Abstract
Objective
Calcitonin (Ct) represents the most important biochemical marker of medullary thyroid cancer (MTC), but has certain limits. We analyzed the performance of procalcitonin (ProCt) in follow-up MTC patients.
Methods
In this monocentric and retrospective study, we consecutively obtained ProCt and Ct values from all MTC patients that we visited during the period from April 2021 to May 2022. Patients were defined as having structural evidence of disease (29/90, 32.2%) irrespective of Ct values or, in its absence, as not evident disease (NED) if Ct was ≤10 ng/L (47/90, 52.2%), or minimal residual disease if Ct was >10 ng/L (14/90, 15.6%).
Results
Ct and ProCt values were highly correlated (r = 0.883, P < 0.01). Median ProCt values differed between NED, minimal residual disease, and structural disease, being 0.04 ng/mL, 0.26 ng/mL, and 1.98 ng/mL, respectively (P < 0.01). ProCt was undetectable (<0.04 ng/mL) in 40/47 (85.1%) of NED patients, in 3/14 (21.4%) patients with minimal residual disease and in none of the patients with a structural disease (P < 0.01). Among the 11 patients with detectable but ≤10 ng/L Ct and undetectable ProCt values, none had a structural disease. The most accurate cut-off of ProCt to distinguish between the presence or absence of a structural disease was >0.12 ng/mL (P < 0.01, area under the curve: 0.963), with the following sensitivity, specificity, positive predictive value, and negative predictive value (NPV): 100%, 83.61%, 74.4%, and 100.0%.
Conclusions
ProCt and Ct have a high correlation in MTC follow-up. ProCt may be useful as an adjunct to Ct, especially for its NPV concerning the structural disease.
Keywords: procalcitonin, calcitonin, medullary thyroid carcinoma, follow-up
Introduction
Medullary thyroid cancer (MTC) originates from parafollicular C-cells and represents 2% of all thyroid malignancies and 0.4–1.4% of all thyroid nodules (1). MTC is sporadic in 75–80% of cases or manifests as a hereditary tumor in the remaining 25%, in the context of multiple endocrine neoplasia 2 (MEN2) syndrome, due to a germline REarranged during Transfection mutation. MTC is treated with a total thyroidectomy and a central lymph node dissection. More extensive surgery is necessary if there is lateral lymph node compartment involvement (2). MTC follow-up is then based on a periodical neck ultrasound (US) and biochemical follow-up, based primarily on calcitonin (Ct) and carcinoembryonic antigen (CEA) measurement. Ct is secreted by parafollicular C cells and is the most sensitive marker for MTC. It is a polypeptide hormone composed of 32 amino acids and synthesized from a 116-amino-acid prohormone procalcitonin (ProCt) (3). Ct is highly sensitive in the diagnosis and follow-up of MTC, but its specificity is not high. Various physiological and pathological conditions other than MTC can be associated with secondary hypercalcitoninemia. Moreover, Ct has many analytical problems: it is highly unstable with a short (15–40 min) and concentration-dependent half-life at room temperature, and thus, it is necessary to be kept on ice after the withdrawal. Furthermore, Ct suffers from many laboratory interferences (4, 5). Additionally, Ct results are not comparable between different laboratories and assays, owing to the great number of different kits available in the market (5). From a post-analytical standpoint, there is no consensus on the Ct cut-off that should be adopted for the diagnosis and follow-up of MTC (2). In particular, regarding MTC follow-up, many authors suggest that a Ct level below the limit of detectability of the laboratory assay is indicative of a cured MTC (6), while others consider the patient cured when a Ct level is lower than 10 ng/L (7, 8). On the other hand, also Ct-negative MTCs have been described, anecdotally (9): since the diagnosis, but particularly during the follow-up, MTC can differentiate and lose the ability to produce and/or secrete mature Ct. As such, many other MTC markers have been proposed in the diagnosis and follow-up of MTC. CEA is rarely useful in the diagnosis of MTC, due to being elevated in many pathological and physiological conditions (tobacco smoking, gastrointestinal tract inflammatory disease, benign lung disease, and other tumors) and is raised at diagnosis only in 60–70% of MTC patients (2, 10, 11, 12). However, CEA is considered particularly useful in MTC follow-up in cases of MTC dedifferentiation: when a rising CEA level is observed, accompanied by a stable or decreasing Ct trend, it is well established that we are seeing a disease progression of a differentiated tumor (2, 13). The Ct precursor ProCt has been proposed as an alternative tumor marker in the diagnosis and follow-up of MTC (4, 14). ProCt has many characteristics that overcome Ct pitfalls, due to ProCt having a half-life of 20–24 h, not dependent on its concentrations and stability at room temperature (15). All the ProCt kits available on the market produce comparable results, being the intellectual property of ProCt assays held by a single company (16, 17). A few reports suggest that ProCt would also be useful in cases of spurious hypercalcitoninemia due to heterophilic antibody interference (18, 19). Its levels are not influenced by gender or by the physiological and pathological conditions or drugs that increase Ct levels (20). Neuroendocrine tumors represent an exception, being able to secrete both. Additionally, although rare, Ct-negative and ProCt-positive histologically proven MTC cases are possible, although very rare (21). A possible limit of ProCt is that its production is not limited to C-cells, but it is also produced by extra-thyroidal tissues not only in response to bacterial sepsis but also by causes of severe systemic inflammatory response syndrome, localized bacterial infections, autoimmune disease, severe trauma, surgery, heat stroke, and cardiogenic shock as well as fungal and parasitic infections (20). However, in the absence of signs of inflammation, ProCt is produced only by parafollicular C-cells and neuroendocrine cells in the lungs and bowel, as well as Ct (22). A good correlation has been found between Ct and ProCt in the pre-surgical setting with an association to MTC tumor burden (14, 23) and also with prognosis (23). Also in the post-surgical setting, Ct and ProCt were found correlated (15, 24), with elevated ProCt levels among the vast majority of recurrent MTCs. A recent metanalysis by Giovanella et al. documented good ProCt performances in this setting, with a sensitivity and specificity of 0.93 (95% CI: 0.85–0.97) and 0.91 (95% CI: 0.20–1.00), respectively. However, only few studies are available in the literature on this issue, and the metanalysis was indeed based on only four studies (4). Thus, the aim of the present study is the analysis of the performance of ProCt, in adjunct to Ct, in the follow-up of MTC patients, in a quite large, consecutive and monocentric series.
Materials and methods
Patients
According to the aims of our study, we enrolled patients already treated for an MTC. We retrospectively and consecutively enrolled MTC patients who followed up at our institution (Endocrinology Unit, University Hospital of Padua) who had been examined during the period from April 2021 to May 2022. All patients had undergone a thyroidectomy with a central neck dissection and a lateral neck dissection when appropriate, depending on pre-operative and intra-operative findings. Patients were assessed as not evident disease (NED) when the imaging was negative, and serum Ct did not exceed basally the upper limit of the assay (≤10 ng/L). Patients with negative imaging results but a serum Ct higher than 10 ng/L were considered as having a minimal residual disease, in the absence of any structural evidence. Patients with a structural evidence of disease (persistence or recurrent disease), regardless of their biochemical status, were defined on the basis of a positive imaging finding provided by US and, when indicated, CT, MRI, and (18) F-FDOPA (6-[18F]-L-fluoro-L-3, 4-dihydroxyphenylalanine)-based PET–CT and/or a Ct measurement in the wash-out fluid of suspected lesions when appropriate. Patients with uncertain imaging results were not included in the series. Patients with any sign of acute infection were excluded. Both sporadic and hereditary MTC cases were included. We enrolled 90 patients. For all patients included in the study, we obtained a Ct and ProCt value at their last follow-up examination and we evaluated their response to therapy based on the aforementioned criteria. CEA levels were available for 83/90 (92.2%) of patients. Disease progression status was defined based on increasing tumor burden, according to RECIST criteria, and/or on Ct doubling times lower than 24 months. Patients were considered with stable disease if Ct-doubling times were higher than 24 months, without increasing disease burden (2). The study was performed in accordance with the guidelines of the Helsinki Declaration; all patients gave their informed consent to the test and the inclusion of results in the present study. The study was approved by the Local Ethical Committee (Padua General Hospital, code number: 296n/AO/22).
Laboratory assays
Ct was measured using a two-site CLIA (Immulite2000; Siemens Diagnostics) with an analytical sensitivity of 1 ng/L. ProCt was measured using a two-site, two-step CLIA LIAISON BRAHMS PCT II GEN (DiaSorin, Saluggia, Italy), with an analytical sensitivity of 0.04 ng/mL. CEA was measured using a CLIA kit (Lumipulse® G CEA-N Immunoreaction Cartridges, Japan), with an analytical sensitivity of 0.096 ng/mL.
Statistical analyses
The Kolmogorov–Smirnov test was used to assess the normal distribution of each variable. As the variables were not distributed normally, data are reported as medians and interquartile ranges (IQR). The Mann–Whitney test for independent non-parametric data was used to analyze median Ct, ProCt, and CEA levels between patients with a locoregional vs metastatic structural disease and between patients with either a stable or progressive disease. The Kruskal–Wallis test for independent non-parametric data was used to analyze the median ProCt and CEA levels in the three groups of patients (NED, minimal residual disease, and structural disease). Correlations between variables were studied with the non-parametric test Spearman rank correlation analysis. Categorical variables (detectable/undetectable ProCt patients and CEA lower or higher than the laboratory with respect to disease outcomes) were compared with the ϰ2 test. In dichotomizing data related to ProCt, we decided to consider the detectable/undetectable value because ProCt is normally undetectable in healthy subjects (20), while CEA levels may be increased by many physiological situations (12), so we decided to dichotomize its values according to the upper limit of the laboratory range (≤4.7 ng/mL). A P-value of <0.05 was considered statistically significant. R package version 2.7-2 (https://socialsciences.mcmaster.ca/jfox/Misc/Rcmdr/) was used for statistical analysis.
Results
The final series included 90 MTC (50 females and 40 males), median age 47.11 years (range: 5.9–83.0 years) patients. 51 had a sporadic MTC and 39 had a hereditary MTC. The median follow-up (from MTC surgery to the last follow-up examination) was 97.5 months, IQR: 30.5–194.5 months. The other clinical and pathological characteristics of the enrolled series are summarized in Table 1. At the end of the follow-up period, 47/90 (52.2%) were NED, 14/90 (15.6%) had a minimal residual disease, and 29/90 (32.2%) had a structural evidence of disease. Owing to the design of the enrollment, no disease-related deaths were documented. Thirteen patients started TKI (tyrosine kinase inhibitors) treatment, among which four (30.8%) had a progressive disease and nine (69.2%) had a stable disease at the last follow-up.
Table 1.
Clinical and pathological characteristics and pre-surgical CEA and Ct values of the enrolled series.
| Group | Overall | Sporadic MTC | Hereditary MTC | |
|---|---|---|---|---|
| n (%)a | 90 | 51 | 39 | |
| T (%) | 1 | 48 (64.0) | 29 (63.0) | 19 (65.5) |
| 2 | 11 (14.7) | 7 (15.2) | 4 (13.8) | |
| 3 | 14 (18.7) | 8 (17.4) | 6 (20.7) | |
| 4 | 2 (2.6) | 2 (4.4) | 0 (0) | |
| NA | 15 | 5 | 10 | |
| n (%) | 0 | 43 (55.8) | 26 (54.2) | 17 (58.6) |
| 1 | 29 (37.7) | 19 (39.6) | 10 (34.5) | |
| X | 5 (6.5) | 3 (6.2) | 2 (6.9) | |
| NA | 13 | 3 | 10 | |
| M (%) | 0 | 57 (81.4) | 33 (78.6) | 24 (85.7) |
| 1 | 6 (8.6) | 5 (11.9) | 1 (3.6) | |
| X | 7 (10.0) | 4 (9.5) | 3 (10.7) | |
| NA | 20 | 9 | 11 | |
| Stage (%) | I | 33 (47.1) | 19 (46.3) | 14 (48.3) |
| II | 8 (11.4) | 4 (9.8) | 4 (13.8) | |
| III | 9 (12.9) | 6 (14.6) | 3 (10.3) | |
| IV | 20 (28.6) | 12 (29.3) | 8 (27.6) | |
| NA | 20 | 10 | 10 | |
| Cancer size (mm), median value (IQR); mean (SD) | 10.00 (1.00–18.5); 14.63 (1.77) |
12.00 (8.00–20.00); 17.12 (2.50) |
6.5 (3–15); 10.69 (2.14) |
|
| Pre-surgical CEA level (ng/mL), median value (IQR); mean (SD) | 13.00 (4.70–50.9); 33.88 (7.65) |
13.00 (6.87); 29.36 (6.87) |
46.20 (3.95–115.75); 59.85 (34–21) |
|
| Pre-surgical Ct level (ng/L), median value (IQR); mean (SD) | 127.00 (44.10–761.25); 1760.01 (620.85) |
134.00 (58.98– 1096.25); 1667.44 (574.27) | 115.00 (33.38– 683.25); 1911.82 (1361.28) |
aPercentages calculated based on available data.
CEA, carcinoembryonic antigen; Ct, calcitonin; IQR, interquartile range; MTC, medullary thyroid cancer; SD, standard deviation.
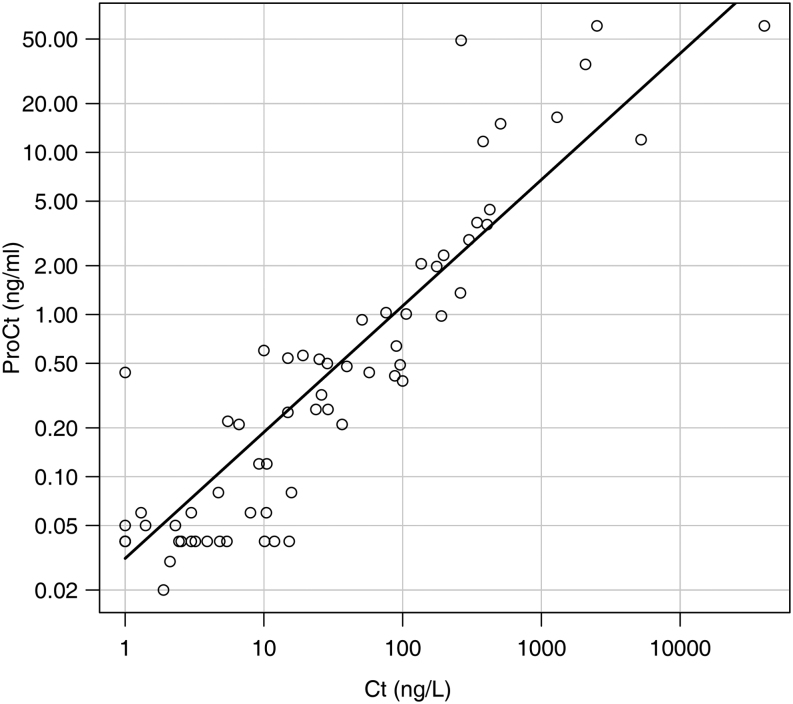
A strong correlation was found between ProCt and Ct values at the last post-surgical follow-up at Spearman rank correlation analysis (r = 0.883, P < 0.01, Fig. 1). Median ProCt values were significantly different among the three groups (NED, minimal residual disease and structural disease), being 0.04 ng/mL (IQR: 0.04–0.04 ng/mL), 0.26 (IQR: 0.06–0.56), and 1.98 ng/mL (IQR: 0.49–11.78), respectively (P < 0.01). ProCt was undetectable (<0.04 ng/mL) in 40/47 (85.1%) of NED patients, in 3/14 (21.4%) of patients with a minimal residual disease and in none of the patients with a structural disease (P < 0.01) (Table 2).
Figure 1.
Correlation between calcitonin (Ct) and procalcitonin (ProCt) (r = 0.883, P < 0.01) at the last post-surgical follow-up. Values are represented in their logarithmic to base 10.
Table 2.
Median procalcitonin (ProCt), calcitonin (Ct), and carcinoembryonic antigen (CEA) levels according to disease response.
| Factor | NED (47/90) (52.2%) | Minimal residual disease (14/90) (15.6%) | Structural evident disease (29/90) (32.2%) | P |
|---|---|---|---|---|
| ProCt (ng/mL) median values (IQR) | 0.04 (0.04–0.04) | 0.26 (0.06–0.56) | 1.98 (0.49– 11.78) | <0.01 |
| Ct (ng/L) median values (IQR) | 1.000 (1.0–2.4) | 17.45 1 (1.90–57.40) | 176.00 (47.70–410.75) | <0.01 |
| CEA (ng/mL) median values (IQR) | 2.00 (0.70–3.40) | 2.40 (1.50–3.40) | 14.35 (4.65–51.25) | <0.01a |
| ProCt ≤0.04, n (%) | 40/47 (85.1%) | 3/14 (21.4%) | 0/29 (0%) | <0.01 |
| Ct ≤10 ng/L, n (%) | 47/47 (100%) | 0/14 (0%) | 2/29 (10%) | <0.01 |
| <1 ng/L: 31/47 (66.0%) | <1 ng/L: 0/29 (0%) | |||
| >1 ng/L and ≤10 ng/L: 16/47 (34.0%) | >1 ng/L and ≤10 ng/L: 2/2 (100%) | |||
| CEA ≤ 4.7 ng/mL, n (%) | 37/41 (90.2%)b | 12/14 (85.7%) | 6/28 (21.4%)c | <0.01a |
aBetween structural evident disease vs minimal residual disease and vs NED, there was no significant difference between NED and minimal residual disease; b Six missing data; c One missing data.
IQR, interquartile range; NED, not evident disease.
In patients with structural evidence of disease, median ProCt values were higher in patients with a metastatic disease than in patients with a locoregional persistence, being 4.44 ng/mL (IQR: 1.04–30.31 ng/mL) and 0.54 ng/mL (IQR: 0.40–2.06 ng/mL), respectively (P < 0.01). A trend toward higher ProCt values was documented in patients taking TKI with a progressive disease than a stable disease, being 25.71 ng/mL (IQR: 14.10–47.61 ng/mL) and 3.59 ng/mL (IQR: 0.59–21.28 ng/mL), respectively, but without reaching the statistical significance, maybe owing to the small number of patients (P = 0.13, with four patients in the former and nine patients in the latter group). None among the patients in TKI treatment had undetectable ProCt values (data not shown).
Median Ct values were different between NED patients and patients with a minimal residual disease for definition, in this study. Ct median values were higher in the presence of a structural evidence of disease than in the group with minimal residual disease, being 176.00 (IQR: 47.70–410.75 ng/L) and 17.45 ng/L (IQR: 11.90–57.40 ng/L), respectively (P < 0.01). Ct was higher than 10 ng/L in 27/29 (93.1%) patients with a structural evidence of disease (Table 2). Indeed, two patients with structural disease persistence had Ct values ≤10 ng/L, being equal to 10 and 5.5 ng/L: both had a metastatic disease and were in therapy with TKI.
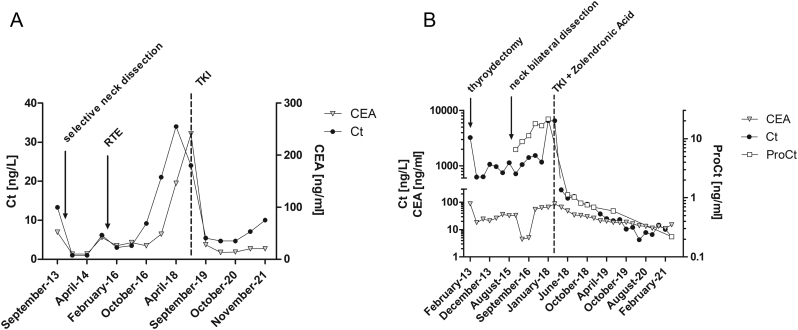
The former (patient A, Fig. 2A) was a 56-year-old female, thyroidectomized in 2010, for a sporadic MTC, 3.3 cm at its largest, N0. During the follow-up, the patient underwent a surgical intervention to remove lateral neck compartment lymph node recurrences, and then she had external radiotherapy with a tumoral marker trend characterized by a slightly increased Ct, but with a sharply elevated CEA. In January 2019, TKI treatment was started for the radiological progression of lung metastases, with subsequent stable disease, oscillating CEA levels and measurable Ct values, yet lower than 10 ng/L. At the last follow-up, both ProCt and CEA were elevated, being 0.6 and 15.3 ng/mL, respectively.
Figure 2.
Trends in calcitonin (Ct) and carcinoembryonic antigen (CEA) values in patient A (A) and trends in Ct, CEA, and procalcitonin (ProCt) in patient B (B). In the latter case, values are represented in their logarithmic to base 10.
The latter (patient B, Fig. 2B) was a 49-year-old MEN2A male, who underwent a thyroidectomy in 2013, with a pre-surgical Ct level of 3225 ng/L and a CEA of 88 ng/mL, for an IVC stage MTC. Six months after surgery, his Ct level dropped to 634 ng/L, CEA to 24 ng/mL, without clear US evidence of disease. The patient underwent a surgical bilateral neck dissection in September 2015. Despite that, shortly after the surgical intervention, Ct levels started to rise again. Starting from April 2016, available ProCt levels were elevated, paralleling the Ct trend. In April 2018, a TKI treatment was initiated after symptomatic bone metastasis, with a drop in Ct and ProCt serum levels: the former oscillating up and down around or under 10 ng/L, and the latter always detectable, with a value of 0.22 ng/mL at the last follow-up. Also, CEA was elevated, being equal to 20 ng/mL. The patient had a stable disease at the latest follow-up.
Moreover, among patients with a structural evidence of disease, higher Ct values were found in presence of a metastatic disease than in locoregional disease, being Ct median values of 379.00 ng/L (IQR: 103.50–1884.50 ng/L) and of 97.95 ng/L (IQR: 28.6–176.00 ng/L), respectively (P = 0.03).
Eighteen patients had a measurable Ct value, but lower than 10 ng/ml. Of these patients, 11/18 had an undetectable ProCt value and 7/18 had a detectable (>0.04 ng/mL) one. Among the 11 patients with detectable but low Ct values and undetectable ProCt values, none had a structural disease. Of seven patients with low Ct but detectable ProCt values, two had structural evidence of disease and five were NED. At the receiver operating characteristic (ROC) curve analysis, the most accurate cut-off of ProCt in order to distinguish between the presence and absence of a structural evident disease was >0.12 ng/mL (P < 0.01, area under the curve: 0.963), with the following sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV): 100%, 83.61%, 74.4%, and 100%.
A trend toward higher ProCt/Ct ratio was found in patients with structural evidence of disease when compared to patients with a minimal residual disease, being 0.01178 (IQR: 0.008066–0.02191) and 0.007392 (IQR: 0.005063–0.01231), respectively, but without reaching the statistical significance (P = 0.09).
CEA and Ct levels were correlated at the Spearman rank correlation analysis, although with less power than Ct and ProCt (r = 0.585, P < 0.01). CEA and ProCt values were correlated (r = 0.628, P < 0.01), too.
CEA median values were higher in cases with structural disease than in those with minimal residual disease and NED, being 14.35 ng/mL (IQR: 4.65–51.25 ng/mL), 2.40 ng/mL (IQR: 1.50–3.40 ng/mL), and 2.00 ng/mL (IQR: 0.70–3.40 ng/mL), respectively (P < 0.01), but levels did not differ between minimal residual disease and NED, contrary to ProCt and Ct. CEA did not exceed the normal range (≤4.7 ng/mL), in 37/41 (90.2%) of NED patients, in 12/14 (85.7%) patients with a minimal residual disease and in 6/28 (21.4%) of patients with a structural disease (Table 2). Among patients with structural disease, CEA median levels were higher in metastatic than in locoregional disease, being 43.05 (IQR: 20.00–139.60 ng/mL) and 5.20 ng/mL (IQR: 3.00–9.60 ng/mL), respectively (P < 0.01).
Overall, among patients in TKI treatment, Ct and ProCt were still correlated, although weakly, at the limits of the statistical significance: r = 0.5619, P = 0.046 (data not shown). On the contrary, CEA and Ct and CEA and ProCt lost their correlation.
Discussion
After surgery, in cases of undetectable or within the normal range Ct/CEA values and negative US findings after the first year, current guidelines (2) recommend that the two tumor markers should be checked yearly, all lifelong. Indeed, there is disagreement in the literature over the more adequate cut-off of Ct which appropriately defines a patient ‘cured’: should Ct be undetectable or within the limits of the laboratory range? On the other hand, in cases of elevated postoperative Ct values less than 150 pg/mL, patients should undergo physical examination, measurement of Ct and CEA and a neck US every 6 months (Recommendation 47, GRADE C, based on expert opinion) (2). This last setting represents a real challenge in clinical practice: the most part of MTC patients have negative imaging for years, in spite of recurrent and useful clinical, biochemical, and radiological assessments. Moreover, MTC can also lose its ability to secrete Ct during the follow-up in rare cases and clinical settings (21, 25). So, we decided to evaluate the ProCt performance in the follow-up of MTC, in a quite large and monocentric series, with the aim to explore the possibility that such a marker could be of help in identifying patients at risk of structural disease.
At first, our results show that cured MTC patients have lower ProCt values when compared to non-cured MTC, among which the highest ProCt values were found in patients with metastatic disease, paralleling the results obtained by other groups (15, 26). Furthermore, ProCt and Ct are highly correlated, suggesting that the secretion of Ct and ProCt is largely in parallel. As such, Ct is generally adequate for the MTC patient’s follow-up. However, ProCt may be useful in MTC follow-up in certain contexts, by our findings. Indeed, at the ROC curve analysis, we found a ProCt cut-off – >0.12 ng/mL – able to detect structural disease with a high sensitivity, around 100%. This data suggest that ProCt could be a good adjunct to Ct in MTC follow-up: irrespective of Ct values, none of the patients with ProCt values under this cut-off had a structural disease. In other words, due to its good NPV, ProCt could be useful to select patients with measurable Ct who do not warrant the bi-annual follow-up recommended by the current guidelines. Our present data are in line with a previous report, based on a comparable although less numerous series of MTC patients in follow-up, where a highly accurate cut-off of ProCt for the identification of the structural disease was identified by ROC curve analysis (26).
Nevertheless, by our data, ProCt was undetectable in 3/14 patients in the minimal residual disease group, raising some issues about the ProCt sensitivity; therefore, the significance of ProCt in this context is to be clarified. However, these ProCt outliers, given the absence of any radiological evidence of disease, in presence of a Ct value > 10 ng/L, could point to falsely increased levels of Ct, paralleling the suggestions of other authors (17). Considering ProCt specificity, not all NED patients had an undetectable ProCt value (only 40/47 patients). In this context, ProCt could help in identifying patients that require closer attention, or, alternatively, although patients with evidence of sepsis were not included, ProCt elevation could be related to other non-identified inflammatory causes. Indeed, in order to fully define the accuracy and reliability of ProCt value in the follow-up of MTC patients, we would need to know the future history of NED patients with detectable ProCt values and that of patients with minimal residual disease with an undetectable ProCt. Certainly, our study has the significant limit of being a retrospective study. Furthermore, our series includes a relatively high prevalence of patients with a structural evidence of disease: being a tertiary level center, we often follow up more complex cases. Maybe the prevalence of patients with a structural evidence of disease does not reflect the real prevalence in the population of follow-up MTC patients and this could have affected PPV and NPV results. Finally, although the numerosity is relatively consistent for a rare disease, the total number of patients enrolled is not sufficient enough to draw definitive conclusions: larger, multicentric, and prospective studies are needed and made feasible due to the high inter-assay comparability of ProCt (16, 17).
Interestingly, two patients with a structural persistence had Ct values lower than 10 ng/L, but both had ProCt and CEA values unequivocally high at the last follow-up. These data are to be taken with caution because both these patients were in TKI treatment. It is well known that during TKI treatment, transient variations in Ct and CEA levels are often documented and such short-term fluctuations may not reflect the responsiveness to therapy (27). Still, analyzing the fluctuations of Ct in these two patients, we can notice that one of the patients showed a lowering of Ct values a long time before TKI initiation, although keeping an unequivocally high CEA and with a high ProCt value at the last follow-up. This behavior can have various explanations. Indeed, MTC that preferentially secrete ProCt instead of Ct due to a defective prohormone processing are possible, with anecdotal cases being described in literature, at diagnosis and in recurrent and metastatic MTCs (21, 25). Moreover, in vitro studies have demonstrated that RNA processing of Ct and ProCt vary during the different stages of growth, with a low Ct expression (and thus a higher proportion of precursors), especially during rapid growth (28, 29, 30). This could increase the ProCt/Ct ratio, data encountered in many studies, in which higher ProCt/Ct value had a prognostic significance, being able to predict a shorter progression-free survival (28). In our series, there was a trend toward a higher ProCt/Ct ratio in the presence of a structural disease than in a minimal residual disease, but without reaching the statistical significance in both cases, maybe owing to numerosity issues.
Further studies are necessary to evaluate the reliability of the ProCt trend in patients treated with TKI.
Moreover, it should be noted that ProCt performs better than CEA, always being detectable in patients with a structural evidence of disease, while CEA is within the normal range missed in 21.4% of cases, at least according to CEA laboratory range limits.
In conclusion, the results obtained by our study reinforce ProCt as an adjunct in MTC follow-up, mainly because it is highly sensitive to the presence of a structural persistent disease: no patients with a structural disease had a negative ProCt value, as opposed to CEA and Ct. Moreover, the identified cut-off of ProCt (>0.12 ng/mL) had a 100% sensitivity for the presence of a structural disease. Given its high NPV, ProCt could be of help in the follow-up of MTC patients with detectable Ct, but <150 ng/L and absence of any radiological evidence of disease, to select patients candidate to a less strict follow-up.
However, its significance in minimal residual disease cases and in NED cases needs further research, possibly coming from large series, comparing Ct and ProCt performances in MTC follow-up.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the manuscript.
Funding
The study did not receive any specific grant from any funding agency in the public, commercial and no-profit sector.
Statement of ethics
The study was performed in accordance with the guidelines of the Helsinki Declaration; all patients gave their informed consent to the test, and to the inclusion of the results in the present study. The retrospective study was notified to the ethical committee of our institution.
Author contribution statement
CM, SC and JM: study conceptualization; TB, IP and GC: data collection; MI, FT and DB: data curation; data analysis: SC, AM and JM; writing-original draft preparation: SC, CM and SZ; supervision: CM and SZ. The final draft article was approved by all the authors.
Acknowledgements
The authors thank Jillian Walton for text editing and also want to thank Diego Faggian for his excellent technical assistance. We thank the DIMAR department (DImed and MAlattie Rare) for the support in the research on rare diseases.
References
- 1.Randle RW, Balentine CJ, Leverson GE, Havlena JA, Sippel RS, Schneider DF, Pitt SC. Trends in the presentation, treatment, and survival of patients with medullary thyroid cancer over the past 30 years. Surgery 2017161137–146. ( 10.1016/j.surg.2016.04.053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wells SA, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini Fet al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 201525567–610. ( 10.1089/thy.2014.0335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker KL, Nylén ES, White JC, Müller B, Snider RH. Clinical review 167: Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. Journal of Clinical Endocrinology and Metabolism 2004891512–1525. ( 10.1210/jc.2002-021444) [DOI] [PubMed] [Google Scholar]
- 4.Giovanella L, Garo ML, Ceriani L, Paone G, Campenni’ A, D’Aurizio F. Procalcitonin as an alternative tumor marker of medullary thyroid carcinoma. Journal of Clinical Endocrinology and Metabolism 20211063634–3643. ( 10.1210/clinem/dgab564) [DOI] [PubMed] [Google Scholar]
- 5.Censi S, Cavedon E, Fernando SW, Barollo S, Bertazza L, Zambonin L, Zaninotto M, Faggian D, Plebani M, Mian C. Calcitonin measurement and immunoassay interference: a case report and literature review. Clinical Chemistry and Laboratory Medicine 2016541861–1870. ( 10.1515/cclm-2015-1161) [DOI] [PubMed] [Google Scholar]
- 6.Pellegriti G, Leboulleux S, Baudin E, Bellon N, Scollo C, Travagli JP, Schlumberger M. Long-term outcome of medullary thyroid carcinoma in patients with normal postoperative medical imaging. British Journal of Cancer 2003881537–1542. ( 10.1038/sj.bjc.6600930) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elisei R, Pinchera A. Advances in the follow-up of differentiated or medullary thyroid cancer. Nature Reviews. Endocrinology 20128466–475. ( 10.1038/nrendo.2012.38) [DOI] [PubMed] [Google Scholar]
- 8.Barbot N, Calmettes C, Schuffenecker I, Saint-André JP, Franc B, Rohmer V, Jallet P, Bigorgne JC. Pentagastrin stimulation test and early diagnosis of medullary thyroid carcinoma using an immunoradiometric assay of calcitonin: comparison with genetic screening in hereditary medullary thyroid carcinoma. Journal of Clinical Endocrinology and Metabolism 199478114–120. ( 10.1210/jcem.78.1.7904611) [DOI] [PubMed] [Google Scholar]
- 9.Trimboli P, Giovanella L. Serum calcitonin negative medullary thyroid carcinoma: a systematic review of the literature. Clinical Chemistry and Laboratory Medicine 2015531507–1514. ( 10.1515/cclm-2015-0058) [DOI] [PubMed] [Google Scholar]
- 10.Wells SA, Haagensen DE, Linehan WM, Farrell RE, Dilley WG. The detection of elevated plasma levels of carcinoembryonic antigen in patients with suspected or established medullary thyroid carcinoma. Cancer 1978421498–1503. (https://doi.org/10.1002/1097-0142(197809)42:3+<1498::aid-cncr2820420821>3.0.co;2-t) [DOI] [PubMed] [Google Scholar]
- 11.Machens A, Ukkat J, Hauptmann S, Dralle H. Abnormal carcinoembryonic antigen levels and medullary thyroid cancer progression: a multivariate analysis. Archives of Surgery 2007142289–293. ( 10.1001/archsurg.142.3.289) [DOI] [PubMed] [Google Scholar]
- 12.Zarkesh M, Arab N, Tavangar SM, Nozhat Z, Fanaei SM, Hedayati M. Utilizing the circulating tumor markers in diagnosis and management of medullary thyroid cancer. Pathology, Research and Practice 2022229153694. ( 10.1016/J.PRP.2021.153694) [DOI] [PubMed] [Google Scholar]
- 13.Barbet J, Campion L, Kraeber-Bodéré F, Chatal JF. & GTE Study Group. Prognostic impact of serum calcitonin and carcinoembryonic antigen doubling-times in patients with medullary thyroid carcinoma. Journal of Clinical Endocrinology and Metabolism 2005906077–6084. ( 10.1210/jc.2005-0044) [DOI] [PubMed] [Google Scholar]
- 14.Censi S, di Stefano M, Repaci A, Benvenuti T, Manso J, Pagotto U, Iacobone M, Barollo S, Bertazza L, Galuppini Fet al. Basal and calcium-stimulated procalcitonin for the diagnosis of medullary thyroid cancers: lights and shadows. Frontiers in Endocrinology (Lausanne) 202112754565. ( 10.3389/FENDO.2021.754565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Algeciras-Schimnich A, Preissner CM, Theobald JP, Finseth MS, Grebe SK. Procalcitonin: a marker for the diagnosis and follow-up of patients with medullary thyroid carcinoma. Journal of Clinical Endocrinology and Metabolism 200994861–868. ( 10.1210/jc.2008-1862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lippi G, Salvagno GL, Gelati M, Pucci M, lo Cascio C, Demonte D, Faggian D, Plebani M. Two-center comparison of 10 fully-automated commercial procalcitonin (PCT) immunoassays. Clinical Chemistry and Laboratory Medicine 20195877–84. ( 10.1515/CCLM-2019-0888) [DOI] [PubMed] [Google Scholar]
- 17.Kratzsch J, Willenberg A, Frank-Raue K, Kempin U, Rocktäschel J, Raue F. Procalcitonin measured by three different assays is an excellent tumor marker for the follow-up of patients with medullary thyroid carcinoma. Clinical Chemistry and Laboratory Medicine 2021591861–1868. ( 10.1515/cclm-2021-0428) [DOI] [PubMed] [Google Scholar]
- 18.Giovanella L, Giordani I, Imperiali M, Orlandi F, Trimboli P. Measuring procalcitonin to overcome heterophilic-antibody-induced spurious hypercalcitoninemia. Clinical Chemistry and Laboratory Medicine 201856e191–e193. ( 10.1515/cclm-2017-0993) [DOI] [PubMed] [Google Scholar]
- 19.Bolstad N, Warren DJ, Bjerner J, Kravdal G, Schwettmann L, Olsen KH, Rustad P, Nustad K. Heterophilic antibody interference in commercial immunoassays; a screening study using paired native and pre-blocked sera. Clinical Chemistry and Laboratory Medicine 2011492001–2006. ( 10.1515/CCLM.2011.702) [DOI] [PubMed] [Google Scholar]
- 20.Schneider HG, Lam QT. Procalcitonin for the clinical laboratory: a review. Pathology 200739383–390. ( 10.1080/00313020701444564) [DOI] [PubMed] [Google Scholar]
- 21.Brutsaert EF, Gersten AJ, Tassler AB, Surks MI. Medullary thyroid cancer with undetectable serum calcitonin. Journal of Clinical Endocrinology and Metabolism 2015100337–341. ( 10.1210/jc.2014-3095) [DOI] [PubMed] [Google Scholar]
- 22.Maruna P, Nedělníková K, Gürlich R. Physiology and genetics of procalcitonin. Physiological Research 200049(Supplement 1) S57–S61. (available at: https://pubmed.ncbi.nlm.nih.gov/10984072/) [PubMed] [Google Scholar]
- 23.Machens A, Lorenz K, Dralle H. Utility of serum procalcitonin for screening and risk stratification of medullary thyroid cancer. Journal of Clinical Endocrinology and Metabolism 2014992986–2994. ( 10.1210/jc.2014-1278) [DOI] [PubMed] [Google Scholar]
- 24. Kaczka K, Mikosiński S, Fendler W, Celnik A, Pomorski L. Calcitonin and procalcitonin in patients with medullary thyroid cancer or bacterial infection. Advance in Clinical and Experimental Medicine. 2012;21:169––178.. [PubMed] [Google Scholar]
- 25.Bugalho MJ, Madureira D, Domingues R, Pereira T, Cortez L. Medullary thyroid carcinoma preferentially secreting procalcitonin. Thyroid 2014241190–1191. ( 10.1089/thy.2013.0664) [DOI] [PubMed] [Google Scholar]
- 26.Trimboli P, Lauretta R, Barnabei A, Valabrega S, Romanelli F, Giovanella L, Appetecchia M. Procalcitonin as a postoperative marker in the follow-up of patients affected by medullary thyroid carcinoma. International Journal of Biological Markers 201833156–160. ( 10.1177/1724600817747518) [DOI] [PubMed] [Google Scholar]
- 27.Kurzrock R, Atkins J, Wheler J, Fu S, Naing A, Busaidy N, Hong D, Sherman S. Tumor marker and measurement fluctuations may not reflect treatment efficacy in patients with medullary thyroid carcinoma on long-term RET inhibitor therapy. Annals of Oncology 2013242256–2261. ( 10.1093/annonc/mdt177) [DOI] [PubMed] [Google Scholar]
- 28.Walter MA, Meier C, Radimerski T, Iten F, Kränzlin M, Müller-Brand J, de Groot JWB, Kema IP, Links TP, Müller B. Procalcitonin levels predict clinical course and progression-free survival in patients with medullary thyroid cancer. Cancer 201011631–40. ( 10.1002/cncr.24738) [DOI] [PubMed] [Google Scholar]
- 29.Nelkin BD, Chen KY, de Bustros A, Roos BA, Baylin SB.Changes in calcitonin gene RNA processing during growth of a human medullary thyroid carcinoma cell line. Cancer Research 1989496949–6952. (available at: https://pubmed.ncbi.nlm.nih.gov/2582437/) [PubMed] [Google Scholar]
- 30.Berger CL, de Bustros A, Roos BA, Leong SS, Mendelsohn G, Gesell MS, Baylin SB. Human medullary thyroid carcinoma in culture provides a model relating growth dynamics, endocrine cell differentiation, and tumor progression. Journal of Clinical Endocrinology and Metabolism 198459338–343. ( 10.1210/jcem-59-2-338) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a