Abstract
Objective
The aim of the study is to evaluate clinical features and outcomes after different therapeutic strategies for ductal prostate adenocarcinoma (DPC), a rare but aggressive subtype of invasive prostate cancer (PCa) accounting for, in the pure and mixed form, 1% or less and 5% or less, respectively, of all the newly diagnosed PCa.
Materials and methods
Patients with a proven diagnosis of DPC undergoing surgery, radiotherapy, and androgen deprivation therapy, alone or in combination, were considered for this multicenter, retrospective study. The study assessed overall survival (OS), disease-free survival (DFS), and age-related disease-specific survival.
Results
Eighty-one patients met the study inclusion criteria. Pure DPC was found in 29 patients (36%) and mixed ductal-acinar-PCa in 52 patients (64%). After a median follow-up of 63 months (range, 3–206 months), 3- and 5-year OS rates were 84% and 67%, respectively, and 3- and 5-year DFS rates were 54% and 34%, respectively. There were no significant differences in OS or DFS between the pure and mixed DPC groups. Pure DPC was associated with a higher rate of metastatic disease at onset. Patients 74 years or younger had better disease-specific survival (p=0.0019). A subgroup analysis favored radiotherapy as the primary treatment for nonmetastatic, organ-confined DPC (3- and 5-year DFS of 80% and 50%, respectively, compared with 5-year DFS of 35% for surgical patients; p = 0.023).
Conclusions
Our study found DPC to be rarer, more aggressive, more likely to metastasize, and have a worse prognosis than the common acinar variant, especially in its pure form. Multicenter series are encouraged to obtain large data sets, or propensity score matching analyses with patients with conventional PCa are desirable to understand the best therapeutic approach and improve outcomes.
Keywords: Androgen deprivation therapy, Mixed ductal-acinar prostate cancer, Pure ductal carcinoma of the prostate, Radiotherapy, Surgery
1. Introduction
Prostate cancer (PCa) is a common disease in developed countries. A broad range of clinical biological behaviors has been identified in this type of tumor, from less to more aggressive patterns, with different prognoses and outcomes.[1] Histological characterization and grading of PCa have become an important component in predicting tumor aggressiveness, thereby optimizing the therapeutic approach.[1,2] In 2016, the World Health Organization Classification of Tumors of the Urinary System and Male Genital Organs recognized rare histological subtypes of PCa other than the most common acinar adenocarcinoma (APC), including neuroendocrine tumors, adenosquamous carcinoma, basal cell carcinoma, and ductal prostate adenocarcinoma (DPC).[3] Such rare histologies, along with some aggressive variants of APC, such as signet ring and sarcomatoid, have been recently described to occur with higher stage at the time of diagnosis and are associated with frequent metastasis, increased mortality, and risk of biochemical recurrence after surgery.[4]
Histologically, DPC can be confused with intraductal carcinoma, but the latter is characterized by the preservation of a layer of p63-positive basal cells, likely representing the retrograde spread of an associated infiltrating high-grade carcinoma into adjacent ductal structures.[5]
Pure DPC is a rare histological subtype of invasive PCa that accounts for less than 1% of all PCa diagnoses,[6–9] while the mixed ductal plus acinar pattern accounts for less than or equal to 5% of cases.[10]
Ductal prostate adenocarcinoma was originally called “endometrial carcinoma of the prostate utricle,” “cribriform,” or “endometrioid” because of the supposed origin from a different precursor than the more common APC, the remnant paramesonephric tissue.[7,9] Melicow and Pachter[11] first described DPC as an exophytic papillary mass projecting into the urethral lumen, with its base in the crater of the utricle or around the verumontanum, histologically resembling female endometrioid adenocarcinoma.
In the original series, the presence of obstructive urinary symptoms and frequent identification of DPC in transurethral resection of the prostate supported the typical, central, transition zone location within the prostate gland of this subtype of PCa. However, additional studies have identified DPC in all prostate gland zones, including peripheral ones.[12,13]
Compared with common APC, which accounts for 90% of cases, DPC usually shows more aggressive behavior. Therefore, it has recently been considered as a high-risk, Gleason pattern 4 or 5 disease, presenting with larger tumor masses and more frequent extraprostatic invasion and lymph node involvement than its acinar counterpart.[9]
Because of the rarity of DPC, only a few published studies are available in the literature. Compared with APC, DPC is reported to have a higher mortality rate, increased risk of metastases, and significantly lower prostate-specific antigen (PSA) levels at diagnosis.[6–9]
Several features of DPC, such as lower PSA secretion; lower expression of ETS-related gene oncoprotein, phosphatase, and tensin homolog; higher expression of tumor suppressor genes (P16 and P53); and decreased expression of steroid-related markers demonstrate differences in the tumorigenesis compared with that of conventional, acinar PCa.[14–16]
There is a paucity of data comparing different treatment modalities and related outcomes in DPC; therefore, an appropriate therapeutic approach is not yet fully defined.
The purpose of this multicenter retrospective study was to evaluate the clinical features and outcomes of patients with a histological diagnosis of DPC managed using different therapeutic strategies.
2. Materials and methods
Records of patients with histologically proven DPC were retrospectively collected. A multicenter research project was designed and involved 3 recruiting institutions (Barcelona, Brescia, and Istanbul) with contributions from pathologists, radiation oncologists, and urologists. Patients with pure or mixed DPC at any stage of disease were included and reclassified according to the AJCC-UICC TNM Classification of Malignant Tumours – Eighth Edition.[17] Patients who underwent surgery, radiotherapy, and androgen deprivation therapy (ADT) alone or in combination were considered eligible for inclusion in the study. The metastatic staging framework was performed using chest and abdominal computed tomography (CT) and bone scans. Patient demographics and clinical and histological data were collected from the individual hospital medical records of each involved center and recorded in a single database. Patients with evidence of ductal variant prostate carcinoma in the pathology report of the prostate biopsy or, when performed, prostatectomy specimens were selected from prospectively maintained institutional clinical pathology databases at each involved center. Before data collection, each participating institution proceeded with pathological revision of the histological slides of the recruited patients and staging according to the 2016 World Health Organization classification of prostate tumors.[3] Before biopsy and after treatment, PSA values and data from clinical follow-up were also recorded. Follow-up information was collected until the patient died or the last clinical examination. The follow-up schedule consisted of clinical examination and PSA detection every 3 months for the first year after primary treatment, then every 6 months until the fifth year, and annual follow-up until the 10th year after treatment. Restaging with CT scan plus bone scan or, only in recent years, functional imaging (11C-choline-PET/CT or 68Ga-PSMA PET/CT) was performed in patients with rising PSA (with a focus on the PSA doubling time) and/or new onset symptoms (urinary retention, hematuria, or bone pain refractory to pain relief). Biochemical recurrence of disease was defined in accordance with the Phoenix criteria (PSA ≥ 0.2 ng/mL after primary surgery, PSA ≥ PSA nadir +2 ng/mL after radiation);[18,19] local recurrence was detected using magnetic resonance; and distant progression of disease was assessed using chest and abdomen CT scan plus bone scan.
Statistical analysis was performed using IBM SPSS software v23.0, and survival analyses were performed using R (R Core Team, 2021, with survival and ggplot2 packages). The optimal cutoff point for continuous variables was detected using the maximally selected rank statistics (“maxstat” R package), providing a cutoff value corresponding to the most significant relationship with the outcome (log-rank test). Kaplan-Meier survival analysis was used to calculate disease-free survival (DFS, defined as the time between the end of primary treatment and the date of disease recurrence and/or progression, taking into account the sum of the events “treatment failure”: biochemical relapse only and/or local recurrence and/or distant progression), overall survival (OS, the time between the end of primary treatment and patient’s death for any cause), and disease-specific survival (DSS, the time between the end of primary treatment and patient’s death because of PCa). P values less than 0.05 were considered statistically significant.
3. Results
From January 1997 to December 2018, 81 consecutive patients with DPC from 3 European institutions were retrospectively evaluated. Pure DPC was recorded in 29 patients (36%), and mixed ductal and acinar adenocarcinoma was observed in 52 patients (64%). The baseline characteristics of the study population are summarized in Table 1.
Table 1.
Characteristics of the population in study (patients layered for pure or mixed form).
| Parameters | Pure DPC 29 patients, n (%) | Mixed DPC 52 patients, n (%) | p |
|---|---|---|---|
| Age, yr | |||
| Median | 71 | 67 | |
| Range | 60–89 | 52–83 | 0.577 |
| Age ≤ 60 yr | 1 (3) | 9 (17) | |
| Age 61–75 yr | 22 (76) | 39 (75) | |
| Age >75 yr | 6 (21) | 4 (8) | |
| Disease stage | 0.169 | ||
| Organ confined (T1–2, N0, M0) | 6 (21) | 10 (19) | |
| Locally advanced (T3–4, N0, M0) | 7 (24) | 22 (42) | |
| Lymph node involvement (any T, N1, M0) | 3 (10) | 11 (21) | |
| Metastatic disease (any T, any N, M1) | 13 (45) | 9 (17) | |
| Bone metastases | 9 (69) | 5 (56) | |
| Visceral metastases | 3 (23) | 1 (11) | |
| Both visceral and bone metastases | 1 (8) | 3 (33) | |
| Pretreatment PSA | 0.214 | ||
| <4 ng/mL | 3 (10) | 3 (6) | |
| 4–10 ng/mL | 10 (34) | 22 (42) | |
| 10–20 ng/mL | 7 (24) | 11 (21) | |
| 20–50 ng/mL | 4 (14) | 8 (15) | |
| >50 ng/mL | 5 (17) | 8 (15) | |
| Therapeutic approach | |||
| Surgery | 3 (10) | 29 (56) | |
| Prostate-confined disease (T1–4, N0M0) | 1 (3) | 19 (36) | |
| Advanced disease (any T, N1M0) | 2 (7) | 9 (17) | |
| Definitive RT | 14 (48) | 13 (25) | 0.145 |
| Prostate-confined disease (T1–4, N0M0) | 11 (38) | 11 (21) | |
| Advanced disease (any T, N1M0) | 0 (0) | 2 (4) | |
| Adjuvant RT | 1 (3) | 13 (25) | |
| Prostate-confined disease (T1–4, N0M0) | 1 (3) | 9 (17) | |
| Advanced disease (any T, N1M0) | 0 (0) | 4 (8) | |
| Exclusive hormone therapy | 11 (38) | 9 (17) | |
| Concomitant hormone therapy | 13 (45) | 19 (36) | |
| Concomitant to radical RT | 12 (41) | 11 (21) | |
| Concomitant to adjuvant RT | 0 (0) | 4 (8) | |
| After surgery (without RT) | 1 (3) | 4 (8) |
DPC = ductal prostate adenocarcinoma; PSA = prostate-specific antigen; RT = radiation therapy.
The median age at diagnosis was 68 years (range, 52–89 years) for the entire population in the study, 71 years (range, 60–89 years) for the pure PCa group, and 67 years (range, 52–83 years) for the mixed ductal and acinar PCa group. The median pretreatment blood PSA level was 10.9 ng/mL (range, 1.34–383 ng/mL) for pure DPC and 12.0 ng/mL (range, 2.4–1540 ng/mL) for mixed DPC.
Localized prostatic disease was diagnosed in 16 patients (20%), 6 (21%) with pure DPC and 10 (19%) with mixed DPC. Twenty-nine patients (36%) presented with locally advanced DPC (7 [24%] pure DPC and 22 [42%] mixed DPC). Fourteen patients (17%) presented with locoregional lymph node involvement. Twenty-two patients (27%) had metastatic disease at presentation, 13 (45%) of the 29 with pure DPC, and 9 (17%) of the 52 with mixed ductal-acinar PCa (p = 0.169).
Surgery was the treatment of choice in 32 patients (40%), mainly in the mixed group (29 [56%]), and consisted of radical prostatectomy with or without pelvic lymphadenectomy. Among these, 11 patients (34%) had histological evidence of nodal involvement, and the remaining had limited or locally advanced disease (pT2–4). Only one patient with bone metastases underwent radical surgery and then started ADT; at the 6-month follow-up visit, stable disease was recorded.
External beam radiotherapy (EBRT) was administered under different settings. First, as a definitive treatment, with or without concomitant ADT (28 patients in total, 35%), the average total dose delivered to the target volume (prostate and seminal vesicles) was 72.15 Gy (range, 56.25–80 Gy) (equivalent dose in 2-Gy fractions). Second, as an adjuvant treatment after radical prostatectomy (14 patients, 17%), 10 patients received a total dose of 70 Gy (35 fractions) to the prostate lodge, while 4 patients received 66 Gy (33 fractions). External beam radiotherapy was also used for palliation of painful symptoms from bone metastases in 13 patients (16%) with metastatic disease at onset. Stereotactic irradiation, with or without concurrent ADT, was offered to 9 patients (11%) with bone/nodal oligorecurrence/oligoprogression; however, stereotactic reirradiation of post-primary EBRT intraprostatic relapse was observed in 1 patient (1%).
An 85-year-old, metastatic, pure DPC patient, presenting with very poor clinical conditions, was considered unsuitable for any active treatment; thus, best supportive care was the chosen approach in this case.
Androgen deprivation therapy with luteinizing hormone-releasing hormone analogues or antiandrogen drugs was used as the primary treatment in 20 patients (25%): 16 (20%) of them had stage IV disease at presentation, 2 (7%) nonmetastatic patients in the pure DPC group, and 2 (4%) in the mixed DPC group, 80 years or older; therefore, they were considered unsuitable for aggressive primary treatment with curative intent (surgery or EBRT). Among the latter, only 1 patient had disease progression at the last follow-up visit, whereas the others still showed biochemical control of the disease.
After a median follow-up of 63 months (range, 3–206 months), the 3- and 5-year OS rates were 84% and 67% for the entire series (Fig. 1A), and 3- and 5-year DFS were 54% and 34%, respectively (Fig. 1B). When we focused on pure DPC patients, we found 3- and 5-year OS of 86% and 63%, and 3- and 5-year DFS of 64% and 24%, respectively. The mixed DPC group had a 3- and 5-year OS of 83% and 69% (p = 0.360), respectively, and 3- and 5-year DFS of 49% and 41%, respectively (p = 0.970; Figs. 1C–D).
Figure 1.

Kaplan-Meier curves for OS (A) and DFS (B) for the entire population and after stratification for pure DPC versus mixed DPC (C–D). DFS = disease-free survival; OS = overall survival; DPC = ductal prostate adenocarcinoma.
The stratification of patients according to disease stage is shown in Figure 2. The 3- and 5-year OS rates were 92% and 76% for prostate-confined disease (independent from localized or locally advanced DPC), 78% and 65% for nodal involvement, and 61% and 34% for metastatic disease at onset, respectively (p = 0.005). The 3- and 5-year DFS rates were 89% and 78% for organ-confined disease, 58% for nodal involvement, and 48% and 27% for metastatic disease, respectively (p = 0.140).
Figure 2.

Kaplan-Meier curves for OS and DFS after patient stratification by disease stage. DFS = disease-free survival; OS = overall survival.
Patients younger than 74 years had higher DSS; the 3- and 5-year DSS were 51% and 38% over the cutoff point, respectively, and 88% and 74% for patients 74 years or younger, respectively (p = 0.002; Fig. 3).
Figure 3.

Log-rank test of age and correlation between age and DSS. DSS = disease-specific survival.
The International Society of Urological Pathology grade group showed no statistically significant correlation with either OS or DFS.
There was no statistically significant correlation between PSA levels and ductal pattern in the prostate specimens (p = 0.313, data not shown).
A subgroup analysis of nonmetastatic patients who underwent curative treatment was also performed. The 3- and 5-year OS rates were 100% and 78% for pure DPC and 91% and 77% for mixed DPC (p = 0.480; Fig. 4A); the 3- and 5-year DFS rates were 63% and 25%, and 50% and 41%, respectively (p = 0.730; Fig. 4B). Surgery alone was associated with worse DFS for the treatment of prostate-confined disease (3- and 5-year DFS both 35%, compared with 80% and 50% for nonsurgical patients, respectively; p = 0.023). These findings were confirmed after patient stratification by disease stage (Figs. 4C–D). We also found 3- and 5-year OS rates of 100% and 82% for surgical patients, respectively, versus 85% and 72% in the EBRT group (p = 0.310). Regardless of the local or radical nature of treatment, the addition of ADT did not have a significant influence on survival in the entire nonmetastatic population or after selective analysis of patients with prostate-confined disease (data not shown).
Figure 4.
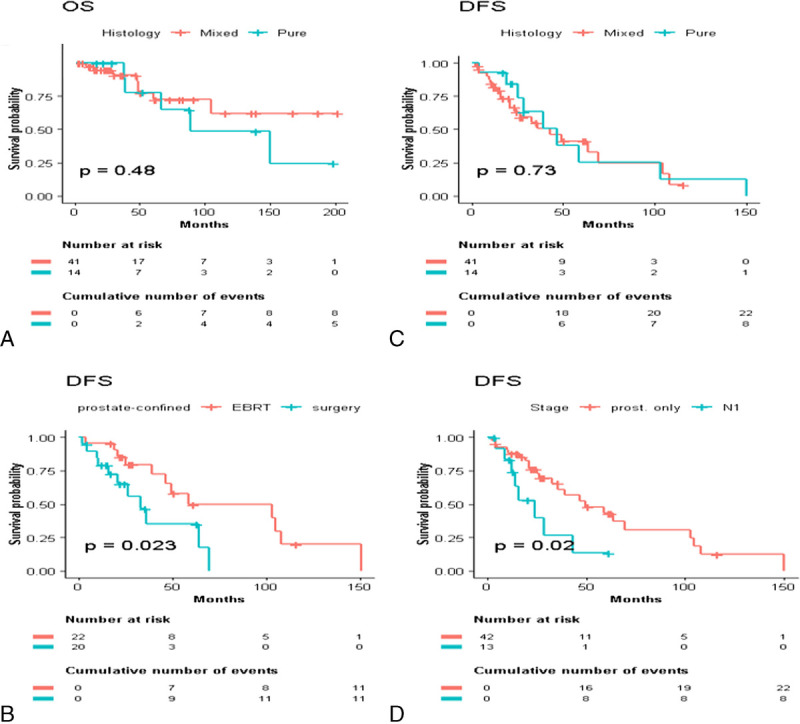
Subgroup analysis of nonmetastatic DPC patients: stratification by pure versus mixed histology (A–B), DFS for radical therapeutic approach (C), and stage of disease (D). DFS = disease-free survival; DPC = ductal adenocarcinoma; EBRT = external beam radiotherapy; OS = overall survival.
4. Discussion
Ductal PCa is a rare, but aggressive subtype of PCa. Sometimes, DPC exhibits unique clinicopathological and radiological features, even extremely rare patterns, which are not clearly appreciable on preoperative imaging or biopsy, as Giganti et al.[20] recently reaffirmed. Most patients with DPC complain of urethral obstruction or hematuria at the time of disease presentation, as this type of tumor often originates from primary periurethral prostate ducts. To date, there is much information regarding the histological findings of DPC[15,20–23]; however, evidence regarding adequate management remains lacking. Because of its rarity, little data are reported in the literature, most of which are case series[15] or small sample studies.[9] The pathological characterization of DPC has been completed in recent years.[10] A discrete spectrum of case series, although retrospective, has been mainly published in the last few years and is listed in Table 2.
Table 2.
Main studies published about DPC.
| Study | No. patients | Pure or mixed DPC | Treatment modality | Outcomes and comments |
|---|---|---|---|---|
| Brinker et al.,[24] 1999 (retrospective) | 58 | 30 pure 28 mixed | 20 RP 38 unspecified | 10% M+ DPC at presentation 2-yr actuarial risk of progression 34% (RP) and 42% (all patients) Shortened average time to progression compared with previously reported acinar carcinoma |
| Eade et al., [25] 2007 (retrospective) | 6 | 3 pure 3 mixed | EBRT + ADT | 1 biochemical recurrence 2 died for metastases No local recurrences 4 disease-free (including metastatic patients) |
| Orihuela et al.,[9] 2008 (retrospective) | 17 | 8 pure 9 mixed | 2 RP 2 EBRT 7 EBRT + ADT 6 ADT (M+ DPC) | 8 (M0) disease-free 2 biochemical recurrences (1 M0, 1 M+) 1 (M0) local relapse 1 (M0) brain mets 4 (M+) patients remained in remission 1 (M+) death for other cause |
| Tu et al.,[6] 2009 (retrospective) | 108 | 25 pure 50 mixed | 76 RP 32 EBRT | RP: PFS 5.8 mo, OS: 11.2 mo, higher survival for pure DPC EBRT: PFS 5.5 mo, OS: 8.2 mo Median time to local progression shorter and median time to metastatization longer for pure DPC |
| Iğdem et al.,[6] 2010 (retrospective) | 31 | 15 pure 16 mixed | 16 RP 14 EBRT 1 ADT | 3 bone mets (2 RP, 1 EBRT) 1 biochemical relapse (EBRT) Good response to local therapy |
| Morgan et al.,[8] 2010 (retrospective, SEER registry) | 371 | 371 pure | ‐ | Compared with acinar PCa more M+ (12% vs. 4%), more poorly differentiated pathology (50% vs. 32%), 30% lower mean baseline PSA |
| Meeks et al.,[12] 2012 (retrospective, SEER registry) | 442,607 | 435 pure Mixed and acinar PCa excluded | 168 RP 80 EBRT 13 EBRT + BRT 9 BRT 42 RP + EBRT | 30% advanced disease for DPC (vs. 7% acinar PCa) 12% DPC cancer death (vs. 4% acinar PCa) Overall DPC OS worse OS and DSS similar for DPC and Gleason 4 + 4 acinar PCa |
| Hiramatsu et al.,[26] 2012 (retrospective) | 7 | 2 pure 5 mixed | 4 ADT 2 RP 1 EBRT + ADT | 3 (M0) no biochemical recurrence 1 M+ underwent DTX 1 M+ lung PD |
| Kan et al.,[7] 2014 (retrospective) | 19 | ‐ | ‐ | DPC locally aggressive High incidence of intraluminal growth and rectal invasion High failure rate after RP or ADT |
| Kim et al.,[10] 2015 (retrospective) | 29 | 5 pure 24 mixed | RP | Poorer prognosis for pure DPC in case of biochemical relapse No difference in positive margins and final pathologic stage Higher % of postoperative Gleason score ≥ 8 for pure DPC |
| Nakamura et al.,[27] 2015 (retrospective) | 41 | 17 RP 19 EBRT + ADT 5 ADT | 29% biochemical relapse after RP 10% biochemical relapse after EBRT + ADT 40% death in the ADT group | |
| Knipper et al.,[28] 2019 (retrospective) | 581 | ‐ | 253 RP 61 EBRT 188 ADT | Higher cancer-specific mortality for M0-RP and overall M+ DPC |
| Wu et al.,[29] 2019 (retrospective) | 35 | ‐ | ADT | DPC not associated with poorer cancer free survival or OS No prognostic difference between high and low % of ductal pattern within prostate specimen |
| Bergamin et al.,[30] 2019 (retrospective) | 27 | 9 pure 18 mixed | EBRT | 4 local and 5 distant failures Propensity to metastases to unusual sites Recurrence at low absolute PSA values and often asymptomatic |
| Bardoscia et al.,[31] 2020 (retrospective) | 81 | 19 pure 63 mixed | 24 RP 21 EBRT 17 RP + EBRT 19 ADT | Pure DPC: 3 local, 4 distant failures Mixed DPC: 6 local, 9 distant failures Significant SHR for cancer specific mortality for monotherapy (1.4) versus multimodal approach (0.2) Multivariate analysis confirmed age and baseline PSA to drive primary treatment choice |
| Ranasinghe et al.,[32] 2021 (retrospective) | 228 | ‐ | 155 RP 34 EBRT ± ADT 39 Other | RP 3- and 5-year MFS 83% and 75% (DPC) vs. 96% and 95% (APC) 3- and 5-year OS 97% and 88% (DPC) vs. 99% and 97% (APC) EBRT 3- and 5-year MFS 84% and 62% (DPC) vs. 100% and 93% (APC) 3- and 5-year OS 93% and 82% (DPC) vs. both 100% (APC) No MFS and OS differences between RP and EBRT |
| Chow et al.,[33] 2021 (retrospective) | 202 | 10 pure 192 mixed | 202 RP | DPC HR 1.57 for BCF Salvage-free survival 8.1 mo (DPC) vs. 22.0 mo (APC) MFS 6.7 mo (DPC) vs. 78.6 mo (APC) |
| Tan et al.,[34] 2021 (retrospective) | 79 | 79 mixed | 79 RP | DPC HR 2.368 for achieving undetectable PSA DPC HR 1.918 for BCF Mean time to BCF 14.3 vs. 19.8 mo for DPC >15% vs. < 15% |
ADT = androgen deprivation therapy; APC = acinar prostate cancer; BCF = biochemical failure; DPC = ductal prostate adenocarcinoma; DSS = disease-specific survival; DTX = docetaxel; EBRT = external beam radiotherapy; HR = hazard ratio; M0 = nonmetastatic DPC; M+ = metastatic DPC; MFS = metastasis-free survival; OS = overall survival; PCa = prostate cancer; PD = progression of disease; PFS = progression-free survival; PSA = prostate specific antigen; RP = radical prostatectomy; SEER = Surveillance, Epidemiology, and End Results; SHR = subdistribution hazard ratio.
Compared with conventional acinar PCa, DPC is usually associated with a worse prognosis. A review of the surveillance epidemiology and results program data revealed a higher percentage of DPC cases in the locally advanced and early metastatic setting, with more distant metastases and higher mortality than acinar PCa. However, the rate of lymph node metastases seemed to be similar for the 2 subgroups, and no differences were identified between mixed and pure DPC in terms of OS (despite the absence of information on morphological pattern, percentage of ductal differentiation, or ductal to acinar ratio).[12,35,36] Although not statistically significant, our study showed that stratification of patients of pure and mixed forms plays a nonnegligible role in clinical practice regarding DPC, given the more aggressive disease at presentation and worse outcomes of the pure variant. In support of this, the log-rank test revealed that patients older than 74 years had a significantly higher incidence of pure histology and worse DSS (Fig. 3). Such findings may seem to be confirmed by genetic analysis, as reported by Seipe et al.[37] In fact, the somatic changes in DPC seem comparable with those in advanced and/or metastatic castration-resistant acinar adenocarcinoma. More recently, the clinical features of DPC have been hypothesized to be driven by genomic instability (associated with RB1 loss), as well as copy number gains in the TAP1, SLC4A2, and EHHADH genes implicated in treatment resistance.[33]
In 2018, Amin[38] emphasized the importance of the Gleason scoring system in predicting the behavior of prostate tumors based on architecture, because of the absence of reliable and specific clinical features to discriminate DPC from usual acinar PCa. Therefore, it is important that pathologists recognize and accurately report the presence and percentage of DPC in prostate specimens.[38] Notably, only 6 of 81 patients in our series presented with PSA levels less than 4 ng/mL, while 13 had PSA levels greater than 50 ng/mL. Many researchers have questioned the Surveillance, Epidemiology, and End Results (SEER) study evidence of DPC having serum PSA levels 30% lower than its acinar counterpart,[12] because they have observed comparable baseline PSA levels between the 2 categories.[35,39,40] Packiam et al.[41] confirmed higher PSA levels in patients with DPC than in patients with APC and a Gleason score of 6–7, and lower PSA levels than in men with Gleason scores of 8–10. In the last year, 2 other retrospective studies of patients undergoing radical prostatectomy for localized PCa have found DPC exhibits a more aggressive tumor biology than conventional, acinar PCa, with extraprostatic extension, seminal vesicle and lymphovascular invasion, higher rates of Gleason Grade groups 4 or 5, multifocal tumors, and larger tumor size.[33,34] Moreover, DPC was independently associated with locally advanced disease and a trend toward positive surgical margins and nodal involvement on multivariate analysis.[34] In our series, baseline PSA levels were extremely heterogeneous in both pure and mixed DPC, and stratification of patients according to ductal pattern did not show any significant correlations.
Although there are no official guidelines, the management of DPC is generally the same as that for acinar PCa. Primary surgery is the preferred option for patients with eradicable disease and life expectancy older than 10–15 years, while definitive EBRT is usually chosen in cases of locally advanced disease, and ADT alone for metastatic patients or those with reduced life expectancy.[27] Recently, Ranasinghe et al.[32] retrospectively reviewed 228 cases of DPC and compared survival outcomes between such cohort of patients (155 treated with primary surgery and 34 undergoing primary EBRT, respectively) and a cohort of high-risk APC patients (163 submitted to primary surgery and 74 to primary EBRT, respectively). They found worse OS and metastasis-free survival for patients with DPC, regardless of the treatment modality, together with poor responses to ADT, probably due to the upregulation of resistance pathways, as the available targeted sequencing data revealed.[32] In particular, the authors reported a 5-year OS of 88% for DPC versus 97% for APC in the surgical group, and 5-year OS of 82% for DPC versus 100% for APC in the EBRT group.
Unfortunately, our study design did not include a direct comparison between patients with DPC and their acinar counterparts. However, although not statistically significant, we reported a 5-year OS of 82% for prostate-confined DPC patients undergoing primary surgery and 72% for their irradiated counterparts. Therefore, in this setting, we may hypothesize an overlap in the 5-year survival rates for DPC between the 2 studies. Indirectly, such findings may confirm that the ductal pattern has a worse prognosis than typical PCa; hence, it is amenable to aggressive, local treatment.
In our series, there was also no clear advantage of the adoption of combination strategies (EBRT plus surgery and EBRT plus ADT) for the treatment of DPC, in line with the abovementioned report from the MD Anderson Cancer Center.[32] Tu and colleagues[39] previously reported OS and progression-free survival rates of 11.2 and 5.8 years for 76 patients treated with surgery, compared with 8.2 and 5.5 years for 32 patients who underwent EBRT, respectively. Nakamura et al.[42] reported a lower recurrence rate in patients who underwent EBRT (10%) than in the surgical group (29%). Bergamin et al.[30] analyzed patients with DPC undergoing radiotherapy exclusively and reported relapses in 9/38, with a propensity of DPC to metastasize at unusual sites.
Notably, our series showed that the primary surgical approach for the treatment of nonmetastatic DPC was significantly associated with less favorable outcomes than prostate irradiation. Further patient selection, with a focus on prostate-confined DPC only, seemed to confirm these findings (Fig. 4C) and allowed us to remove the selection bias of advanced, node-positive, mixed DPC patients who underwent extended radical surgery (Table 1) and unavoidably progressed after primary treatment. To some extent, radiotherapy might be offered primarily to patients with histological evidence of ductal adenocarcinoma in prostate specimens, especially in cases of localized or locally advanced disease, as it is a reliable treatment option for improving outcomes.
In this scenario, multidisciplinary discussion of patients with histologically confirmed diagnoses of pure or mixed DPC among urologists, radiation oncologists, medical oncologists, radiologists, and pathologists may represent a milestone in choosing the most adequate, patient-tailored treatment option.
Unfortunately, despite the use of a multicenter database, the relatively small number of patients in our series did not allow us to draw significant conclusions on the true influence of pure ductal histology on survival, especially because it was unavoidably influenced by the stage of disease at the time of diagnosis. However, our data are in line with the available literature and previous reports. A recent Italian, monoinstitutional, and multidisciplinary retrospective experience also supported the role of a definitive, local therapeutic approach to achieve better local disease control, and improve disabling symptoms and long-term survival, together with the need for a better understanding of the role of PSA surveillance in the setting of DPC (despite baseline PSA levels seemed to drive primary treatment choice).[31]
The role of ADT is one of the most debated topics in DPC management. In our series, adding hormone therapy to primary local treatment of nonmetastatic DPC did not show any advantage in terms of OS or DFS, while ADT alone was mainly prescribed to de novo metastatic DPC patients, or to elderly nonmetastatic patients unsuitable for aggressive radical treatment. Therefore, this result is subject to bias. Reports of DPC that do not respond to ADT are available in the literature. In this regard, DPC has been reported to produce higher levels of estrogen receptor B and to be associated with a lower expression of 5-α-reductase-2 and squalene epoxidase. Such observations may suggest a correlation between DPC and lower sex hormone levels, and hence, higher castration resistance than usual histology.[43]
Recently, the prognostic value of the ductal pattern in de novo metastatic PCa has been investigated without evidence that the presence of DPC is related to adverse clinical outcomes.[44] Similar to other studies,[29,45] our findings showed a high frequency of metastatic disease at the time of diagnosis (more than 25% of patients in total, just under half in the pure DPC group). Despite the small number of cases, combining local EBRT of the primary tumor with ADT seemed to improve outcomes in metastatic onset, among which all but one DPC patient showed relatively stable disease and good biochemical control at the last follow-up. These findings are in line with recent evidence from the HORRAD trial,[46] the STAMPEDE trial,[47] and the STOPCAP systematic review and meta-analysis,[48] reporting improved time to PSA progression and better OS with the addition of prostate EBRT to first-line ADT for the treatment of low-burden, metastatic PCa at onset. However, none of these studies discriminated between APC and DPC.
Importantly, our series revealed that stereotactic irradiation, with or without concurrent ADT, was effectively offered to DPC patients with bone and/or nodal oligorecurrence/oligoprogression after primary treatment, as well as for one case of intraprostatic relapse reirradiation after primary prostate EBRT. Involved-node stereotactic radiotherapy has gained an established role as a safe and effective treatment option for oligorecurrent/oligoprogressive PCa.[49,50] Advances in radiation delivery techniques, such as volumetric modulated arc therapy, have resulted in focal, ablative, high-conformal radiation doses to the target volume with contemporary maximum sparing of the surrounding normal tissues and organs owing to improved time to disease progression and delay of systemic treatment.[51] Finally, radiation therapy was confirmed to be a wide-spectrum therapeutic approach given its renewed efficacy for the palliation of painful symptoms from metastases.[52–54]
To our knowledge, the present study represents one of the largest collections of multicenter data on DPC with long-term follow-up.
The main limitation of our study is its retrospective nature because of the unavoidable shortcomings related to the study design. Another possible limitation is that the percentage of the ductal pattern in the mixed DPC cases was not known; therefore, the true influence of the ductal component on clinical outcomes for mixed ductal-acinar PCa could not be fully assessed. Unfortunately, we could not conduct a centralized review of the histological preparations by an expert referent. We are aware that the quality of the histological tests in our series depended on the anatomic pathology department of each involved center, and this may be a third limitation.[35] Furthermore, because of the relatively wide observation period, data on DPC patients with heterogeneous disease stages and clinical features were collected, causing typical and unavoidable biases in the statistical analysis. Finally, the overall numbers categorized by treatment options were limited and did not allow for reliable multivariate analysis. Propensity score matching analysis with patients with typical histology may be desirable because it is likely to improve the statistical power of our results.
Overall, our study had significant strengths, such as a relatively large sample size despite the rarity of DPC and the availability of information regarding age, PSA, stage, treatment, and survival, which confirmed the role of aggressive, local treatment in improving outcomes.
5. Conclusions
Ductal prostate adenocarcinoma remains an unusual subtype of PCa with morphological features and, in some cases, unique clinical features. The pure form seemed to exhibit more aggressive behavior in view of its more frequent metastatic onset and shorter time to treatment failure than mixed acinar-ductal pattern disease. Our findings are in line with the available literature and support radical, local treatments, such as radiation therapy and surgery, to achieve good disease control and improve survival of nonmetastatic patients, regardless of concurrent ADT. External beam radiotherapy might be offered primarily to patients with histological evidence of ductal adenocarcinoma in prostate specimens, especially for the treatment of localized or locally advanced disease, as a reliable therapeutic option to improve the outcomes of such a rare but aggressive type of PCa. In a metastatic setting, the addition of local tumor treatment (EBRT) to primary systemic pharmacological therapy may improve local disease control, quality of life, and outcomes. External beam radiotherapy was also confirmed to be safe and effective for the palliation of painful symptoms from bone metastases. Multidisciplinary discussion of these patients is recommended to choose the most appropriate patient-tailored treatment option.
Because of the rarity of DPC worldwide, prospective studies are not conceivable. Therefore, retrospective data collection with large international data sets is desirable to better understand the management and role of different therapeutic strategies.
Conflict of interest statement
The authors have declared they have no conflicts of interest.
Acknowledgments
None.
Statement of ethics
Ethical review and approval were not required by the Institutional Review Board, due to the retrospective study design. Informed consent was obtained from all subjects involved in the study. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding source
This study was partially supported by Italian Ministry of Health — Ricerca Corrente.
Author contributions
SC contributed the research design and participated in the writing of themanuscript. LB participated in the performance of research, contributed analytic tools, and participated in the writing of the manuscript. MN participated in data analysis and participated in the writing of the paper. SI participated in the performance of research. LT contributed analytic tools and participated in the writing of the manuscript. SMM. contributed critical revision for important intellectual content. AB participated in data analysis. FG contributed critical revision for important intellectual content. LM contributed critical revision for important intellectual content. PC contributed analytic tools. CI contributed critical revision for important intellectual content. CG participated in the performance of research and contributed critical revision for important intellectual content.
All Authors approved the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
How to cite this article: Cozzi S, Bardoscia L, Najafi M, Igdem S, Triggiani L, Magrini SM, Botti A, Guedea F, Melocchi L, Ciammella P, IottiC, Gutierrez C. Ductal prostate cancer: Clinical features and outcomes from a multicenter retrospective analysis and overview of the current literature. Curr Urol 2022;16(4):218–226. doi: 10.1097/CU9.0000000000000118.
Contributor Information
Salvatore Cozzi, Email: salvatore.cozzi@ausl.re.it.
Masoumeh Najafi, Email: najafi.mas@iums.ac.ir.
Sefik Igdem, Email: sefik.igdem@florence.com.tr.
Luca Triggiani, Email: triggioluca@hotmail.it.
Stefano Maria Magrini, Email: stefano.magrini@unibs.it.
Andrea Botti, Email: andrea.botti@ausl.re.it.
Ferran Guedea, Email: fguedea@mit.edu.
Laura Melocchi, Email: laura.melocchi@poliambulanza.it.
Patrizia Ciammella, Email: patrizia.ciammella@ausl.re.it.
Cinzia Iotti, Email: cinzia.iotti@ausl.re.it.
Cristina Gutierrez, Email: cgutierrezm@iconcologia.net.
References
- 1.Bell KJL, Del Mar C, Wright G, Dickinson J, Glasziou P. Prevalence of incidental prostate cancer: A systematic review of autopsy studies. Int J Cancer 2015;137(7): 1749–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Center MM Jemal A Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol 2012;61(6):1079–1092. [DOI] [PubMed] [Google Scholar]
- 3.Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of tumours of the urinary system and male genital organs—part B: Prostate and bladder tumours. Eur Urol 2016;70(1):106–119. [DOI] [PubMed] [Google Scholar]
- 4.Bronkema C Arora S Sood A, et al. Rare histological variants of prostate adenocarcinoma: A national cancer database analysis. J Urol 2020:204(2):260–266. [DOI] [PubMed] [Google Scholar]
- 5.Guo CC, Epstein JI. Intraductal carcinoma of the prostate on needle biopsy: Histologic features and clinical significance. Mod Pathol 2006;19(12):1528–1535. [DOI] [PubMed] [Google Scholar]
- 6.Iğdem S Spiegel DY Efstathiou J, et al. Prostatic duct adenocarcinoma: clinical characteristics, treatment options, and outcomes—A rare cancer network study. Onkologie 2010;33(4):169–173. [DOI] [PubMed] [Google Scholar]
- 7.Kan RWM, Kan CF, Wong JHM, Fu KKF, Ng CF, Chan SWH. Ductal adenocarcinoma of the prostate: A Hong Kong case series. Int Urol Nephrol 2014;46(11):2133–2137. [DOI] [PubMed] [Google Scholar]
- 8.Morgan TM, Welty CJ, Vakar-Lopez F, Lin DW, Wright JL. Ductal adenocarcinoma of the prostate: Increased mortality risk and decreased serum prostate specific antigen. J Urol 2010; 184(6):2303–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orihuela E, Green JM. Ductal prostate cancer: Contemporary management and outcomes. Urol Oncol 2008;26(4):368–371. [DOI] [PubMed] [Google Scholar]
- 10.Kim A Kwon T You D, et al. Clinicopathological features of prostate ductal carcinoma: Matching analysis and comparison with prostate acinar carcinoma. J Korean Med Sci 2015;30(4):385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melicow MM, Pachter MR. Endometrial carcinoma of proxtatic utricle (uterus masculinus). Cancer 1967;20(10):1715–1722. [DOI] [PubMed] [Google Scholar]
- 12.Meeks JJ Zhao LC Cashy J, et al. Incidence and outcomes of ductal carcinoma of the prostate in the USA: Analysis of data from the Surveillance, Epidemiology, and End Results program. BJU Int 2012;109(6):831–834. [DOI] [PubMed] [Google Scholar]
- 13.Christensen WN, Steinberg G, Walsh PC, Epstein JI. Prostatic duct adenocarcinoma. Findings at radical prostatectomy. Cancer 1991;67(8):2118–2124. [DOI] [PubMed] [Google Scholar]
- 14.Herawi M, Epstein JI. Immunohistochemical antibody cocktail staining (p63/HMWCK/AMACR) of ductal adenocarcinoma and Gleason pattern 4 cribriform and noncribriform acinar adenocarcinomas of the prostate. Am J Surg Pathol 2007;31(6):889–894. [DOI] [PubMed] [Google Scholar]
- 15.Tomlins SA Rhodes DR Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005; 310:644–648. [DOI] [PubMed] [Google Scholar]
- 16.Morais CL Herawi M Toubaji A, et al. PTEN loss and ERG protein expression are infrequent in prostatic ductal adenocarcinomas and concurrent acinar carcinomas. Prostate 2015;75:1610–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brierley JD Gospodarowicz MK Wittekind C, et al. eds. TNM Classification of Malignant Tumours. 8th ed. Oxford: Wiley Blackwell; 2017. [Google Scholar]
- 18.Amling CL, Bergstralh EJ, Blute ML, Slezak JM, Zincke H. Defining prostate specific antigen progression after radical prostatectomy: What is the most appropriate cut point? J Urol 2001;165(4);1146–1151. [PubMed] [Google Scholar]
- 19.Buyyounouski MK Hanlon AL Eisenberg DF, et al. Defining biochemical failure after radiotherapy with and without androgen deprivation for prostate cancer. Int J Radiat Oncol Biol Phys 2005;63(5):1455–1462. [DOI] [PubMed] [Google Scholar]
- 20.Giganti F Allen C Sridhar A, et al. Mixed acinar and macrocystic ductal prostatic adenocarcinoma. Lancet Oncol 2021;22(1):e37. [DOI] [PubMed] [Google Scholar]
- 21.Seipel AH Wiklund F Wiklund NP, et al. Histopathological features of ductal adenocarcinoma of the prostate in 1,051 radical prostatectomy specimens. Virchows Arch 2013;462:429–436. [DOI] [PubMed] [Google Scholar]
- 22.Dube VE, Farrow GM, Greene LF. Prostatic adenocarcinoma of ductal origin. Cancer 1973;32(2):402–409. [DOI] [PubMed] [Google Scholar]
- 23.Sanati S, Watson MA, Salavaggione AL, Humphrey PA. Gene expression profiles of ductal versus acinar adenocarcinoma of the prostate. Mod Pathol 2009;22(10):1273–1279. [DOI] [PubMed] [Google Scholar]
- 24.Brinker DA, Potter SR, Epstein JI. Ductal adenocarcinoma of the prostate diagnosed on needle biopsy: Correlation with clinical and radical prostatectomy findings and progression. Am J Surg Pathol 1999;23(12):1471–1479. [DOI] [PubMed] [Google Scholar]
- 25.Eade TN Al-Saleem T Horwits EM, et al. Role of radiotherapy in ductal (endometrioid) carcinoma of the prostate. Cancer 2007;109(10):2011–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiramatsu K Tsuzaka Y Kaneko T, et al. Ductal adenocarcinoma of the prostate: A report of 7 cases. Nihon Hinyokika Gakkai Zasshi 2012;103(5):671–674. [DOI] [PubMed] [Google Scholar]
- 27.Mohler JL Antonarakis ES Armstrong AJ, et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2019;17(5):479–505. [DOI] [PubMed] [Google Scholar]
- 28.Knipper S Preisser F Mazzole E, et al. Contemporary comparison of clinicopathologic characteristics and survival outcomes of prostate ductal carcinoma and acinar adenocarcinoma: A population-based study. Clin Genitourin Cancer 2019;17(3):231–237.e2. [DOI] [PubMed] [Google Scholar]
- 29.Jang WS Shin SJ Yoon CY, et al. Prognostic significance of the proportion of ductal component in ductal adenocarcinoma of the prostate. J Urol 2017;197(4):1048–1053. [DOI] [PubMed] [Google Scholar]
- 30.Bergamin S Eade T Kneebone A, et al. Ductal carcinoma of the prostate: An uncommon entity with atypical behaviour. Clin Oncol (R Coll Radiol) 2019;31(2):108–114. [DOI] [PubMed] [Google Scholar]
- 31.Bardoscia L Triggiani L Sandri M, et al. Non-metastatic ductal adenocarcinoma of the prostate: Pattern of care from an uro-oncology multidisciplinary group. World J Urol 2021;39(4):1161–1170. [DOI] [PubMed] [Google Scholar]
- 32.Ranasinghe W Shapiro DD Hwang H, et al. Ductal prostate cancers demonstrate poor outcomes with conventional therapies. Eur Urol 2021;79(2):298–306. [DOI] [PubMed] [Google Scholar]
- 33.Chow K Bedő J Ryan A, et al. Ductal variant prostate carcinoma is associated with a significantly shorter metastasis-free survival. Eur J Cancer 2021;148:440–450. [DOI] [PubMed] [Google Scholar]
- 34.Tan YG Khalid F Huang HH, et al. Prostatic ductal adenocarcinoma variant predicts worse pathological and oncological outcomes: Insight from over 1000 consecutive patients from a large prospective uro-oncology registry. Prostate 2021;81(4):242–251. [DOI] [PubMed] [Google Scholar]
- 35.Amin A, Epstein JI. Pathologic stage of prostatic ductal adenocarcinoma at radical prostatectomy: Effect of percentage of the ductal component and associated grade of acinar adenocarcinoma. Am J Surg Pathol 2011;35(4):615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Epstein JI Allsbrook WC Jr Amin MB Egevad LL, ISUP Grading Committee . The 2005 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol 2005;29(9):1228–1242. [DOI] [PubMed] [Google Scholar]
- 37.Seipel AH Whitington T Delahunt B, et al. Genetic profile of ductal adenocarcinoma of the prostate. Hum Pathol 2017;69:1–7. [DOI] [PubMed] [Google Scholar]
- 38.Amin A. Prostate ductal adenocarcinoma. Appl Immunohistochem Mol Morphol 2018;26(7):514–521. [DOI] [PubMed] [Google Scholar]
- 39.Tu SM Lopez A Leibovici D, et al. Ductal adenocarcinoma of the prostate: Clinical features and implications after local therapy. Cancer 2009;115(13):2872–2880. [DOI] [PubMed] [Google Scholar]
- 40.Samaratunga H, Duffy D, Yaxley J, Delahunt B. Any proportion of ductal adenocarcinoma in radical prostatectomy specimens predicts extraprostatic extension. Hum Pathol 2010; 41(2):281–285. [DOI] [PubMed] [Google Scholar]
- 41.Packiam VT Patel SG Pariser JJ, et al. Contemporary population-based comparison of localized ductal adenocarcinoma and high-risk acinar adenocarcinoma of the prostate. Urology 2015;86(4):777–782. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura K, Terada N, Kobayashi T. Clinical characteristics of prostate ductal adenocarcinoma in Kyoto University Hospital. Thinyokika Kiyo 2015;61(12):487–491. [PubMed] [Google Scholar]
- 43.Jardel P Debiais C Godet J, et al. Ductal carcinoma of the prostate shows a different immunophenotype from high grade acinar cancer. Histopathology 2013;63(1):57–63. [DOI] [PubMed] [Google Scholar]
- 44.Wu T Zhao J Liu Z, et al. Does ductal adenocarcinoma of the prostate (DA) have any prognostic impact on patients with de novo metastatic prostate cancer? Prostate 2019;79(14):1673–1682. [DOI] [PubMed] [Google Scholar]
- 45.Vinceneux A Bruyère F Haillot O, et al. Ductal adenocarcinoma of the prostate: Clinical and biological profiles. Prostate 2017;77(12):1242–1250. [DOI] [PubMed] [Google Scholar]
- 46.Boeve LMS Hulshof M Vis AN, et al. Effect on survival of androgen deprivation therapy alone compared to androgen deprivation therapy combined with concurrent radiation therapy to the prostate in patients with primary bone metastatic prostate cancer in a prospective randomised clinical trial: Data from the HORRAD trial. Eur Urol 2019;75(3):410–418. [DOI] [PubMed] [Google Scholar]
- 47.James ND Sydes MR Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomized controlled trial. Lancet 2016;387(10024):1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burdett S Boeve LM Ingleby FC, et al. Prostate radiotherapy for metastatic hormone-sensitive prostate cancer: A STOPCAP systematic review and meta-analysis. Eur Urol 2019;76(1):115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Bleser E Jereczek-Fossa BA Pasquier D, et al. Metastasis-directed therapy in treating nodal oligorecurrent prostate cancer: A multi-institutional analysis comparing the outcome and toxicity of stereotactic body radiotherapy and elective nodal radiotherapy. Eur Urol 2019;76(6):732–739. [DOI] [PubMed] [Google Scholar]
- 50.Cozzi S Botti A Timon G, et al. Prognostic factors, efficacy, and toxicity of involved-node stereotactic body radiation therapy for lymph node oligorecurrent prostate cancer: An investigation of 117 pelvic lymph nodes. Strahlenther Onkol 2021. doi: 10.1007/s00066-021-01871-5. [DOI] [PubMed] [Google Scholar]
- 51.Cornford P van den Bergh RCN Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II-2020 update: Treatment of relapsing and metastatic prostate cancer. Eur Urol 2021;79(2):263–282. [DOI] [PubMed] [Google Scholar]
- 52.Mizumoto M Harada H Asakura H, et al. Radiotherapy for patients with metastases to the spinal column: A review of 603 patients at Shizuoka Cancer Center Hospital. Int J Radiat Oncol Biol Phys 2011;79(1):208–213. [DOI] [PubMed] [Google Scholar]
- 53.Howell DD James JL Hartsell WF, et al. Single-fraction radiotherapy versus multifraction radiotherapy for palliation of painful vertebral bone metastases-equivalent efficacy, less toxicity, more convenient: A subset analysis of radiation therapy oncology group trial 97-14. Cancer 2013;119(4):888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nguyen QN Chun SG Chow E, et al. Single-fraction stereotactic vs conventional multifraction radiotherapy for pain relief in patients with predominantly nonspine bone metastases: A randomized phase 2 trial. JAMA Oncol 2019;5(6):872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]


