Abstract
Purpose of Review
Olfactory dysfunction contributes to the psychopathology of mental illness. In this review, we describe the neurobiology of olfaction, and the most common olfactory alterations in several mental illnesses. We also highlight the role, hitherto underestimated, that the olfactory pathways play in the regulation of higher brain functions and its involvement in the pathophysiology of psychiatric disorders, as well as the effect of inflammation on neurogenesis as a possible mechanism involved in olfactory dysfunction in psychiatric conditions.
Recent Findings
The olfactory deficits present in anxiety, depression, schizophrenia or bipolar disorder consist of specific alterations of different components of the sense of smell, mainly the identification of odours, as well as the qualifications of their hedonic valence (pleasant or unpleasant). Epidemiological findings have shown that both environmental factors, such as air pollutants, and inflammatory disease of the upper respiratory tract, can contribute to an increased risk of mental illness, at least in part, due to peripheral inflammatory mechanisms of the olfactory system.
Summary
In this review, we describe the neurobiology of olfaction, and the most common olfactory function alterations in several psychiatric conditions and its role as a useful symptom for the differential diagnosis. We also highlight the effect of inflammation on neurogenesis as a possible mechanism involved in olfactory dysfunction in these psychiatric conditions.
Keywords: Olfaction, Mental illness, Anxiety, Depression, Schizophrenia, Bipolar disorder, Neurogenesis, Olfactory neuroepithelium, Olfactory bulbs
Introduction
Mental illnesses such as anxiety, depression, schizophrenia or bipolar disorder are a worldwide problem of increasingly frequent, being estimated that one in every three people will suffer from one of these disorders during his life. In addition, these diseases have a high degree of comorbidity, so that they can present at the same time in the same individual.
Olfactory disturbances contribute to psychopathology of mental illness [1••, 2••, 3]. The sense of smell is crucial in many aspects of life and health. It is important for feeding, alerting of potential danger and orienting towards pleasant stimuli as well as for social relationships. Loss of smell, therefore, has relevant implications for quality of life and physical safety, and is often associated with social isolation. Subjects with loss of smell are more likely to suffer from anxiety, depression and even have a greater risk of death, because of their inability to respond to dangerous odours, such as toxic chemicals, rotting food and leaking gas [4••, 5].
Olfactory deficits present in mental illness consist of specific alterations of different components of the sense of smell as perception, identification and discrimination, as well as the qualification of its hedonic valence (pleasant or unpleasant) [6]. Recent evidences have highlighted the role, hitherto underestimated, of the olfactory pathway in the regulation of higher brain functions and its involvement in the pathophysiology of psychiatric disorders. In this sense, an overlap has been described between the neural connections of the olfactory system, temporo-limbic and frontal functions that are associated with higher brain functions such as cognition, memory and emotion, which are the ones that are altered in psychiatric disorders [1••, 7]. Accordingly, it is currently considered that alterations of the peripheral and central olfactory system contribute to an altered emotional process of olfactory stimulation in patients with psychiatric disorders. Epidemiological findings have shown that environmental factors, such as air pollutants, and exposure to inflammatory upper airway diseases may contribute to increased risk of suffering from mental illness, perhaps at least partly due to peripheral inflammatory mechanisms of the olfactory system [1••].
Olfactory dysfunction can be considered potential marker for psychiatric disorders, and may constitute either a factor of vulnerability or a consequence of these disorders [8]. Moreover, olfactory dysfunction may be a significant pathological hallmark in the early stages of disease progression, including first episode psychosis [1••, 3, 9]. However, although the pathological implication of olfactory impairments in psychiatric disorders, how olfactory dysfunction affects the neural mechanisms involved in higher brain functions remains poorly understood.
Anatomy and Physiology of Olfactory System
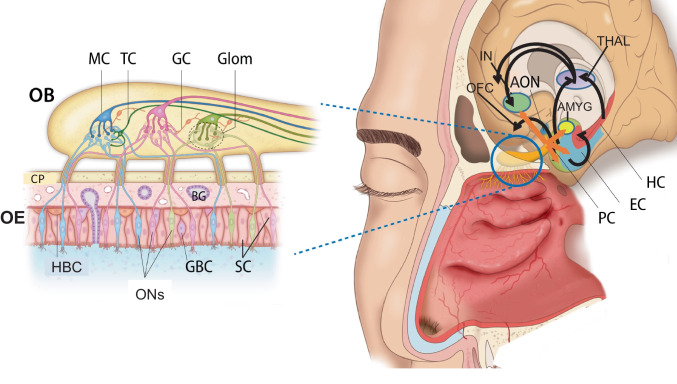
Odour information is initially perceived through the olfactory neurons (ONs) in the olfactory epithelium (OE) and transmitted to the central olfactory system, which is composed of the olfactory bulbs (OB) which in turn projects to a variety of secondary olfactory structures including the anterior olfactory nucleus (AON), piriform cortex olfactory tubercle, the lateral entorhinal cortex and para-amygdaloid complex [10–13] (Fig. 1). Neurons within these secondary olfactory structures project to tertiary olfactory structures, which include the orbitofrontal cortex (OFC), the insular cortex and the dorsal hippocampus [14] (Fig. 1).
Fig. 1.
Peripheral and central olfactory system. Odour information is initially perceived through the olfactory neurons in the olfactory epithelium and transmitted to the central olfactory system, which is composed of the olfactory bulbs which in turn projects to a variety of secondary olfactory structures including the anterior olfactory nucleus, piriform cortex olfactory tubercle, the lateral entorhinal cortex and para-amygdaloid complex. Neurons within these secondary olfactory structures project to tertiary olfactory structures, which include the orbitofrontal cortex, the insular cortex and the dorsal hippocampus. An overlap has been described between the neural connections of the olfactory system, temporo-limbic and frontal functions that are associated with higher brain functions such as cognition, memory and emotion, which are the ones that are altered in psychiatric disorders. AMYG, amygdala complex; AON, anterior olfactory nucleus; BG, Bowman’s gland; CP, cribriform plate; EC, enthorrinal cortex; GBC, globose basal cells; GC, glomerular cells; Glom, glomerulus; HBC, horizontal basal cells; HC, hippocampus; IN, insular cortex; MC, mitral cells; OB, olfactory bulb; OE, olfactory epithelium; OFC, orbitofrontal cortex; ONs, olfactory neurons; PC, piriform cortex; SC, sustentacular cells; THAL, thalamus; TC, tufted cells
The OE is a pseudostratified epithelium located in the dorsal and posterior portion of the nasal cavity, extending along the nasal septum and turbinates, containing different cell populations. The sustentacular cells surround the cell bodies of the dendritic projection of the mature ONs. Below this layer are the immature neurons and the progenitor basal cells (horizontal and globose cells) (Fig. 1). The half-life of ONs is of 30 days and the OE has ability to regenerate, with normal ONs production through globose cell proliferation and differentiation [15]. In case of severe OE damage, the quiescent horizontal basal cells are activated and differentiating not only in ONs, but also in sustentacular cells and in globose basal cells, mechanisms by which attempts are made to maintain OE homeostasis.
ONs are bipolar cells that have dendrites extending to the nasal cavity that contain the sensory cilia, and an axon that passes through the cribriform plate to reach the OB (Fig. 1). ONs express a type of olfactory receptor and project to the glomeruli of the OB that contains axons with the same olfactory receptor. Once the odorant interacts with the olfactory receptors of the ONs, action potentials in the ON axon are generated relaying odorant information into OBs. In addition to the ONs, the OE contains sustentacular or supporting cells for stability of the epithelium, and basal cells that provide regenerative capacity.
At OB level, axons from ONs synapse with the dendrites of secondary olfactory projection neurons, the mitral (MCs) and tufted cells (TCs), forming a structure called glomerulus (Fig. 1). MCs and TCs are the primary efferent projection neurons of the OB. The incoming axons from the ONs also synapse on local gamma-aminobutyric acid (GABA)ergic and dopaminergic interneurons (periglomerular cells). Glomeruli are the first synaptic relay in the olfactory pathway and play a basic role in smell perception [16]. MCs and TCs have different physiological responses to odours, including odour intensity coding [17].
Smell deficits are found in around 20% of the general population [18]. The most common smell disturbances include anosmia (complete loss of olfactory ability) and hyposmia (decreased olfactory ability). Hyperosmia (heightened olfactory ability) is less common. Aspects of smell commonly tested in clinical populations include odour detection/threshold, discrimination and identification [19]. Odour threshold refers to the smallest concentration of an odour required to produce the percept of smell. Odour discrimination is the ability to distinguish between two or more different odours. Odour identification, the most commonly used test, is the ability to detect, identify and name a scent [19]. Both odour identification and odour discrimination tests rely more on cognitive functions (specifically semantic memory) than odour threshold tests, given they require knowledge of a particular scent.
Olfactory Dysfunction in Mental Illness
The neuroanatomic overlap of the olfactory system and limbic brain regions makes the olfactory system of particular relevance to psychiatric conditions marked by significant mood disturbance and negative symptoms including anxiety, major depression, schizophrenia and bipolar disorder [20, 21••, 22–24].
Anxiety
Anxiety is an unpleasant and sometimes prevalent emotional state impacting in several spheres of life. From milder manifestations of anxiety, like mild perfectionism, to more severe disorders such as post-traumatic stress disorder or agoraphobia, anxiety influences performance in tasks involving various sensory modalities. Generalized anxiety disorder (GAD) includes excessive and persistent anxiety, and worry with apprehensive expectations of events in association with physical symptoms, including feeling keyed up or on edge, and having difficulty concentrating.
Olfactory dysfunction has been demonstrated in patients with GAD consisting in odour threshold, discrimination and identification deficits [25, 26]. When considered the moderate/severe anxiety subgroup, olfactory discrimination and identification, but not odour threshold, scores were significantly and inversely correlated with the anxiety score, suggesting that poorer olfactory function is correlated with greater severity of somatic anxiety [25]. These findings correspond to that the olfactory and affective processes overlap in the cerebral anatomy structure—the cortical amygdala nuclei [27]. The amygdala and the OFC are the primary implicated neurocircuitries of anxiety disorders [28]. The primary olfactory cortex, including the cortical amygdala nuclei, transports odour information to other parts of the amygdala [29]. Dysfunction in the overlapping structure, the amygdala, may therefore be the crucial factor in the relationship. Taking in consideration that odour threshold is a predominant peripheral sensory process, which is related to olfactory receptors at the OE level, while odour discrimination and identification are central sensory processes that are related to the primary olfactory cortex [29], it is possible to speculate that this is reason why odour discrimination is related to anxiety symptom severity, whereas odour threshold is not [25].
In addition, the influence of trait or sustained anxiety on human olfactory function is consistent with a consistent shift in odour sensitivity toward certain odorants and away from others [30, 31, 32••]. For example, in post-traumatic stress disorder, an increased sensitivity toward potentially dangerous odorants has been described [32••, 33]. These specific fear-olfactory relationships are considered to be driven by the close link in associative memory existing between emotional trauma and the odours present during these experiences. In addition, it has been suggested that odour hedonics may play a critical role in threat-related changes in olfactory function in anxious subjects that process unpleasant odorants differentially [34]. In this line, it has been described a positive correlation between state anxiety levels, a transitory emotional state characterized by subjective perceived feelings of tension and apprehension fluctuating over time, and unpleasant odour discrimination accuracy [35]. Other clinical and preclinical data suggest that trigeminal properties of odorants may be relevant to contextual threat and anxiety-related odour sensitivities [36]. In this line, patients suffering from post-traumatic stress disorder tend to perform better in odour identification tests and respond faster to CO2, an unpleasant stinging gas which acts on the trigeminal system [34]. Similarly, an association between increased anxiety sensitivity and increased sensitivity for guaiacol, a smoke-like odorant with high trigeminal properties, has been described [30, 32••]. Given that the intranasal trigeminal system functions, in part, to detect irritants and potentially harmful airborne chemicals in the environment increased sensitivity to odorants with high trigeminal properties is consistent with numerous other safety behaviours that are enhanced in those with anxiety-related traits and disorders [32••].
An olfactory dysfunction has been also described in panic disorder consisting in a lower odour threshold that has been found correlated with the severity of symptoms [37]. Moreover, Clepce et al. [26] found poor odour discrimination in patients with panic disorder, social phobia and agoraphobia. However, other studies did not find significant differences between anxiety patients and controls [38].
In addition to the different olfaction tests used in the different studies, they key symptoms of different anxiety disorders may be also account for these conflicting results [25]. Further olfactory studies in terms of anxiety disorders focused on key symptoms are required. A better understanding of how the olfactory system modulates odorants in response to stress/anxiety, and how specific odour factors such as hedonic valence and trigeminal properties influence the stress/anxiety-olfactory relationship is needed.
Depression
Depression is one of the most common mental health conditions, with a prevalence of 17% in the general population. Symptoms include loss of interest for activities that in the past were pleasurable (anhedonia), feelings of worthlessness, difficulty concentrating and sleep and suicidal ideation. Increasing evidence has revealed the existence of a relationship between the presence of alterations of smell and depression [2••, 24, 39, 40, 41••].
In this line, patients with olfactory loss are more likely to exhibit symptoms of depression [42, 43]. Moreover, a number of studies have been undertaken to assess olfactory function in populations suffering from depression. Depressed patients express impaired odour threshold, discrimination and identification [2••, 22, 24, 39, 41••, 44–48], being olfactory threshold the most significant feature in depression [49, 50].
Various clinical studies indicate a correlation of olfactory dysfunction with the severity of depression, worsening in turn the quality of life of the patient and with the suicidal ideation [24, 51, 52]. In detail, recurrent depressive disorders have been found associated with reduced odour identification [48], while the longer duration of disease is negatively related to odour threshold [53]. All these observations support the concept that depression is not uniformly related to olfactory impairment, but depends on duration and course of the disease [53]. It has been suggested that reduced olfactory identification might be caused by cognitive impairment in recurrent depression, while the relation between olfactory threshold and depression might be partially caused by a cumulative damage of the olfactory epithelium after frequent respiratory diseases, as later described. However, some studies do not report reduction of olfactory function in any of the measured domains or found only small differences in olfactory function between depressive patients and controls [54, 55]. Variations in the results may be due to different populations, variance in the olfaction measures, small patient cohorts and some studies using patients with primary olfactory dysfunction [47] while others using patients with primary depression [19].
Hedonic rating of smells may be affected by depression since depressed patients over-evaluated the pleasantness of positive odours, suggesting a functional bias in brain processing of pleasantness in depressive states [44, 46]. Moreover, the hedonic rating of unpleasant odour is also affected since patients tend to perceive unpleasant odours as more unpleasant than controls [51, 56]. The impairment in hedonic perception tends to disappear after mood improvement [51]. Depressed patients also exhibit “olfactory anhedonia”, since they cannot discriminate between different concentrations of pleasant odours. This anhedonia may be a possible state marker of depression [51, 56].
Taking all these observations together, several studies identified olfactory dysfunction as a potential biomarker of depression [41••, 44, 45, 51, 56–58]. Successful treatment of depression has been shown to reverse olfactory dysfunction since olfactory threshold, discrimination and central olfactory processing appear to normalize [24, 39, 46, 51, 59]. Interesting is the finding that olfactory training improves depressive symptoms [60], although controversial results have been found in a more recent study [61].
Despite all these observations, the mechanisms underlying loss of smell in depression are not fully known. Given the prevalence and the high morbidity and mortality rates associated with depression [62] and olfactory alterations [5], future studies are required in order to investigate the use of the evaluation of the loss of smell in the detection of individuals with high risk for depression.
Schizophrenia
Schizophrenia is a chronic and debilitating mental illness, with a prevalence of 0.3% and heterogeneous symptomatology usually including positive symptoms such as hallucinations or delusions, disorganized speech, negative symptoms such as speech poverty and cognitive deficits including deficits in attention, memory and functional executives. The disease is commonly associated with deficiencies in psychosocial functions. Although many etiological factors remain to be still elucidated, the interactions between genetic susceptibility and stress environment in the early stages of life are considered relevant in the development of schizophrenia.
Olfactory dysfunction in schizophrenia is a relatively little studied compared to other cognitive alterations. Smell is the sensory modality most closely related in their neuroanatomical substrates with cognitive and emotional disturbances characteristic of this disorder. Various studies have revealed several features of olfactory dysfunction, particularly impaired the identification of odours in patients with schizophrenia, with an early onset of psychosis or individuals at high risk of psychosis [1••, 3, 63–65]. Odour discrimination has been also described impaired in schizophrenia patients, although only a small number of studies investigated odour discrimination [66••, 67, 68]. However, some authors found no deficits in odour identification in patients with schizophrenia [69, 70]. These discrepancies might be due to shorter duration of illness, younger age or milder negative symptomatology in the studied patient groups.
In patients with schizophrenia or in subjects with risk of suffering from it, olfactory alterations are frequently associated with common deficits in motivational and affective capacities known as negative symptoms, such as loss of ability of feeling or experiencing emotions, loss of motivation, difficulties in enjoying things and decreased of verbal expression [64, 65, 71, 72], as well as impaired cognitive and social functions and depressive symptoms [1••, 22, 41••, 43, 64, 71]. Along this line, patients with prominent negative symptoms have olfactory deficits of greater magnitude than those without negative symptoms [72].
Early-onset schizophrenia, defined as disease that has its onset before the age of 18, is a more severe and chronic schizophrenia phenotype that presents with more negative symptoms and cognitive impairment than adult-onset schizophrenia. The degree of severity of smell loss in early-onset schizophrenia is more severe than in adult [65].
Despite the growing number of studies on the olfactory hedonic judgment in schizophrenia, the evidence is still inconsistent. Some studies found that individuals with schizophrenia judge stimuli with valence positive as less pleasant than healthy controls [73]. However, Clepce et al. [66••] described that schizophrenia patients with psychotic symptoms active judge olfactory stimuli with positive valence as more pleasant, and those of negative valence as more unpleasant. In this study, the increased range of hedonic judgment in individuals with schizophrenia was correlated with the severity of schizophrenia symptoms. In contrast, the study performed by Walsh-Messinger et al. [74] showed that individuals with schizophrenia and controls made comparable affective ranges to the stimulus olfactory of different valences. More recently, Lui et al. [72] have shown that individuals with schizophrenia and prominent negative symptoms qualify the olfactory stimuli with positive valence as less pleasant and stimuli with negative valence as less unpleasant relative to the controls. Gender has not been found to have significant effects on hedonic olfactory judgment in individuals with schizophrenia with prominent negative symptoms [72]. However, antipsychotic drugs modify judgment altered hedonic in relation to stimuli with valence positive, so that after antipsychotic treatment, no differences are observed between individuals with schizophrenia and control cases [72].
A possible explanation for these inconsistent findings on olfactory hedonic judgment may be related with the phenomenon called “ambivalence affective” in schizophrenic patients, which postulates that positive and negative emotions are processed in different systems of the human brain, and that the activation simultaneous of both pleasant and unpleasant emotions it is more common in individuals with schizophrenia. In this sense, it has been described that the processing of pleasant olfactory stimuli is supported by reward-related neural circuits, while unpleasant olfactory stimuli are supported by neural circuits related to emotions, suggesting that these two dimensions of odour may offer a window into the study of differential mechanisms underlying olfactory alterations in schizophrenia [71, 72].
It should be noted that although some patients with schizophrenia have olfactory hallucinations, not an association has been shown between these and alterations of smell [75], which suggests that these symptoms may be mediated by different neural mechanisms.
Although the olfactory deficits in schizophrenia have been described as independent of the effects of smoking and treatment with antipsychotic drugs [76], a meta-analysis has revealed that smoking is paradoxically associated with small deficits in olfactory function in schizophrenia [23]. This study also revealed that individuals on a first-generation antipsychotic regimen showed greater olfactory deficits than those treated with second-generation antipsychotics [23].
Given that the alteration in the identification of odours, in addition to being present in individuals diagnosed with schizophrenia, has also been described among first-degree relatives of patients with schizophrenia, in young people at high risk of suffering from psychosis, and in the first episode of schizophrenia [3, 60, 64, 72], it has been suggested that alteration in olfactory function may be a potential clinical biomarker of schizophrenia already in the early stages of the disease, as well as its progression. The use of olfactory deficit as a biomarker would allow an early diagnosis of the disease and the establishment of therapeutic strategies, among which olfactory training may be considered.
Bipolar Disorder
Bipolar disorder is characterized by the recurrence of mood episodes, either depressive or manic alternating with periods of remission [77]. Psychotic symptoms during acute phases lead to some confusion with schizophrenia diagnostic, while the recurrence of depressive episodes conducts to misdiagnosis with major depressive disorder.
Only few studies assessed olfactory function in bipolar disorders and, generally, these studies include a small number of patients [6]. Bipolar patients or individuals with bipolar spectrum may have an impaired sense of smell compared to controls [78••, 79–81]. Olfactory deficits, such as a lowered odour threshold [82] and reduced odour identification [81, 83], have been reported in patients with bipolar disorder even in euthymic phases [83], although some controversial results have been obtained. In a recent study, only patients in the acute phase of a mood episode showed impaired olfactory threshold [81]. Clinical symptoms were negatively correlated with odour sensitivity but not odour identification ability [81]. No difference in odour discrimination has been found [6, 38].
Thus, impairment of olfactory threshold might be a potential marker for distinguishing between bipolar disorder patients in acute phase vs remission [81]. However, few data exist comparing olfactory function between subjects in manic state and remission. Recently, it has been described patients with manic episodes showing deficits in identifying positive odours [78••]. These patients evaluated these smells as less pleasant and less emotional compared to remitted bipolar subjects and healthy controls. Indeed, patients in manic states have more severe deficits in olfactory identification compared with the euthymic bipolar patients who also have an altered sense of identification compared to healthy controls. However, when considering the olfactory threshold, no significant differences were observed between manic patients compared to euthymic and control subjects [78••].
These olfactory dysfunctions may constitute potential indicator of manic episodes. The persistence of olfactory dysfunction in remission phase (deficit in the olfactory identification of positive odours compared to healthy controls) may constitute a potential trait indicator of bipolarity.
Pathophysiological Mechanisms Involved in Loss of Smell in Mental Illness
Several areas of the brain associated with processing of emotions overlap with the olfactory pathway. Thus, the link between loss of smell and mental illness may be due to effects on common anatomical structures [2••, 39]. Although the underlying pathophysiological mechanisms of the relationship between olfactory dysfunction and mental illness are still unknown, it has been suggested that various processes may be involved such as inflammation, alterations in the neurogenesis of the peripheral and central structures of the olfactory system, and structural and functional cerebral alterations.
Inflammation Associated with Loss of Smell in Mental Illness
There is large body of clinical evidence indicating that inflammatory processes are involved in the pathophysiology of depression and schizophrenia [84, 85]. Preclinical studies have pointed out aberrant systemic and brain immune systems as potential mechanisms underlying neuroinflammation leading to behavioural outcomes relevant to psychiatric disorders [86, 87]. This notion is supported by epidemiological findings that environmental factors such as air pollutants and viral infections contribute to the risk for psychiatric diseases perhaps, at least in part via nasal inflammatory mechanisms [81, 88–90].
Although less is known about the specific pathological role of OE inflammation for neurobehavioral consequences and psychiatric disorders pathophysiology, it is known that chronic inflammation damages the OE and inhibits its regeneration [91, 92]. It is conceivable the notion that OE inflammation could impact central olfactory neural structures. Both inflammatory sinonasal diseases and neurodegenerative and psychiatric diseases are found to involve OB volume loss [41••, 93–95]. Furthermore, recent evidences suggest that inflammatory sinonasal diseases are associated with an increased risk of psychiatric symptoms such as depression, anxiety and cognitive dysfunction [96••, 97, 98].
Depression
Several studies have shown that depression is associated with an inflammatory response [62, 99], which also has a fundamental role in the pathophysiology of suicide [100]. Activation of inflammatory pathways in the brain is an important inducer of excitotoxicity that contributes to the neural damage observed in depression [101]. This activation is considered to occur mainly due to the presence of proinflammatory cytokines.
Knowledge of the contribution of proinflammatory cytokines in depression derives from clinical and preclinical observations which have shown that patients with depression have greater inflammatory activity in the central nervous system manifested by the increase in circulating interleukin-6 (IL-6) and C-reactive protein, the presence of IL-1β and neural adhesion molecules in the cerebrospinal fluid, and by the increased gene expression of various cytokines and interferon gamma (IFN-γ) [99].
Depressive symptoms are markedly prevalent in patients with loss of smell due to inflammatory diseases of the upper respiratory tract, such as chronic rhinosinusitis (CRS) and acute rhinosinusitis of viral origin. CRS is a complex disease characterized by persistent inflammation of the paranasal sinuses, affecting 10–15% of general population. CRS is classified according to the presenting phenotype, that is, CRS with (CRSwNP) and without (CRSsNP) nasal polyps, which has clinical implications regarding treatment outcomes, recurrence rates and severity of symptoms [102]. The loss of smell is present in 56–78% of CRS patients and has a progressive and fluctuating course [102].
Depressive symptoms constitute one of the main comorbidities of CRS, with a range of prevalence between 11 and 40% [103, 104]. In fact, it has been suggested that depression is underdiagnosed in many patients with CRS [103, 104]. The pathophysiological mechanisms underlying depressive symptoms in CRS are still unknown. However, a key mechanism in the pathophysiology of the loss of smell in inflammatory disease of the upper respiratory tract associated with depression is inflammation. In inflammatory diseases of the respiratory tract, three inflammatory endotypes have been described: type 1, type 2 and type 3, which are distinguished by their cellularity and release of different proinflammatory cytokines. The RSCwNP is predominantly a type 2 inflammation in which activated T helper (Th2) cells produce cytokines such as IL-4, IL-5 and IL-13 in the sinonasal mucosa, leading to the production of IgE antibodies and the recruitment of eosinophils [102].
Among the factors associated with depression, there is also the exposure to viral diseases. Influenza infections are associated with an increased risk of develop depression, severe enough to require antidepressant treatment, increasing the risk of depression by 40% with the number of previous infections [105]. Loss of smell is one of the first among other neurological manifestations of SARS-CoV-2 infection (severe acute respiratory coronavirus-2 syndrome). It has been described that anosmia in people with COVID-19 infection is associated with depression, which tends to persist even up to 6 months after resolution of symptoms (postviral) [40, 106]. Although the pathophysiological mechanisms underlying depressive symptoms in postviral illnesses are still unknown, the types 1/3 inflammatory endotypes are present in the epithelial cells of the airways. This inflammation is characterized by the release of interleukins such as IL-17a, IL-8, IL-22, IFN-γ and tumor necrosis factor alpha (TNF-α) [102].
Schizophrenia
The risk of schizophrenia is associated with a variety of environmental and genetic factors, including associated with immunity and inflammation. Substantial epidemiologic evidences suggest that maternal, perinatal, childhood and adult infections can increase the risk of a diagnosis of schizophrenia [107]. Several organisms and different types of infection have been linked to the risk of schizophrenia; however, exposure to the influenza virus appears to play a relevant role [107].
In agreement with preclinical studies suggesting that respiratory infections can lead to inflammation in the central nervous system or have access to it via the olfactory route, recent large cohort studies have shown complications such as psychosis and cognitive alterations in COVID-19 patients [108]. All these observations suggest that, although the mechanism of the association between nasal viral infections and schizophrenia remains unknown, nasal inflammation caused by infections may contribute to olfactory disturbances in psychopathology of schizophrenia. In this line, a recent molecular study based on RNA sequencing has shown that certain molecular pathways involved in the immune/inflammatory system, such as the NF- κB signalling pathways, are altered in the ONs in the OE, and that these changes correlate with the volume of OB in patients with the first episode of psychosis [109••].
These observations support the notion that inflammation in the OE can alter the maturity of the neuronal circuit in the olfactory system and cerebral cortical areas involved in the regulation of higher brain functions that are relevant in schizophrenia. However, future research is required on the molecular, cellular and functional mechanisms that underlie the effects of OE inflammation on the role of relevant brain areas in pathophysiology of mental illness.
Alteration in the Process of Neurogenesis
The process of neurogenesis is one of the strongest links between olfactory alterations and mental illness [110]. Neurogenesis occurs primarily in the dentate gyrus in the hippocampus, in the subventricular zone (SVZ) and in the OE [111]. The neural stem cells of the SVZ and the hippocampus continually differentiate into functional neurons necessary to regulate olfactory functions and antidepressant behaviours, respectively. Neural progenitor cells of SVZ migrate to the OBs, where they differentiate into dopaminergic interneurons in the periglomerular area, acquiring a role relevant in the olfactory function. In experimental models of depression, the reduction in the volume of the OB has been associated with a decrease in the thickness of the OE, to a decrease in the number of mature and immature NO and basal stem cells suggesting an alteration of neurogenesis and, therefore, of the turnover of olfactory neurons in the OE that could be the basis of the olfactory alterations that occur in depression [112].
On the other hand, hippocampal atrophy that occurs in depression causes a large number of not working connections of the olfactory tract. These structural changes limit the development of central and peripheral ONs [60], which leads to impaired olfactory function. The relevance of altered neurogenesis in loss of smell and depression has also been pointed out after the improvement of the olfactory alterations and restoration of hippocampal neurogenesis that has been described after antidepressant treatments [113].
In post-mortem studies of individuals with schizophrenia, neural and molecular changes in the OE have been described. In this line, a decrease in the number of basal progenitor cells and an increase in immature ONs have been shown, suggesting impaired neurogenesis and development of ONs [114].
Structural Brain Alterations
Neuroimaging studies have revealed the presence of structural and functional abnormalities in various regions of the brain involved in olfactory processing in individuals with mental illness. These brain areas include the OB and primary cortices, which have connectivity with higher brain regions such as the frontal and OFC that regulate processing of higher order information [7].
While anxiety has enhancing effects on certain elements of olfactory function, they may simultaneously be related to deficits in olfactory structure and function [115, 116]. Thus, combat Veterans with post-traumatic stress, compared with healthy combat Veterans, have less grey matter volume of both primary and secondary olfactory cortices [115], impaired odour identification ability and reduced detection sensitivity to a specific odorant, phenyl ethyl alcohol (PEA) [116]. Interestingly, PEA, a rose-like odorant, is relatively selective to the olfactory nerve/circuit, having little, if any action on the trigeminal circuit [32••].
Studies suggest that the OB and their central connections have a crucial function in emotional behaviour [117]. In patients with depression, schizophrenia or psychosis, a reduction in the volume of the OB has been observed [40, 93]. Moreover, preclinical research has demonstrated that removing OBs induces depression-like behaviour in rodents [118], that is associated with changes in neurotransmission, endocrine and immune responses that have similarities to those observed in humans with depression [119].
Individuals with schizophrenia have a decrease in the depth of the olfactory sulcus in comparison with control cases [120], although the measure of the olfactory sulcus does not correlate with clinical variables such as the age of onset of the disease, medication or severity of symptoms. A more recent study has shown that in psychotic patients, the values of the left olfactory sulcus were significantly lower than those on the right side [121]. Interestingly, a positive correlation between BO volume and the depth of the olfactory sulcus has been described [121]. These structural abnormalities of the olfactory system have been not only observed in individuals with schizophrenia, but also in high-risk young individuals who developed schizophrenia, as well as in unaffected first-degree relatives [3]. These findings suggest that the depth of the olfactory sulcus and OB volume may be markers of vulnerability for schizophrenia, including in the prodromal stages before the onset of symptoms and reflecting an alteration in the early neural development [120].
In bipolar disorders, white matter abnormalities have been found especially in the OFC and different parts of the limbic system, with the most severe alterations found in patients recruited during mania [78••, 122].
Conclusions
Alterations of smell constitute a symptom common in mental illnesses such as anxiety, depression schizophrenia and bipolar disorders.
In anxiety, olfactory dysfunction consists in odour threshold, discrimination and identification deficits. When considered the moderate/severe anxiety, poorer olfactory function is correlated with greater severity of somatic anxiety. The influence of trait or sustained anxiety on olfactory function is consistent with a durable shift in odour sensitivity toward particular odorants and away from others. Odour hedonics may play a critical role in threat-related changes in olfactory function in anxious subjects that process unpleasant odorants differentially. Trigeminal properties of odorants may be also relevant to contextual threat and anxiety-related odour sensitivities.
Depressed patients express impaired odour threshold, discrimination and identification, being olfactory threshold the most significant feature in depression. A correlation of olfactory dysfunction with the severity of depression and with the suicidal ideation has been described. Depression is not uniformly related to olfactory impairment, but depends on duration and course of depression. Hedonic rating of smells is also affected by depression since depressed patients over-evaluated the pleasantness of positive odours, and tend to perceive unpleasant odours as more unpleasant.
In schizophrenia, the identification of odours is particularly impaired, being also present in patients with an early onset of psychosis or in individuals at high risk of psychosis. Thus, it has been suggested that alteration in olfactory function may be a potential clinical biomarker of schizophrenia already in the early stages of the disease, as well as of its progression. Patients with prominent negative symptoms have deficits olfactory disorders of greater magnitude than those without negative symptoms. The degree of severity of smell loss in early-onset schizophrenia is more severe than in adult. Despite the growing number of studies on the olfactory hedonic judgment in schizophrenia, the evidence is still inconsistent.
A lowered odour threshold and reduced odour identification have been reported in patients with bipolar disorders even in euthymic phases, although some controversial results have been obtained.
Several areas of the brain associated with processing of emotions overlap with the olfactory pathway. Thus, the link between loss of smell and mental illness may be due to effects on common anatomical structures. Although the underlying pathophysiological mechanisms of the relationship between olfactory dysfunction and mental illness are still unknown, it has been suggested that various processes may be involved such as inflammation, alterations in the neurogenesis of the peripheral and central structures of the olfactory system, and structural and functional cerebral alterations.
Compliance with Ethical Standards
Conflict of Interest
C. Marin declares that she has no conflict of interest. I. Alobid has received speaker and consultancy honoraria from Viatris, Roche, Sanofi, GSK, MSD, Menarini, Salvat and Novartis. M. Fuentes declares that she has no conflict of interest. M. López-Chacón declares that he has no conflict of interest. J. Mullol has received speaker and consultancy honoraria, and grants from Sanofi-Genzyme & Regeneron, Novartis, Viatris, Uriach Group, Mitsubishi-Tanabe, Menarini, UCB, AstraZeneca, GSK and MSD.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Concepció Marin, Email: cmarin@recerca.clinic.cat.
Joaquim Mullol, Email: jmullol@clinic.cat.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.•• Hasegawa Y, Ma M, Sawa A, Lane AP, Kamiya A. Olfactory impairment in psychiatric disorders: does nasal inflammation impact disease psychophysiology? Transl Psych. 2022; 12: 314. COMMENT:Review about the role of nasal inflammation in the pathophysiology of psychiatric disorders. [DOI] [PMC free article] [PubMed]
- 2.Croy I, Hummel T. Olfaction as a marker for depression. J Neurol. 2017;264:631–638. doi: 10.1007/s00415-016-8227-8. [DOI] [PubMed] [Google Scholar]
- 3.Moberg PJ, Kamath V, Marchetto DM, Calkins ME, Doty RL, Hahn CG, Borgmann-Winter KE, Kohler CG, Gur RE, Turetsky BI. Meta-analysis of olfactory function in schizophrenia, first-degree family members, and youths at risk for psychosis. Schizophr Bull. 2014;40:50–59. doi: 10.1093/schbul/sbt049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS ONE. 2014;9:e107541. doi: 10.1371/journal.pone.0107541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi JS, Jang SS, Kim J, Hur K, Ference E, Wrobel B. Association between olfactory dysfunction and mortality in US adults. JAMA Otolaryngol Head Neck Surg. 2021;147:49–55. doi: 10.1001/jamaoto.2020.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry C, Meyrel M, Bigot M, Alonso M, Lledo PM, Dárgel AA. Can olfactory dysfunction be a marker of trait of states of bipolar disorders. J Affect Dis. 2020;266:498–502. doi: 10.1016/j.jad.2020.01.081. [DOI] [PubMed] [Google Scholar]
- 7.Bhattarai JP, Etyemez S, Jaaro-Peled H, Janke E, Leon Tolosa UD, Kamiya A, Gottfried JA, Sawa A, Ma M. Olfactory modulation of the medial prefrontal cortex circuitry: implications for social cognition. Semin Cell Dev Biol. 2022;129:31–39. doi: 10.1016/j.semcdb.2021.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Hage W. Olfaction as a marker for psychiatric and neurological diseases. Brain Sci. 2021;12:23. doi: 10.3390/brainsci12010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Xu J, Li B, Guo W, Zhang J, Hu J. Olfactory impairment in first-episode schizophrenia: a case-control study, and sex dimorphism in the relationship between olfactory impairment and psychotic symptoms. BMC Psychiatry. 2018;18:199. doi: 10.1186/s12888-018-1786-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su CY, Menuz K, Carlson JR. Olfactory perception: receptors, cells and circuits. Cell. 2009;129:45–59. doi: 10.1016/j.cell.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson DA, Sullivan RM. Cortical processing of odor subjects. Neuron. 2011;72:506–519. doi: 10.1016/j.neuron.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misiak M, Hipolito MM, Ressom HW, Obisesan TO, Manaye KF, Nwlia EA. Apo E4 alleles and impaired olfaction as predictors of Alzheimer’s Disease. Clin Exp Psychol. 2017;3:169. doi: 10.4172/2471-2701.1000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imamura F, Ito A, LaFever BJ. Subpopulations of projection neurons in the olfactory bulb. Front Neural Circuits. 2020;14:561822. doi: 10.3389/fncir.2020.561822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doty RL, Philip S, Reddy K, Kerr KL. Influences of antihypertensive and antihyperlipidemic drugs on the senses of taste and smell: a review. J Hypertens. 2003;21:1805–1813. doi: 10.1097/00004872-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Kanaya K, Kondo K, Suzukawa K, Sakamoto T, Kikuta S, Okada K, Yamasoba T. Innate immune responses and neuroepithelial degeneration and regeneration in the mouse olfactory mucosa induced by intranasal administration of Poly (I:C) Cell Tissue Res. 2014;357:279–299. doi: 10.1007/s00441-014-1848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Attems J, Walker L, Jellinger KA. Olfaction and aging: a mini-review. Gerontology. 2015;61:485–490. doi: 10.1159/000381619. [DOI] [PubMed] [Google Scholar]
- 17.Nagayama S, Takakhashi YK, Yoshihara Y, Mori K. Mitral and tufted cells differ in the decoding manner of odor maps in the rat olfactory bulb. J Neurophysiol. 2004;91:2532–2540. doi: 10.1152/jn.01266.2003. [DOI] [PubMed] [Google Scholar]
- 18.Mullol J, Alobid I, Mariño-Sánchez F, Quintó L, de Haro J Bernal-Sprekelsen M, Valero A, Picado C, Marin C. Furthering the understanding of olfaction, prevalence of loss of smell and risk factors: a population-based survey (OLFACAT study). MBJ Open. 2012; 2: e001256. [DOI] [PMC free article] [PubMed]
- 19.Taalman H, Wallace C, Milev R. Olfactory functioning and depression: a systemic review. Front Psychiatry. 2017;8:190. doi: 10.3389/fpsyt.2017.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malaspina D, Coleman E. Olfaction and social-drive in schizophrenia. Arch Gen Psychiatry. 2003;60:578–584. doi: 10.1001/archpsyc.60.6.578. [DOI] [PubMed] [Google Scholar]
- 21.Moberg PJ, Arnold SE, Doty RL, Gur RE, Balderston CC, Roalf DR, Gur RC, Kohler CG, Kanes SJ, Siegel SJ, Turetsky BI. Olfactory functioning in schizophrenia: relationship to clinical neuropsychological, and volumetric MRI measures. J Clin Exp Neuropsychol. 2006;28:1444–1461. doi: 10.1080/13803390500434409. [DOI] [PubMed] [Google Scholar]
- 22.Clepce M, Gossler A, Reich K, Kornhuber J, Thuerauf N. The relation between, depression, anhedonia and olfactory hedonic estimates-a pilot study in major depression. Neurosci Lett. 2010;471:139–143. doi: 10.1016/j.neulet.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 23.Ishizuka K, Tajinda K, Colantuoni C, Morita M, Winicki J, Le C, Lin S, Schretlen D, Sawa A, Cascella NG. Negative symptoms of schizophrenia correlate with impairment on the University of Pennsylvania smell identification test. Neurosci Res. 2010;66:106–110. doi: 10.1016/j.neures.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Croy I, Symmank A, Schellong J, Hummel C, Gerber J, Joraschky P, Hummel T. Olfaction as a marker for depression in humans. J Affect Disord. 2014;160:80–86. doi: 10.1016/j.jad.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Guo W, Yu L, Luo D, Xie L, Xu J. Association between anxious symptom severity and olfactory impairment in young adults with generalized anxiety disorder: a case-control study. Neuropsychiatr Dis Treat. 2021;17:2977–2883. doi: 10.2147/NDT.S314857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clepce M, Reich K, Gossler A, Kornhuber J, Thuerauf N. Olfactory abnormalities in anxiety disorders. Neurosci Lett. 2012;511:43–46. doi: 10.1016/j.neulet.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 27.Good KP, Sullivan RK. Olfactory function in psychotic disorders: insights from neuroimaging studies. World J Psychiatry. 2015;5:210–221. doi: 10.5498/wjp.v5.i2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milad MR, Rauch SL. The role of the orbitofrontal cortex in anxiety disorders. Ann NY Acad Sci. 2010;1121:546–561. doi: 10.1196/annals.1401.006. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen AD, Shenton ME, Levitt JJ. Olfactory dysfunction in schizophrenia: a review of neuroanatomy and psychophysiological measurements. Harv Rev Psychiatry. 2010;18:279–292. doi: 10.3109/10673229.2010.511060. [DOI] [PubMed] [Google Scholar]
- 30.Houghton DC, Howard SK, Uhde TW, Paquet C, Schlosser RJ, Cortese RM. Odor sensitivity impairment: a behavioral marker for psychological distress? CNS Spectr. 2019;24:404–412. doi: 10.1017/S1092852918001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Houghton DC, Stein DJ, Cortese BM. Review: Exteroceptive sensory abnormalities in childhood and adolescent anxiety and obsessive-compulsive disorder: a critical review. J Am Acad Child Adolesc Psychiatry. 2020;59:78–87. doi: 10.1016/j.jaac.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 32.•• Cortese BM, Uhde TW, Schumann AY, McTeague LM, Sege CT, Calhoun CD, Danielson CK. Anxiety-related shifts in smell function in children and adolescents. Chem Senses. 2021; 46: bjab051. COMMENT: Shifts in olfactory function in anxiety. [DOI] [PMC free article] [PubMed]
- 33.Cortese BM, Schumann AY, Howell AN, McConnell PA, Yang QX, Uhde TW. Preliminary evidence for differential olfactory and trigeminal processing in combat veterans with and without PTSD. Neuroimage Clin. 2018;17:378–387. doi: 10.1016/j.nicl.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.La Buisonnière-Ariza V, Lepore F, Kojok KM, Frasnelli J. Increased odor detection speed in highly anxious healthy adults. Chem Senses. 2013;38:577–584. doi: 10.1093/chemse/bjt028. [DOI] [PubMed] [Google Scholar]
- 35.Krusemark EA, Li W. Enhanced olfactory sensory perception of threat in anxiety: an event-related fMRI study. Chemosens Percept. 2012;5:37–45. doi: 10.1007/s12078-011-9111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galliot E, Laurent L, Hacquemand R, Purié G, Millot JL. Fear-like behavioral responses in mice in different odorant environments: trigeminal versus olfactory mediation under low doses. Behav Processes. 2012;90:161–166. doi: 10.1016/j.beproc.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Burón E, Bulbena A, Bulbena-Cabré A. Olfactory functioning in panic disorder. J Affect Disord. 2015;175:292–298. doi: 10.1016/j.jad.2015.01.049. [DOI] [PubMed] [Google Scholar]
- 38.Kamath V, Paksarian D, Cui L, Moberg PJ, Turetsky BI, Merikangas KR. Olfactory processing in bipolar disorder, major depression, and anxiety. Bipolar Disord. 2018;20:547–555. doi: 10.1111/bdi.12625. [DOI] [PubMed] [Google Scholar]
- 39.Athanassi A, Doncel RD, Bath KG, Madairon N. Relationship between depression and olfactory sensory function: a review. Chem Senses. 2021;46:1–12. doi: 10.1093/chemse/bjab044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dudine L, Canaletti C, Giudici F, Lunardelli A, Abram G, Santini I, Baroni V, Paris M, Peavento V, Manganotti P, Ronchese F, Gregoretti B, Negro C. Investigation on the loss of taste and smell and consequent psychological effects: a cross-sectional study on healthcare workers who contracted the COVID-19 infection. Front Public Health. 2021;9:666442. doi: 10.3389/fpubh.2021.666442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Negoias S, Croy I, Gerber J, Puschmann S, Petrowski K, Joraschky P, Hummel T. Reduced olfactory bulb volume and olfactory sensitivity in patients with acute major depression. Neuroscience. 2010;169:415–421. doi: 10.1016/j.neuroscience.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 42.Temmel AFP, Quint Ch, Schickinger-Fischer B, Klimek L, Stoller E, Hummel T. Characteristics of olfactory disorders in relation to major causes of olfactory loss. Arch Otolaryngol Head Neck Surg. 2002;128:635–641. doi: 10.1001/archotol.128.6.635. [DOI] [PubMed] [Google Scholar]
- 43.Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life-an updated review. Chem Senses. 2014;29:185–194. doi: 10.1093/chemse/bjt072. [DOI] [PubMed] [Google Scholar]
- 44.Lombion-Pouthier S, Vandel P, Nezelof S, Haffen E, Millot JL. Odor perception in patients with mood disorders. J Affect Disord. 2006;90:187–191. doi: 10.1016/j.jad.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Kazour F, Richa S, Char AC, Surget A, Elhage W, Atanasova B. Olfactory markers for depression: differences between bipolar and unipolar patients. PLoS ONE. 2020;15:e0237565. doi: 10.1371/journal.pone.0237565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pause BM, Miranda A, Göder R, Aldenhoff JB, Ferstl R. Reduced olfactory performance in patients with major depression. J Psychiatr Res. 2001;35:271–277. doi: 10.1016/S0022-3956(01)00029-2. [DOI] [PubMed] [Google Scholar]
- 47.Kohli P, Soler ZM, Nguyen SA, Muus JS, Schlosser RJ. The association between olfaction and depression: a systematic review. Chem Sense, s. 2016;41:479–486. doi: 10.1093/chemse/bjw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khil I, Rahe C, Wellmann J, Baune BT, Wersching H, Berger K. Association between major depressive disorder and odor identification impairment. J Affect Disord. 2016;203:332–338. doi: 10.1016/j.jad.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 49.Marine N, Boriana A. Olfactory markers of depression and Alzheimer’s disease. Neurosci Biobehav Rev. 2014;45:262–270. doi: 10.1016/j.neubiorev.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 50.Chen B, Klarmann R, Israel M, Ning Y, Colle R, Hummel T. Difference of olfactory deficit in patients with acute episode of schizophrenia and major depressive episode. Schizo Res. 2019;212:99–106. doi: 10.1016/j.schres.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Naudin M, El-Hage W, Gomes M, Gaillard P, Belzung C, Atanasova B. State and trait olfactory markers o major depression PLoS One. 2012;7:e46938. doi: 10.1371/journal.pone.0046938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zucco GM, Bollini F. Odour recognition memory and dour identification in patients with mild and severe major depressive disorders. Psychiatry Res. 2011;190:217–220. doi: 10.1016/j.psychres.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 53.Pabel LD, Hummel T, Weidner K, Croy I. The impact of severity, course and duration of depression on olfactory function. J Affect Disord. 2018;238:194–203. doi: 10.1016/j.jad.2018.05.033. [DOI] [PubMed] [Google Scholar]
- 54.Swiecicki L, Zatorski P, Bzinkowska D, Sienkiewicz-Jarosz H, Szyndler J, Scinska A. Gustatory and olfactory function in patients with unipolar and bipolar depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:827–834. doi: 10.1016/j.pnpbp.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 55.Postolache TT, Doty RL, Wehr TA, Jimma LA, Han L, Turner EH, Matthews JR, Neumeister A, No C, Kroger H, Bruder GE, Rosenthal NE. Monorhinal odor identification and depression scores in patients with seasonal affective disorder. J Affect Disord. 1999;56:27–35. doi: 10.1016/S0165-0327(99)00027-0. [DOI] [PubMed] [Google Scholar]
- 56.Atanasova B, El-Hage W, Chabanet C, Gaillard P, Belzung C, Camus V. Olfactory anhedonia and negative olfactory alliesthesia in depressed patients. Psychiatry Res. 2010;176:190–196. doi: 10.1016/j.psychres.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 57.Pollatos O, Albrecht J, Kopietz R, Linn J, Schoepf V, Kleemann AM, Schreder T, Schandry R, Wiesmann M. Reduced olfactory sensitivity in subjects with depressive symptoms. J Affect Disord. 2007;102:101–108. doi: 10.1016/j.jad.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 58.Atanasova B, Graux J, El Hage W, Hommet C, Camus V, Belzung C. Olfaction: a potential cognitive marker of psychiatric disorders. Neurosci Biobehav. 2008;32:1315–1325. doi: 10.1016/j.neubiorev.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Pause BM, Raack N, Sojka B, Göder R, Aldenhoff JB, Ferstl R. Convergent and divergent effects of odors and emotions in depression. Psychophysiology. 2003;40:209–225. doi: 10.1111/1469-8986.00023. [DOI] [PubMed] [Google Scholar]
- 60.Wegener BA, Croy I, Hähner A, Hummel T. Olfactory training with older people. Int J Geriatr Psychiatry. 2018;33:212–220. doi: 10.1002/gps.4725. [DOI] [PubMed] [Google Scholar]
- 61.Pabel LD, Murr J, Weidner K, Hummel T, Croy I. Null effect of olfactory training with patients suffering from depressive disorders-an exploratory randomized controlled clinical trial. Front Psychiatry. 2020;11:593. doi: 10.3389/fpsyt.2020.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wislowska A, Kolowska K, Maciejak P. Neurobiological basis of increased risk for suicidal behaviour. Cells. 2021;10:2519. doi: 10.3390/cells10102519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carnemolla SE, Hsieh JW, Sipione R, Landis BN, Kumfor F, Piguet O, Manuel AL. Olfactory dysfunction in frontotemporal dementia and psychiatric disorders: A systematic review. Neurosci Biobehav Rev. 2020;118:588–611. doi: 10.1016/j.neubiorev.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Kamath C, Crawford J, DuBois S, Nucifora FC, Nestadt G, Sawa A, Schretlen D. Contributions of olfactory and neuropsychological assessment to the diagnosis of first-episode schizophrenia. Neuropsychology. 2019;33:203–211. doi: 10.1037/neu0000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Z, Li S, Wen J, Zhang X, Hummel T, Zou L. Early-onset schizophrenia showed similar but more severe olfactory identification impairment than adult-onset schizophrenia. Front Psychiatry. 2020;11:626. doi: 10.3389/fpsyt.2020.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clepce M, Reich K, Gossler A, Kornhuber J, Thuerauf N. Olfactory perception in schizophrenia: the rating range for hedonic judgements is increased during acute episodes. Psychiatry Res. 2013;208:81–83. doi: 10.1016/j.psychres.2012.09.052. [DOI] [PubMed] [Google Scholar]
- 67.Urban-Kowalczyk M, Strzelecki D, Smigielski J, Kotlicka-Antczak M. Odor perception and hedonics in chronic schizophrenia and in first episode psychosis. Neuropsychiatr Dis Treat. 2019;15:647–654. doi: 10.2147/NDT.S192523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Minovi A, Dombrowski T, Brüne M, Dazert S, Juckel G. Olfactory function and morphology of olfactory epithelium in an adult population with schizophrenia. Schizophr Res. 2015;16:513–514. doi: 10.1016/j.schres.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 69.Cieslak K, Walsh-Messinger J, Standford A, Vaez-Azizi L, Antonius D, Harkavy-Friedman J, Goetz D, Goetz RR, Malaspina D. Olfactory performance segregates effects of anhedonia and anxiety on social function in patients with schizophrenia. J Psychiatry Neurosci. 2015;40:387–393. doi: 10.1503/jpn.140268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malaspina D, Goetz R, Keller A, Messinger JW, Bruder G, Goetz D, Opler M, Harlap S, Harkavy-Friedman J, Antonius D. Olfactory processing, sex effects and heterogeneity in schizophrenia. Schizophr Res. 2012;135:144–151. doi: 10.1016/j.schres.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Larson EM, Donaldson KR, Jonas KG, Lian W, Bromet EJ, Kotov R, Mohanty A. Pleasant and unpleasant odor identification ability is associated with distinct dimensions of negative symptoms transdiagnostically in psychotic disorders. Schizophr Res. 2022;248:183–193. doi: 10.1016/j.schres.2022.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lui SS, Chui MWY, Chui WWH, Wong JOY, Man CMY, Cheung EFC, Chan RCK. Impaired olfactory identification and hedonic judgement in schizophrenia patients with prominent negative symptoms. Cognitive Neurospychiatry. 2020;25:126–138. doi: 10.1080/13546805.2019.1704709. [DOI] [PubMed] [Google Scholar]
- 73.Strauss GP, Alle DN, Ross SA, Duke LA, Schwartz J. Olfactory hedonic judgement in patients with deficit syndrome schizophrenia. Schizophr Bull. 2010;36:860–868. doi: 10.1093/schbul/sbn178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walsh-Messinger J, Wong PS, Antonius D, McMahon K, Opler LA, Ramirez PM, Malaspina D. Sex differences in hedonic judgement of odors in schizophrenia cases and healthy controls. Psychiatry Res. 2018;269:345–353. doi: 10.1016/j.psychres.2018.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arguedas D, Langdon R, Stevenson R. Neuropsychological characteristics associated with olfactory hallucinations in schizophrenia. J Int Neuropsychol Res. 2012;18:799–808. doi: 10.1017/S1355617712000471. [DOI] [PubMed] [Google Scholar]
- 76.Robabeh S, Mohammad JM, Reza A, Mahan B. The evaluation of olfactory function in patients with schizophrenia. Glob J Health Sci. 2015;7:319–330. doi: 10.5539/gjhs.v7n6p319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fifth ed. American Psychiatric Association, Arlington, VA, USA. 2013.
- 78.Kazour F, Atanasova B, Mourad M, El Hachem Ch, Desmidt T, Richa S, El-Hage W. Mania associated olfactory dysfunction: a comparison between bipolar subjects in mania and remission. J Psychiatr Res. 2022;156:330–338. doi: 10.1016/j.jpsychires.2022.10.038. [DOI] [PubMed] [Google Scholar]
- 79.Kazour F, Richa S, Desmidt T, Lemaire M, Atanasova B, El Hage W. Olfactory and gustatory functions in bipolar disorders: a systematic review. Neurosci Biobehav. 2017;80:69–79. doi: 10.1016/j.neubiorev.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 80.Lovdahl H, Boen E, Malt EA, Malt UF. Somatic and cognitive symptoms as indicators of potential endophenotypes in bipolar spectrum disorders: an exploratory and proof-of-concept study comparing bipolar II disorder with recurrent brief depression and healthy controls. J Affect Disord. 2014;166:59–70. doi: 10.1016/j.jad.2014.04.056. [DOI] [PubMed] [Google Scholar]
- 81.Li SB, Li ZT, Lyu ZH, Zhang XY, Zou LQ. Odour identification impairment is a trait but not a disease-specific marker for bipolar disorders: comparisons of bipolar disorder with different episodes, major depressive disorder and schizophrenia. Aust N Z J Psychiatry. 2022; 56. 71–80. [DOI] [PubMed]
- 82.Krüger S, Frasneli J, Bräunig P, Hummel T. Increased olfactory sensitivity in euthymic patients with bipolar disorder with event-related episodes compared with patients with bipolar disorder without such episodes. J Psychiatry Neurosci. 2006;31:263–270. [PMC free article] [PubMed] [Google Scholar]
- 83.Lahera G, Ruiz-Murugarren S, Fernandez-Liria A, Saiz-Ruiz J, Buck BE, Penn DL. Relationship between olfactory function and social cognition in euthymic bipolar patients. CNS Spectr. 2016;21:53–59. doi: 10.1017/S1092852913000382. [DOI] [PubMed] [Google Scholar]
- 84.Khandaker G, Cousinin L, Deakin J, Lennoz BR, Yolken R, Jones PB. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry. 2015;2:258–270. doi: 10.1016/S2215-0366(14)00122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hodes GE, Kana V, Menard C, Merad M, Russo SJ. Neuroimmune mechanism of depression. Nat Neurosci. 2015;18:1386–1393. doi: 10.1038/nn.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gumusoglu SB, Stevens HE. Maternal inflammation and neurodevelopmental programming: a review of preclinical outcomes and implications for translational psychiatry. Biol Psychiatry. 2019;85:107–121. doi: 10.1016/j.biopsych.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 88.Braithwaite I, Zhang S, Kirkbride JB, Osborn DPJ, Hayes JF. Air pollution (particulate matter) exposure and associations with depression anxiety, bipolar, psychosis and suicide risk: a systematic review and meta-analysis. Environ Heatlh Perspect. 2019;127:126002. doi: 10.1289/EHP4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Attademo L, Bernardini F, Garinella R, Compton MT. Environmental pollution and risk of psychotic disorders: a review of the science to date. Schizophr Res. 2017;181:55–59. doi: 10.1016/j.schres.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 90.Gladka A, Rymaszewska J, Zatonski T. Impact of air pollution on depression and suicide. Int J Occup Med Environ Heath. 2018;31:711–721. doi: 10.13075/ijomeh.1896.01277. [DOI] [PubMed] [Google Scholar]
- 91.Chen M, Reed RR, Lane AP. Acute inflammation regulates neuroregeneration through the NF-kappaB pathway in olfactory epithelium. Proc Natl Acad Sci USA. 2017;114:8089–8094. doi: 10.1073/pnas.1620664114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen M, Reed RR, Lane AP. Chronic inflammation directs and olfactory stem cell functional switch from neuroregeneration to immune defense. Cell Stem Cell. 2019;25:501–13.e5. doi: 10.1016/j.stem.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Turetsky BI, Moberg PJ, Yousem DM, Doty RL, Arnold SE, Gur RE. Reduced olfactory bulb volume in patients with schizophrenia. Am J Psychiatry. 2000;157:828–830. doi: 10.1176/appi.ajp.157.5.828. [DOI] [PubMed] [Google Scholar]
- 94.Rottstadt F, Han P, Weidner K, Schellong J, Wolff-Stephan S, Strauss T, Kitzler H, Hummel T, Croy I. Reduced olfactory bulb volume in depression-a structural moderator analysis. Hum Brain Mapp. 2018;39:2573–2582. doi: 10.1002/hbm.24024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rombaux P, Potier H, Bertrand B, Duprez T, Hummel T. Olfactory bulb volume in patients with sinonasal disease. Am J Rhinol. 2008;22:598–601. doi: 10.2500/ajr.2008.22.3237. [DOI] [PubMed] [Google Scholar]
- 96.Kim JY, Ko I, Kim MS, Yu MS, Cho BJ, Kim DK. Association of chronic rhinosinusitis with depression and anxiety in a nationwide insurance population. JAMA Otolaryngol Head Neck Surg. 2019;145:313–319. doi: 10.1001/jamaoto.2018.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schlosser RJ, Storck K, Cortese BM, Uhde TW, Rudmik L, Soler ZM. Depression in chronic rhinosinusitis: a controlled cohort study. Am J Rhinol Allergy. 2016;30:128–133. doi: 10.2500/ajra.2016.30.4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Soler ZM, Eckert MA, Storck K, Schlosser RJ. Cognitive function in chronic rhinosinusitis: a controlled clinical study. Int Forum Allergy Rhinol. 2015;5:1010–1017. doi: 10.1002/alr.21581. [DOI] [PubMed] [Google Scholar]
- 99.Latham R, Kieling C, Arseneault L, Kohrt BA, Moffitt TE, Rasmussen LJH, Rocha TB, Mondelli V, Fisher HL. Longitudinal associations between adolescents’ individualised risk for depression and inflammation in a UK cohort study. Brain Behav Immun. 2022;101:78–83. doi: 10.1016/j.bbi.2021.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Amitai M, Taler M, Lebow M, Ben-Baruch R, Apter A, Fenning S, Weizman A, Chen A. Eur Neuropsychopharmacol. 2020;40:61–69. doi: 10.1016/j.euroneuro.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 101.Labra-Ruiz NA, Santamaría-del Angel D, Osnaya-Brizuela N, Valenzuela-Peraza A, Juárez-Olguín H, Punzo-Soto M, Calderón-Guzmán D. Inflammatory process and immune system in major depressive disorder. Int J Neuropsychopharmacol. 2022;25:46–53. doi: 10.1093/ijnp/pyab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fokkens WJ, Lund VS, Hopkins C, Helings PW, Kern R, Reitsma S, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020; 58 (Suppl S29): 1–464. [DOI] [PubMed]
- 103.Liu DT, Bartosik TJ, Campion NJ, Bayer K, Tu A, Victoria S, Besser G, Mueller CA, Gangl K, Eckl-Dorna J, Schneider S. Chronic rhinosinusitis symptoms differentially impact the likehood of major depressive disorders. Laryngoscope Investig Otolaryngol. 2022;7:29–35. doi: 10.1002/lio2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vandelaar LJ, Jiang ZY, Saini A, YAo WC, Luong AU, Citardi MJ. PHQ-9 and SNOT-22: elucidating the prevalence of depression in chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2020; 162: 142–147. [DOI] [PubMed]
- 105.Bornand D, Toovey S, Jick SS, Meier CR. The risk of new onset depression in association with influenza-a population-based observational study. Brain Behav Immun. 2016;53:131–137. doi: 10.1016/j.bbi.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 106.Taboada M, Cariñena A, Moreno E, Rodriguez N, Dominguez MJ, Casal A, Riveiro V, Diaz-Vieito M, Valdés L, Álvarez J, Seoane-Pillado T. Post-COVID-19 functional status six-months after hospitalization. J Infect. 2021;82:e31–e33. doi: 10.1016/j.jinf.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kepinska AP, Lyegbe CO, Vernon AC, Yolken R, Murray RM, Pollak TA. Schizophrenia and influenza at the centenary of the 1918–1919 Spanish influenza pandemic: mechanism of psychosis risk. Front Psychiatry. 2020;11:72. doi: 10.3389/fpsyt.2020.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Varatharaj A, Thomas N, Ellul MA, Davies NWS, Pollak TA, Tenorio EL, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang K, Hua J, Etyemez S, Paez A, Prasad N, Ishizuka K, Sawa A, Kamath V. Volumetric alteration of olfactory bulb and immune-related molecular changes in olfactory epithelium in first episode psychosis patients. Schizophrenia Res. 2021;235:9–11. doi: 10.1016/j.schres.2021.07.016. [DOI] [PubMed] [Google Scholar]
- 110.Ren G, Xue P, Wu B, Yang F, Wu X. Intranasal treatment of lixisenatide attenuated emotional and olfactory symptoms via CREB-mediated adult neurogenesis in mouse depression model. Aging. 2021;13:3898–3908. doi: 10.18632/aging.202358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Avaro V, Hummel T, Calegari F. Scent of stem cells: how can neurogenesis make us smell better? Front Neurosci. 2022;16:964395. doi: 10.3389/fnins.2022.964395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li Q, Yang D, Wang J, Liu K, Feng G, Li J, Liao J, Wei Y, Li Z. Reduced amount of olfactory receptor neurons in the rat model of depression. Neurosci Lett. 2015;603:48–54. doi: 10.1016/j.neulet.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 113.Rochet M, El-Hage W, Richa S, Kazour F, Atanasova B. Depression, olfaction, and quality of life: a mutual relationship. Brain Sci. 2018;8:80. doi: 10.3390/brainsci8050080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arnold SE, Han LY, Moberg PJ, Turetsky BI, Gur RE, Trojanoswski JQ, Hahn CG. Dysregulation of olfactory receptor neuron lineage in schizophrenia. Arch Gen Psychiatry. 2001;58:829–835. doi: 10.1001/archpsyc.58.9.829. [DOI] [PubMed] [Google Scholar]
- 115.Cortese BM, McConnell PA, Froeliger B, Leslie K, Uhde TW. Burning odor-elicited anxiety in OEF/OIF combat veterans: Inverse relationship to gray matter volume in olfactory cortex. J Psychiatr Res. 2015;70:58–66. doi: 10.1016/j.jpsychires.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wilkerson AK, Uhde TW, Leslie K, Freemand WC, LaRowe SD, Schumann A, Cortese BM. Paradoxical olfactory function in combat veterans: the role of PTSD and odor factors. Mil Psychol. 2018;30:120–130. doi: 10.1080/08995605.2018.1425063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu D, Cai X, Wang L, Yi F, Liao W, Huang R, Fang C, Chen J, Zhou J. Comparative proteomics of rat olfactory bulb reveal insights into susceptibility and resiliency to chronic-stress-induced depression or anxiety. Neuroscience. 2021;473:29–43. doi: 10.1016/j.neuroscience.2021.08.012. [DOI] [PubMed] [Google Scholar]
- 118.Yuan TF, Slotnick BM. Role of olfactory system dysfunction in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:26–30. doi: 10.1016/j.pnpbp.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 119.Song C, Leonard BE. The olfactory bulbectomized rat as a model of depression. Neurosci Biobehav Rev. 2005;29:627–647. doi: 10.1016/j.neubiorev.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 120.Takahashi T, Nakamura Y, Nakamura K, ikeda E, Furuichi A, Kido M, et al. ALtered depth of the olfactory sulcus in first-episode schizophrenia. Prog NeuroPsychopharmacol Biol Psychiatry. 2013; 40: 167–172. [DOI] [PubMed]
- 121.Asal N, Bayar M, Inal M, Sahan MH, Dogan A, Buturak SV. Olfactory bulbs volume and olfactory sulcus depth in psychotic patients and patients with anxiety disorder/depression. Eur Arch Otorhinolaryngol. 2018;275:3017–3024. doi: 10.1007/s00405-018-5187-x. [DOI] [PubMed] [Google Scholar]
- 122.Magioncalda P, Martino M. A unified model of the pathophysiology of bipolar disorder. Mol Psychiatr. 2022;27:202–211. doi: 10.1038/s41380-021-01091-4. [DOI] [PubMed] [Google Scholar]