Abstract
OBJECTIVES
Low-income, first-time mothers generally breastfeed exclusively and, overall, for a shorter average duration than high-income, multiparous mothers. A potential barrier to breastfeeding success is access to a breast pump for home use. In this pilot study, we estimated the effect of providing a manual breast pump during birth hospitalization for home use on any/exclusive breastfeeding and investigated participant attitudes about manual pumps and their breastfeeding experiences.
METHODS
Sixty low-income, first-time mothers were enrolled in a pilot randomized controlled trial. One-half received a manual breast pump and the other half received an attention control. Breastfeeding exclusivity, duration, and use of the manual pump were assessed at 6 and 12 weeks. Qualitative interviews regarding the breastfeeding experience were completed. Thirty-one women answered 13 questions that were then transcribed, coded, and grouped into themes.
RESULTS
Participants who were randomized to manual breast pump receipt during birth hospitalization had increased manual pump use at 6 weeks (13/19 [68%] versus controls 5/17 [29%]), there was no effect of pump receipt on any nor exclusive breastfeeding at 12 weeks. In qualitative analysis of the overall breastfeeding experience, participants expressed a need for additional support and had conflicting attitudes regarding breastfeeding and the pumping experience.
CONCLUSIONS
Manual breast pump receipt in hospital among low-income, first-time mothers did not affect breastfeeding exclusivity or duration. Participants reported that early and ongoing lactation support is essential. Strategies to improve breastfeeding outcomes low-income, first-time mothers are needed.
Exclusive breastfeeding is the optimal form of infant nutrition and is associated with many health benefits for infants and lactating individuals,1,2 especially among low-income women who are eligible for the Special Supplemental Nutrition Program for Women, Infants and Children (WIC) in the United States.3 WIC-eligible women are 19% less likely to ever breastfeed and by 6 months postpartum 31% less likely continue breastfeeding compared with those ineligible for WIC.4 Suboptimal breastfeeding initiation and duration in low-income, WIC-eligible women is troubling and points to a need to improve breastfeeding outcomes in this group.
Manual pump use may help improve breastfeeding outcomes. Low-cost manual pumps may be as effective in breastmilk removal as more costly electric breast pumps.5,6 A prior, quasi-experimental study explored the effect of hospital discharge bags in a 3-arm trial where bags that contained (1) breastfeeding information and supplies (BF-info); (2) breastfeeding information, supplies, and a manual breast pump (PUMP); or (3) formula samples were given to dyads at birth hospitalization. Provision of BF-info and PUMP discharge bags resulted in higher rates of exclusive breastfeeding at 12 weeks compared with the formula sample discharge bag group. However, there was no significant difference in exclusive breastfeeding rates at 12 weeks between the PUMP and BF-info groups (62% vs 54%). Notably, there was differential attrition between groups: the BF-info group received several supplies not routinely provided (eg, DVD on latching, nipple pads, and nipple cream), and more than 85% of the study sample was not WIC-eligible.7 Access to pumps has been found to be associated with decreased formula use in WIC-eligible women8; however, the literature is not conclusive. Bream and colleagues demonstrated in a retrospective study that Black WIC-eligible women with access to a manual breast pump had lower breastfeeding rates.9
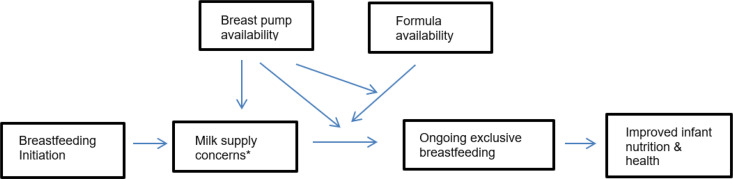
Although most US birth hospitalization bags no longer routinely contain formula, mailed samples of formula and formula coupons are often sent to pregnant and postpartum individuals, which can reduce exclusive breastfeeding rates.10 Instead, if hospital discharge bags contained a manual breast pump and instructions for when to use it, that could potentially improve breastfeeding rates. In groups with a high risk for suboptimal breastfeeding outcomes, such as low-income women, having a hospital discharge bag with a manual breast pump may preempt use of formula and improve milk supply (Fig 1). In this pilot randomized controlled trial, we sought to estimate the effect of manual breast pump provision to WIC-eligible women for home use after hospital discharge to estimate a future sample size and utility for a larger trial (Fig 1). Participant attitudes and opinions regarding the manual breast pump intervention were examined through qualitative methods to determine best practices associated with successful implementation of a breast pump intervention.
FIGURE 1.
Conceptual model of potential mediating and moderating effects of going home from the hospital with a manual breast pump on ongoing exclusive breastfeeding and milk supply concerns. *Milk supply concerns may include low milk supply as well as short-term maternal separation from the infant.
Methods
Setting
This pilot randomized controlled trial took place in a 625-bed teaching hospital in California from 2017 to 2019, in which 40% of the patient population in the hospital setting are at or below 200% of the federal poverty line and 38% of the approximately 1500 women delivering at the study hospital per year are publicly insured. In this birth hospital setting, approximately 89% of birthing persons designate English as their preferred language to communicate health care information, 6% communicate in Spanish, and 5% prefer to communicate health care information in other languages. At the time of the study, the hospital was in the process of becoming Baby-Friendly designated.11,12 International Board Certified Lactation Consultants (IBCLC) were available to see all participants in the hospital during the birth hospitalization. Outpatient lactation support was provided in a free, weekly breastfeeding drop-in support group with IBCLC. Local WIC services included routine provision of manual pumps after in-person evaluation, an expanded food package for breastfeeding women, ongoing lactation support, and counseling against unnecessary formula supplementation.
Outcomes
The primary outcome of this pilot study was to estimate plausible values for the intervention’s effect on exclusive breastfeeding at 12 weeks (defined by intake of only human milk in the previous 24 hours) to estimate the sample size for a future multicenter trial.13 The intervention group received a manual breast pump during the birth hospitalization and instructions about when to use it after hospital discharge. Control group participants received an attention control of a board book and instructions about shared reading with the infant. This trial was approved by the university’s institutional review board. Secondary outcomes included exclusive breastfeeding at 6 weeks, exclusive breastfeeding through 12 weeks, and any breastfeeding at 6 and 12 weeks. Information regarding manual and electric pump use was collected at 6 and 12 weeks. No participants were eligible to request or receive electric pumps directly from our study. Qualitative methods were used to determine best practices associated with implementation of a breast pump intervention to improve breastfeeding rates among low-income, first-time mothers.
Inclusion/Exclusion Criteria
Inclusion criteria were WIC-eligible (income below 185% the federal poverty level), primiparous women who had given birth at our hospital to liveborn infants admitted to the well newborn nursery, had initiated breastfeeding, and were 12 to 96 hours of age at the time of enrollment. Admission criteria to the newborn nursery were a minimum gestational age of 35 weeks 0 days and birth weight of at least 2300 g. Exclusion criteria were maternal age <18 years, maternal incarceration, non–English-speaking or reading, multiple birth, or infants with conditions that preclude breastfeeding exclusivity (eg, cleft lip and palate). Potential participants were approached for study consent by a research team member examining the electronic medical record unit lists for the newborn nursery and identifying dyads who met inclusion and exclusion criteria. An in-person eligibility screening with prospective participants was completed that asked if the infant was the mother’s first child. Women with older adopted children, older stepchildren, or previous pregnancies that did not result in a liveborn infant were eligible for inclusion. WIC eligibility was derived from maternal self-report of household size and total household income.
Randomization and In-Hospital Phase
Before study enrollment, a randomization sequence was generated by a statistician. An assistant not involved with the study filled opaque randomization envelopes with index cards indicating group assignment. Participants were allocated 1:1 to the intervention or control. After enrollment, baseline data were collected and participants were randomized. Baseline data included demographic information, prenatal history, intention to breastfeed, breastfeeding self-efficacy,14 and planned use of breast pumps (Supplemental Table 5). Participants were notified of their randomization arm after enrollment and were given the intervention Medela Harmony manual breast pump or the attention control board book. The intervention also included instructions for manual breast pump use called, “Reasons to Use the Manual Breast Pump” and control group received instructions of equal length called, “Reading to Baby.” There was not specific coaching about the handout; however, IBCLC were available to see the women and infants at bedside by request if there were questions about how to use the device.
Follow-Up
The follow-up included phone or e-mail surveys at 6 weeks and 12 weeks (Supplemental Table 6) that assessed breastfeeding, manual pump use, any electric pump use, use of hands to express human milk, receipt of formula, and receipt of donor or shared human milk. There was no participant remuneration.
Sample Size/Statistical Methods
This pilot study enrolled 60 participants. Statistical analysis was conducted in SPSS and SAS statistical software. The primary objective of this pilot study was to estimate plausible values for the intervention’s effect on exclusive breastfeeding at 12 weeks to estimate the effect size for planning a future definitive study. Effect sizes are expressed as between-group differences in percentages along with a 95% Wald confidence interval (CI). We assumed that the control group rate of exclusive breastfeeding at 12 weeks in WIC-eligible women would be 20%.15 The sample size of 30 per group with complete data would ensure that CIs would be no wider than 50 percentage points. Such a width would imply that the true difference in proportions for which there would be 80% power would be at least 35 percentage points.
Data collection and storage
Data were collected on paper forms and transcribed to Microsoft Excel spreadsheets that were stored on a secure research drive. Study survey data were collected and managed using REDCap electronic data capture tools hosted at the study institution.16,17
Qualitative Methods
At 12 weeks’ follow up, all participants were invited to complete a qualitative interview that included 13 open-ended questions regarding the breastfeeding experience, human milk expression practices, infant feeding decisions, and reasons for formula use. The qualitative interviews were done over the phone with a trained research assistant asking the participant open-ended questions and transcribing their response during the live interview. If phone interviews were declined, participants had the option to free text their answers in an electronic written survey in REDCap. Written responses to each question were deidentified and transcribed. The transcriptions were analyzed according to the answers to each of the 13 questions using inductive thematic analysis with a constant-comparative approach. At least 2 investigators coded each narrative response.
Results
Quantitative Results
A total of 708 women were screened for this study, 288 were approached, 74 were eligible, and 60 (81%) of the eligible participants consented, enrolled, and were randomized (Table 1). Thirty were randomized to the intervention and 29 received the intervention (1 infant was transferred to the neonatal ICU amid the consent process) and 30 to control (Supplemental Fig 2). The mean age of participants was 24 ± 5 years, 33/59 (56%) were white, 9/59 (15%) Black, 5/59 (8%) Asian and/or Pacific Islander, 4/59 (7%) American Indian/Alaska Native, and 8/59 (13%) self-identified their race as other. A total of 21/59 (36%) participants were of Hispanic ethnicity. Fifty-five of 59 (85%) participants were married or had a live-in partner and 43/59 (73%) delivered vaginally. Thirty-seven of 59 participants (62%) completed the 6-week follow-up and 31/56 participants (52%) completed the 12-week follow-up. Individuals who did not complete a survey were lost to follow-up.
TABLE 1.
Participant Demographic and Clinical Characteristics (N = 59)
| Characteristics of Participants | Pump, Intervention (n = 30), n (%)a | Book, Attention Control (n = 29), n (%)a |
|---|---|---|
| Race | ||
| White | 19 (63) | 14 (48) |
| Black/African American | 4 (13) | 5 (17) |
| Asian | 2 (7) | 0 (0) |
| Pacific Islander | 1 (3) | 2 (7) |
| American Indian/Alaska Native | 1 (3) | 3 (10) |
| Other | 4 (13) | 4 (14) |
| Hispanic ethnicity | 10 (33) | 11 (38) |
| Education | ||
| 8th grade or less | 0 (0) | 1 (3) |
| Some high school | 4 (13) | 3 (10) |
| High school grad or GED | 9 (30) | 8 (28) |
| Vocational or trade school | 1 (3) | 1 (3) |
| Some college or associate’s degree | 11 (37) | 9 (31) |
| College graduate | 3 (10) | 6 (21) |
| Graduate school | 2 (7) | 0 (0) |
| Marital status | ||
| Single/never married | 15 (50) | 15 (51) |
| Single/divorced/separated | 1 (3) | 1 (3) |
| Married/live-in partner | 12 (40) | 13 (45) |
| Other | 1 (3) | 0 (0) |
| Declined to answer | 1 (3) | 0 (0) |
| Insurance | ||
| No insurance | 1 (3) | 1 (3) |
| Private insurance | 4 (13) | 10 (34) |
| Medicaid/MediCal | 18 (60) | 15 (51) |
| Military insurance | 3 (10) | 1 (3) |
| Other | 3 (10) | 2 (7) |
| Vaginal delivery | 20 (67) | 23 (79) |
| Smokes tobacco | 1 (3) | 0 (0) |
| Smoke exposure in household | 11 (37) | 10 (34) |
| Diabetes | 3 (10) | 4 (14) |
| Infant born late preterm | 1 (3) | 2 (7) |
Percentages may not add up to 100 because of rounding errors.
At 6 weeks, 13/19 (68%) of intervention group participants and 5/17 (29%) of control participants reported use of the manual pump (Table 2). Follow-up at this time also included questions regarding electric pump use to gauge all potential pump use. There were no between-group differences in electric pump use, hand expression, or direct breastfeeding for intervention or control groups at 6 weeks. Provision of breast milk was similar between groups at 6 weeks; 15/19 (79%) participants in the manual pump intervention group and 14/18 (78%) participants in the control group were providing any breastmilk to their infants (Table 3). Exclusive breastfeeding rates at 6 week were 72% (13/18) in the control and 42% (8/19) in the intervention groups.
TABLE 2.
Breast Milk Expressiona Practices by Study Arm
| Follow-up | Pump Group n (%) | Book Group n (%) | Difference in Percentages (95% CI) | P valueb |
|---|---|---|---|---|
| 6-wk follow-up (n = 37) | ||||
| Manual pumpc | 13/19 (68) | 5/172 (29) | 39 (5.5-65.2) | .04 |
| Electric pump | 5/19 (26) | 10/18 (56) | −29.2 (–56.4 to 3.2) | .1 |
| Hand expression | 8/19 (42) | 5/18 (28) | 14.3 (–17.1 to 44.2) | .5 |
| 12-wk follow-up (n = 31) | ||||
| Manual pump | 8/16 (50) | 5/15 (33) | 16.7 (–19.2 to 48.6) | .5 |
| Electric pump | 5/16 (31) | 9/15 (60) | −28.8 (–59.3 to 7.9) | .2 |
| Hand expression | 5/16 (31) | 6/15 (40) | −8.8 (–41.6 to 25.7) | .7 |
Participants could choose more than 1 human milk expression method.
Estimated by Fisher exact test.
One participant completed 6-wk questionnaire but left this item blank.
TABLE 3.
Human Milk Outcomes by Study Arm
| Pump Group n (%) | Book Group n (%) | Difference in proportion (95% CI) | P valuea | |
|---|---|---|---|---|
| 6 wk (N = 37) | ||||
| DBF | 14/19 (74) | 13/18 (72) | 1.5 (−28.1 to 31.6) | 1.0 |
| Any breastmilk | 15/19 (79) | 14/18 (78) | 1.2 (−26.9 to 29.9) | 1.0 |
| EBM | 8/19 (42) | 13/18 (72) | −30.1 (−58.4 to 2.6) | .1 |
| 12 wk (N = 31) | ||||
| DBF | 9/16 (56) | 10/15 (67) | −10.4 (−42.5 to 25.1) | .7 |
| Any breast milk | 10/16 (63) | 11/15 (73) | −10.8 (−42.2 to 22.5) | .7 |
| EBM at 12 wkb | 5/16 (31) | 7/15 (47) | −15.4 (−47.6 to 20) | .5 |
| EBM through 12 wk | 5/16 (31) | 5/15 (33) | −2.1 (−35.2 to 30.5) | 1.0 |
DBF, direct breast feeding (ie, breastfed at the breast); EBM, exclusively breastmilk fed.
Fisher exact test P value.
EBM feeding at 12 wk was defined as 24-h recall of no liquid other than breastmilk, EBM through 12 wk estimated by participant recall of introduction of other liquids or solids at any time in their infant’s lifetime.
There were no differences in manual, electric, or hand expression of milk at 12 weeks, nor direct breastfeeding between the intervention and control groups (Table 2, Table 3). The number of infants exclusively breastfed at 12 weeks were 7/15 (47%) in the control group and 5/16 (31%) intervention group (difference in proportions, –15.4 with 95% CI, –48.4 to 20.7). There was no difference in exclusive breastfeeding rates at 12 weeks and the CI suggests that the manual pump group would not be significantly improved even if all participants completed the study.
Qualitative Results
Thirty-one women (52%) responded to the 13 open-ended questions. Three themes emerged regarding the breastfeeding and pumping experience in our participants: (1) the breastfeeding experience was mixed, (2) additional support is needed for breastfeeding women, and (3) expressing breastmilk with manual pump has challenges and rewards. Interviews were conducted after 12 weeks of participation in the randomized trial. See Table 4 for quotations from the participants.
TABLE 4.
Qualitative Themes Regarding the Breastfeeding Experience of Participants
| Theme | Selected Quotations |
|---|---|
| Mixed breastfeeding experience | “Nice during the time…soothing and comforting while breastfeeding” (Participant 13) “…mostly it was easy. He won’t eat if he is tired so I have to watch for his cues very carefully and feed him before he gets tired” (Participant 51) |
| Need for ongoing breastfeeding support | “When I was having a hard time getting baby to latch, the doctor said to supplement…I wish I went to a lactation consultant instead” (Participant 35) “I wished there was more help with the lactation consultant at the hospital” (Participant 36) |
| Expressing milk with manual pump has challenges and rewards | “I pump for my partner to feed the baby at night so I can sleep” (Participant 30) “The manual pump takes a long time and sometimes the handle pops off” (Participant 8) “I used the manual breast pump to pump my breasts when they are too full, and my baby is not hungry at the time” (Participant 9) |
Theme 1: Mixed Breastfeeding Experience
Breastfeeding was described as a stressful but rewarding experience that led to bonding and closeness with the newborn infant. One participant responded that breastfeeding was: …rewarding at first, we have grown so close. My son knows me and feels safe.” (Participant 8)
Another noted that breastfeeding provided a close bond, but at a significant cost: “…very time consuming and sacrificial, but great bonding experience. If I’m not breastfeeding, then I was pumping, and it took too much time.” (Participant 34)
Challenges with breastfeeding were noted to negatively influence mental health: “I had to give up on it because it was adding to my anxiety a lot.” (Participant 12) Maternal mental health was not measured in our study but was attributed by this participant as a reason to stop breastfeeding.
Theme 2: Need for Ongoing Breastfeeding Support
Mothers participating in our study identified that they needed additional support for breastfeeding, especially in the immediate postpartum day and ongoing medically accurate support in the outpatient setting. One participant noted during birth hospitalization that she needed help early on and felt that institutional support was lacking: “I wish that I was still able to breastfeed…and that as soon as I was to feed my baby when she was born someone would have been there to help. The hospital just left me on my own for the first few feeds resulting in lots of pain and discouragement.” (Participant 14)
Another participant noted that support after leaving the hospital was important and was perceived as inadequate: “When I was having a hard time getting baby to latch, the doctor said to supplement…I wish I went to a lactation consultant instead.” (Participant 35)
Theme 3: Expressing Breastmilk With a Manual Pump has Challenges and Rewards
Manual pumps were well-received and noted as a convenient, but time-consuming, method to store milk and were used for various reasons. One participant used the manual pump to relieve engorgement: “I have my manual pump if I only want to take the pressure off, but not pump a full session. It is extremely helpful.” (Participant 51)
Manual pump access was also noted as a convenient way to express breastmilk: “…it [manual pump] was faster than setting up the electric one….” (Participant 25)
Expressing milk with the manual pump was also used to get additional rest during nighttime feeds: “…yes, it [manual pump] was helpful so I didn’t have to get up every night to feed, so that dad can help….” (Participant 34)
Discussion
Historically, formula companies have marketed to birthing individuals during birth hospitalization through gift bags and free formula. This practice is harmful and is associated with decreased breastfeeding duration and exclusivity,18,19 even after adjusting for the strength of the prenatal breastfeeding intention.20 We aimed to estimate plausible values for the effect that provision of manual pumps, in a similar fashion to the historically provided formula company discharge bags, had on breastfeeding outcomes in low-income, WIC-eligible women at risk for suboptimal breastfeeding rates.
Our cohort had relatively high rates of exclusive breastfeeding in both the intervention and control groups (31% and 47%, respectively, at 12 weeks) with lower rates at 12 weeks in the intervention group. In the qualitative arm, participants remarked that manual pumps at hospital discharge were helpful. Although this study was not powered to detect differences in breastfeeding rates, participants who were in the manual pump arm had exclusive breastfeeding rates that trended in a negative fashion at 6 weeks, which is similar to results of an observational study by Bream and colleagues.9 Based on the wide CIs in this pilot work, we estimate that there was only a 2.5-percentage point improvement in exclusive breastfeeding and as much as a 58-percentage point deterioration of exclusive breastfeeding rates with the provision of a manual pump.
Participants in our study did comment on the need for ongoing education and appropriate lactation services after birth hospitalization, the lack of which they felt led to not meeting their breastfeeding goals. It has been described in WIC-eligible women that early problems with latch contribute to discontinuing breastfeeding before 1 month, especially in primiparous women.21,22 Additionally, food insecurity, living at or below 75% of the federal poverty level, perceived insufficient milk supply, and marital status being single are all associated with early breastfeeding cessation in this group.21,22
Strengths and Limitations
A major limitation of this pilot study was that we excluded persons who did not speak English because both our study and control interventions included English writing (pump instruction booklets, library flyer, children’s book, and study instructions). Thus, the utility of distributing a manual pump to individuals who did not communicate in English was not assessed. Additionally, we did not collect information regarding the employment and lactation policies of the participants’ workplaces nor the frequency and duration of manual or electric pump use. It has been reported that the workplace environment can affect breastfeeding duration and exclusivity when women return to work.23,24 Because the frequency and duration of manual and electric pump use was not assessed, it is challenging to infer if this information would have made a difference in our study because prior work among low-income, WIC-eligible women has demonstrated no difference in breastfeeding outcomes when using a manual versus electric breast pump.5 Last, our pilot study had 48% of participants lost to follow-up, making interpretation of our estimates and the detectable effects about 40% larger. Although our study attrition rate had no differences across groups and was a strong response rate compared with other studies in WIC-eligible populations,7 a definitive future trial would require sample size planning that adjusts for the attrition rate. Demographic information for participants who enrolled versus completed the study is available in the table in the supplement (Supplemental Table 7). It is possible that the loss to follow-up rate may have introduced bias and limited our precision in estimating the intervention’s effects.
Conclusions
Manual breast pumps given to low-income, first-time mothers during birth hospitalization for home use led to an increased use of the manual pump at 6 weeks postpartum and were well received by the participants but did not improve breastfeeding exclusivity or duration at 12 weeks. Interventions to improve breastfeeding rates in low-income, first-time parents are needed.
Supplementary Material
Acknowledgments
We thank research coordinators Althea Crichlow, MS, and Iesha Miller, MHA, for their assistance with data collection and management along with the Center for Healthcare Policy and Research at the University of California at Davis.
Footnotes
FUNDING: This project was funded by the Academic Pediatric Association Nutrition in Underserved Communities Young Investigator Grant (principal investigator: Dr Kair). The funders/sponsor did not participate in the work. Dr Hoyt-Austin’s work is supported by the Quality, Safety, and Comparative Effectiveness Research Training in Primary Care (QSCERT-PC) Program funded by HRSA T32HP30037. Drs Hoyt-Austin and Kair’s work is also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860. Dr Kair’s effort was also supported by a Building Interdisciplinary Research Careers in Women’s Health award (K12 HD051958, principal investigator Dr Nancy Lane) funded by the National Institute of Child Health and Human Development (NICHD), Office of Research on Women’s Health, Office of Dietary Supplements, and the National Institute of Aging. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health.
CONFLICT OF INTEREST DISCLOSURES: The authors have indicated they have no potential conflicts of interest to disclose.
CLINICAL TRIALS INFORMATION: This clinical trial was registered at clinicaltrials.gov (NCT03192241).
DATA SHARING STATEMENT: Requests for data sharing will be considered on reasonable request to the corresponding author.
Dr Hoyt-Austin carried out initial data analysis of quantitative and qualitative data, drafted the initial manuscript, and approved the final manuscript as submitted. Dr Kair conceptualized and designed the study, carried out analysis of quantitative and qualitative data, reviewed and revised the manuscript, and approved the final manuscript as submitted. Dr Chantry assisted with the design of the study, assisted with interpretation of qualitative and quantitative results, and critically revised the manuscript and approved the final manuscript as submitted. Dr Tancredi assisted with the design of the study, conducted data analysis and interpretation of results, critically revised the manuscript, and approved the final manuscript as submitted. Drs Moua and Cheng conducted data collection, undertook initial analysis of quantitative and qualitative data, reviewed and revised the manuscript, and approved the final manuscript as submitted.
References
- 1. Meek JY, Noble L, Section on Breastfeeding; Policy Statement: Breastfeeding and the use of human milk. Pediatrics. 2022;150(1):e2022057988. [DOI] [PubMed] [Google Scholar]
- 2. Ip S, Chung M, Raman Get al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep). 2007;153:1–186 [PMC free article] [PubMed] [Google Scholar]
- 3. Oliveira V, Frazao E. The WIC program: background, trends, and economic issues, 2015 edition. Available at: https://www.ers.usda.gov/publications/pub-details/?pubid=43927. Accessed February 12, 2021
- 4. Zhang Q, Lamichhane R, Wright M, McLaughlin PW, Stacy B. Trends in breastfeeding disparities in US infants by WIC eligibility and participation. J Nutr Educ Behav. 2019;51(2):182–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hayes DK, Prince CB, Espinueva V, Fuddy LJ, Li R, Grummer-Strawn LM. Comparison of manual and electric breast pumps among WIC women returning to work or school in Hawaii. Breastfeed Med. 2008;3(1):3–10 [DOI] [PubMed] [Google Scholar]
- 6. Becker GE, Smith HA, Cooney F. Methods of milk expression for lactating women. Cochrane Database Syst Rev. 2016;9(9):CD006170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bai Y, Wunderlich SM, Kashdan R. Alternative hospital gift bags and breastfeeding exclusivity. ISRN Nutr. 2013;2013:560810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meehan K, Harrison GG, Afifi AA, Nickel N, Jenks E, Ramirez A. The association between an electric pump loan program and the timing of requests for formula by working mothers in WIC. J Hum Lact. 2008;24(2):150–158 [DOI] [PubMed] [Google Scholar]
- 9. Bream E, Li H, Furman L. The effect of breast pump use on exclusive breastfeeding at 2 months postpartum in an inner-city population. Breastfeed Med. 2017:12:149–155 [DOI] [PubMed] [Google Scholar]
- 10. Waite WM, Christakis D. The impact of mailed samples of infant formula on breastfeeding rates. Breastfeed Med. 2016;11(1):21–25 [DOI] [PubMed] [Google Scholar]
- 11. Pérez-Escamilla R, Martinez JL, Segura-Pérez S. Impact of the baby-friendly hospital initiative on breastfeeding and child health outcomes: a systematic review. Matern Child Nutr. 2016;12(3):402–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Implementation guidance: protecting, promoting and supporting breastfeeding in facilities providing maternity and newborn services – the revised Baby-Friendly hospital initiative. Geneva: World Health Organization; 2018 [PubMed] [Google Scholar]
- 13. Greiner T. Exclusive breastfeeding: measurement and indicators. Int Breastfeed J. 2014;9(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dennis CL. The breastfeeding self-efficacy scale: psychometric assessment of the short form. J Obstet Gynecol Neonatal Nurs. 2003;32(6):734–744 [DOI] [PubMed] [Google Scholar]
- 15. WIC. WIC breastfeeding data local agency report, fiscal year 2016. Washington, DC: US Department of Agriculture; 2017 [Google Scholar]
- 16. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harris PA, Taylor R, Minor BLet al. ; REDCap Consortium. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feldman-Winter L, Grossman X, Palaniappan Aet al. Removal of industry-sponsored formula sample packs from the hospital: does it make a difference? J Hum Lact. 2012;28(3):380–388 [DOI] [PubMed] [Google Scholar]
- 19. Sadacharan R, Grossman X, Matlak S, Merewood A. Hospital discharge bags and breastfeeding at 6 months: data from the infant feeding practices study II. J Hum Lact. 2014;30(1):73–79 [DOI] [PubMed] [Google Scholar]
- 20. Chantry CJ, Dewey KG, Peerson JM, Wagner EA, Nommsen-Rivers LA. In-hospital formula use increases early breastfeeding cessation among first-time mothers intending to exclusively breastfeed. J Pediatr. 2014;164(6):1339–45.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hornsby PP, Gurka KK, Conaway MR, Kellams AL. Reasons for early cessation of breastfeeding among women with low income. Breastfeed Med. 2019;14(6):375–381 [DOI] [PubMed] [Google Scholar]
- 22. Gallo S, Kogan K, Kitsantas P. Racial and ethnic differences in reasons for breastfeeding cessation among women participating in the Special Supplemental Nutrition Program for Women, Infants, and Children. J Midwifery Womens Health. 2019;64(6):725–733 [DOI] [PubMed] [Google Scholar]
- 23. Kim JH, Shin JC, Donovan SM. Effectiveness of workplace lactation interventions on breastfeeding outcomes in the United States: an updated systematic review. J Hum Lact. 2019;35(1):100–113 [DOI] [PubMed] [Google Scholar]
- 24. Dinour LM, Szaro JM. Employer-based programs to support breastfeeding among working mothers: a systematic review. Breastfeed Med. 2017;12(3):131–141 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.