Abstract
Objective
Deleted in liver cancer 1 (DLC1) is a GTPase-activating protein that is reported as a suppressor in certain human cancers. However, the detailed biological function of DLC1 is still unclear in human prostate cancer (PCa). In the present study, we aimed to explore the function of DLC1 in PCa cells.
Methods
Silencing and overexpression of DLC1 were induced in an androgen-sensitive PCa cell line (LNCaP) using RNA interference and lentiviral vector transduction. The Cell Counting Kit-8 assay was performed to determine cell proliferation. The cell cycle was examined by performing a propidium iodide staining assay.
Results
Our results indicated that DLC1 overexpression markedly suppressed the proliferation and cell cycle progression of LNCaP cells. Moreover, DLC1 expression was negatively correlated with Rho-associated protein kinase (ROCK) expression in LNCaP cells. Importantly, this study showed that the ROCK inhibitor Y27632 restored the function of DLC1 in LNCaP cells and reduced the tumorigenicity of LNCaP cells in vivo.
Conclusion
Our results indicated that DLC1 overexpression markedly suppressed the proliferation and cell cycle progression of PCa cells and negatively correlated with ROCK expression in PCa cells and tissue.
Keywords: Cell cycle, Deleted in liver cancer 1, Proliferation, Prostate cancer, Rho-associated protein kinase
1. Introduction
Prostate cancer (PCa) is a heterogeneous tumor with various properties and a common cancer for males worldwide [1, 2]. Although traditional strategies for PCa have progressed in recent years, the five-year survival rate of PCa patients is far from satisfactory. Therefore, gaining deep insight into the pathogenesis of PCa is a critical step for developing novel therapies.
Deleted in liver cancer 1 (DLC1) belongs to the Rho guanosine triphosphatase (GTPase) activating protein (GAP) family and is located on human chromosome 8p21-22 [3, 4]. A previous study demonstrated that downregulation of DLC1 was associated with poor prognosis in patients with gastric cancer [5]. Moreover, DLC1 suppresses the progression of hepatocellular carcinoma by inhibiting the Rho and Rho-associated protein kinase (ROCK) signaling pathway [3]. Further, DLC1 markedly suppresses the invasion of PCa cells via regulating the Rho pathway [6]. However, the underlying molecular network of DLC1 in PCa cells still needs to be further explored.
DLC1 catalyzes the transformation of Rho GTPase from the GTP-bound active state to the GDP-bound inactive state [7]. ROCK is one of the most well-known downstream effectors of RhoA. ROCK1 and ROCK2 are two homologs of RhoA in humans that have been mapped to chromosomes 18q11 and 2p24, respectively [8]. Moreover, ROCK is upregulated in human PCa and breast cancer [9, 10]. A previous study has reported that DLC1 possesses the activity of Rho GAP, which is specific for RhoA [11]. However, the detailed correlation between DLC1 and ROCK is still unclear in PCa cells.
β-catenin has been identified as a transcriptional coactivator, which is induced by Wnt and recognized as a target for cancer therapy [12,13]. Dysregulation of Wnt/β-catenin signaling is identified as a biomarker for several human malignancies [[14], [15], [16], [17]]. Moreover, DLC1 interacts with α-catenin to enhance its anti-oncogene activity [18]. However, the role of DLC1 in the Wnt/β-catenin signaling pathway has not been identified in PCa cells.
The key protein in G0/G1 phase of cell cycle—cyclin D1 has been reported as an essential regulator in the process of the cell cycle [19]. Abnormal expression of cyclin D1 influences the progression of the cell cycle. The upregulation of cyclin D1 contributes to tumorigenesis [20]. Hence, cyclin D1 has functioned as a useful prognostic marker for cancers. Moreover, previous report indicated that β-catenin positively correlated with cyclin D1 [21]. However, the detailed molecular network of cyclin D1 is still unclear in PCa cells.
To further investigate the biological function of DLC1 and its possible targets in PCa cells, we induced DLC1 silencing and overexpression in PCa cells using RNA interference (RNAi) and lentiviral vector transduction, respectively.
2. Materials and methods
2.1. Bioinformatics analysis
Data were collected from RNA-sequencing data of the PCa cohort of the Cancer Genome Atlas (TCGA, https://tcga-data.nci.nih.gov/tcga/tcga/), and microarray gene expression profile dataset GSE55945 (Gene Expression Omnibus, http://www.ncbi.nlm.nih.gov/geo) were used to determine the expression of DLC1. A gene set enrichment analysis algorithm was used to identify the pathways that were significantly different between DLC1-high and -low PCa samples.
2.2. Tissue specimens and cell culture
A total of 25 paired PCa samples and adjacent-matched noncancerous tissue were obtained from Shanghai East Hospital, Shanghai, China. Samples were snap-frozen in liquid nitrogen and stored at −80 °C for further analysis. The PCa cell line used in this study was LNCaP cells. Human normal prostate epithelial cell line (RWPE-1 cells) was used as control. Both of them were purchased from the Cell Bank of Shanghai Biology Institute (Shanghai, China). All culture media were mixed with 10% fetal bovine serum (GIBCO, Carlsbad, CA, USA) containing 2 mM glutamine and 1% penicillin and streptomycin (Solarbio, Beijing, China). LNCaP and RWPE-1 cells were grown in Dulbecco's modified Eagle's medium (Trueline, Corning, NY, USA) and maintained in a 5% CO2 atmosphere at 37 °C. All patients were informed and gave their written consents. This study and the experimental procedures were approved by the independent ethics committee of Shanghai East Hospital, School of Medicine, Tongji University, Shanghai, China (No: 2013-DF21), and strictly obeyed the Declaration of Helsinki. The handling of mice and all animal experiments were performed according to the Institutional Animal Care and Use Committee guidelines and the institute's guidelines of Shanghai East Hospital, School of Medicine, Tongji University, Shanghai, China.
2.3. RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA from different samples was extracted using TRIzol reagent (Invitrogen, CA, USA). Then, RNA was reverse transcribed into complementary DNA (cDNA) using a cDNA synthesis kit (Fermentas, Ontario, Canada) according to the manufacturer's instructions. qRT-PCR conditions were as follows: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 45 s, and then normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The relative gene expression was calculated by the 2−ΔΔCt method [22]. All data represented the average of three replicates. The primers used in this study are listed in Supplementary Table 1.
2.4. Lentiviral-mediated RNA interference and overexpression of DLC1
Three short-interfering RNAs (siRNA) targeting the human gene DLC1 (NM_001164271.1; siDLC1-1, siDLC1-2, and siDLC1-3) were synthesized (Major, Shanghai, China). A nonspecific scramble siRNA sequence was treated as a negative control (siNC). Moreover, lentiviral plasmid (pLVX-puro) containing the full length of human DLC1 cDNA sequence (oeDLC1) was synthesized by Genewiz Company (Suzhou, China). The mock plasmid was functioned as a negative control (oeNC). All of the constructs were transiently transfected into LNCaP cells by Lipofectamine 2000 (Invitrogen, CA, USA) according to the manufacturer's instructions. Assays were performed 48 h after transfection. Detailed information about siDLC1 sequences is provided in Supplementary Table 2.
2.5. Western blot analysis
Whole protein lysates of the indicated samples were extracted by RIPA lysis buffer (JRDUN, Shanghai, China) with ethylene diamine tetraacetic acid (EDTA)-free protease inhibitor cocktail (Roche, Mannheim, Germany). The protein concentration was estimated by using an enhanced bicinchoninic acid protein assay kit (Thermo Fisher, Cleveland, OH, USA). Equal amounts of total protein (25 μg) were fractionated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to nitrocellulose membranes (Millipore, Billerica, MA, USA) overnight. After being blocked with 5% nonfat dry milk (P0216, Beyotime, Shanghai, China) for 1 h at room temperature, the membranes were probed at 4 °C overnight with the primary antibodies (ROCK1 [1:2000; Abcam, Cambridge, UK], ROCK2 [1:1000; Abcam, UK], DLC-1 [1:1000; Abcam, UK], β-catenin [1:5000; Abcam, UK], cyclin D1 [1:10,000; Abcam, UK], and GAPDH [1:2000; Cell Signaling Technology, Beverly, MA, USA]), and then the secondary anti-mouse IgG antibody (1:1000; Beyotime, Shanghai, China) for 1 h at 37 °C. An enhanced chemiluminescence system (Tanon, Guangzhou, China) was used to detect the protein expression value. The protein levels were normalized to GAPDH.
2.6. Cell proliferation assay
Cell Counting Kit-8 (CCK-8) assay (SAB, Pearland, Texas, USA) was used to measure the cell proliferation profile according to the manufacturer's protocol. Briefly, cells transfected as indicated were seeded in 96-well plates and cultured for 0 h, 24 h, 48 h, and 72 h. CCK-8 solution (10 μL in 100 μL Dulbecco’s modified Eagle's medium) was added to each well and incubated for 1 h. Optical density values at a wavelength of 450 nm were measured by a microplate reader (Pulangxin, Beijing, China). Triplicates measurements were performed at each time point.
2.7. Cell cycle assay
Propidium iodide staining was used to determine the DNA content. Cells with or without treatment were harvested and resuspended in phosphate buffer saline (PBS, Solarbio, Beijing, China). Then the cells were fixed with 70% ethanol, −20 °C for 2 h. Each group was treated with RNase A (Solarbio, Beijing, China) at 37 °C for 15 min. Then, propidium iodide (7Sea Biotech, Shanghai, China) was added to the cells. The cells were incubated at room temperature in darkness for 30 min. A flow cytometer (Becton Dickinson & Company, San Diego, CA, USA) was used to analyze the DNA content. The FlowJo cell cycle analysis program (Tree Star, San Carlos, CA, USA) was used to analyze the percentage of cells at Gap phase 0 (G0)/G1, synthesis phase, and G2/mitotic phases.
2.8. Xenograft model
The assay was carried out according to the institute's guidelines for animal experiments and was approved by the independent ethics committee of Shanghai East Hospital (NO. 2016-016). An equal number of PCa cells transfected with siNC and siDLC1 (n=2×106) were subcutaneously injected into the right flank of 4–6-week-old BALB/c nude mice (Shanghai Laboratory Animal Company, Shanghai, China). Mice were sacrificed by CO2 inhalation followed by cervical dislocation. The length and width of the tumors were examined every 3 days for 33 days at Day 12 after the injection.
2.9. Statistical analysis
GraphPad Prism software version 7.0 (GRAPH PAD software Inc, CA, USA) was used for statistical analyses. Data were displayed as the mean ± standard deviation (SD) of at least three samples. Statistical significance was determined by analysis of variance (ANOVA) for multiple comparisons, and Pearson's correlation analysis was used to examine the relationship between DLC1 and ROCK1 (ROCK2). The p<0.05 was accepted to indicate statistical significance.
3. Results
3.1. DLC1 was downregulated in PCa tissue and cells
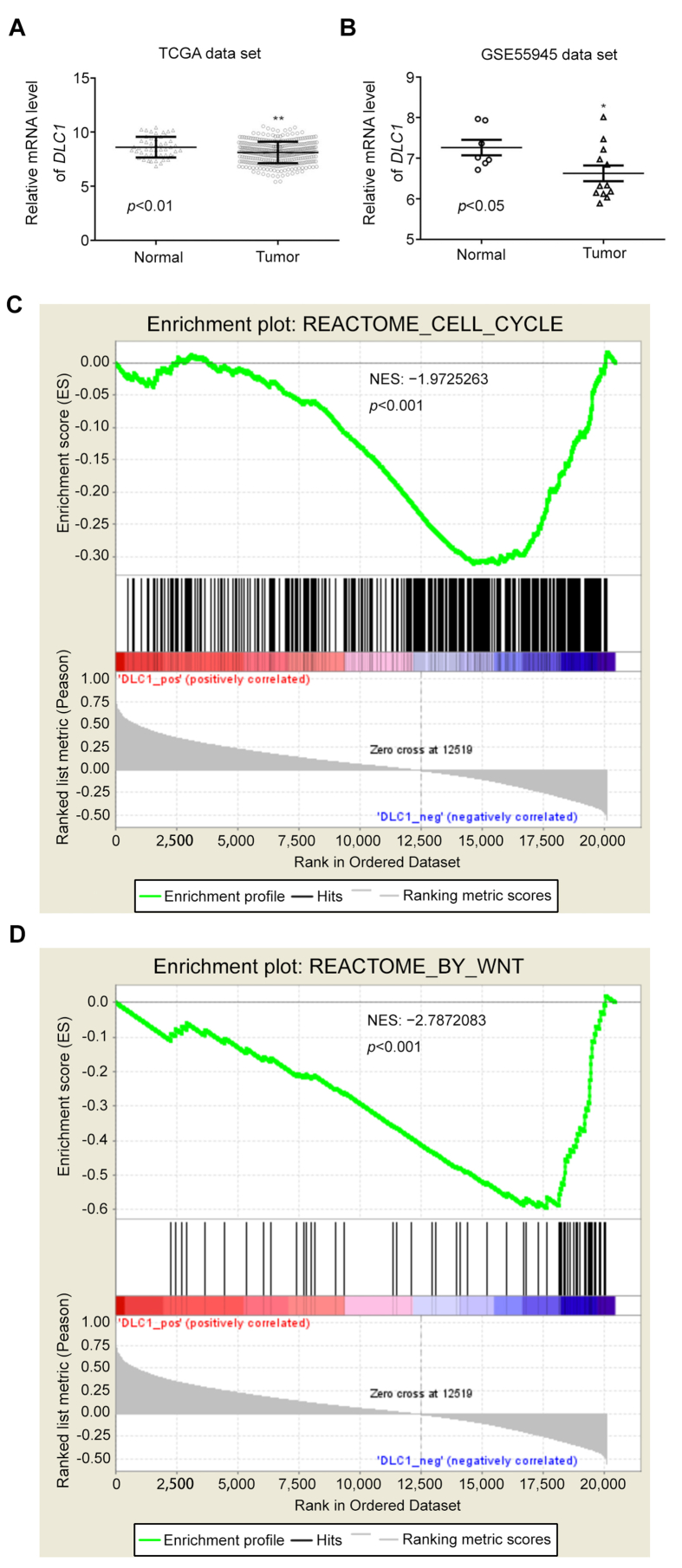
To assess the expression of DLC1 in PCa tissue, we collected its expression profile from two publicly available data sets, including the PCa data set from the Cancer Genome Atlas (TCGA) and GSE55945 data set from the Gene Expression Omnibus database. DLC1 was clearly downregulated in PCa tissue compared with normal tissue in these two datasets (Supplementary Fig. 1A,B). Moreover, DLC1 was indicated as a suppressor of the cell cycle and Wnt pathway in PCa tissue (Supplementary Fig. 1C,D).
Next, qRT-PCR was used to examine the relative mRNA levels of DLC1, ROCK1, and ROCK2 in 25 pairs of human PCa and matched paracancerous tissue. As shown in Fig. 1A, the relative mRNA level of DLC1 was much lower in PCa tissue than in paracancerous tissue, which was opposite to that of ROCK1 and ROCK2.
Figure 1.
DLC1 was downregulated in PCa tissue and PCa cells. (A) The mRNA levels of DLC1, ROCK1, and ROCK2 were examined in PCa tissues (n=25 in each group). (B) The relative mRNA levels of DLC1, ROCK1, and ROCK2 in LNCaP and RWPE-1 cells were presented, respectively. (C) The relative protein levels of DLC1, ROCK1, and ROCK2 in LNCaP and RWPE-1 cells were presented, respectively. (D) DLC1 mRNA was negatively correlated with ROCK1 mRNA or ROCK2 mRNA in PCa tissue, n=25 for each group. DLC1, deleted in liver cancer 1; PCa, prostate cancer; ROCK, Rho-associated protein kinase; GAPDH, Glyceraldehyde-3-phosphate dehydrogenase. ∗∗ p<0.01, ∗∗∗ p<0.001.
Then, we compared the relative mRNA levels of DLC1, ROCK1, and ROCK2 between PCa cell LNCaP and normal prostate epithelial cell RWPE-1. The relative mRNA and protein levels of DLC1 were significantly decreased in LNCaP cells compared with that in RWPE-1 cells. However, the relative mRNA and protein levels of ROCK1 and ROCK2 were much higher in LNCaP cells than in RWPE-1 cells (Fig. 1B,C). Furthermore, correlation analysis indicated that DLC1 was negatively correlated with ROCK1and ROCK2 in LNCaP cells (Fig. 1D).
3.2. Knockdown and overexpression of DLC1 in LNCaP cells
To further assess the function of DLC1 in LNCaP cells, we induced DLC1 knockdown and overexpression in LNCaP cells. For overexpression, the full-length DLC1 cDNA was inserted into a lentiviral vector. Then, the recombinant vector (oeDLC1) and mock plasmid (oeNC) were transfected into LNCaP cells. The untreated cells acted as a blank control (Blank). Marked overexpression of DLC1 was clearly identified in oeDLC1-transfected cells (Fig. 2A,B).
Figure 2.
Overexpression and knockdown of DLC1 in LNCaP cells. (A) The mRNA levels of DLC1 in oeDLC1-transfected cells were examined using qRT-PCR. (B) The protein levels of DLC1 in oeDLC1-transfected cells were examined using Western blot. (C) The relative mRNA levels of DLC1 in siDLC1-transfected cells were examined using qRT-PCR. (D) The relative protein levels of DLC1 in siDLC1-transfected cells were examined using Western blot. DLC1, deleted in liver cancer 1; PCa, prostate cancer; siNC, negative control siRNA; oeNC, negative control of overexpression; oeDLC1, overexpression of DLC1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; qRT-PCR, quantitative real-time real-time polymerase chain reaction. ∗∗∗ p<0.001.
For the silencing assay, three siRNAs targeting human DLC1 (siDLC1-1, siDLC1-2 and siDLC1-3) and nonspecific scramble siRNA (siNC) were synthesized and transfected into LNCaP cells. DLC1 siRNAs strongly reduced the endogenous expression of DLC1. Moreover, siDLC1-1 showed a stronger effect than siDLC1-2 and siDLC1-3 (Fig. 2C,D). Therefore, oeDLC1 transfected and siDLC1-1-transfected cells were chosen for the following analyses.
3.3. DLC1 overexpression inhibited the proliferation and cell cycle of LNCaP cells
CCK-8 assay was performed to examine cell proliferation. As shown in Fig. 3A, the cell proliferation of the Blank and NC groups showed no significant difference. However, the proliferation rate markedly decreased in oeDLC1-transfected cells, whereas it was significantly upregulated in siDLC1-transfected cells. These results demonstrated that DLC1 was an anti-proliferative factor in PCa cells. Moreover, overexpression of DLC1 deeply decreased the colony formation of human LNCaP cells, while the opposite results was obtained in siDLC1 transfected cells (Fig. 3B).
Figure 3.
DLC1 overexpression suppressed the proliferation and cell cycle of LNCaP cells. (A) Cell proliferation was detected 12 h, 24 h, 48 h, and 72 h after transfection with NC (both siNC and oeNC), oeDLC1, and siDLC1.(B) Colony formation assay was performed in cells as indicated above. (C) Cell cycle profiles of cells transfected with NC, oeDLC1, or siDLC1 were examined by using flow cytometry. (D) The protein levels of ROCK1, ROCK2, β-catenin, cyclin D1, and cyclin B in different transfected cells. DLC1, deleted in liver cancer 1; PCa, prostate cancer; ROCK, Rho-associated protein kinase; OD450, optical density 450; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NC, negative control; oeDLC1, overexpression of DLC1; siDLC1, DLC1 siRNA; S, synthesis phase; G1, gap phase 1; G2, gap phase 2. ∗ p<0.05; ∗∗ p<0.01; ∗∗∗ p<0.001.
Next, flow cytometry was utilized to assess the cell cycle in different cells as indicated. As shown in Fig. 3C, the G0/G1 phase cell population was significantly decreased in siDLC1-transfected cells compared with that in NC-transfected cells. Moreover, DLC1 siRNA prolonged the G2/M phase in LNCaP cells, whereas the opposite results were obtained in oeDLC1 transfected cells.
Moreover, Western blot analysis was used to quantify the protein levels of ROCK1, ROCK2, β-catenin, cyclin D1, and cyclin B (the key protein of G2/M phase) in cells as indicated. Importantly, the protein levels of ROCK1 and ROCK2 markedly decreased in oeDLC1-transfected cells but increased in siDLC1-transfected cells (Fig. 3D). Interestingly, DLC1 overexpression also significantly inhibited the expression of β-catenin and cyclin D1, while promoted the expression of cyclin B in PCa cells.
3.4. The ROCK inhibitor Y27632 rescued the function of DLC1 in LNCaP cells
To further analyze the connection between DLC1 and ROCK, a specific ROCK inhibitor Y27632 (10 mmol/L; ACS-3030; ATCC, VA, USA) was used to treat siNC- and siDLC1-transfected cells. As shown in Fig. 4A, Y27632 significantly inhibited the cell proliferation rate of siNC- and siDLC1-transfected cells and deeply reduced the colony formation in siDLC1-transfected cells as well (Fig. 4B). Moreover, the function of siDLC1 in the cell cycle was abolished by Y27632 (Fig. 4C). In addition, we also examined the protein levels of β-catenin and cyclin D1 in siDLC1-transfected cells as indicated. As shown in Fig. 4D, Y27632 markedly suppressed the expression of β-catenin and cyclin D1, but promoted cyclin B expression in siDLC1-transfected cells. Taken together, these results demonstrated that the ROCK inhibitor Y27632 played a key role in rescuing the function of DLC1 in PCa cells.
Figure 4.
The ROCK inhibitor Y27632 rescued the function of DLC1 in LNCaP cells. (A) Cell proliferation was detected at 12 h, 24 h, 48 h, and 72 h in cells transfected with siNC, siDLC1, or siNC and treated with Y27632. (B) Colony formation assay was performed in cells as indicated above. (C) Y27632 reduced the effect of siDLC1 in PCa cell cycle regulation. (D) Western blot was used to examine the protein levels of β-catenin, cyclin D1, and cyclin B in cells as indicate above. ROCK, Rho-associated protein kinase; PCa, prostate cancer; siNC, negative control siRNA; DLC1, deleted in liver cancer 1; siDLC1, DLC1 siRNA; G1, Gap phase 1; G2, Gap phase 2; S, synthesis phase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. ∗ p<0.05; ∗∗ p<0.01; ∗∗ p<0.001.
3.5. Knockdown of DLC1 promoted the tumorigenicity of LNCaP cells in vivo
To further examine the effect of DLC1 on tumorigenicity in vivo, a PCa model was established in nude mice. An equal number of LNCaP cells that were transfected with siNC and siDLC1 were hypodermically injected into nude mice. Then, the mice were treated with Y27632 once the tumor formed. Subsequently, the tumor was determined every 3 days for 33 days (starting at Day 12). All of the injected cells were able to clearly develop tumors in nude mice. However, both the tumor volume and weight were significantly increased by injection with siDLC1-transfected cells compared to injection with siNC-transfected cells. Interestingly, Y27632 abolished the tumorigenicity of PCa cells transfected with siDLC1 (Fig. 5A,B).
Figure 5.
The ROCK inhibitor Y27632 inhibited the tumorigenicity of LNCaP cells that transfecting with siDLC1 in vivo. (A and B) The tumor volume and weight in nude mice injected with PCa cells transfected with siNC or siDLC1, with or without Y27632 treatment (n=6 in each group). (C) The relative protein levels of DLC1, ROCK1, ROCK2, β-catenin, and cyclin D1 in different tumors as indicated. ROCK, Rho-associated protein kinase; PCa, prostate cancer; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; DLC1, deleted in liver cancer 1; siDLC1, DLC1 siRNA; siNC, negative control siRNA. ∗∗∗ p<0.001.
Further, the protein level of DLC1 was also increased by Y27632 in vivo. (Fig. 5C) Meanwhile, the levels of β-catenin and cyclin D1 were significantly decreased by Y27632. These findings were consistent with those in vitro. Therefore, the ROCK inhibitor Y27632 was a promising agent in the treatment of PCa.
4. Discussion
Testosterone is needed for PCa cells to maintain growth and progression during its early stages. Hence, androgen-deprivation therapy has been identified as a useful approach in the treatment of PCa at its initial phase [23]. However, this method is not effective for androgen depletion-independent cells. Therefore, a novel useful biomarker for PCa is urgently needed.
In the current study, we investigated the biological function of DLC1 in LNCaP cells. Silencing and overexpression of DLC1 were induced by using RNAi and lentiviral vector transduction in LNCaP cells. The results obtained from these two experimental sections were consistent, which make our results more robust.
A previous study reported that DLC1 suppressed the progression of hepatocellular carcinoma cells via inhibiting ROCK [3]. Moreover, overexpression of DLC1 significantly inhibits the proliferation of cutaneous squamous cell carcinoma [24]. Moreover, a similar result was obtained in Burkitt's lymphoma cells [25]. In the current research, our results demonstrated that DLC1 was downregulated in PCa tissues and cells. Moreover, DLC1 overexpression markedly suppressed the proliferation and cell cycle progression of LNCaP cells. Therefore, our findings indicated that DLC1 was an anti-proliferation and cell cycle factor in PCa cells. Importantly, our results not only elucidated that DLC1 was a tumor suppressor in LNCaP cells, but also demonstrated its potential value as a biomarker for PCa diagnosis. Furthermore, our analysis indicated a negative correlation between DLC1 and ROCK in PCa tissue and cells. Therefore, DLC1 was involved in the ROCK pathway in PCa tissues and cells. DLC1 might suppress the growth of PCa cells by inhibiting ROCK1 and ROCK2.
A previous report stated that DLC1 suppressed the growth of colon cancer cells by inhibiting Wnt/β-catenin signaling [26]. In the present study, DLC1 overexpression markedly inhibited the expression of β-catenin in LNCaP cells. It has been confirmed that ROCK is the upstream factor of β-catenin in breast cancer and colon carcinoma cells [27, 28]. Therefore, β-catenin might be a component in the DLC1/ROCK signaling pathway in PCa cells. Our findings firstly illustrated a negative correlation between DLC1 and β-catenin in PCa cells.
Cyclin D1 belongs to the G1 cyclin family and is identified as a positive regulator of cell cycle progression [21]. Moreover, a previous report demonstrated that DLC1 suppressed the growth of renal carcinoma cells by regulating cyclin D1 [29]. Importantly, our studies also obtained similar results. Therefore, DLC1 might inhibit the cell cycle of PCa cells by suppressing cyclin D1. Moreover, DLC1-knockdown significantly promoted the tumorigenicity of LNCaP cells in vivo, which was markedly suppressed in the presence of Y27632, the ROCK inhibitor. Therefore, Y27632 is a potential agent for the treatment of PCa. Targeting the ROCK signaling pathway might provide a novel perspective for the treatment of PCa.
5. Conclusion
In the present study, our results indicated that DLC1 overexpression markedly suppressed the proliferation and cell cycle progression of PCa cells and negatively correlated with ROCK expression in PCa cells and tissue. Our findings not only deepen the understanding of DLC1 in PCa cells but also provide novel insight into PCa therapy.
Author contributions
Study concept and design: Weihua Chen.
Data acquisition: Hua Gong, Kang Chen.
Data analysis: Lan Zhou, Yongchao Jin.
Drafting of manuscript: Hua Gong.
Critical revision of the manuscript: Weihua Chen.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgement
This study was supported by the Key Scientific Research Project of Shanghai Municipal Commission of Health and Family Planning (No. 201640014), the project of Natural Science Foundation of Jiangxi (No. 20171BAB205019), and the Special Diseases Program of Pudong New Area Health System (No. PWZzb2017-06). All authors sincerely acknowledged the support given by Shanghai University of Medicine and Health Sciences Affiliated Zhoupu Hospital and Shanghai East Hospital, School of Medicine, Tongji University, Shanghai, China.
Footnotes
Peer review under responsibility of Tongji University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajur.2021.12.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig S1.
DLC1 was downregulated in PCa tissues. (A and B) The relative mRNA level of DLC1 was downregulated in PCa tissues. Data were collected from the PCa data set from TCGA and GSE55945 data set from Gene Expression Omnibus data. (C) Enrichment plot of REACTOME_CELL_CYCLE with enrichment score −1.97, p<0.001. (D) Enrichment plot of REACTOME_SIGNALING_BY_WNT with enrichment score −2.79, p<0.001. DLC1 is predicated that negatively involved in cell cycle and Wnt signaling pathway in PCa tissues. TCGA, The Cancer Genome Atlas data. PCa, prostate cancer.
References
- 1.Siemens D.R., Hoare D., Skinner T., Black A. Serum follicle-stimulating hormone levels predict time to development of castration resistant prostate cancer. Can Urol Assoc J. 2015;9:122–127. doi: 10.5489/cuaj.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sebesta E.M., Anderson C.B. The surgical management of prostate cancer. Semin Oncol. 2018;44:347–357. doi: 10.1053/j.seminoncol.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Wong C.C., Wong C.M., Ko F.C., Chan L.K., Ching Y.P., Yam J.W., et al. Deleted in liver cancer 1 (DLC1) negatively regulates Rho/ROCK/MLC pathway in hepatocellular carcinoma. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basak P., Dillon R., Leslie H., Raouf A., Mowat M.R. The deleted in liver cancer 1 (Dlc1) tumor suppressor is haploinsufficient for mammary gland development and epithelial cell polarity. BMC Cancer. 2015;15:630. doi: 10.1186/s12885-015-1642-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su Y., Lin L., Zhang J., Jiang Y., Pan C., Sun L., et al. Low expression of DLC1 is predictive of poor therapeutic efficiency of fluoropyrimidine and oxaliplatin as adjuvant chemotherapy in gastric cancer. Mol Med Rep. 2015;12:5771–5779. doi: 10.3892/mmr.2015.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tripathi V., Popescu N.C., Zimonjic D.B. DLC1 induces expression of E-cadherin in prostate cancer cells through Rho pathway and suppresses invasion. Oncogene. 2014;33:724–733. doi: 10.1038/onc.2013.7. [DOI] [PubMed] [Google Scholar]
- 7.Hall A. Signal transduction through small GTPasesA—tale of two GAPs. Cell. 1992;69:369–391. doi: 10.1016/0092-8674(92)90441-e. [DOI] [PubMed] [Google Scholar]
- 8.Amin E., Dubey B.N., Zhang S.C., Gremer L., Dvorsky R., Moll J.M., et al. Rho-kinase: regulation, (dys)function, and inhibition. Biol Chem. 2013;394:1399–1410. doi: 10.1515/hsz-2013-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsubara M., Bissell M.J. Inhibitors of rho kinase (ROCK) signaling revert the malignant phenotype of breast cancer cells in 3D context. Oncotarget. 2016;7:31602–31622. doi: 10.18632/oncotarget.9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mu D., Zhou G., Li J., Su B., Guo H. Ursolic acid activates the apoptosis of prostate cancer via ROCK/PTEN mediated mitochondrial translocation of cofilin-1. Oncol Lett. 2018;15:3202–3206. doi: 10.3892/ol.2017.7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong C.M., Lee J.M.F., Ching Y.P., Jin D.Y., Ng I.O.L. Genetic and epigenetic alterations of DLC-1 gene in hepatocellular Carcinoma. Cancer Res. 2003;63:7646–7651. [PubMed] [Google Scholar]
- 12.Yang J., Mowry L.E., Nejak-Bowen K.N., Okabe H., Diegel C.R., Lang R.A., et al. β-catenin signaling in murine liver zonation and regeneration: a Wnt-Wnt situation. Hepatology. 2014;60:964–976. doi: 10.1002/hep.27082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui C., Zhou X., Zhang W., Qu Y., Ke X. Is β-catenin a druggable target for cancer therapy? Trends Biochem Sci. 2018;43:623–634. doi: 10.1016/j.tibs.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Xu J., Prosperi J.R., Choudhury N., Olopade O.I., Goss K.H. Beta-catenin is required for the tumorigenic behavior of triple-negative breast cancer cells. PLoS One. 2015;10 doi: 10.1371/journal.pone.0117097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong L., Deng J., Sun Z.M., Pan A.P., Xiang X.J., Zhang L., et al. Interference with the beta-catenin gene in gastric cancer induces changes to the miRNA expression profile. Tumour Biol. 2015;36:6973–6983. doi: 10.1007/s13277-015-3415-1. [DOI] [PubMed] [Google Scholar]
- 16.Francis J.C., Thomsen M.K., Taketo M.M., Swain A. β-catenin is required for prostate development and cooperates with Pten loss to drive invasive carcinoma. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White B.D., Chien A.J., Dawson D.W. Dysregulation of Wnt/beta-catenin signaling in gastrointestinal cancers. Gastroenterology. 2012;142:219–232. doi: 10.1053/j.gastro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tripathi V., Popescu N.C., Zimonjic D.B. DLC1 interaction with alpha-catenin stabilizes adherens junctions and enhances DLC1 antioncogenic activity. Mol Cell Biol. 2012;32:2145–2159. doi: 10.1128/MCB.06580-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka T., Kubota M., Shinohara K., Yasuda K., Kato J-y. In vivo analysis of the cyclin D1 promoter during early embryogenesis in Xenopus. Cell Struct Funct. 2003;28:165–177. doi: 10.1247/csf.28.165. [DOI] [PubMed] [Google Scholar]
- 20.Liang S., Mu K., Wang Y., Zhou Z., Zhang J., Sheng Y., et al. CyclinD1, a prominent prognostic marker for endometrial diseases. Diagn Pathol. 2013;8:138. doi: 10.1186/1746-1596-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H., Wang H., Zhou X., Makki M.S., Wang J. Overexpression of β-catenin and cyclin D1 predicts a poor prognosis in ovarian serous carcinomas. Int J Clin Exp Pathol. 2014;7:264–271. [PMC free article] [PubMed] [Google Scholar]
- 22.Livak K., Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods. 2000;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Jeong J.H., Bhatia A., Toth Z., Oh S., Inn K.S., Liao C.P., et al. TPL2/COT/MAP3K8 (TPL2) activation promotes androgen depletion-independent (ADI) prostate cancer growth. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang C., Wu D., Jia J., Liu D., Li Z., Zhang C., et al. DLC1 as a regulator of proliferation, invasion, cell cycle, and apoptosis in cutaneous squamous cell carcinoma. Tumour Biol. 2013;34:2633–2643. doi: 10.1007/s13277-013-0813-0. [DOI] [PubMed] [Google Scholar]
- 25.Feng M., Huang B., Du Z., Xu X., Chen Z. DLC-1 as a modulator of proliferation, apoptosis and migration in Burkitt's lymphoma cells. Mol Biol Rep. 2011;38:1915–1920. doi: 10.1007/s11033-010-0311-z. [DOI] [PubMed] [Google Scholar]
- 26.Wang C., Wang J., Liu H., Fu Z. Tumor suppressor DLC-1 induces apoptosis and inhibits the growth and invasion of colon cancer cells through the Wnt/beta-catenin signaling pathway. Oncol Rep. 2014;31:2270–2278. doi: 10.3892/or.2014.3057. [DOI] [PubMed] [Google Scholar]
- 27.Guerra F.S., Oliveira R.G., Fraga C.A.M., Mermelstein C.D.S., Fernandes P.D. ROCK inhibition with Fasudil induces beta-catenin nuclear translocation and inhibits cell migration of MDA-MB 231 human breast cancer cells. Sci Rep. 2017;7:13723. doi: 10.1038/s41598-017-14216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song G.L., Jin C.C., Zhao W., Tang Y., Wang Y.L., Li M., et al. Regulation of the RhoA/ROCK/AKT/β-catenin pathway by arginine-specific ADP-ribosytransferases 1 promotes migration and epithelial-mesenchymal transition in colon carcinoma. Int J Oncol. 2016;49:646–656. doi: 10.3892/ijo.2016.3539. [DOI] [PubMed] [Google Scholar]
- 29.Zhang T., Zheng J., Jiang N., Wang G., Shi Q., Liu C., et al. Overexpression of DLC-1 induces cell apoptosis and proliferation inhibition in the renal cell carcinoma. Cancer Lett. 2009;283:59–67. doi: 10.1016/j.canlet.2009.03.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.