Abstract
The therapeutic potential of sotrovimab in the treatment of coronavirus disease 2019 (COVID‐19) is a controversial issue. The aim of this study was to evaluate the efficacy and safety of sotrovimab in COVID‐19 patients. To this end, PubMed, Cochrane Library, Embase, Web of Science, medRxiv, and Google Scholar were searched up to 15 August 2022. The reference lists of key studies were also scanned to find additional records. Meta‐analysis was performed using Comprehensive Meta‐Analysis. Seventeen studies involving 27,429 patients were included. A significant difference was observed in mortality rate (odds ratio [OR] = 0.40; 95% CI: 0.25–0.63, p = 0.00), hospitalisation rate (OR = 0.53; 95% CI: 0.43–0.65. p = 0.00), hospital or death rate (OR = 0.43; 95% CI: 0.25–0.73, p = 0.00), the need for mechanical ventilation (OR = 0.57; 95% CI: 0.33–0.96, p = 0.03), and ICU admission (OR = 0.33; 95% CI: 0.17–0.67, p = 0.00) of the sotrovimab‐receiving group compared to those having no sotrovimab. However, no significant difference was observed between the two groups in terms of disease progression (OR = 0.45; 95% CI: 0.16–1.24, p = 0.12) and emergency department visit (OR = 1.01; 95% CI: 0.83–1.24, p = 0.87). The two groups had no significant difference in terms of incidence of adverse events (OR = 0.98; 95% CI: 0.78–1.23, p = 0.88). The findings of the present meta‐analysis support that sotrovimab could be an effective and safe treatment option to reduce mortality and hospitalisation rate in both Delta and Omicron Variants of COVID‐19.
Keywords: COVID‐19, SARS‐CoV‐2, sotrovimab
Abbreviations
- CI
confidence interval
- COVID‐19
Coronavirus disease 2019
- ED
emergency department
- EU
European Union
- FDA
food and drug administration
- mAb
monoclonal antibody
- MHRA
medicines and healthcare products regulatory agency
- NOS
newcastle–ottawa scale
- PCR
polymerase chain reaction
- RCT
randomized clinical trial
- RR
risk ratio
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus‐2
1. INTRODUCTION
Since the beginning of the coronavirus disease 2019 (COVID‐19) pandemic, many therapeutic interventions have been introduced and evaluated. 1 , 2 , 3 The current evidence shows the effectiveness of monoclonal antibody (mAb) therapy in the prevention and treatment of COVID‐19. 4 Several anti‐severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) mAb therapies, such as casirivimab/imdevimab, 5 , 6 , 7 bamlanivimab, 8 bamlanivimab/etesevimab, 9 , 10 , 11 and sotrovimab 4 , 12 have been proposed as promising potential treatments for patients with COVID‐19. Their antiviral activities have been shown by binding and neutralising SARS‐CoV‐2. 13 Sotrovimab is a recombinant human mAb developed by Vir Biotechnology and GlaxoSmithKline 14 which is approved by the UK's Medicines and Healthcare Products Regulatory Agency (MHRA) for the treatment of high‐risk patients with mild to moderate COVID‐19 symptoms. 15 On June 2021, the US Food and Drug Administration (FDA) authorised the administration of sotrovimab (a 500‐mg single dose intravenously) in high‐risk patients with COVID‐19. 16 On December 2021, the European Union (EU) granted the use of sotrovimab based on the result of the COMET‐ICE phase III trial. 17 Recently, WHO recommended the use of sotrovimab in patients with non‐severe COVID‐19. 18 Despite several published clinical and observational studies 12 , 19 , 20 showing the therapeutic potential of sotrovimab for COVID‐19 in reducing hospitalisation and mortality rate, there are some controversies over using sotrovimab for COVID‐19 patients. 21 Therefore, the aim of this study is to evaluate the efficacy and safety of sotrovimab in COVID‐19 patients.
2. METHODS
The Preferred Reporting Items for Systematic reviews and Meta‐Analysis—Rapid Review (PRISMA‐RR) was used in this research. 22
2.1. Search strategy
Two researchers independently searched PubMed, Cochrane Library, Embase, Web of Science, medRxiv, and Google Scholar to identify the relevant evidence up to 15 August 2022. The reference lists of key studies and relevant systematic reviews were also scanned to discover additional citations. No language restriction was considered. The search terms key included 2019‐novel coronavirus, SARS‐CoV‐2, COVID‐19, 2019‐nCoV, Sotrovimab, and monoclonal antibody. The following search strategy was used to identify the relevant evidence in PubMed: ((((((((Coronavirus[Title/Abstract]) OR (Coronavirus[MeSH Terms])) OR (COVID‐19[Title/Abstract])) OR (SARS‐CoV‐2[Title/Abstract])) OR (COVID‐19[MeSH Terms])) OR (SARS‐CoV‐2[MeSH Terms])) OR (2019 novel coronavirus infection[Title/Abstract])) OR (2019‐nCoV infection[Title/Abstract])) AND ((Sotrovimab [Title/Abstract]) OR (monoclonal antibody [MeSH Terms]).
2.2. Study selection
The inclusion criteria were as follows: (1) population: patients with positive COVID‐19 polymerase chain reaction (PCR) test, (2) intervention: sotrovimab, (3) control: any therapeutic intervention, and (4) outcomes: primary efficacy outcomes (mortality rate, hospitalisation rate, and hospitalisation or death rate), secondary efficacy outcomes (ICU admission, disease progression, need for mechanical ventilation and emergency department (ED) visits), and safety outcomes (the incidence of adverse events). Studies conducted on animal models, case reports, case series, and commentary were excluded.
2.3. Data extraction and quality assessment
The risk of bias in nonrandomised studies of interventions (ROBINS‐I) 23 and Cochrane RoB 24 tools were used to assess the RoB of observational and randomized controlled trials, respectively. Two researchers independently extracted the following data: (1) information of the study (first author, year of publication, country, and design), (2) patient characteristics (sample size, sex, and mean age), (3) interventions (sample size, treatment dose, and treatment duration), and (4) efficacy and safety outcomes.
2.4. Evidence synthesis
The Comprehensive Meta‐Analysis software (version 3) was used to compare the efficacy and safety of sotrovimab versus control. The odds ratio with a 95% confidence interval (CI) was considered to analyse the dichotomous variables. The heterogeneity was defined as p < 0.10 or I 2>50%. The random‐effects model was used for highly heterogeneous studies. Otherwise, the fixed‐effects model was applied.
3. RESULTS
3.1. Study characteristics
Figure 1 shows the flow diagram of study selection according to its title, abstract, and full text. After removing duplicated records, seventeen studies, 19 , 20 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 (two RCTs and fifteen observational studies) with 27,429 patients were included in the meta‐analysis. The main characteristics of included studies are listed in Table 1.
FIGURE 1.

PRISMA flow diagram of the included studies in the meta‐analysis
TABLE 1.
Characteristics of included studies
| First author, year | Place | Design | COVID‐19 variant | N (M/F) | Intervention (N) | Control (N) |
|---|---|---|---|---|---|---|
| Gupta, 2022 29 | International a | RCT | NA | 1057 (485/572) | Sotrovimab (528) | Placebo (529) |
| Self, 2022 20 | International b | RCT | NA | 536 (308/228) | Sotrovimab (182) | Placebo (178); BRII‐196 plus BRII‐198 group (176) |
| Aggarwal, 2022 25 | USA | OS | Delta | 2085 (912/1173) | Sotrovimab (522) | mAb‐untreated (1563) |
| Aggarwal 2022 26 | USA | OS | Omicron | 5205 (2058/3147) | Sotrovimab (1542) | Untreated (3663) |
| Huang, 2022 31 | USA | OS | Delta | 2357 | Sotrovimab (311) | No treatment (2046) |
| Ong, 2022 19 | Singapore | OS | NA | 94 (59/35) | Sotrovimab (19) | Untreated 75 |
| Sharif‐Askari, 2022 35 | UAM | OS | NA | 10,882 (5492/5390) | Sotrovimab (2653) | Favipiravir (7094), sotrovimab/favipravir (2653) |
| Gleeson 2022 28 | UK | OS | Omicron | 122 | Sotrovimab (47) | No treatment (48), Molnupiravir (21), unknown (5), Molnupiravir + sotrovimab (1) |
| Vora 2022 37 | USA | OS | Omicron | 66 | Sotrovimab (16) | Nirmatrelvir/Ritonavir (12), remdesivir (38) |
| Zheng, 2022 39 | UK | OS | Omicron | 5951 (2451/3500) | Sotrovimab (3288) | Molnupiravir (2663) |
| Yetmar, 2022 38 | USA | OS | Omicron | 361 (229/139) | Sotrovimab (269) | Bebtelovimab (92) |
| Radcliffe 2022 34 | USA | OS | Omicron | 122 (70/52) | Sotrovimab (24) | No therapy (48), Molnupiravir (49), Nirmatrelvir/Ritonavir (1) |
| Piccicacco 2022 33 | USA | OS | Omicron | 260 (127/133) | Sotrovimab (88) | Control (90), remdesivir (82) |
| Solera 2022 36 | Canada | OS | Omicron | 300 (171/129) | Sotrovimab (109) | No sotrovimab (191) |
| Hedvat 2022 30 | USA | OS | Omicron | 154 | Sotrovimab (51) | No treatment (75), Nirmatrelvir/ritonavir (28) |
| Martin‐Blondel 2022 32 | France | OS | Omicron, Delta | 249 | Sotrovimab (116) | Casirivimab/Imdevimab (133) |
| Chavarot 2022 27 | France | OS | Omicron | 125 (75/50) | Sotrovimab (25) | No sotrovimab (100) |
Abbreviations: F, female; M, male; N, number; NOS, Newcastle Ottawa Scale; OS, observational study; RCT, randomized controlled trial; RoB, Risk of bias.
Brazil; Canada, Peru, Spain, and USA.
USA, Denmark, Switzerland, and Poland.
3.2. Risk of bias assessment
The methodological quality of included RCTs was high (Figure 2). The observational studies demonstrated acceptable quality using the ROBINS‐I tool (Table S1).
FIGURE 2.

Risk of bias assessment of the included trials
3.3. Efficacy outcomes
3.3.1. Primary outcomes
The results of the meta‐analysis showed a significant difference between the sotrovimab and no sotrovimab groups in terms of mortality rate (OR = 0.40; 95% CI: 0.25–0.63, p = 0.00, fixed‐effects model), hospitalisation rate (OR = 0.53; 95% CI: 0.43–0.65. p = 0.00, random‐effects model), and hospital or death rate (OR = 0.43; 95% CI: 0.25–0.73, p = 0.00, random‐effects model) (Figure 3).
FIGURE 3.

Forest plot of sotrovimab versus control for primary efficacy outcomes. (a) mortality rate. (b) hospitalisation rate. (c) Hospitalisation or death rate.
3.3.2. Secondary outcomes
Based on the findings of the meta‐analysis, sotrovimab and control groups showed no significant difference in terms of disease progression (OR = 0.45; 95% CI: 0.16–1.24, p = 0.12, random‐effects model), and ED visits (OR = 1.01; 95% CI: 0.83–1.24, p = 0.87, fixed‐effects model) (Figure 4). However, a significant difference was detected between the two groups in terms of need for mechanical ventilation (OR = 0.57; 95% CI: 0.33–0.96, p = 0.03, fixed‐effects model) and ICU admission (OR = 0.33; 95% CI: 0.17–0.67, p = 0.00, fixed‐effects model) (Figure 4).
FIGURE 4.

Forest plot of sotrovimab versus control for secondary efficacy outcomes. (a) ICU admission (b) mechanical ventilation (c) disease progression (d) ED visits.
3.3.3. Safety outcomes
The pooled estimate indicated no significant difference between the sotrovimab and control groups in terms of incidence of adverse events (OR = 0.98; 95% CI: 0.78–1.23, p = 0.88, fixed‐effects model) (Figure 5).
FIGURE 5.
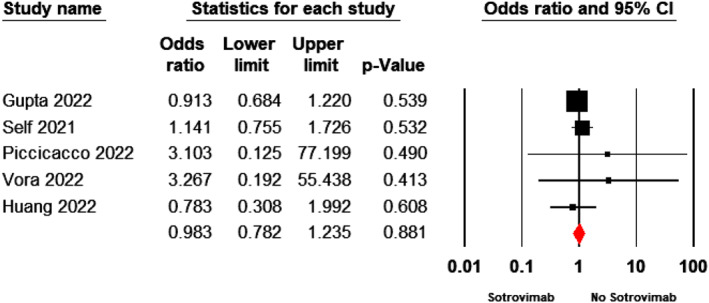
Forest plot of sotrovimab versus control for incidence of adverse events
3.4. Sensitivity analysis and subgroup analysis
A subgroup analysis was carried out based on the dominant variant of SARS‐CoV‐2 and study design for outcomes of mortality and hospitalisation rate (Table 2). The results showed a significant difference between the sotrovimab and control groups in terms of mortality rate based on Delta variant (OR = 0.07; 95% CI: 0.01–0.51, p = 0.00, fixed‐effects model) and Omicron variant (OR = 0.27; 95% CI: 0.14–0.53, p = 0.00, fixed‐effects model). Although the mortality rate was statistically significant for observational studies (OR = 0.24; 95% CI: 0.14–0.43, p = 0.00, fixed‐effects model), but was not significant for RCTs (OR = 0.95; 95% CI: 0.44–2.03, p = 0.90, fixed‐effects model). Moreover, the hospitalisation rate was statistically significant based on COVID‐19 variants and study design (p < 0.05).
TABLE 2.
Subgroup analysis and sensitivity analysis
| Analysis | No. of studies | Sample size | Point estimate (95% CI) | p‐value | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| Q‐value | p‐value | I‐squared | |||||
| Sensitive analysis | |||||||
| Mortality rate | 15 | 26,588 | 0.40 [0.25, 0.63] | 0.00 | 12.15 | 0.59 | 00.00 |
| Hospitalisation rate | 13 | 20,315 | 0.48 [0.34, 0.67] | 0.00 | 23.85 | 0.02 | 49.69 |
| Hospitalisation or death rate | 4 | 9491 | 0.40 [0.22, 0.72] | 0.00 | 8.81 | 0.03 | 65.95 |
| Disease progression | 5 | 9930 | 0.45 [0.16, 1.24] | 0.12 | 11.01 | 0.02 | 63.68 |
| ICU admission | 6 | 7488 | 0.33 [0.17, 0.67] | 0.00 | 2.48 | 0.77 | 0.00 |
| ED visits | 5 | 7606 | 1.00 [0.69, 1.45] | 0.96 | 6.40 | 0.17 | 37.51 |
| The need for mechanical ventilation | 6 | 4014 | 0.39 [0.16, 0.91] | 0.03 | 7.51 | 0.18 | 33.43 |
| Adverse events | 5 | 5211 | 0.98 [0.78, 1.23] | 0.88 | 2.15 | 0.70 | 0.00 |
| Subgroup analysis | |||||||
| Mortality rate by COVID‐19 variant | |||||||
| Delta | 2 | 4442 | 0.07 [0.01, 0.51] | 0.00 | 0.08 | 0.76 | 00.00 |
| Omicron | 9 | 12,406 | 0.27 [0.14, 0.51] | 0.00 | 1.52 | 0.99 | 00.00 |
| Mortality rate by study design | |||||||
| RCT | 2 | 1417 | 0.95 [0.44, 2.03] | 0.90 | 1.08 | 0.29 | 7.77 |
| Observational | 13 | 25,171 | 0.24 [0.14, 0.43] | 0.00 | 3.35 | 0.99 | 0.00 |
| Hospitalisation rate by COVID‐19 variant | |||||||
| Delta | 2 | 4442 | 0.07 [0.01, 0.51] | 0.00 | 0.08 | 0.76 | 00.00 |
| Omicron | 9 | 12,406 | 0.27 [0.14, 0.51] | 0.00 | 1.52 | 0.99 | 00.00 |
| Hospitalisation rate by study design | |||||||
| RCT | 1 | 1057 | 0.19 [0.08, 0.48] | 0.00 | 0.00 | 1.00 | 0.00 |
| Observational | 11 | 19,133 | 0.57 [0.46, 0.71] | 0.00 | 18.12 | 0.05 | 44.83 |
Abbreviations: CI, confidence interval; ED, emergency department; RCT, randomized controlled trial.
Furthermore, a sensitivity analysis was performed to compare the fixed and random effects estimates of the pooled estimate. A random‐effects model was used for all outcomes regardless of their I2 and p values which showed no change in the pooled estimate for efficacy and safety outcomes including, mortality rate (OR = 0.40; 95% CI: 0.25–0.63, p = 0.00, random‐effects model), hospitalisation rate (OR = 0.48; 95% CI: 0. 34–0. 67, p = 0.00, random‐effects model), hospitalisation or death rate (OR = 0.40; 95% CI: 0.22–0.72, p = 0.00, random‐effects model), disease progression (OR = 0.45; 95% CI: 0.16–1.24, p = 0.12, random‐effects model), ICU admission (OR = 0.33; 95% CI: 0.17–0.67, p = 0.00, random‐effects model), ED visits (OR = 1.00; 95% CI: 0.69–1.45, p = 0.96, random‐effects model), need for mechanical ventilation (OR = 0.39; 95% CI: 0.16–0.91, p = 0.03, random‐effects model), and adverse events (OR = 0.98; 95% CI: 0.78–1.23, p = 0.88, random‐effects model).
3.5. Publication bias
No publication bias was found in pooled estimate of hospitalisation rate (Begg test, p = 0.44; Egger test, p = 0.24). However, publication bias was detected based on Egger's test in pooled estimate of mortality rate (p = 0.001), but the Begg test showed no evidence of publication bias for pooled estimate of mortality rate (p = 0.36).
4. DISCUSSION
The purpose of this study was to examine the efficacy and safety of sotrovimab in the treatment of COVID‐19 infection. Sotrovimab is mainly used to treat patients with mild to moderate COVID‐19 symptoms and prevent the disease progression. 40 However, its efficacy against Omicron variants is unknown. 31
Our result indicated that sotrovimab was associated with a significantly lower mortality rate in patients with COVID‐19 compared with no sotrovimab. In line with our findings, a recent systematic review and meta‐analysis 41 found that neutralising mABs were associated with a significantly lower rate of death in nonhospitalised patients with COVID‐19. However, the meta‐analysis conducted by Ao et al. 42 reported no significant advantages in the use of sotrovimab for the treatment of patients with COVID‐19 infection in terms of mortality rate. This difference in results may be due to more studies included in our meta‐analysis.
The hospitalisation rate was also significantly lower in the sotrovimab‐receiving patients compared to the control. In agreement with our results, a recently published meta‐analysis 43 showed that sotrovimab may reduce the hospitalisation rate in patients with non‐severe COVID‐19. Overall, mAb therapies can effectively reduce the hospitalisation rate caused by COVID‐19 disease. 7 , 9 , 44 , 45 , 46 , 47 , 48 Moreover, a significantly lower rate of hospitalisation or death was observed among sotrovimab‐receiving patients. Note that this finding should be interpreted with caution due to a high degree of heterogeneity between studies.
Nowadays, the therapeutic potential of sotrovimab in treating COVID‐19 infection caused by the Omicron variant is disputed. 49 Recently, FDA suspended the approval of sotrovimab for the treatment of COVID‐19 patients due to the rise in the proportion of BA.2 sub‐variant. 49 Huygens et al. 21 reported sotrovimab resistance and viral persistence in immunocompromised patients with the Omicron variant of SARS‐CoV‐2. Furthermore, current data show the persistence of SARS‐CoV‐2 and spike gene mutations after sotrovimab infusion. 50 However, recent studies 51 , 52 have shown that sotrovimab retains its activity against the Omicron BA.1 variant. Based on VanBlargan et al. 52 unlike other mAbs, neutralising activity of sotrovimab against the Omicron BA.1 variant was minimally affected. 52 Moreover, Mader et al. found that sotrovimab retains its potential in prophylaxis and treatment of the Omicron variant. 51 Our subgroup analysis showed that sotrovimab was effective for patients infected with the SARS‐CoV‐2 Omicron variant in terms of reducing mortality and hospitalisation rate compared to those receiving no sotrovimab.
Our meta‐analysis showed no significant benefit in favour of sotrovimab for secondary outcomes including, disease progression, need for ventilation, and ED visits. In accordance with our results, a meta‐analysis of three studies showed no significant clinical benefits in using sotrovimab in patients with COVID‐19 in terms of disease progression. 42 However, other mAbs were associated with lower risk of disease progression, 6 and ED visits. 41
Based on the present meta‐analysis, sotrovimab treatment was associated with a reduced risk of ICU admission compared with the control. Studies revealed less frequent need for mechanical ventilation 48 and ICU 53 admission among patients treated with anti–SARS‐CoV‐2 mAbs compared with those receiving no mAbs.
According to the findings of our meta‐analysis, treatment with sotrovimab was not associated with higher adverse events compared with those receiving no sotrovimab. The meta‐analysis of Lin et al. 41 showed that neutralising mABs are safe and not associated with a higher risk of adverse events compared to placebo. Anti‐SARS‐CoV‐2 mABs therapies are generally well‐tolerated in patients suffering from COVID‐19. 47 , 54 , 55 , 56 , 57
Our study has some important limitations. First, a significant publication bias was detected in the present meta‐analysis. Second, most studies included in the meta‐analysis are retrospective and subject to sources of bias or confounding. Third, distinct types of pharmaceutical interventions were used as comparison in the present meta‐analysis which can prone the results to imprecision, heterogeneity, and reported effect size. Fourth, some studies included in our analysis did not report the type of COVID‐19 variant in patients; therefore, they were excluded from sub‐group analysis. Fifth, the COVID‐19 patients showed high heterogeneity in terms of COVID‐19 vaccination, treatment protocols, and commodity diseases. Finally, a limited number of studies reported adverse events in the intervention and control treatment groups.
5. CONCLUSION
The findings of the present meta‐analysis indicated the efficacy of sotrovimab in reducing mortality and hospitalisation rate in patients with mild to moderate COVID‐19 compared with no‐sotrovimab groups. The subgroup analysis also revealed that sotrovimab could effectively reduce mortality and hospitalisation rate in patients infected with Omicron or Delta variants of SARS‐CoV‐2. Compared with the control group, sotrovimab was associated with reduced need for mechanical ventilation and ICU admission and hospitalisation or death rate. The findings of this meta‐analysis also showed the safety of sotrovimab. Nonetheless, it was not effective in terms of prevention of disease progression and reduced ED visits.
AUTHOR CONTRIBUTIONS
Conceptualisation and project administration: Bahman Amani and Behnam Amani. Literature searching: Behnam Amani and Bahman Amani. Data extraction and quality assessment: Bahman Amani and Behnam Amani. Data Analysis: Bahman Amani and Behnam Amani. Writing – original draft: Behnam Amani. Writing – review & editing: Bahman Amani and Behnam Amani.
CONFLICTS OF INTEREST
There is no conflict of interest.
Supporting information
Supporting Information S1
Amani B, Amani B. Efficacy and safety of sotrovimab in patients with COVID‐19: a rapid review and meta‐analysis. Rev Med Virol. 2022;32(6):e2402. 10.1002/rmv.2402
DATA AVAILABILITY STATEMENT
Data are available online for the included studies. 19 , 20 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39
REFERENCES
- 1. Akinbolade S, Coughlan D, Fairbairn R, et al. Combination therapies for COVID‐19: an overview of the clinical trials landscape. Br J Clin Pharmacol. 2022;88(4):1590‐1597. 10.1111/bcp.15089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Selvarajan S, Anandaradje A, Shivabasappa S, Melepurakkal Sadanandan D, Nair NS, George M. Efficacy of pharmacological interventions in COVID‐19: a network meta‐analysis. Br J Clin Pharmacol. 2022;88(9):4080‐4091. 10.1111/bcp.15338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yamani AH, Alraddadi BM, Almaghrabi RS, et al. Early use of tocilizumab in solid organ transplant recipients with COVID‐19: a retrospective cohort study in Saudi Arabia. Immun Inflamm Dis. 2022;10(3):e587. 10.1002/iid3.587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta A, Gonzalez‐Rojas Y, Juarez E, et al. Early treatment for Covid‐19 with SARS‐CoV‐2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385(21):1941‐1950. 10.1056/nejmoa2107934 [DOI] [PubMed] [Google Scholar]
- 5. Abani O, Abbas A, Abbas F, et al. Casirivimab and imdevimab in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised, controlled, open‐label, platform trial. Lancet. 2022;399(10325):665‐676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O’Brien MP, Forleo‐Neto E, Sarkar N, et al. Effect of subcutaneous casirivimab and imdevimab antibody combination vs placebo on development of symptomatic COVID‐19 in early asymptomatic SARS‐CoV‐2 infection: a randomized clinical trial. JAMA. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suzuki Y, Shibata Y, Minemura H, et al. Real‐world Clinical Outcomes of Treatment with Casirivimab‐Imdevimab Among Patients with Mild‐To‐Moderate Coronavirus Disease 2019 during the Delta Variant Pandemic. medRxiv; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohen MS, Nirula A, Mulligan MJ, et al. Effect of bamlanivimab vs placebo on incidence of COVID‐19 among residents and staff of skilled nursing and assisted living facilities: a randomized clinical trial. JAMA. 2021;326(1):46‐55. 10.1001/jama.2021.8828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus etesevimab in mild or moderate Covid‐19. N Engl J Med. 2021;385(15):1382‐1392. 10.1056/nejmoa2102685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID‐19: a randomized clinical trial. JAMA. 2021;325(7):632‐644. 10.1001/jama.2021.0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiang H, He B, Li Y, Cheng X, Zhang Q, Peng W. Bamlanivimab plus etesevimab treatment have a better outcome against COVID‐19: a meta‐analysis. J Med Virol. 2022;94(5):1893‐1905. 10.1002/jmv.27542 [DOI] [PubMed] [Google Scholar]
- 12. Gupta A, Gonzalez‐Rojas Y, Juarez E, et al. Effect of sotrovimab on hospitalization or death among high‐risk patients with mild to moderate COVID‐19: a randomized clinical trial. JAMA. 2022;327(13):1236. 10.1001/jama.2022.2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taylor PC, Adams AC, Hufford MM, De La Torre I, Winthrop K, Gottlieb RL. Neutralizing monoclonal antibodies for treatment of COVID‐19. Nat Rev Immunol. 2021;21(6):382‐393. 10.1038/s41577-021-00542-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heo Y‐A. Sotrovimab: first approval. Drugs. 2022;82(4):477‐484. 10.1007/s40265-022-01690-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahase E. Covid‐19: UK Approves Monoclonal Antibody Sotrovimab for over 12s at High Risk. British Medical Journal Publishing Group; 2021. [DOI] [PubMed] [Google Scholar]
- 16. FDA News Release . Coronavirus (COVID‐19) Update: FDA Authorizes Additional Monoclonal Antibody for Treatment of COVID‐19. AahblS. [Google Scholar]
- 17. Gupta A, Gonzalez‐Rojas Y, Juarez E, et al. Effect of the Neutralizing SARS‐CoV‐2 Antibody Sotrovimab in Preventing Progression of COVID‐19: A Randomized Clinical Trial. medRxiv; 2021. [Google Scholar]
- 18. Kmietowicz Z. Covid‐19: WHO Recommends Baricitinib and Sotrovimab to Treat Patients. British Medical Journal Publishing Group; 2022. [DOI] [PubMed] [Google Scholar]
- 19. Ong SW, Ren D, Lee PH, et al. Real‐World use of sotrovimab for pre‐emptive treatment in high‐risk hospitalized COVID‐19 patients: an observational cross‐sectional study. Antibiotics. 2022;11(3):345. 10.3390/antibiotics11030345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Self WH, Sandkovsky U, Reilly CS, et al. Efficacy and Safety of Two Neutralising Monoclonal Antibody Therapies, Sotrovimab and BRII‐196 Plus BRII‐198, for Adults Hospitalised with COVID‐19 (TICO): A Randomised Controlled Trial. The Lancet Infectious Diseases; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huygens S, Munnink BO, Gharbharan A, Koopmans M, Rijnders B. High Incidence of Sotrovimab Resistance and Viral Persistence after Treatment of Immunocompromised Patients Infected with the SARS‐CoV‐2 Omicron Variant. medRxiv; 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stevens A, Garritty C, Hersi M, Moher D. Developing PRISMA‐RR, a reporting guideline for rapid reviews of primary studies (Protocol). EQUATOR Network. 2018. https://www.equator‐network.org/wp‐content/uploads/2018/02/PRISMA‐RR‐protocol.pdf [Google Scholar]
- 23. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aggarwal N, Beatty L, Bennett TD, et al. Real‐World Evidence of the Neutralizing Monoclonal Antibody Sotrovimab for Preventing Hospitalization and Mortality in COVID‐19 Outpatients. medRxiv; 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aggarwal NR, Beaty LE, Bennett TD, Carlson NE, Ginde AA. Change in Effectiveness of Sotrovimab for Preventing Hospitalization and Mortality in COVID‐19 Outpatients during the Omicron Phase. medRxiv; 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chavarot N, Melenotte C, Amrouche L, et al. Early treatment with sotrovimab monoclonal antibody in kidney transplant recipients with Omicron infection. Kidney Int. 2022;101(6):1290‐1293. 10.1016/j.kint.2022.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gleeson S, Martin P, Thomson T, et al. Kidney Transplant Recipients and Omicron: Outcomes, Effect of Vaccines and the Efficacy and Safety of Novel Treatments. medRxiv; 2022. [Google Scholar]
- 29. Gupta A, Gonzalez‐Rojas Y, Juarez E, et al. Effect of sotrovimab on hospitalization or death among high‐risk patients with mild to moderate COVID‐19: a randomized clinical trial. JAMA. 2022;327(13):1236‐1246. 10.1001/jama.2022.2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hedvat J, Lange NW, Salerno DM, et al. COVID‐19 Therapeutics and Outcomes Among Solid Organ Transplant Recipients during the Omicron BA. 1 Era. American Journal of Transplantation; 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang DT, McCreary EK, Bariola JR, et al. Effectiveness of Casirivimab and Imdevimab, and Sotrovimab during Delta Variant Surge: A Prospective Cohort Study and Comparative Effectiveness Randomized Trial. medRxiv; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martin‐Blondel G, Marcelin A.‐G, Soulié C, et al. Outcome of very high‐risk patients treated by Sotrovimab for mild‐to‐moderate COVID‐19 Omicron, a prospective cohort study (the ANRS 0003S COCOPREV study). J Infect. 2022;84(6):e101‐e104. 10.1016/j.jinf.2022.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Piccicacco N, Zeitler K, Ing A, et al. Real‐world effectiveness of early remdesivir and sotrovimab in the highest‐risk COVID‐19 outpatients during the Omicron surge. J Antimicrob Chemother. 2022;77(10):2693‐2700. 10.1093/jac/dkac256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Radcliffe C, Palacios CF, Azar MM, Cohen E, Malinis M. Real‐world experience with available, outpatient COVID‐19 therapies in solid organ transplant recipients during the Omicron surge. Am J Transplant. 2022;22(10):2458‐2463. 10.1111/ajt.17098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saheb Sharif‐Askari F, Ali Hussain Alsayed H, Tleyjeh I, et al. Sotrovimab lowers the risk of COVID‐19 related hospitalization or death in a large population cohort in the United Arab Emirates. Clin Pharmacol Ther. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Solera JT, Árbol BG, Alshahrani A, et al. Impact of vaccination and early monoclonal antibody therapy on COVID‐19 outcomes in organ transplant recipients during the Omicron wave. Clin Infect Dis official Publ Infect Dis Soc Am. 2022:ciac324. 10.1093/cid/ciac324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vora SB, Englund JA, Trehan I, et al. Monoclonal Antibody and Antiviral Therapy for Treatment of Mild‐To‐Moderate COVID‐19 in Pediatric Patients. medRxiv; 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yetmar ZA, Beam E, O'Horo JC, et al. Outcomes of bebtelovimab and sotrovimab treatment of solid organ transplant recipients with mild‐to‐moderate coronavirus disease 2019 during the Omicron epoch. Transpl Infect Dis. 2022;24(4):e13901. 10.1111/tid.13901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zheng B, Green AC, Tazare J, et al. Comparative Effectiveness of Sotrovimab and Molnupiravir for Prevention of Severe COVID‐19 Outcomes in Non‐hospitalised Patients: An Observational Cohort Study Using the OpenSAFELY Platform. medRxiv; 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Elesdoudy A. Sotrovimab: is it effective in early treatment of mild and moderate COVID‐19 infections? A retrospective study. Egypt J Bronchology. 2021;15(1):1‐6. 10.1186/s43168-021-00104-8 [DOI] [Google Scholar]
- 41. Lin WT, Hung SH, Lai CC, Wang CY, Chen CH. The impact of neutralizing monoclonal antibodies on the outcomes of COVID‐19 outpatients: a systematic review and meta‐analysis of randomized controlled trials. J Med Virol. 2022;94(5):2222‐2229. 10.1002/jmv.27623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ao G, Li A, Wang Y, Tran C, Qi X. Lack of efficacy for sotrovimab use in patients with COVID‐19: a meta‐analysis. J Infect. 2022;85(1):e10‐e12. 10.1016/j.jinf.2022.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Siemieniuk RA, Bartoszko JJ, Martinez JPD, et al. Antibody and cellular therapies for treatment of Covid‐19: a living systematic review and network meta‐analysis. BMJ. 2021:374. 10.1136/bmj.n2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chilimuri S, Mantri N, Gurjar H, et al. Implementation and outcomes of monoclonal antibody infusion for COVID‐19 in an inner‐city safety net hospital: a South‐Bronx experience. J Natl Med Assoc. 2022;113(6):701‐705. 10.1016/j.jnma.2021.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Piccicacco N, Zeitler K, Montero J, et al. Effectiveness of Severe Acute Respiratory Syndrome Coronavirus 2 Monoclonal Antibody Infusions in High‐Risk Outpatients. Oxford University Press US. 2021:ofab292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Razonable RR, Pawlowski C, O'Horo JC, et al. Casirivimab–Imdevimab treatment is associated with reduced rates of hospitalization among high‐risk patients with mild to moderate coronavirus disease‐19. EClinicalMedicine. 2021;40:101102. 10.1016/j.eclinm.2021.101102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Webb BJ, Buckel W, Vento T, et al. Real‐world Effectiveness and Tolerability of Monoclonal Antibody Therapy for Ambulatory Patients with Early COVID‐19. Oxford University Press US; 2021:ofab331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wynia MK, Beaty LE, Bennett TD, et al. Real World Evidence of Neutralizing Monoclonal Antibodies for Preventing Hospitalization and Mortality in COVID‐19 Outpatients. medRxiv; 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. https://www.fda.gov/drugs/drug‐safety‐and‐availability/fda‐updates‐sotrovimab‐emergency‐use‐authorization
- 50. Rockett R, Basile K, Maddocks S, et al. Resistance mutations in SARS‐CoV‐2 Delta variant after sotrovimab use. N Engl J Med. 2022;386(15):1477‐1479. 10.1056/nejmc2120219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mader A.‐L, Tydykov L, Glück V, et al. Omicron's binding to Sotrovimab, Casirivimab, Imdevimab, CR3022, and sera from previously infected or vaccinated individuals. Iscience. 2022;25(4):104076. 10.1016/j.isci.2022.104076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. VanBlargan LA, Errico JM, Halfmann PJ, et al. An infectious SARS‐CoV‐2 B. 1.1. 529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat Med. 2022;28(3):1‐6. 10.1038/s41591-021-01678-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gueguen J, Colosio C, Del Bello A, et al. Early administration of anti–SARS‐CoV‐2 monoclonal antibodies prevents severe COVID‐19 in kidney transplant patients. Kidney Int Rep. 2022;7(10):2319‐2320. 10.1016/j.ekir.2022.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Farcy DA, Dalley MT, Miro G, et al. A comparison of SARS‐COV‐2 neutralizing antibody therapies in high‐risk patients with mild to moderate COVID‐19 disease at a single academic hospital. J Emerg Med. 2022;62(1):83‐91. 10.1016/j.jemermed.2021.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Goldin L, Elders T, Werhane L, Korwek K, Poland R, Guy J. Reactions and COVID‐19 disease progression following SARS‐CoV‐2 monoclonal antibody infusion. Int J Infect Dis. 2021;112:73‐75. 10.1016/j.ijid.2021.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Joy AP, Augustine AT, Karattuthodi MS, et al. The impact of casirivimab‐imdevimab antibody cocktail in patients amidst and post COVID 19 treatment: a retro‐prospective comparative study in India. Clin Epidemiol Global Health. 2022;14:100967. 10.1016/j.cegh.2022.100967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Santos JDL, Bhisitkul D, Carman M, et al. The use of monoclonal antibody therapy in pediatric patients with COVID‐19: a retrospective case series. Int J Emerg Med. 2022;15(1):1‐7. 10.1186/s12245-022-00414-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
Data are available online for the included studies. 19 , 20 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39


