Abstract
The uropathogenic Escherichia coli strain CFT073 has multiple iron acquisition systems, including heme and siderophore transporters. A tonB mutant derivative of CFT073 failed to use heme as an iron source or to utilize the siderophores enterobactin and aerobactin, indicating that transport of these compounds in CFT073 is TonB dependent. The TonB− derivative showed reduced virulence in a mouse model of urinary tract infection. Virulence was restored when the tonB gene was introduced on a plasmid. To determine the importance of the individual TonB-dependent iron transport systems during urinary tract infections, mutants defective in each of the CFT073 high-affinity iron transport systems were constructed and tested in the mouse model. Mouse virulence assays indicated that mutants defective in a single iron transport system were able to infect the kidney when inoculated as a pure culture but were unable to efficiently compete with the wild-type strain in mixed infections. These results indicate a role for TonB-dependent systems in the virulence of uropathogenic E. coli strains.
The virulence of pathogenic Escherichia coli is dependent upon the ability to multiply in host tissues (14). Iron is an essential nutrient for growth of E. coli, but the availability of this element within the host is limited. The bulk of the iron in humans is intracellular, predominately found in heme proteins or sequestered in ferritin, while the smaller amount of extracellular iron is tightly bound to high-affinity iron-binding proteins (30, 39). Pathogenic E. coli bacteria have evolved a variety of mechanisms to acquire iron from host sources (14). One mechanism is the synthesis and transport of siderophores, low-molecular-weight iron chelators with a high affinity for iron (8). Most E. coli strains, and other enteric bacteria, produce the catechol siderophore enterobactin (11). Additionally, aerobactin, a hydroxamate siderophore, is produced by many E. coli strains isolated from patients with urinary tract infection (UTI), bacteremia, or other extraintestinal infections (22). The precise contribution to virulence of either aerobactin or enterobactin is not well established (14). Another mechanism for iron acquisition in pathogenic E. coli is the direct utilization of host iron compounds, particularly heme or hemoglobin (25, 26). We showed previously that a chromosomal locus encoding heme utilization genes is widely distributed among pathogenic E. coli and Shigella dysenteriae strains (32, 51, 55).
Transport of iron associated with siderophores or heme requires specific outer membrane receptors and depends upon TonB and its accessory proteins, ExbB and ExbD (5, 40). The TonB protein, which is anchored in the cytoplasmic membrane, provides energy to the outer membrane receptors for the transport of iron compounds (5). The contribution of TonB-dependent iron transport systems in virulence has been assessed with several gram-negative pathogens. Mutations in tonB of S. dysenteriae (41), Salmonella enterica serovar Typhimurium and Salmonella enterica serovar Typhi (13, 52), Vibrio cholerae (19, 45), Pseudomonas aeruginosa (49), or Haemophilus influenzae (21) resulted in an avirulent phenotype in animal models. However, it is not clear whether the loss of virulence is due to a loss of all TonB-dependent transport systems or the loss of a particular system. With some host-pathogen interactions it has been shown that mutations in one TonB-requiring system attenuate the pathogen even though the pathogen possesses other iron transport systems. For example, loss of the TonB-dependent siderophore receptor in Vibrio anguillarum (8) or the TonB-dependent hemoglobin receptors in Neisseria meningitidis (48) and Haemophilus ducreyi (47) was associated with a loss of virulence. In S. dysenteriae, however, the loss of virulence associated with tonB mutation was not reproduced by mutations affecting siderophore biosynthesis and heme uptake systems (41), indicating that there are other TonB-dependent systems that are required for virulence in Shigella.
This study was undertaken to identify the role of TonB and the TonB-dependent iron uptake systems in uropathogenic E. coli (UPEC) using a mouse model of UTI. UTI is the most common form of extraintestinal infection due to E. coli (14), and E. coli is one of the most common causative agents of all types of UTIs (22). A variety of virulence factors contributing to uropathogenesis of E. coli have been recognized, including specific fimbriae, hemolysin production, presence of colicin V plasmids, certain capsular and lipopolysaccharide antigens (22), and genes encoded within a pathogenicity island (16, 17). The production of hemolysin suggests that heme utilization might be associated with uropathogenesis. However, relatively few studies have examined the roles of iron and heme transport systems in UTIs.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Strains and plasmids are listed in Table 1. E. coli was grown in LB (Luria broth) and was incubated at 37°C. When necessary, the following antibiotics were used: chloramphenicol (Cm) (30 μg/ml), kanamycin (Km) (50 μg/ml), and carbenicillin (Cb) (250 μg/ml). The abilities of E. coli strains to utilize various iron sources were tested as described previously (51). For growth in iron-restricted conditions, the iron chelator ethylenediamine di(o-hydroxyphenylacetic acid) (EDDA) (Sigma Chemical Co.) was added at a concentration of 1 mg/ml. The hemin (Sigma) concentration was 8 μM. Plasmids were introduced into E. coli by electroporation as described by Dower et al. (10).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant properties | Reference or source |
|---|---|---|
| E. coli | ||
| DH5α | endA1 hsdR17(rK−mK−) supE44 thi-1 recA1 gyrA relA1 Δ(lacZYA-argF) U169deoR[f80dlac Δ(lacZ)M15] | 44 |
| SM10 | thi thr leuB tonA lacY supE recA::RP4-2-Tc::Mu-Km Kmr | 46 |
| CFT073 | UTI clinical isolate | 33 |
| CFT073-TB | tonB | This study |
| CFT073-LH | chuA::cam | This study |
| CFT073-ENT | entF::kan | This study |
| CFT073-IUC | iucB::cam | This study |
| CFT073-ENT/IUC | entF::kan iucB::cam | This study |
| CFT073-IUT | iutA::cam | This study |
| Plasmids | ||
| pBluescript SK | Cloning vector; Cbr | Stratagene |
| pWKS30 | Cloning vector; Cbr | 53 |
| pWSc-1 | pWKS30 containing sacB gene; Cbr Sucs | 38 |
| pHM5 | Suicide vector; Cbr Sucs | 42 |
| pCVD442 | Suicide vector; Apr Sucs | 9 |
| pUC-4K | kan cassette | Pharmacia |
| pMA9 | cam resistance gene | 20 |
| pYUK1 | tonB in pACYC177; Cmr | This study |
| pUCHUA::Cm | chuA::cam in pHM5 | This study |
| pIUCB::Cm | iucB::cam in pWSc-1 | This study |
| pIUTA::Cm | iutA::cam in pCVD442 | This study |
| pUENTF::Km | entF::kan in pWSc-1 | This study |
Isolation of an E. coli tonB mutant.
A spontaneous tonB mutant of CFT073 was obtained by screening colonies resistant to a 1-mg/ml concentration of Pirazmonam (S. J. Lucania, Bristol-Myers Squibb Co.) for loss of high-affinity iron transport as described previously (24, 51).
Construction of isogenic iron transport system mutants by allelic exchange.
The chuA, entF, iucB, and iutA mutants of CFT073 were generated by allelic exchange. The genes were PCR amplified as described below and cloned into pBluescript SK (Stratagene). An antibiotic resistance cassette was inserted into the gene, which was then subcloned into the indicated vector. Each of the following constructs was introduced into CFT073 by electroporation or, for plasmid pCVD442 constructs, by conjugation using the donor strain SM10 (λ pir). Putative mutants were screened for the appropriate resistance patterns, for loss of the plasmid, for the presence of the interrupted gene in the chromosome by PCR, and for the correct phenotype.
The chuA mutation in CFT073-LH was constructed as follows: the chuA gene from CFT073 was amplified by PCR using the primers 5′-CCCAGAGATATCGAGGCTTGCA-3′ and 5′-TCACGGATATCGCCGCGCATC-3′ with Taq polymerase (Fisher Scientific). The product was digested with EcoRV and cloned into pBluescript SK. A cam cassette was introduced into the chuA gene at the internal NruI site (51), and the chuA::cam EcoRV fragment was subcloned into pHM5.
The aerobactin synthesis (iucB) mutant, CFT073-IUC, was constructed by amplification and cloning of a fragment containing the last 1,496 bp of iucA, the iucB gene, and the first 810 bp of iucC using the primers 5′-TATCCTGTCGACTTTACCAC-3′ and 5′-GCAGCTTGATATCCAGCCC-3′. The product was digested with SalI and EcoRV and cloned into pWSc-1. A cam cassette was introduced into the internal SmaI site of the iucB gene (29).
The entF mutants, CFT073-ENT and CFT073-ENT/IUC, were constructed following amplification and cloning of the CFT073 entF gene using the primers 5′-CCGCGGATCCGGCGCT-3′ and 5′-GACGATAGATATCCAGCTC-3′. The kan cassette from pUC-4K was introduced into an NsiI site within entF (43). The SmaI/XhoI fragment containing entF::kan was inserted into pWSc-1.
The aerobactin transport mutant, CFT073-IUT, was constructed by PCR amplification and cloning of a fragment containing the iutA gene with the primer pair 5′-CCTCTAGATGATGCGCAAAAAGTATATGC-3′ and 5′-GGAAGCTTCAGAACAGCACTGAGTAGTT-3′. The cam cassette was inserted into the EcoRV site within iutA, and the disrupted gene was amplified and cloned into the SmaI site of pCVD442 (9).
Mouse virulence assays.
A modification of the mouse model of ascending UTI originally described by Hagberg et al. (18) was used for this study. Swiss-Webster mice were used as the test animals. Cultures of CFT073 and their isogenic mutants were prepared by picking single colonies from plates and inoculating 40 ml of LB in a 500-ml flask. These cultures were grown at 37°C without agitation for 2 days. The cultures were then diluted to an A600 of 0.8, 40 ml of the culture was centrifuged, and the pellet was resuspended in 0.250 ml of phosphate-buffered saline. Mice were injected transurethrally in the bladders with 0.025 ml of the suspension, giving an inoculum containing approximately 109 CFU. Mice were sacrificed 2 days after challenge; bladder and kidneys were dissected and homogenized, and each was cultured on Luria (L) agar plates. Both kidneys from each mouse were combined. Viable counts were determined as CFU per gram of bladder or kidney for each mouse. Medians were calculated for all the mice used for a given set of inoculations. P values of the number of CFU per gram were calculated by the Mann-Whitney test.
For competition experiments, CBA female mice were used as the test animals. Mixed cultures of CFT073 and its isogenic mutants were grown in LB at 37°C without agitation for 2 days. The cultures were diluted and cultured another 2 days in static LB broth. Finally the strains were diluted in 40 ml of LB broth in a 500-ml flask and incubated two more days. Thirty milliliters of culture was centrifuged for 10 min at 10,000 × g, and the pellet was resuspended to an A600 of 0.6. Eighteen milliliters (each) of the wild-type and mutant strains were mixed and centrifuged for 10 min at 7,000 rpm and resuspended in 0.5 ml of phosphate-buffered saline. Mice were injected transurethrally in the bladders with 0.05 ml of the suspension. The initial inoculum was determined by plating serial dilutions of the mixed culture and replica plating on L agar containing the appropriate antibiotic. Mice were sacrificed, and samples were processed as described for the single infections; and the ratio of mutant to wild-type bacteria was determined by replica plating. The competitive index was calculated from each mouse with a positive bladder or kidney infection and is defined as the ratio of output mutant to wild-type bacteria (recovered from the bladder or kidneys) divided by the ratio of input mutant to wild-type bacteria (inoculated into the mouse). A competitive index of <1 indicates that the strain was recovered in lower numbers than the wild type. The mean competitive index was calculated for each group of mice, and P values were calculated by Student's t test.
To determine whether the mutants had reverted to the wild type in vivo, bacteria recovered from the mice were plated on L agar and replica plated onto media containing antibiotics corresponding to the resistance cassette used to create the mutation. No reversion to the wild type was detected in vivo or in vitro for any of the mutants used in this study.
Growth in urine.
Pooled human urine was sterilized by filtration and stored at 4°C. Overnight LB cultures of CFT073 and the iron transport mutants were diluted into urine to give a cell density of approximately 107 CFU/ml and incubated at 37°C. These cultures were diluted 1:100 into urine for two more passages. Growth was monitored by plating samples on L agar to determine the number of CFU/ml of urine.
RESULTS
Identification of iron transport systems in E. coli CFT073.
This study was undertaken to determine the role of iron transport systems in E. coli UTI. CFT073, a UPEC strain isolated from the blood and urine of a patient with acute pyelonephritis, was chosen for this study, because it has been shown to be virulent in a mouse model of ascending UTI (33, 34). This allows us to assess the effects of iron transport system mutations on virulence in the mouse model. We first characterized the iron transport systems expressed by CFT073. Supernatants of iron-restricted cultures of this strain tested positive for both catechol and hydroxamate siderophores as determined by the Arnow assay (1) and ferric perchlorate assay (2), respectively. Bioassays confirmed that CFT073 synthesized and transported the catechol enterobactin and the hydroxamate siderophore aerobactin (Table 2). This strain also was able to transport the fungal hydroxamate siderophore ferrichrome and used hemin, myoglobin, hemoglobin, heme-albumin, and hemoglobin-haptoglobin as sources of iron (Table 2 and data not shown).
TABLE 2.
Iron transport systems in E. coli CFT073 and its isogenic iron uptake mutants
| Straina | FeSO4 utilizationb | Hemin utilizationb | Aerobactin
|
Enterobactin
|
||
|---|---|---|---|---|---|---|
| Synthesis | Transportb | Synthesis | Transportb | |||
| CFT073 | + | + | + | + | + | + |
| CFT073-TB | + | − | + | − | + | − |
| CFT073-TB(pYUK1) | + | + | + | + | + | + |
| CFT073-LH | + | −c | + | + | + | + |
| CFT073-ENT | + | + | + | + | − | + |
| CFT073-IUC | + | + | − | + | + | + |
| CFT073-ENT/IUC | + | + | − | + | − | + |
| CFT073-IUT | + | + | + | − | + | + |
Abbreviations: -TB, tonB; -LH, chuA; -ENT, entF; -IUC, iucB; -ENT/IUC, entF iucB; -IUT, iutA; pYUK1, TonB+ plasmid.
Bioassays were performed by seeding the strain in L-EDDA agar and spotting the indicated compound on the surface of the plate. + indicates a zone of growth around the iron compound. − indicates no stimulation of growth. Synthesis of siderophores was determined by chemical assays and bioassays.
A small amount of background growth was observed on occasion.
Isolation and characterization of a CFT073 tonB mutant.
The high-affinity iron transport systems identified in CFT073 have been shown in other E. coli strains to require TonB for transport of the ligand across the outer membrane (6, 11, 51). Therefore, a TonB− derivative of CFT073 was isolated to functionally eliminate all of these iron transport systems and thereby determine whether any of these systems is required for virulence in the mouse model of UTI. A tonB mutant was isolated on medium containing Pirazmonam, an antibiotic for which resistance in E. coli is associated with tonB mutations (24). The putative tonB mutant, designated CFT073-TB, was unable to use hemin or the siderophores aerobactin, enterobactin, and ferrichrome as iron sources, but it grew normally when iron salts were added to the medium (Table 2 and data not shown). When CFT073-TB was transformed with pYUK1, a plasmid containing the wild-type E. coli tonB gene, the transport of all of these iron sources was restored (Table 2). Restoration of wild-type function by the tonB plasmid indicates that the mutant phenotype was likely due to a mutation in the tonB gene and that tonB is required for the transport of iron compounds through these uptake systems.
Virulence of the tonB mutant in the mouse model of ascending UTI.
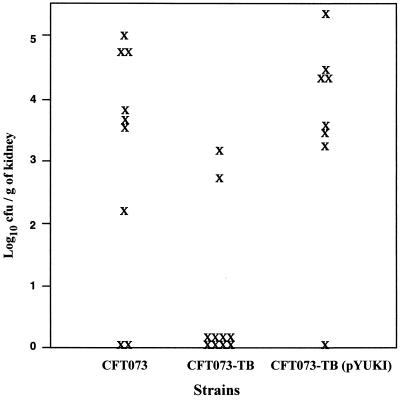
A mouse model of pyelonephritis was used to assess the contribution of TonB-mediated iron uptake in the virulence of UPEC. Recovery of the tonB mutant from the kidneys of transurethrally infected mice was compared with that of the wild-type strain. As shown in Fig. 1 and Table 3, bacteria were recovered from the kidneys of 78% of the mice infected with the wild-type strain but only 20% of those inoculated with the tonB mutant strain. None of the positive kidney cultures from mice infected with the tonB mutant strain had more than 104 CFU/g (Fig. 1). In contrast, the median number of bacteria recovered from mice infected with the TonB+ complemented strain was >104 CFU/g of kidney (Table 3). This indicates that the ability of the tonB mutant to colonize the kidney at the inoculum tested was significantly reduced over the 48-h postinoculation period. To confirm that the tonB mutation was responsible for the reduced ability of the strain to infect the kidneys, the TonB− strain complemented with the wild-type tonB gene on a plasmid (pYUK1) was tested in the mouse model (Fig. 1). The complemented strain was recovered from the kidneys in numbers similar to those for the wild-type strain (Table 3). The kidneys of 87.5% of these mice were infected, and the median number of bacteria was >104 CFU/g of kidney.
FIG. 1.
Recovery of bacteria from the kidneys of mice infected with TonB+ and TonB− strains. Quantitative bacterial counts are shown. Each X symbol represents CFU per gram of tissue from an individual mouse. Values near the x axes indicate no detectable bacteria in the kidney.
TABLE 3.
Infectivity rates and median no. of CFU per gram of kidney from animals with positive kidney cultures
| Strain | Phenotype | Infectivity ratea | Median no. of CFU/g of kidney |
|---|---|---|---|
| CFT073 | TonB+ | 7/9 | 5.2 × 103 |
| CFT073-TB | TonB− | 2/10 | 0b |
| CFT073-TB(pYUK1) | TonB+ | 7/8 | 1.3 × 104 |
Number of animals with positive kidney cultures/number of animals inoculated.
P = 0.01 in comparison with CFT073 and CFT073-TB(pYUK1), as determined by the Mann-Whitney test.
The ability of the tonB mutant to colonize the mouse urinary tract was further assessed in competition with the wild-type strain in the mouse. By coinfecting each mouse with a mixture of the wild-type and mutant strains, the ability of the mutant to directly compete with the wild type in colonization and multiplication in the tissues of the animal can be measured. As shown in Table 4, the tonB mutant strain was unable to infect the kidneys and colonized the bladder very poorly in the presence of the wild-type strain. The wild-type bacteria were recovered from the bladder and kidneys of all the mice with a positive infection. When the TonB− strain was complemented with pYUK1, the ability to compete successfully with the wild type and infect the bladder and kidneys was restored (Table 4). The wild-type and complemented TonB+ strains were recovered from the bladder and kidneys of mice in similar numbers. These data indicate that inactivation of all high-affinity iron transport systems due to the loss of TonB significantly reduces the infectivity and virulence of CFT073. However, it was not known whether the reduced infectivity is due to the loss of all of these transport systems or to the loss of one specific system.
TABLE 4.
Mouse competition assay with CFT073 and tonB derivatives
| CFT073 vs.: | No. of mice | Competitive indexa
|
|
|---|---|---|---|
| Bladder | Kidney | ||
| CFT073-TB | 7b | 0.02 ± 0.017c | 0 |
| CFT073-TB(pYUK1) | 3 | 0.64 ± 0.43d | 1.11 ± 0.09d |
Competitive index is the ratio of output mutant to wild-type bacteria (recovered from the bladder or kidneys) divided by the ratio of input mutant to wild-type bacteria (inoculated into the mouse). A competitive index of <1 indicates that the strain was recovered in lower numbers than the wild type. Zero indicates that no mutant bacteria were recovered from that site. P values were calculated with Student's t test.
Bacteria were not recovered from the kidneys of one of the mice.
P < .001.
P > .1.
Construction of isogenic mutants affecting iron uptake.
To determine the roles of individual TonB-dependent iron transport systems during UTIs, mutants of CFT073 defective in heme iron transport or in siderophore-mediated iron transport were constructed, and the effects of these mutations on virulence were tested in the mouse model. Two aerobactin mutants, one defective in synthesis, CFT073-IUC, and one defective in transport, CFT073-IUT, were constructed by disrupting the iucB and iutA genes, respectively. CFT073-IUC failed to secrete aerobactin but maintained the ability to transport the compound (Table 2), while CFT073-IUT was able to synthesize but not transport aerobactin (Table 2). Bioassays confirmed that the other high-affinity transport systems were functional in these mutants (Table 2). Enterobactin synthesis mutants were constructed by allelic exchange in both the wild-type and IucB− backgrounds (Table 2). The entF single mutant, CFT073-ENT, and the iucB entF double mutant, CFT073-ENT/IUC, were analyzed for iron transport systems. These mutants were unable to produce one or both siderophores but retained the ability to use exogenous enterobactin and aerobactin for iron transport (Table 2).
In addition to its siderophore-mediated iron transport systems, CFT073 can use heme or heme compounds (Table 2). Sequences with homology to the chuA heme transport receptor gene of S. dysenteriae and E. coli O157:H7 were observed previously in this strain (51, 55). To determine if chuA encodes a functional heme receptor and to generate a heme transport mutant, chuA was disrupted by marker exchange. The chuA mutant, CFT073-LH, synthesized and transported both enterobactin and aerobactin, but it grew poorly when hemin was the iron source (Table 2). This indicates that chuA encodes the heme receptor in CFT073. A small amount of growth of CFT073-LH was seen on L-EDDA agar containing hemin as the iron source, most likely because the mutant was able to use its two siderophores to scavenge iron from the medium even in the presence of EDDA.
Effects of mutations in the iron transport systems on mouse virulence.
To define whether the synthesis and transport of siderophores or the expression of the heme receptor was involved in virulence, the wild-type CFT073 strain and the isogenic iron utilization mutants were compared using the mouse model. The single siderophore synthesis mutants, CFT073-IUC and CFT073-ENT, and the heme transport mutant, CFT073-LH, infected both the kidney (Table 5) and bladder (data not shown). The mutants were able to infect the kidneys of the majority of the mice, and the numbers of bacteria isolated from the kidneys were similar to that for the wild type (P > 0.5) (Table 5). These data suggest that the synthesis of either of the siderophores, perhaps in conjunction with the heme uptake system, provides sufficient iron for the multiplication of the E. coli bacteria in the bladders and kidneys.
TABLE 5.
Recovery of iron transport mutants of CFT073 from the kidneys of infected mice
| Strain | Phenotype | Infectivity ratea | No. of CFU/g of kidneyb |
|---|---|---|---|
| Trial 1 | |||
| CFT073 | Wild type | 9/9 | 5.6 × 103 (1.1 × 102–6.7 × 104) |
| CFT073-IUC | Iuc− | 8/10 | 3.5 × 104 (2.1 × 102–1.8 × 105) |
| Trial 2 | |||
| CFT073 | Wild type | 5/7 | 1.2 × 104 (1.1 × 103–5.6 × 104) |
| CFT073-LH | ChuA− | 6/9 | 2.8 × 104 (1.4 × 102–7.0 × 104) |
| Trial 3 | |||
| CFT073 | Wild type | 13/14 | 3.8 × 104c (7.0 × 102–1.4 × 105) |
| CFT073-ENT | Ent− | 14/14 | 4.1 × 103 (2.1 × 102–9.2 × 104) |
| CFT073-IUC/ENT | Iuc− Ent− | 13/17 | 1.1 × 103c (2.8 × 102–4.7 × 104) |
Number of animals with positive kidney cultures/number of animals inoculated.
Median (range) of the number of CFU/g for all animals with positive cultures.
P = 0.02 (Mann-Whitney); for all other comparisons, P > 0.05.
To determine whether any siderophore was required for UTI, CFT073-IUC/ENT, the siderophore synthesis double mutant, was compared to the wild-type strain. The number of bacteria in the bladders of mice infected with the mutant strain was modestly, but significantly (P < 0.05), reduced compared with the wild type strain (50; data not shown). When the kidneys of infected mice were analyzed, the disadvantage was more apparent; the number of bacteria in the kidney was 10-fold lower for the Ent− Iuc− mutant strain (Table 5) (P = 0.02). The fact that the double mutant showed a reduced ability to invade or colonize the mouse kidney suggests that siderophores are required for growth in this environment (Table 5). If this hypothesis is correct, then it should be possible to rescue the siderophore synthesis mutant by coinfection with a wild-type strain that is secreting the siderophores. Mice were infected with a mixed culture containing the wild-type strain and the double-mutant strain, and in this competition assay, the double mutant was able to survive and colonize the bladders and the kidneys of mice (Table 6). The numbers of Ent− Iuc− bacteria were equal to the numbers of the wild-type bacteria in the mixed infection (Table 6), suggesting that the exogenous siderophores synthesized by the wild-type strain are sufficient to suppress the effect of the mutations.
TABLE 6.
Mouse competition assay with CFT073 and its isogenic iron utilization strains
| CFT073 vs.: | No. of mice | Competitive indexa
|
|
|---|---|---|---|
| Bladders | Kidneys | ||
| CFT073-ENT/IUC | 6b | 0.90 ± 0.38c | 1.11 ± 0.52c |
| CFT073-IUT | 7d | 0.008 ± 0.06e | 0 |
| CFT073-LH | 7d | 0.026 ± 0.003e | 0.058 ± 0.006c |
Competitive index is the ratio of output mutant to wild-type bacteria (recovered from the bladder or kidney) divided by the ratio of input mutant to wild-type bacteria (inoculated into the mouse). A competitive index of <1 indicates that the strain was recovered in lower numbers relative to the wild type.
Bacteria were not recovered from the kidneys of two of the mice.
P ≥ 0.1.
Bacteria were not recovered from the kidneys of one of the mice.
P < .001.
Although the mutants with defects in single iron transport systems were able to colonize the bladders and kidneys when tested individually, the competition assay is a more sensitive indicator of ability to survive in vivo compared to the wild-type strain. Mutants defective in aerobactin and heme transport were tested in the mouse competition assay. These two systems were selected for analysis because aerobactin has been shown to be an important virulence factor for some extraintestinal E. coli isolates (54), and heme transport systems are commonly found in pathogenic strains. Because the siderophore synthesis mutant could be rescued by coinfection with the wild-type strain, an aerobactin receptor mutant, CFT073-IUT, that was unable to use aerobactin (Table 2) was tested in the competition assay. As shown in Table 6, the IutA− mutant strain was severely reduced in its ability to compete with the wild type for colonization of the bladders of infected mice. In the kidneys, the IutA− mutant was unable to colonize in competition with the wild-type strain, and none of the iutA mutant bacteria were recovered from any of the kidneys.
Similarly, CFT073-LH, the heme receptor mutant of CFT073, showed a disadvantage when compared with the wild-type strain in the competition assay for the ability to colonize the bladder and kidneys of mice (Table 6). The ChuA− strain was recovered from bladders and kidneys of only three of the seven infected mice, and the competitive indices indicated that this strain was significantly diminished in its ability to colonize and grow in these organs (Table 6).
Growth of iron transport mutants in urine.
To determine whether urine represents an iron-restricted environment and could influence the need for one or more iron transport systems in the human urinary tract, we measured growth of the strains in human urine in vitro. All the strains tested had similar growth rates and reached approximately the same final density in the first passage in urine (A650 = 0.5 to 0.6). The strains were subcultured in urine in case a growth defect of the mutants due to iron limitation was suppressed by iron storage during growth in broth prior to the initial inoculation into urine. The mutants and the wild-type strain had equivalent growth rates and reached the same final density (A650 = 0.5 to 0.6) following the second and third passage in urine. Thus, urine does not appear to be an iron-limiting environment for growth of an E. coli UTI strain.
DISCUSSION
This study examined the role of TonB and TonB-mediated iron uptake systems in the ability of UPEC to infect the urinary tract of the mouse. TonB is known to be required by E. coli for high-affinity transport and utilization of several nutrients including chelated iron and vitamin B12 (3, 11, 35). The fact that the tonB mutant of the UPEC strain CFT073 was attenuated for infection of the bladder and kidney indicates that CFT073 requires TonB in vivo, most likely to mediate the uptake of iron during colonization and multiplication within the urinary tract.
A contribution of tonB in host colonization and virulence has been shown for other pathogens, such as Salmonella serovar Typhimurium (52), H. influenzae (21) and S. dysenteriae (41). Tsolis et al. (52) showed that a tonB mutant of Salmonella enterica serovar Typhimurium was attenuated for infection in mice. They proposed that TonB-mediated iron uptake in Salmonella serovar Typhimurium was required for colonization of extraintestinal tissues (52). In S. dysenteriae, TonB is required for growth in the intracellular environment of host cells; a tonB mutant retained its ability to invade host cells but failed to multiply intracellularly and did not spread to adjacent cells (41). These studies, however, do not indicate which TonB-dependent systems are required in the various stages of infections.
To determine the relative contributions of individual TonB-dependent iron transport systems in UPEC, mutants of CFT073 defective in each of the characterized high-affinity iron transporters were analyzed in a mouse model of UTI. When administered as pure cultures, the mutants with defects in enterobactin or aerobactin were still able to infect the bladder and kidney. This suggests that production of either siderophore is sufficient to allow acquisition of iron in the urinary tract. This is in agreement with previous studies by Montgomerie et al. (36) and Miles and Khimji (31) showing that neither aerobactin nor enterobactin production correlated with an enhanced ability to cause UTI. Synthesis of at least one siderophore appears to be required in vivo; a mutant unable to produce either of the siderophores showed reduced infection of the mouse kidney. The ability of a siderophore-producing wild-type strain to compensate for the attenuation of the double siderophore synthesis mutant in the competition assay indicates that siderophores are synthesized and secreted in vivo and promote colonization.
While a single siderophore-mediated iron transport system may be sufficient for colonization, it seemed likely that the presence of additional systems could improve efficiency of growth in the host by allowing the pathogen to take advantage of multiple iron sources. The two different types of siderophores produced by CFT073 may function in different environments within the host or at different times during the course of an infection. Brock et al. (7) have presented evidence suggesting that enterobactin and aerobactin fulfill somewhat different roles in that they may acquire iron in vivo from different sources: enterobactin by scavenging predominantly extracellular, transferrin-bound iron and aerobactin by obtaining iron preferentially from host intracellular iron complexes. Heme is the most abundant iron source in vivo, and the presence of a heme transport system in CFT073 may be important for the acquisition of iron from heme or hemoglobin. Recent evidence in support for the role of the chu heme transport system in virulence of extraintestinal E. coli strains was presented by Bonacorsi et al. (4). The authors identified the chu locus as one of the specific chromosomal regions associated with the ability of E. coli K1 strains to invade the meninges of neonates. Although utilization of heme may not be essential for the initial colonization of the bladder by UPEC strains, it could play a role in the later stages of disease when there is hemolysis of host cells and release of heme and hemoglobin. Many of the UPEC strains, including CFT073, produce one or more hemolysins (12, 23), and expression of hemolysin (15), like the heme transport system (51), is increased under conditions of iron starvation. Furthermore, it has been recently shown that ChuA expression is influenced by RfaH in the UPEC strain 536 (37). RfaH is a positive effector of transcription of hemolysin, as well as lipopolysaccharide biosynthetic genes (27, 28). Thus, the hemolysin and heme transport systems may work in concert to take advantage of the abundant supply of heme as an iron source within the host.
The potential benefit to the uropathogen of having heme and aerobactin iron transport systems, in addition to enterobactin, was assessed by measuring direct competition between the heme or aerobactin mutants and wild-type bacteria. Under these conditions, the mutants were found to be at a severe disadvantage compared to the wild type. The presence of multiple iron transport systems allows the wild-type strain to grow more rapidly in vivo and outcompete the strains lacking heme or aerobactin-mediated iron transport. This most likely reflects growth in the tissues of the urinary tract rather than in urine, since none of the mutants showed any reduction in the growth rate relative to the wild type in human urine in vitro.
It is likely that there are additional TonB-dependent iron transport systems in pathogenic E. coli that contribute to iron acquisition in vivo. A potential iron transport system was found by DNA sequence analysis of a pathogenicity island of CFT073 (17). Two open reading frames (L5 and R4) within the island showed homology to TonB-dependent outer membrane receptors. Further characterization is required to determine whether either of these open reading frames, or other unidentified iron transport systems, plays a role in iron transport by UPEC and contributes to the loss of infectivity associated with the tonB mutation. While the loss of any one of these iron transporters may reduce the fitness of the pathogen in vivo, the redundancy in iron transport systems may partially compensate for the loss of one or more systems. The severe defect associated with the tonB mutation likely reflects the loss of all of these systems, although it is possible that TonB also may be required in vivo for a function other than iron transport.
ACKNOWLEDGMENTS
This work was supported by grants AI16935 to S.M.P., AI39000 to R.A.W., and AI01583 to P.R. from the National Institutes of Health. A.G.T. was supported by a research supplement for underrepresented minorities from the National Institute of Allergy and Infectious Diseases and by a David and Lucile Packard Foundation Fellowship from the National Hispanic Scholarship Fund.
We thank the Bristol-Myers Squibb Company for generously providing Pirazmonam. We thank Yuki Gleason and Ana-Maria Valle for construction of the tonB plasmid and expert technical assistance and Elizabeth Wyckoff and Stephanie Reeves for critical reading of the manuscript.
REFERENCES
- 1.Arnow L E. Colorimetric determination of the components of 3,4-dihydroxyphenylalanine-tyrosine mixtures. J Biol Chem. 1937;118:531–537. [Google Scholar]
- 2.Atkin C L, Neilands J B, Phaff H J. Rhodotorulic acid from species of Leucosporidium, Rhodosporidium, Rhodotorula, Sporidiobolus, and Sporobolomyces, and a new alanine-containing ferrichrome from Cryptococcus melibiosum. J Bacteriol. 1970;103:722–733. doi: 10.1128/jb.103.3.722-733.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassford P J, Jr, Bradbeer C, Kadner R J, Schnaitman C A. Transport of vitamin B12 in tonB mutants of Escherichia coli. J Bacteriol. 1976;128:242–247. doi: 10.1128/jb.128.1.242-247.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonacorsi S P P, Clermont O, Tinsley C, LeGall I, Beaudoin J C, Elion J, Nassif X, Bingen E. Identification of regions of the Escherichia coli chromosome specific for neonatal meningitis-associated strains. Infect Immun. 2000;68:2096–2101. doi: 10.1128/iai.68.4.2096-2101.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun V. Energy-coupled transport and signal transduction through the gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol Rev. 1995;16:295–307. doi: 10.1111/j.1574-6976.1995.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 6.Braun V, Hantke K, Köster W. Bacterial iron transport: mechanisms, genetics, and regulation. In: Sigel A, Sigel H, editors. Metal ions in biological systems. Iron transport and storage in microorganisms, plants and animals. New York, N.Y: Marcel Dekker; 1998. [PubMed] [Google Scholar]
- 7.Brock J H, Williams P H, Licéaga J, Woodridge K G. Relative availability of transferrin-bound iron and cell-derived iron to aerobactin-producing and enterochelin-producing strains of Escherichia coli and to other microorganisms. Infect Immun. 1991;59:3185–3190. doi: 10.1128/iai.59.9.3185-3190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crosa J H. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol Rev. 1989;53:517–530. doi: 10.1128/mr.53.4.517-530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnenberg M S, Kaper J B. Construction of a eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dower W J, Miller J F, Ragsdale C W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988;16:6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Earhart C F. Uptake and metabolism of iron and molybdenum. In: Neidhardt F C, editor. Escherichia coli and Salmonella. Washington, D.C.: ASM Press; 1996. pp. 1075–1090. [Google Scholar]
- 12.Felmlee T, Pellett S, Welch R A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985;163:94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorbacheva V Y, Faundez G, Godfrey H P, Cabello F C. Restricted growth of ent(-) and tonB mutants of Salmonella enterica serovar Typhi in human Mono Mac 6 monocytic cells. FEMS Microbiol Lett. 2001;196:7–11. doi: 10.1111/j.1574-6968.2001.tb10532.x. [DOI] [PubMed] [Google Scholar]
- 14.Griffiths E. Iron and the virulence of Escherichia coli. In: Sussman M, editor. Escherichia coli mechanisms of virulence. Cambridge, United Kingdom: Cambridge University Press; 1997. pp. 331–371. [Google Scholar]
- 15.Gruenig H M, Rutschi D, Schoch C, Lebek G. The chromosomal fur gene regulates the extracellular haemolytic activity encoded by certain Hly plasmids. Zentbl Bakteriol Hyg A. 1987;266:231–238. doi: 10.1016/s0176-6724(87)80036-6. [DOI] [PubMed] [Google Scholar]
- 16.Guyer D M, Henderson I R, Nataro J P, Mobley H L. Identification of Sat, an autotransporter toxin produced by uropathogenic Escherichia coli. Mol Microbiol. 2000;38:53–66. doi: 10.1046/j.1365-2958.2000.02110.x. [DOI] [PubMed] [Google Scholar]
- 17.Guyer D M, Kao J-S, Mobley H L. Genetic analysis of a pathogenicity island in uropathogenic Escherichia coli CFT073: distribution of homologous sequences among isolates from patients with pyelonephritis, cystitis, and catheter-associated bacteriuria and from fecal samples. Infect Immun. 1998;66:4411–4417. doi: 10.1128/iai.66.9.4411-4417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagberg L, Engberg I, Freter R, Lam J, Olling S, Svanborg E C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983;40:273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henderson D P, Payne S M. Vibrio cholerae iron transport systems: roles of heme and siderophore iron transport in virulence and identification of a gene associated with multiple iron transport systems. Infect Immun. 1994;62:5120–5125. doi: 10.1128/iai.62.11.5120-5125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong M, Payne S M. Effect of mutations in Shigella flexneri chromosomal and plasmid-encoded lipopolysaccharide genes on invasion and serum resistance. Mol Microbiol. 1997;24:779–791. doi: 10.1046/j.1365-2958.1997.3731744.x. [DOI] [PubMed] [Google Scholar]
- 21.Jarosik G P, Sanders J D, Cope L D, Muller-Eberhard U, Hansen E J. A functional tonB gene is required for both utilization of heme and virulence expression by Haemophilus influenzae type b. Infect Immun. 1994;62:2470–2477. doi: 10.1128/iai.62.6.2470-2477.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson J R. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4:80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kao J-S, Stucker D M, Warren J W, Mobley H L T. Pathogenicity island sequences of pyelonephritogenic Escherichia coli CFT073 are associated with virulent uropathogenic strains. Infect Immun. 1997;65:2812–2820. doi: 10.1128/iai.65.7.2812-2820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsen R A, Thomas M G, Wood G E, Postle K. Partial suppression of an Escherichia coli TonB transmembrane domain mutation (delta V17) by a missense mutation in ExbB. Mol Microbiol. 1994;13:627–640. doi: 10.1111/j.1365-2958.1994.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 25.Law D, Kelly J. Use of heme and hemoglobin by Escherichia coli O157 and other Shiga-like-toxin-producing E. coli serogroups. Infect Immun. 1995;63:700–702. doi: 10.1128/iai.63.2.700-702.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Law D, Wilkie K M, Freeman R, Gould F K. The iron uptake mechanisms of enteropathogenic Escherichia coli: the use of haem and haemoglobin during growth in an iron-limited environment. J Med Microbiol. 1992;37:15–21. doi: 10.1099/00222615-37-1-15. [DOI] [PubMed] [Google Scholar]
- 27.Leeds J A, Welch R A. Enhancing transcription through the Escherichia coli hemolysin operon, hlyCABD: RfaH and upstream JUMPStart DNA sequences function together via a postinitiation mechanism. J Bacteriol. 1997;179:3519–3527. doi: 10.1128/jb.179.11.3519-3527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leeds J A, Welch R A. RfaH enhances elongation of Escherichia coli hlyCABD mRNA. J Bacteriol. 1996;178:1850–1857. doi: 10.1128/jb.178.7.1850-1857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez J L, Herrero M, de Lorenzo V. The organization of intercistronic regions of the aerobactin operon of pColV-K30 may account for the differential expression of the iucABCD iutA genes. J Mol Biol. 1994;238:288–293. doi: 10.1006/jmbi.1994.1290. [DOI] [PubMed] [Google Scholar]
- 30.Mietzner T A, Morse S A. The role of iron-binding proteins in the survival of pathogenic bacteria. Annu Rev Nutr. 1994;14:471–493. doi: 10.1146/annurev.nu.14.070194.002351. [DOI] [PubMed] [Google Scholar]
- 31.Miles A A, Khimji P L. Enterobacterial chelators of iron: their occurrence, detection, and relation to pathogenicity. J Med Microbiol. 1975;8:477–490. doi: 10.1099/00222615-8-4-477. [DOI] [PubMed] [Google Scholar]
- 32.Mills M, Payne S M. Genetics and regulation of heme iron transport in Shigella dysenteriae and detection of an analogous system in Escherichia coli O157:H7. J Bacteriol. 1995;177:3004–3009. doi: 10.1128/jb.177.11.3004-3009.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mobley H L, Green D M, Trifillis A L, Johnson D E, Chippendale G R, Lockatell C V, Jones B D, Warren J W. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun. 1990;58:1281–1289. doi: 10.1128/iai.58.5.1281-1289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mobley H L, Jarvis K G, Elwood J P, Whittle D I, Lockatell C V, Russell R G, Johnson D E, Donnenberg M S, Warren J W. Isogenic P-fimbrial deletion mutants of pyelonephritogenic Escherichia coli: the role of alpha Gal(1–4) beta Gal binding in virulence of a wild-type strain. Mol Microbiol. 1993;10:143–155. doi: 10.1111/j.1365-2958.1993.tb00911.x. [DOI] [PubMed] [Google Scholar]
- 35.Moeck G S, Coulton J W. TonB-dependent iron acquisition: mechanism of siderophore-mediated active transport. Mol Microbiol. 1998;28:675–681. doi: 10.1046/j.1365-2958.1998.00817.x. [DOI] [PubMed] [Google Scholar]
- 36.Montgomerie J Z, Bindereif A, Neilands J B, Kalmanson G M, Guze L B. Association of hydroxamate siderophore (aerobactin) with Escherichia coli isolated from patients with bacteremia. Infect Immun. 1984;46:835–838. doi: 10.1128/iai.46.3.835-838.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagy G, Dobrindt U, Kupfer M, Emody L, Karch H, Hacker J. Expression of hemin receptor molecule ChuA is influenced by RfaH in uropathogenic Escherichia coli strain 536. Infect Immun. 2001;69:1924–1928. doi: 10.1128/IAI.69.3.1924-1928.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Occhino D A, Wyckoff E E, Henderson D P, Wrona T J, Payne S M. Vibrio cholerae iron transport: haem transport genes are linked to one of two sets of tonB, exbB, exbD genes. Mol Microbiol. 1998;29:1493–1507. doi: 10.1046/j.1365-2958.1998.01034.x. [DOI] [PubMed] [Google Scholar]
- 39.Otto B R, Verweij-van A M J J, MacLaren D M. Transferrins and heme-compounds as iron sources for pathogenic bacteria. Crit Rev Microbiol. 1992;18:217–233. doi: 10.3109/10408419209114559. [DOI] [PubMed] [Google Scholar]
- 40.Postle K. TonB protein and energy transduction between membranes. J Bioenerg Biomembr. 1993;25:591–601. doi: 10.1007/BF00770246. [DOI] [PubMed] [Google Scholar]
- 41.Reeves S A, Torres A G, Payne S M. TonB is required for intracellular growth and virulence of Shigella dysenteriae. Infect Immun. 2000;68:6329–6336. doi: 10.1128/iai.68.11.6329-6336.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Runyen-Janecky L J, Hong M, Payne S M. The virulence plasmid-encoded impCAB operon enhances survival and induced mutagenesis in Shigella flexneri after exposure to UV radiation. Infect Immun. 1999;67:1415–1423. doi: 10.1128/iai.67.3.1415-1423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rusnak F, Sakaitani M, Drueckhammer D, Reichert J, Walsh C T. Biosynthesis of the Escherichia coli siderophore enterobactin: sequence of the entF gene expression and purification of EntF, and analysis of covalent phosphopantetheine. Biochemistry. 1991;30:2916–2927. doi: 10.1021/bi00225a027. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 45.Seliger S, Mey A, Valle A, Payne S. The two TonB systems of Vibrio cholerae: redundant and specific functions. Mol Microbiol. 2001;39:801–812. doi: 10.1046/j.1365-2958.2001.02273.x. [DOI] [PubMed] [Google Scholar]
- 46.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 47.Stevens M K, Porcella S, Klesney-Tait J, Lumbley S, Thomas S E, Norgard M V, Radolf J D, Hansen E J. A hemoglobin-binding outer membrane protein is involved in virulence expression by Haemophilus ducreyi in an animal model. Infect Immun. 1996;64:1724–1735. doi: 10.1128/iai.64.5.1724-1735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stojiljkovic I, Hwa V, de Saint Martin L, O'Gaora P, Nassif X, Heffron F, So M. The Neisseria meningitidis haemoglobin receptor: its role in iron utilization and virulence. Mol Microbiol. 1995;15:531–541. doi: 10.1111/j.1365-2958.1995.tb02266.x. [DOI] [PubMed] [Google Scholar]
- 49.Takase H, Nitanai H, Hoshino K, Otani T. Requirement of the Pseudomonas aeruginosa tonB gene for high-affinity iron acquisition and infection. Infect Immun. 2000;68:4498–4504. doi: 10.1128/iai.68.8.4498-4504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torres A G. Ph.D. dissertation. Austin: The University of Texas at Austin; 1999. [Google Scholar]
- 51.Torres A G, Payne S M. Haem iron-transport system in enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol. 1997;23:825–833. doi: 10.1046/j.1365-2958.1997.2641628.x. [DOI] [PubMed] [Google Scholar]
- 52.Tsolis R M, Baumler A J, Heffron F, Stojiljkovic I. Contribution of TonB- and Feo-mediated iron uptake to growth of Salmonella typhimurium in the mouse. Infect Immun. 1996;64:4549–4556. doi: 10.1128/iai.64.11.4549-4556.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang R F, Kushner S R. Construction of versatile low-copy number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]
- 54.Williams P H. Novel iron uptake system specified by ColV plasmids: an important component in the virulence of invasive strains of Escherichia coli. Infect Immun. 1979;26:925–932. doi: 10.1128/iai.26.3.925-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wyckoff E E, Duncan D, Torres A G, Mills M, Maase K, Payne S M. Structure of the Shigella dysenteriae haem transport locus and its phylogenetic distribution in enteric bacteria. Mol Microbiol. 1998;28:1139–1152. doi: 10.1046/j.1365-2958.1998.00873.x. [DOI] [PubMed] [Google Scholar]