Abstract
Objective
Multiple endocrine neoplasia type 4 (MEN4) is caused by a CDKN1B germline mutation first described in 2006. Its estimated prevalence is less than one per million. The aim of this study was to define the disease characteristics.
Methods
A systematic review was performed according to the PRISMA 2020 criteria. A literature search from January 2006 to August 2022 was done using MEDLINE® and Web of ScienceTM.
Results
Forty-eight symptomatic patients fulfilled the pre-defined eligibility criteria. Twenty-eight different CDKN1B variants, mostly missense (21/48, 44%) and frameshift mutations (17/48, 35%), were reported. The majority of patients were women (36/48, 75%). Men became symptomatic at a median age of 32.5 years (range 10–68, mean 33.7 ± 23), whereas the same event was recorded for women at a median age of 49.5 years (range 5–76, mean 44.8 ± 19.9) (P = 0.25). The most frequently affected endocrine organ was the parathyroid gland (36/48, 75%; uniglandular disease 31/36, 86%), followed by the pituitary gland (21/48, 44%; hormone-secreting 16/21, 76%), the endocrine pancreas (7/48, 15%), and the thyroid gland (4/48, 8%). Tumors of the adrenal glands and thymus were found in three and two patients, respectively. The presenting first endocrine pathology concerned the parathyroid (27/48, 56%) and the pituitary gland (11/48, 23%). There were one (27/48, 56%), two (13/48, 27%), three (3/48, 6%), or four (5/48, 10%) syn- or metachronously affected endocrine organs in a single patient, respectively.
Conclusion
MEN4 is an extremely rare disease, which most frequently affects women around 50 years of age. Primary hyperparathyroidism as a uniglandular disease is the leading pathology.
Keywords: multiple endocrine neoplasia type 4, CDKN1B, parathyroid gland, pituitary gland
Introduction
Multiple endocrine neoplasia (MEN) is a rare group of autosomal dominant disorders with a wide spectrum of endocrine and non-endocrine manifestations (Table 1). Five different types of MEN have been described so far: MEN1, MEN2 (formerly MEN2A), MEN3 (formerly MEN2B), the recently identified MEN4 (1, 2, 3, 4), and MEN5 (5, 6). The penetrance is varied and the phenotypic expression is heterogeneous, thereby leading to different manifestations of the syndrome even within members of the same family (2, 5, 7, 8).
Table 1.
Multiple endocrine neoplasia (MEN) type 1–5.
| MEN1 | MEN2 | MEN3 | MEN4 | MEN5 | |
|---|---|---|---|---|---|
| Alternative nomenclature | Wermer syndrome | MEN2A, Sipple syndrome | MEN2B, Gorlin-Vickers/Williams-Pollock/Wagenmann–Froboese syndrome | --- | --- |
| OMIM® | #131100 | #171400 | #162300 | #610755 | --- |
| Gene | MEN1 | RET | RET | CDKN1B | MAX |
| Location | 11q13.1 | 10q11.21 | 10q11.21 | 12p13.1 | 14q23.3 |
| Inheritance | Autosomal-dominant | Autosomal-dominant | Autosomal-dominant | Autosomal-dominant | Autosomal-dominant |
| Encoded protein | Menin | RET | RET | p27 | MAX |
| Leading tumor (prevalence %) | PHPT (85%) | Medullary thyroid cancer (95–100%) | Medullary thyroid cancer (95–100%) | PHPT (75%) | Pheochromocytoma |
| Further manifestations | pNET: gastrinoma, insulinoma (30–80%) Pituitary adenoma (30–50%) |
Pheochromocytoma (50%) | Pheochromocytoma (50%) | Pituitary adenoma (44%) pNET: non-functional, gastrinoma Papillary thyroid cancer Tumors of the adrenal gland and the thymus |
Paraganglioma Pituitary adenoma Ganglioneuroma Ganglioneuroblastoma PHPT |
| PHPT (20–30%) FMTC |
Ganglioneuroma of the lips, tongue, and colon, marfanoid habitus (95%) |
OMIM®, Online Mendelian Inheritance in Man®; RET, rearranged in transfection (proto-oncogene); CDKN1B, cyclin-dependent kinase (CDK) inhibitor 1b gene (tumor suppressor gene); MAX, MYC-associated factor X (tumor suppressor gene); PHPT, primary hyperparathyroidism; pNET, pancreatic neuroendocrine tumor; FMTC, familial medullary thyroid cancer.
The prevalence of MEN1 is estimated to lie between 1/10,000 and 1/30,000, whereas the prevalence of MEN2 is approximately 1/35,000 (9, 10) (https://www.orpha.net/consor/cgi-bin/index.php). MEN3 is about 20 times less frequent than MEN2, which amounts to an estimated prevalence of 1/500,000 (11, 12). The newly described MEN4 syndrome is extremely rare and its prevalence is probably less than one per million (https://www.orpha.net/consor/cgi-bin/index.php). MEN5 presents another very rare syndrome, the incidence of which has not yet been established (5).
MEN1 is the most frequent syndrome. The underlying germline mutation is a heterozygous loss-of-function of the tumor suppressor gene MEN1. Affected patients present with primary hyperparathyroidism (PHPT), functional or non-functional pancreatic neuroendocrine tumors, and pituitary adenomas (13, 14). However, about 10–30% of patients with a MEN1-like phenotype do not show any alterations of the MEN1 gene (7, 15, 16).
In 2002, Fritz and co-workers first described an autosomal recessive MEN-like syndrome in the rat. Animals exhibiting the mutant phenotype spontaneously developed multiple neuroendocrine malignancies within the first year of life with high penetrance. These included bilateral adrenal pheochromocytoma, multiple extra-adrenal pheochromocytomas, bilateral medullary thyroid cell neoplasia, bilateral parathyroid hyperplasia, and pituitary adenoma. The appearance of these tumors was preceded by the development of bilateral juvenile cataracts. All animals tested negative for mutations of the MEN1 and RET gene. The causative genetic defect initially remained unknown and the syndrome was termed MENX (17).
Eventually, in 2006, Pellegata and co-workers discovered the underlying mutation of this novel MEN syndrome (1). The mutation was not only described in rats, but the first human case was reported. The syndrome is caused by an inactivating germline mutation of the cyclin-dependent kinase (CDK) inhibitor 1b gene (CDKN1B), a gene coding for the nuclear protein p27kip1, commonly referred to as p27 or KIP1. It is a putative tumor suppressor gene regulating cell cycle progression, notably the progression from the G1 to the S phase. Mutations of CDKN1B result in a truncated p27 protein, which is unstable and rapidly degraded (18, 19). It exhibits a reduced binding capacity to interacting partners and its concentration within the nucleus is reduced (2). Immunohistochemical staining of the tissue of affected patients often shows a delocalization from the nucleus to the cytoplasm or they completely fail to detect expression of the p27 protein (1, 18, 20, 21, 22, 23, 24, 25). The incidence of CDKN1B mutations in patients with a MEN1-like phenotype, testing negative for the MEN1 and the RET gene, is likely to be in the range of 1.5–3.7% (20, 21, 26). The CDKN1B germline mutation has an autosomal dominant inheritance pattern in humans.
In 2008, the novel human MENX syndrome, caused by the CDKN1B germline mutation, was renamed MEN4 during the 11th International Workshop on Multiple Endocrine Neoplasia in Delphi, Greece (4). Up until 2017, only 19 cases of MEN4 had been reported in the medical literature (2). The clinical penetrance and precise tumor spectrum of MEN4 are still poorly defined.
The present study constitutes a systematic review of MEN4. A patient from our hospital was included.
Materials and methods
Case presentation
A 54-year-old woman from our hospital underwent medical therapy for macroprolactinoma, focused parathyroidectomy for PHPT, and total thyroidectomy for multifocal papillary thyroid carcinoma. The patient displayed a MEN1-like phenotype; however, no pathogenic MEN1 gene mutation was found. Genetic analysis identified a previously unreported variant in exon 1 of the CDKN1B gene (c.349C>T, p.P117S) (27) (https://www.ncbi.nlm.nih.gov/clinvar/variation/493111/?new_evidence=true). So far, it was classified as a variant of unknown clinical significance according to the standards and guidelines of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology (28). In the present clinical context, the diagnosis of MEN4 was established.
Systematic review
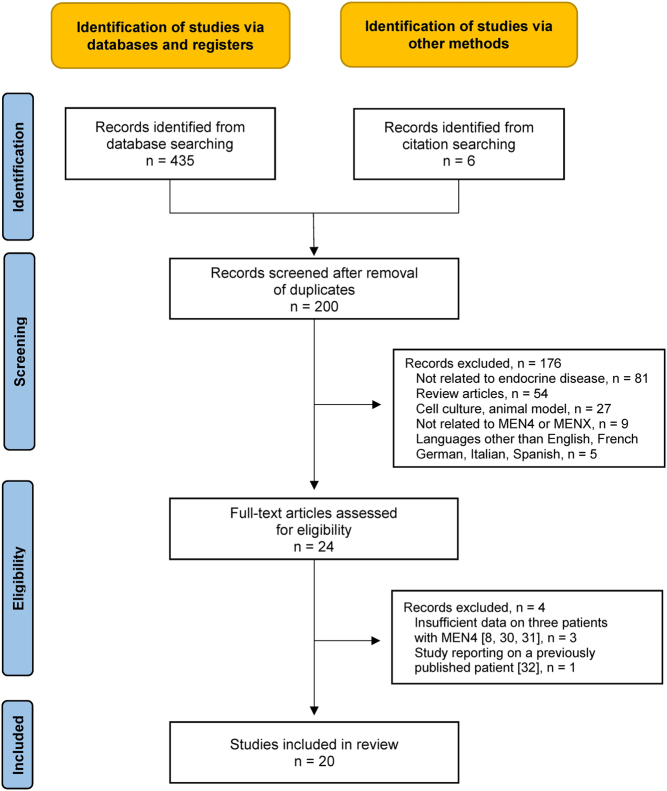
A literature search (MEDLINE®, https://pubmed.ncbi.nlm.nih.gov/, Web of ScienceTM) on MEN4 was carried out. A systematic review was performed in accordance with the PRISMA 2020 criteria (29). Figure 1 provides the PRISMA flow chart (8, 30, 31, 32). The search terms ‘MEN4’, ‘MENX’, and ‘CDKN1B’ were used. The full search strategy is shown in Supplementary Table 1 (see section on supplementary materials given at the end of this article) (supporting information). All records from 1 January 2006 to 31 August 2022 were scrutinized. The start of the search predates the first description of MEN4 in humans. The last search update was performed on 1 September 2022. Additional records were identified from citation searching. After excluding duplicates, the records were screened by two reviewers independently (H.S., P.R.), according to the inclusion and exclusion criteria shown in Supplementary Table 2 (supporting information). The two reviewers independently analyzed the remaining records, as full-text articles. Twenty studies were eventually included in the review. Data concerning phenotype and genotype of all published symptomatic cases of MEN4 patients, as well as of all asymptomatic carriers of the pathogenic CDKN1B mutations, were collected. The characteristics of the included studies are detailed in Supplementary Table 3 (supporting information). Table A (supplemental online material) provides detailed information on the data retrieved. The collected data were cross-checked by the two assessors.
Figure 1.
PRISMA flow chart for the literature search. MEN, multiple endocrine neoplasia.
Statistical methods
Data were captured on a spreadsheet (Microsoft Excel®). Categorical data were presented as frequencies and percentages. Age was presented as median with range and mean ± s.d. Age was compared between asymptomatic carriers and symptomatic patients, female and male patients at first presentation, and symptomatic female and symptomatic male patients with a Welch t-test (R Statistics version 4.2.2).
Results
From 2006 to August 2022, 20 publications with 64 patients met the inclusion criteria (1, 15, 18, 20, 21, 22, 26, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45). Including the patient from our hospital, 65 cases were available for further analysis. Table A (supplemental online material) provides detailed information on the published data.
There were 48 symptomatic patients (median age 43.5 years, range 5–76, mean 42.2 ± 20.8) and 17 asymptomatic carriers (median age 47.5 years, range 16–76, mean 49.3 ± 17.8) (P = 0.26). All asymptomatic carriers were first-degree relatives of and shared the same pathogenic CDKN1B variants with the related symptomatic index patients. Twenty-four symptomatic patients were described as isolated sporadic cases, whereas the remaining 24 symptomatic patients were reported in families with at least 2 members carrying the same genetic variant. The latter 24 symptomatic patients were found in a total of 11 families. In each family, a different CDKN1B variant was described. The median number of affected family members was three (range 2–13).
There were 47 women (47/65, 72%) and 16 men (16/65, 25%). In two instances, no gender information was available (2/65, 3%). The age at first presentation for symptomatic patients and the age at the time of diagnosis for asymptomatic carriers were provided for women in 35 instances (median 49 years, range 5–76, mean 46.3 ± 19.2) and for men in 11 instances (median 34 years, range 10–70, mean 36.9 ± 22.2) (P = 0.22). There were 28 different CDKN1B variants. The majority were missense (29/65, 45%) and frameshift (22/65, 34%) mutations. To a lesser extent, nonsense alterations (8/65, 12%) and small deletions (6/65, 9%) were found. In one case report, the underlying CDKN1B mutation was not specified (1/65, 2%).
For further analysis, all asymptomatic carriers of a CDKN1B variant were excluded. Table 2 provides an overview of the remaining 48 symptomatic MEN4 patients with a proven underlying CDKN1B mutation.
Table 2.
Genotype and phenotype of published symptomatic cases of CDKN1B-mutated MEN4.
| Ref. | M/F | Agea | Mutation | Typeb | Parathyroid gland | Pituitary gland | Endocrine pancreas | Thyroid gland |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 30 | c.227G>A, p.W76* | NS | PHPT (46) | Adenoma, GH (30) | ||
| 20 | F | 45 | c.59_77dup19 | FS | PHPT (47) | Adenoma, ACTH (46) | ||
| 26 | F | 61 | c.-7G>C | MS | PHPT (61) | |||
| 26 | F | 50 | c.283c>T, p.P95S | MS | PHPT, mg (50) | Gastrinoma, ZES (50) | ||
| 26 | F | 50 | c.595T>C, p.*199Qext60 | NS | PHPT, mg (50) | |||
| 26 | F | 66 | c.595T>C, p.*199Qext60 | NS | PHPT, ug (66) | |||
| 21 | F | 64 | c.678C>T, p.P69L | MS | PHPT (67) | Adenoma, nf (79) | Papillary cancer (64) | |
| 36 | M | 68 | c.25G>A, p.G9R | MS | PHPT (68) | |||
| 36 | F | 53 | c.397C>A, p.P133T | MS | PHPT (53) | |||
| 15 | F | 42 | c.163G>A, p.A55T | MS | PHPT (51) | Gastrinoma, m, ZES (42) | ||
| 37 | F | NA | c.286A>C, p.K96Q | MS | Adenoma, PRL (NA) | |||
| 37 | F | NA | c.356C>T, p.I119T | MS | Adenoma, GH (NA) | |||
| 33 | F | 69 | c.-32_-29delGAGA | SD | PHPT (74) | |||
| 22 | F | 62 | c.-456_-453delCCTT | SD | Adenoma, GH (62) | NET, nf (62) | ||
| 18 | F | 41 | c.374_375delCT, p.S125* | FS | PHPT, mg, r (41, 50, 55) | Hyperprolactinemia (56) | Gastrinoma, m, ZES (50) | |
| 38 | F | 15 | c.378G>C, p.E126D | MS | PHPT (15) | |||
| 41 | F | 5 | c.-29_-26delAGAG | SD | Adenoma, GH (5) | |||
| 39 | F | 56 | c.397C>A, p.P133T | MS | PHPT (56) | Papillary cancer, mf (56) | ||
| 42 | M | 38 | c.-80C>7 | MS | PHPT, mg (38) | |||
| 42 | F | 61 | c.-29_-26delAGAG | SD | PHPT, ug (61) | |||
| 42 | Fc | 49 | c.397C>A, p.P133T | MS | PHPT, ug (49) | |||
| 34 | F | NA | c.121_122delTT, p.L41Nfs*83 | FS | PHPT (NA) | |||
| 34 | F | NA | c.121_122delTT, p.L41Nfs*83 | FS | PHPT (NA) | NET, nf (67) | ||
| 34 | F | NA | c.121_122delTT, p.L41Nfs*83 | FS | PHPT (NA) | Adenoma, nf (66) | ||
| 34 | F | NA | c.121_122delTT, p.L41Nfs*83 | FS | PHPT (NA) | |||
| 34 | M | NA | c.121_122delTT, p.L41Nfs*83 | FS | PHPT (NA) | Adenoma, nf (64) | ||
| 34 | F | NA | c.121_122delTT, p.L41Nfs*83 | FS | PHPT (NA) | |||
| 34 | M | NA | c.121_122delTT, p.L41Nfs*83 | FS | PHPT (NA) | Adenoma, nf (46) | ||
| 34 | F | NA | c.121_122delTT, p.L41Nfs*83 | FS | PHPT (NA) | |||
| 34 | F | 36 | c.121_122delTT, p.L41Nfs*83 | FS | PHPT (36) | Adenoma, ACTH (37) | ||
| 34 | M | NA | c.121_122delTT, p.L41Nfs*83 | FS | PHPT (NA) | |||
| 34 | F | NA | c.121_122delTT, p.L41Nfs*83 | FS | PHPT (NA) | |||
| 34 | F | NA | c.121_122delTT, p.L41Nfs*83 | FS | PHPT (NA) | |||
| 34 | M | NA | c.121_122delTT, p.L41Nfs*83 | FS | PHPT (NA) | |||
| 35 | M | 65 | c.285dupC, p.K96Qfs*29 | FS | PHPT, ug (65) | |||
| 40 | M | 11 | c.407A>G, p.D136G | MS | Adenoma, ACTH (11) | |||
| 40 | F | 9 | c.376G>C, p.E126Q | MS | Adenoma, ACTH (9) | |||
| 40 | F | 10 | c.356T>C, p.I119T | MS | Adenoma, ACTH (10) | |||
| 40 | M | 13 | c.320delA, p.Q107Rfs*12 | FS | Adenoma, ACTH (12) | |||
| 40 | M | 10 | c.-29_-26delAGAG | SD | Adenoma, ACTH (10) | |||
| 45 | F | 35 | c.281C>T, p.P94L | MS | PHPT (51) | Hyperprolactinemia (NA) | Papillary cancer, mf (55) | |
| 45 | F | 66 | c.206C>T, p.P69L | MS | PHPT (66) | Adenoma, nf (66) | ||
| 43 | M | 34 | c.356T>C, p.I119T | MS | ||||
| 43 | F | 76 | c.482C>G, p.S161C | MS | NET, nf, mf, (NA) | |||
| 44 | F | 33 | c.179G>A, p.W60* | NS | PHPT, mg, r (33, 45) | Adenoma, PRL (39) | NET, nf, mf (47) | |
| 44 | F | 26 | c.475G>A, p.D159N | MS | PHPT (26) | |||
| 44 | M | 31 | c.374_375delCT, p.S125c | NS | ||||
| PC | F | 54 | c.349C>T, p.P117S | MS | PHPT (59) | Adenoma, PRL (54) | Papillary cancer, mf (59) |
aAge at the time of presentation of the first endocrine disease.
bType of mutation.
cSimultaneous MEN1 germline mutation: c.1621G>A, p.A541T.
Data is presented in order of year of publication. Age at time of presentation is indicated in parentheses.
Ref., reference; M, male; F, female; MS, missense mutation; FS, frameshift mutation; NS, nonsense mutation; SD, small deletion; NA, not available; PC, present case; PHPT, primary hyperparathyroidism; mg, multiglandular; ug, uniglandular; r, recurrent; GH, growth hormone; ACTH, adrenocorticotropic hormone; PRL, prolactin; nf, non-functioning; mf, multifocal; ZES, Zollinger Ellison syndrome; m, metastatic; NET, neuroendocrine tumor.
The majority of patients were women (36/48, 75%). The median age for the presentation of the first endocrine disorder was 43.5 years (range 5–76). Men became symptomatic at a median age of 32.5 years (range 10–68, mean 33.7 ± 23), whereas the same event was recorded for women at a median age of 49.5 years (range 5–76, mean 44.8 ± 19.9) (P = 0.25). There were one (27/48, 56%), two (13/48, 27%), three (3/48, 6%), or four (5/48, 10%) syn- or metachronously affected endocrine organs in a single patient.
The most frequently affected endocrine organ was the parathyroid gland (75%), followed by the pituitary gland (44%), the endocrine pancreas (15%), the thyroid gland (8%), the adrenal gland (6%), and the thymus (4%).
Thirty-six patients presented with PHPT as the leading endocrine disorder (36/48, 75%). In five patients, either multi-glandular (5/36, 14%) and/or recurrent disease (2/36, 6%) was diagnosed. Most patients were women (29/36, 81%), and the median age at diagnosis of PHPT was 51 years (range 15–74).
Twenty-one patients presented with an adenoma of the pituitary gland (21/48, 44%). The adenoma was non-functioning in 5 patients and hormone-secreting in 16 patients (7× ACTH, 5× prolactin, and 4× growth hormone). Ten patients presented with a pituitary microadenoma (<1 cm), five patients with a pituitary macroadenoma, and in six instances, the size of the adenoma was not specified. Most patients were women (16/21, 76%), and the median patient age at presentation was 39 years (range 5–79).
Nine patients (9/48, 19%) presented with gastroenteropancreatic neuroendocrine tumors (GEP-NET). There were four patients with a non-functional NET of the pancreas (1× metastatic, 1× non-metastatic, and 2x non-metastatic multifocal), three patients with gastrinoma of the pancreas (Zollinger-Ellison syndrome, 2× metastatic, 1× non-metastatic), and two patients with a NET of the stomach (non-metastatic) and ileum (metastatic), respectively.
Four patients presented with papillary thyroid carcinoma (4/48, 8%), of which three were multifocal. All patients were women, and the median age was 57.5 years (range 55–64).
There were three patients with tumors of the adrenal gland (1× bilateral nonfunctioning, 1× bilateral with suspicion of cyclic cortisol secretion, and 1x unilateral with subclinical cortisol secretion) and two patients with tumors of the thymus (1× thymic hyperplasia causing myasthenia gravis, 1× atypical carcinoid tumor causing ectopic Cushing's syndrome). Finally, one patient presented with a carcinoid tumor of the lungs (metastatic) and an angiomyolipoma of the kidney was found in two patients.
The presenting endocrine disease for each patient was reviewed. The leading first endocrine pathologies concerned the parathyroid (27/48, 56%) and the pituitary gland (11/48, 23%). In two instances, the thymus was the presenting organ (2/48, 4%). In one patient each, the first affected organ was the thyroid gland, the stomach, the ileum, and the pancreas (4/48, 8%). Finally, in four patients, two or more endocrine organs were simultaneously diagnosed as being diseased (4/48, 8%).
Discussion
The 2022 WHO Classification of Tumors of the Endocrine Organs describes 15 syndromes associated with endocrine lesions and tumors (5). Three new syndromes have been added to the last edition of 2017, namely MEN4, MEN5, and MAFA-related insulinomatosis. MEN syndromes now include five entities, MEN1–5.
Clinical definition of MEN
Patients with tumors of two or more endocrine organs fulfill the diagnostic criteria of MEN. Furthermore, a familial MEN is defined by the presence of an index patient and the identification of at least one first-degree relative with a tumor in one or more endocrine organs or tissues, known to be classically affected in MEN syndromes (26).
Genetic definition of MEN
There is an ever-expanding understanding of the genetic basis of diseases. This also holds true for MEN. MEN1 is caused by a germline mutation of the tumor suppressor gene MEN1, which is composed of 10 exons encoding the 610 amino acid protein menin (15). A germline mutation of the proto-oncogene RET (rearranged in transfection), on the other hand, is responsible for the MEN2 and MEN3 syndromes. In contrast to MEN1, the type of RET mutation strongly correlates with its phenotype. Additionally, there is MEN4, which is the result of a germline mutation of a tumor suppressor gene called CDK inhibitor 1b (CDKN1B). Finally, MEN5 is related to a germline mutation of a tumor suppressor gene named MYC-associated factor X (MAX) (6).
The rationale for a systematic review
Pellegata and co-workers described the first human case of CDKN1B-related MEN4 in 2006 (1). Since then, there has been a constant trickle of case reports only slowly increasing our understanding of this new entity.
In 2019, Frederiksen et al. published a literature review on MEN4. They identified 30 mutation-positive patients representing 16 different pathogenic CDKN1B variants. The authors added a further 13 mutation-positive members of a large Danish family to the list (34).
The present study is the first systematic review of MEN4 according to the PRISMA 2020 criteria (29). We identified 65 individuals displaying 28 different pathogenic CDKN1B variants. There were 17 asymptomatic carriers of the mutation and 48 symptomatic patients with at least 1 endocrine disorder.
Phenotypic similarities between MEN1 and MEN4
There is substantial phenotypic overlap between MEN1 and MEN4. Prior to the identification of the underlying CDKN1B mutation in MEN4, most patients were probably misclassified as MEN1. In 10–30% of clinical MEN1 cases, no variant of more than 400 MEN1 mutations can be found (15, 16). Identifiable germline mutations of the MEN1 gene can be found in approximately 80% of familial MEN1 syndromes but only in about 30% of sporadic MEN1 syndromes (26). The remainder of MEN1 patients, being negative for the MEN1 mutation, are due to other still-to-be-identified tumor-susceptibility gene mutations. Approximately 3% of patients displaying a MEN1-like phenotype but testing negative for MEN1 mutations are reported to have mutations in the causative CDKN1B gene (1, 15, 18, 20, 21, 22, 26, 32, 33), reclassifying these patients as belonging to the MEN4 syndrome (32, 44).
Clinical presentation of MEN1 and MEN4
The phenotypic characteristics of MEN4 are still ill-defined due to the limited number of published cases. The multitude of underlying genetic mutations may further contribute to clinical diversity. In fact, the present systematic review listed no less than 28 different CDKN1B variants in 48 symptomatic MEN4 patients.
Parathyroid adenomas are the most frequent tumors in both MEN1 and MEN4, with a prevalence of about 85% and 80%, respectively. In MEN1, PHPT manifests in early adulthood with an almost even gender distribution (3, 7, 8, 14). In contrast, MEN4 not only displays a clear female predominance (81%) but also manifests about two decades later (1, 18, 20, 21, 22, 26, 33). The median age of presentation of 51 years for women coincides with the hormonal changes of menopause, which have been implicated in the pathogenesis of PHPT by altering p27kip1 levels (18, 46). PHPT in MEN1 is an overwhelmingly multiglandular disease with a high recurrence rate, even after routine subtotal (three-and-a-half) gland resection (3, 7, 8, 14). In the present systematic review of MEN4, only a minority of patients presented with multiglandular (14%) or recurrent disease (6%). Simple excision of the single enlarged parathyroid gland (focused parathyroidectomy) might therefore be an appropriate surgical approach for MEN4 patients.
Pituitary adenomas are the second and third most frequent endocrine tumors in MEN4 and MEN1, respectively. For MEN1, this percentage amounts to 20% for clinically apparent adenomas and up to 40% if adenomas detected by hormonal testing or MRI screening are included (3, 7, 8, 14, 47). With regards to MEN4, the present systematic review reported pituitary adenomas in 44% of patients. In MEN1 patients, the pituitary adenomas are mostly lactotroph, followed by somatotroph, corticotroph, and gonadotroph as well as non-functioning. A similar hormonal distribution is found for MEN4 patients in the present systematic review.
Pituitary adenomas in MEN1 are mostly macroadenomas (80%), whereas in MEN4, we report a 2 to 1 ratio in favor of pituitary microadenomas. In MEN1, pituitary adenomas display an aggressive biologic behavior. Normalization of hormonal hypersecretion after treatment occurs in less than 50% of patients (3, 8, 9, 14, 47). Given the paucity of data, no conclusion concerning treatment and treatment success in MEN 4 patients can be drawn.
Pancreatic islet cell and gastroduodenal neuroendocrine tumors are the second most common endocrine pathology in MEN1 (13). They become manifest in 30–80% of patients. GEP-NETs often present with multifocality and a propensity to metastasize. Surgery is often extensive with a high risk of recurrence. Due to their malignant potential, they are the leading pathology determining the patient’s outcome quoad vitam (13, 14, 34).
As for MEN4, the present systematic review found GEP-NETs in just 19% of patients. However, four out of nine patients presented with metastatic disease.
Two different diagnostic pathways
MEN syndromes are clinically defined by the affection of two or more endocrine organs in one specific individual. Such a cluster of endocrine diseases in a single person will eventually raise the suspicion of an underlying genetic mutation. A positive family history will further underline the need for genetic testing.
The CDKN1B mutation as a causative factor for MEN was first described in humans in 2006 (1). Since then, genetic testing for the CDKN1B mutation was also performed for patient cohorts with just a single endocrine pathology and a negative family history (36, 37, 40, 42). These are patients, who do not fulfill the clinical criteria for MEN syndromes. Such ‘screening’ studies led to the identification of additional MEN4 patients, who were included in the present systematic review.
Costa-Guda et al. studied somatic mutations and germline sequence abnormalities in the CDKN1B gene in 86 patients with sporadic parathyroid adenomas (36). Two germline mutations were identified. A similar study by Borsari et al. investigated 147 patients with sporadic parathyroid adenomas for CDKN1B gene mutations finding 3 pathologic germline variants (42). Tichomirowa et al. performed a CDKN1B germline analysis in 124 AIP mutation-negative familial isolated pituitary adenoma kindreds (37). Two CDKN1B germline mutation carriers were identified. Finally, Chasseloup et al. studied germline CDKN1B loss-of-function variants in mostly pediatric Cushing’s disease patients with or without a MEN4 phenotype (40). Five variants of interest were found.
Genetic testing for CDKN1B mutations
A distinction has to be made between patients clinically presenting with MEN and their asymptomatic relatives. All patients with the clinical diagnosis of MEN should be tested for mutations of the MEN1 and RET genes, in accordance with the leading endocrine tumors. If negative, then testing of the CDKN1B and MAX genes should be considered (next-generation sequencing). Genetic testing for asymptomatic relatives should be stratified according to the mutation found in the symptomatic index patient (Sanger sequencing). In general, genetic testing can be offered if it confers a substantial benefit to the asymptomatic carrier of the genetic alteration in terms of disease prevention or prognosis. As for MEN1, such a benefit has only been shown following prophylactic thymectomy for thymic NET (2, 14). With regards to MEN2 and MEN3, the potential benefit of germline RET testing has been proven far greater. Prophylactic surgery for medullary thyroid carcinoma and pheochromocytoma provides a clear survival benefit to carriers of certain RET germline mutations (48). Due to the paucity of data on MEN4 and MEN5, no guidelines currently exist with regard to the genetic testing of asymptomatic relatives.
In general, genetic testing and counseling for MEN should be performed by an experienced and specially qualified team. The MEN syndromes are transmitted in an autosomal dominant fashion. Therefore, each sibling carries a 50% risk of having the mutation. A negative genetic test result will offer reassurance to those who do not carry the mutation and prevents unnecessary clinical, biochemical, and radiological screenings (2). A positive genetic test result, on the other hand, should ensure inclusion into a surveillance program according to the risk profile of the respective MEN syndrome.
Strengths and weaknesses of the systematic review
Despite the comprehensive literature search, only 48 symptomatic MEN4 patients were available for the final analysis. Due to the paucity of data, the results and conclusions drawn have to be interpreted with caution.
First, it has to be noted that, of this whole collective, 13 patients belong to 1 large Danish family (13/48, 27%). This relative preponderance of one single family may have led to a certain distortion of the data (34).
Second, some further bias may result from the fact that a quarter of all patients (12/48, 25%) were derived from large CDKN1B ‘screening’ studies of populations with a single endocrine pathology and a negative family history (36, 37, 40, 42). The inclusion of patients with a single endocrine neoplasm may have had an influence on the prevalence data concerning the respective endocrine pathologies.
Despite all the shortcomings, the present study allows for a more precise description of the phenotypic manifestations of MEN4. In particular, it enabled a more detailed elaboration of the clinical differences to the somewhat similar MEN1 syndrome. Eventually, it also provides a listing of the underlying genetic variants.
Conclusions
MEN4, first described in 2006, is a very rare disease with only a few dozen cases reported in the literature. The underlying genetic alternations and the phenotypic manifestations are still poorly defined. Therefore, the establishment of evidence-based management guidelines remains difficult. The present systematic review showed that MEN4 most frequently affects women around 50 years of age with uniglandular PHPT presenting as the leading pathology. A MEN4 registry and future larger scale systematic reviews are needed.
Supplementary Material
Declaration of interest
No conflict of interest.
Funding
The authors received no financial funding.
Registration
The study was not registered.
Consent
Written informed consent was obtained from the patient.
References
- 1.Pellegata NS, Quintanilla-Martinez L, Siggelkow H, Samson E, Bink K, Höfler H, Fend F, Graw J, Atkinson MJ. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. PNAS 200610315558–15563. ( 10.1073/pnas.0603877103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alrezk R, Hannah-Shmouni F, Stratakis CA. MEN4 and CDKN1B mutations: the latest of the MEN syndromes. Endocrine-Related Cancer 201724T195–T208. ( 10.1530/ERC-17-0243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonnell JE, Gild ML, Clifton-Bligh RJ, Robinson BG. Multiple endocrine neoplasia: an update. Internal Medicine Journal 201949954–961. ( 10.1111/imj.14394) [DOI] [PubMed] [Google Scholar]
- 4.Alevizaki M, Stratakis CA. Multiple endocrine neoplasias: advances and challenges for the future. Journal of Internal Medicine 20092661–4. ( 10.1111/j.1365-2796.2009.02108.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nosé V, Gill A, Teijeiro JMC, Perren A, Erickson L. Overview of the 2022 WHO classification of familial endocrine tumor syndromes. Endocrine Pathology 202233197–227. ( 10.1007/s12022-022-09705-5) [DOI] [PubMed] [Google Scholar]
- 6.Seabrook AJ, Harris JE, Velosa SB, Kim E, McInerney-Leo AM, Dwight T, Hockings JI, Hockings NG, Kirk J, Leo PJet al. Multiple endocrine tumors associated with germline MAX mutations: multiple endocrine neoplasia Type 5? Journal of Clinical Endocrinology and Metabolism 20211061163–1182. ( 10.1210/clinem/dgaa957) [DOI] [PubMed] [Google Scholar]
- 7.Thakker RV.Multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4). Molecular and Cellular Endocrinology 20143862–15. ( 10.1016/j.mce.2013.08.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu X, Guan J, Wang Y, Shi S, Song C, Li ZP, Feng ST, Chen J, Luo Y. A narrative review of multiple endocrine neoplasia syndromes: genetics, clinical features, imaging findings, and diagnosis. Annals of Translational Medicine 20219 944. ( 10.21037/atm-21-1165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Salameh A, Cadiot G, Calender A, Goudet P, Chanson P. Clinical aspects of multiple endocrine neoplasia type 1. Nature Reviews. Endocrinology 202117207–224. ( 10.1038/s41574-021-00468-3) [DOI] [PubMed] [Google Scholar]
- 10.Mathiesen JS, Kroustrup JP, Vestergaard P, Stochholm K, Poulsen PL, Rasmussen ÅK, Feldt-Rasmussen U, Schytte S, Pedersen HB, Hahn CHet al. Incidence and prevalence of multiple endocrine neoplasia 2A in Denmark 1901–2014: a nationwide study. Clinical Epidemiology 2018101479–1487. ( 10.2147/CLEP.S174606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathiesen JS, Kroustrup JP, Vestergaard P, Madsen M, Stochholm K, Poulsen PL, Krogh Rasmussen Å, Feldt-Rasmussen U, Schytte S, Pedersen HBet al. Incidence and prevalence of multiple endocrine neoplasia 2B in Denmark: a nationwide study. Endocrine-Related Cancer 201724L39–L42. ( 10.1530/ERC-17-0122) [DOI] [PubMed] [Google Scholar]
- 12.Znaczko A, Donnelly DE, Morrison PJ. Epidemiology, clinical features, and genetics of multiple endocrine neoplasia type 2B in a complete population. Oncologist 2014191284–1286. ( 10.1634/theoncologist.2014-0277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marini F, Giusti F, Tonelli F, Brandi ML. Pancreatic neuroendocrine neoplasms in multiple endocrine neoplasia Type 1. International Journal of Molecular Sciences 202122 4041. ( 10.3390/ijms22084041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR, Melmed S, Sakurai A, Tonelli F, Brandi MLet al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). Journal of Clinical Endocrinology and Metabolism 2012972990–3011. ( 10.1210/jc.2012-1230) [DOI] [PubMed] [Google Scholar]
- 15.Belar O, De La Hoz C, Pérez-Nanclares G, Castaño L, Gaztambide S. & Spanish MEN1 Group. Novel mutations in MEN1, CDKN1B and AIP genes in patients with multiple endocrine neoplasia type 1 syndrome in Spain. Clinical Endocrinology 201276719–724. ( 10.1111/j.1365-2265.2011.04269.x) [DOI] [PubMed] [Google Scholar]
- 16.Georgitsi MMEN.MEN-4 and other multiple endocrine neoplasias due to cyclin-dependent kinase inhibitors (p27(Kip1) and p18(INK4C)) mutations. Best Practice and Research. Clinical Endocrinology and Metabolism 201024425–437. ( 10.1016/j.beem.2010.01.001) [DOI] [PubMed] [Google Scholar]
- 17.Fritz A, Walch A, Piotrowska K, Rosemann M, Schäffer E, Weber K, Timper A, Wildner G, Graw J, Höfler Het al. Recessive transmission of a multiple endocrine neoplasia syndrome in the rat. Cancer Research 2002623048–3051. [PubMed] [Google Scholar]
- 18.Tonelli F, Giudici F, Giusti F, Marini F, Cianferotti L, Nesi G, Brandi ML. A heterozygous frameshift mutation in exon 1 of CDKN1B gene in a patient affected by MEN4 syndrome. European Journal of Endocrinology 2014171K7–K17. ( 10.1530/EJE-14-0080) [DOI] [PubMed] [Google Scholar]
- 19.Lee M, Pellegata NS. Multiple endocrine neoplasia syndromes associated with mutation of p27. Journal of Endocrinological Investigation 201336781–787. ( 10.3275/9021) [DOI] [PubMed] [Google Scholar]
- 20.Georgitsi M, Raitila A, Karhu A, van der Luijt RB, Aalfs CM, Sane T, Vierimaa O, Mäkinen MJ, Tuppurainen K, Paschke Ret al. Germline CDKN1B/p27Kip1 mutation in multiple endocrine neoplasia. Journal of Clinical Endocrinology and Metabolism 2007923321–3325. ( 10.1210/jc.2006-2843) [DOI] [PubMed] [Google Scholar]
- 21.Molatore S, Marinoni I, Lee M, Pulz E, Ambrosio MR, degli Uberti EC, Zatelli MC, Pellegata NS. A novel germline CDKN1B mutation causing multiple endocrine tumors: clinical, genetic and functional characterization. Human Mutation 201031E1825–E1835. ( 10.1002/humu.21354) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Occhi G, Regazzo D, Trivellin G, Boaretto F, Ciato D, Bobisse S, Ferasin S, Cetani F, Pardi E, Korbonits Met al. A novel mutation in the upstream open reading frame of the CDKN1B gene causes a MEN4 phenotype. PLoS Genetics 20139 e1003350. ( 10.1371/journal.pgen.1003350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marinoni I, Pellegata NS. P27Kip1: a new multiple endocrine neoplasia gene? Neuroendocrinology 20119319–28. ( 10.1159/000320366) [DOI] [PubMed] [Google Scholar]
- 24.Wiedemann T, Pellegata NS. Animal models of multiple endocrine neoplasia. Molecular and Cellular Endocrinology 201642149–59. ( 10.1016/j.mce.2015.07.004) [DOI] [PubMed] [Google Scholar]
- 25.Pellegata NS.MENX and MEN4. Clinics (Sao Paulo) 201267(Supplement 1) 13–18. ( 10.6061/clinics/2012(sup0104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal SK, Mateo CM, Marx SJ. Rare germline mutations in cyclin-dependent kinase inhibitor genes in multiple endocrine neoplasia type 1 and related states. Journal of Clinical Endocrinology and Metabolism 2009941826–1834. ( 10.1210/jc.2008-2083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nykamp K, Anderson M, Powers M, Garcia J, Herrera B, Ho YY, Kobayashi Y, Patil N, Thusberg J, Westbrook Met al. Sherloc: a comprehensive refinement of the ACMG-AMP variant classification criteria. Genetics in Medicine 2017191105–1117. ( 10.1038/gim.2017.37) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector Eet al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in Medicine 201517405–424. ( 10.1038/gim.2015.30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SEet al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021372 n71. ( 10.1136/bmj.n71) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talbot JN, Zhang-Yin J, Kerrou K, Aveline C, Vagne B, Bélissant O, Tassart M, Périé S, Bouchard P, Christin-Maitre Set al. Multiple endocrine neoplasia type 1 or 4: detection of hyperfunctioning parathyroid glands with 18F-fluorocholine PET/CT, illustrative cases and pitfalls. Quarterly Journal of Nuclear Medicine and Molecular Imaging 202266130–140. ( 10.23736/S1824-4785.22.03440-9) [DOI] [PubMed] [Google Scholar]
- 31.Watanabe A, Wiseman SM. Multiple endocrine neoplasia type 4 & primary hyperparathyroidism: what the surgeon needs to know. American Journal of Surgery 20222241017–1018. ( 10.1016/j.amjsurg.2022.04.025) [DOI] [PubMed] [Google Scholar]
- 32.Pardi E, Mariotti S, Pellegata NS, Benfini K, Borsari S, Saponaro F, Torregrossa L, Cappai A, Satta C, Mastinu Met al. Functional characterization of a CDKN1B mutation in a Sardinian kindred with multiple endocrine neoplasia type 4 (MEN4). Endocrine Connections 201541–8. ( 10.1530/EC-14-0116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malanga D, De Gisi S, Riccardi M, Scrima M, De Marco C, Robledo M, Viglietto G. Functional characterization of a rare germline mutation in the gene encoding the cyclin-dependent kinase inhibitor p27Kip1 (CDKN1B) in a Spanish patient with multiple endocrine neoplasia-like phenotype. European Journal of Endocrinology 2012166551–560. ( 10.1530/EJE-11-0929) [DOI] [PubMed] [Google Scholar]
- 34.Frederiksen A, Rossing M, Hermann P, Ejersted C, Thakker RV, Frost M. Clinical features of multiple endocrine neoplasia Type 4: novel pathogenic variant and review of published cases. Journal of Clinical Endocrinology and Metabolism 20191043637–3646. ( 10.1210/jc.2019-00082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brock P, Bustamante Alvarez J, Mortazavi A, Roychowdhury S, Phay J, Khawaja RA, Shah MH, Konda B. Co-occurrence of multiple endocrine neoplasia type 4 and spinal neurofibromatosis: a case report. Familial Cancer 202019189–192. ( 10.1007/s10689-019-00152-6) [DOI] [PubMed] [Google Scholar]
- 36.Costa-Guda J, Marinoni I, Molatore S, Pellegata NS, Arnold A. Somatic mutation and germline sequence abnormalities in CDKN1B, encoding p27Kip1, in sporadic parathyroid adenomas. Journal of Clinical Endocrinology and Metabolism 201196E701–E706. ( 10.1210/jc.2010-1338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tichomirowa MA, Lee M, Barlier A, Daly AF, Marinoni I, Jaffrain-Rea ML, Naves LA, Rodien P, Rohmer V, Faucz FRet al. Cyclin-dependent kinase inhibitor 1B (CDKN1B) gene variants in AIP mutation-negative familial isolated pituitary adenoma kindreds. Endocrine-Related Cancer 201219233–241. ( 10.1530/ERC-11-0362) [DOI] [PubMed] [Google Scholar]
- 38.Elston MS, Meyer-Rochow GY, Dray M, Swarbrick M, Conaglen JV. Early onset primary hyperparathyroidism associated with a novel germline mutation in CDKN1B. Case Reports in Endocrinology 20152015 510985. ( 10.1155/2015/510985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bugalho MJ, Domingues R. Uncommon association of cerebral meningioma, parathyroid adenoma and papillary thyroid carcinoma in a patient harbouring a rare germline variant in the CDKN1B gene. BMJ Case Reports 20162016bcr2015213934. ( 10.1136/bcr-2015-213934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chasseloup F, Pankratz N, Lane J, Faucz FR, Keil MF, Chittiboina P, Kay DM, Tayeb TH, Stratakis CA, Mills JLet al. Germline CDKN1B loss-of-function variants cause pediatric Cushing's disease with or without an MEN4 phenotype. Journal of Clinical Endocrinology and Metabolism 20201051983–2005. ( 10.1210/clinem/dgaa160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambugaro S, Di Ruvo M, Ambrosio MR, Pellegata NS, Bellio M, Guerra A, Buratto M, Foschini MP, Tagliati F, degli Uberti Eet al. Early onset acromegaly associated with a novel deletion in CDKN1B 5'UTR region. Endocrine 20154958–64. ( 10.1007/s12020-015-0540-y) [DOI] [PubMed] [Google Scholar]
- 42.Borsari S, Pardi E, Pellegata NS, Lee M, Saponaro F, Torregrossa L, Basolo F, Paltrinieri E, Zatelli MC, Materazzi Get al. Loss of p27 expression is associated with MEN1 gene mutations in sporadic parathyroid adenomas. Endocrine 201755386–397. ( 10.1007/s12020-016-0941-6) [DOI] [PubMed] [Google Scholar]
- 43.Lavezzi E, Brunetti A, Smiroldo V, Nappo G, Pedicini V, Vitali E, Trivellin G, Mazziotti G, Lania A. Case report: new CDKN1B mutation in multiple endocrine neoplasia Type 4 and brief literature review on clinical management. Frontiers in Endocrinology (Lausanne) 202213 773143. ( 10.3389/fendo.2022.773143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seabrook A, Wijewardene A, De Sousa S, Wong T, Sheriff N, Gill AJ, Iyer R, Field M, Luxford C, Clifton-Bligh Ret al. MEN4, the MEN1 Mimicker: A Case Series of three Phenotypically Heterogenous Patients with Unique CDKN1B Mutations. Journal of Clinical Endocrinology and Metabolism 20221072339–2349. ( 10.1210/clinem/dgac162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chevalier B, Odou MF, Demonchy J, Cardot-Bauters C, Vantyghem MC. Multiple Endocrine Neoplasia Type 4: novel CDNK1B variant and immune anomalies. Annales d’Endocrinologie 202081124–125. ( 10.1016/j.ando.2020.04.002) [DOI] [PubMed] [Google Scholar]
- 46.Huang KT, Pavlides SC, Lecanda J, Blank SV, Mittal KR, Gold LI. Estrogen and progesterone regulate p27Kip1 levels via the ubiquitin-proteasome system: pathogenic and therapeutic implications for endometrial cancer. PLoS One 20127 e46072. ( 10.1371/journal.pone.0046072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vergès B, Boureille F, Goudet P, Murat A, Beckers A, Sassolas G, Cougard P, Chambe B, Montvernay C, Calender A. Pituitary disease in MEN type 1 (MEN1): data from the France-Belgium MEN1 multicenter study. Journal of Clinical Endocrinology and Metabolism 200287457–465. ( 10.1210/jcem.87.2.8145) [DOI] [PubMed] [Google Scholar]
- 48.Wells SA, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini Fet al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 201525567–610. ( 10.1089/thy.2014.0335) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a