Summary
Background
The COVID‐19 pandemic offered a unique opportunity to understand inflammatory bowel disease (IBD) management during unexpected disruption. This could help to guide practice overall.
Aims
To compare prescribing behaviour for IBD flares and outcomes during the early pandemic with pre‐pandemic findings
Methods
We performed an observational cohort study comprising patients who contacted IBD teams for symptomatic flares between March and June 2020 in 60 National Health Service trusts in the United Kingdom. Data were compared with a pre‐pandemic cohort after propensity‐matching for age and physician global assessment of disease activity.
Results
We included 1864 patients in each of the pandemic and pre‐pandemic cohorts. The principal findings were reduced systemic corticosteroid prescription during the pandemic in Crohn's disease (prednisolone: pandemic 26.5% vs. 37.1%; p < 0.001) and ulcerative colitis (UC) (prednisolone: pandemic 33.5% vs. 40.7%, p < 0.001), with increases in poorly bioavailable oral corticosteroids in Crohn's (pandemic 15.6% vs. 6.8%; p < 0.001) and UC (pandemic 11.8% vs. 5.2%; p < 0.001). Ustekinumab (Crohn's and UC) and vedolizumab (UC) treatment also significantly increased. Three‐month steroid‐free remission in each period was similar in Crohn's (pandemic 28.4% vs. 32.1%; p = 0.17) and UC (pandemic 36.4% vs. 40.2%; p = 0.095). Patients experiencing a flare and suspected COVID‐19 were more likely to have moderately‐to‐severely active disease at 3 months than those with a flare alone.
Conclusions
Despite treatment adaptations during the pandemic, steroid‐free outcomes were comparable with pre‐pandemic levels, although concurrent flare and suspected COVID‐19 caused worse outcomes. These findings have implications for IBD management during future pandemics and for standard practice.
PREPARE‐IBD—treatment adaptations to treat IBD flares during the COVID‐19 pandemic compared to an age‐ and disease activity‐matched pre‐pandemic cohort.

1. INTRODUCTION
Crohn's disease and ulcerative colitis (UC) represent the two principal forms of inflammatory bowel disease (IBD). Both are characterised by mucosal and extraintestinal immune dysregulation. Given that the cornerstone of treatment involves effective immune suppression, it became clear early in the coronavirus infectious disease (COVID)‐19 pandemic that IBD clinicians' normal practice may become disrupted, with potential effects on patient outcomes. 1 While conventional pre‐pandemic treatment paradigms are effective at inducing disease remission in IBD, many therapies are associated with an increased risk of infections requiring hospitalisation and/or development of opportunistic infections, in particular, thiopurines and tofacitinib. 2 The immune suppressive effects of many of these drugs may last for weeks or months following treatment discontinuation, with international guidelines advising intervals of up to 6 months before administering live vaccines. 3 Even in the absence of immune‐directed treatments, IBD patients have a higher seasonal influenza risk and are more likely to be hospitalised. 4 High dose corticosteroids and uncontrolled IBD disease activity are now important risk factors for severe COVID‐19 infection and many clinicians harboured grave concerns regarding the safety of immunosuppressive therapies during the start of the pandemic. 5 , 6 , 7 It is unknown if this translated to modification of IBD flare management strategies and if subsequent disease outcomes were impacted. Thus, the onset of the COVID‐19 pandemic offered a unique opportunity to observe the consequences of perturbations to conventional healthcare pathways.
In March 2020, we established the multicentre cohort “Physician Responses to disease flares and Patient Adaptation in Relation to Events in Inflammatory Bowel Disease during the COVID‐19 pandemic (PREPARE‐IBD)”. We collated data relating to management of active disease during the first wave of the pandemic from across the UK and compared these with a pre‐pandemic control group. We assessed how treatment behaviour changed during this challenging period and the subsequent impact on 3‐month outcomes to guide future practice within and outside of a pandemic setting.
2. MATERIALS AND METHODS
2.1. Aims
We aimed to identify differences in UK prescribing practices for treating IBD flares during the first wave of the COVID‐19 pandemic compared with a pre‐pandemic cohort, and the impact on patient outcomes.
2.2. Study design
We conducted an observational cohort study in 60 National Health Service Trusts in the United Kingdom. Patients over 16 years‐old were recruited if they had a flare of IBD symptoms and/or acquired COVID‐19 infection between 1st March and 30th June 2020: the ‘pandemic cohort’. A ‘pre‐pandemic cohort’ comparator group comprised patients suffering IBD flares between 1st January and 30th June 2019. Three‐month follow‐up data were collected for both cohorts until 30th September 2020. Patients were identified through hospital admission documentation, outpatient clinics or IBD helplines. Data were collected at each site by healthcare professionals and entered pseudo‐anonymously into a Research Electronic Data Capture (REDCap) database hosted by the Exeter IBD group. 8 The REDCap system provides a secure database accessed through a password verified by two‐factor authentication at each log in session.
2.3. Patients
Patients had histologically confirmed IBD, encompassing Crohn's disease, UC or IBD‐unclassified (IBD‐U); UC and IBD‐U patients were analysed together. Patients were included if they had contact with their IBD service because of IBD symptoms in keeping with a disease flare. Baseline characteristics included patient demographics, disease behaviour, location and duration according to the Montreal classification, 9 weight, body mass index (BMI), smoking status, medication history and presence of extra‐intestinal manifestations. For patients with active disease, changes in treatment or adjustments to pre‐existing treatment were recorded. Disease severity was determined by physician global assessment (PGA) as inferred by researchers from contemporaneous clinical notes. To minimise confounders, the cohorts underwent propensity‐score matching for age and PGA‐defined disease activity before further analysis. If an individual had two or more flares during the study period, the first was captured and subsequent episodes were noted within the 3‐month follow‐up data. For those diagnosed with COVID‐19 infection, the SARS‐CoV‐2 PCR (polymerase chain reaction) swab date and result were collected, along with serology, symptoms, treatment and outcome. During this phase of the UK pandemic, community testing for SARS‐CoV‐2 was not widespread. Some patients were diagnosed following a positive PCR test while others reported typical symptoms but were not formally tested; given that the latter would have affected IBD management decision‐making, these patients have been included within the relevant analyses.
2.4. Outcomes
The primary outcome measure was corticosteroid‐free remission at 3 months (as defined by PGA) during the two study periods. Secondary outcome measures included hospital attendance and length of stay during the index IBD flare, and flare incidence, management, hospital admission and need for surgery for subsequent IBD flare episodes at 3 months. COVID‐19‐related outcomes included incidence, hospitalisation, need for respiratory support, intensive care unit (ICU) admission and death.
2.5. Statistical methods
The study was analysed and reported according to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) methodology 10 and Statistical Analysis and Methods in the Published Literature (SAMPL). 11 Statistical analysis was performed using R 4.1.0 (R Foundation for Statistical Computing). We have summarised continuous variables using medians and interquartile ranges, with comparisons done using the Mann–Whitney U test. We report categorical variables as percentages and have used Fisher's exact test for comparisons. All p values are reported without correction for multiple testing.
We conducted propensity matching of the pre‐pandemic and pandemic cohorts with the matchit package in R using nearest neighbour matching of the age and disease activity, as assessed by PGA. Pre‐ and post‐matching quantile‐quantile (QQ) plots are shown in Figure S1.
2.6. Ethical considerations
This study was registered with research governance teams at all hospital sites to approve access to patient records. The study was approved by the Leeds and Bradford ethics committee (IRAS No: 284920, REC reference: 20/HRA/2731) and Protocol listed in ClinicalTrials.gov Identifier: NCT04410484.
3. RESULTS
3.1. Whole cohort
Data from 5220 patients were collected, of whom 2683 (51.4%) were female. The pandemic cohort comprised 3226 patients, including those suffering from a flare of IBD (2855 patients, 88.5%), proven/suspected COVID‐19 infection (306 patients, 9.5%) or both (65 patients, 2.0%). There were 1994 patients in the pre‐pandemic cohort. The flow diagram (Figure 1) illustrates cohorts within the study. Demographic and IBD phenotypic details are summarised in Table S1.
FIGURE 1.
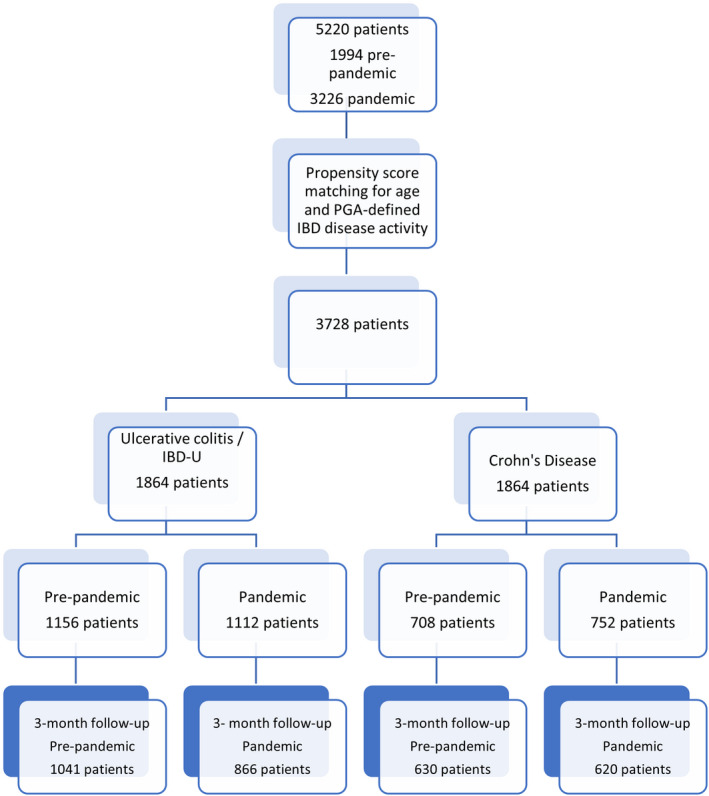
Flow diagram demonstrating the number of patients initially recruited, those remaining after propensity score matching and the final numbers of patients included in each group of analyses. IBD‐U, inflammatory bowel disease‐unclassified.
A lower proportion of the pandemic IBD flare cohort was judged to have PGA‐defined severe disease at flare onset compared with the pre‐pandemic cohort (575/2748, 20.9% vs. 494/1864, 26.5%; p < 0.001), while a greater proportion suffered from a mild flare (755/2748, 27.5% vs. 405/1864, 21.7%; p < 0.001).
3.2. Matched IBD flare cohorts
The pandemic and pre‐pandemic flare cohorts were subsequently matched for age and PGA‐defined IBD disease activity (n = 3728 in total, 1864 patients in each matched cohort). Disease‐specific patient demographics, disease phenotypes and activity, medical therapies and clinical outcomes for these matched Crohn's disease and UC/IBD‐U cohorts are discussed in the following sections and summarised in Tables S2 and S3.
3.3. Matched Crohn's disease flare cohort
3.3.1. Patient demographics
The pre‐pandemic and pandemic cohorts comprised 708 and 752 patients, respectively (Table S2). There were insignificant differences with regards to gender, body weight, BMI, ethnicity, smoking status and number of comorbidities. The mean age of the groups was 34.0 years.
3.3.2. Disease phenotype
There were no significant differences in Montreal classification between the cohorts, with ileocolonic disease the most common phenotype, other than a higher proportion of patients with perianal disease within the pre‐pandemic group (163/672, 24.3% vs. 130/720, 18.1%; p = 0.0047).
3.3.3. Baseline therapies
At baseline, a significantly higher proportion of patients in the pre‐pandemic group was being treated with infliximab (76/708, 10.7% vs. 53/752, 7.0%; p = 0.016) and/or thiopurines (162/708, 22.9% vs. 134/752, 17.8%; p = 0.019). In the pandemic cohort, there was increased use of budesonide (21/708, 3.0% vs. 49/752, 6.5%; p = 0.0020) and vedolizumab (28/708, 4.0% vs. 50/752, 6.6%; p = 0.026). Notably, there was no difference in the baseline use of oral prednisolone.
3.3.4. Disease activity
Harvey‐Bradshaw index (HBI) was recorded for 59.3% and 64.4% of patients in the matched pandemic and pre‐pandemic groups, respectively. The mean score was 8.0, with most patients suffering from a moderate disease flare as evaluated by the PGA. The mean C‐reactive protein (CRP) at time of flare was higher in the pre‐pandemic cohort (35.0 mg/L [9.3–90.0] vs. 22.0 mg/L [6.0–79.0]; p = 0.0032), with no significant difference in baseline CRP level. There was no significant difference in flare faecal calprotectin between the two groups (623.5 mcg/g [264.2–1800.0] vs. 735.0 mcg/g [266.5–1800.0]; p = 1.0). Over 80% of patients underwent full blood count and CRP testing, and over 30% had faecal calprotectin level checked (Tables S4 and S5). During the pandemic, there was increased reliance on faecal calprotectin (222/752, 29.5% vs. 139/708, 19.6%; p < 0.001) and reduced use of CRP (358/752, 47.6% vs. 394/708, 55.6%; p = 0.0024) to determine active Crohn's disease. The proportion of flares diagnosed radiologically (299/752, 39.8% vs. 289/708, 40.8%; p = 0.71) or with endoscopy (141/752, 18.8% vs. 156/708, 22.0%; p = 0.13) was comparable in the two cohorts.
3.3.5. Treatment adaptations
New therapies
The approach to treating active disease differed in these two matched cohorts (Figure 2A). In the pandemic group, a lower proportion of patients was prescribed intravenous corticosteroids (147/752, 19.5% vs. 232/708, 32.8%; p < 0.001) or oral prednisolone (199/752, 26.5% vs. 263/708, 37.1%; p < 0.001) compared with the pre‐pandemic group. A higher proportion was given oral budesonide (117/752, 15.6% vs. 48/708, 6.8%; p < 0.001). Furthermore, in the pandemic group, a higher percentage of patients was treated with ustekinumab (78/752, 10.4% vs. 40/708, 5.6%; p = 0.0010). There were no differences between the two groups regarding acute treatment with enteral nutrition, anti‐tumor necrosis factor (TNF) monotherapy, anti‐TNF/thiopurine combination therapy, or vedolizumab (Figure 2B). Thiopurine monotherapy treatment was numerically lower in the pandemic group but the effect did not reach statistical significance (30/752, 4.0% vs. 43/708, 6.1%; p = 0.072). Adjustment of pre‐existing treatment during an acute flare was comparable between the two groups.
FIGURE 2.
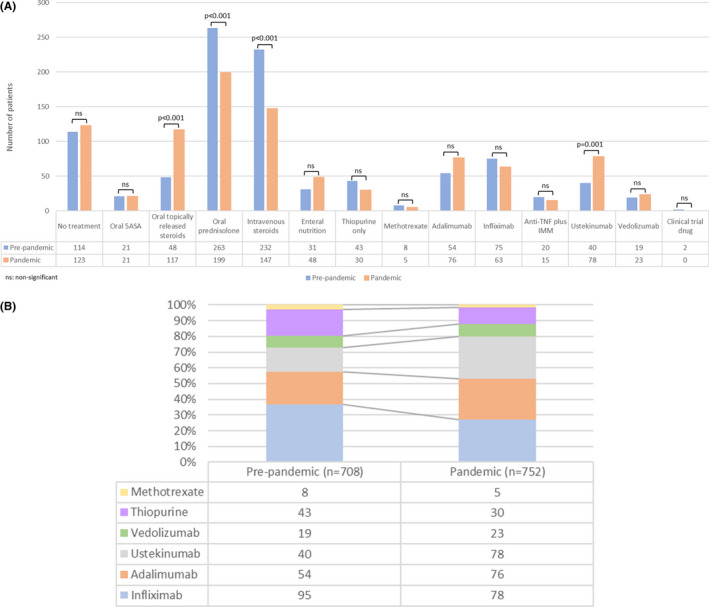
(A) Medications used to treat Crohn's disease flares in the pre‐pandemic and pandemic cohorts, following propensity score matching for age and physician global assessment of disease activity. (B) Proportion of patients with a Crohn's disease flare treated with different biologics and IMMs in the pre‐pandemic and pandemic cohorts, following propensity score matching for age and physician global assessment of disease activity. “n” numbers represent the total number of patients in each cohort. 5‐ASA , 5‐aminosalicylate; IMM, immunomodulator; TNF, tumour necrosis factor; ns, not significant.
Initial outcome
A lower proportion of patients attended the Emergency Department because of their flare in the pandemic group (331/743, 44.5% vs. 389/704, 55.3%; p < 0.001). Similarly, fewer patients were admitted to hospital (321/746, 43.0% vs. 443/705, 62.8%; p < 0.001). In those admitted, there was no difference in length of stay or mortality.
Three‐month follow‐up
There was no difference in steroid‐free remission (defined by PGA) between the pandemic and pre‐pandemic groups (175/616, 28.4% vs. 195/608, 32.1%; p = 0.17) following Crohn's disease flare episodes (Figure 4). Patients' disease activity status was also comparable, as was the proportion of patients who experienced a subsequent flare within this period (pandemic: 194/668, 29.0% vs. pre‐pandemic: 161/663, 24.3%; p = 0.11) (Figure 5). The flares were more commonly diagnosed radiologically (58/194, 29.9% vs. 26/161, 16.1%; p = 0.0026) and/or with raised faecal calprotectin (47/194, 24.2% vs. 18/161, 11.2%; p = 0.0015) during the pandemic period. There was no statistical difference in the percentage of patients diagnosed endoscopically (22/194, 11.3% vs. 26/161, 16.1%; p = 0.21). Furthermore, there was no difference in the proportion of patients undergoing endoscopy who had active disease (59/84, 70.2% vs. 94/133, 70.7%; p = 1.0).
FIGURE 4.
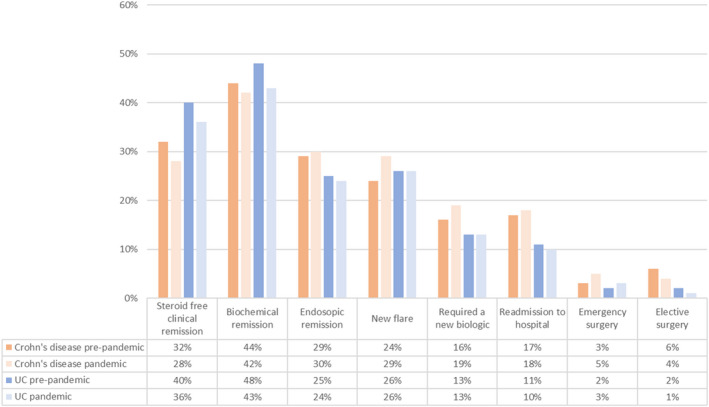
Physician global assessment (PGA) of disease activity 3 months post‐flare in ulcerative colitis and Crohn's disease in the pre‐pandemic and pandemic cohorts, which had been propensity matched for age and initial PGA. “n” numbers depict the total number in the group following propensity matching but follow‐up data are not available for all patients. The differences in disease activity between the groups are not statistically significant using Fisher's exact test.
FIGURE 5.
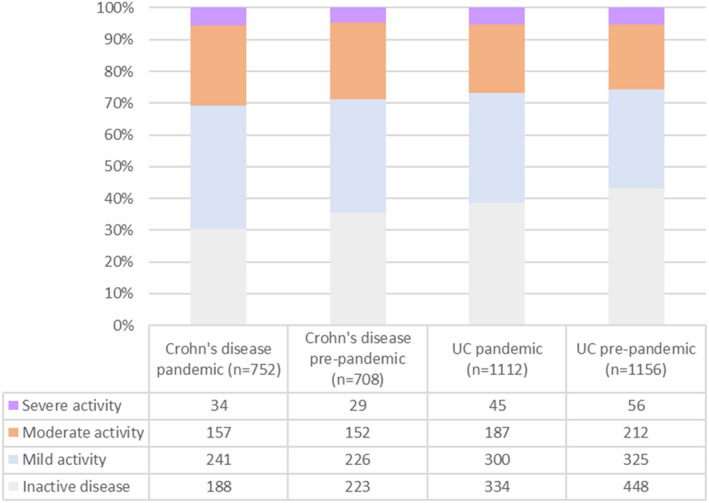
Clinical outcomes in the Crohn's disease and ulcerative colitis/IBD‐unclassified pre‐pandemic groups following propensity score matching for age and initial physician global assessment for disease activity. None of the comparisons between the disease‐specific pre‐pandemic and pandemic groups were statistically significant using Fisher's exact test. IBD, inflammatory bowel disease.
More patients received acute budesonide treatment within the 3‐month follow‐up period during the pandemic compared with the pre‐pandemic group (22/194, 11.3% vs. 6/161, 3.7%; p = 0.0094) whereas fewer commenced systemic corticosteroid therapy (73/104, 70.2% vs. 72/82, 87.8%; p = 0.004) and thiopurines (3/194, 1.5% vs. 9/161, 5.6%; p = 0.042). There was no difference in hospital admission rates or the need for elective or emergency surgery (Table S2).
3.4. Matched UC/IBD‐unclassified flare cohort
3.4.1. Patient demographics
The UC/IBD‐U flare patients were also matched for disease activity and age (Table S3). The pandemic and pre‐pandemic groups comprised 1112 and 1156 patients, respectively, with a median age of 38.0 and 39.0 years. Over 90% of the patients in each group had a diagnosis of UC. The groups were comparable with respect to median BMI, ethnicity, smoking status and comorbidities.
3.4.2. Disease phenotype
There were no differences in disease extent between the groups, with left‐sided disease the most common phenotype.
3.4.3. Baseline therapies
In the pandemic group, a larger proportion of patients was treated with a poorly bioavailable steroid (39/1112, 3.5% vs. 11/1156, 1.0%; p < 0.001), infliximab (89/1112, 8.0% vs. 66/1156, 5.7%; p = 0.037) or tofacitinib (29/1112, 2.6% vs. 14/1156, 1.2%; p = 0.020) at baseline, compared with the pre‐pandemic group. In contrast, a lower proportion was on no treatment (220/1112, 19.8% vs. 276/1156, 23.9%; p = 0.019). There was no difference in oral prednisolone use at baseline between the groups.
3.4.4. Disease activity
Partial Mayo score was calculated for 70.3% and 69.6% of patients in the matched pandemic and pre‐pandemic groups, respectively. The median score was 6.0, with the largest number of patients in each group suffering from moderate disease activity from PGA evaluation. The median peak CRP was lower in the pandemic group (16.0 mg/L [4.0–58.0] vs. 21.0 mg/L [5.0–67.4]; p = 0.0078), while the median peak faecal calprotectin (1138 mcg/g [489.0–2000.0] vs. 982 mcg/g [403.0–1800.0]; p = 0.0045) was significantly higher. Overall, over 80% of patients had full blood count and CRP checked during the flare and over 35% had faecal calprotectin measured (Tables S5 and S6). There were significant differences in the mode of diagnosing active UC between the two groups. During the pandemic, faecal calprotectin measurement (416/1112, 37.4% vs. 282/1156, 24.4%; p < 0.001) and imaging (127/1112, 11.4% vs. 102/1156, 8.8%; p = 0.043) were preferred, compared with reductions in the use of CRP (495/1112, 44.5% vs. 601/1156, 52.0%; p < 0.001) and endoscopy (364/1112, 32.7% vs. 564/1156, 48.8%; p < 0.001).
3.4.5. Treatment adaptations
New therapies
In the pandemic group, a higher percentage of patients was prescribed poorly bioavailable corticosteroids (131/1112, 11.8% vs. 60/1156, 5.2%; p < 0.001), vedolizumab (76/1112, 6.8% vs. 44/1156, 3.8%; p = 0.0014) or ustekinumab (14/1112, 1.3% vs. 1/1156, 0.1%; p < 0.0001) to treat active disease, compared with the disease activity‐matched pre‐pandemic group (Figure 3A,B). In contrast, a lower proportion of patients in the pandemic group was managed with oral prednisolone (372/1112, 33.5% vs. 470/1156, 40.7%, p < 0.001), intravenous corticosteroids (342/1112, 30.8% vs. 502/1156, 43.4%; p < 0.001) or thiopurines (54/1112, 4.9% vs. 82/1156, 7.1%; p = 0.027). Patient concern about COVID‐19 risk prompted treatment change in 13/1112 (1.2%) of cases, while physician concern was the reason for 30/1112 (2.7%) patients.
FIGURE 3.
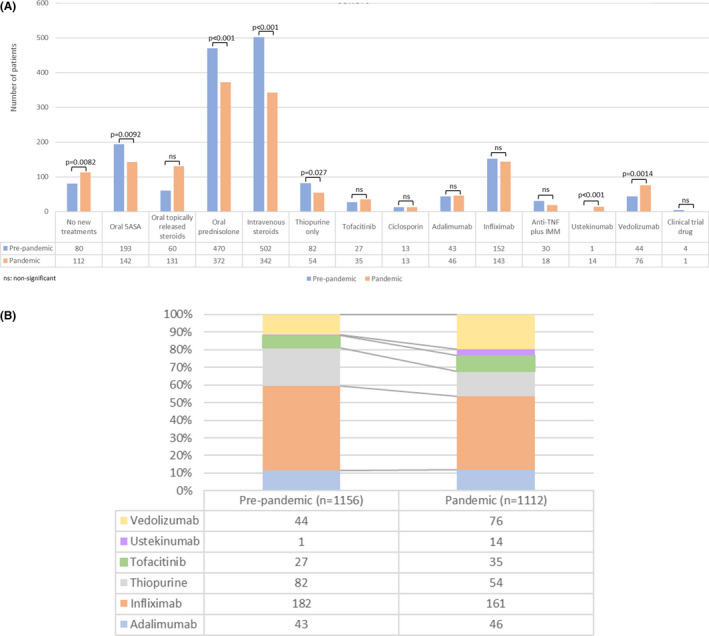
(A) Medications used to treat ulcerative colitis or IBD‐U flares in the pre‐pandemic and pandemic cohorts, following propensity score matching for age and physician global assessment of disease activity. (B) Proportion of patients with a Crohn's disease flare treated with different biologics and IMMs in the pre‐pandemic and pandemic cohorts, following propensity score matching for age and physician global assessment of disease activity. “n” numbers represent the total number of patients in each cohort. 5‐ASA, 5‐ aminosalicylate; IBD‐U, inflammatory bowel disease‐unspecified; IMM, immunomodulator; ns, not significant; TNF, tumour necrosis factor.
Initial outcome
There was a significant reduction in patients with acute flares attending the Emergency Department in the pandemic group (412/1104, 37.3% vs. 541/1142, 47.4%; p < 0.001). Similarly, a lower proportion of patients was admitted for hospital treatment (470/1107, 42.5% vs. 645/1146, 56.3%; p < 0.001). Three hundred and twenty‐six patients were admitted for acute severe UC, as determined by the Truelove and Witts criteria. A detailed description of these patients' outcomes has been published by our group in the PROTECT‐IBD study. 12
Three‐month follow‐up
The rates of steroid‐free remission (defined by PGA) were comparable between the two groups (pandemic: 312/858, 36.4% vs. pre‐pandemic: 404/1006, 40.2%; p = 0.095) (Figure 4). There were also no significant differences in disease activity between the two groups at 3‐month follow‐up or the proportion of patients who suffered from a flare (Figure 5). There were 634/866 (73.2%) patients with inactive or mild disease in the pandemic cohort compared with 773/1041 (74.2%, p = 0.23) in the pre‐pandemic cohort. A similar percentage of patients was in biochemical (140/704, 58.2% vs. 528/838, 63.0%, p = 0.059) and endoscopic remission (33/139, 23.7% vs. 61/249, 24.5%; p = 0.90) (Table S3). There was no statistical distinction between the proportion of patients who experienced a further flare of disease within this 3‐month period (250/951, 26.3% vs. 280/1091, 25.7%, p = 0.53). In the pandemic group, a higher proportion of patients was identified by a raised faecal calprotectin level (100/250, 40.0% vs. 59/280, 21.1%; p < 0.001), while fewer patients were diagnosed endoscopically (53/250, 21.2% vs. 93/280, 33.2%; p = 0.0025).
The treatment strategies employed for these flares were broadly similar between the two groups, including the use of oral prednisolone (83/250, 33.2% vs. 88/280, 31.4%; p = 0.71). However, more patients in the pandemic group were prescribed poorly bioavailable corticosteroids (20/250, 8.0% vs. 6/280, 2.1%; p = 0.021), and ustekinumab (5/250, 2.0% vs. 0/280, 0.0%; p = 0.023). There were no significant differences in rates of readmission to hospital or surgical intervention.
4. COVID‐19 COHORT
A total of 371 patients reported suspected COVID‐19 infection during the study period, of whom 194 (52%) had a positive SARS‐CoV‐2 PCR swab. When SARS‐CoV‐2 first affected the UK population, COVID‐19 testing was not widely available and patients were instructed to isolate for possible infection. Fifty‐six (15%) patients had negative swabs, but given concerns of false‐negative results, 30 continued to be managed as presumed COVID‐19 due to symptoms in keeping with acute infection. Sixty‐five patients from the COVID‐19 cohort also reported an IBD flare during the study period. Data relating to the timeline of IBD flare and COVID‐19 infection during the study period are incomplete. Of the 36 patients where this is available, 28 developed COVID‐19 after a flare of IBD, with a median interval of 2 weeks; 22 COVID‐19 infections followed an admission to hospital.
About 92.4% of the PCR‐positive COVID‐19 patients had typical symptoms, including fever, cough and breathlessness, which were reported by 69%, 63% and 41% of patients, respectively. Symptoms started a median of 6 (IQR 2–10) days prior to testing; 22.1% of patients suffered with gastrointestinal symptoms, with 15%, 8.8% and 4.1% reporting diarrhoea, abdominal pain and nausea and/or vomiting, respectively. There was no association between gastrointestinal symptoms and severe COVID outcomes.
COVID‐19 patients had a significantly higher mean age compared with those without COVID‐19 (51.0 years vs. 39.0 years, p = 0.001), with no significant difference in gender distribution. Patients with COVID‐19 had a higher prevalence of systemic comorbidities. There was no significant difference in disease phenotype between patients with COVID‐19 and those without. However, patients who suffered with COVID‐19 had a longer IBD disease duration compared with the pandemic or pre‐pandemic IBD flare cohorts (9.0 years vs. 4.0 years vs. 3.0 years, p = 0.001). No differences in PGA‐defined IBD disease activity were observed, with median HBI and pMayo scores for patients with Crohn's disease and UC/IBD‐U almost identical between the two groups.
COVID‐19 pneumonitis is associated with laboratory markers of inflammation, including a reduced haemoglobin, lymphocyte count and albumin, and raised CRP. These changes were magnified in patients who suffered with an IBD flare and suspected COVID‐19 infection during the study period, though the COVID‐19 data include patients in the community who would not have routinely undergone blood tests (Figure 6; Table S7).
FIGURE 6.
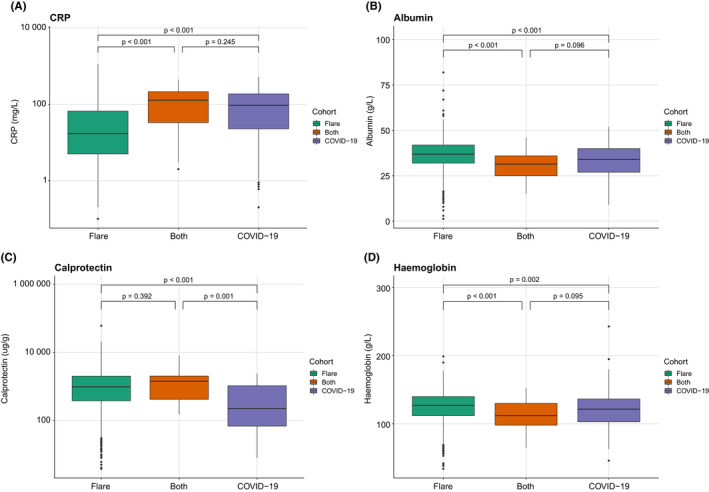
Comparison of selected laboratory markers, including CRP, albumin, faecal calprotectin and haemoglobin, between patients suffering an IBD flare, COVID‐19 infection or both, using Kruskal–Wallis test. CRP, C‐reactive protein; IBD, inflammatory bowel disease.
Compared with patients with an IBD flare only, those with an IBD flare and suspected COVID‐19 were more likely to attend the Emergency Department (26/42, 61.9% vs. 1054/2814, 37.5%; p = 0.003) or be admitted to hospital (44/64, 68.8% vs. 1132/2825, 40.1%; p < 0.001) for all causes: 54/65 patients with an IBD flare and suspected COVID‐19 underwent PCR testing, of which 29 (53.7%) were positive; 61/371 patients with suspected COVID‐19 infection had their IBD therapy changed due to concerns about COVID‐19 infection. Overall, there were no significant changes in treatment for an IBD flare between those patients who had suspected COVID‐19 during the study period and those who did not.
A total of 141 patients were admitted to hospital for COVID‐19 pneumonitis, of whom 24 were admitted to ICU and 20 required invasive ventilation. Patients with COVID‐19 during the study period were more likely to require admission to an ICU for any reason compared with those with IBD flares alone (35/223, 15.7% vs. 34/1123, 3.0%; p < 0.001): 35 patients died from COVID‐19 pneumonitis and associated complications. Patients with IBD flares without COVID‐19 infection who required ICU admission were predominantly post‐operative. Further details of this group are available in previous publications. 12
At 3 months, compared with patients who suffered with a flare only, those who flared and had suspected COVID‐19 infection were less likely to be in biochemical remission (9/32, 28.1% vs. 979/2419, 40.5%; p = 0.01) and were more likely to have moderate‐to‐severely active disease (15/29, 51.7% vs. 616/2206, 27.9%; p = 0.026). There was no statistical difference in steroid‐free remission, further IBD flares or requirement for steroid therapy. At 3 months, admission rates were higher amongst patients who suffered with both an IBD flare and suspected COVID‐19 infection initially (303/2410, 12.6% vs. 11/32, 34.4%; p = 0.005); it was not possible to determine the cause of admission due to paucity of data.
5. DISCUSSION
This large, national, multicentre observational cohort study has demonstrated important findings regarding the UK's response to IBD flares, treatment adaptations and patients' outcomes during the first wave of the COVID‐19 pandemic. Furthermore, to our knowledge, this represents one of the largest cohorts detailing IBD flares with patient‐level information internationally and provides key general observations and lessons about contemporary IBD management. Most significantly, and reassuringly, despite the various adaptations to standard treatment employed, there was no difference in steroid‐free remission, disease activity or risk of further IBD flares at 3 months between age‐ and disease activity‐matched pandemic and pre‐pandemic cohorts for both Crohn's disease and UC/IBD‐U. These adaptations included an increased use of poorly bioavailable corticosteroids or ustekinumab during the pandemic to treat both active Crohn's disease and UC/IBD‐U and more vedolizumab treatment for active UC/IBD‐U. There was reduced prescription of systemic corticosteroids in both groups and of thiopurines in the UC/IBD‐U group. During the pandemic, a higher proportion of patients was diagnosed with active disease using non‐endoscopic modalities, though the percentage of patients with active endoscopic disease was similar between the two cohorts. When compared with the uninfected pandemic group, patients with both active IBD and suspected COVID‐19 infection during the study period were less likely to be in biochemical remission, were more likely to have moderate‐to‐severely active disease and were at higher risk of hospital admission at 3 months.
The rapid onset and progression of the COVID‐19 pandemic in early 2020 generated much uncertainty and concern for patients with IBD and other chronic immune‐mediated inflammatory conditions. The initial lack of knowledge about the pathophysiology of COVID‐19 raised concerns regarding the potentially fatal consequences of immunosuppressive treatment on patients' ability to fight viral infections. Simultaneously, effective disease control would reduce disease flares, IBD complications and admission to overwhelmed hospitals that presented a risk of nosocomial COVID‐19 transmission. The continued emergence of novel variants with uncertain coverage from the current vaccines and unpredictable levels of pathogenicity and infectivity remains likely, so the fine immunosuppressive balance between disease control and the risk of COVID‐19 (and other respiratory infections) will continue to play a role in treatment decision‐making. 13 While the focus was largely on short‐term IBD goals during the first pandemic wave, it seems increasingly clear that acute COVID‐19 infection risk will become a chronic issue, fluctuating as public health intervention policies and seasons change. Accordingly, IBD specialists will need to tailor individuals' acute and maintenance treatment, aiming for prolonged, steroid‐free disease remission and subsequent complication‐free survival, while balancing their risk of COVID‐19 infection depending on factors such as age, comorbidity and place of work. 14 This is particularly apt given the UK‐based CLARITY study which demonstrates that anti‐spike neutralising antibody levels decay quickly after vaccination in patients taking infliximab, implying a vital role for timely booster doses. Furthermore, some patients mount undetectable B and/or T cell responses, where even boosting may not be beneficial. 15 This is supported by the bioarchived OCTAVE study looking at vaccine response with immunosuppression for a range of diseases. 16
Reassuringly, many drugs commonly used in IBD appear not to increase the risk of COVID‐19 infection and hospitalisation. 17 , 18 However, data from the SECURE‐IBD registry, including 525 patients internationally with IBD and COVID‐19 infection, and analyses of 600 patients with rheumatic disease from the Global Rheumatology Alliance registry, suggest that systemic corticosteroids increase the risk of severe COVID. 19 , 20 , 21 Our primary finding that 3‐month steroid‐free outcomes were comparable pre‐ and mid‐pandemic despite the shift towards poorly bioavailable steroid flare induction treatment is therefore important and reassuring. Biologics can then be safely instituted, notwithstanding the potential effect on vaccine efficacy, which may influence the timing of biologic commencement, as we move towards consideration of long‐term disease management in the context of COVID‐19 risk.
Specific treatment adaptations in IBD during the pandemic have been described, including cessation of immunomodulatory and anti‐TNF therapy and increased use of biologics in thiopurine‐naive patients. 22 However, the full range of adaptations and their impact on IBD outcomes have not previously been reported. Meanwhile, discontinuation of biologic therapy has been associated with risk of flares during the pandemic. 23 , 24 Together with the findings that SARS‐CoV‐2 acquisition or severe disease appear no more likely in patients with IBD than the general population, these studies largely favour IBD therapy continuation and escalation based on standard algorithms. 25 , 26 , 27
During the first pandemic wave, a higher proportion of patients contacting secondary care were judged to be experiencing a mild flare. It is possible that concerns regarding nosocomial COVID‐19 acquisition led to reduced thresholds for seeking specialist input or even over‐reporting of symptoms. The switch to virtual consultations may also have facilitated earlier clinical review. These initial modifications to the patient's journey are reflected in fewer blood tests and increased use of faecal calprotectin to confirm active IBD, and a reduction in endoscopic diagnosis of active UC, presumably to minimise hospital visits. Upon matching for disease activity across both Crohn's disease and UC, the most consistent treatment adaptation was the increased use of poorly bioavailable oral corticosteroids, both as a baseline therapy and new treatment for current and future flares within 3 months. This was accompanied by a significant reduction in the use of systemic corticosteroids (both intravenous and oral) for flare management, both initially and at 3 months. The use of vedolizumab (UC) and ustekinumab (UC and Crohn's disease) increased during the pandemic, which we assume was due to the better safety profile (poorly available oral corticosteroids, vedolizumab) and/or reduced need to attend hospital for infusions (ustekinumab). However, the UK approved ustekinumab for moderate‐to‐severe UC during the pandemic (June 2020), which may have contributed to this rise. Oral mesalazine prescriptions for UC patients experiencing a flare decreased, apart from in patients who suffered an IBD flare and COVID‐19 infection, where mesalazine dosing increased. This is presumed to be an attempt to avoid corticosteroid therapy, as these patients were managed before the efficacy of steroid treatment for COVID‐19 pneumonitis was established. 28 Thiopurine use decreased, presumably related to concerns of increased risk of severe viral infection. Crucially, despite these treatment adaptations, steroid‐free remission, disease activity and risk of further IBD flare at 3 months were no different between matched pandemic and pre‐pandemic cohorts of both CD and UC. This suggests that IBD teams adapted management appropriately for specific patients. This may also suggest that the preferential use of poorly bioavailable corticosteroids over conventional systemic steroid therapy for IBD flares in selected patients has been an underused strategy in the UK pre‐pandemic, with implications for side‐effect risk; in our pre‐pandemic cohort, 972/1156 (84.1%) patients with a UC flare and 495/708 (69.9%) of those suffering from a Crohn's disease flare were treated with intravenous or oral systemic corticosteroids. 29 , 30 , 31 However, the longer‐term effects of this strategy on our patient cohort are undefined; the feasibility of further follow‐up is being explored.
A lower proportion of patients suffering an IBD flare during the pandemic attended an Emergency Department or were admitted to hospital compared with the pre‐pandemic period. In the UK, people were actively encouraged to isolate and avoid face‐to‐face encounters unless necessary, to reduce viral transmission. This, alongside fear of the rising case and fatality rates and the desire not to add to the burden of an increasingly stretched health service, would have influenced patient decision‐making, leading them to contact hospital departments via telephone for advice or self‐manage their condition at home. This change has been seen across the world, with tele‐medicine use increasing exponentially during the COVID‐19 pandemic. 32 Similarly, access to endoscopy services in the UK during the first wave was significantly reduced due to concerns over potential SARS‐CoV‐2 exposure, intra‐procedural transmission and staff redeployment. 33 , 34 In line with this, we found that patients experiencing a further UC/IBD‐U flare at baseline and within 3 months were less likely to undergo endoscopic evaluation and more likely to have non‐invasive assessment of disease activity such as faecal calprotectin.
The number of reported COVID‐19 cases in IBD patients seemed low given the population served by the hospitals involved in the study. However, we recognise further cases may have been unreported or asymptomatic. Only 67.9% of the 371 patients with suspected COVID‐19 underwent PCR testing, reflecting the reduced access when the pandemic first emerged; 22.6% of patients reported gastrointestinal symptoms with their suspected COVID‐19 infection, which is a low incidence compared with published rates of 53%. 35 We conjecture this is due to IBD patients having a higher threshold at which they report gastrointestinal symptoms or an assumption that symptoms were related to IBD or functional overlay. Twenty‐two patients developed COVID‐19 symptoms following hospital admission, which represents only 1.6% (22/1335) of IBD hospital admissions in this study.
Although some patients had their IBD therapy changed due to suspected COVID‐19 infection, there were no significant differences in therapeutic interventions in these two cohorts. Patients who had an IBD flare and suspected COVID‐19 infection during the pandemic were more likely to have active disease at 3 months, and a flare in the subsequent 3 months. This is not explained by differences in treatment adaptations and may relate to compliance during the pandemic. In the event of further waves of COVID‐19 infection, consideration should be given to enhanced monitoring of this group after the index presentation. The low numbers in this group, the relatively poor sensitivity of PCR testing early in the pandemic and reduced reporting may have reduced the statistical strength of this finding.
This study has several strengths, most notably the considerable sample size across many hospitals, together with inclusion of a propensity matched control group from a pre‐pandemic period which mitigated against the differences in disease activity between the two cohorts. To our knowledge, this is the largest published cohort of patients suffering with an IBD flare, both within and outside of the pandemic. Other reports have dealt with the effects of COVID‐19 infection in patients with IBD. In contrast, this study also reports management of IBD flares in patients without COVID‐19 infection and the adaptations made during the pandemic, alongside medium‐term outcomes. These findings have implications for flare treatment outside the pandemic setting, particularly with regards to systemic corticosteroid use. We accept that our study also has limitations. PREPARE‐IBD was a retrospective study and therefore carries inherent risks of missing data points. This was particularly relevant for collection of 3‐month outcome data. Furthermore, propensity matching did not match for hospitals, and consequently did not account for inter‐provider differences in therapeutic approach. However, hospitals in the UK largely follow British Society of Gastroenterology guidelines, so this effect is not likely to be relevant. 27 We recognise that this study reflects prescribing practice, drug availability and resource in the UK which may differ from other countries, though we believe the findings remain relevant for clinical decision‐making in other healthcare settings. While a large overall cohort of patients was recruited, it was not possible for clinical staff to provide detailed data for all eligible patients from their centres due to time capacity during the height of the pandemic, which may have led to selection bias. This may partly explain the high proportion of patients suffering an IBD flare who presented to the Emergency Department or were admitted to hospital in this study. Similarly, adverse events may not have been captured if management was community‐based or IBD teams were not informed, causing potential under‐reporting. The disease activity stratification of patients within this study relies heavily on PGA, which needed to be inferred by researchers from clinical notes. Particularly during the early pandemic, the usual objective tools for determining ‘true’ inflammatory flares were largely unavailable: PGA, informed partly by clinical measures, such as Harvey‐Bradshaw Index and Mayo scores, was therefore the predominant way for clinicians—and, subsequently, researchers—to evaluate the need and nature of escalated therapy. A set of strict criteria involving examination, biomarker or endoscopy findings for active disease was therefore not included in the protocol. While we accept that some patients will have been treated despite having non‐inflammatory symptoms, this simply reflects the unconventional patient and physician behaviours provoked by the pandemic, which we have described. In any case, regarding this study, the same researchers extracted data and recorded PGA for both the pandemic and pre‐pandemic cohorts, providing a degree of internal validity.
6. CONCLUSION
PREPARE‐IBD, a multicentre observational cohort study, has demonstrated a range of treatment adaptations of IBD flares in the UK during the COVID‐19 pandemic. Most notably, these include a reduction of systemic corticosteroids and increased use of poorly bioavailable corticosteroids. While the long‐term effect of these adaptations on disease control is unknown, IBD outcomes at 3 months were not compromised, which is testament to the agility of IBD clinicians in the UK during this unprecedented time. Aside from the pandemic, this large dataset suggests that systemic corticosteroids to manage IBD flares may be safely avoided in more patients than previously supposed, and this strategy could be considered more widely post‐pandemic. Future work will interrogate this large multicentre IBD cohort more deeply to explore current IBD management practices and consider strategies to improve care.
AUTHOR CONTRIBUTIONS
Aamir Saifuddin: Writing – original draft (lead); writing – review and editing (lead). Alexandra J. Kent: Conceptualization (equal); methodology (equal); visualization (lead); writing – original draft (lead); writing – review and editing (lead). Shameer J. Mehta: Conceptualization (equal); methodology (equal); writing – original draft (lead); writing – review and editing (lead). Lucy C. Hicks: Conceptualization (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Haidee A. Gonzalez: Conceptualization (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Jonathan P. Segal: Conceptualization (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Matthew J. Brookes: Conceptualization (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Sreedhar Subramanian: Conceptualization (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Neeraj Bhala: Conceptualization (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Thomas E. Conley: Conceptualization (equal); methodology (equal). Kamal V. Patel: Conceptualization (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Christopher A. Lamb: Conceptualization (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Gareth J. Walker: Conceptualization (equal); formal analysis (supporting); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Nicholas A. Kennedy: Conceptualization (equal); data curation (lead); formal analysis (lead); methodology (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Shaji Sebastian: Conceptualization (lead); data curation (supporting); methodology (lead); project administration (lead); resources (lead); supervision (lead); writing – original draft (equal); writing – review and editing (equal).
AUTHORSHIP
All authors have approved the final manuscript.
Guarantor of article: Shaji Sebastian.
FUNDING INFORMATION
The study was funded by Hull University Teaching Hospitals NHS Trust.
CONFLICTS OF INTEREST
SS holds research grants from Biogen, Takeda, AbbVie, Tillotts Pharma, Ferring and Biohit; served on the advisory boards of Takeda, AbbVie, Merck, Ferring, Pharmacocosmos, Warner Chilcott, Janssen, Falk Pharma, Biohit, TriGenix, Celgene and Tillots Pharma; and has received speaker fees from AbbVie, Biogen, AbbVie, Janssen, Merck, Warner Chilcott and Falk Pharma. GJW has served as a speaker and/or advisory board member for AbbVie, Falk and Janssen. He has had support to attend meetings from AbbVie, Falk, Janssen and Norgine. His department has received research funding from Tillotts. NAK has served as a speaker and/or advisory board member for Allergan, Falk, Janssen, Mylan, Pharmacosmos, Takeda and Tillotts. He has had support to attend meetings from AbbVie, Falk, Janssen and Norgine. His department has received research funding from AbbVie, Celgene, Celtrion, MSD, Napp, Pfizer, Pharmacosmos and Takeda. SrS has received speaker fees from MSD, Actavis, Abbvie, Dr Falk pharmaceuticals, Shire and received educational grants from MSD, Abbvie, Actavis and is an advisory board member for Celltrion, Dr Falk pharmaceuticals and Vifor pharmaceuticals. CAL has received research support and/or has received fees for delivery of non‐promotional education from: Genentech, Janssen, Takeda, Abbvie, Dr Falk, AstraZeneca, Eli Lilly, Orion, Pfizer, Roche, Sanofi Aventis, Ferring, UCB and Biogen. MJB has received research grants from Vifor International, Pharmacosmos and Tillots Pharma; has received speaker fee from Abbvie and Vifor International; has been an advisory board member for Tillots Pharma, Vifor International; and received travel/conference expenses from Vifor International, Abbvie and Tillots Pharma. AJK has served on the advisory boards for Abbvie, Janssen and BMS Celgene and has received speaker fees from Takeda, Pfizer and Janssen; and received travel/conference expenses from Tillotts, Janssen, Abbvie and Shield Therapeutics. KP has received honoraria for educational meetings and speaker fees from Abbvie, Janssen, Takeda, DrFalk and Ferring. KP has received Advisory Board fees from Abbvie and Janssen. TEC has received speaker fees from Celltrion. LCH, HAG, SJM, AS have no conflicts of interest to declare.
Supporting information
Appendix S1
ACKNOWLEDGEMENTS
We are grateful to the clinical and research teams in the participating sites for identification of patients, data collection and data entry. UK gastroenterology trainees and trainee networks (MaGNET: Mersey Gastroenterology Network, GLINT: Gastro London Investigative Network for Trainees, WMRIG: West Midlands Research in Gastroenterology, GasTRIN NoW: Gastroenterology Trainee Research and Improvement Network North‐West, OxYGEN: The Oxford and Thames Valley Young Gastroenterologists Network, TReNDD NI: Trainee Research Network in Digestive Diseases Northern Ireland) were integral in data collection for this study. We appreciate support from Crohn's & Colitis UK and the British Society of Gastroenterology for promotion of this study.
Contributors (PREPARE‐IBD collaborators)
Shukri Abdale, Abdulla Abbesi, Anwar Abusrewil, Precious Aghimien, Saeed Ahmed, Akram Ali, Amjad Ali, Jad Alkhouri, Patrick Allen, Ammar Al‐Rifaie, Richard Appleby, Ramesh Arasaradnam, Naila Arebi, Bradley Arms‐Williams, Muteeb Ashraf, Andrea Au, Tamar Avades, Homira Ayubi, Samantha Baillie, Sharmili Balarajah, Aaron Bancil, Abdul Basit, Murad Bayati, Andrew Bell, Alexander Berry, Neeraj Bhala, Shivaram Bhat, Joya Bhattacharyya, Sophia Bishop, Laura Blackmore, Ashley Bond, Simon Borg‐Bartolo, Emma Botwright, Sonia Bouri, Stephen Boyle, Neil Bradley, Fiona Brailsford, Ewen Brennan, Deborah Britton, Matthew Brooks, Caitlin Brown, Rhys Butcher, Jeff Butterworth, Rachel Campbell, Roisin Campbell, Iona Campbell, Ruth Carr, Josiah Carter, Peter Cartlidge, Rajiv Chandy, Kelly Chatten, Rakesh Chaudhary, Desmond Chee, Jonathan Chessbrough, Andrea Churchhouse, Jennie Clough, Alexander Cole, Thomas Conley, Johannah Cook, Rachel Cooney, Sarah Cotton, Tamsin Critchlow, Frederic Cuison, Chris Curran, Ana‐Marie Darie, Robin Dart, Pantong Davwar, Kasamu Kabiru Dawa, Anjan Dhar, Shahida Din, Kok Leong Diong, Benjamin Disney, Emma Dooks, Louise Downey, Anita D'Souza, Lovesh Dyall, Mary Elias, Richard Felwick, Michael Finegan, Paul Flanagan, Rishi Fofaria, Steven Fong, Richard Fox, Aileen Fraser, Christian Frunza, Alhasssan Ghodeif, Nivedita Ghosh, Leah Gilroy, Haidee Aleman Gonzalez, Larissa Good, John Gordon, Nicola Grasso, Aurelian Gueroult, James Gulliver, Sarah Guthrie, Markus Gwiggner, Mina Hanna, Christopher Harlow, Wendy Harrison, Ailsa Hart, Barney Hawthorne, Lucy Hicks, Patrick Hooper, Willow Howard, Nassir Hussain, Thomas Hutton, Aye Mya Htun, Peter Irving, Reema Jagdish, Anun Javed, Asima Javed, Nishani Jayasooriya, Matthew Johnson, Emma Johnston, Gareth‐Rhys Jones, Cynthia Kanagasundaram, Foteini Karagkouni, Karen Kemp, Nick Kennedy, Alexandra Kent, Hesham Khalil, Najeebullah Khan, Zahid Khan, Mais Khasawneh, Andy King, Beverley Kirkham, Fiona Kirkham, Flora Kokwaro, Mohammed Korani, Ioannis Koumoutsos, Aditi Kumar, Anish John Kuriakose Kuzhiyanjal, Martyn Lakeland, Christopher Lamb, Sophie Laverick, Charlie Lees, Emma Levell, Scott Levison, Samuel Lim, Yuen‐Hui Lim, Jimmy Limdi, James Lindsay, Jessica Lisle, Alan Lobo, Raphael Luber, Laura Lucacui, Holly Lyne, Jonathan MacDonald, Aarani Mahalingam, Sara Mahgoub, Ridhima Malakar, Fenella Marley, Joy Mason, Ali Masri, Zia Mazhar, Hannah McCaughan, Tracy McCort, Adam McCulloch, Stuart McIlwaine, Nirmol Meah, Leila Mebarek, Shameer Mehta, Mike Mendall, Nassir Mir, Victoria Moffatt, Gordon Moran, Liam Morris, Gary Morrison, Graham Morrison, Robert Mulligan, Charles Murray, Jenny Murray, Sally Myers, Pineshwari Naeck‐Boolauky, Andres Naranjo, Janu Navaratnam, Deanna Naylor, Emma Nixon, Kirsty Nixon, Jonathan Nolan, Olaolu Olabintan, Elaine Ong Ming San, Hayley Owen, Christopher Palmer‐Jones, Kamal Patel, Kalyan Peddada, Leon Pee, Mohammed Peerally, Rebecca Perkins, Frank Phillips, Keith Pohl, Richard Pollok, Nick Powell, Farah Qayyum, Maria Quarashi, Mohammed Nabil Quraishi, Elizabeth Radcliffe, Shellie Radford, Sohail Rahmany, Hanin Ramadan, Arvind Ramadas, Anne Reddington, Christy Riggott, Tom Riley, Peter Rimmer, Susan Ritchie, Konstantina Rosiou, Siobhan Rowland, Joseph Sabine, Aamir Saifuddin, Shahzad Sarwar, Ayodele Sasegbon, Jayne Saunders, Shaji Sebastian, Gregory Sebepos‐Rogers, John Paul Seenan, Jonathan Segal, Christian Selinger, Solange Serna, Sonika Sethi, Matthew Shale, Richard Shenderey, Achuth Shenoy, Yousuf Sherifat, Roosy Sheth, Spyros Siakavellas, Rafid Sikafi, Amar Singh, Salil Singh, Updesh Singh, Ganesh Sivaji, Philip Smith, Ally Speight, Andy Spence, Cath Stansfield, Helen Steed, Sreedhar Subramanian, Kishaani Suseehran, Maria Tabuso, Donatas Taucius, Joanne Taylor, Amit Thakor, Tony Tham, Gill Townsend, Tristan Townsend, Thomas Troth, Ruth Tunney, Kelly Turner, Nosheen Umar, Vithushan Vakeeswarasarma, Ajay Verma, Gareth Walker, Hazel Wallace, Hannah Walton, Bo Wang, Eleanor Warner, Callum Watson, Susie Wen, Monika Widlak, Maureen Williams, Amy Woods, Lisa Younge, Mansoor Zafar.
Saifuddin A, Kent AJ, Mehta SJ, Hicks LC, Gonzalez HA, Segal JP, et al. Treatment adaptations and outcomes of patients experiencing inflammatory bowel disease flares during the early COVID‐19 pandemic: the PREPARE‐IBD multicentre cohort study. Aliment Pharmacol Ther. 2022;56:1460–1474. 10.1111/apt.17223
Aamir Saifuddin, Alexandra J. Kent and Shameer J. Mehta contributed equally.
The Handling Editor for this article was Professor Richard Gearry, and it was accepted for publication after full peer‐review.
**PREPARE‐IBD Collaborators—see Appendix.
Contributor Information
Aamir Saifuddin, Email: m.saifuddin@nhs.net.
PREPARE‐IBD Collaborators:
Shukri Abdale, Abdulla Abbesi, Anwar Abusrewil, Precious Aghimien, Saeed Ahmed, Akram Ali, Amjad Ali, Jad Alkhouri, Patrick Allen, Ammar Al‐Rifaie, Richard Appleby, Ramesh Arasaradnam, Naila Arebi, Bradley Arms‐Williams, Muteeb Ashraf, Au Andrea, Tamar Avades, Homira Ayubi, Samantha Baillie, Sharmili Balarajah, Aaron Bancil, Abdul Basit, Murad Bayati, Andrew Bell, Alexander Berry, Neeraj Bhala, Shivaram Bhat, Joya Bhattacharyya, Sophia Bishop, Laura Blackmore, Ashley Bond, Simon Borg‐Bartolo, Emma Botwright, Sonia Bouri, Stephen Boyle, Neil Bradley, Fiona Brailsford, Ewen Brennan, Deborah Britton, Matthew Brooks, Caitlin Brown, Rhys Butcher, Jeff Butterworth, Rachel Campbell, Roisin Campbell, Iona Campbell, Ruth Carr, Josiah Carter, Peter Cartlidge, Rajiv Chandy, Kelly Chatten, Rakesh Chaudhary, Desmond Chee, Jonathan Chessbrough, Andrea Churchhouse, Jennie Clough, Alexander Cole, Thomas Conley, Johannah Cook, Rachel Cooney, Sarah Cotton, Tamsin Critchlow, Frederic Cuison, Chris Curran, Ana‐Marie Darie, Robin Dart, Pantong Davwar, Kasamu Kabiru Dawa, Anjan Dhar, Shahida Din, Kok Leong Diong, Benjamin Disney, Emma Dooks, Louise Downey, Anita D’Souza, Lovesh Dyall, Mary Elias, Richard Felwick, Michael Finegan, Paul Flanagan, Rishi Fofaria, Steven Fong, Richard Fox, Aileen Fraser, Christian Frunza, Alhasssan Ghodeif, Nivedita Ghosh, Leah Gilroy, Haidee Aleman Gonzalez, Larissa Good, John Gordon, Nicola Grasso, Aurelian Gueroult, James Gulliver, Sarah Guthrie, Markus Gwiggner, Mina Hanna, Christopher Harlow, Wendy Harrison, Ailsa Hart, Barney Hawthorne, Lucy Hicks, Patrick Hooper, Willow Howard, Nassir Hussain, Thomas Hutton, Aye Mya Htun, Peter Irving, Reema Jagdish, Anun Javed, Asima Javed, Nishani Jayasooriya, Matthew Johnson, Emma Johnston, Gareth‐Rhys Jones, Cynthia Kanagasundaram, Foteini Karagkouni, Karen Kemp, Nick Kennedy, Alexandra Kent, Hesham Khalil, Najeebullah Khan, Zahid Khan, Mais Khasawneh, Andy King, Beverley Kirkham, Fiona Kirkham, Flora Kokwaro, Mohammed Korani, Ioannis Koumoutsos, Aditi Kumar, Anish John Kuriakose Kuzhiyanjal, Martyn Lakeland, Christopher Lamb, Sophie Laverick, Charlie Lees, Emma Levell, Scott Levison, Samuel Lim, Yuen‐Hui Lim, Jimmy Limdi, James Lindsay, Jessica Lisle, Alan Lobo, Raphael Luber, Laura Lucacui, Holly Lyne, Jonathan MacDonald, Aarani Mahalingam, Sara Mahgoub, Ridhima Malakar, Fenella Marley, Joy Mason, Ali Masri, Zia Mazhar, Hannah McCaughan, Tracy McCort, Adam McCulloch, Stuart McIlwaine, Nirmol Meah, Leila Mebarek, Shameer Mehta, Mike Mendall, Nassir Mir, Victoria Moffatt, Gordon Moran, Liam Morris, Gary Morrison, Graham Morrison, Robert Mulligan, Charles Murray, Jenny Murray, Sally Myers, Pineshwari Naeck‐Boolauky, Andres Naranjo, Janu Navaratnam, Deanna Naylor, Emma Nixon, Kirsty Nixon, Jonathan Nolan, Olaolu Olabintan, Elaine Ong Ming San, Hayley Owen, Christopher Palmer‐Jones, Kamal Patel, Kalyan Peddada, Leon Pee, Mohammed Peerally, Rebecca Perkins, Frank Phillips, Keith Pohl, Richard Pollok, Nick Powell, Farah Qayyum, Maria Quarashi, Mohammed Nabil Quraishi, Elizabeth Radcliffe, Shellie Radford, Sohail Rahmany, Hanin Ramadan, Arvind Ramadas, Anne Reddington, Christy Riggott, Tom Riley, Peter Rimmer, Susan Ritchie, Konstantina Rosiou, Siobhan Rowland, Joseph Sabine, Aamir Saifuddin, Shahzad Sarwar, Ayodele Sasegbon, Jayne Saunders, Shaji Sebastian, Gregory Sebepos‐Rogers, John Paul Seenan, Jonathan Segal, Christian Selinger, Solange Serna, Sonika Sethi, Matthew Shale, Richard Shenderey, Achuth Shenoy, Yousuf Sherifat, Roosy Sheth, Spyros Siakavellas, Rafid Sikafi, Amar Singh, Salil Singh, Updesh Singh, Ganesh Sivaji, Philip Smith, Ally Speight, Andy Spence, Cath Stansfield, Helen Steed, Sreedhar Subramanian, Kishaani Suseehran, Maria Tabuso, Donatas Taucius, Joanne Taylor, Amit Thakor, Tony Tham, Gill Townsend, Tristan Townsend, Thomas Troth, Ruth Tunney, Kelly Turner, Nosheen Umar, Vithushan Vakeeswarasarma, Ajay Verma, Gareth Walker, Hazel Wallace, Hannah Walton, Bo Wang, Eleanor Warner, Callum Watson, Susie Wen, Monika Widlak, Maureen Williams, Amy Woods, Lisa Younge, and Mansoor Zafar
REFERENCES
- 1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirchgesner J, Lemaitre M, Carrat F, Zureik M, Carbonnel F, Dray‐Spira R. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology. 2018;155(2):337–346.e10. 10.1053/J.GASTRO.2018.04.012 [DOI] [PubMed] [Google Scholar]
- 3. Kucharzik T, Ellul P, Greuter T, Rahier JF, Verstockt B, Abreu C, et al. ECCO guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohn's Colitis 2021;15(6):879–913. 10.1093/ECCO-JCC/JJAB052 [DOI] [PubMed] [Google Scholar]
- 4. Tinsley A, Navabi S, Williams ED, Liu G, Kong L, Coates MD, et al. Increased risk of influenza and influenza‐related complications among 140,480 patients with inflammatory bowel disease. Inflamm Bowel Dis. 2019;25(2):369–76. 10.1093/IBD/IZY243 [DOI] [PubMed] [Google Scholar]
- 5. Kennedy NA, Jones GR, Lamb CA, Appleby R, Arnott I, Beattie RM, et al. British Society of Gastroenterology guidance for management of inflammatory bowel disease during the COVID‐19 pandemic. Gut. 2020;69(6):984–90. 10.1136/GUTJNL-2020-321244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ungaro RC, Brenner EJ, Agrawal M, Zhang X, Kappelman MD, Colombel JF, et al. Impact of medications on COVID‐19 outcomes in inflammatory bowel disease: analysis of more than 6000 patients from an international registry. Gastroenterology. 2022;162(1):316–319.e5. 10.1053/J.GASTRO.2021.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ricciuto A, Lamb CA, Benchimol EI, Walker GJ, Kennedy NA, Kuenzig ME, et al. Inflammatory bowel disease clinical activity is associated with COVID‐19 severity especially in younger patients. J Crohns Colitis. 2022;16(4):591–600. 10.1093/ECCO-JCC/JJAB172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)‐a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. 10.1016/J.JBI.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a working party of the 2005 Montreal world congress of gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5A–36A. 10.1155/2005/269076 [DOI] [PubMed] [Google Scholar]
- 10. Gharaibeh A, Koppikar S, Bonilla‐Escobar FJ. Strengthening the reporting of observational studies in epidemiology (STROBE) in the International Journal of Medical Students. Int J Med Stud. 2014;2(2):36–7. 10.5195/IJMS.2014.76 [DOI] [Google Scholar]
- 11. Lang TA, Altman DG. Basic statistical reporting for articles published in biomedical journals: the “statistical analyses and methods in the published literature” or the SAMPL guidelines. Int J Nurs Stud. 2015;52(1):5–9. 10.1016/J.IJNURSTU.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 12. Sebastian S, Walker GJ, Kennedy NA, Conley TE, Patel KV, Subramanian S, et al. Assessment, endoscopy, and treatment in patients with acute severe ulcerative colitis during the COVID‐19 pandemic (PROTECT‐ASUC): a multicentre, observational, case‐control study. Lancet Gastroenterol Hepatol. 2021;6(4):271–81. 10.1016/S2468-1253(21)00016-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. WHO Coronavirus (COVID‐19) Dashboard . WHO Coronavirus (COVID‐19) Dashboard with Vaccination Data [Internet]. Available from: https://covid19.who.int/ Accessed 18 May 2022.
- 14. Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, et al. STRIDE‐II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat‐to‐target strategies in IBD. Gastroenterology. 2021;160(5):1570–83. 10.1053/j.gastro.2020.12.031 [DOI] [PubMed] [Google Scholar]
- 15. Lin S, Kennedy NA, Saifuddin A, Reynolds CJ, Seoane RC, Kottoor SH, et al. Antibody decay, T cell immunity and breakthrough infections following two SARS‐CoV‐2 vaccine doses in inflammatory bowel disease patients treated with infliximab and vedolizumab. Nat Commun. 2022;13(1):1–14. 10.1038/s41467-022-28517-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kearns P, Siebert S, Willicombe M, Gaskel C, Kirkham A, Pirrie S, et al. Examining the Immunological Effects of COVID‐19 Vaccination in Patients with Conditions Potentially Leading to Diminished Immune Response Capacity – The OCTAVE Trial. Available at SSRN: https://ssrn.com/abstract=3910058 or 10.2139/ssrn.3910058 [DOI]
- 17. Meyer A, Semenzato L, Zureik M, Weill A, Carbonnel F, Dray‐Spira R. Risk of severe COVID‐19 in patients treated with IBD medications: a French nationwide study. Aliment Pharmacol Ther. 2021;54(2):160–6. 10.1111/APT.16410 [DOI] [PubMed] [Google Scholar]
- 18. Ungaro RC, Brenner EJ, Gearry RB, Kaplan GG, Kissous‐Hunt M, Lewis JD, et al. Effect of IBD medications on COVID‐19 outcomes: results from an international registry. Gut. 2021;70(4):725–32. 10.1136/GUTJNL-2020-322539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gianfrancesco M, Hyrich KL, Al‐Adely S, Carmona L, Danila MI, Gossec L, et al. Characteristics associated with hospitalisation for COVID‐19 in people with rheumatic disease: data from the COVID‐19 global rheumatology Alliance physician‐reported registry. Ann Rheum Dis. 2020;79(7):859–66. 10.1136/ANNRHEUMDIS-2020-217871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lamb CA, Sebastian S, Kent AJ, Segal JP, Gonzalez HA, Brookes MJ, et al. Letter: risk of severe COVID‐19 outcomes associated with inflammatory bowel disease medications—reassuring insights from the United Kingdom PREPARE‐IBD multicentre cohort study. Aliment Pharmacol Ther. 2021;53(11):1236–40. 10.1111/APT.16349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brenner EJ, Ungaro RC, Gearry RB, Kaplan GG, Kissous‐Hunt M, Lewis JD, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID‐19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159(2):481–491.e3. 10.1053/J.GASTRO.2020.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sharma E, Meade S, D'Errico F, Pavlidis P, Luber R, Zeki S, et al. The effects of COVID‐19 on IBD prescribing and service provision in a UK tertiary centre. Gastrohep. 2020;2(6):318–26. 10.1002/YGH2.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen J, Peng X, Zhang M, Zhi M. Impact of medication discontinuation on patients with inflammatory bowel disease during the COVID‐19 outbreak. Gastroenterology. 2021;160(6):2223. 10.1053/J.GASTRO.2020.05.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rizzello F, Calabrese C, Salice M, Calandrini L, Privitera H, Melotti L, et al. COVID‐19 in IBD: the experience of a single tertiary IBD center. Dig Liver Dis. 2021;53(3):271–6. 10.1016/J.DLD.2020.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khan N, Patel D, Xie D, Pernes T, Lewis J, Yang YX. Are patients with inflammatory bowel disease at an increased risk of developing SARS‐CoV‐2 than patients without inflammatory bowel disease? Results from a Nationwide Veterans' Affairs cohort study. Am J Gastroenterol. 2021;116(4):808–10. 10.14309/AJG.0000000000001012 [DOI] [PubMed] [Google Scholar]
- 26. Ludvigsson JF, Axelrad J, Halfvarson J, Khalili H, Larsson E, Lochhead P, et al. Inflammatory bowel disease and risk of severe COVID‐19: a nationwide population‐based cohort study in Sweden. United European Gastroenterol J. 2021;9(2):177–92. 10.1002/UEG2.12049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–s106. 10.1136/gutjnl-2019-318484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Group TRC . Dexamethasone in hospitalized patients with Covid‐19. N Engl J Med. 2020;384(8):693–704. 10.1056/NEJMOA2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. IBD UK . Steroid Management [Internet]. Available from: https://ibduk.org/ibd‐standards/flare‐management/steroid‐management Accessed 18 May 2022.
- 30. Selinger CP, Parkes GC, Bassi A, Fogden E, Hayee B, Limdi JK, et al. A multi‐centre audit of excess steroid use in 1176 patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46(10):964–73. 10.1111/APT.14334 [DOI] [PubMed] [Google Scholar]
- 31. Blackwell J, Selinger C, Raine T, Parkes G, Smith MA, Pollok R. Steroid use and misuse: a key performance indicator in the management of IBD. Frontline Gastroenterol. 2021;12(3):207–13. 10.1136/FLGASTRO-2019-101288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lees CW, Regueiro M, Mahadevan U. Innovation in inflammatory bowel disease care during the COVID‐19 pandemic: results of a global telemedicine survey by the International Organization for the study of inflammatory bowel disease. Gastroenterology. 2020;159(3):805–808.e1. 10.1053/J.GASTRO.2020.05.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rees CJ, Rutter MD, Sharp L, Hayee B, East JE, Bhandari P, et al. COVID‐19 as a barrier to attending for gastrointestinal endoscopy: weighing up the risks. Lancet Gastroenterol Hepatol. 2020;5(11):960–2. 10.1016/S2468-1253(20)30268-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hayee B, Thoufeeq M, Rees CJ, Penman I, East J. Safely restarting GI endoscopy in the era of COVID‐19. Gut. 2020;69(12):2063–70. 10.1136/GUTJNL-2020-321688 [DOI] [PubMed] [Google Scholar]
- 35. Elmunzer BJ, Spitzer RL, Foster LD, Merchant AA, Howard EF, Patel VA, et al. Digestive manifestations in patients hospitalized with coronavirus disease 2019. Clin Gastroenterol Hepatol. 2021;19(7):1355–1365.e4. 10.1016/J.CGH.2020.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


