Abstract
Recent reports revealed an increased rate of hospitalization and mortality of coronavirus disease 2019 (COVID‐19) among patients with psychiatric disorders. On the other hand, there is a link between latent infections, including Toxoplasma gondii, herpes simplex virus type 1 (HSV‐1) and cytomegalovirus (CMV) with psychiatric disorders. We individually assessed data regarding 1) the mortality rate of COVID‐19 among individuals with psychiatric disorders; 2) the association of latent infections in COVID‐19 patients and 3) the association between latent infections and psychiatric disorders. We developed the hypothesis that latent infection could increase the risk of severe COVID‐19 among patients with psychiatric disorders. Cumulative evidence proposed that infection with toxoplasmosis, CMV and HSV‐1 could increase the risk of severe acute respiratory syndrome coronavirus 2 (SARS‐Co‐V2) infections among patients with psychiatric disorders probably by induction of hyperinflammatory conditions. These infections are also associated with hyperinflammation and T cell exhaustion, which has also been observed in both schizophrenia and COVID‐19. This hypothesis provides new insights into the role of latent infections in increasing the mortality rates of COVID‐19 among individuals with psychiatric disorders. Strategies for screening, early diagnosis and treatment of these infections could be recommended for COVID‐19 patients with a background of psychiatric disorders.
Keywords: COVID‐19, latent infections, psychiatric disorders, SARS‐CoV‐2
1. BACKGROUND
1.1. Opportunistic infections increase mortality rates of patients with coronavirus disease 2019 (COVID‐19)
Immune disturbances, including hyperinflammation and cytokine storm syndrome (CSS), are key players in developing severe sequels of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, such as acute respiratory distress syndrome and multiorgan failure and death. Immunomodulatory therapies (by corticosteroids or other agents) have shown promising outcomes for the control of CSS among patients with COVID‐19 and decreased mortality rates. 1 However, immunomodulatory therapy can increase the risk of reactivation of latent toxoplasmosis, cytomegalovirus (CMV) and herpes simplex virus type (HSV)‐infections. 1 In this regard, several cases of severe and fatal toxoplasmosis, CMV and HSV‐1 infections have been reported among COVID‐19 patients, most of which received immunomodulatory therapies (Reviewed in).1 A higher incidence of CMV and HSV‐1 were reported among severe COVID‐19 cases. 2 Latent CMV infection was associated with an increased risk of COVID‐19‐related hospitalization. 3 Critically ill patients with severe COVID‐19 were reported to be at a higher risk for CMV and epstein‐barr virus (EBV) reactivation. 4 Toxoplasmosis could be a risk factor for the severity of SARS‐CoV‐2 infection. 5
1.2. Increased mortality rate of COVID‐19 among individuals with psychiatric disorders
The global COVID‐19 pandemic has presented a complicated challenge among patients with psychiatric disorders, while higher hospitalization and mortality rates have been reported among these patients. 6 , 7 The results of a recent meta‐analysis revealed that the mortality rate of COVID‐19 is 1.75‐time higher among patients with mental health disorders compared to infected patients without mental health disorders (odds ratio [OR] = 1.75 8 ; p < .05). As such, the COVID‐19 patients with severe mental health disorders had an increased mortality rate than patients without severe mental health disorders (OR: 2.26 [95% confidence interval [CI], 1.18–4.31])). 8
1.3. Association between neurotropic infections and psychiatric disorders
Neurotropic infections (toxoplasmosis, CMV and HSV‐1) are highly prevalent infections among the general population, and their infections are usually asymptomatic (latent) in immunocompetent individuals; however, immunocompromised patients or pregnant women may have severe sequels with fatal outcome. Nevertheless, several neuropsychiatric disorders have been linked to these latent infections. 9 Studies indicated the link between schizophrenia and psychiatric disorders with Toxoplasma (T). gondii, CMV and HSV‐1 infection. 9 Hamdani et al. 10 have shown significant associations between seropositivity to T. gondii, HSV‐1, HSV‐2, and CMV with lower cognitive functions among patients with schizophrenia and bipolar disorder. 10 Shirts et al. 11 found that seropositivity to HSV‐1 and CMV was significantly associated with impaired cognitive function among patients with schizophrenia disorder. 11 Increased seropositivity to T. gondii infection was reported among treatment‐resistant schizophrenia patients. 12 Holub et al. 13 found that hospitalization of schizophrenia patients with T. gondii infection was increased during their last admission compared to T. gondii‐seronegative schizophrenia patients (p = 0.003; mean difference 32.9 days). As such, the T. gondii seropositive patients had higher levels in the Positive Subscale of Positive and Negative Symptom Scale (PANSS); as well, as the concentrations of anti‐T. gondii antibodies were negatively associated with the PANSS scores. 13 The result of a meta‐analysis revealed that T. gondii‐IgG seropositivity was significantly increased among patients with bipolar disorder (OR 1.52, p = 0.02), schizophrenia (OR 1.81, p < 0.00001), addiction (OR 1.91, p < 0.00001) and OCD (OR 3.4, p < 0.001). 14 Taken together, cumulative evidence revealed an association between these neurotropic infections and psychiatric disorders.
2. THE HYPOTHESIS/THEORY
It is a hypothesis that infection with toxoplasmosis, CMV, and HSV could increase the risk of severe COVID‐19 among patients with psychiatric disorders.
3. EVALUATION OF THE HYPOTHESIS/IDEA
Neurotropic infections like toxoplasmosis, CMV, and HSV augment inflammatory reactions. 9 It is also proposed that co‐infection of these pathogens synergically augments the level of inflammatory reactions more than their single infection. 9 Therefore, these infections could have an indirect role in increasing the mortality rate of COVID‐19, probably by induction of CSS. On the other hand, there is a strong connection between the severity of neuropsychiatric disorders with inflammatory immune responses. 9 While, anti‐inflammatory therapies have been prescribed as an adjuvant in patients with major depressive disorder in a number of studies. 15 , 16 Anti‐inflammatory drugs have been widely used for the modulation of CCS in patients with severe COVID‐19. 1 Hence, anti‐inflammatory therapy in schizophrenia and COVID‐19 could increase the risk of severe toxoplasmosis, CMV, or HSV infection.
Recent reports revealed immune disturbances in COVID‐19 patients, including T cell exhaustion, and a marked increase of proinflammatory and inflammatory cytokines, such as interferon‐γ, tumour necrosis factor‐a, interleukin (IL)‐2 and IL‐17. 17 T cell exhaustion has also linked to neurological manifestations of COVID‐19. 18 Remarkably, there is also a strong link between T. gondii, CMV, and HSV‐1 infection with T cell (especially CD8 T cells) exhaustion. 19 , 20 , 21 Indeed, the altered T cell function has been reported among patients with schizophrenia 22 , 23 and in animal models of mental disorders. 24 Hence, these latent infections could have a risk factor for COVID‐19 severity, especially among schizophrenia patients. Figure 1 presents a hypothetical scheme linking COVID‐19, psychiatric disorders and neurotropic infections.
FIGURE 1.
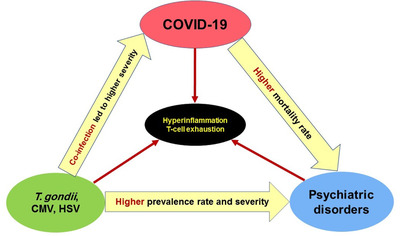
A hypothetical scheme linking COVID‐19, psychiatric disorders, and neurotropic infections
4. CONSEQUENCES OF THE HYPOTHESIS AND DISCUSSION
In conclusion, T. gondii, CMV, and HSV‐1 infections may have an indirect role in increasing the mortality rate of COVID‐19 among patients with psychiatric disorders. Co‐infection of these pathogens may also increase the mortality rate due to their synergistic effects. Screening, early diagnosis and treatment of these neurotropic infections might mitigate the severe sequel of COVID‐19 among patients with a background of psychiatric disorders.
AUTHOR CONTRIBUTIONS
Dr. Abdoli conceived the idea. Dr. Mofazzal and Dr. Abdoli contributed to the preparation and collection of original literatures and figures and the writing and editing of manuscript. Dr. Sefidfard, Dr Taghipour, Dr. Roustazadeh, Dr. Matin, Dr. Mir, Dr. Badri and Dr. Bahrami were responsible for the structural designs, scientific quality and writing.
FUNDING INFORMATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest. The paper was handled by editors and has undergone a rigorous peer‐review process. Dr. Amir Abdoli was not involved in the journal's review of/or decisions related to this manuscript.
ETHICAL APPROVAL
Not applicable.
ACKNOWLEDGEMENTS
Not applicable.
Mofazzal Jahromi MA, Sefidfard M, Taghipour A, et al. Latent infections, coronavirus disease 2019 and psychiatric disorders: The friend of my enemy. Clin Transl Disc. 2022;2:e141. 10.1002/ctd2.141
[Correction added on November 5, 2022 after first online publication: the Author Contributions, Acknowledgments, Funding, Conflicts of Interest, Data Availability Statement and Ethical approval has been updated].
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Abdoli A, Falahi S, Kenarkoohi A. COVID‐19‐associated opportunistic infections: a snapshot on the current reports. Clin Exp Med. 2022;22(3):327‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shrock E, Fujimura E, Kula T, et al. Viral epitope profiling of COVID‐19 patients reveals cross‐reactivity and correlates of severity. Science. 2020;370(6520):eabd4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alanio C, Verma A, Mathew D, et al. Cytomegalovirus latent infection is associated with an increased risk of COVID‐19‐related hospitalization. J Infect Dis. 2022;226(3):463‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Naendrup J‐H, Garcia Borrega J, Eichenauer DA, Shimabukuro‐Vornhagen A, Kochanek M, Böll B. Reactivation of EBV and CMV in severe COVID‐19—epiphenomena or trigger of hyperinflammation in need of treatment? A large case series of critically ill patients. J Intensive Care Med. 2022;37(9):1152‐1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Flegr J. Toxoplasmosis is a risk factor for acquiring SARS‐CoV‐2 infection and a severe course of COVID‐19 in the Czech and Slovak population: a preregistered exploratory internet cross‐sectional study. Parasit Vectors. 2021;14(1):508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nemani K, Li C, Olfson M, et al. Association of psychiatric disorders with mortality among patients with COVID‐19. JAMA Psychiatry. 2021;78(4):380‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tzur Bitan D, Kridin K, Cohen AD, Weinstein O. COVID‐19 hospitalisation, mortality, vaccination, and postvaccination trends among people with schizophrenia in Israel: a longitudinal cohort study. Lancet Psychiatry. 2021;8(10):901‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fond G, Nemani K, Etchecopar‐Etchart D, et al. Association between mental health disorders and mortality among patients with covid‐19 in 7 countries: a systematic review and meta‐analysis. JAMA Psychiatry. 2021;78(11):1208‐1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abdoli A, Taghipour A, Pirestani M, et al. Infections, inflammation, and risk of neuropsychiatric disorders: the neglected role of “co‐infection. Heliyon. 2020;6(12):e05645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamdani N, Daban‐Huard C, Godin O, et al. Effects of cumulative Herpesviridae and Toxoplasma gondii infections on cognitive function in healthy, bipolar, and schizophrenia subjects. J Clin Psychiatry. 2017;78(1):0. [DOI] [PubMed] [Google Scholar]
- 11. Shirts BH, Prasad KM, Pogue‐Geile MF, Dickerson F, Yolken RH, Nimgaonkar VL. Antibodies to cytomegalovirus and Herpes Simplex Virus 1 associated with cognitive function in schizophrenia. Schizophr Res. 2008;106(2):268‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vlatkovic S, Sagud M, Svob Strac D, et al. Increased prevalence of Toxoplasma gondii seropositivity in patients with treatment‐resistant schizophrenia. Schizophr Res. 2018;193:480‐481. [DOI] [PubMed] [Google Scholar]
- 13. Holub D, Bankovská Motlová L, Dragomirecká E, et al. Clinical differences between Toxoplasma gondii seropositive and seronegative schizophrenia patients in czech and international studies. Eur Psychiatry. 2011;26(S2):2125. [Google Scholar]
- 14. Sutterland AL, Fond G, Kuin A, et al. Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: systematic review and meta‐analysis. Acta Psychiatr Scand. 2015;132(3):161‐179. [DOI] [PubMed] [Google Scholar]
- 15. Çakici N, van Beveren NJM, Judge‐Hundal G, Koola MM, Sommer IEC. An update on the efficacy of anti‐inflammatory agents for patients with schizophrenia: a meta‐analysis. Psychol Med. 2019;49(14):2307‐2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cho M, Lee TY, Kwak YB, Yoon YB, Kim M, Kwon JS. Adjunctive use of anti‐inflammatory drugs for schizophrenia: a meta‐analytic investigation of randomized controlled trials. Aust N Z J Psychiatry. 2019;53(8):742‐759. [DOI] [PubMed] [Google Scholar]
- 17. De Biasi S, Meschiari M, Gibellini L, et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID‐19 pneumonia. Nat Commun. 2020;11(1):3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heming M, Li X, Räuber S, et al. Neurological manifestations of COVID‐19 feature T cell exhaustion and dedifferentiated monocytes in cerebrospinal fluid. Immunity. 2021;54(1):164‐175. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van den Berg SPH, Pardieck IN, Lanfermeijer J, et al. The hallmarks of CMV‐specific CD8 T‐cell differentiation. Med Microbiol Immunol. 2019;208(3):365‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bellon M, Nicot C. Telomere dynamics in immune senescence and exhaustion triggered by chronic viral infection. Viruses. 2017;9(10):289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roe K. The link between Toxoplasma gondii infections and higher mortality in COVID‐19 patients having schizophrenia. European Archives of Psychiatry and Clinical Neuroscience. 2021. [DOI] [PMC free article] [PubMed]
- 22. Steiner J, Jacobs R, Panteli B, Brauner M, Schiltz K, Bahn S, et al. Acute schizophrenia is accompanied by reduced T cell and increased B cell immunity. Eur Arch Psychiatry Clin Neurosci. 2010;260(7):509‐518. [DOI] [PubMed] [Google Scholar]
- 23. Craddock RM, Lockstone HE, Rider DA, et al. Altered T‐cell function in schizophrenia: a cellular model to investigate molecular disease mechanisms. PLoS One. 2007;2(8):e692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Nat Acad Sci USA. 2004;101(21):8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


