Abstract
High levels of Treponema denticola in subgingival dental plaque are associated with severe periodontal disease. T. denticola, along with Porphyromonas gingivalis and Bacteroides forsythus, are the only cultivatable oral microorganisms that produce significant amounts of “trypsin-like” peptidase activity. The ability of subgingival plaque to hydrolyze N-α-benzoyl-dl-arginine-2-naphthylamide (BANA) is associated with high levels of one or more of these organisms. The purpose of this study was to identify the gene encoding trypsin-like activity in T. denticola and thus facilitate molecular-level studies of its potential role in disease. Using published peptide sequences of a T. denticola surface-associated oligopeptidase with BANA-hydrolyzing activity, we identified the gene, designated opdB, in an apparently noncoding region of the T. denticola genome unannotated contigs (11/2000; http://www.tigr.org). The opdB gene begins with a TTG start codon and encodes a 685-residue peptide with high homology to the oligopeptidase B family in prokaryotes and eukaryotes. An isogenic T. denticola opdB mutant was constructed by allelic replacement mutagenesis using an ermF/AM gene cassette. The mutant lacked BANA-hydrolyzing activity and had a slightly slower growth rate than the parent strain. This mutant will be used in future studies of interactions of T. denticola with host cells and tissue.
The presence in subgingival plaque of elevated levels of a specific complex of three anaerobic organisms is associated with periodontal disease severity (35). These bacteria (Treponema denticola, Porphyromonas gingivalis, and Bacteroides forsythus) possess enzymes that hydrolyze chromogenic substrates for trypsin-like peptidase activity (18). The presence of high proportions of one or more of these organisms in subgingival plaque can be determined by the ability of the plaque to hydrolyze N-α-benzoyl-dl-arginine-2-naphthylamide (BANA) and N-α-benzoyl-l-arginine-p-nitroanilide (BApNA). The relationship between high levels of these organisms in plaque, elevated levels of this bacterial enzyme in plaque, and active periodontal disease (4, 5) suggested that BANA-hydrolyzing activity could be a useful diagnostic indicator (19). While the association of disease severity with these specific organisms and with high levels of BANA-hydrolyzing activity is well established, no studies have demonstrated a direct function for this enzyme activity of T. denticola in periodontal pathogenesis.
BANA-hydrolyzing enzymes have been identified in T. denticola and P. gingivalis. A BANA-hydrolyzing peptidase of T. denticola has been isolated, and its biochemical activity has been characterized (21, 23, 25). The activity profile of the outer membrane-associated enzyme showed it to be an oligopeptidase capable of hydrolyzing ester, amide, and peptide bonds involving the carboxyl group arginine and lysine and, combined with partial peptide sequences (22), suggested that it belongs to the superfamily of prolyl oligopeptidases that includes Escherichia coli oligopeptidase B (EC 3.4.21.83). Various forms of the arginine-specific (Rgp) and lysine-specific (Kgp) proteases of P. gingivalis account for most of its trypsin-like activity in vitro (30). While an enzyme that hydrolyzes one arginine-nitroanilide substrate has been identified in B. forsythus, the PrtH enzyme did not hydrolyze BApNA (31), and no BANA- or BApNA-hydrolyzing enzymes have been characterized. There is little information available on the cellular localization of the PrtH enzyme or on its contribution to the overall proteolytic or peptidolytic activity of the organism.
The purpose of this study was to identify the gene encoding this trypsin-like or BANA-hydrolyzing peptidase in T. denticola and thus facilitate molecular-level studies of this enzyme and its potential role in disease. It will be particularly interesting to determine whether this T. denticola enzyme activity, conserved among periodontal pathogens, has effects that complement or enhance those of the chymotrypsin-like protease (CTLP) of this organism (7, 8, 13, 38). Recent advances in molecular-biology techniques have facilitated genetic and molecular analysis of oral spirochetes. The T. denticola genome project under way at The Institute of Genome Research will be a major contribution to the knowledge base. Using these resources and peptide sequences derived from a surface-associated BANA-hydrolyzing oligopeptidase of T. denticola (22, 23), we demonstrate here that a single conserved gene encodes T. denticola trypsin-like enzyme activity.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
T. denticola ATCC 35405 was grown and maintained in NOS broth medium as previously described (14). For allelic replacement, mutants were selected on NOS/GN plates (6) containing erythromycin (EM; 40 μg ml−1) as described previously (10, 17). Cultures were examined by phase-contrast microscopy for purity and typical strain morphology before use. For studies of growth kinetics, 3-day cultures were adjusted to an absorbance at 600 nm (A600) of 0.35 in NOS broth. Tubes containing 10 ml of NOS broth were inoculated with 0.5 ml of cells and incubated under anaerobic conditions. At each time point, three tubes per strain were removed from the anaerobic chamber for A600 readings.
E. coli JM109 (39) was used as a host strain for routine subcloning and plasmid preparations. E. coli TOP10F′ (Invitrogen) was used as a host strain for direct TA cloning of PCR fragments in plasmid vector pCR3.1 (Invitrogen). Plasmid vector pSY103, a derivative of pTZ19R (39) in which the 17-bp SacI-AccI fragment was deleted, was used for further subcloning. E. coli strains were grown in Luria-Bertani broth or agar medium supplemented with ampicillin (50 μg ml−1), kanamycin (50 μg ml−1), and EM (200 μg ml−1) as appropriate.
Chemicals.
Unless otherwise noted, chemicals were purchased at the highest available purity from Sigma Chemical Co. (St. Louis, Mo.) or Fisher Scientific (Chicago, Ill.).
Protein electrophoresis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was done as described previously (9). Cells were harvested by centrifugation at 10,000 × g (10 min, 4°C) and resuspended in phosphate-buffered saline at an A600 of 0.25. One-milliliter samples were then centrifuged briefly at 10,000 × g, and the pellets were resuspended in 50 μl of sample buffer containing dithiothreitol. Samples were heated at 70°C for 10 min prior to electrophoresis. Samples were separated in a 10% BIS-Tris gel in MES (morpholineethanesulfonic acid) running buffer (Novex). The gel was stained with Coomassie blue R-250. Gelatin zymography was performed using a precast polyacrylamide gel containing gelatin (Novex) in accordance with the manufacturer's instructions. For gelatin zymography, cells were grown to an A600 of 0.25, harvested by centrifugation, suspended in 1/10 volume of sample buffer, and incubated for 10 min at room temperature before electrophoresis.
Recombinant DNA methods.
Unless stated otherwise, standard methods as described by Ausubel et al. (2) or Sambrook et al. (32) were followed. DNA fragments were eluted from agarose gels using the Gene Clean kit (Bio101, La Jolla, Calif.). Genomic DNAs and plasmid DNAs were isolated using the Wizard genomic DNA purification kit and Wizard Plus SV minipreps kit (Promega, Madison, Wis.), respectively. For Southern blot analysis, HindIII-digested genomic DNAs separated on 0.7% agarose gel were transferred to nylon membranes (Immobilon-Ny; Millipore) and hybridized with biotin-labeled DNA probes, followed by incubation of the blots with streptavidin, biotinylated alkaline phosphatase, and chemiluminescence detection reagent (New England Biolabs) using the manufacturers' instructions. Chemiluminescence was detected using a Fluor-S Multi-Imager (Bio-Rad).
DNA sequence analysis.
Templates for DNA sequencing included plasmid DNA and PCR products. Sequencing reactions performed using ABI PRISM BigDye terminator cycle sequencing kits with fluorescence-labeled dideoxynucleoside triphosphates (Applied Biosystems Inc., Foster City, Calif.) and sequence-derived primers, according to the manufacturer's instructions. DNA sequences were resolved using an Applied Biosystems model 310 automated DNA sequencer. Both strands of the DNA sequence reported here were sequenced in their entirety. Analysis of DNA sequence data was performed using SeqEd 1.0 (Applied Biosystems Inc.), DNA Strider (Service de Biochimie, Department de Biologie, Institut de Recherche Fondamentale, Commissariat a l'Energie Atomique, France), and MacVector 7.0 (Genetics Computer Group). The nonredundant SWISS-PROT, PIR, EMBL, and GenBank databases were searched for homologous peptide and nucleotide sequences using the BLAST (1) network service at the National Center for Biotechnology Information, National Institutes of Health.
Allelic replacement mutagenesis.
An isogenic defined opdB mutant was constructed using the method of Li et al. (17) by electroporation of T. denticola with the selectable ermF/AM gene cassette (11) cloned between fragments of the target sequence, as described previously (10).
Enzymatic activity assays.
Enzymatic activities of T. denticola parent and mutant strains were tested in several ways. Hydrolysis of chromogenic substrates BApNA and succinyl-l-alanyl-l-alanyl-l-prolyl-l-phenylalanine-p-nitroanilide (SAApNA) were assayed as described by Uitto et al. (37). Four-day cultures were adjusted to an A600 of 0.25 in NOS broth and then diluted 1:8 in deionized water for assays. NOS broth diluted 1:8 in deionized water served as a negative control. Activities of a battery of enzymes were assayed using the API-ZYM test strip (bioMerieux, Inc., Hazelwood, Mo.). For this assay, cells were grown to a MacFarland standard turbidity of 5 and processed according to the manufacturer's instructions. Gelatinolytic activity was tested by zymography in a gelatin-containing polyacrylamide gel (NOVEX) using the manufacturer's instructions. Zymogram gels were stained with Coomassie blue R-250.
Nucleotide sequence accession number.
The nucleotide sequence of T. denticola opdB has been assigned GenBank accession no. AF355459.
RESULTS
Identification of a gene encoding BANA-hydrolyzing activity.
Previous studies characterized a peptidase enzyme from T. denticola that hydrolyzes -X-Arg-p-nitroaniline peptides between arginine and the chromogen (21, 23, 25). Using N-terminal and internal amino acid sequences determined from the native peptidase (22), we searched six-frame translation products of the preliminary unannotated contigs of the T. denticola genome (http://www.tigr.org) for the gene encoding these peptide sequences. The peptide sequences aligned with translations from three reading frames within 3 kb of each other in the contigs. Using the preliminary genome sequences, oligonucleotide primers CX213 (5′ CTT AGA AGC TGC CTC ATA C 3′) and CX216 (5′ TTT CGA TAG TTC ACG CCG 3′) were designed to amplify a 4.6-kb PCR product from T. denticola DNA containing the putative peptidase gene. The PCR product was cloned into TA cloning vector pCR3.1, yielding pSY102. The entire DNA sequence of both strands of the putative peptidase gene in pSY102 was determined using sequence-derived primers. Sequence analysis showed that an apparent open reading frame that could encode a protein of the expected size (685 residues) (23) was present, though it was not evident in the preliminary sequence releases. As shown in Fig. 1, the peptide sequences derived from the native protein were contained in the deduced amino acid sequence of the peptidase. The predicted coding region begins with a TTG start codon, a relatively rare feature in prokaryotic genes. While the previously published amino acid sequence identified the N-terminal residue as methionine, DNA sequencing from independently isolated template DNAs confirmed the TTG codon at this position.
FIG. 1.
T. denticola opdB DNA sequence, with deduced amino acid sequence of the OpdB peptide beneath the nucleotide sequence. The predicted ribosome binding site upstream of the TTG start codon is underlined, as are locations of the amino acid sequences determined from the native peptidase (22). These sequences were used to localize the opdB coding region in the preliminary T. denticola genome sequence. The conserved domain common to members of the prolyl oligopeptidase family is shaded. Dashes, Gly-X-Ser-X-Gly consensus sequence of serine proteases (residues 535 to 539); asterisks, putative catalytic triad residues S-537, D-622, and H-657. Numbering of amino acids (in parentheses) and nucleotides is shown to the right of each line.
As suggested by the peptide sequence data used to identify the gene, the deduced OpdB peptide showed significant homology to E. coli oligopeptidase B and other members of a highly conserved family of prolyl oligopeptidases found in numerous prokaryotes and eukaryotes, including many pathogenic gram-negative bacteria, pathogenic protozoa, humans, and plants. Prokaryote members of this group average approximately 80 kDa in size, and the catalytic domain is contained in the C-terminal region (29). As shown in Fig. 1, OpdB contains the conserved peptidase domain of this group (residues 463 to 542), with an E value of 1.3 × 10−33 using the Pfam, 3.1, protein domain family database multiple alignment tool (3) on the BLAST network server. T. denticola OpdB has 42% peptide identity and 61% similarity with the 686-residue E. coli homologue oligopeptidase B. BLAST searches showed similarly significant homology (E values less than 1 × 10−100) between T. denticola OpdB and peptidases of numerous human pathogens, including Salmonella enterica, Pseudomonas spp., Mycobacterium spp., Rickettsia prowazekii, Trypanosoma spp., and Leishmania major.
Construction of an opdB mutant by allelic replacement.
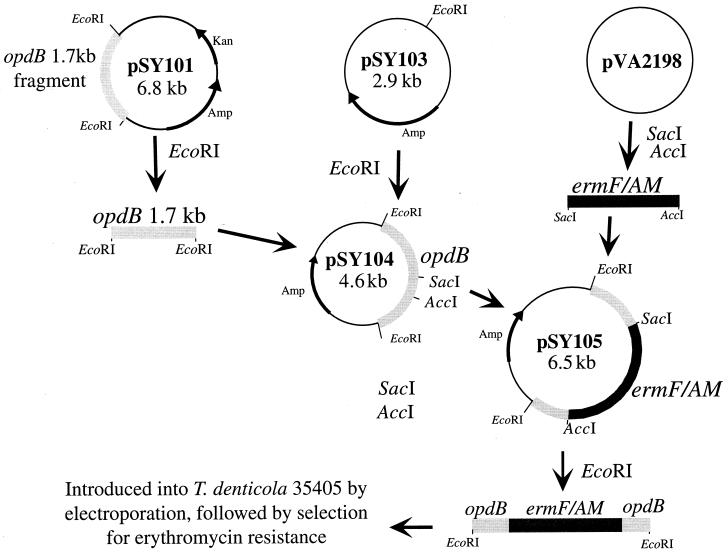
To determine the contribution of OpdB to T. denticola trypsin-like activity and overall behavior, we constructed a defined mutation in the opdB locus using an ermF/AM cassette. A 1.7-kb internal fragment of opdB was amplified by PCR using oligonucleotide primers CX203 (5′ TTG AAA AGC CGC CTA TTG CG 3′) and CX204 (5′ GGG AGA ATA TGA AAG CAT GT 3′). The PCR product was cloned in the TA cloning site of pCR3.1 as described above. As shown in Fig. 2, the fragment was then excised with EcoRI and inserted in the EcoRI site of pSY103, which lacks recognition sites for SacI and AccI. The resulting plasmid, pSY104, was digested with SacI and AccI, which recognize unique sites in the opdB locus at bp 1193 and 1426, respectively. The ermF/AM cassette was excised from pVA2198 (11) with SacI and AccI and ligated to the 4.4-kb SacI-AccI fragment of pSY104. The resulting plasmid, pSY105, was digested with EcoRI, and the 3.6-kb opdB-ermF/AM fragment was gel purified and used to electroporate T. denticola, as described previously (10). After 3 weeks of incubation under anaerobic conditions, a total of 30 EM-resistant colonies were obtained from three electroporations.
FIG. 2.
Strategy for mutagenesis of opdB. The 1.7-kb opdB fragment carried on pSY101 was inserted in the EcoRI site of pSY103. The resulting plasmid, pSY104, digested with SacI and AccI, was ligated with the SacI-AccI ermF/AM cassette excised from pVA2198 (11). The resulting plasmid, pSY105, was digested with EcoRI, and the 3.6-kb opdB-ermF/AM fragment was used to electroporate T. denticola, as described previously (10). EM-resistant transformants were selected and screened for the expected allelic replacement event.
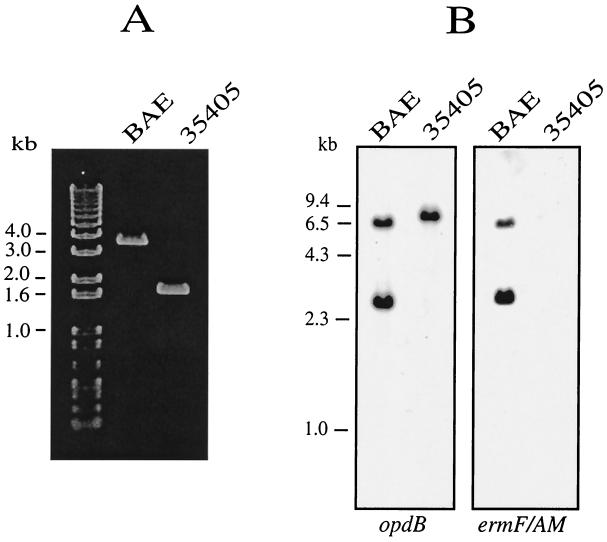
To confirm the construction of the mutant strain, genomic DNA isolated from the parent strain and six EM-resistant transformants was subjected to PCR analysis with combinations of primers flanking the intended target sites in opdB and within ermF. Three isolates, one of which was designated BAE, had the 2.1-kb ermF/AM cassette in the disrupted target gene and had identical enzymatic activities (data not shown). Figure 3A shows the results of PCR using CX203 and CX204, whose targets are separated by approximately 1.7 kb in strain ATCC 35405. As expected the PCR product in mutant strain BAE was 3.6 kb. Similar results were obtained using CX213 and CX216, which recognize sites 5′ and 3′ to opdB (data not shown). To further confirm these results, Southern blot analysis of HindIII-digested genomic DNA was performed using as probes the CX203-CX204 PCR product and the 2.1-kb ermF/AM cassette (Fig. 3B). These results demonstrate that strain BAE has acquired a single copy of the ermF/AM cassette in the opdB locus.
FIG. 3.
Confirmation of opdB mutant construction. (A) PCR amplification of opdB in T. denticola ATCC 35405 and isogenic opdB mutant BAE. Genomic DNAs were subjected to PCR using primers CX203 and CX204. (B) Southern blot analysis of T. denticola ATCC 35405 and isogenic opdB mutant BAE. Genomic DNAs were digested with HindIII and hybridized with a biotinylated internal fragment of opdB or the SacI-AccI fragment of ermF/AM. The ermF/AM cassette contains a single HindIII site.
Growth pattern of opdB mutant strain.
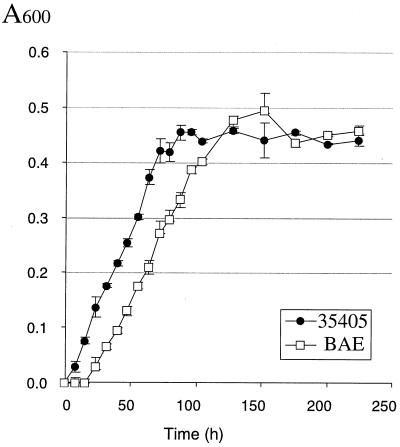
The fact that a viable opdB mutant was obtained demonstrated that the gene was not essential for growth of T. denticola. To determine the contribution of the OpdB peptidase to in vitro growth of T. denticola, the growth of the parent and mutant strains was assayed over a 10-day period. The results of one study are shown in Fig. 4. The growth rates of the parent and mutant strains were very similar, and both strains reached a final A600 of approximately 0.45. While the maximum growth rate of BAE appeared be slightly lower than that of ATCC 35405, the only obvious difference was the longer lag period before the A600 of BAE began to increase. This lengthier lag phase was also observed when the growth study was repeated (data not shown).
FIG. 4.
Growth of T. denticola ATCC 35405 and isogenic opdB mutant BAE. Bacteria were inoculated in NOS media and incubated under anaerobic conditions. A600 was measured every 8 h for 5 days and every 24 h for the next 5 days. Error bars represent the range of A600 values obtained from triplicate samples.
Enzymatic activity of opdB mutant strain.
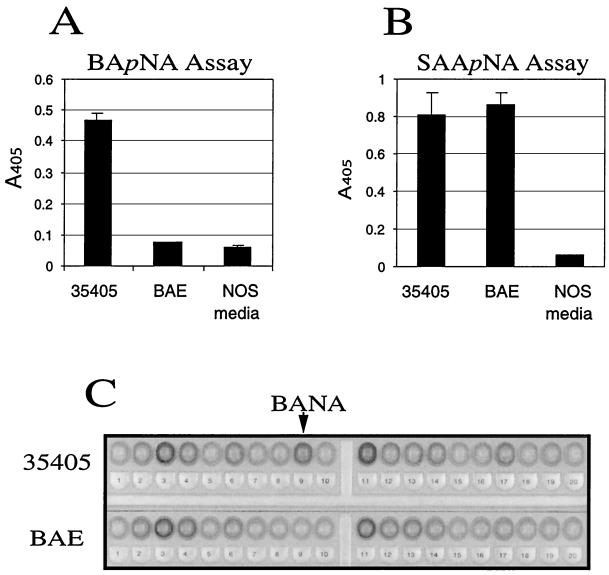
Assays of enzymatic activities in ATCC 35405 and BAE are shown in Fig. 5. There was no detectable hydrolysis of BApNA by T. denticola BAE (Fig. 5A). These data suggest that opdB encodes all of the trypsin-like activity of T. denticola. In contrast, Fig. 5B shows that the activity of the SAApNA-degrading CTLP of this organism is unchanged in strain BAE. To assay a range of enzyme activities in the parent and mutant strains, API-ZYM tests were performed on ATCC 35405 and BAE. As shown in Fig. 5C, the only detectable difference between the two strains was the lack of detectable trypsin-like BANA-hydrolyzing activity in BAE. It is also worth noting that N-glutaryl-phenylalanine-2-nitroanilide, the substrate for α-chymotrypsin used in this assay, did not detect the activity of T. denticola CTLP. Gelatin zymography of ATCC 35405 and BAE (not shown) revealed no differences in gelatinolytic activity between the parent and mutant strains. Combined with the results of the SAApNA hydrolysis assay, this suggests that neither the peptidase nor the protease activities of CTLP were diminished in the mutant strain.
FIG. 5.
Enzymatic activities of T. denticola ATCC 35405 and opdB mutant BAE. (A) Trypsin-like activity measured by BApNA hydrolysis. (B) Chymotrypsin-like activity measured by SAApNA hydrolysis. NOS media, culture medium negative control. Vertical bars show standard deviations of triplicate samples. (C) API-ZYM strip. Tested enzymes: 1, control; 2, alkaline phosphatase; 3, esterase; 4, esterase lipase; 5, lipase; 6, leucine arylamidase; 7, valine arylamidase; 8, cystine arylamidase; 9, trypsin; 10, α-chymotrypsin; 11, acid phosphatase; 12, naphthol-AS-BI-phosphohydrolase; 13, α-galactosidase; 14, β-galactosidase; 15, β-glucuronidase; 16, α-glucosidase; 17, β-glucosidase; 18, N-acetyl-β-glucosaminidase; 19, α-mannosidase; 20, α-fucosidase. Arrow, substrate for the trypsin assay (BANA).
Protein profile of opdB mutant strain.
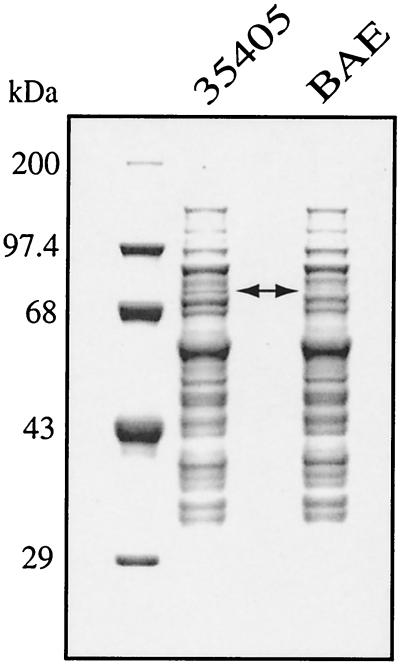
The predicted molecular mass of the full-length OpdB peptide is 78 kDa. The size of the BANA-hydrolyzing peptidase has been reported as 78 kDa by SDS-PAGE (23), as 69 kDa (25) by tube gel SDS-PAGE, and as approximately 50 kDa by gel filtration (25). As shown in Fig. 6, a protein band migrating at approximately 78 kDa in ATCC 35405 appears to be missing in BAE. These data combined with N-terminal sequence data (23) suggest that, unlike the CTLP of this organism encoded by prtP (16) and the trypsin-like Rgp and Kgp enzymes of P. gingivalis (27, 28), the OpdB peptidase does not contain a signal peptide and does not undergo further prepeptide processing at the N terminus.
FIG. 6.
SDS-PAGE analysis of T. denticola ATCC 35405 and opdB mutant BAE whole-cell extracts. Molecular mass standards are shown in the left lane. Arrow, band at approximately 78 kDa that is present in ATCC 35405 and that appears to be missing in BAE. This is consistent with the predicted molecular mass of the OpdB peptide.
DISCUSSION
Trypsin-like activity of periodontal pathogens, detectable by the ability of subgingival dental plaque from periodontal lesions to hydrolyze chromogenic peptide substrates, has been the basis for development of diagnostic tests for anaerobic periodontal infections (18, 33). In the oral environment, this trypsin-like activity is secreted primarily by the three members of a specific complex of cultivatable anaerobes most highly associated with severe periodontal disease: T. denticola, P. gingivalis, and B. forsythus (35). The major trypsin-like enzyme complexes of P. gingivalis (Rgp and Kgp) and their associated hemagglutinin domains are strongly implicated in various stages of periodontal pathogenesis, including fimbria-mediated adherence to cells and degradation of host proteins. To date, no studies have linked the trypsin-like peptidase activity of T. denticola with periodontal cytopathology. This area of investigation does not appear to have been pursued recently, even though, in our view, the strong association of the peptidase activity with periodontal pathogens makes it an obvious candidate for investigation as a potential virulence factor.
In the present study we characterized the opdB gene encoding trypsin-like peptidase activity in T. denticola and showed that it encodes all trypsin-like activity detectable under standard in vitro conditions. By searching amino acid translations of the preliminary T. denticola genome sequence with short peptide sequences that had been determined from the native protein, we identified a potential genetic locus for the peptidase gene. DNA sequencing showed that an open reading frame that could encode a protein of the expected size was present, though it was not apparent in the preliminary sequence releases. As determined from our sequence data, the restriction enzyme pattern of the opdB locus bears no resemblance to that of a cloned T. denticola gene reported to have trypsin-like activity in E. coli (20) (data not shown).
The predicted opdB gene product showed significant homology to members of a superfamily of prolyl oligopeptidases in both prokaryotic and eukaryotic organisms. These include the OpdB of trypanosomes, which is involved in cellular invasion by these pathogenic protozoans (24). Recent biochemical studies of this group of enzymes in prokaryotes, focusing on the E. coli oligopeptidase B, suggest that these enzymes may function as “convertases,” whose function is to activate specific precursor proteins (29). In eukaryotes, cleavage of precursor proteins (such as prohormones and neuropeptide precursors) is required for activation, and many of these convertases function by cleavage at arginine residues. It is intriguing to speculate that T. denticola OpdB might have a role in modulating inflammatory response pathways by cleavage of specific host precursor proteins. Studies planned in our laboratory will address this issue.
The predicted opdB coding region begins with a TTG start codon, which, though quite rare, is found in several prokaryotic systems including the highly conserved cya locus of enterobacteria and related facultative anaerobes (36), as well as a variety of other genes in archaebacteria (12), chloroplasts (15), and mitochondria (26). In at least some of these systems, the use of this translation initiation codon is believed to contribute to posttranscriptional control of expression of the protein. At the present time we have no information as to the specific significance of the TTG start codon in this T. denticola gene. In this regard, it may be of interest that previous studies suggested that the BANA-hydrolyzing activity of oral spirochetes (expressed as the BANA hydrolysis detection limit in numbers of spirochetes present) was much higher in subgingival plaque samples containing high levels of spirochetes (4) than in strains grown in broth culture (18). The identification of the opdB gene will facilitate analysis of OpdB expression in vivo to determine whether this gene is upregulated in periodontal disease (34).
An isogenic T. denticola opdB mutant was constructed by allelic replacement mutagenesis. The opdB mutant, designated BAE, carries a deletion of 233 bp in the opdB gene, into which the ermF/AM gene cassette was introduced. Construction of the mutant was confirmed by both PCR and Southern blot analysis. Growth studies showed that the T. denticola parent and mutant strains grew similarly in NOS broth medium and reached the same final optical density, although the mutant had a very slightly lower maximum growth rate and a longer lag time before entering logarithmic growth phase. T. denticola strain BAE lacks all detectable BApNA- and BANA-hydrolyzing activity, suggesting that OpdB is responsible for all of the trypsin-like enzyme activity in this organism. Further analysis of the mutant will help to determine whether other trypsin-like enzymes are activated under different environmental conditions. Other enzyme activities appeared to be the same in both strains, and there were no apparent differences in expression of proteins other than OpdB. This isogenic T. denticola mutant will be used in planned studies examining the biological role of OpdB in the interactions between T. denticola and host tissue in periodontal diseases.
ACKNOWLEDGMENTS
We thank Walter Loesche for encouragement and helpful discussions.
This work was supported by Public Health Service grant DE13565 from the National Institute of Dental and Craniofacial Research (J.C.F.). Partial support for S.Y.L. was provided by Kangnung National University, Kangnung, Korea (2000).
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 1. New York, N.Y: Wiley-Interscience; 1995. [Google Scholar]
- 3.Bateman A, Birney E, Durbin R, Eddy S R, Finn R D, Sonnhammer E L. Pfam 3.1: 1313 multiple alignments and profile HMMs match the majority of proteins. Nucleic Acids Res. 1999;27:260–262. doi: 10.1093/nar/27.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bretz W A, Loesche W J. Characteristics of trypsin-like activity in subgingival plaque samples. J Dent Res. 1987;66:1668–1672. doi: 10.1177/00220345870660111301. [DOI] [PubMed] [Google Scholar]
- 5.Bretz W A, Lopatin D E, Loesche W J. Benzoyl-arginine naphthylamide (BANA) hydrolysis by Treponema denticola and/or Bacteroides gingivalis in periodontal plaques. Oral Microbiol Immunol. 1990;5:275–279. doi: 10.1111/j.1399-302x.1990.tb00425.x. [DOI] [PubMed] [Google Scholar]
- 6.Chan E C S, DeCiccio A, McLaughlin R, Klitorinos A, Siboo R. An inexpensive solid medium for obtaining colony-forming units of oral spirochetes. Oral Microbiol Immunol. 1997;12:372–376. doi: 10.1111/j.1399-302x.1997.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 7.Ellen R P, Ko K S, Lo C M, Grove D A, Ishihara K. Insertional inactivation of the prtP gene of Treponema denticola confirms dentilisin's disruption of epithelial junctions. J Mol Microbiol Biotechnol. 2000;2:581–586. [PubMed] [Google Scholar]
- 8.Fenno J C, Hannam P M, Leung W K, Tamura M, Uitto V-J, McBride B C. Cytopathic effects of the major surface protein (Msp) and the chymotrypsinlike protease (CTLP) of Treponema denticola. Infect Immun. 1998;66:1869–1877. doi: 10.1128/iai.66.5.1869-1877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenno J C, Müller K-H, McBride B C. Sequence analysis, expression and binding activity of recombinant major outer sheath protein (Msp) of Treponema denticola. J Bacteriol. 1996;178:2489–2497. doi: 10.1128/jb.178.9.2489-2497.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenno J C, Wong G W K, Hannam P M, McBride B C. Mutagenesis of outer membrane virulence determinants of the oral spirochete Treponema denticola. FEMS Microbiol Lett. 1998;163:209–215. doi: 10.1111/j.1574-6968.1998.tb13047.x. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher H M, Schenkein H A, Morgan R M, Bailey K A, Berry C R, Macrina F L. Virulence of a Porphyromonas gingivalis W83 mutant defective in the prtH gene. Infect Immun. 1995;63:1521–1528. doi: 10.1128/iai.63.4.1521-1528.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golderer G, Dlaska M, Grobner P, Piendl W. TTG serves as an initiation codon for the ribosomal protein MvaS7 from the archaeon Methanococcus vannielii. J Bacteriol. 1995;177:5994–5996. doi: 10.1128/jb.177.20.5994-5996.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grenier D, Uitto V-J, McBride B C. Cellular location of a Treponema denticola chymotrypsinlike protease and importance of the protease in migration through the basement membrane. Infect Immun. 1990;58:347–351. doi: 10.1128/iai.58.2.347-351.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haapasalo M, Singh U, McBride B C, Uitto V-J. Sulfhydryl-dependent attachment of Treponema denticola to laminin and other proteins. Infect Immun. 1991;59:4230–4237. doi: 10.1128/iai.59.11.4230-4237.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirose T, Ideue T, Wakasugi T, Sugiura M. The chloroplast infA gene with a functional UUG initiation codon. FEBS Lett. 1999;445:169–172. doi: 10.1016/s0014-5793(99)00123-4. [DOI] [PubMed] [Google Scholar]
- 16.Ishihara K, Miura T, Kuramitsu H K, Okuda K. Characterization of the Treponema denticola prtP gene encoding a prolyl-phenylalanine-specific protease (dentilisin) Infect Immun. 1996;64:5178–5186. doi: 10.1128/iai.64.12.5178-5186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Ruby J, Charon N, Kuramitsu H. Gene inactivation in the oral spirochete Treponema denticola: construction of an flgE mutant. J Bacteriol. 1996;178:3664–3667. doi: 10.1128/jb.178.12.3664-3667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loesche W J, Bretz W A, Kerschensteiner D, Stoll J, Socransky S S, Hujoel P, Lopatin D E. Development of a diagnostic test for anaerobic periodontal infections based on plaque hydrolysis of benzoyl-dl-arginine-naphthylamide. J Clin Microbiol. 1990;28:1551–1559. doi: 10.1128/jcm.28.7.1551-1559.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loesche W J, Syed S A, Stoll J. Trypsin-like activity in subgingival plaque. A diagnostic marker for spirochetes and periodontal disease? J Periodontol. 1987;58:266–273. doi: 10.1902/jop.1987.58.4.266. [DOI] [PubMed] [Google Scholar]
- 20.MacDougall J H, Beighton D, Russell R R. Cloning and expression of protease genes from Treponema denticola in Escherichia coli. Oral Microbiol Immunol. 1991;6:270–274. doi: 10.1111/j.1399-302x.1991.tb00490.x. [DOI] [PubMed] [Google Scholar]
- 21.Mäkinen K K, Chen C Y, Mäkinen P L, Ohta K, Loesche W J. The benzoylarginine peptidase from Treponema denticola (strain ASLM), a human oral spirochaete: evidence for active-site carboxyl groups. Mol Microbiol. 1990;4:1413–1417. doi: 10.1111/j.1365-2958.1990.tb00721.x. [DOI] [PubMed] [Google Scholar]
- 22.Mäkinen K K, Mäkinen P L. The peptidolytic capacity of the spirochete system. Med Microbiol Immunol. 1996;185:1–10. doi: 10.1007/s004300050008. [DOI] [PubMed] [Google Scholar]
- 23.Mäkinen K K, Mäkinen P L, Loesche W J, Syed S A. Purification and general properties of an oligopeptidase from Treponema denticola ATCC 35405—a human oral spirochete. Arch Biochem Biophys. 1995;316:689–698. doi: 10.1006/abbi.1995.1092. [DOI] [PubMed] [Google Scholar]
- 24.Morty R E, Lonsdale-Eccles J D, Morehead J, Caler E V, Mentele R, Auerswald E A, Coetzer T H, Andrews N W, Burleigh B A. Oligopeptidase B from Trypanosoma brucei, a new member of an emerging subgroup of serine oligopeptidases. J Biol Chem. 1999;274:26149–26156. doi: 10.1074/jbc.274.37.26149. [DOI] [PubMed] [Google Scholar]
- 25.Ohta K, Mäkinen K K, Loesche W J. Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infect Immun. 1986;53:213–220. doi: 10.1128/iai.53.1.213-220.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okimoto R, MacFarlane J L, Wolstenholme D R. Evidence for the frequent use of TTG as the translation initiation codon of mitochondrial protein genes in the nematodes Ascaris suum and Caenorhabditis elegans. Nucleic Acids Res. 1990;18:6113–6118. doi: 10.1093/nar/18.20.6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pavloff N, Pemberton P A, Potempa J, Chen W C, Pike R N, Prochazka V, Kiefer M C, Travis J, Barr P J. Molecular cloning and characterization of Porphyromonas gingivalis lysine-specific gingipain. A new member of an emerging family of pathogenic bacterial cysteine proteinases. J Biol Chem. 1997;272:1595–1600. doi: 10.1074/jbc.272.3.1595. [DOI] [PubMed] [Google Scholar]
- 28.Pavloff N, Potempa J, Pike R N, Prochazka V, Kiefer M C, Travis J, Barr P J. Molecular cloning and structural characterization of the Arg-gingipain proteinase of Porphyromonas gingivalis. Biosynthesis as a proteinase-adhesin polyprotein. J Biol Chem. 1995;270:1007–1010. doi: 10.1074/jbc.270.3.1007. [DOI] [PubMed] [Google Scholar]
- 29.Polgar L. A potential processing enzyme in prokaryotes: oligopeptidase B, a new type of serine peptidase. Proteins. 1997;28:375–379. [PubMed] [Google Scholar]
- 30.Potempa J, Pike R, Travis J. The multiple forms of trypsin-like activity present in various strains of Porphyromonas gingivalis are due to the presence of either Arg-gingipain or Lys-gingipain. Infect Immun. 1995;63:1176–1182. doi: 10.1128/iai.63.4.1176-1182.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saito T, Ishihara K, Kato T, Okuda K. Cloning, expression, and sequencing of a protease gene from Bacteroides forsythus ATCC 43037 in Escherichia coli. Infect Immun. 1997;65:4888–4891. doi: 10.1128/iai.65.11.4888-4891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 33.Seida K, Saito A, Yamada S, Ishihara K, Naito Y, Okuda K. A sensitive enzymatic method (SK-013) for detection of Treponema denticola, Porphyromonas gingivalis and Bacteroides forsythus in subgingival plaque samples. J Periodontal Res. 1992;27:86–91. doi: 10.1111/j.1600-0765.1992.tb01808.x. [DOI] [PubMed] [Google Scholar]
- 34.Shelburne C E, Gleason G R, Germaine B H, Mullally B H, Wolff L F, Coulter W A, Lopatin D E. Measurement of Porphyromonas gingivalis gene activation in vivo. J Dent Res. 2001;80:S52. [Google Scholar]
- 35.Socransky S S, Haffajee A D, Cugini M A, Smith C, Kent R L. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 36.Trotot P, Sismeiro O, Vivares C, Glaser P, Bresson-Roy A, Danchin A. Comparative analysis of the cya locus in enterobacteria and related gram-negative facultative anaerobes. Biochimie. 1996;78:277–287. doi: 10.1016/0300-9084(96)82192-4. [DOI] [PubMed] [Google Scholar]
- 37.Uitto V-J, Grenier D, Chan E C, McBride B C. Isolation of a chymotrypsinlike enzyme from Treponema denticola. Infect Immun. 1988;56:2717–2722. doi: 10.1128/iai.56.10.2717-2722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uitto V-J, Pan Y M, Leung W K, Larjava H, Ellen R P, Finlay B B, McBride B C. Cytopathic effects of Treponema denticola chymotrypsin-like proteinase on migrating and stratified epithelial cells. Infect Immun. 1995;63:3401–3410. doi: 10.1128/iai.63.9.3401-3410.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]