Letter to the Editor
Hematopoietic cell transplantation-comorbidity index (HCT-CI) was developed to evaluate comorbidity burden before HCT and to provide prognostic information about transplant outcomes1. Development and internal validation of the HCT-CI included both pediatric and adult patients having a median age 44.8 (range 0.8–72.7) years. Subsequently, the model was prospectively validated in 7,171 adult and 944 pediatric patients with malignant diseases and separately in 888 adult and 3,195 pediatric patients with non-malignant diseases given allogeneic HCT2,3. In both studies, the HCT-CI provided valuable prognostic information for all patients except those with hemoglobinopathies. However, many pediatric patients were classified with scores of 0, leading to uncertainty about accurately capturing all comorbidities in this population3,4. Additionally, anecdotal reports from pediatric providers have raised question about utilization of the HCT-CI in clinical practice for children and adolescents. This has led to significant interest in re-evaluating the HCT-CI in younger patients utilizing registry data. To determine the potential impact of such studies, we developed a brief survey aimed at understanding the current utilization and limitations of the HCT-CI in younger population, which was distributed by email in July 2020 to the members of the Regimen Related Toxicity & Supportive Care and Pediatric Cancer Working Committees of the Center for International Blood and Marrow Transplant Research (CIBMTR®), and to Medical Directors of transplant centers. The survey included a description of the objective, which was to understand the patterns of HCT-CI use and to determine if practical modifications to the HCT-CI could change its utilization in clinical practice. A link to complete the survey was included in the email with a reminder email sent four weeks later. The survey was kept open for two months following the initial email. Responses were recorded anonymously.
All respondents were given each of the first 2 multiple choice questions independently and sequentially: 1) Which patient population do you primarily treat? 2) How often do you use the HCT-CI for pre-transplant planning/counseling? While our intent was to evaluate practice specifically in the pediatric population, both adult and pediatric physicians participate in the groups emailed; thus, our first question sought to determine the respondent’s primary patient population treated. Then, those who responded to question 2 noting use of the HCT-CI <50% of the time, were then asked to respond to question 3 and 4: 3) If the HCT-CI is used <50% of the time, what limits you from using this tool? 4) How likely are you to use a new comorbidity tool if designed for children? The absolute number and frequency of responses were described from all responders and then separated by patient population into pediatric or adult transplant physicians caring for patients ≤18 years old (yo) or >18 yo, respectively, or both.
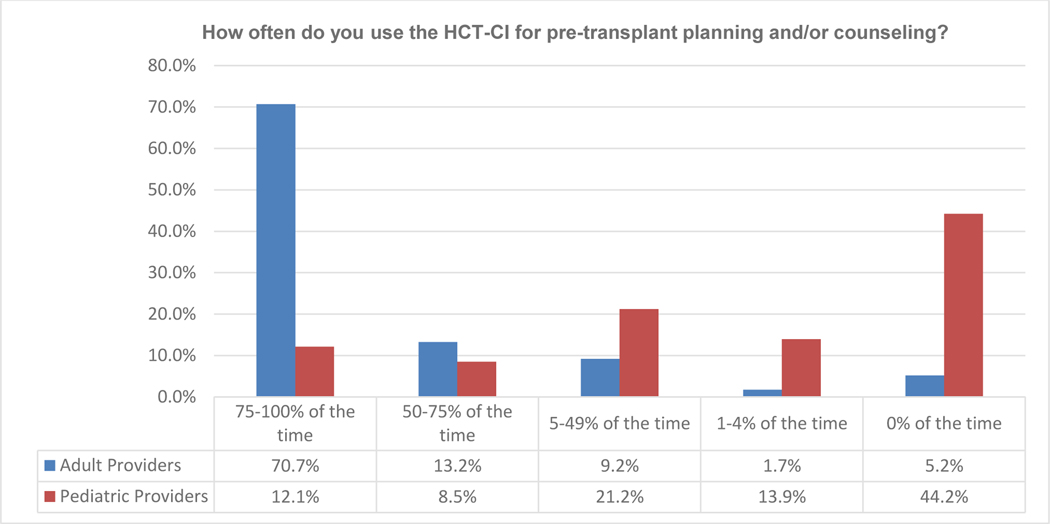
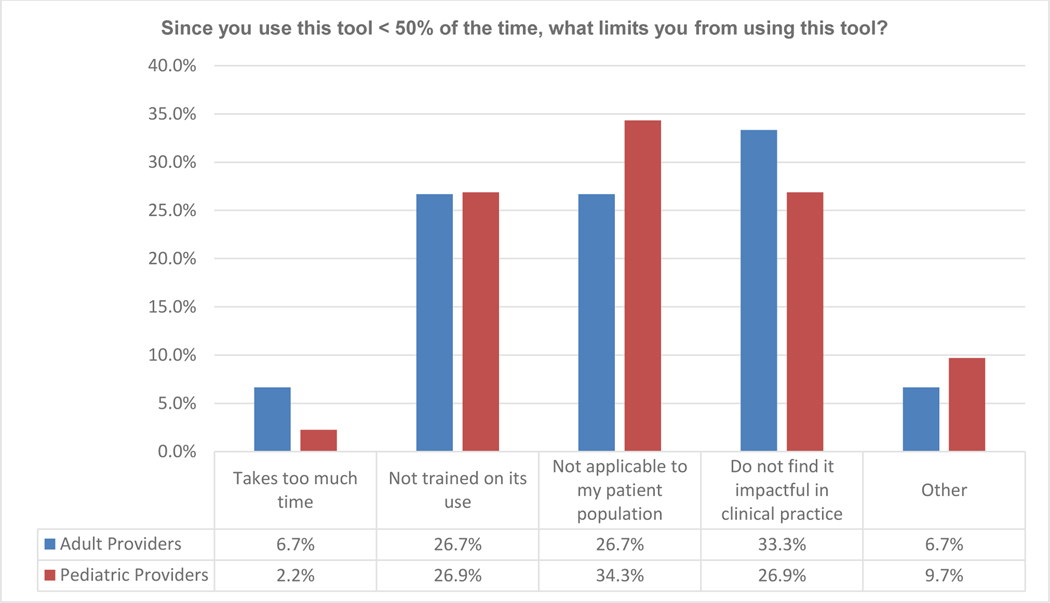
The survey was distributed to 1,221 physicians and 341 responses were received, providing a response rate of 28%. Fifty percent of the responders self-identified as primarily treating pediatric patients, whereas 39.6% primarily treated adult patients, and 11.4% cared for both pediatric and adult patients; providers who treated both adult and pediatric patients were groups together with those treating adults only in the analysis. When asked how often the HCT-CI was used for pre-transplant planning and/or counseling, 42.2% of all responders reported using it 75–100% of the time and 11% using it 50–75% of the time. However, the response to this question varied by the population of patients treated; 70.7% of physicians managing adults and 12.1% managing pediatric patients report using the HCT-CI 75–100% of the time, with an additional 13.2% of adult and 8.5% of pediatric physicians using the HCT-CI 50–75% of the time. Interestingly, only 5% of adult transplant physicians versus 44.2% of pediatric transplant physicians reported never using the HCT-CI (Figure 1A). Of respondent pediatric transplant physicians, 79% indicated use of the HCT-CI <50% of the time and their reasonings included “not applicable to my patient population” (34.3%), “do not find it impactful in clinical practice” (26.9%), or “not trained on its use” (26.8%). The frequency of these responses was similar in both adult as well as pediatric transplant physicians (Figure 1B), despite the lower frequency of “use of the HCT-CI <50% of the time” response among adult physician respondents (14%). Additionally, 93% of the pediatric providers noted that they would be more likely to use a new score if it was developed specifically for children and young adults.
Figure 1A.
Response to Survey Question 2, Stratified by primary patient population treated
Figure 1B.
Response to Survey Question 3, Stratified by primary patient population treated
Although the HCT-CI has been integrated into routine clinical care for adults, the results of our survey suggest that the adoption of this risk tool is lagging amongst pediatric providers. To date, the HCT-CI is the most widely utilized tool used to assess a patient’s risk of transplant-related mortality. However, 80% of pediatric physicians who responded to our survey reported using the HCT-CI <50% of the time, noting that they do not feel that the HCT-CI is applicable to their patient population or impactful in their clinical practice. Pediatric-focused transplant physicians have thus highlighted a practical management difference compared to their adult colleagues which needs to be further explored. Previously reported studies have noted challenges in applying the HCT-CI in children4–7. The assessment of organ function in children differs from adults, especially for renal disease, obesity, and pulmonary disease. These definitions can potentially be enhanced, e.g., renal disease may be assessed using estimated glomerular filtration rate which aligns with pre-transplant assessments done in children, obesity assessed using growth percentile curves, and additional parameters for assessment of pulmonary disease in younger patients who have difficulty performing spirometry. Furthermore, additional hitherto unrecognized comorbidities may be present in this younger population which may help better capture comorbidity burden than the current HCT-CI. Our survey results support a desire by the pediatric transplant community for expansion of the HCT-CI to be more applicable to a younger population, with over 90% of pediatric respondents more likely to adopt a pediatric-specific tool. We acknowledge the limitations in survey responses, including potential bias arising from the physicians who chose to respond to our survey. We may have inadvertently introduced bias in the email communication to physicians alluding to the development of a more practical/modified HCT-CI, and so may have elicited responses from those with a particular interest in the development of a new iteration of the HCT-CI. Therefore, our results may be an overestimation of those who do not use the HCT-CI routinely and are open to its revision. A total of 167 pediatric transplant physicians (28%) responded, highlighting that there is a large group of pediatric physicians who would welcome age-appropriate adjustments to broaden the use of the HCT-CI for younger population.
In conclusion, the results of our survey show, for the first time, that HCT-CI has not been adopted by pediatric physicians as widely as their adult counterparts. There is an unmet need in the pediatric population for a pre-transplant risk score that can be utilized by pediatric providers to predict transplant-related mortality. Future studies will need to focus on expanding comorbidity definitions and identifying additional pre-transplant risk factors or biomarkers that impact outcomes of pediatric and young adult patients. Additionally, given the heterogeneity of indications for transplantation in children and young adults, there should be consideration for separate risk scores for patients with malignant and non-malignant diseases reflecting the differences in disease pathobiology, and pre-transplant exposures. Ultimately, the goal of enhancing the performance of the HCT-CI in a younger population will aid pediatric providers in better estimating individual risk of transplant-associated complications for children and their families.
Acknowledgment:
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); HHSH250201700006C from the Health Resources and Services Administration (HRSA); and N00014-20-1-2705 and N00014-20-1-2832 from the Office of Naval Research; Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: AbbVie; Accenture; Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies Corporation; Adienne SA; Allovir, Inc.; Amgen, Inc.; Astellas Pharma US; bluebird bio, inc.; Bristol Myers Squibb Co.; CareDx; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; Eurofins Viracor; ExcellThera; Fate Therapeutics; Gamida-Cell, Ltd.; Genentech Inc; Gilead; GlaxoSmithKline; Incyte Corporation; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Karyopharm Therapeutics; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Magenta Therapeutics; Medac GmbH; Merck & Co.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; Oncopeptides, Inc.; Orca Biosystems, Inc.; Pfizer, Inc.; Pharmacyclics, LLC; Sanofi Genzyme; Seagen, Inc.; Stemcyte; Takeda Pharmaceuticals; Tscan; Vertex; Vor Biopharma; Xenikos BV.
Conflicts of interest:
Dr. Schiller reports grants or contracts for clinical trials from AbbVie, Actininium, Actuate, Arog, Astellas, BMS/Celgene, Celator, Constellation, Daliichi-Sankyo, Deciphera, Delta-Fly, Forma, Fujifilm, Gamida, Genentech-Roche, Geron, Incyte, Karyopharm, Kite/Gilead, Mateon, Onconova, Pfizer, PrECOG, Regimmune, Samus, Sangamo, Sellas, Stemline, Takeda, Agios, Amgen, Jazz, Elevate, Bio, Ono-UK, Novartis, Sanofi; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Amgen, Jazz, Stemline, kite, BMS, Sanofi, Astellas; leadership or fiduciary in other board, society, committee or advocacy group, paid or unpaid from ASH foundation Chair-unpaid; stock or stock options BMS, Amgen, Johnson & Johnson. Dr. Pasquini reports research support from Novartis, Kite, BMS, Amgen (completed 2019), Consultancy (Iisted as the professional providing insight), BMS (CAR T cell Steering Committee - former as of June 2021). Dr. Chhabra reports institutional research funding from Amgen, Janssen and Sanofi; and honoraria (for advisory boards) from GSK and Sanofi.
Data Use Statement:
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.
References
- 1.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorror ML, Logan BR, Zhu X, et al. Prospective validation of the predictive power of the hematopoietic cell transplantation comorbidity index: A Center for International Blood and Marrow Transplant Research study. Biol Blood Marrow Transplant. 2015;21(8):1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thakar MS, Broglie L, Logan B, et al. The Hematopoietic Cell Transplant Comorbidity Index predicts survival after allogeneic transplant for non-malignant diseases. Blood. 2019;133(7):754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith AR, Majhail NS, MacMillan ML, et al. “Hematopoietic Cell Transplantation Comorbidity Index predicts transplantation outcomes in pediatric patients”. Blood. 2011;117(9):2728–2734. [DOI] [PubMed] [Google Scholar]
- 5.Friend BD, Tang K, Markovic D, et al. Identifying risk factors associated with worse outcomes in adolescents and young adults undergoing hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2019;66(12):e27940. [DOI] [PubMed] [Google Scholar]
- 6.Broglie L, Ruiz J, Jin Z, et al. Limitations of applying the Hematopoietic Cell Transplantation Comorbidity Index in pediatric patients receiving allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2021;27(1):74.e1–74.e9. [DOI] [PubMed] [Google Scholar]
- 7.Wood W, Deal A, Whitley J, et al. Usefulness of the hematopoietic cell transplantation-specific comorbidity index (HCT-CI) in predicting outcomes for adolescents and young adults with hematologic malignancies undergoing allogeneic stem cell transplant. Pediatr Blood Cancer. 2011;57(3):499–50. [DOI] [PubMed] [Google Scholar]