whereas Omicron, the current prevalent variant exhibited the most significant ability to escape neutralizing activity. Omicron had a 15.7‐fold decrease in NT50 compared with the PS pseudovirus (Figure 4F). In addition, Beta also showed a significant ability to escape neutralization (6.82‐fold decrease in NT50) (Figure 4C). Consistent with the fold decrease in NT50, 25%, 60%, and 90% of these serum samples lost their neutralizing activity against the Alpha, Delta, and Omicron pandemic variants, respectively (Figure 4G). Thus, our results indicate that the virus has continued to evolve its ability to escape vaccine‐induced nAb responses during the COVID‐19 pandemic.
Figure 4.
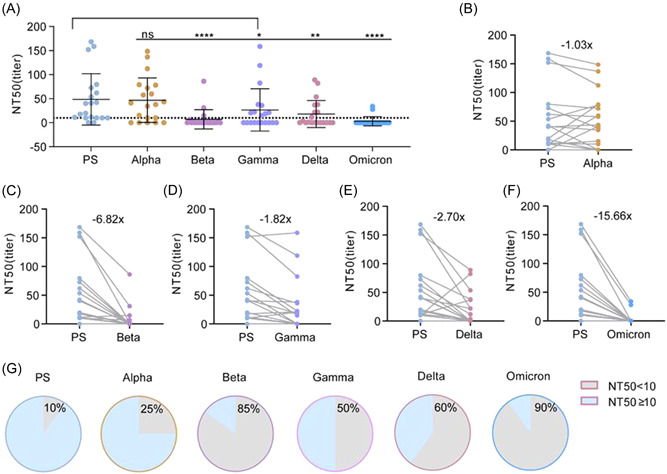
Comparison of neutralization potency of WIBP‐CorV vaccinated sera against SARS‐CoV‐2 VOCs pseudoviruses (n = 20). Neutralizing antibody titers of 20 WIBP‐CorV vaccinated sera samples were expressed as NT50 and calculated by nonlinear regression using SPSS. (A) Comparison of NT50 values of sera against PS and VOCs. Each point represents a serum sample and is expressed as mean ± SD, and the dotted line indicates NT50 = 10. (B–F) Comparison of sera NT50 between PS and Alpha, Beta, Delta, Gamma, and Omicron levels. Gray lines connect the same serum sample, and the number on the scatter plot indicates the mean reduction multiple of the NT50. (G) The proportion of serum samples with an NT50 below 1:10 and above 1:10 against PS and Alpha, Beta, Delta, Gamma, and Omicron, respectively. Multiple comparisons Friedman test with Dunn's post hoc test was used to compare p values between PS and VOCs. p > 0.05, not significant (ns), *p < 0.05, **p < 0.01, ****p < 0.0001. PS, prototype strain; VOCs, variants of concern.
3.5. SARS‐CoV‐2 variant enhances the ability to escape nAb response acquired by natural infection during the pandemic
As the pandemic continues, an increasing number of individuals have been infected and have acquired nAbs in their sera. To compare the escape capacity of VOCs from the COVID‐19 pandemic to the pre‐existing nAb responses, 65 convalescent serum samples (Supporting Information: Table S2) and their neutralizing activities against different pseudoviruses were detected. Comparatively, the NT50 values of these convalescent sera were much higher than those of immunized sera, regardless of PS or VOCs pseudovirus platforms. Interestingly, convalescent sera showed varying degrees of decreased neutralizing activity against VOCs, except for Alpha (Figure 5A,B). Compared to the PS pseudovirus control, Beta, Gamma, and Delta showed a significant ability to escape neutralization, with 2.05‐, 1.43‐, and 2.28‐fold decreases in NT50, respectively (Figure 5C–E). Omicron exhibited the most significant ability to escape the neutralizing activity of all the VOCs, with a 3.71‐fold decrease in NT50 to PS pseudovirus (Figure 5F). Consistent with the fold decrease in NT50, 3%, 15%, and 43% of these serum samples lost their neutralizing activity against Alpha, Delta, and Omicron, respectively (Figure 5G). In addition, factors such as patient age, sex, and clinical severity did not significantly affect the degree of decrease in the neutralizing activity against VOCs (Supporting Information: Figure S1). Therefore, our results suggest that the SARS‐CoV‐2 variant enhances the ability of the virus to escape the nAb response acquired by natural infection during the pandemic.
Figure 5.
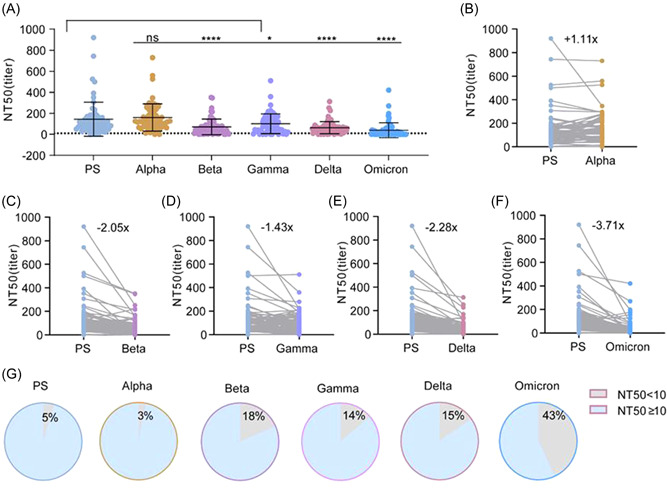
Comparison of neutralization potency of convalescent sera against SARS‐CoV‐2 PS and VOCs pseudoviruses (n = 65). Neutralizing antibody titers of 65 convalescent sera samples were expressed as NT50 and calculated by nonlinear regression using SPSS. (A) Comparison of NT50 values of sera against PS and VOCs. Each point represents a serum sample and is expressed as mean ± SD, and the dotted line indicates NT50 = 10. (B–F) Comparison of sera NT50 between PS and Alpha, Beta, Delta, Gamma, and Omicron levels. Gray lines connect the same serum sample, and the number on the scatter plot indicates the mean reduction multiple of the NT50. (G) The proportion of serum samples with an NT50 below 1:10 and above 1:10 against PS and Alpha, Beta, Delta, Gamma, and Omicron, respectively. Multiple comparisons Friedman test with Dunn's post hoc test was used to compare p values between PS and VOCs. p > 0.05, not significant (ns), *p < 0.05, **p < 0.01, ****p < 0.0001. PS, prototype strain; VOCs, variants of concern.
3.6. SARS‐CoV‐2 variant evolves to abolish the effects of therapeutic mAbs during the pandemic
To evaluate the effects of therapeutic mAbs against VOCs, the neutralizing activities of imdevimab, casirivimab, bamlanivimab, and etesevimab against PS and VOCs pseudoviruses were determined. Early pandemic strains, such as PS and Alpha, could be neutralized by four mAbs, even if Alpha was partially neutralized by etesevimab (Figure 6A–D). Beta and Gamma were also resistant to neutralization by bamlanivimab and etesevimab (Figure 6A–E). Although Delta was completely resistant to the neutralizing activity of bamlanivimab, it was still neutralized by imdevimab, casirivimab, and etesevimab (Figure 6A–D). Notably, Omicron was completely resistant to the neutralizing activities of all four mAbs (Figure 6A–D). Therefore, our findings demonstrate that SARS‐CoV‐2 has evolved to abolish the effects of therapeutic mAbs against the COVID‐19 pandemic.
Figure 6.
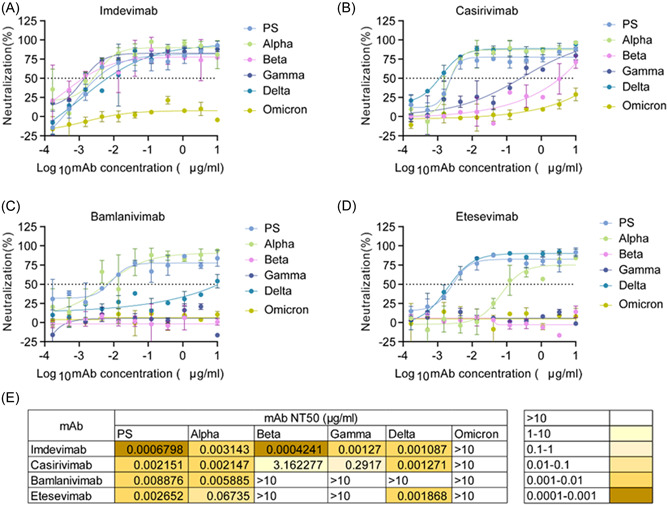
Comparison of neutralizing potency of therapeutic mAbs against SARS‐CoV‐2 PS and VOCs pseudoviruses. Neutralization curves of four therapeutic mAbs, imdevimab (A), casirivimab (B), bamlanivimab (C), and etesevimab (D), against SARS‐CoV‐2 PS and VOCs pseudoviruses. (E) NT50 values of different mAbs against PS and VOCs. The dotted line represents a 50% neutralization rate. Data are shown as the mean ± SD. mAb, monoclonal antibodies; PS, prototype strain; VOCs, variants of concern.
4. DISCUSSION
This study successfully established lentivirus‐based pseudovirus neutralization assay platforms for detecting the neutralizing activity of sera and therapeutic mAbs against SARS‐COV‐2 PS and VOCs variants. New SARS‐COV‐2 variants causing local outbreaks or pandemics showed different degrees of ability to escape neutralization, elicited by nAb responses following natural infection, vaccination, or the application of therapeutic mAbs. In particular, our study demonstrated that newly emerging and pandemic variants enhance infectivity and exhibit a strong ability to escape these nAb responses during the pandemic.
Several important factors can influence viral infectivity, such as the viral receptor, the infectious dose, viral virulence, and the immune status of the infected individuals with or without underlying diseases. 23 To detect the neutralizing activity based on the cell models and pseudovirus, MOI was determined to play key roles in this study. Because hACE2 is the main receptor for SARS‐COV‐2 entering cells, 24 HEK293T cells were constructed to express this receptor on the cell surface and were used in these assay platforms. Different pseudoviruses showed different infectivity in the cell, which might be caused by different mutations in the RBD region of the S protein of different VOCs. However, other potential viral receptors may also participate in the entrance of cells, such as CD147, 25 neuropilin‐1, 26 sialic acid, 27 acetyl heparan sulfate, 28 tyrosine‐protein kinase receptor UFO (AXL), 29 LDLRAD3, TMEM30A, CLEC4G, 30 and C‐type lectins (DC‐SIGN, L‐SIGN, LSECtin, ASGR1, CLEC10A, and Tweety family member 2) and others. 31 These receptors specifically interact with the NTD or glycan‐dependent binding partners of the S protein. 26 , 27 , 28 , 29 , 30 , 31 , 32 However, the effects of these viral receptors on the assay platforms remain to be investigated. Although cell models do not fully reflect SARS‐COV‐2 infectivity in humans, the neutralizing activity assay is still a sensitive assay protocol for responding to viral immune evasion, as evidenced by the consistent results obtained from neutralization assays based on live viruses in this study.
Previous studies also reported the fold reduction of nAbs induced by SARS‐COV‐2 natural infection and vaccination against VOCs, although these magnitude values differed significantly from our results. 16 , 17 , 18 , 20 The main reason might be that these previous studies of neutralizing resistance to VOCs were not conducted on the same platform. Therefore, our unified assay platforms provide a good opportunity to analyze the mutation trends and neutralization‐escaping ability of VOCs during the pandemic. Although Beta did not cause the pandemic, it exhibited significant antineutralization effects on the sera in this study. Beta also showed neutralizing resistance to messenger RNA (mRNA) (BNT162b2 or mRNA1273) vaccine‐induced sera, consistent with our results. 16 , 18 The combined role of K417N/E484K/N501Y mutations in the RBD of Beta might be attributed to the resistance. 4 , 32 However, Alpha, with N501Y substitution, did not show neutralization escaping ability. 6 , 18 Therefore, E484K and K417N could play an important role in the escape ability. Consistent with previous reports, 32 , 33 Gamma had a worse escaping ability than Beta by replacing K417N with the K417T mutation. Delta exhibited a moderate degree of escaping neutralization, which resulted in two new mutations, L452R and T478K, in the RBD region. 34 L452R was shown to be associated with the escaping neutralization of several monoclonal and polyclonal antibodies. 35 Simultaneously, T478K enhanced the binding ability with ACE2. 36 Omicron, showed the strongest resistance to neutralization of all VOCs, accumulating more than 15 mutations in the RBD. 8 , 37 More importantly, several mutations in Omicron were inherited from previous VOCs, such as K417N, T478K, and N501Y. Although E484K is absent, new mutations in the RBD, such as G446S, S477N, E484A, Q493R, G496S, and Q498R, might also contribute to the neutralization escaping ability of Omicron. 38 Interestingly, the increased prevalence of Omicron sublineages BA.4/5 has made infections more transmissible and resistant to immunity. 39 This is attributed to the emerging L452R and F486V mutations found in BA.4/5. 40
In conclusion, the ongoing evolution of SARS‐CoV‐2 is resulting in the emergence of new variants that are able to evade neutralization by nAbs to varying degrees. Among these variants, Omicron appears to be the most resistant to neutralization by both serum and mAbs. The evolution of Omicron is achieved by mutating based on existing favorable mutations to gain stronger immune escape ability during the COVID‐19 pandemic. It is predicted that the virus will evolve and break through the immune barrier established by infection or vaccination. Therefore, global surveillance of new viral variants, early identification and quarantine of infected individuals, and development of more effective vaccines are important measures to control the COVID‐19 pandemic.
AUTHOR CONTRIBUTIONS
Xionglin Fan designed all the experiments. Leyong Yuan collected sera from convalescent individuals, Yandi Zhang collected sera from healthy individuals after vaccination. Yandi Zhang and Jo‐Lewis constructed the pseudo‐neutralization assay platform and performed experiments with Hongyan Hou, Feng Wang, Xiaosong Lin, Mengze Gan, Zongjie Yao, Hui Fu, and Jinge Cao. Yandi Zhang and Xiaosong Lin collected data and drafted the manuscript. Xionglin Fan revised the manuscript accordingly.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Supplementary information.
ACKNOWLEDGMENTS
This study was supported by grants from the National Key R&D Project (2021YFC2600200), the Emergency Key Program of Guangzhou Laboratory (EKPG21‐30), the Applied Basic Research Key Project of Wuhan Municipal Bureau of Science and Technology (020020601012218), and the Fundamental Research Funds for the Central Universities (HUST COVID‐19 Rapid Response Call No. 2020kfyXGYJ040).
Zhang Y, Ndzouboukou J‐LB, Lin X, et al. SARS‐CoV‐2 evolves to reduce but not abolish neutralizing action. J Med Virol. 2022;95:e28207. 10.1002/jmv.28207
Yandi Zhang, Jo‐Lewis B. Ndzouboukou, and Xiaosong Lin contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Zhang Y, Banga Ndzouboukou JL, Gan M, Lin X, Fan X. Immune evasive effects of SARS‐CoV‐2 variants to COVID‐19 emergency used vaccines. Front Immunol. 2021;12:771242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. FDA . Emergency Use Authorization. 2022. Accessed September 23, 2022. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#coviddrugs
- 3. Korber B, Fischer WM, Gnanakaran S, et al. Tracking changes in SARS‐CoV‐2 spike: evidence that D614G increases infectivity of the COVID‐19 virus. Cell. 2020;182(4):812‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yuan M, Huang D, Lee CD, et al. Structural and functional ramifications of antigenic drift in recent SARS‐CoV‐2 variants. Science. 2021;373(6556):818‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harvey WT, Carabelli AM, Jackson B, et al. SARS‐CoV‐2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19(7):409‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andrew Rambaut NL, Oliver Pybus, Wendy Barclay, Jeff Barrett, Alesandro Carabelli, Tom Connor, Tom Peacock, David L Robertson, Erik Volz, on behalf of COVID‐19 Genomics Consortium UK (CoG‐UK) . Preliminary genomic characterisation of an emergent SARS‐CoV‐2 lineage in the UK defined by a novel set of spike mutations. ARTIC Network. 2020. https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563 [Google Scholar]
- 7. Dhar MS, Marwal R, Vs R, et al. Genomic characterization and epidemiology of an emerging SARS‐CoV‐2 variant in Delhi, India. Science. 2021;374(6570):995‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Viana R, Moyo S, Amoako DG, et al. Rapid epidemic expansion of the SARS‐CoV‐2 Omicron variant in Southern Africa. Nature. 2022;603(7902):679‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cao Y, Yisimayi A, Jian F, et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022;608(7923):593‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malato J, Ribeiro RM, Leite PP, et al. Risk of BA.5 infection among persons exposed to previous SARS‐CoV‐2 variants. N Engl J Med. 2022;387(10):953‐954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA‐1273 COVID‐19 vaccine efficacy clinical trial. Science. 2022;375(6576):43‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lei Q, Hou H, Yu C, et al. Kinetics of neutralizing antibody response underscores clinical COVID‐19 progression. J Immunol Res. 2021;2021:9822706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hou H, Zhang Y, Tang G, et al. Immunologic memory to SARS‐CoV‐2 in convalescent COVID‐19 patients at 1 year postinfection. J Allergy Clin Immunol. 2021;148(6):1481‐1492 e1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Collier AY, Yu J, McMahan K, et al. Differential kinetics of immune responses elicited by Covid‐19 vaccines. N Engl J Med. 2021;385(21):2010‐2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu J, Mao Q, Wu X, et al. Considerations for the feasibility of neutralizing antibodies as a surrogate endpoint for COVID‐19 vaccines. Front Immunol. 2022;13:814365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chemaitelly H, Yassine HM, Benslimane FM, et al. mRNA‐1273 COVID‐19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID‐19 disease in Qatar. Nat Med. 2021;27(9):1614‐1621. [DOI] [PubMed] [Google Scholar]
- 17. Tseng HF, Ackerson BK, Luo Y, et al. Effectiveness of mRNA‐1273 against SARS‐CoV‐2 Omicron and Delta variants. Nat Med. 2022;28(5):1063‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bates TA, Leier HC, Lyski ZL, et al. Neutralization of SARS‐CoV‐2 variants by convalescent and BNT162b2 vaccinated serum. Nat Commun. 2021;12(1):5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen RE, Zhang X, Case JB, et al. Resistance of SARS‐CoV‐2 variants to neutralization by monoclonal and serum‐derived polyclonal antibodies. Nat Med. 2021;27(4):717‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen Y, Shen H, Huang R, Tong X, Wu C. Serum neutralising activity against SARS‐CoV‐2 variants elicited by CoronaVac. Lancet Infect Dis. 2021;21(8):1071‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lei Q, Li Y, Hou HY, et al. Antibody dynamics to SARS‐CoV‐2 in asymptomatic COVID‐19 infections. Allergy. 2021;76(2):551‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Y, Ma ML, Lei Q, et al. Linear epitope landscape of the SARS‐CoV‐2 spike protein constructed from 1,051 COVID‐19 patients. Cell Rep. 2021;34(13):108915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aleem A, Akbar SA, Slenker AK. Emerging Variants of SARS‐CoV‐2 and Novel Therapeutics Against Coronavirus (COVID‐19). StatPearls Publishing; 2022. [Google Scholar]
- 24. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280 e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang K, Chen W, Zhang Z, et al. CD147‐spike protein is a novel route for SARS‐CoV‐2 infection to host cells. Signal Transduct Target Ther. 2020;5(1):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cantuti‐Castelvetri L, Ojha R, Pedro LD, et al. Neuropilin‐1 facilitates SARS‐CoV‐2 cell entry and infectivity. Science. 2020;370(6518):856‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morniroli D, Gianni ML, Consales A, Pietrasanta C, Mosca F. Human sialome and coronavirus disease‐2019 (COVID‐19) pandemic: an understated correlation. Front Immunol. 2020;11:1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clausen TM, Sandoval DR, Spliid CB, et al. SARS‐CoV‐2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020;183(4):1043‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang S, Qiu Z, Hou Y, et al. AXL is a candidate receptor for SARS‐CoV‐2 that promotes infection of pulmonary and bronchial epithelial cells. Cell Res. 2021;31(2):126‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu S, Liu Y, Zhou Z, et al. Genome‐wide CRISPR activation screen identifies candidate receptors for SARS‐CoV‐2 entry. Sci China: Life Sci. 2022;65(4):701‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lu Q, Liu J, Zhao S, et al. SARS‐CoV‐2 exacerbates proinflammatory responses in myeloid cells through C‐type lectin receptors and Tweety family member 2. Immunity. 2021;54(6):1304‐1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoffmann M, Arora P, Groß R, et al. SARS‐CoV‐2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell. 2021;184(9):2384‐2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khan A, Zia T, Suleman M, et al. Higher infectivity of the SARS‐CoV‐2 new variants is associated with K417N/T, E484K, and N501Y mutants: an insight from structural data. J Cell Physiol. 2021;236(10):7045‐7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Planas D, Veyer D, Baidaliuk A, et al. Reduced sensitivity of SARS‐CoV‐2 variant Delta to antibody neutralization. Nature. 2021;596(7871):276‐280. [DOI] [PubMed] [Google Scholar]
- 35. McCallum M, Bassi J, De Marco A, et al. SARS‐CoV‐2 immune evasion by the B.1.427/B.1.429 variant of concern. Science. 2021;373(6555):648‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang Y, Liu C, Zhang C, et al. Structural basis for SARS‐CoV‐2 Delta variant recognition of ACE2 receptor and broadly neutralizing antibodies. Nat Commun. 2022;13(1):871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carreño JM, Alshammary H, Tcheou J, et al. Activity of convalescent and vaccine serum against SARS‐CoV‐2 Omicron. Nature. 2022;602(7898):682‐688. [DOI] [PubMed] [Google Scholar]
- 38. McCallum M, Czudnochowski N, Rosen LE, et al. Structural basis of SARS‐CoV‐2 Omicron immune evasion and receptor engagement. Science. 2022;375(6583):864‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhou Y, Zhi H, Teng Y. The outbreak of SARS‐CoV‐2 Omicron lineages, immune escape, and vaccine effectivity. J Med Virol. 2022. Published September 12, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang Q, Guo Y, Iketani S, et al. Antibody evasion by SARS‐CoV‐2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature. 2022;608(7923):603‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


