Abstract
The human body is inhabited by numerous bacteria, fungi, and viruses, and each part has a unique microbial community structure. The gastrointestinal tract harbors approximately 100 trillion strains comprising more than 1000 bacterial species that maintain symbiotic relationships with the host. The gut microbiota consists mainly of the phyla Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria. Of these, Firmicutes and Bacteroidetes constitute 70–90% of the total abundance. Gut microbiota utilize nutrients ingested by the host, interact with other bacterial species, and help maintain healthy homeostasis in the host. In recent years, it has become increasingly clear that a breakdown of the microbial structure and its functions, known as dysbiosis, is associated with the development of allergies, autoimmune diseases, cancers, and arteriosclerosis, among others. Metabolic diseases, such as obesity and diabetes, also have a causal relationship with dysbiosis. The present review provides a brief overview of the general roles of the gut microbiota and their relationship with metabolic disorders.
Keywords: obesity, diabetes, metabolism
Physiological role of the gut microbiota
Nutrient metabolism and absorption
Degradation of indigestible polysaccharides
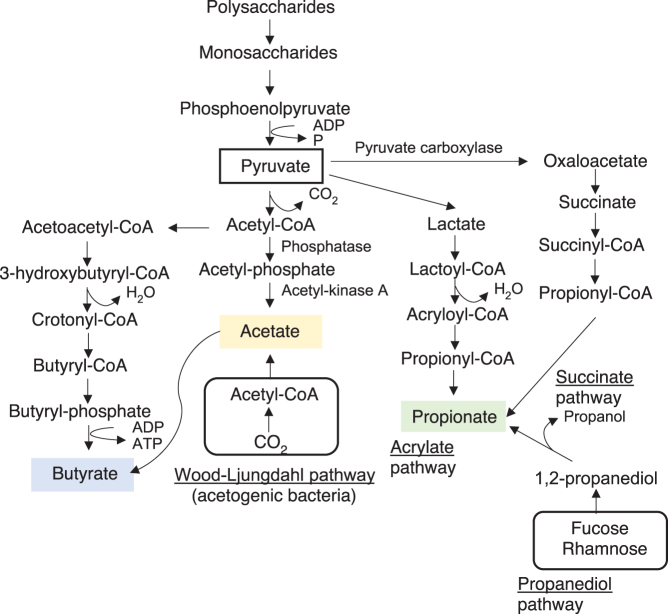
Short-chain fatty acids (SCFAs) are metabolites generated from the fermentation of insoluble dietary fiber and indigestible polysaccharides by the gut microbiota. Linear monovalent carboxylic acids with fewer than six carbons, such as acetate (C2), propionate (C3), and butyrate (C4), are the most abundant SCFAs, with average ratios of ~60%:20%:20%. Total intestinal SCFA concentrations can reach 100 mM (Cummings et al. 1987). Butyrate is the main nutrient source for colonocytes. Acetate has the highest intestinal concentration of all SCFAs. It enters the liver via the portal vein, undergoes oxidation, and is used mainly by hepatocytes. Propionate participates in hepatic gluconeogenesis. These SCFAs maintain the barrier function of the intestinal tract, are bioactive substances in energy metabolism (Morrison and Preston 2016), regulate immunocyte development, and are anti-inflammatory (Louis et al. 2014, Richards et al. 2016). Various pathways are involved in SCFA synthesis (Koh et al. 2016). Acetate is synthesized from pyruvate during glycolysis and by acetogenic bacteria via the Wood–Ljungdahl pathway, which produces acetyl-CoA from CO2. Acetoacetyl-CoA is converted to butyryl-CoA, which, in turn, is transformed into butyrate. Major butyrate-producing bacterial taxa include the Ruminococcaceae, Lachnospiraceae, Erysipelotrichaceae, and Clostridiaceae of the Firmicutes phylum (Barcenilla et al. 2000, Louis et al. 2004). Clostridium spp. (such as C. butyricum) and Butyrivibrio spp. (such as B. fibrisolvens) also produce butyrate. Acetobacter spp. and Gluconobacter spp. produce acetate (Louis et al. 2014, Knip & Siljander 2016, Koh et al. 2016). SCFAs are interconverted, and 24% of all acetate is converted to butyrate (Boets et al. 2017). Pathways involved in propionate synthesis include the acrylate pathway mediated by lactate metabolism, the succinate pathway mediated by succinate, and the propanediol pathway utilizing deoxy sugars such as fucose and rhamnose (Fig. 1) (Louis & Flint 2017). Akkermansia muciniphila and other species are propionate-producing, mucin-degrading bacteria (Derrien et al. 2004). SCFAs are important not only for energy and glucose metabolism, inflammation, and immune function regulation (Koh et al. 2016) but also for intestinal environment maintenance. For example, butyrate is important for maintaining an anaerobic environment in the intestinal tract and activating PPARγ by promoting mitochondrial β-oxidation in intestinal epithelial cells (IECs), maintaining hypoxia for epithelial cells and inhibiting oxygen transfer into the intestinal lumen (Rivera-Chavez et al. 2016, Byndloss et al. 2017). Acetate and propionate produced in the intestine also play an important role in intestinal homeostasis. They induce colonic Treg to protect against experimentally induced colitis (Smith et al. 2013). Another group reported that acetate suppresses intestinal inflammation via G protein-coupled receptor (GPR) 43 signaling expressed on neutrophils (Maslowski et al. 2009). It also enhances intestinal barrier function and contributes to defense against pathogens (Fukuda et al. 2011).
Figure 1.
Short-chain fatty acid (SCFA) biosynthesis pathways.
Bile acids and lipid metabolism
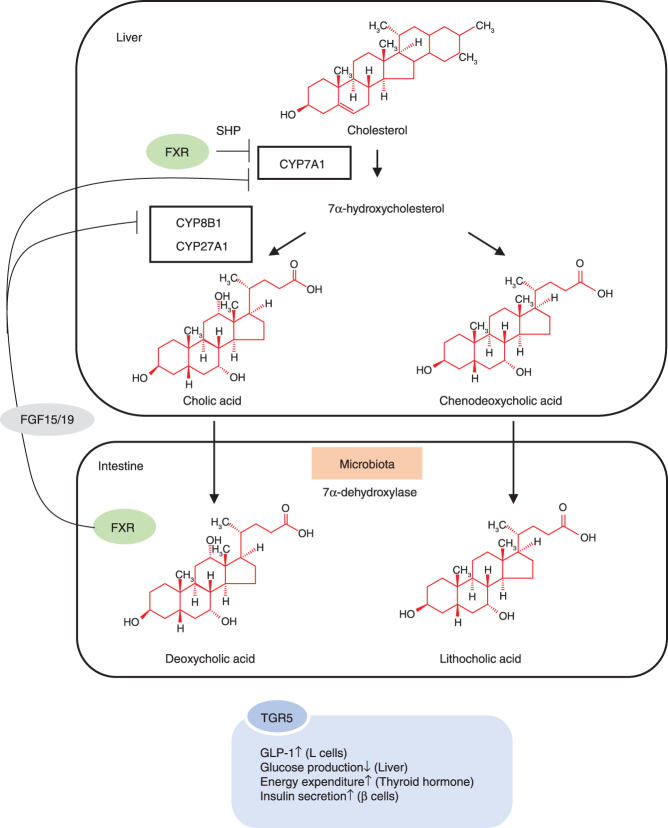
Secondary bile acids are major bacterial metabolites affecting host physiology. Hepatocytes produce cholic acid and chenodeoxycholic acid from cholesterol. These lipid-soluble bile acids are conjugated to glycine or taurine to form water-soluble bile salts and are stored in the gallbladder and released into the intestine during digestion. Gut bacteria dehydroxylate primary bile salts into secondary bile salts. These bile acids are actively resorbed along the proximal and distal ileum into the hepatic portal circulation. Bacteria also deconjugate certain primary and secondary conjugated bile salts into lipid-soluble bile acids that are passively absorbed into the hepatic portal circulation. Approximately 95% of bile acids delivered to the duodenum are recycled via enterohepatic circulation. Bile acids emulsify and facilitate the absorption of dietary lipids. They are also ligands for bile acid receptors, such as transmembrane G protein-coupled receptor 5 (TGR5) and farnesoid X receptor (FXR), which regulate energy and cholesterol metabolism and bile acid transporter gene expression (Wahlstrom et al. 2016) (Fig. 2). Deoxycholic acid is associated with hepatocellular carcinoma (Yoshimoto et al. 2013) and the inhibition of bacterial overgrowth. Recently, centenarians were found to have a distinct gut microbiome enriched in Parabacteroides merdae, Odoribacter laneus, and Odoribacteraceae. These microbiota synthesize isoallolithocholic acid, which has antibacterial efficacy against Gram-positive bacteria (Sato et al. 2021). Hence, this gut bacteria-derived bile acid might be conducive to human longevity.
Figure 2.
Bile acid metabolism. The microbiota dehydroxylate primary bile acids, such as cholic acid and chenodeoxycholic acid, to secondary bile acids with 7a-dehydroxylase. Activation of the bile acid receptor FXR suppresses the expression of CYP7A1, a bile acid rate-limiting enzyme, via the induction of small heterodimer partner (SHP) expression in the liver to control bile acid synthesis. In addition, FXR activation in the intestine suppresses CYP7A1 and CYP8B1 expression via the induction of FGF15/19 expression.
Additionally, gut microbiota produces lipids. The genes encoding sphingolipid biosynthesis mediators are distributed mainly in eukaryotes. Nevertheless, certain bacterial species in the Bacteroides phylum also have genes that regulate sphingolipid biosynthesis. Bacteroides fragilis produces α-galactosylceramide, which suppresses intestinal natural killer T (NKT) cell activation (An et al. 2014). Patients with inflammatory bowel disease (IBD) present with relatively low fecal Bacteroides sphingolipid concentrations and are negatively correlated with inflammation. The colonization of germ-free (GF) mice by genetically engineered Bacteroides thetaiotaomicron, which lacks sphingolipid synthesis, results in intestinal inflammation and altered host ceramide pools (Brown et al. 2019). Thus, sphingolipids produced by Bacteroides might maintain intestinal homeostasis.
Gut bacteria also saturate dietary unsaturated fatty acids. Lactobacillus plantarum enzymatically metabolizes unsaturated fatty acids via catalysts of hydration and catalysts of dehydration to produce hydroxy-, oxo-, and conjugated fatty acids and partially saturated trans-fatty acids (Kishino et al. 2013). Linoleic acid administration activates the arachidonic acid cascade, which induces chronic inflammation in adipose tissue. Lactobacillus salivarius and Lactobacillus gasseri produce 10-hydroxy-cis-12-octadecenoic acid via linoleic acid hydroxylation, which improves obesity, increases GLP-1 secretion, and improves glucose metabolism (Miyamoto et al. 2019). Dietary nutrients are major drivers that determine microbial composition. Plasma lipidomic analysis of GF or colonized mice fed different nutrient compositions revealed that the microbiota have dynamic effects on systemic lipid profiles, and the magnitude of this influence is related to metabolic disorders (Watanabe et al. 2021). Thus, the gut microbiota comprises a regulator of bile acid metabolism, lipid absorption, inflammatory signals via lipid synthesis, and energy metabolism. Therefore, its disruption can cause metabolic disorders through these mechanisms, which will be discussed in more detail later in this article.
Vitamins
Vitamins are either fat soluble (A, D, E, and K) or water soluble (B-complex and C). Fat-soluble vitamins are transported to various tissues and act as signaling molecules and bioactive effectors, whereas water-soluble vitamins act as coenzymes. Vitamin administration alters gut bacteria, increases their biodiversity, and elevates SCFA levels (Pham et al. 2021). However, gut bacteria themselves synthesize vitamins (Steinert et al. 2020).
Humans cannot synthesize any vitamin, except D. Thus, the human vitamin supply depends on diet and gut bacteria. Gut microbiota produce C, K, and B-complex vitamins. Thiele et al. analyzed the microbial genome and assessed the ability of bacteria to synthesize thiamine (B1), riboflavin (B2), niacin (B3), pantothenate (B5), pyridoxine (B6), biotin (B7), folate (B9), and cobalamin (B12). Approximately 40–65% of 256 human commensal bacteria possess a B-complex synthesis pathway (Magnusdottir et al. 2015). Interactions occur between vitamins and gut microbiota, and the former affect microbial community structures (Pham et al. 2021). Vitamin A administration to neonates within 2 days after birth affects the gut bacterial composition in a sex-dependent manner. It increases Bifidobacterium abundance in boys but increases Actinobacteria and A. muciniphila abundance in girls (Huda et al. 2019). Dietary vitamin A intake and plasma concentrations affect the gut bacterial composition (Wu et al. 2011, Li et al. 2017), which could aid in protection against various pathogens (Long et al. 2007, 2011).
Vitamin D also modifies the gut microbial community structure, and supplementation is associated with significant changes in Firmicutes, Actinobacteria, and Bacteroidetes abundances. Veillonellaceae and Oscillospiraceae (Firmicutes) are negatively associated with 25(OH)-D levels and vitamin D supplementation (Bellerba et al. 2021). Vitamin D may protect against IBD by suppressing immunocyte activation, strengthening intestinal barrier functions, and enhancing antibacterial peptide production (Pham et al. 2021). Certain bacterial species regulate vitamin D receptors (VDRs). Wu et al. reported that the probiotics Lactobacillus rhamnosus and L. plantarum increase VDR in the IECs and protect against colitis in a VDR signaling-dependent manner (Wu et al. 2015). A genome-wide association analysis revealed that variations in VDR affect β-diversity and Parabacteroides abundance (Wang et al. 2016). This evidence suggests that gut microbiota regulate vitamin D signaling and help to maintain intestinal homeostasis.
Amino acid metabolism
Gut microbiota degrade proteins, produce amino acids (AAs), and metabolize the choline and l-carnitine in red meat to trimethylamine (TMA). The latter enters circulation and is metabolized in the liver to trimethylamine-N-oxide (TMAO). In an animal model, TMAO was found to promote atherosclerosis. Plasma TMAO could increase cardiovascular disease (CVD) risk and mortality in a dose-dependent manner (Koeth et al. 2013, Tang et al. 2013, Schiattarella et al. 2017). Wang et al. showed that a structural analog of choline, 3,3-dimethyl-1-butanol (DMB), inhibits microbial TMA production. Oral DMB administration reduces TMAO levels and inhibits choline diet-induced macrophage foam cell formation to suppress the development of arteriosclerosis in apolipoprotein E knockout (KO) mice. Therefore, microbial intervention might help to prevent arteriosclerosis (Wang et al. 2015).
Gut microbiota also metabolize other AAs. Plasma levels of the branched-chain amino acids (BCAAs) valine, leucine, and isoleucine are correlated with insulin resistance in diabetic patients (Newgard et al. 2009, Batch et al. 2013, Jang et al. 2016). Pedersen et al. integrated analyses of serum metabolomics and metagenomics and showed that elevated BCAA levels in insulin-resistant individuals are associated with microbiota, which are enriched in BCAA biosynthesis pathways. Prevotella copri and Bacteroides vulgatus are the major BCAA synthesizers. The oral administration of P. copri to mice fed a high-fat diet results in increased serum BCAAs and aggravates glucose intolerance. Thus, BCAAs produced by gut microbiota could contribute to insulin resistance (Pedersen et al. 2016).
The aromatic AAs tyrosine, phenylalanine, and tryptophan are correlated with diabetes risk (Wang et al. 2011, Chen et al. 2016). Bacteria harbor genes encoding mediators of aromatic AA metabolism. Antibiotic administration strongly affects these metabolic pathways. Hence, aromatic AA metabolism by gut microbiota affects the host AA profile (Fujisaka et al. 2018).
Immune function maintenance and protection against pathogens
SCFAs exert various bioactive effects that help maintain the immune system (Shibata et al. 2017). Butyrate is an energy source for IECs and has anti-inflammatory and immunomodulatory effects (den Besten et al. 2013). Butyrate interacts with IECs, induces antimicrobial peptide and cytokine production, inhibits pathogen overgrowth, and strengthens intestinal barrier functions (Raqib et al. 2006, Singh et al. 2014, Shibata et al. 2017). The predominant phylum Firmicutes consists mainly of butyrate-producing Clostridium clusters IV and XIV (Eckburg et al. 2005). Clostridiales bacteria induce regulatory T-cell differentiation in the intestinal mucosa, which helps suppress the immune response (Atarashi et al. 2011). Furusawa et al. reported that butyrate derived from Clostridiales leads to butyrate-enhanced histone H3 acetylation in both the promoter and conserved non-coding sequence regions of Foxp3 (Furusawa et al. 2013), suggesting that butyrate is an epigenetic modifier for Treg induction.
Bifidobacteria are major producers of acetate, which has immunoregulatory effects. Acetate fortifies the gut barrier function against Escherichia coli O157 (Fukuda et al. 2011). Acetate induces apoptosis via neutrophil GPR43 and suppresses colitis in a mouse colorectal cancer model (Maslowski et al. 2009). Acetate also alleviates allergic airway disease (AAD) in a mouse model. The oral administration of a high-fiber diet or acetate to pregnant mice delays the onset of AAD in their offspring, possibly by inducing Tregs via Foxp3 acetylation at its promoter and HDAC9 inhibition (Thorburn et al. 2015). Succinate, produced by neonate intestinal bacteria, promotes gut microbiota maturation by establishing Clostridiales in the intestinal tract, thereby preventing Salmonella and pathogenic E. coli growth (Kim et al. 2017). Thus, certain bacteria suppress immunocyte activation. In contrast, other bacteria can activate certain immunocytes. Atarashi et al. reported that salivary Klebsiella spp. ectopically colonizing the intestine activate the Th1 immune response and exacerbate intestinal inflammation in a mouse IBD model (Atarashi et al. 2017). In rodents, segmented filamentous bacteria strongly induce Th17cells (Atarashi et al. 2015), trigger the production of the proinflammatory cytokines IL-17 and IL-22, and promote antimicrobial peptide production in IECs (Shale et al. 2013). In contrast, some bacteria such as Gordonibacter pamelaeae, Eggerthella lenta, and Raoultibacter massiliensis negatively regulate Th17 cell differentiation by producing the secondary bile acid metabolites 3-oxolithocholic acid (3-oxoLCA) and isolithocholic acid (isoLCA). These bile acids inhibit retinoic acid receptor-related orphan nuclear receptor-γ t (RORγt), a key transcription factor that promotes Th17 cell differentiation. Since 3-oxoLCA and isoLCA levels are reduced in patients with IBD and inversely correlated with Th17 cell/IL-17-related genes, decreased 3-oxoLCA and isoLCA in dysbiosis could contribute to IBD pathophysiology (Paik et al. 2022). In general, Tregs have anti-inflammatory effects, whereas Th17 cells act in a pro-inflammatory manner in IBD, and the Th17/Treg balance is important to maintain intestinal immune homeostasis, with its dysregulation contributing to IBD development (Ueno et al. 2018). Thus, although the mechanisms are complex, it is important to elucidate the interaction between gut commensal bacteria and immunocyte functions.
Regulation of intestinal motility
Intestinal motility is vital to digestion, absorption, and excretion. Gut bacteria regulate intestinal motility by helping to develop and maintain the intestinal nervous system. Intestinal motility is seldom observed in fetuses with little or no intestinal bacteria (Kien 1996). GF mice present with decreased colonic neuron density and intestinal motility compared with those in specific pathogen-free (SPF) mice. Fecal microbiota transplantation promotes serotonin (5-HT) production in the intestinal mucosa and neurons and increases neuron density and motility (De Vadder et al. 2018). Deoxycholic acid produced by spore-forming bacteria enhances 5-HT biosynthesis from colonic enterochromaffin cells and activate platelet functions and intestinal peristalsis (Yano et al. 2015). Obata et al. compared RNA sequencing of the intestinal tracts of conventionally raised mice with GF mice and found a transcription factor, aryl hydrocarbon receptor (AhR), was induced by microbiota. AhR was expressed in colonic neurons and promoted peristalsis of the intestinal tract, indicating a close link between microbiota and the enteric nervous system (Obata et al. 2020). These findings show that intestinal microbiota help to regulate intestinal motility. Therefore, gut dysbiosis can cause chronic constipation and irritable bowel syndrome, which are associated with dysregulated peristalsis. In summary, the gut microbiota play an important role in maintaining systemic homeostasis through the metabolism of nutrients, such as indigestible polysaccharides, lipids, vitamins, and AAs, and regulation of the immune system and intestinal function. In the latter part of this article, we outline the relationship between dysbiosis and obesity/glucose metabolism.
History of GF animals
Next-generation sequencing technologies such as 16S rRNA sequencing and shotgun metagenomic sequencing have been developed to obtain microbial information. Details of these technologies are described in other reviews (Wensel et al. 2022). The transplantation of gut bacteria into GF animals is a powerful tool to investigate the effects of the gut microbiota of interest on biological functions and disease. In this section, we briefly introduce the significance and history of the development of GF animals.
GF animals harbor no detectable microorganisms and can help clarify the roles and significance of the gut microbiota. Gnotobiotic animals are GF and colonized by a single strain or a specific bacterial community comprising various species. They help demonstrate the physiological effects of certain bacteria on the host.
The development of GF animals began in 1885 through the advocacy of Louis Pasteur in France (Pasteur 1885). In 1895, Nuttal and Thierfelder obtained Guinea pigs via aseptic Caesarean section and maintained them in a sterile environment for 13 days (Nuttal & Thierfelder 1985). In 1989, Schottelius et al. created the first reported GF chickens. In the early 1900s, Kuster et al. established GF goats. Later, GF rats, chickens, and guinea pigs were bred as experimental animals.
GF mice were first weaned in 1954 (Reyniers et al. 1946, Reyniers et al. 1949, Miyakawa et al. 1954). The goal of early GF animal research was to determine whether bacterial symbiosis was beneficial or detrimental to the survival of host organisms. GF animal research is used in physiology, nutrition, bacteriology, and immunology. Current microbiota study tools and techniques have revealed close associations between bacteria and metabolic diseases.
However, maintenance of a gnotobiotic status is expensive and requires experienced staff, and the available facilities for GF animals are limited. Furthermore, as GF animals have not innately experienced immunological stimulation by microorganisms, their nutritional status and immune systems are quite different from those of SPF animals. Therefore, we should be cautious in interpreting results of such studies.
Gut microbiota in obesity
Obesity is one of the most serious and common medical conditions in modern society. According to World Health Organization data, the obese population is increasing annually. In 2016, 39% of all adults (1.9 billion people) were overweight and 13% of all adults (650 million people) were obese. Obesity causes metabolic disorders, hypertension, glucose intolerance, dyslipidemia, and hyperuricemia and is a risk factor for atherosclerosis, ischemic heart disease, and CVD. Therefore, the pathogenesis, prevention, and treatment of obesity merit further investigation. Recent studies have revealed a close relationship between gut microbiota and obesity. The latter alters the gut microbiota, which is in turn closely related to obesity pathogenesis, as it affects host immunity and metabolism. Thus, interventions involving gut microbiota could be used to prevent and treat obesity.
The gut microbiota is inextricably linked to obesity
Bäckhed et al. first found that fecal microbiota transplantation of normal microbiota from the cecum of conventionally raised mice into GF C57/BL6 mice increased fat mass and insulin resistance. The increase in fat storage was not due to an increased food intake but a decreased expression of the fasting-induced adipose factor (Fiaf), a fat accumulation inhibitory factor (Backhed et al. 2004). Several years later, they showed the mechanisms by which GF mice are resistant to obesity compared to conventionally raised mice. They found that (1) GF mice had elevated intestinal Fiaf levels, which activated PGC1α in the skeletal muscle, and (2) AMPK in skeletal muscle is activated in GF mice independently of Fiaf signaling. These two phenotypes can lead to enhanced fatty acid oxidation in GF animals, suggesting that the gut microbiota plays an important role in promoting energy accumulation (Backhed et al. 2007). Subsequently, several studies showed that the transplantation of gut microbiota from obese human or mouse models into GF mice results in obesity (Turnbaugh et al. 2006, Vijay-Kumar et al. 2010, Ridaura et al. 2013). Furthermore, microbial perturbation via antibiotic administration in childhood has been shown to elevate the risk of obesity in adulthood (Cox et al. 2014, Cox & Blaser 2015, Schwartz et al. 2016). This led us to recognize that disruption of the microbiota (dysbiosis) is a cause, and not a consequence, of obesity and is closely associated with metabolic diseases (Le Chatelier et al. 2013). Thus, the gut microbiota is inextricably linked to obesity.
In 2006, it was first shown that gut bacteria differ with obesity. These findings showed a relative increase in the Firmicutes/Bacteroides (F/B) ratio in obese humans and rodents (Ley et al. 2006, Turnbaugh et al. 2006). Since then, numerous studies have reported on the gut microbiota of obese patients to clarify this dysbiosis among various ethnic groups and different geographic regions (Table 1). However, the pattern of microbial structure changes with obesity is not constant, and some reports show no change or a decrease in the F/B ratio (Schwiertz et al. 2010, Tims et al. 2013). Interactions among multiple factors such as race, region, diet, and cultural background might account for these discrepancies. Some human studies have revealed that Oscillospira is reduced with obesity (Konikoff & Gophna 2016, Yang et al. 2021) and is expected to be a candidate for next-generation probiotics as it produces SCFAs such as butyrate. However, several reports showed that this bacterium is associated with gallstones and chronic constipation, suggesting complex interactions between the bacterium and host physiology (Yang et al. 2021). Goodrich et al. analyzed the gut microbiota of 416 pairs of twins and found that the abundance of Christensenellaceae was correlated with low BMI and that the transplantation of Christensenella minuta into GF mice reduces weight gain (Goodrich et al. 2014). Everard et al. showed that the relative abundance of A. muciniphila, which has been linked to a favorable effect on glucose metabolism, is reduced in obese and diabetic mice and humans (Everard et al. 2013). The metabolic ameliorating effects of A. muciniphila are described later in the ‘Impaired gut barrier function’ section.
Table 1.
Characteristics of microbiota associated with obesity among different countries. Characteristics of the microbial composition in obese individuals vary by race and region.
| Age | Number | Country | Obese vs normal (excerpted bacteria is shown) | Other phenotype | References | |
|---|---|---|---|---|---|---|
| Adults | 10 obese/20 lean | Japan | ↑Firmicutes ↑Fusobacteria ↑Alistipes ↑Anaerococcus ↑Corpococcus ↑Fusobacterium ↑Parvimonas |
↓Bacteroides ↓Desulfovibrio ↓Faecalibacterium ↓Lachnoanaerobaculum ↓Olsenella ↓Faecalibacterium prausnitzii |
Bacteroidetes/Firmicutes (B/F) ratio not significant | Andoh et al. (2016) |
| Adults | 20 normal weight/20 obese/9 anorexic | France | ↑Lactobacillus (not significant) | ↓Bacteroidetes | Firmicutes data are similar in the three categories | Armougom et al. (2009) |
| Adults | 20 obese/20 normal weight | Italy | ↑Veillonellaceae ↑Dialister spp. |
↓Oscillospira genus | ↓a-diversity | Borgo et al. (2013) |
| Adults | 17 obese/25 obese with metabolic syndrome/ 25 healthy | Mexico | ↑Faecalibacterium ↑Roseburia ↑Lachnospira ↑Coprococcus ↑Bilophila |
↓Erysipelotrichaceae | Chávez-Carbajal et al. (2019) | |
| Adults | 3 obese/24 overweight/106 normal weight/7 underweight | Italy | ↑Selenomonas ↑Megasphaera ↑Streptococcus ↑Dorea ↑Lachnobacterium ↑Jannaschia ↑Dialister ↑Eubacterium |
↓Paraprevotella | Firmicutes/Bacteroidetes (F/B) ratio not significant | Gallè et al. (2020) |
| Adults | 24 obese/28 overweight/31 normal weight/21 underweight | Saudi Arabia | ↑Lentisphaerae | Harakeh et al. (2020) | ||
| Adults | 1674 subjects | USA | ↑Acidaminococcus ↑Megasphaera ↑Catenibacterium ↑Prevotella ↑Streptococcus |
↓Oscillospira ↓Cloacibacillus ↓Anaerotruncus ↓Ruminococcus ↓Coprobacillus ↓Eggerthella |
Kaplan et al. (2019) | |
| Adults | 33 obese/23 non-obese | Japan | ↑Blautia hydrogenotorophica ↑Coprococcus catus ↑Eubacterium ventriosum ↑Ruminococcus bromii ↑Ruminococcus obeum |
↓Bacteroides ↓Bacteroides faecichinchillae ↓Bacteroides thetaiotaomicron ↓Blautia wexlerae ↓Clostridium bolteae ↓Flavonifractor plautii |
↑F/B ratio, ↑Shannon–Wiener index | Kasai et al. (2015) |
| Adults | 167 normal/396 overweight or obese with different BMI history | Finland | ↑Roseburia ↑Blautia |
↓Rikenellaceae ↓Oscillospira |
↓Shannon index | Loftfield et al. (2020) |
| Adults | 52 African American/46 Caucasian American | USA | Bacteroidetes numbers not significant | Mai et al. (2009) | ||
| Adults | 170 HIV-negative women | South Africa | ↑Prevotella | Oduaran et al. (2020) | ||
| Adults | 531 subjects (132 obese/100 normal weight) | Finland | ↑Tissierellacea ↑Blautia |
↓Archaea (Methanobrevibacter) | F/B ratio not significant | Org et al. (2017) |
| Adults | 248 subjects (83 obese or overweight/83 normal weight/82 underweight) | Bangladesh | ↑Acidaminococcus | ↓Oscillospira | ↓Chao1 richness, ↓Shannon diversity index | Osborne et al. (2020) |
| Adults | 767 subjects | Japan | ↓Alistipes ↓Clostridium XlVb ↓Erysipelotrichaceae incertae sedis ↓Lactobacillus |
Blautia hansenii and Blautia producta were negatively associated with changes in VFA | Ozato et al. (2022) | |
| Adults | 1001 subjects | Japan | ↑Prevotella in men ↑Clostridium sensu stricto, Roseburia ↑Ruminococcus, and Megasphaera in women |
↓Blautia and Bifidobacterium in men ↓Blautia, Bifidobacterium, Eggerthella, Sutterella, and Erysipelotrichaceae incertae sedis in women |
Blautia was the only microbiota significantly and negatively associated with VFA, regardless of sex | Ozato et al. (2019) |
| Adults | 5 obese/5 surgically treated/5 obese/5 lean | India | ↑Bacteroides | Patil et al. (2012) | ||
| Adults | 599 subjects ( 142 obese/246 overweight/211 normal weight) | USA | ↑Bacilli ↑Streptococcaceae ↑Lactobacillaceae |
↓Clostridia including Christensenellaceae ↓Clostridiaceae and Dehalobacteriaceae |
↓Number of OTUs, ↓Shannon index | Peters et al. (2018) |
| Adults | 32 obese/32 normal weight | Mexico | ↑Clostridum leptum ↑Lactobacillus |
↓Prevotella ↓Escherichia coli |
Radilla-Vazquez et al. (2016) | |
| Adults | 60 subjects (25 obese with T2D/25 obese non-diabetic/5 non-obese with T2D/5 control) | Egypt | ↑Prevotella ↑Clostridium ↑Faecalibacterium ↑Staphylococcus |
↓Akkermansia | ↑F/B ratio | Salah et al. (2019) |
| Adults | 33 obese/35 overweight/30 normal weight | Germany | ↑Bacteroidetes | ↓Bifidobacterium ↓Methanobrevibacter spp. ↓Ruminococcus flavefaciens subgroup |
↓F/B ratio | Schwiertz et al. (2010) |
| Adults | 20 twin pairs | Finland | The abundance and diversity of the bacterial groups not significant | Simoes et al. (2013) | ||
| Adults | 1280 subjects (633 lean non-diabetic/494 obese non-diabetic/153 obese with T2D) | Germany | ↑Bacteroides thetaiotaomicron | ↓Faecalibacterium prausnitzii | Thingholm et al. (2019) | |
| Adults | 20 concordant/20 discordant BMI twin pairs | Netherlands | ↑Eubacterium ventriosum, ↑Roseburia intestinalis | ↓Oscillospiraguillermondii | B/F ratio not significant | Tims et al. (2013) |
| Adults | 52 obese/52 normal weight | China | ↓Clostridium perfringens, ↓Bacteroides | Zuo et al. (2011) | ||
| Children | 15 obese/13 normal weight | India | ↑Fecalibacterium prausntzi | Balamurugan et al. (2010) | ||
| Children | 25 overweight/7 obese/24 normal weight | Finland | ↑Staphylococcus aureus | ↓Bifidobacteria | Kalliomaki et al. (2008) | |
| Children | 15 obese/15 normal weight | Swiss | No significant quantitative differences | Payne et al. (2011) | ||
| Children | 138 subjects | Flanders | ↑Bacteroides fragilis | ↓Staphylococcus | Vael et al. (2011) | |
More recently, metabolomics has been used to elucidate the effects of metabolites produced by the gut microbiota. In China, a metagenome-wide association analysis and serum metabolomic profiling revealed that the abundance of glutamate-fermenting B. thetaiotaomicron is reduced with obesity and associated with an elevation in serum glutamine (Gln). Bariatric surgery and B. thetaiotaomicron administration decrease Gln levels in mice. Hence, B. thetaiotaomicron affects AA cycling and has anti-obesity effects (Liu et al. 2017). In contrast, other researchers reported that B. thetaiotaomicron upregulates intestinal lipid absorption transporters, promotes hepatic lipid biosynthesis, and causes obesity and impaired glucose tolerance in high-fat diet-fed mice (Cho et al. 2022). Further research is required to identify the mechanism through which gut bacteria help to improve obesity and metabolic disorders.
Obesity treatment alters the gut microbiota
Bariatric surgery is a well-established obesity treatment. It promotes weight loss and improves obesity-related metabolic disorders such as hypertension, diabetes, and dyslipidemia (Courcoulas et al. 2018) by reducing food intake and altering gut hormone and bile acid levels, energy metabolism, and the gut microbiota (Debedat et al. 2019). Several studies have shown that the microbial community structure is altered after bariatric surgery. Nevertheless, the results and conclusions were inconsistent among reports possibly because of differences in study designs, sample sizes, subject races, geography, and diet (Table 2). Ryan et al. demonstrated that the metabolic effects of sleeve gastrectomy are abolished in FXR-disrupted mice, suggesting that FXR signaling contributes to body weight reduction and improvement in glycemic control after bariatric surgery (Ryan et al. 2014). Another group elucidated the mechanism by which Roux-en-Y gastric bypass (RYBG) improves metabolism in rodents. Specifically, it decreases bile acids and increases glucagon-like peptide 1 (GLP-1) via L-cell proliferation. The decrease in bile acids, associated with a decline in Lactobacillus abundance, results in intestinal L-cell proliferation, an increase in GLP-1 secretory capacity, and improvements in glycemic control (Dang et al. 2021). Torsten et al. analyzed 40 pre-RYBG and post-RYBG cases and showed that RYBG improved chronic inflammation but increased proinflammatory Proteobacteria. Lipopolysaccharide (LPS) and flagellin-specific immunoglobulin A (IgA) were increased, but the total fecal IgA content remained unchanged. Hence, an increase in intestinal IgA after bariatric surgery neutralized immunogenic bacteria and their components, leading to the improved systemic inflammation (Scheithauer et al. 2022). Thus, metabolic improvements mediated by bariatric surgery are mainly associated with changes in incretin secretion and immune activities that are mediated by microbial alterations.
Table 2.
Characteristics of microbiota after bariatric surgery in different countries.
| Operation | Number | Country | Major bacterial changes after surgery | Diversity after surgery | References | |
|---|---|---|---|---|---|---|
| Roux-en-Y gastric bypass | 14 | Brazil | ↓F/B ratio | ↑OTUs richness | Al Assal et al. (2020) | |
| Gastric banding or Roux-en-Y gastric bypass | 24 | France | ↑Butyricimonas virosa , 11 altered after Roux-en-Y and 2 altered after gastric banding | ↑Microbial gene richness | Aron-Wisnewsky et al. (2019) | |
| Roux-en-Y gastric bypass or sleeve gastrectomy | 53 | China | 33 altered after sleeve and 19 altered after Roux-en-Y | ↑Richness and evenness | Chen et al. (2020) | |
| Roux-en-Y gastric bypass | 24 | China | ↑Bacteroidetes ↑Bifidobacterium |
Chen et al. (2017) | ||
| Roux-en-Y gastric bypass or sleeve gastrectomy | 197 | France, Switzerland, USA | ↑Akkermansia muciniphila | ↑Shannon index and gene richness | Farin et al. (2020) | |
| Sleeve gastrectomy | 10 | Japan | ↑Bacteroidetes ↑Fusobacteria |
↑Faith PD ↑Chao1 ↑Shannon index |
Fukuda et al. (2022) | |
| Roux-en-Y gastric bypass | 30 | France | ↑Bacteroides/Prevotella ↑E. coli |
↓Bifidobacterium/ Lactobacillus/ Leuconostoc ↓Pediococcus |
Furet et al. (2010) | |
| Roux-en-Y gastric bypass or sleeve gastrectomy | 31 | Korea | ↑Streptococcus ↑Oscillospira ↑Akkermansia |
↓Prevotella ↓Turicibacter ↓Bifidobacterium |
↑Observed species | Han et al. (2022) |
| Sleeve gastrectomy or sleeve with duodenojejunal bypass or gastric banding | 44 | Japan | ↑Bacteroidetes ↑Lactobacillales ↑Enterobacteriales |
Kikuchi et al. (2018) | ||
| Roux-en-Y gastric bypass | 30 | France | ↑Proteobacteria ↑Bacteroides ↑Escherichia |
↓Lactobacillus ↓Dorea ↓Blautia ↓Bifidobacterium |
↑Richness | Kong et al. (2013) |
| Sleeve gastrectomy | 23 | China | ↑α-diversity | Liu et al. (2017) | ||
| Roux-en-Y gastric bypass or sleeve gastrectomy | 28 | Spain | (Sleeve) ↑Akkermansia ↑Haemophilus (Roux-en-Y) ↑Clostridium ↑Fusobacterium |
(Sleeve) ↓Anaerostip ↓Bifidobacterium (Roux-en-Y) ↓Bifidobacterium ↓Collinsella |
Sanchez-Alcoholado et al. (2019) | |
| Roux-en-Y gastric bypass or sleeve gastrectomy | 45 | Poland | ↑Bacteroidetes ↑Bacteroidales ↑Bacteroidia ↑Prevotellaceae ↑Rikenellaceae |
↓Firmicutes ↓Clostridiales ↓Clostridia ↓Lachnospiraceae ↓Blautia |
Stefura et al. (2022) | |
| Roux-en-Y gastric bypass | 40 | Netherlands | ↑Proteobacteria ↑Akkermansia |
↓Roseburia ↓Bacteroides ↓Faecallibacterium |
↑α-diversity | Scheithauer et al. (2022) |
Gut microbiota and glucose metabolism
Research on the relationships among gut microbiota, diabetes, and obesity has progressed. Gut microbiota differ by nationality and race in patients with type 2 diabetes (T2DM). A metagenomic analysis of a Chinese T2DM cohort conducted by Qin et al. revealed decreased butyrate-producing bacteria, increased methane metabolism and hydrogen sulfide production, and upregulation of the expression of genes regulating oxidative stress resistance (Qin et al. 2012). A shotgun metagenomic analysis conducted by Karlsson et al. on a cohort of 145 European women with a normal status, impaired glucose tolerance, and T2DM characterized compositional and functional alterations in the gut microbiota. They established a random forest model based on the metagenomic data that could be applied to patients with impaired glucose tolerance. However, this model could not be consistently applied to the cohort of Qin et al. (Karlsson et al. 2013). Hence, metagenomic profiles differ between diabetic and healthy individuals and can be a good predictive tool for impaired glucose metabolism, but regional and racial differences in gut microbiota must be considered. There is nonetheless a strong link between microbial dysfunction and impaired glucose metabolism. Moreover, gut dysbiosis is already present at the impaired glucose tolerance stage, even before T2DM onset. A metagenomic analysis conducted by Wu et al. on ~1500 Swedish subjects showed that prediabetes and T2DM are characterized by alterations in the gut microbiota and their functional genes and decreases in the abundance of butyrate-producing bacteria. The authors constructed a machine learning model using a random forest algorithm to distinguish individuals with prediabetes or diabetes and showed that bacterial compositional/functional alterations are associated with insulin resistance at the impaired glucose tolerance stage (Wu et al. 2020). Gut microbiota analysis could help diagnose early-stage glucose intolerance and improve early intervention. Based on these studies, the construction of a machine learning model to predict the development of T2DM in healthy and prediabetic patients has been challenging (Aasmets et al. 2021). However, it could serve as a predictive marker for individual risks of metabolic disorders.
Gut microbiota are also implicated in type 1 diabetes (T1DM). Gavin et al. proteomically analyzed stool samples of patients with T1DM and detected proteins associated with intestinal inflammation, reduced intestinal barrier functions, and changes in gut microbiota even before disease onset (Gavin et al. 2018). Tracking the gut microbiota of children congenitally predisposed to T1DM revealed reduced microbial diversity and transient spikes in Ruminococcus gnavus and Streptococcus infantarius abundance before disease onset (Kostic et al. 2015). A metagenomic analysis of 74 T1DM patients and 296 healthy controls showed microbial differences between groups; P. copri and Eubacterium siraeum were more common in T1DM, whereas Firmicutes and Faecalibacterium prausnitzii were more prevalent in controls. In this study, altered bacterial species and metabolic pathways were associated with host glycemic control (Shilo et al. 2022). Thus, changes in gut microbiota and the ambient environment might be associated with T1DM development. As an immunological mechanism, the commensal gut bacterium Parabacteroides distasonis has an epitope similar to the insulin beta chain, the target protein of the autoimmune response in T1DM. The presence of this bacteria could cause the production of autoimmune antibodies leading to the pathogenesis of T1DM (Girdhar et al. 2022). However, some reports suggest that the microbiota inhibit the development of T1DM. MyD88-KO NOD mice develop T1DM in a GF environment but not in the presence of intestinal bacteria (Wen et al. 2008). Shimokawa et al. reported that Ruminococcus spp. might prevent the onset of T1DM by inducing CD8+ regulatory T cells (Shimokawa et al. 2020), suggesting a close interaction between the microbiota and immune system and the onset of T1DM. This evidence clearly implicates the involvement of microbiota in glucose metabolism. The mechanisms by which microbiota contribute to glucose metabolism are described in the following sections.
Short-chain fatty acids
SCFAs exert their metabolic effects through G protein-coupled fatty acid receptors such as GPR41 and GPR43. GPRs are involved in GLP-1 and peptide YY (PYY) secretion in intestinal L cells (Samuel et al. 2008, Tolhurst et al. 2012) to regulate insulin secretion by stimulating pancreatic β-cells and energy balance (Natarajan & Pluznick 2014, McNelis et al. 2015). Mice lacking these receptors exhibit reduced GLP-1 and PYY secretion after SCFA administration (Psichas et al. 2015, Koh et al. 2016, Brooks et al. 2017). Butyrate enhances thermogenesis in skeletal muscle and brown adipose tissue (BAT), improves glucose metabolism by upregulating PGC-1α, AMPK, and p38 expression (Gao et al. 2009), promotes energy expenditure via GPR41 in sympathetic ganglia, and contributes to in vivo energy homeostasis (Inoue et al. 2012). Kimura et al. also reported that SCFAs regulate energy metabolism by promoting sympathetic activation via GPR41 signaling (Kimura et al. 2011). SCFA-mediated GPR43 activation in adipocytes inhibits insulin signaling by suppressing Akt phosphorylation to reduce fat accumulation (Kimura et al. 2013). This evidence suggests that increasing SCFAs in the body might be metabolically beneficial. In addition to the intake of non-digestible polysaccharides, physical activity (Magzal et al. 2022) and cold stimuli (Ichikawa et al. 2021) are implicated in SCFA production. Prior attempts have been made to ameliorate metabolic diseases using SCFA-related interventions. Emanuel et al. reported that infusions of SCFAs, such as acetate, butyrate, and propionate, into the distal colon increase fasting fat oxidation, resting energy expenditure, and PYY concentrations in men (Canfora et al. 2017).
FXR/TGR5/bile acids
FXR (NR1H4) is a member of the nuclear receptor superfamily of TFs that senses and regulates bile acid, lipid, and glucose metabolism (Matsubara et al. 2013, Sayin et al. 2013). Gut microbiota regulate FXR signaling by reducing the FXR antagonist muricholic acid (Sayin et al. 2013). Mice lacking FXR signaling exhibit dyslipidemia (Sinal et al. 2000). The FXR agonists 6-ethyl-chenodeoxycholic acid, fexaramine, and GW 4064 and FXR overexpression improve the metabolic profile in a mouse obesity model (Zhang et al. 2006, Cipriani et al. 2010, Fang et al. 2015). In contrast, FXR-KO mice display improvements in metabolic disturbances induced by a high-fat diet (Prawitt et al. 2011) and increased GLP-1 secretion (Trabelsi et al. 2015). Jiang et al. reported that oral administration of the selective high-affinity FXR inhibitor glycine-β-muricholic acid ameliorates obesity, insulin resistance, and fatty liver (Jiang et al. 2015). These conflicting results suggest that FXR plays complex roles in the etiology of metabolic dysfunction and that its effects on metabolism vary in an organ-dependent manner. Future research should aim to clarify the significance of FXR in glucose metabolism.
TGR5 is a membrane-bound or G-protein bile acid-activated receptor (Kawamata et al. 2003). TGR5 expression is ubiquitous in human and rodent tissues and is upregulated in the lungs, liver, gallbladder, spleen, adipose tissue, CNS, and intestine (Duboc et al. 2014). Bile acids stimulate the secretion of intestinal hormones that regulate blood glucose and appetite (Adrian et al. 2012), such as GLP-1 and PYY. The latter is a centrally acting appetite suppressant (Kuhre et al. 2018). Bile acids also affect metabolism via TGR5 receptors in BAT. In mice, bile acid-induced TGR5 activation in BAT increases energy expenditure by inducing the cAMP-dependent thyroid hormone-activating enzyme known as type 2 iodothyronine deiodinase (D2), leading to improved obesity tolerance and metabolic disease. This increase in TGR5-mediated energy expenditure is abolished in D2−/− mice. The authors also confirmed that bile acid induces D2 expression and elevated oxygen consumption in a TGR-dependent fashion in human skeletal muscle cells. Hence, bile acid-TGR5-cAMP-D2 signaling might be protective against obesity in humans (Watanabe et al. 2006).
In vitro, bile acids could decrease endoplasmic reticulum (ER) stress, which is increased in obesity and closely related to insulin resistance etiology. ER stress blocks insulin signaling by overactivating c-Jun N-terminal kinase and promoting the serine phosphorylation of insulin receptor substrate-1 (Ozcan et al. 2004), inducing β-cell dysfunction and diabetes onset. Ozcan et al. reported that taurine-bound ursodeoxycholic acid reduces ER stress in cultured cells and whole animals. The administration of this conjugated bile acid to obese and diabetic mice normalizes hyperglycemia, restores systemic insulin sensitivity, improves fatty liver, and enhances insulin actions in liver, muscle, and adipose tissues (Ozcan et al. 2006). Alterations to the bile acid profile through microbiota modification have been investigated for the improvement of metabolic disorders. Jielong et al. reported that phenolic blueberry extracts increase energy expenditure in BAT and improve hepatic lipid metabolism via TGR5 and FXR. These effects are strongly correlated with bile acid regulation, reduction of the FXR inhibitors TαMCA and TβMCA, and expansion of the gut microbiota, including Bifidobacterium spp. and Lactobacillus spp. Antibiotic administration attenuates the aforementioned metabolic effects (Guo et al. 2019).
Chronic antibiotic treatments such as vancomycin or metronidazole change bile acid composition due to a loss of intestinal bacteria harboring bile acid-converting enzymes and resulted in the inhibition of inflammatory secondary bile acids production. Furthermore, the changes in the bile acid composition induced anti-inflammatory TGR5 in the liver. As a result, antibiotic treatment mitigated high-fat diet-induced systemic inflammation in C57BL6 mice. However, these effects were not observed in 129S1 or 129S6 mice. Hence, antibiotic modification of the gut microbiota and changes in bile acid and inflammatory signaling can improve glucose metabolism. However, these effects vary by host genetic background and inflammatory potential (Fujisaka et al. 2016).
Imidazole propionate
Imidazole propionate (ImP) is a histidine (His) metabolite produced by the gut microbiota. A study on 1990 subjects in 3 European countries showed elevated ImP levels in the serum of prediabetic and T2DM patients. Nevertheless, there was no correlation between His consumption and ImP levels, suggesting that the gut microbiota is largely responsible for increases in ImP (Molinaro et al. 2020). ImP exacerbates insulin resistance by activating the mTOR pathway and inhibiting insulin signaling (Koh et al. 2018). Thus, ImP might contribute to metabolic disease progression. Clostridium bolteae, Cenarchaeum symbiosum, and R. gnavus might affect ImP levels. High saturated fatty acid, low fiber, and low unsaturated fatty acid diets are associated with elevated ImP. Furthermore, ImP levels are high in patients with poorly controlled T2DM, and ImP inhibits the action of metformin. The glucose-lowering effect of metformin is abolished in mice pretreated with ImP, via the inhibition of AMPK phosphorylation in a p38γ-dependent manner (Koh et al. 2020). Thus, metabolites produced from AAs by gut microbiota not only modulate insulin signaling but also influence drug action.
Impaired gut barrier function
Dysbiosis causes so-called ‘leaky gut’ syndrome wherein the intestinal barrier function is impaired, with increased permeability, via decreases in intestinal mucus and tight junction protein content in the intestinal epithelium. With increased intestinal permeability, the bacterial cell wall component endotoxin enters circulation and causes hyper-endotoxemia. Endotoxin activates Toll-like receptor (TLR) 4, which in turn promotes chronic inflammation in the adipose tissue and liver and exacerbates obesity-induced insulin resistance (Amar et al. 2008, Gummesson et al. 2011, Lassenius et al. 2011). Impaired barrier functions can be treated by microbial intervention. Amer et al. found that administration of the probiotic Bifidobacterium animalis prevents LPS invasion into circulation and improves the inflammatory and metabolic status of diet-induced obese mice (Amar et al. 2011). In humans, supplementation with the probiotics Bacillus indicus, Bacillus subtilis, Bacillus coagulans, Bacillus licheniformis, and Bacillus clausii reduces serum endotoxin levels by 42% (McFarlin et al. 2017). Thus, intervention with gut bacteria, to strengthen gut barrier functions, is a novel therapeutic approach.
A. muciniphila is an oval, nonmotile, Gram-negative bacterium of phylum Verrucomicrobia. In 2004, Derrien isolated it from healthy human feces inoculated on media containing mucin as the nutrient source (Derrien et al. 2004). The genus Akkermansia (of A. muciniphila) is derived from that of the Dutch microbial ecologist Antoon Akkermans. In both obese humans and rodents, reductions in A. muciniphila abundance have been reported (Everard et al. 2013, Le Chatelier et al. 2013). By contrast, therapeutic interventions that increase A. muciniphila are associated with improvements in obesity or glucose metabolism. For example, when mice fed a high-fat, high-sucrose diet are treated with polyphenol-rich cranberry extract, the abundance of A. muciniphila is increased, which is associated with improved obesity, insulin resistance, and intestinal inflammation (Anhe et al. 2015). Bofutsushosan is a Chinese herbal medicine that improves insulin resistance by increasing A. muciniphila and improving intestinal barrier functions (Fujisaka et al. 2020). Metformin also modulates the gut microbiota, promotes A. muciniphila growth, and improves glucose metabolism (Shin et al. 2014, de la Cuesta-Zuluaga et al. 2017). Prebiotics such as isomalto-oligosaccharides and red pitaya betacyanin significantly increase A. muciniphila and improve glucose metabolism (Song et al. 2016, Singh et al. 2017). A. muciniphila helps maintain intestinal and mucosal epithelial cell functions and suppresses intestinal inflammation leading to improved metabolism in diabetic and obese patients (Everard et al. 2013, Plovier et al. 2017, Ansaldo et al. 2019). Crala et al. reported that pasteurized A. muciniphila administration decreases body weight in a mouse diet-induced obesity model by increasing systemic energy expenditure and fecal energy excretion (Depommier et al. 2020). In a randomized, double-blind, controlled trial on A. muciniphila in humans, A. muciniphila transplantation improved weight loss, total cholesterol levels, and insulin resistance compared to those with a placebo. Furthermore, pasteurized A. muciniphila had higher efficacy than live A. muciniphila. Hence, A. muciniphila administration might be both a safe and effective treatment (Plovier et al. 2017, Depommier et al. 2019). Kim et al. demonstrated that whereas live A. muciniphila suppresses diet-induced fatty liver (Kim et al. 2020), pasteurized A. muciniphila improves intestinal barrier functions but does not impede the progression of nonalcoholic steatohepatitis (Morrison et al. 2022). These results suggest that live and pasteurized A. muciniphila are beneficial for obesity-related metabolic disorders but have different effects on the host, and further research is required to clarify them.
Gut microbiota and hepatic diseases
The liver receives >70% of its blood flow from the portal vein and is continuously exposed to nutrients and bacteria-related substances from the intestine. Therefore, compositional and functional changes in microbiota play important roles in hepatic disease pathogenesis. A healthy intestinal barrier maintains its function and prevents gut microbiota from reaching the liver. However, when the barrier is impaired by various environmental factors, such as an unbalanced diet, gut bacteria and/or their components can be translocated to the liver. TLRs and pathogen recognition receptors, such as the inflammasome, recognize bacterial antigens and enhance the production of proinflammatory cytokines by activating hepatic macrophages and the immune response (Regnier et al. 2021). Dysbiosis is associated with the pathological conditions of various hepatic diseases such as alcoholic hepatitis, nonalcoholic fatty liver disease (NAFLD), cirrhosis, and hepatocellular carcinoma (HCC). Chronic alcohol intake has been reported to cause dysbiosis and impair intestinal barrier functions in animals and humans (Yan et al. 2011, Mutlu et al. 2012). Enterococcus faecalis is increased in alcoholic hepatitis patients and secretes cytolysin, which causes hepatocyte death and liver injury. In one study, bacteriophages targeting cytolysin-producing E. faecalis abolished ethanol-induced liver damage (Duan et al. 2019). Future research should establish whether this approach improves alcoholic hepatitis in humans.
Loomba et al. assessed hepatic fibrosis severity based on biopsies of 86 American patients with NAFLD. Whole-genome shotgun sequencing of stool samples revealed that advanced hepatic fibrosis is associated with increased Proteobacteria and decreased Firmicutes, Ruminococcus obeum, and Eubacterium rectale abundance (Loomba et al. 2017). In contrast, Boursier et al. showed that the relative abundances of Bacteroides and Ruminococcus were increased and that of Prevotella was decreased in French patients with advanced hepatic fibrosis (Boursier et al. 2016). Race, geographical factors, diet, and patient enrollment criteria also substantially influence gut microbial responses and must be considered. However, changes in gut microbiota can cause hepatic fibrosis. In the future, gut microbiota could be used to predict the risk of hepatic diseases.
Gut microbiota are also implicated in the development of HCC. Deoxycholic acid is increased in obesity-induced gut dysbiosis, induces the senescence-associated secretory phenotype in hepatic stellate cells, and is associated with various proinflammatory and tumor-promoting factors in the liver. These processes promote HCC development in mice exposed to chemical carcinogens (Yoshimoto et al. 2013). Ma et al. demonstrated that hepatic CXCR-positive NKT cells have antitumor efficacy in mouse spontaneous HCC and metastatic liver cancer models. Primary bile acids upregulate, whereas bacteria-derived secondary bile acids downregulate, CXCL16 expression, which in turn induces hepatic NKT cells. Clostridium scindens produces secondary bile acids that inhibit hepatic NKT cells (Ma et al. 2018). These studies demonstrate that the gut microbiota is vital in regulating hepatocarcinogenesis through bile acid signaling.
Conclusions
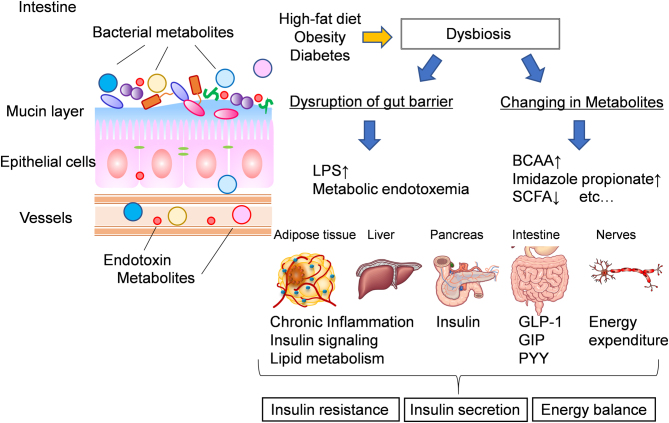
In recent years, as our understanding of the physiological role of the gut microbiota has advanced, the mechanisms by which its dysfunction can cause metabolic disorders have gained clarity (Fig. 3). Future research should aim to elucidate the complex roles of the gut microbiota in these processes. These discoveries might lead to the application of the gut microbiota and their metabolites for the future treatment of metabolic diseases.
Figure 3.
Mechanisms of dysbiosis-induced impaired glucose metabolism. There are two major mechanisms of impaired glucose metabolism mediated by dysbiosis. One is the disruption of the intestinal barrier function, which causes LPS to enter circulation, inducing chronic inflammation and exacerbating insulin resistance, and the other is the effect of microbial metabolites. Increased branched-chain amino acids (BCAAs), imidazole propionate, and decreased short-chain fatty acids, such as butyrate, can affect insulin resistance in various organs, insulin secretion, and energy expenditure.
Declaration of interest
K.T. received lecture fees/grants from Mitsubishi Tanabe Pharma Corporation., MSD K.K., Novo Nordisk Pharma Ltd., Daiichi Sankyo Co. Ltd., Takeda Pharmaceutical Co. Ltd., Suntory Global Innovation Center Ltd., Mitsubishi Tanabe Pharma Corporation, Asahi Kasei Pharma Corporation, and the Mitsubishi Foundation.
Funding
Our lab is supported by grants from the JSPS (Japan Society for the Promotion of Science), KAKENHI grant numbers 17K09821 and 20K08882 to S.F., grants from AMED PRIME (JP18gm6010023h0001) to S.F., grants from AMED-FORCE (JP21gm4010014h0001) to K.T., and grants from the Japan Diabetes Foundation, Research Funding Granted by the President of the University of Toyama, Research Funding Granted by the Japan Society for the Study of Obesity (JASSO) and Novo Nordisk Pharma Ltd. and Takeda Science Foundation to S.F. Y.W. received grants from the JSPS (Japan Society for the Promotion of Science) KAKENHI grant numbers 21K20896 and 22K16424, grants from the Lotte Foundation, and the Yakult Bio-Science Foundation.
Acknowledgements
The authors are grateful to Mrs Keiko Honda, Mrs Yurie Iwakuro, and Mrs Kumi Sassa for technical assistance to maintain the lab.
References
- Aasmets O, Lull K, Lang JM, Pan C, Kuusisto J, Fischer K, Laakso M, Lusis AJ, Org E.2021Machine learning reveals time-varying microbial predictors with complex effects on glucose regulation. mSystems 6 e01191-20. ( 10.1128/mSystems.01191-20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian TE, Gariballa S, Parekh KA, Thomas SA, Saadi H, Al Kaabi J, Nagelkerke N, Gedulin B, Young AA.2012Rectal taurocholate increases L cell and insulin secretion, and decreases blood glucose and food intake in obese type 2 diabetic volunteers. Diabetologia 552343–2347. ( 10.1007/s00125-012-2593-2) [DOI] [PubMed] [Google Scholar]
- Al Assal K Prifti E Belda E Sala P Clement K Dao MC Dore J Levenez F Taddei CR Fonseca DC,. et al. 2020Gut microbiota profile of obese diabetic women submitted to Roux-en-Y gastric bypass and its association with food intake and postoperative diabetes remission. Nutrients 12278. ( 10.3390/nu12020278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, Chamontin B, Ferrieres J.2008Energy intake is associated with endotoxemia in apparently healthy men. American Journal of Clinical Nutrition 871219–1223. ( 10.1093/ajcn/87.5.1219) [DOI] [PubMed] [Google Scholar]
- Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermudez-Humaran LG, Smirnova N, Berge M, Sulpice T, Lahtinen Set al. 2011Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Molecular Medicine 3559–572. ( 10.1002/emmm.201100159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- An D, Oh SF, Olszak T, Neves JF, Avci FY, Erturk-Hasdemir D, Lu X, Zeissig S, Blumberg RS, Kasper DL.2014Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell 156123–133. ( 10.1016/j.cell.2013.11.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh A Nishida A Takahashi K Inatomi O Imaeda H Bamba S Kito K Sugimoto M & Kobayashi T. 2016Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population. Journal of Clinical Biochemistry and Nutrition 5965–70. ( 10.3164/jcbn.15-152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anhe FF, Roy D, Pilon G, Dudonne S, Matamoros S, Varin TV, Garofalo C, Moine Q, Desjardins Y, Levy Eet al. 2015A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 64872–883. ( 10.1136/gutjnl-2014-307142) [DOI] [PubMed] [Google Scholar]
- Ansaldo E, Slayden LC, Ching KL, Koch MA, Wolf NK, Plichta DR, Brown EM, Graham DB, Xavier RJ, Moon JJet al. 2019Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science 3641179–1184. ( 10.1126/science.aaw7479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armougom F Henry M Vialettes B Raccah D & Raoult D. 2009Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS One 4e7125. ( 10.1371/journal.pone.0007125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron-Wisnewsky J Prifti E Belda E Ichou F Kayser BD Dao MC Verger EO Hedjazi L Bouillot JL Chevallier JM,. et al. 2019Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut 6870–82. ( 10.1136/gutjnl-2018-316103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Suda W, Luo C, Kawaguchi T, Motoo I, Narushima S, Kiguchi Y, Yasuma K, Watanabe E, Tanoue Tet al. 2017Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science 358359–365. ( 10.1126/science.aan4526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Ando M, Kamada N, Nagano Y, Narushima S, Suda W, Imaoka A, Setoyama H, Nagamori Tet al. 2015Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 163367–380. ( 10.1016/j.cell.2015.08.058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Yet al. 2011Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331337–341. ( 10.1126/science.1198469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI.2004The gut microbiota as an environmental factor that regulates fat storage. PNAS 10115718–15723. ( 10.1073/pnas.0407076101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Manchester JK, Semenkovich CF, Gordon JI.2007Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. PNAS 104979–984. ( 10.1073/pnas.0605374104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balamurugan R George G Kabeerdoss J Hepsiba J Chandragunasekaran AM & Ramakrishna BS. 2010Quantitative differences in intestinal Faecalibacterium prausnitzii in obese Indian children. British Journal of Nutrition 103335–338. ( 10.1017/S0007114509992182) [DOI] [PubMed] [Google Scholar]
- Barcenilla A, Pryde SE, Martin JC, Duncan SH, Stewart CS, Henderson C, Flint HJ.2000Phylogenetic relationships of butyrate-producing bacteria from the human gut. Applied and Environmental Microbiology 661654–1661. ( 10.1128/AEM.66.4.1654-1661.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batch BC, Shah SH, Newgard CB, Turer CB, Haynes C, Bain JR, Muehlbauer M, Patel MJ, Stevens RD, Appel LJet al. 2013Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolism: Clinical and Experimental 62961–969. ( 10.1016/j.metabol.2013.01.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellerba F, Muzio V, Gnagnarella P, Facciotti F, Chiocca S, Bossi P, Cortinovis D, Chiaradonna F, Serrano D, Raimondi Set al. 2021The association between vitamin D and gut microbiota: a systematic review of human studies. Nutrients 13 3378. ( 10.3390/nu13103378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boets E, Gomand SV, Deroover L, Preston T, Vermeulen K, De Preter V, Hamer HM, Van den Mooter G, De Vuyst L, Courtin CMet al. 2017Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. Journal of Physiology 595541–555. ( 10.1113/JP272613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgo F Garbossa S Riva A Severgnini M Luigiano C Benetti A Pontiroli AE Morace G & Borghi E. 2018Body mass index and sex affect diverse microbial niches within the gut. Frontiers in Microbiology 9213. ( 10.3389/fmicb.2018.00213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LAet al. 2016The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 63764–775. ( 10.1002/hep.28356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks L, Viardot A, Tsakmaki A, Stolarczyk E, Howard JK, Cani PD, Everard A, Sleeth ML, Psichas A, Anastasovskaj Jet al. 2017Fermentable carbohydrate stimulates FFAR2-dependent colonic PYY cell expansion to increase satiety. Molecular Metabolism 648–60. ( 10.1016/j.molmet.2016.10.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EM, Ke X, Hitchcock D, Jeanfavre S, Avila-Pacheco J, Nakata T, Arthur TD, Fornelos N, Heim C, Franzosa EAet al. 2019Bacteroides-derived sphingolipids are critical for maintaining intestinal homeostasis and symbiosis. Cell Host and Microbe 25 668–680.e7. ( 10.1016/j.chom.2019.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byndloss MX, Olsan EE, Rivera-Chavez F, Tiffany CR, Cevallos SA, Lokken KL, Torres TP, Byndloss AJ, Faber F, Gao Yet al. 2017Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357570–575. ( 10.1126/science.aam9949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfora EE, van der Beek CM, Jocken JWE, Goossens GH, Holst JJ, Olde Damink SWM, Lenaerts K, Dejong CHC, Blaak EE.2017Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Scientific Reports 7 2360. ( 10.1038/s41598-017-02546-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Carbajal A Nirmalkar K Perez-Lizaur A Hernandez-Quiroz F Ramirez-Del-Alto S Garcia-Mena J & Hernandez-Guerrero C. 2019Gut Microbiota and Predicted Metabolic Pathways in a Sample of Mexican Women Affected by Obesity and Obesity Plus Metabolic Syndrome. International Journal of Molecular Science 20438. ( 10.3390/ijms20020438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Zheng X, Ma X, Bao Y, Ni Y, Hu C, Rajani C, Huang F, Zhao A, Jia Wet al. 2016Tryptophan predicts the risk for future Type 2 diabetes. PLoS One 11 e0162192. ( 10.1371/journal.pone.0162192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H Qian L Lv Q Yu J Wu W & Qian H. 2017Change in gut microbiota is correlated with alterations in the surface molecule expression of monocytes after Roux-en-Y gastric bypass surgery in obese type 2 diabetic patients. American Journal of Translational Research 91243–1254. [PMC free article] [PubMed] [Google Scholar]
- Chen G Zhuang J Cui Q Jiang S Tao W Chen W Yu S Wu L Yang W Liu F,. et al. 2020. Two bariatric surgical procedures differentially alter the intestinal microbiota in obesity patients. Obesity Surgery302345–2361. ( 10.1007/s11695-020-04494-4) [DOI] [PubMed] [Google Scholar]
- Cho SH, Cho YJ, Park JH.2022The human symbiont Bacteroides thetaiotaomicron promotes diet-induced obesity by regulating host lipid metabolism. Journal of Microbiology 60118–127. ( 10.1007/s12275-022-1614-1) [DOI] [PubMed] [Google Scholar]
- Cipriani S, Mencarelli A, Palladino G, Fiorucci S.2010FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. Journal of Lipid Research 51771–784. ( 10.1194/jlr.M001602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courcoulas AP, King WC, Belle SH, Berk P, Flum DR, Garcia L, Gourash W, Horlick M, Mitchell JE, Pomp Aet al. 2018Seven-year weight trajectories and health outcomes in the longitudinal assessment of bariatric surgery (LABS) study. JAMA Surgery 153427–434. ( 10.1001/jamasurg.2017.5025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LM, Blaser MJ.2015Antibiotics in early life and obesity. Nature Reviews. Endocrinology 11182–190. ( 10.1038/nrendo.2014.210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana Det al. 2014Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158705–721. ( 10.1016/j.cell.2014.05.052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, Velasquez-Mejia EP, Carmona JA, Abad JM, Escobar JS.2017Metformin is associated with higher relative abundance of mucin-degrading akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care 4054–62. ( 10.2337/dc16-1324) [DOI] [PubMed] [Google Scholar]
- Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT.1987Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 281221–1227. ( 10.1136/gut.28.10.1221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang JT, Mocanu V, Park H, Laffin M, Tran C, Hotte N, Karmali S, Birch DW, Madsen K.2021Ileal microbial shifts after Roux-en-Y gastric bypass orchestrate changes in glucose metabolism through modulation of bile acids and L-cell adaptation. Scientific Reports 11 23813. ( 10.1038/s41598-021-03396-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vadder F, Grasset E, Manneras Holm L, Karsenty G, Macpherson AJ, Olofsson LE, Backhed F.2018Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. PNAS 1156458–6463. ( 10.1073/pnas.1720017115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debedat J, Clement K, Aron-Wisnewsky J.2019Gut microbiota dysbiosis in human obesity: impact of bariatric surgery. Current Obesity Reports 8229–242. ( 10.1007/s13679-019-00351-3) [DOI] [PubMed] [Google Scholar]
- den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM.2013The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of Lipid Research 542325–2340. ( 10.1194/jlr.R036012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NMet al. 2019Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nature Medicine 251096–1103. ( 10.1038/s41591-019-0495-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depommier C, Van Hul M, Everard A, Delzenne NM, De Vos WM, Cani PD.2020Pasteurized Akkermansia muciniphila increases whole-body energy expenditure and fecal energy excretion in diet-induced obese mice. Gut Microbes 111231–1245. ( 10.1080/19490976.2020.1737307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien M, Vaughan EE, Plugge CM, de Vos WM.2004Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. International Journal of Systematic and Evolutionary Microbiology 541469–1476. ( 10.1099/ijs.0.02873-0) [DOI] [PubMed] [Google Scholar]
- Duan Y, Llorente C, Lang S, Brandl K, Chu H, Jiang L, White RC, Clarke TH, Nguyen K, Torralba Met al. 2019Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 575505–511. ( 10.1038/s41586-019-1742-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboc H, Tache Y, Hofmann AF.2014The bile acid TGR5 membrane receptor: from basic research to clinical application. Digestive and Liver Disease 46302–312. ( 10.1016/j.dld.2013.10.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA.2005Diversity of the human intestinal microbial flora. Science 3081635–1638. ( 10.1126/science.1110591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NMet al. 2013Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. PNAS 1109066–9071. ( 10.1073/pnas.1219451110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Yet al. 2015Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nature Medicine 21159–165. ( 10.1038/nm.3760) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farin W Onate FP Plassais J Bonny C Beglinger C Woelnerhanssen B Nocca D Magoules F Le Chatelier E Pons N,. et al. 2020Impact of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy on gut microbiota: a metagenomic comparative analysis. Surgery for Obesity and Related Diseases: Official Journal of the American Society for Bariatric Surgery 16852–862. ( 10.1016/j.soard.2020.03.014) [DOI] [PubMed] [Google Scholar]
- Fujisaka S, Avila-Pacheco J, Soto M, Kostic A, Dreyfuss JM, Pan H, Ussar S, Altindis E, Li N, Bry Let al. 2018Diet, genetics, and the gut microbiome drive dynamic changes in plasma metabolites. Cell Reports 223072–3086. ( 10.1016/j.celrep.2018.02.060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaka S, Ussar S, Clish C, Devkota S, Dreyfuss JM, Sakaguchi M, Soto M, Konishi M, Softic S, Altindis Eet al. 2016Antibiotic effects on gut microbiota and metabolism are host dependent. Journal of Clinical Investigation 1264430–4443. ( 10.1172/JCI86674) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaka S, Usui I, Nawaz A, Igarashi Y, Okabe K, Furusawa Y, Watanabe S, Yamamoto S, Sasahara M, Watanabe Yet al. 2020Bofutsushosan improves gut barrier function with a bloom of Akkermansia muciniphila and improves glucose metabolism in mice with diet-induced obesity. Scientific Reports 10 5544. ( 10.1038/s41598-020-62506-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki Tet al. 2011Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469543–547. ( 10.1038/nature09646) [DOI] [PubMed] [Google Scholar]
- Fukuda N Ojima T Hayata K Katsuda M Kitadani J Takeuchi A Goda T Ueda Y Iwakura H Nishi M,. et al. 2022Laparoscopic sleeve gastrectomy for morbid obesity improves gut microbiota balance, increases colonic mucosal-associated invariant T cells and decreases circulating regulatory T cells. Surgical Endoscopy 367312–7324. ( 10.1007/s00464-022-09122-z) [DOI] [PubMed] [Google Scholar]
- Furet JP Kong LC Tap J Poitou C Basdevant A Bouillot JL Mariat D Corthier G Dore J Henegar C,. et al. 2010Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 593049–3057. ( 10.2337/db10-0253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato Tet al. 2013Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504446–450. ( 10.1038/nature12721) [DOI] [PubMed] [Google Scholar]
- Galle F Valeriani F Cattaruzza MS Gianfranceschi G Liguori R Antinozzi M Mederer B Liguori G & Romano Spica V. 2020Mediterranean diet, physical activity and gut microbiome composition: a cross-sectional study among healthy young italian adults. Nutrients 122164. ( 10.3390/nu12072164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J.2009Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 581509–1517. ( 10.2337/db08-1637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin PG Mullaney JA Loo D Cao KL Gottlieb PA Hill MM Zipris D & Hamilton-Williams EE. 2018Intestinal metaproteomics reveals host-microbiota interactions in subjects at risk for type 1 diabetes. Diabetes Care 412178–2186. ( 10.2337/dc18-0777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdhar K, Huang Q, Chow IT, Vatanen T, Brady C, Raisingani A, Autissier P, Atkinson MA, Kwok WW, Kahn CRet al. 2022A gut microbial peptide and molecular mimicry in the pathogenesis of type 1 diabetes. PNAS 119 e2120028119. ( 10.1073/pnas.2120028119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JTet al. 2014Human genetics shape the gut microbiome. Cell 159789–799. ( 10.1016/j.cell.2014.09.053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gummesson A, Carlsson LM, Storlien LH, Backhed F, Lundin P, Lofgren L, Stenlof K, Lam YY, Fagerberg B, Carlsson B.2011Intestinal permeability is associated with visceral adiposity in healthy women. Obesity 192280–2282. ( 10.1038/oby.2011.251) [DOI] [PubMed] [Google Scholar]
- Guo J, Han X, Tan H, Huang W, You Y, Zhan J.2019Blueberry extract improves obesity through regulation of the gut microbiota and bile acids via pathways involving FXR and TGR5. iScience 19676–690. ( 10.1016/j.isci.2019.08.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y Kim G Ahn E Jung S Jung Y Kim Y Ha E Heo Y Ryu DH Park H,. et al. 2022Integrated metagenomics and metabolomics analysis illustrates the systemic impact of the gut microbiota on host metabolism after bariatric surgery. Diabetes, Obesity & Metabolism 241224–1234. ( 10.1111/dom.14689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harakeh S Angelakis E Karamitros T Bachar D Bahijri S Ajabnoor G Alfadul SM Farraj SA Al Amri T Al-Hejin A,. et al. 2020Impact of smoking cessation, coffee and bread consumption on the intestinal microbial composition among Saudis: a cross-sectional study. PLoS One 15e0230895. ( 10.1371/journal.pone.0230895) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huda MN, Ahmad SM, Kalanetra KM, Taft DH, Alam MJ, Khanam A, Raqib R, Underwood MA, Mills DA, Stephensen CB.2019Neonatal vitamin A supplementation and vitamin A status are associated with gut microbiome composition in Bangladeshi infants in early infancy and at 2 years of age. Journal of Nutrition 1491075–1088. ( 10.1093/jn/nxz034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa N, Sasaki H, Lyu Y, Furuhashi S, Watabe A, Imamura M, Hayashi K, Shibata S.2021Cold exposure during the active phase affects the short-chain fatty acid production of mice in a time-specific manner. Metabolites 12 20. ( 10.3390/metabo12010020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue D, Kimura I, Wakabayashi M, Tsumoto H, Ozawa K, Hara T, Takei Y, Hirasawa A, Ishihama Y, Tsujimoto G.2012Short-chain fatty acid receptor GPR41-mediated activation of sympathetic neurons involves synapsin 2b phosphorylation. FEBS Letters 5861547–1554. ( 10.1016/j.febslet.2012.04.021) [DOI] [PubMed] [Google Scholar]
- Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, Rhee J, Hoshino A, Kim B, Ibrahim Aet al. 2016A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nature Medicine 22421–426. ( 10.1038/nm.4057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Xie C, Lv Y, Li J, Krausz KW, Shi J, Brocker CN, Desai D, Amin SG, Bisson WHet al. 2015Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nature Communications 6 10166. ( 10.1038/ncomms10166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliomaki M Collado MC Salminen S & Isolauri E. 2008Early differences in fecal microbiota composition in children may predict overweight. American Journal of Clinical Nutrition 87534–538. ( 10.1093/ajcn/87.3.534) [DOI] [PubMed] [Google Scholar]
- Kaplan RC Wang Z Usyk M Sotres-Alvarez D Daviglus ML Schneiderman N Talavera GA Gellman MD Thyagarajan B Moon JY,. et al. 2019Gut microbiome composition in the Hispanic Community Health Study/Study of Latinos is shaped by geographic relocation, environmental factors, and obesity. Genome Biology 20219. ( 10.1186/s13059-019-1831-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson FH, Tremaroli V, Nookaew I, Bergstrom G, Behre CJ, Fagerberg B, Nielsen J, Backhed F.2013Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 49899–103. ( 10.1038/nature12198) [DOI] [PubMed] [Google Scholar]
- Kasai C Sugimoto K Moritani I Tanaka J Oya Y Inoue H Tameda M Shiraki K Ito M Takei Y,. et al. 2015Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterology 15100. ( 10.1186/s12876-015-0330-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Yet al. 2003A G protein-coupled receptor responsive to bile acids. Journal of Biological Chemistry 2789435–9440. ( 10.1074/jbc.M209706200) [DOI] [PubMed] [Google Scholar]
- Kien CL.1996Digestion, absorption, and fermentation of carbohydrates in the newborn. Clinics in Perinatology 23211–228. ( 10.1016/S0095-5108(1830239-2) [DOI] [PubMed] [Google Scholar]
- Kikuchi R Irie J Yamada-Goto N Kikkawa E Seki Y Kasama K & Itoh H. 2018The impact of laparoscopic sleeve gastrectomy with duodenojejunal bypass on intestinal microbiota differs from that of laparoscopic sleeve gastrectomy in Japanese patients with obesity. Clinical Drug Investigation 38545–552. ( 10.1007/s40261-018-0638-0) [DOI] [PubMed] [Google Scholar]
- Kim S, Lee Y, Kim Y, Seo Y, Lee H, Ha J, Lee J, Choi Y, Oh H, Yoon Y.2020Akkermansia muciniphila prevents fatty liver disease, decreases serum triglycerides, and maintains gut homeostasis. Applied and Environmental Microbiology 86e03004-19. ( 10.1128/AEM.03004-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YG, Sakamoto K, Seo SU, Pickard JM, Gillilland MG, Pudlo NA, Hoostal M, Li X, Wang TD, Feehley Tet al. 2017Neonatal acquisition of Clostridia species protects against colonization by bacterial pathogens. Science 356315–319. ( 10.1126/science.aag2029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G.2011Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). PNAS 1088030–8035. ( 10.1073/pnas.1016088108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani Tet al. 2013The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nature Communications 4 1829. ( 10.1038/ncomms2852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishino S, Takeuchi M, Park SB, Hirata A, Kitamura N, Kunisawa J, Kiyono H, Iwamoto R, Isobe Y, Arita Met al. 2013Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. PNAS 11017808–17813. ( 10.1073/pnas.1312937110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knip M, Siljander H.2016The role of the intestinal microbiota in type 1 diabetes mellitus. Nature Reviews. Endocrinology 12154–167. ( 10.1038/nrendo.2015.218) [DOI] [PubMed] [Google Scholar]
- Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li Let al. 2013Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nature Medicine 19576–585. ( 10.1038/nm.3145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong LC Tap J Aron-Wisnewsky J Pelloux V Basdevant A Bouillot JL Zucker JD Dore J & Clement K. 2013Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. American Journal of Clinical Nutrition 9816–24. ( 10.3945/ajcn.113.058743) [DOI] [PubMed] [Google Scholar]
- Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F.2016From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 1651332–1345. ( 10.1016/j.cell.2016.05.041) [DOI] [PubMed] [Google Scholar]
- Koh A, Manneras-Holm L, Yunn NO, Nilsson PM, Ryu SH, Molinaro A, Perkins R, Smith JG, Backhed F.2020Microbial imidazole propionate affects responses to metformin through p38gamma-dependent inhibitory AMPK phosphorylation. Cell Metabolism 32 643–653.e4. ( 10.1016/j.cmet.2020.07.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh A, Molinaro A, Stahlman M, Khan MT, Schmidt C, Manneras-Holm L, Wu H, Carreras A, Jeong H, Olofsson LEet al. 2018Microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell 175 947–961.e17. ( 10.1016/j.cell.2018.09.055) [DOI] [PubMed] [Google Scholar]
- Konikoff T, Gophna U.2016Oscillospira: a central, enigmatic component of the human gut microbiota. Trends in Microbiology 24523–524. ( 10.1016/j.tim.2016.02.015) [DOI] [PubMed] [Google Scholar]
- Kostic AD Gevers D Siljander H Vatanen T Hyotylainen T Hamalainen AM Peet A Tillmann V Poho P Mattila I,. et al. 2015The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe 17260–273. ( 10.1016/j.chom.2015.01.0010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhre RE, Wewer Albrechtsen NJ, Larsen O, Jepsen SL, Balk-Moller E, Andersen DB, Deacon CF, Schoonjans K, Reimann F, Gribble FMet al. 2018Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas. Molecular Metabolism 1184–95. ( 10.1016/j.molmet.2018.03.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassenius MI, Pietilainen KH, Kaartinen K, Pussinen PJ, Syrjanen J, Forsblom C, Porsti I, Rissanen A, Kaprio J, Mustonen Jet al. 2011Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care 341809–1815. ( 10.2337/dc10-2197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy Set al. 2013Richness of human gut microbiome correlates with metabolic markers. Nature 500541–546. ( 10.1038/nature12506) [DOI] [PubMed] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI.2006Microbial ecology: human gut microbes associated with obesity. Nature 4441022–1023. ( 10.1038/4441022a) [DOI] [PubMed] [Google Scholar]
- Li L, Krause L, Somerset S.2017Associations between micronutrient intakes and gut microbiota in a group of adults with cystic fibrosis. Clinical Nutrition 361097–1104. ( 10.1016/j.clnu.2016.06.029) [DOI] [PubMed] [Google Scholar]
- Liu R, Hong J, Xu X, Feng Q, Zhang D, Gu Y, Shi J, Zhao S, Liu W, Wang Xet al. 2017Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nature Medicine 23859–868. ( 10.1038/nm.4358) [DOI] [PubMed] [Google Scholar]
- Loftfield E Herzig KH Caporaso JG Derkach A Wan Y Byrd DA Vogtmann E Mannikko M Karhunen V Knight R,. et al. 2020Association of body mass index with fecal microbial diversity and metabolites in the Northern Finland Birth Cohort. Cancer Epidemiolology, Biomarkers & Prevention 292289–2299. ( 10.1158/1055-9965.EPI-20-0824) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long KZ, Garcia C, Santos JI, Rosado JL, Hertzmark E, Dupont HL, Ko G.2007Vitamin A supplementation has divergent effects on Norovirus infections and clinical symptoms among Mexican children. Journal of Infectious Diseases 196978–985. ( 10.1086/521195) [DOI] [PubMed] [Google Scholar]
- Long KZ, Santos JI, Rosado JL, Estrada-Garcia T, Haas M, Al Mamun A, DuPont HL, Nanthakumar NN.2011Vitamin A supplementation modifies the association between mucosal innate and adaptive immune responses and resolution of enteric pathogen infections. American Journal of Clinical Nutrition 93578–585. ( 10.3945/ajcn.110.003913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, Dulai PS, Caussy C, Bettencourt R, Highlander SKet al. 2017Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metabolism 251054–1062.e5. ( 10.1016/j.cmet.2017.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P, Duncan SH, McCrae SI, Millar J, Jackson MS, Flint HJ.2004Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. Journal of Bacteriology 1862099–2106. ( 10.1128/JB.186.7.2099-2106.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P, Flint HJ.2017Formation of propionate and butyrate by the human colonic microbiota. Environmental Microbiology 1929–41. ( 10.1111/1462-2920.13589) [DOI] [PubMed] [Google Scholar]
- Louis P, Hold GL, Flint HJ.2014The gut microbiota, bacterial metabolites and colorectal cancer. Nature Reviews. Microbiology 12661–672. ( 10.1038/nrmicro3344) [DOI] [PubMed] [Google Scholar]
- Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako Vet al. 2018Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360eaan5931. ( 10.1126/science.aan5931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusdottir S, Ravcheev D, de Crecy-Lagard V, Thiele I.2015Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Frontiers in Genetics 6 148. ( 10.3389/fgene.2015.00148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magzal F, Shochat T, Haimov I, Tamir S, Asraf K, Tuchner-Arieli M, Even C, Agmon M.2022Increased physical activity improves gut microbiota composition and reduces short-chain fatty acid concentrations in older adults with insomnia. Scientific Reports 12 2265. ( 10.1038/s41598-022-05099-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai V McCrary QM Sinha R & Glei M. 2009Associations between dietary habits and body mass index with gut microbiota composition and fecal water genotoxicity: an observational study in African American and Caucasian American volunteers. Nutrition Journal 849. ( 10.1186/1475-2891-8-49) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis Det al. 2009Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 4611282–1286. ( 10.1038/nature08530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T, Li F, Gonzalez FJ.2013FXR signaling in the enterohepatic system. Molecular and Cellular Endocrinology 36817–29. ( 10.1016/j.mce.2012.05.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlin BK, Henning AL, Bowman EM, Gary MA, Carbajal KM.2017Oral spore-based probiotic supplementation was associated with reduced incidence of post-prandial dietary endotoxin, triglycerides, and disease risk biomarkers. World Journal of Gastrointestinal Pathophysiology 8117–126. ( 10.4291/wjgp.v8.i3.117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNelis JC, Lee YS, Mayoral R, van der Kant R, Johnson AM, Wollam J, Olefsky JM.2015GPR43 potentiates beta-cell function in obesity. Diabetes 643203–3217. ( 10.2337/db14-1938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa M, Iijima R, Kobayashi R, Tajima M, Isomura N, Shimizu T, Kobayashi I, Asano M.1954Report on success of long duration rearing of Germfree Guinea Pigs. Trans Society of Pathology Japan 43450–452. [Google Scholar]
- Miyamoto J, Igarashi M, Watanabe K, Karaki SI, Mukouyama H, Kishino S, Li X, Ichimura A, Irie J, Sugimoto Yet al. 2019Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nature Communications 10 4007. ( 10.1038/s41467-019-11978-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinaro A, Bel Lassen P, Henricsson M, Wu H, Adriouch S, Belda E, Chakaroun R, Nielsen T, Bergh PO, Rouault Cet al. 2020Imidazole propionate is increased in diabetes and associated with dietary patterns and altered microbial ecology. Nature Communications 11 5881. ( 10.1038/s41467-020-19589-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison DJ, Preston T.2016Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7189–200. ( 10.1080/19490976.2015.1134082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison MC, Gart E, Duyvenvoorde WV, Snabel J, Nielsen MJ, Leeming DJ, Menke A, Kleemann R.2022Heat-inactivated Akkermansia muciniphila improves gut permeability but does not prevent development of non-alcoholic steatohepatitis in diet-induced obese Ldlr-/-.Leiden mice. International Journal of Molecular Sciences 232325. ( 10.3390/ijms23042325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A.2012Colonic microbiome is altered in alcoholism. American Journal of Physiology. Gastrointestinal and Liver Physiology 302G966–G978. ( 10.1152/ajpgi.00380.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan N, Pluznick JL.2014From microbe to man: the role of microbial short chain fatty acid metabolites in host cell biology. American Journal of Physiology. Cell Physiology 307C979–C985. ( 10.1152/ajpcell.00228.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CAet al. 2009A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metabolism 9311–326. ( 10.1016/j.cmet.2009.02.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttal GHF & Thierfelder H. 1895Thierisches leben ohne bakterien im verdauungskanal. Zeitschrift für Physiologische Chemie 21109.( 10.1515/bchm2.1897.23.3.231) [DOI] [Google Scholar]
- Obata Y, Castano Á, Boeing S, Bon-Frauches AC, Fung C, Fallesen T, de Aguero MG, Yilmaz B, Lopes R, Huseynova Aet al. 2020Neuronal programming by microbiota regulates intestinal physiology. Nature 578284–289. ( 10.1038/s41586-020-1975-8) [DOI] [PubMed] [Google Scholar]
- Oduaran OH Tamburini FB Sahibdeen V Brewster R Gomez-Olive FX Kahn K Norris SA Tollman SM Twine R Wade AN,. et al. 2020Gut microbiome profiling of a rural and urban South African cohort reveals biomarkers of a population in lifestyle transition. BMC Microbiology 20330. ( 10.1186/s12866-020-02017-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Org E Blum Y Kasela S Mehrabian M Kuusisto J Kangas AJ Soininen P Wang Z Ala-Korpela M Hazen SL,. et al. 2017Relationships between gut microbiota, plasma metabolites, and metabolic syndrome traits in the METSIM cohort. Genome Biology 1870. ( 10.1186/s13059-017-1194-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne G Wu F Yang L Kelly D Hu J Li H Jasmine F Kibriya MG Parvez F Shaheen I,. et al. 2020The association between gut microbiome and anthropometric measurements in Bangladesh. Gut Microbes 1163–76. ( 10.1080/19490976.2019.1614394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato N Saito S Yamaguchi T Katashima M Tokuda I Sawada K Katsuragi Y Kakuta M Imoto S Ihara K,. et al. 2019Blautia genus associated with visceral fat accumulation in adults 20-76 years of age. NPJ Biofilms and Microbiomes 528. ( 10.1038/s41522-019-0101-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato N Yamaguchi T Mori K Katashima M Kumagai M Murashita K Katsuragi Y Tamada Y Kakuta M Imoto S,. et al. 2022Two blautia species associated with visceral fat accumulation: a one-year longitudinal study. Biology (Basel) 11318. ( 10.3390/biology11020318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS.2004Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306457–461. ( 10.1126/science.1103160) [DOI] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS.2006Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 3131137–1140. ( 10.1126/science.1128294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik D, Yao L, Zhang Y, Bae S, D'Agostino GD, Zhang M, Kim E, Franzosa EA, Avila-Pacheco J, Bisanz JEet al. 2022Human gut bacteria produce TauEta17-modulating bile acid metabolites. Nature 603907–912. ( 10.1038/s41586-022-04480-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasteur L.1885Observations relatives a la note precooente de M. Duclaux. Comptes Rendus de l’Académie des Sciences, Paris 100 68. [Google Scholar]
- Patil DP Dhotre DP Chavan SG Sultan A Jain DS Lanjekar VB Gangawani J Shah PS Todkar JS Shah S,. et al. 2012Molecular analysis of gut microbiota in obesity among Indian individuals. Journal of Biosciences 37647–657. ( 10.1007/s12038-012-9244-0) [DOI] [PubMed] [Google Scholar]
- Payne AN Chassard C Zimmermann M Muller P Stinca S & Lacroix C. 2011The metabolic activity of gut microbiota in obese children is increased compared with normal-weight children and exhibits more exhaustive substrate utilization. Nutrition & Diabetes 1e12. ( 10.1038/nutd.2011.8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F, Prifti E, Falony Get al. 2016Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535376–381. ( 10.1038/nature18646) [DOI] [PubMed] [Google Scholar]
- Peters BA Shapiro JA Church TR Miller G Trinh-Shevrin C Yuen E Friedlander C Hayes RB & Ahn J. 2018A taxonomic signature of obesity in a large study of American adults. Scientific Reports 89749. ( 10.1038/s41598-018-28126-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham VT, Dold S, Rehman A, Bird JK, Steinert RE.2021Vitamins, the gut microbiome and gastrointestinal health in humans. Nutrition Research 9535–53. ( 10.1016/j.nutres.2021.09.001) [DOI] [PubMed] [Google Scholar]
- Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein Let al. 2017A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nature Medicine 23107–113. ( 10.1038/nm.4236) [DOI] [PubMed] [Google Scholar]
- Prawitt J, Abdelkarim M, Stroeve JH, Popescu I, Duez H, Velagapudi VR, Dumont J, Bouchaert E, van Dijk TH, Lucas Aet al. 2011Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes 601861–1871. ( 10.2337/db11-0030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, Ghatei MA, Bloom SR, Frost G.2015The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. International Journal of Obesity 39424–429. ( 10.1038/ijo.2014.153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen Det al. 2012A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 49055–60. ( 10.1038/nature11450) [DOI] [PubMed] [Google Scholar]
- Radilla-Vazquez RB Parra-Rojas I Martinez-Hernandez NE Marquez-Sandoval YF Illades-Aguiar B & Castro-Alarcon N. 2016Gut microbiota and metabolic endotoxemia in young obese mexican subjects. Obesity Facts 91–11. ( 10.1159/000442479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raqib R, Sarker P, Bergman P, Ara G, Lindh M, Sack DA, Nasirul Islam KM, Gudmundsson GH, Andersson J, Agerberth B.2006Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. PNAS 1039178–9183. ( 10.1073/pnas.0602888103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnier M, Van Hul M, Knauf C, Cani PD.2021Gut microbiome, endocrine control of gut barrier function and metabolic diseases. Journal of Endocrinology 248R67–R82. ( 10.1530/JOE-20-0473) [DOI] [PubMed] [Google Scholar]
- Reyniers JA, Trexler PC, Ervin RF.1946Rearing germ-free albino rats. Lobund Reports 11–84. [PubMed] [Google Scholar]
- Reyniers JA, Trexler PC, Ervin RF, Wagner M, Luckey TD, Gordon HA. Rearing germfree chickens. Lobund Report. 1949;2:1-–116.. [Google Scholar]
- Richards JL, Yap YA, McLeod KH, Mackay CR, Marino E.2016Dietary metabolites and the gut microbiota: an alternative approach to control inflammatory and autoimmune diseases. Clinical and Translational Immunology 5 e82. ( 10.1038/cti.2016.29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JRet al. 2013Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341 1241214. ( 10.1126/science.1241214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Chavez F, Zhang LF, Faber F, Lopez CA, Byndloss MX, Olsan EE, Xu G, Velazquez EM, Lebrilla CB, Winter SEet al. 2016Depletion of butyrate-producing Clostridia from the gut microbiota drives an aerobic luminal expansion of Salmonella. Cell Host and Microbe 19443–454. ( 10.1016/j.chom.2016.03.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Perez HE, Sandoval DA, Kohli R, Backhed Fet al. 2014FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 509183–188. ( 10.1038/nature13135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salah M Azab M Ramadan A & Hanora A. 2019New insights on obesity and aiabetes from gut microbiome alterations in Egyptian adults. OMICS 23477–485. ( 10.1089/omi.2019.0063) [DOI] [PubMed] [Google Scholar]
- Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa Met al. 2008Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. PNAS 10516767–16772. ( 10.1073/pnas.0808567105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Alcoholado L Gutierrez-Repiso C Gomez-Perez AM Garcia-Fuentes E Tinahones FJ & Moreno-Indias I. 2019Gut microbiota adaptation after weight loss by Roux-en-Y gastric bypass or sleeve gastrectomy bariatric surgeries. Surgery for Obesity and Related Diseases: Official Journal of the American Society for Bariatric Surgery 151888-1895.( 10.1016/j.soard.2019.08.551) [DOI] [PubMed] [Google Scholar]
- Sato Y, Atarashi K, Plichta DR, Arai Y, Sasajima S, Kearney SM, Suda W, Takeshita K, Sasaki T, Okamoto Set al. 2021Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature 599458–464. ( 10.1038/s41586-021-03832-5) [DOI] [PubMed] [Google Scholar]
- Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, Angelin B, Hyotylainen T, Oresic M, Backhed F.2013Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metabolism 17225–235. ( 10.1016/j.cmet.2013.01.003) [DOI] [PubMed] [Google Scholar]
- Scheithauer TPM, Davids M, Winkelmeijer M, Verdoes X, Aydin Ö, de Brauw M, van de Laar A, Meijnikman AS, Gerdes VEA, van Raalte Det al. 2022Compensatory intestinal antibody response against pro-inflammatory microbiota after bariatric surgery. Gut Microbes 14 2031696. ( 10.1080/19490976.2022.2031696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, Trimarco B, Esposito G, Perrino C.2017Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. European Heart Journal 382948–2956. ( 10.1093/eurheartj/ehx342) [DOI] [PubMed] [Google Scholar]
- Schwartz BS, Pollak J, Bailey-Davis L, Hirsch AG, Cosgrove SE, Nau C, Kress AM, Glass TA, Bandeen-Roche K.2016Antibiotic use and childhood body mass index trajectory. International Journal of Obesity 40615–621. ( 10.1038/ijo.2015.218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiertz A, Taras D, Schafer K, Beijer S, Bos NA, Donus C, Hardt PD.2010Microbiota and SCFA in lean and overweight healthy subjects. Obesity 18190–195. ( 10.1038/oby.2009.167) [DOI] [PubMed] [Google Scholar]
- Shale M, Schiering C, Powrie F.2013CD4(+) T-cell subsets in intestinal inflammation. Immunological Reviews 252164–182. ( 10.1111/imr.12039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata N, Kunisawa J, Kiyono H.2017Dietary and microbial metabolites in the regulation of host immunity. Frontiers in Microbiology 8 2171. ( 10.3389/fmicb.2017.02171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo S Godneva A Rachmiel M Korem T Bussi Y Kolobkov D Karady T Bar N Wolf BC Glantz-Gashai Y,. et al. 2022The gut microbiome of Adults with type 1 diabetes and its association with the host glycemic control. Diabetes Care 45555–563. ( 10.2337/dc21-1656) [DOI] [PubMed] [Google Scholar]
- Shimokawa C Kato T Takeuchi T Ohshima N Furuki T Ohtsu Y Suzue K Imai T Obi S Olia A,. et al. 2020CD8(+) regulatory T cells are critical in prevention of autoimmune-mediated diabetes. Nature Communications 111922. ( 10.1038/s41467-020-15857-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, Bae JW.2014An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 63727–735. ( 10.1136/gutjnl-2012-303839) [DOI] [PubMed] [Google Scholar]
- Simoes CD Maukonen J Kaprio J Rissanen A Pietilainen KH & Saarela M. 2013Habitual dietary intake is associated with stool microbiota composition in monozygotic twins. Journal of Nutrition 143417–423. ( 10.3945/jn.112.166322) [DOI] [PubMed] [Google Scholar]
- Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ.2000Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102731–744. ( 10.1016/s0092-8674(0000062-3) [DOI] [PubMed] [Google Scholar]
- Singh DP, Singh J, Boparai RK, Zhu J, Mantri S, Khare P, Khardori R, Kondepudi KK, Chopra K, Bishnoi M.2017Isomalto-oligosaccharides, a prebiotic, functionally augment green tea effects against high fat diet-induced metabolic alterations via preventing gut dysbacteriosis in mice. Pharmacological Research 123103–113. ( 10.1016/j.phrs.2017.06.015) [DOI] [PubMed] [Google Scholar]
- Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DHet al. 2014Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40128–139. ( 10.1016/j.immuni.2013.12.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly M, Glickman JN, Garrett WS.2013The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341569–573. ( 10.1126/science.1241165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Chu Q, Yan F, Yang Y, Han W, Zheng X.2016Red pitaya betacyanins protects from diet-induced obesity, liver steatosis and insulin resistance in association with modulation of gut microbiota in mice. Journal of Gastroenterology and Hepatology 311462–1469. ( 10.1111/jgh.13278) [DOI] [PubMed] [Google Scholar]
- Stefura T Zapala B Gosiewski T Skomarovska O Pedziwiatr M & Major P. 2022Changes in the composition of oral and intestinal microbiota after sleeve gastrectomy and Roux-En-Y gastric bypass and their impact on outcomes of bariatric surgery. Obesity Surgery 321439–1450. ( 10.1007/s11695-022-05954-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert RE, Lee YK, Sybesma W.2020Vitamins for the gut microbiome. Trends in Molecular Medicine 26137–140. ( 10.1016/j.molmed.2019.11.005) [DOI] [PubMed] [Google Scholar]
- Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL.2013Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. New England Journal of Medicine 3681575–1584. ( 10.1056/NEJMoa1109400) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thingholm LB Ruhlemann MC Koch M Fuqua B Laucke G Boehm R Bang C Franzosa EA Hubenthal M Rahnavard A,. et al. 2019Obese individuals with and without type 2 diabetes show different gut microbial functional capacity and composition. Cell Host & Microbe 26 252–264 e210. ( 10.1016/j.chom.2019.07.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn AN, McKenzie CI, Shen S, Stanley D, Macia L, Mason LJ, Roberts LK, Wong CH, Shim R, Robert Ret al. 2015Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nature Communications 6 7320. ( 10.1038/ncomms8320) [DOI] [PubMed] [Google Scholar]
- Tims S, Derom C, Jonkers DM, Vlietinck R, Saris WH, Kleerebezem M, de Vos WM, Zoetendal EG.2013Microbiota conservation and BMI signatures in adult monozygotic twins. ISME Journal 7707–717. ( 10.1038/ismej.2012.146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM.2012Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61364–371. ( 10.2337/db11-1019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabelsi MS, Daoudi M, Prawitt J, Ducastel S, Touche V, Sayin SI, Perino A, Brighton CA, Sebti Y, Kluza Jet al. 2015Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nature Communications 6 7629. ( 10.1038/ncomms8629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI.2006An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 4441027–1031. ( 10.1038/nature05414) [DOI] [PubMed] [Google Scholar]
- Ueno A, Jeffery L, Kobayashi T, Hibi T, Ghosh S, Jijon H.2018Th17 plasticity and its relevance to inflammatory bowel disease. Journal of Autoimmunity 8738–49. ( 10.1016/j.jaut.2017.12.004) [DOI] [PubMed] [Google Scholar]
- Vael C Verhulst SL Nelen V Goossens H & Desager KN. 2011Intestinal microflora and body mass index during the first three years of life: an observational study. Gut Pathogens 38. ( 10.1186/1757-4749-3-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT.2010Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 32822, 8–231. ( 10.1126/science.1179721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom A, Sayin SI, Marschall HU, Backhed F.2016Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metabolism 2441–50. ( 10.1016/j.cmet.2016.05.005) [DOI] [PubMed] [Google Scholar]
- Wang J, Thingholm LB, Skieceviciene J, Rausch P, Kummen M, Hov JR, Degenhardt F, Heinsen FA, Ruhlemann MC, Szymczak Set al. 2016Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nature Genetics 481396–1406. ( 10.1038/ng.3695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez Cet al. 2011Metabolite profiles and the risk of developing diabetes. Nature Medicine 17448–453. ( 10.1038/nm.2307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MKet al. 2015Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 1631585–1595. ( 10.1016/j.cell.2015.11.055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama Tet al. 2006Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439484–489. ( 10.1038/nature04330) [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Fujisaka S, Ikeda K, Ishikawa M, Yamada T, Nawaz A, Kado T, Kuwano T, Nishimura A, Bilal Met al. 2021Gut microbiota, determined by dietary nutrients, drive modification of the plasma lipid profile and insulin resistance. iScience 24 102445. ( 10.1016/j.isci.2021.102445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JAet al. 2008Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 4551109–1113. ( 10.1038/nature07336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensel CR, Pluznick JL, Salzberg SL, Sears CL.2022Next-generation sequencing: insights to advance clinical investigations of the microbiome. Journal of Clinical Investigation 132. ( 10.1172/JCI154944) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight Ret al. 2011Linking long-term dietary patterns with gut microbial enterotypes. Science 334105–108. ( 10.1126/science.1208344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Tremaroli V, Schmidt C, Lundqvist A, Olsson LM, Kramer M, Gummesson A, Perkins R, Bergstrom G, Backhed F.2020The gut microbiota in prediabetes and diabetes: a population-based cross-sectional study. Cell Metabolism 32 379–390.e3 e373. ( 10.1016/j.cmet.2020.06.011) [DOI] [PubMed] [Google Scholar]
- Wu S, Yoon S, Zhang YG, Lu R, Xia Y, Wan J, Petrof EO, Claud EC, Chen D, Sun J.2015Vitamin D receptor pathway is required for probiotic protection in colitis. American Journal of Physiology. Gastrointestinal and Liver Physiology 309G341–G349. ( 10.1152/ajpgi.00105.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan AW, Fouts DE, Brandl J, Starkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B.2011Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 5396–105. ( 10.1002/hep.24018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Li Y, Wen Z, Liu W, Meng L, Huang H.2021Oscillospira - a candidate for the next-generation probiotics. Gut Microbes 13 1987783. ( 10.1080/19490976.2021.1987783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY.2015Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161264–276. ( 10.1016/j.cell.2015.02.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori Met al. 2013Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 49997–101. ( 10.1038/nature12347) [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM, Edwards PA.2006Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. PNAS 1031006–1011. ( 10.1073/pnas.0506982103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo HJ Xie ZM Zhang WW Li YR Wang W Ding XB & Pei XF. 2011Gut bacteria alteration in obese people and its relationship with gene polymorphism. World Journal of Gastroenterology 171076–1081. ( 10.3748/wjg.v17.i8.1076) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a