Abstract
During sleep, reduced brain energy demands provide an opportunity for biosynthetic processes like protein synthesis. Sleep is required for some forms of memory consolidation which requires de novo protein synthesis. We measured regional cerebral protein synthesis rates (rCPS) in human subjects to ascertain how rCPS is affected during sleep. Subjects underwent three consecutive L-[1-11C]leucine PET scans with simultaneous polysomnography: 1. rested awake, 2. sleep-deprived awake, 3. sleep. Measured rCPS were similar across the three conditions. Variations in sleep stage times during sleep scans were used to estimate rCPS in sleep stages under the assumption that measured rCPS is the weighted sum of rCPS in each stage, with weights reflecting time and availability of [11C]leucine in that stage. During sleep scans, subjects spent most of the time in N2, N3, and awake and very little time in N1 and REM; rCPS in N1 and REM could not be reliably estimated. When stages N1 and N2 were combined [N1,N2], estimates of rCPS were more robust. In selective regions, estimated rCPS were statistically significantly higher (30–39%) in [N1,N2] compared with N3; estimated rCPS in N3 were similar to values measured in sleep-deprived awake scans. Results indicate increased rates of protein synthesis linked to [N1,N2] sleep.
Keywords: Leucine, N2, positron emission tomography, protein synthesis, sleep
Introduction
Energy demands are significantly reduced during non-rapid eye movement (non-REM) sleep 1 providing an opportunity for biosynthetic processes such as protein synthesis to occur. 2 Studies in rats and rhesus monkeys suggest that protein synthesis rates in brain are indeed increased during non-REM sleep.3,4 Protein synthesis plays a role in cortical plasticity during sleep in kittens,5,6 and sleep deprivation of mice impairs memory consolidation through an effect on mechanistic target of rapamycin complex 1 (mTORC1), a regulator of protein synthesis. 7 We asked if rates of brain protein synthesis change during sleep and sleep-deprived awake states compared to rested awake.
We used the L-[1-11C]leucine positron emission tomography (PET) method in human subjects. Each subject was scanned three times: 1. rested awake, 2. sleep-deprived awake, and sleep. Based on previous studies in animals,3,4 we hypothesized that rCPS would be higher during slow-wave sleep in multiple regions of interest. We further hypothesized that rCPS would remain unchanged during sleep-deprived wakefulness compared to rested wakefulness. Given that brain activity decreases during sleep deprivation, 8 this provided an important negative control to exclude the possibility that decreases in brain activity during non-REM sleep9 alone would passively increase protein synthesis. Our study has important implications for neurodevelopmental disorders and neurodegenerative diseases considering the roles that sleep abnormalities and protein synthesis dysregulation play in their pathophysiology.10–12
Material and methods
Subjects
All procedures were conducted in accordance with the ethical standards of the Helsinki Declaration of 1975 (and as revised in 1983) and were approved by the National Institutes of Health Combined Neurosciences Institutional Review Board (09-M-N230, NCT00884702), the Walter Reed Army Institute of Research Institutional Review Board, the National Institutes of Health Radioactive Drug Research Committee, and the National Institutes of Health Radiation Safety Committee. The subjects were typically undergraduate students at local universities or post-baccalaureate fellows at the National Institutes of Health. All subjects gave written informed consent. The subjects were monetarily compensated for research-related discomforts and inconveniences.
Subjects were required to be 18–28 years of age with normal or corrected-to-normal vision, as self-reported. Both male and female subjects were included. They were screened for a clinical history of sleep disorders and for their likelihood of falling asleep during simultaneous polysomnography and PET. Subjects with difficulty sleeping supine or outside their home environment were excluded. A clinical team collected a comprehensive medical and psychiatric history and performed a physical examination with associated laboratory tests. Subjects were excluded if they had a significant medical condition (e.g., anemia), a positive urine drug screen, a contraindication for sleep deprivation (e.g., seizure disorder), a contraindication for PET (e.g., pregnancy), or a contraindication for magnetic resonance imaging (e.g., ferromagnetic implants) or if they had a family history of a monogenetic neurological disorder. Additional psychiatric screening was performed using an abbreviated version of the Structured Clinical Interview for DSM-IV-TR. 13 Subjects were excluded if they had a current or past Axis I disorder. Sex, ethnicity, and race were determined by self-report.
A total of 28 subjects were scheduled. One subject was excluded due to a failure to comply with study instructions during the home-monitoring phase, and one subject withdrew before study procedures were complete due to a headache. Five subjects were excluded due to technical problems with one or more scans: radiochemical synthesis failure (2 subjects), radiochemical dose infiltration (1 subject), reconstruction and alignment failure (2 subjects). An additional two subjects were excluded because they were awake for ≥99% of the sleep scan. Our initial analysis of the rCPS data was a comparison of rCPS in sleep with rCPS in awake and awake sleep-deprived using a within subject design. For this analysis we included only subjects who had slept more than 70% weighted time during the sleep scan (16 of the 19 subjects). Within this group of 16, we identified one subject as a statistical outlier in both the awake and sleep scans (>2* SD from respective group means). The final group size for this within subject analysis was 15 subjects. For the regression analysis that considered all sleep stages simultaneously we added the three subjects who slept between 18 and 25% during the sleep scan. The final group size for regression analyses was 18 subjects. For both the 15- and 18-subject groups the Kolmogorov-Smirnov test was used to test for non-normality of the distribution of the rCPS data.
Procedures
A schematic overview and timeline of the entire study can be found in the Supplemental_Figure file. For two weeks immediately prior to the laboratory phase, subjects underwent a sleep-extension protocol at home to eliminate any pre-existing sleep debt that might have accumulated from voluntary sleep deprivation to ensure the rested wakefulness scan represented a fully rested condition. Subjects were required to obtain a continuous period of 10 hr in bed at approximately the same time every night, and they were not allowed to nap. Compliance with this regimen was verified with wrist actigraphy (Mini Motionlogger, Ambulatory Monitoring, Ardsley, New York, USA) and with a voicemail log of in-to-bed and out-of-bed times. During the first 11 days, subjects were required to consume no more than one caffeinated beverage per day, to consume no more than one alcoholic beverage per day, and to discontinue their use of over-the-counter medications. During the last 3 days, they were required to abstain from all caffeine, stimulants, sleeping aids, tobacco, and alcohol.
During the laboratory phase, subjects were admitted to an inpatient unit at the National Institutes of Health Clinical Center. Actigraphy and voicemail log data were reviewed, and subjects who did not follow instructions were withdrawn from the study. Subjects were tested again for abuse of drugs by analyzing a urine sample and excluded accordingly. Subjects underwent three 90-min PET scans. The first was conducted during rested wakefulness at approximately 9:30 am. All subjects then underwent 24 h of sleep deprivation. The second PET scan was conducted during sleep-deprived wakefulness at approximately 9:30 the next morning. During the period of sleep deprivation and during the sleep-deprived awake scan, subjects were kept awake by monitoring them closely and eliciting responses to direct questions. During the scan, responses were non-verbal to minimize head motion. No stimulants were provided or allowed. The third PET scan was conducted during sleep at approximately 12:30 pm of the same day as the second scan. Sleep deprivation continued between the second and third scans.
Polysomnography. Electrode placement followed American Academy of Sleep Medicine standards. 14 Eight electroencephalography channels were employed. Data were recorded and analyzed using Easy EEG III hardware and software (Easy EEG III, Cadwell, Kennewick, Washington, USA). Sleep staging was performed manually from C3 according to standard procedures and criteria. 14 A band-pass filter of 0.5–35.0 Hz was used for the electroencephalography and electro-oculography channels. For the electromyography channel, a high-pass filter at 10 Hz, a low-pass filter at 70 Hz, and a notch filter at 60 Hz was used. EEG was recorded during each PET scan.
L-[11C]leucine PET. Subjects consumed an energy bar, or a comparable meal, with a total of at least 20 g of protein, prior to going to bed the night before the first scan and at midnight the night before the second scan. Subsequently, subjects were food-deprived for 8 hr before each scan so that circulating levels of amino acids would reach a steady state. L-[1-11C]leucine was prepared from H11CN with a modified Strecker-Bucherer reaction as previously described. 15 A venous catheter was inserted into a vein in the antecubital area of one arm for injection of tracer, and an arterial catheter was placed in the radial artery of the non-dominant arm for collection of timed arterial blood samples. The scanner table consisted of a medical-grade, Tempur-Med® mattress. Studies were performed on an ECAT High Resolution Research Tomograph (CPS Innovations, Knoxville, Tennessee, USA). A headband with targets for an optical head-tracking system (Polaris, Northern Digital, Waterloo, Ontario, Canada) was positioned, and subjects were placed in the scanner for a 3-minute transmission scan to determine optimal table positioning within the scanner field of view. This was followed by a 6-minute transmission scan for attenuation correction. A post-emission transmission scan was performed and used as appropriate when subjects exhibited a large movement during the emission scan. Head position information was continuously recorded throughout the scan by the head-tracking system, which has an accuracy of 0.5–0.6 mm, for motion correction of the emission data during image reconstruction. 16 The 90-min dynamic emission scans were then initiated coincident with the intravenous two-minute infusion of 11.1 MBq/kg L-[1-11C]leucine (10–50 MBq/nmol) in 2–10 ml normal saline at a constant rate as previously described. 15
PET data processing. Data were acquired in list mode and reconstructed by means of the motion-compensated 3D ordinary Poisson ordered subset expectation maximization algorithm (30 subsets, 2 iterations). 16 Spatial resolution after reconstruction was approximately 2.6 mm full width at half maximum in radial and transverse directions. 17 Three-dimensional data were reconstructed to 207 slices 1.23 mm thick with a pixel size of 1.21 × 1.21 mm. Images were reconstructed as 42 frames of data (16 × 15 sec, 4 × 30 sec, 4 × 60 sec, 4 × 150 sec, 14 × 300 sec). With this reconstruction, motion-correction was based on position data collected throughout the scan with the Polaris system, 18 and the data were corrected for attenuation based on a single attenuation-correction map (MuMap) applied to all frames of data. In scans with large abrupt subject motion events that had not been fully corrected by this procedure, we proceeded as follows: (1) Summed Frames 1–17 (0–4.5 min post-injection) and aligned the summed image to the MuMap; (2) Determined 3D rigid body spatial transformation matrices for Frames 18–42 (4.5–90 min post-injection) by aligning each frame to the summed image; (3) Applied the inverse of each transformation from the previous step to the original MuMap to obtain individual MuMaps for Frames 18–42; (4) Reconstructed the data again utilizing the original motion-correction together with individual MuMaps for each frame; (5) Aligned Frames 18–42 with the sum of the first 17 frames using the transformations defined in Step 2. These steps assured not only that the data from each frame were aligned to the initial frames, but also that an accurate attenuation correction was applied. The reconstructed volume was resliced so that it aligned with the magnetic resonance imaging volume by use of the Flexible Image Registration Toolbox 19 with a 3D rigid body transformation. The resliced average 30 to 60 min PET image was visually reviewed for correct alignment with the magnetic resonance imaging volume by use of the Volume Imaging in Neurological Research, CoRegistration and ROIs Included software (Max Planck Institute for Neurological Research, Cologne, North Rhine-Westphalia, Germany). The transformation parameters were then applied to each frame of the PET volume (without prior smoothing) to effect its alignment with the magnetic resonance imaging volume.
The kinetic model for the behavior of leucine in brain has been described.20,21 The parameters of the model were estimated for each voxel in the whole brain volume by means of the Basis Function Method 21 with a slightly modified algorithm to avoid negative parameter estimates. 22 rCPS was computed from the model parameters. Images of rCPS were constructed, and regions of interest drawn on magnetic resonance imaging volumes were transferred to rCPS images to compute average rCPS values for each region of interest. No smoothing was applied when computing average values.
Although the scans were 90 min, we analyzed only the first 60 min of the PET data. Subsequent to the PET scanning for this study, we had examined the effects of shortened scan durations and found that estimates of rCPS were very stable between 60 and 90 min, i.e., inclusion of time points beyond 60 min changes computed values of rCPS only negligibly. 23
Analysis of blood samples. Arterial blood sampling was initiated concurrently with the start of L-[1-11C]leucine infusion to determine the time courses of the concentrations of unlabeled and labeled leucine in arterial plasma and total 11C and 11CO2 activities in arterial whole blood. Timed blood samples were hand drawn continuously (∼1 sample/10 s) for the first 4 minutes and at increasing intervals thereafter for a total of ∼40 samples per scan. Blood samples were processed as previously described. 15 Activities of 11C (half-life 20.38 min) in all samples were decay corrected to injection time.
Magnetic resonance imaging
Subjects underwent a non-contrast, T1-weighted, turbo field echo magnetic resonance imaging scan of the brain for drawing regions of interest. Data were collected using an 8-channel head coil on a 3-Telsa scanner (Phillips, Cleveland, Ohio, USA) and using the following parameters: sagittal plane, echo time = 3.7 ms, flip angle = 8°, in-plane field of view = 240 mm, matrix =256 × 256, slice thickness = 1.0 mm, and 192 slices with a 1.0-mm gap. Images were interpolated to isotropic voxel dimensions of 0.94 mm3 or 0.98 mm3.
Regions of interest were drawn with custom software written in Matlab (Mathworks, Natick, Massachusetts, USA). Drawing was manually performed in native space using neuroanatomical atlases as guides.24,25 Left and right portions of each region were drawn separately but combined into a single bilateral region of interest. The drawing procedure for caudate nucleus, putamen, corona radiata, thalamus, parietal cortex, and primary visual cortex have been described previously. 26 The motor cortex was defined as the uttermost posterior gyrus separated from the parietal lobe by the central sulcus and from the temporal lobe by the lateral fissure. We analyzed approximately 50 slices of frontal cortex. It was caudally limited at the disappearance of the medial orbital cortex, coincident laterally with the lateral curvature of the frontal lobe and separated from the parietal lobe by the central sulcus and from the temporal lobe by the lateral fissure. Anterior cingulate cortex was drawn in the coronal plane and comprised 32–40 slices, depending on individual anatomy. It was defined as the medial aspect of the cortex lying immediately above the corpus callosum and separated from the cortex by the cingulate sulcus. It was drawn in the rostro-caudal direction from the first appearance to the projection of the central sulcus. It grossly encompassed Brodmann Areas 24, 32, and 33. The cerebellum was drawn in the transverse plane caudo-rostrally. It was defined as the first appearance in the posterior cranial fossa and foramen magnum until separation by the dura mater from the fourth ventricle, with the pons and medulla in front of and overlying it. Both vermis and the two hemispheres were included. It consisted of 56-69 slices, depending on individual anatomy. The medial orbital cortex was defined as a pre-frontal neocortical region consisting of Brodmann Areas 10, 11, and 47. It included the anterior orbital gyrus, posterior orbital gyrus, lateral orbital gyrus, and olfactory sulcus. It was drawn caudo-rostrally in the transverse plane. It consisted of approximately 30 slices. The whole-brain region of interest was drawn without the ventricles. It was marked in the transverse plane caudo-rostrally from the slice where the basal surface of the cortex first appeared in the focus of the sphenoid bone until the outer border of the brain where it touched the parietal bone. It consisted of 95–120 slices, depending on individual anatomy. The hippocampus and amygdala were drawn in the sagittal plane. From lateral to medial, the hippocampus began at the appearance of the tail of the caudate, under the inferior horn of the lateral ventricle, and continued medially about 25 mm. Similarly, the amygdala began at about 2 mm medial to the first appearance of the hippocampus. It is rostral to the hippocampus and sandwiched between the claustrum and the inferior horn of the lateral ventricle. Further medial, the amygdala lies between the lateral sulcus and the hippocampus and below the globus pallidus. The amygdala extends about 22 mm in the sagittal plane.
Statistics and analyses
The statistical power analysis was based on variation observed in a previous L-[1-11C]leucine PET study in humans. 15 Mean inter-individual gray matter coefficients of variation in rCPS ranged from 6-9%, depending on the structure examined. With a typical gray matter regional coefficient of variation of 8% and the repeated-measures design, it was estimated that rCPS changes of 12%, 9.5%, or 8.4% could be detected (α = 0.05) with statistical power of 90%, 80%, or 70%, respectively, with 15 subjects. In an L-[1-14C]leucine autoradiography study of rCPS during slow-wave sleep in rhesus monkeys, coefficients of variation as large as 29% occurred in some brain regions. 4 Given the effects of partial volume in PET studies 27 and with the aid of computer simulations, it was predicted that the measured effect of sleep on rCPS with PET would be smaller.
We used IBM SPSS Statistics (IBM, Armonk, New York, USA) for analysis of rCPS data by means of a mixed-model analysis of variance with brain region (frontal cortex, anterior cingulate cortex, motor cortex, parietal cortex, medial orbital cortex, primary visual cortex, cerebellum, corona radiata, caudate, putamen, thalamus, hippocampus, amygdala, pons, medulla) and condition (rested awake, sleep-deprived awake, sleep) as within subject variables. In whole brain, rCPS were analyzed by means of ANOVA with condition as a within subject variable. The assumption of sphericity was assumed to be violated, and the Greenhouse-Geisser correction was applied for all main effects and interactions. However, we report degrees of freedom with the assumption of sphericity. All values are means ± SDs.
Estimation of mean rCPS during stages of sleep. As noted previously, the amount of time in each sleep stage that occurred during the sleep scan varied widely among subjects. We utilized the sleep stage data to estimate the mean rCPS of each sleep stage for the subjects in our study. We assumed that measured rCPS could be represented as the sum of rCPS in each sleep stage (appropriately weighted). The weights need to reflect two factors: (1) the total amount of time in a particular sleep stage, and (2) a factor that represents the amount of [11C]leucine in the brain available for incorporation into protein while the subject was in that sleep stage. After injection of [11C]leucine into the vein, [11C]leucine activity in the brain rises until reaching a peak around 3–5 min post injection; then slowly clears. rCPS measured over the 60 min scanning interval is therefore more heavily influenced by conditions during the earlier period of the scan and less by conditions later in the scan. To reflect both time and tissue tracer availability in weighting time in each sleep stage we use the cumulative amount of [11C]leucine in the brain during all intervals in that particular sleep stage divided by the cumulative amount of [11C]leucine in the brain over the 60 min time interval. We define this ratio (expressed as a percentage) as the weighted percent time in each sleep stage. Both numerator and denominator of the ratio are determined by integrating the concentration of [11C]leucine in the brain computed from the tracer kinetic model over the relevant time intervals. Then
| (1) |
where rCPS(S) is measured rCPS in the sleep scan, rCPSA, rCPSN1, etc are rates of cerebral protein synthesis in sleep stages A (awake), N1, etc during the sleep scan (to be estimated), and wA, wN1, etc are the weighted percent time during the sleep scan in A, N1, etc. We note that the above equation cannot be used for estimating rCPS in all the sleep stages because wA, wN1, wN2, wN3 and wREM are not independent; by definition they sum to 100%. We don’t need to estimate rCPSA however; we can utilize rCPS(SD), the measurement of rCPS in the sleep-deprived awake scan. Therefore, rCPSN1, rCPSN2, rCPSN3, and rCPSREM can be estimated based on the equation
| (2) |
Equation (2) is the equation for what we define as Model 4P (four parameter model) with which we estimate rCPS in each stage of sleep. Other models in which we constrained one or two parameters are presented in Results. Briefly, for Model 3P (three parameter model) we assumed that rCPS during N1 is the same as rCPS during N2, and we designate the combined stage as [N1,N2]. We estimated rCPS in [N1,N2], N3, and REM. For Model 2P (two parameter model), we additionally assumed that that rCPS during REM is the same as rCPS sleep-deprived awake, and we estimated rCPS in [N1,N2] and N3. For Model 1P (one parameter model), we assumed that rCPS in all stages of nonREM sleep are the same, and we estimated rCPS in [N1,N2,N3]. In Models 4P, 3P, and 2P we computed the variance inflation factor (VIF) to quantify how much the variance of each variable is inflated due to collinearity with the other variables in the model. Multicollinearity is considered to be high when VIF > 528,29
For the ith variable, VIF was computed as
| (3) |
where R i 2 is the coefficient of determination when the ith variable is regressed on the remaining variables in the model. For Model 4P we also examined the mean and standard error of the difference between estimated rCPS in N2 and N3 by reparameterizing the model in terms of rCPSN1, rCPSN2, [rCPSN2–rCPSN3], and REM. Similarly for Models 3P and 2P the difference between estimated rCPS in [N1,N2] and N3 was estimated by replacing the parameter rCPSN3 by [rCPSN1,N2 – rCPSN3]. All regressions were performed in Excel.
Results
Demographic, sleep stage, and radiochemical data for all subjects
The average age of the 18 participants was 23.4 years (Table 1). Most participants were right-handed, non-white, and non-Hispanic. Eight were male, and ten were female. On average, participants were slightly overweight (body mass index >25). Injected tracer dose was similar across the three scans. Arterial plasma leucine concentrations were higher (10%) in sleep-deprived awake v. sleep scan. Whereas this difference in plasma leucine concentration may be of interest, it is unlikely to have had any effects on rCPS measurements.
Table 1.
Subjects (n = 18).
| Age (years) | 23.4 ± 2.5 |
| Body mass index | 26.4 ± 5.1 |
| L-[1-11C]Leucine dose (MBq/kg) | |
| Rested awake study | 11.7 ± 0.93 |
| Sleep-deprived awake study | 11.6 ± 1.10 |
| Sleep study | 11.5 ± 1.10 |
| Plasma leucine concentration (µM) | |
| Rested awake study | 108.8 ± 19.5 |
| Sleep-deprived awake study | 116.6 ± 21.6 |
| Sleep study | 105.8 ± 28.0 |
Each value is the mean ± SD for the number of subjects indicated in parentheses.
Leucine concentrations during the three scans were compared by ANOVA with condition as a within subject variable. The effect of condition (F2,34 = 4.474; p = 0.023) was statistically significant. Plasma leucine concentrations were 10% higher during sleep-deprived awake v. sleep scans (p = 0.008).
To compare rCPS in the sleep scan with rates in the two awake scans we looked at a subset of 15 subjects who slept between 73 and 100% weighted time during the analysis interval, i.e., the first 60 min, of the sleep PET scan. (The remaining three subjects slept between 18 and 25% of weighted time during the scan and were included in the fitting analyses (Figure 1(c) (filled triangle symbols)). The weighted percent times in the different stages of sleep in the three scans (Figure 1(a) to (c) (filled circle symbols)) indicate that, during the first 60 min of the PET scan, subjects were awake during the rested awake and sleep-deprived awake scans and were asleep for, on average, 94% of the time during the sleep scan. In most subjects, most of the weighted time was in N3 likely due to the prior sleep-deprivation. Very little time was in N1 and REM, and an intermediate time was in N2.
Figure 1.
Distribution of weighted percent time in sleep states during the 60 min rested awake (a), sleep-deprived awake (b), and sleep (c) scans in 18 subjects. Each point represents the weighted percent time in the state in a single subject. Triangles represent the three subjects who slept less than 25% of weighted time during the sleep scan; these subjects were included for the fitting procedures. The figure illustrates the consistent awake states in the rested awake and sleep-deprived awake scans and the wide spread of weighted percent times in N2 and N3 and the very narrow spread and low values for weighted percent time in N1 and REM during the sleep scan.
Protein synthesis rates across conditions
Representative parametric rCPS images do not show any remarkable differences among the three scans (Figure 2). The distribution of rCPS in all conditions and regions was not significantly different from the normal distribution (Kolmogorov-Smirnov test, p > 0.33). Mean rCPS across all regions (Table 2) in the subset of 15 subjects who slept at least 73% of weighted time during the sleep scan appear similar during the three scans: rested awake, sleep-deprived awake, and sleep. Neither the condition × region interaction (F28, 392 = 0.887, p = 0.53, f 2 = 0.06) nor the main effect of condition (F2, 28 = 0.252, p = 0.749, f 2 = 0.018) was statistically significant (Table 3). Whole brain rCPS was considered separately, and the effect of condition (F2, 28 = 0.656; p = 0.502) was not statistically significant.
Figure 2.
Representative rCPS images (a–c) and MRI indicating the level and plane of PET image (d). For this figure only, a 2.6 mm full-width-at-half-maximum smoothing was applied. No smoothing was applied when computing average values within regions of interest.
Table 2.
Regional rates of cerebral protein synthesis (nmol leucine incorporated/g tissue wet weight/min) across conditions (n = 15).
| Rested awake | Sleep-deprived awake | Sleep | |
|---|---|---|---|
| Whole brain | 1.65 ± 0.11 | 1.62 ± 0.16 | 1.65 ± 0.10 |
| Frontal cortex | 1.80 ± 0.17 | 1.79 ± 0.24 | 1.78 ± 0.21 |
| Cingulate cortex | 1.70 ± 0.18 | 1.66 ± 0.25 | 1.63 ± 0.21 |
| Medial orbital cortex | 1.61 ± 0.18 | 1.63 ± 0.25 | 1.62 ± 0.23 |
| Motor cortex | 1.90 ± 0.19 | 1.86 ± 0.27 | 1.90 ± 0.25 |
| Parietal cortex | 1.91 ± 0.18 | 1.86 ± 0.26 | 1.89 ± 0.24 |
| Primary visual cortex | 2.12 ± 0.22 | 2.07 ± 0.33 | 2.07 ± 0.26 |
| Thalamus | 1.52 ± 0.14 | 1.48 ± 0.21 | 1.48 ± 0.20 |
| Caudate | 1.07 ± 0.13 | 1.04 ± 0.17 | 1.04 ± 0.13 |
| Putamen | 1.27 ± 0.13 | 1.26 ± 0.18 | 1.25 ± 0.17 |
| Hippocampus | 1.45 ± 0.17 | 1.48 ± 0.23 | 1.46 ± 0.19 |
| Amygdala | 1.31 ± 0.17 | 1.33 ± 0.21 | 1.33 ± 0.22 |
| Cerebellum | 1.83 ± 0.20 | 1.80 ± 0.28 | 1.81 ± 0.26 |
| Pons | 1.91 ± 0.32 | 1.85 ± 0.33 | 1.87 ± 0.31 |
| Medulla | 1.22 ± 0.19 | 1.23 ± 0.22 | 1.20 ± 0.04 |
| Corona radiata | 0.74 ± 0.10 | 0.72 ± 0.15 | 0.76 ± 0.15 |
Each value is the mean ± SD for the number of subjects indicated in parentheses. These results represent a subset of 15 of the 18 subjects that slept for more than 73% of weighted time during the sleep scan. Neither the condition × region interaction (F28, 392 = 0.887, p = 0.53, f 2 = 0.06) nor the main effect of condition (F2,28) = 0.252, p = 0.749, f 2 = 0.018) was statistically significant. In whole brain rCPS was considered separately, and the effect of condition (F2,28=0.656; p = 0.502) was not statistically significant.
Table 3.
Estimates of rCPS [N1,N2] and rCPSN3 (Model 2P).
| rCPS (nmol leucine incorporated/g tissue wet
weight/min) |
Estimated rCPS[N1,N2] minus rCPSN3 | |||
|---|---|---|---|---|
| Measured rCPS awake (sleep-deprived) | Estimated rCPS[N1,N2] | Estimated rCPSN3 | ||
| Whole brain | 1.53 ± 0.21 | 1.87 ± 0.16 | 1.47 ± 0.07 | 0.40 ± 0.22§ |
| Frontal cortex | 1.76 ± 0.23 | 2.16 ± 0.18 | 1.66 ± 0.08 | 0.49 ± 0.23* |
| Cingulate cortex | 1.63 ± 0.24 | 1.83 ± 0.18 | 1.57 ± 0.08 | 0.26 ± 0.24 |
| Medial orbital cortex | 1.61 ± 0.23 | 1.84 ± 0.19 | 1.55 ± 0.09 | 0.29 ± 0.22 |
| Motor cortex | 1.83 ± 0.25 | 2.34 ± 0.20 | 1.76 ± 0.09 | 0.59 ± 0.27* |
| Parietal cortex | 1.83 ± 0.25 | 2.32 ± 0.19 | 1.75 ± 0.09 | 0.57 ± 0.25* |
| Primary visual cortex | 2.04 ± 0.31 | 2.37 ± 0.23 | 1.98 ± 0.10 | 0.38 ± 0.30 |
| Caudate | 1.03 ± 0.16 | 1.20 ± 0.12 | 0.99 ± 0.05 | 0.21 ± 0.16 |
| Putamen | 1.23 ± 0.18 | 1.41 ± 0.15 | 1.19 ± 0.07 | 0.22 ± 0.20 |
| Thalamus | 1.46 ± 0.20 | 1.86 ± 0.15 | 1.35 ± 0.07 | 0.50 ± 0.20* |
| Hippocampus | 1.45 ± 0.22 | 1.55 ± 0.16 | 1.43 ± 0.07 | 0.12 ± 0.21 |
| Amygdala | 1.33 ± 0.20 | 1.54 ± 0.25 | 1.24 ± 0.11 | 0.30 ± 0.33 |
| Cerebellum | 1.78 ± 0.23 | 2.26 ± 0.21 | 1.64 ± 0.10 | 0.60 ± 0.28* |
| Pons | 1.79 ± 0.33 | 2.06 ± 0.27 | 1.81 ± 0.12 | 0.25 ± 0.35 |
| Medulla | 1.21 ± 0.21 | 1.29 ± 0.15 | 1.16 ± 0.07 | 0.13 ± 0.22 |
| Corona radiata | 0.70 ± 0.15 | 0.88 ± 0.13 | 0.73 ± 0.06 | 0.16 ± 0.19 |
Values for the sleep-deprived awake state are means ± SDs measured in 18 subjects.
Values for rCPS[N1,N2] and rCPSN3 are the means ± standard errors of the estimates in 18 subjects; all estimates statistically significantly different from zero (p < 0.001, t-test, df = 16).
Difference between estimates of rCPS[N1,N2] and rCPSN3 statistically significantly different from zero (*, p ≤ 0.05; §, 0.05 < p ≤ 0.10; t-test, df = 16).
Independent variables only minimally correlated (VIF = 1.2).
Regression model statistically significant in all regions and in whole brain (r2 ≥ 0.95; p < 0.001).
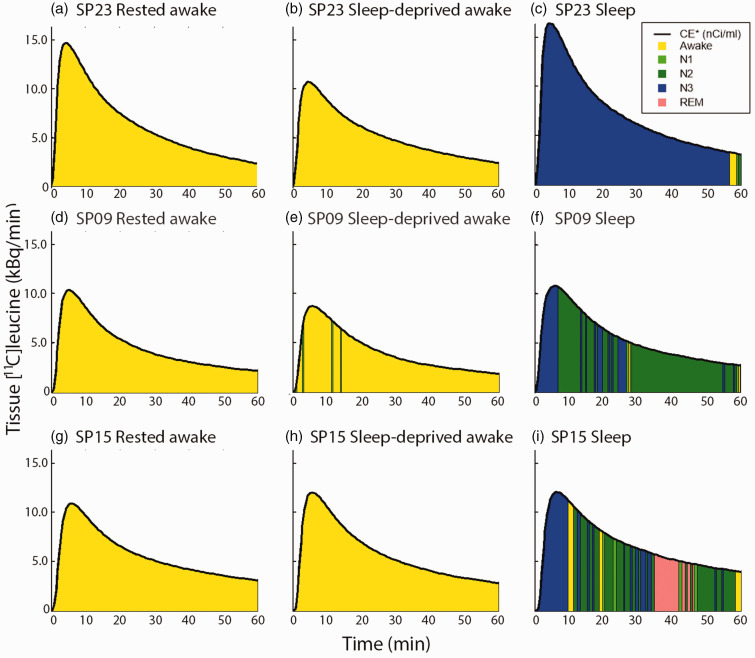
Whereas the rested awake and sleep-deprived awake scans were fairly homogeneous with respect to sleep stage (i.e., subjects were awake throughout most of the scan), the sleep scans varied with respect to duration and frequency of sleep stages (Figure 1(a) to (c)). For illustration we have plotted three subjects’ sleep stage profiles on the time course of [11C]leucine activity in the brain (Figure 3). During the sleep scan, subject SP23 (Figure 3(c)) remained in N3 for most of the sleep scan (97% of weighted time), whereas subject SP09 (Figure 3(f)) was in N2 for 67% of weighted time and SP15 (Figure 3(i)) spent 40 and 34% weighted time in N2 and N3, respectively.
Figure 3.
Time courses of the tissue [11C]leucine activity (kBq/ml) in the three scans for three different subjects. The area under the curves is color-coded to indicate the sleep state of the subject during the scan according to the legend in c. The sum of the areas under the curve in a particular state divided by the total area under the curve represents the weighted percent time in that state. In the rested awake (a, d, g) and the sleep-deprived awake scans (b, e, h), all three subjects were awake for more than 96% of the scan. In the sleep scans (c, e, i), the pattern of sleep states was variable. Subject SP23 (c) remained in N3 for most of the scan (97% weighted time). Subject SP15 (f) spent 8%, 5%, 40%, 34% and 12% weighted time in awake, N1, N2, N3, and REM, respectively. Subject SP09 (i) spent 1%, 2%, 67%, 29%, and 0% weighted time in awake, N1, N2, N3, and REM, respectively. The legend in C. applies to all panels.
We used these variable profiles in 18 subjects to find the best-fitting values of rCPS in the four stages of sleep (Figure 4(a), Model 4P, Supplemental Table 1). For this group of 18 subjects, the distribution of rCPS in all conditions and regions was not significantly different from the normal distribution (Kolmogorov-Smirnov test, p > 0.27). We used each subject’s measured rCPS in the sleep-deprived awake scan as the awake value. Predictably, errors were very large for estimates of rCPSN1 and rCPSREM because weighted % time in these stages was low across our subjects (3 ± 2% (mean ± SD), regional range 0–9% for N1 and 4 ± 6%, regional range 0–20% for REM) (Figure 4a). Estimates of rCPSN1 and rCPSREM were not statistically different from zero (p > 0.29 for N1; for REM (0.05 < p ≤ 0.10) in frontal cortex, medial orbital cortex and amygdala; all other regions p > 0.1; t-test, df = 14). Fitted values for rCPSN2 were higher than measured sleep-deprived awake values (110–139%) and fitted values for rCPSN3 were similar to sleep-deprived awake values (95-109%) across regions. In whole brain, fitted values for rCPSN2 and rCPSN3 were 127 and 98% of measured awake values, respectively. Estimated rCPSN2 was 11-40% higher than rCPSN3 across regions. Differences between rCPSN2 and rCPSN3 ranged from 0.15 to 0.62 nmol/g tissue/min. The difference was statistically significant in thalamus and parietal cortex (p < 0.05, t-test, df = 14) and approached statistical significance in whole brain, frontal cortex, motor cortex and cerebellum (0.05 <p < 0.10, t-test, df = 14).
Figure 4.
Best-fitting values for rCPS in different sleep states in whole brain, frontal cortex, thalamus, and hippocampus. (a) Model 4 P: rCPS in all states (N1, N2, N3, and REM) were estimated; (b) Model 3 P: rCPS in states [N1, N2], N3, and REM were estimated; [N1,N2] was a combination of the two states assuming that rCPSN1 will have a trivial effect on estimated values because subjects spent so little weighted time in N1; (c) Model 2 P: rCPS in states [N1, N2] and N3 were estimated (it was assumed that rCPSREM = rCPS sleep-deprived awake) and (d) Model 1 P: rCPS[N1, N2, N3] was estimated. We assumed that rCPSREM = rCPS sleep-deprived awake. rCPS in nonREM sleep was estimated assuming that rCPS is the same in N1, N2, and N3. Each bar for awake, represents mean ± SD for the 18 subjects. All other bars represent the means ± standard errors of the estimates in the 18 subjects. The maximum VIF for any variable in Models 4 P, 3 P, and 2 P was 1.3, 1.3, and 1.2 respectively; this indicates that independent variables in all models are only minimally correlated.
We tried making simplifying assumptions to avoid fitting so many parameters as follows: Model 3P (Figure 4(b), Supplemental Table 2). We assumed that rCPSN1 is the same as rCPS N2. Percent time in N1 for the 18 subjects ranged from 0% to 9% with a mean of 3%. Consequently, this assumption likely has a trivial effect on the results. Results with this model were similar to Model 4P, i.e., fitted values for rCPS[N1,N2] were higher than measured sleep-deprived awake values (109–131%) and fitted values for rCPS in N3 were similar to sleep-deprived awake values (91–105%) across regions. In whole brain, fitted values of rCPS [N1,N2] and rCPSN3 were 124 and 97% of measured sleep-deprived awake values, respectively. Estimated rCPS[N1,N2] was 10-39% higher than rCPSN3. Differences between rCPS[N1,N2] and rCPSN3 (0.14–0.61 nmol/g tissue/min) were statistically significant in thalamus and parietal cortex (p < 0.05, t-test, df = 15) and approached statistical significance in whole brain, frontal cortex, motor cortex and cerebellum (0.05 < p < 0.10, t-test, df = 15). Fitted values for rCPSREM varied widely likely due to the low weighted % time in REM in most of our subjects. Estimates of rCPSREM were not statistically significantly different from zero (p > 0.10, t-test, df = 15).
Model 2P (Figure 4(c), Table 3). We continued to assume that the effect of N1 will be trivial when combined with N2 as we had for Model 3P. Moreover, we assumed that the rCPSREM is the same as that in the sleep-deprived awake state. This assumption is based on the observation that the rate of energy metabolism during REM is similar to that during wakefulness.9,28 Results with this model were similar to Models 4P and 3P, i.e., fitted values for rCPSN2 (rCPS[N1,N2] in Model 3P) were higher than measured sleep-deprived awake values (107–128%) and fitted values for rCPSN3 were similar to sleep-deprived awake values (93–109%) across regions. In whole brain, fitted values of rCPS[N1,N2] and rCPSN3 were 122 and 96% of measured sleep-deprived awake values, respectively. Estimated values of rCPS[N1,N2] relative to measured awake values varied regionally. For example, in cerebellum, parietal cortex, motor cortex, thalamus and frontal cortex rCPS[N1,N2] estimates were 22–28% higher than awake values, whereas in hippocampus and medulla estimates were 7% higher. That rCPS[N1,N2] in hippocampus was less affected than other cortical regions is noteworthy. Estimated rCPS[N1,N2] was 9–36% higher than rCPSN3. Differences between rCPS[N1,N2] and rCPSN3 (0.13–0.60 nmol/g tissue/min) were statistically significant in frontal cortex, motor cortex, parietal cortex, thalamus, and cerebellum (p < 0.05, t-test, df = 16) and approached statistical significance in whole brain (0.05 < p < 0.10, t-test, df = 16).
Model 1P. In this model, we assumed that rCPSREM is similar to sleep-deprived awake and that rCPS during all stages of nonREM (N1,N2,N3) sleep are the same (Figure 4(d); Supplemental Table 3). Across regions estimates of rCPS in nonREM sleep are similar to measured sleep-deprived awake values and are consistent with results of the comparisons across conditions presented in Table 2. In other words, if we don’t account for the stage of sleep, at least distinguishing between rCPS[N1,N2] and rCPSN3, rCPS during general sleep looks much like the awake state. For all four models, regressions were statistically significant in all regions examined (Table 3 and Supplemental Tables 1–3).
Discussion
To our knowledge, our study is the first study in which measurements of regional rates of cerebral protein synthesis in human subjects were made during sleep and compared with measurements in the awake state in the same subject. Sleep-deprivation did not have a measurable effect on rCPS. Moreover, in the sleep scans taken as a whole, rCPS were not different from awake, but a breakdown of the sleep scan into sleep stages revealed significant effects. Contrary to our hypothesis, our results indicate that rCPS in selective brain regions is increased in stage [N1,N2], not during N3. Due to the very small amount of time subjects spent in N1 and REM, we could not reliably estimate rCPS during these stages. Our results may alter our view of how stages of sleep are involved in plasticity and memory consolidation. Moreover, our study may have implications for neurodevelopmental disorders and neurodegenerative diseases, which have both protein synthesis and sleep abnormalities.
Strengths and weaknesses of the current study
In addition to the interest in the sleep-deprived wakefulness condition itself, sleep deprivation was employed to increase the amount of N3 sleep during the subsequent sleep scan to examine if rCPS is increased in N3 sleep in keeping with our hypothesis. The necessity for sleep-deprivation also precluded the possibility of counterbalancing the order of the conditions with an appropriate wash-out period in between each measurement. The use of prior sleep deprivation necessarily means the sleep state measured was recovery sleep. It is unknown whether recovery sleep, and the altered sleep architecture associated with it, confer the same functional benefits of sleep as sleep after a normal day of wakefulness. Although concerning, it is equally possible that recovery sleep exhibits no qualitative differences and may have only exaggerated any effect of consciousness state and sleep deprivation state on protein synthesis.
The current study extends the L-[1-14C]leucine autoradiographic study of protein synthesis during slow-wave sleep in rats and rhesus monkeys3,4 by measuring this fundamental biological process in the human brain under three conditions with a validated and fully quantitative method. We have demonstrated that, in human subjects, low variance and highly reproducible measures of regional protein synthesis rates can be made with the L-[1-11C]leucine PET method. 15 Moreover, the method takes into account a correction for reincorporation of unlabeled leucine derived from protein breakdown in the tissue.20,27,30 These advantages are important to consider when comparing this in vivo method for measuring protein synthesis to other methods e.g., 31 in which the tracer may affect the process being measured and in which the reliability across cell types and energy states may be problematic.32,33 Moreover, the L-[1-11C]leucine PET method is the only quantitative method adapted and validated for use in human subjects. The within-subject design of our study in which we measure rCPS in each subject under the three different conditions is also a strength for the analysis of results. We used an individual’s rCPS in the sleep-deprived awake state in the equation, minimizing assumptions. Our results demonstrate the power of the L-[1-11C]leucine PET method to answer fundamental questions about protein biosynthesis under different physiological conditions.
rCPS during sleep
We had hypothesized that rCPS would be increased during N3 compared to measurements made during the awake sleep-deprived scan. Our hypothesis was based on results in rat and non-human primate studies in which time in slow-wave sleep was positively correlated with rCPS in multiple regions of interest. Many of the regions affected were small non-neocortical regions easily analyzed in an autoradiographic study, but beyond the spatial resolution of our [11C]leucine PET study. Moreover, in our previous study we used a different approach correlating rCPS with weighted percent time in each stage of sleep. Our previous analysis did not consider all stages of sleep simultaneously. Our approach in the present study was to use all data collected from 18 subjects to find the best-fitting values for rCPS in different phases of sleep in 15 brain regions and the brain as a whole. We reasoned that because the weighted percent times in different phases of sleep varied widely, analysis by group comparisons was less appropriate. We tried finding best-fitting values for all four stages of sleep (N1, N2, N3, and REM), but we had designed our study to maximize N3 sleep, so weighted percent time in other stages (particularly N1 and REM) was low. Consequently, the standard errors of the estimates for rCPSN1 and rCPSREM were very large. We tried several simplifying assumptions, namely that rCPSREM was similar to that in the sleep-deprived awake state and that the contribution of rCPSN1 was small so that N1 could be combined with N2. In all cases, fitted values of rCPSN2 or rCPS[N1,N2] were on average 20% higher than measured awake values and estimates of rCPSN3 were similar to (or slightly less than) awake values. Effects on rCPSN2 or rCPS[N1,N2] were most noteworthy in regions of neocortex, thalamus, and cerebellum. Effects on rCPSN2 or rCPS[N1,N2] in hippocampus were less striking and lower in magnitude. This pattern was consistent across models. Our study cannot address this issue directly, but we note that an EEG/fMRI study in human subjects indicates increased functional connectivity between hippocampus and regions of neocortex during sleep spindles. 34 This connectivity indicates increased capacity for information transfer between brain regions and the possibility that following transfer remodeling takes place in the neocortex.
Numerous studies of human subjects support a role for sleep in memory consolidation.35–37 Even naps are thought to promote improvement on procedural learning and declarative memory formation.38–40 Nap studies in human subjects point to a relationship between improved performance and spindle density and slow oscillations40–42 characteristic of N2. Moreover, memory consolidation is dependent on de novo protein synthesis such that protein synthesis inhibitors prevent consolidation. 43 Our previous L-[1-11C]leucine PET studies in human subjects trained on a visual texture discrimination task indicate a regionally selective and sleep-dependent effect on rates of protein synthesis. 26 Studies in animals also provide evidence that protein synthesis during sleep plays a role in cortical plasticity and memory consolidation.5,44 Our present results are consistent with these relationships and further point to N2 as the sleep stage in which protein synthesis is involved.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221121873 for Increased rates of brain protein synthesis during [N1,N2] sleep: L-[1-11C]leucine PET studies in human subjects by Dante Picchioni, Kathleen C Schmidt, Inna Loutaev, Adriana J Pavletic, Carrie Sheeler, Shrinivas Bishu, Thomas J Balkin and Carolyn B Smith in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-2-jcb-10.1177_0271678X221121873 for Increased rates of brain protein synthesis during [N1,N2] sleep: L-[1-11C]leucine PET studies in human subjects by Dante Picchioni, Kathleen C Schmidt, Inna Loutaev, Adriana J Pavletic, Carrie Sheeler, Shrinivas Bishu, Thomas J Balkin and Carolyn B Smith in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting true views of the Department of the Army or the Department of Defense. A limited subset of these data from one region of interest for two of the conditions was previously published as a negative control in a separate study on memory. 26 We gratefully acknowledge the following individuals for their help: Burlin, T., Duyn, J., Ethridge, S., Evans, B., Friedman, R., Howell, G., Huang, T., Lee, B., Loomba, N., McWhirter, K., Miao, N., Morrow, A., Nadel, J., Nichols, D., Qin, M., Saré, R., Sheeler, C., Speer, A., Turetsky, K., Vesselinovitch, D., Xia, Z., and Zametkin, A.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by ZIA-MH002936 from the Intramural Research Program of the National Institute of Mental Health (Crossref Funder ID: 100000025), ZIA-NS003027 from the Intramural Research Program of the National Institute of Neurological Disorders and Stroke (Crossref Funder ID: 100000065), and W81XWH-08-D-0047 from the Military Operational Medicine Research Program of the United States Army Medical Research and Materiel Command (Crossref Funder ID: 100000182).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: DP, SB, TJB and CBS and made substantial contributions to the study design.
DP, KCS, TJB and CBS made substantial contributions to data interpretation.
KCS, IL, AJP, CS, and CBS made substantial contributions to the data analysis.
DP, KCS, and CBS drafted the manuscript.
KCS and CBS critically revised the article.
All authors approved the final draft submitted for publication.
Supplemental material: Supplemental material for this article is available online.
ORCID iD
Thomas J Balkin https://orcid.org/0000-0002-4177-7719
References
- 1.Kennedy C, Gillin JC, Mendelson W, et al. Local cerebral glucose utilization in non-rapid eye movement sleep. Nature 1982; 297: 325–327. [DOI] [PubMed] [Google Scholar]
- 2.Vyazovskiy VV, Harris KD. Sleep and the single neuron: the role of global slow oscillations in individual cell rest. Nat Rev Neurosci 2013; 14: 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramm P, Smith CT. Rates of cerebral protein-synthesis are linked to Slow-Wave sleep in the rat. Physiol Behav 1990; 48: 749–753. [DOI] [PubMed] [Google Scholar]
- 4.Nakanishi H, Sun Y, Nakamura RK, et al. Positive correlations between cerebral protein synthesis rates and deep sleep in Macaca mulatta. Eur J Neurosci 1997; 9: 271–279. [DOI] [PubMed] [Google Scholar]
- 5.Seibt J, Dumoulin MC, Aton SJ, et al. Protein synthesis during sleep consolidates cortical plasticity in vivo. Curr Biol 2012; 22: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Donnell C, Sejnowski TJ. Selective memory generalization by spatial patterning of protein synthesis. Neuron 2014; 82: 398–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tudor JC, Davis EJ, Peixoto L, et al. Sleep deprivation impairs memory by attenuating mTORC1-dependent protein synthesis. Sci Signal 2016; 9: ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res 2000; 9: 335–352. [DOI] [PubMed] [Google Scholar]
- 9.Braun AR, Balkin TJ, Wesenten NJ, et al. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain 1997; 120: 1173–1197. [DOI] [PubMed] [Google Scholar]
- 10.Hafycz JM, Naidoo NN. Sleep, aging, and cellular health: aged-related changes in sleep and protein homeostasis converge in neurodegenerative diseases. Front Aging Neurosci 2019; 11: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picchioni D, Reith RM, Nadel JL, et al. Sleep, plasticity and the pathophysiology of neurodevelopmental disorders: the potential roles of protein synthesis and other cellular processes. Brain Sci 2014; 4: 150–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zoghbi HY, Bear MF. Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb Perspect Biol 2012; 4: a009886–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.First MB, Spitzer RL, Gibbon M, et al. Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patient edition (SCID-I/NP). New York: Biometrics Research, New York State Psychiatric Institute, 2002. [Google Scholar]
- 14.American Academy of Sleep Medicine. AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. Westchester, IL: Author, 2007. [Google Scholar]
- 15.Bishu S, Schmidt KC, Burlin T, et al. Regional rates of cerebral protein synthesis measured with L-[1-(11)C]leucine and PET in conscious, young adult men: normal values, variability, and reproducibility. J Cereb Blood Flow Metab 2008; 28: 1502–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carson RE, Barker WC, Liow JS, et al. Design of a motion-compensation OSEM list-mode algorithm for resolution-recovery reconstruction for the HRRT. In: IEEE nuclear science symposium conference record 2003; 5: 3281–3285.
- 17.Wienhard K, Schmand M, Casey ME, et al. The ECAT HRRT: performance and first clinical application of the new high resolution research tomograph. IEEE Trans Nucl Sci 2002; 49: 104–110. [Google Scholar]
- 18.Bloomfield PM, Spinks TJ, Reed J, et al. The design and implementation of a motion correction scheme for neurological PET. Phys Med Biol 2003; 48: 959–978. [DOI] [PubMed] [Google Scholar]
- 19.Fischer B, Modersitzki J. Intensity-based image registration with a guaranteed one-to-one point match. Methods Inf Med 2004; 43: 327–330. [PubMed] [Google Scholar]
- 20.Schmidt KC, Cook MP, Qin M, et al. Measurement of regional rates of cerebral protein synthesis with L-[1-(11)C]leucine and PET with correction for recycling of tissue amino acids: I. Kinetic modeling approach. J Cereb Blood Flow Metab 2005; 25: 617–628. [DOI] [PubMed] [Google Scholar]
- 21.Tomasi G, Bertoldo A, Bishu S, et al. Voxel-based estimation of kinetic model parameters of the L-[1-(11)C]leucine PET method for determination of regional rates of cerebral protein synthesis: validation and comparison with region-of-interest-based methods. J Cereb Blood Flow Metab 2009; 29: 1317–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veronese M, Bertoldo A, Tomasi G, et al. Impact of tissue kinetic heterogeneity on PET quantification: case study with the L-[1-(11)C]leucine PET method for cerebral protein synthesis rates. Sci Rep 2018; 8: 931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomasi G, Veronese M, Bertoldo A, et al. Effects of shortened scanning intervals on calculated regional rates of cerebral protein synthesis determined with the L-[1-11C]leucine PET method. PLoS One 2018; 13: e0195580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duvernoy HM. The human brain: surface, three-dimensional sectional anatomy with MRI, and blood supply. 2nd ed. New York: Springer-Verlag Wien, 1999. [Google Scholar]
- 25.Mai JK, Paxinos G, Voss T. Atlas of the human brain. 3rd ed. Amsterdam: Academic Press, 2007. [Google Scholar]
- 26.Picchioni D, Schmidt KC, McWhirter KK, et al. Rates of cerebral protein synthesis in primary visual cortex during sleep-dependent memory consolidation, a study in human subjects. Sleep 2018; 41: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith CB, Schmidt KC, Qin M, et al. Measurement of regional rates of cerebral protein synthesis with L-[1-11C]leucine and PET with correction for recycling of tissue amino acids: II. Validation in rhesus monkeys. J Cereb Blood Flow Metab 2005; 25: 629–640. [DOI] [PubMed] [Google Scholar]
- 28.Reivich M, Isaacs G, Evarts E, et al. Effect of slow wave sleep and rem sleep on regional cerebral blood flow in cats. J Neurochem 1968; 15: 301–301. [DOI] [PubMed] [Google Scholar]
- 29.Sheather SJ. A modern approach to regression with R introduction. Springer Texts Stat 2009; 14–27. [Google Scholar]
- 30.Smith CB, Deibler GE, Eng N, et al. Measurement of local cerebral protein synthesis in vivo: influence of recycling of amino acids derived from protein degradation. Proc Natl Acad Sci U S A 1988; 85: 9341–9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt EK, Clavarino G, Ceppi M, et al. SUnSET, a nonradioactive method to monitor protein synthesis. Nat Methods 2009; 6: 275–277. [DOI] [PubMed] [Google Scholar]
- 32.Hidalgo San Jose L, Signer RAJ. Cell-type-specific quantification of protein synthesis in vivo. Nat Protoc 2019; 14: 441–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marciano R, Leprivier G, Rotblat B. Puromycin labeling does not allow protein synthesis to be measured in energy-starved cells. Cell Death Dis 2018; 9: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andrade KC, Spoormaker VI, Dresler M, et al. Sleep spindles and hippocampal functional connectivity in human NREM sleep. J Neurosci 2011; 31: 10331–10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diekelmann S, Wilhelm I, Born J. The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev 2009; 13: 309–321. [DOI] [PubMed] [Google Scholar]
- 36.Stickgold R. Sleep-dependent memory consolidation. Nature 2005; 437: 1272–1278. [DOI] [PubMed] [Google Scholar]
- 37.Peigneux P, Laureys S, Delbeuck X, et al. Sleeping brain, learning brain. The role of sleep for memory systems. Neuroreport 2001; 12: A111–A124. [DOI] [PubMed] [Google Scholar]
- 38.Lau H, Tucker MA, Fishbein W. Daytime napping: effects on human direct associative and relational memory. Neurobiol Learn Mem 2010; 93: 554–560. [DOI] [PubMed] [Google Scholar]
- 39.Backhaus J, Junghanns K. Daytime naps improve procedural motor memory. Sleep Med 2006; 7: 508–512. [DOI] [PubMed] [Google Scholar]
- 40.Farhadian N, Khazaie H, Nami M, et al. The role of daytime napping in declarative memory performance: a systematic review. Sleep Med 2021; 84: 134–141. [DOI] [PubMed] [Google Scholar]
- 41.Stickgold R, Walker MP. Memory consolidation and reconsolidation: what is the role of sleep? Trends Neurosci 2005; 28: 408–415. [DOI] [PubMed] [Google Scholar]
- 42.Ruch S, Markes O, Duss SB, et al. Sleep stage II contributes to the consolidation of declarative memories. Neuropsychologia 2012; 50: 2389–2396. [DOI] [PubMed] [Google Scholar]
- 43.Agranoff BW. Learning and memory: biochemical approaches. In: Siegel GJ, Albers W, Agranoff BW, et al. (eds) Basic neurochemistry. 3rd ed. Boston: Little Brown & Co., 1981, pp.801–820.
- 44.Abel T, Havekes R, Saletin JM, et al. Sleep, plasticity and memory from molecules to whole-brain networks. Curr Biol 2013; 23: R774–R788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221121873 for Increased rates of brain protein synthesis during [N1,N2] sleep: L-[1-11C]leucine PET studies in human subjects by Dante Picchioni, Kathleen C Schmidt, Inna Loutaev, Adriana J Pavletic, Carrie Sheeler, Shrinivas Bishu, Thomas J Balkin and Carolyn B Smith in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-2-jcb-10.1177_0271678X221121873 for Increased rates of brain protein synthesis during [N1,N2] sleep: L-[1-11C]leucine PET studies in human subjects by Dante Picchioni, Kathleen C Schmidt, Inna Loutaev, Adriana J Pavletic, Carrie Sheeler, Shrinivas Bishu, Thomas J Balkin and Carolyn B Smith in Journal of Cerebral Blood Flow & Metabolism