Abstract
Cerebral autoregulation (CA) refers to the control of cerebral tissue blood flow (CBF) in response to changes in perfusion pressure. Due to the challenges of measuring intracranial pressure, CA is often described as the relationship between mean arterial pressure (MAP) and CBF. Dynamic CA (dCA) can be assessed using multiple techniques, with transfer function analysis (TFA) being the most common. A 2016 white paper by members of an international Cerebrovascular Research Network (CARNet) that is focused on CA strove to improve TFA standardization by way of introducing data acquisition, analysis, and reporting guidelines. Since then, additional evidence has allowed for the improvement and refinement of the original recommendations, as well as for the inclusion of new guidelines to reflect recent advances in the field. This second edition of the white paper contains more robust, evidence-based recommendations, which have been expanded to address current streams of inquiry, including optimizing MAP variability, acquiring CBF estimates from alternative methods, estimating alternative dCA metrics, and incorporating dCA quantification into clinical trials. Implementation of these new and revised recommendations is important to improve the reliability and reproducibility of dCA studies, and to facilitate inter-institutional collaboration and the comparison of results between studies.
Keywords: Cerebral hemodynamics, consensus guidelines, reference values, transfer function analysis
2022 Update summary
| RECa # | Status | Stage/Topic | Resumé | Related RECs |
|---|---|---|---|---|
| 1 | Unchanged | Data acquisition | Experimental conditions | 2, 19, 20, 22, 23 |
| 2 | Revised | Physiological measurements | 20, 22 | |
| 3 | Revised | Duration | 11, 12, 19, 20 | |
| 4 | Unchanged | Sampling frequency | ||
| 5 | Unchanged | Data pre-processing | Hemodynamic signal format | 6 |
| 6 | Revised | Visual inspection and replacement | ||
| 7 | Revised | Resampling interpolation | 13 | |
| 8 | Unchanged | Detrending | ||
| 9 | Unchanged | Low- and high-pass filtering | 6, 15 | |
| 10 | Revised | TFA methodology | Windowing | 11 |
| 11 | Revised | Window duration | 3, 14 | |
| 12 | Unchanged | Welch’s method: superposition and number of windows | 3, 10, 11 | |
| 13 | Unchanged | Additional spectral smoothing: use of a triangular window | 2, 14, 18 | |
| 14 | Revised | TFA reporting | Thresholding (or rejecting) data based on coherence | 17 |
| 15 | Revised | Reporting of units for gain and phase | ||
| 16 | Unchanged | Avoidance of phase ‘wrap around’ | 17 | |
| 17 | Revised | Reporting of selected frequency bands | 11 | |
| 18 | New | Alternative metrics | CBv Step Response and Autoregulation Index (ARI) | 9, 14, 16, 17 |
| 19 | New | Alternative protocols | Protocols to enhance BP variability | 2, 14, 16, 17 |
| 20 | New | Clinical studies/trials | Considerations for clinical settings and applications | 1, 2, 3, 6, 22 |
| 21 | New | Alternative models | Time-domain methods | |
| 22 | New | Alternative measurement techniques | CBF measurement techniques as alternatives to TCD | |
| 23 | New | Normative data and thresholds | Normative values for TFA parameters | 20 |
Recommendation.
Introduction
Cerebral autoregulation (CA), the ability of the brain’s vasculature to attenuate changes in cerebral blood flow (CBF), following changes in arterial blood pressure (BP), was initially defined based on measurements of CBF of low temporal resolution, such as indicator-dilution methods. Those assessments are now regarded as ‘static’ CA, as compared to more recent methods that allow much higher temporal resolution for measurements of CBF, giving rise to the concept of ‘dynamic’ CA (dCA). Dynamic CA is the transient response of CBF to an acute change in BP, lasting only a few seconds.1,2 Most modern physiological and clinical studies of CA tend to be dominated by the dynamic modality, as relatively shorter recordings can be used, without the need of inducing long lasting changes in BP, which are generally dependent on the infusion of vasoactive drugs. 3 Indeed, in its original formulation, dCA involved recordings of CBF with transcranial Doppler ultrasound (TCD), lasting shorter than a minute, following the sudden release of inflated thigh cuffs. 1 The demonstration that dCA could also be assessed from spontaneous fluctuations in BP, was a major advancement, facilitating examination of dCA without any interventions that could interfere with the physiological determinants of dCA.4–6 The use of spontaneous fluctuations in BP as the stimulus (or input) to induce corresponding changes in CBF (or output), paved the way for the use of transfer function analysis (TFA) to represent dCA as a frequency-dependent phenomenon and this approach has received considerable attention in the literature.7–10 Although the main focus of the white paper is the study of dCA in humans, it is important to note that TFA has also been used in animal studies 11 and these would also benefit from many of the recommendations described below.
The 2016 white paper from the Cerebrovascular Research Network (CARNet) proposed a set of recommendations to standardize the application of TFA to study dCA. 7 The primary motivation for that original paper was the lack of methodological consistency observed in the TFA literature, in which large discrepancies can be found in measurement protocols, parameter settings, and reporting of results.9,12,13 Since its original publication, the white paper has had a noticeable impact, reflected by an increasing number of citations and greater methodological convergence in the field, which has facilitated the comparison of outcomes in multi-center studies and promoted collaborative efforts.14–19
Despite the intention of making recommendations based on well-established evidence, the original paper was somewhat constrained by the limited existence of comparative studies assessing alternative measurement protocols, parameter sensitivity, reproducibility, and the disparity of clinical outcomes. For this reason, many of the recommendations could not be supported by evidence; instead, they were based on common practice and expert opinion. In the last five years, however, this evidence gap has been partially filled, allowing for a stronger endorsement of more rigorous recommendations. Moreover, measurement protocols have evolved considerably, broadening the application of TFA beyond the use of spontaneous fluctuations in BP, and these approaches have been extended to different areas of cerebrovascular physiology and clinical applications. These recent developments warrant an update and revision of the original white paper to maintain continuous improvement in scientific rigour and promote cohesion in this vibrant field of research.
This revision has followed an open process of consultation with the entire CARNet membership (Appendix). The notion was first proposed to members during the 10th Annual Meeting of the network that took place during April 2021. All members were invited to submit suggestions for recommendations that would benefit from revision, as well as for novel recommendations that considered recent advances. The writing group amalgamated these suggestions into succinct themes, which were then delegated to working groups for revisions or preparation of new content. Subsequent drafts of the complete, revised manuscript were circulated to the entire membership for review and approval (see Appendix). This process resulted in the revision of 9 of the original 17 recommendations, based on recent evidence. Furthermore, 6 novel recommendations, particularly relevant for clinical studies, are now included herein.
The main aim of this white paper is to provide concise guidelines and not present a systematic review of the literature. For this reason, citations are limited to key references from the peer-reviewed literature that provided evidence to support the revision of existing recommendations or inclusion of new ones. To ensure this new version of the white paper is as succinct as possible, only brief reference is made to those recommendations that have not been substantially amended.
CARNet recommendations
Data acquisition
Environmental conditions
RECOMMENDATION #1 (Revised)
The following controls for each assessment should be considered:
• With the exception of studies where sex differences are not pertinent (e.g., pregnancy), it is highly recommended that study designs include a balanced number of participants of both sexes, to consider the influence of sex on outcomes and also to generate representative data for subsequent clinical studies,
• Assessments should occur in an environmentally controlled location (e.g., temperature, humidity, acoustic noise or other disturbances) and unusual settings (e.g. high altitude, zero gravity) should be clearly stated, giving details (e.g., barometric pressure) where possible,
• Assessments should occur at a standardized time of day within a study and across repeated measures,
• Sensory stimuli (e.g., noise, lights) should be minimized unless designed as an experimental stimulant,
• Participants should refrain from caffeine, nicotine, chocolate, and alcohol for at least 12 h prior to measurement,
• Participants should refrain from moderate (or more vigorous) exercise for at least 6 h prior to measurement,
• Participants should refrain from high calorie meals for at least 4 h prior to measurement,
• Chronic and acute medications should be noted,
• Body position should be clearly noted. For resting measures, a 15-min period of rest with uncrossed legs should precede the assessment, and
• Departures from these recommendations should be discussed in the manuscript.
CBF is responsive to a wide range of stimuli. As such, control over the environmental conditions for performing measurements 20 and participant preparation protocols is paramount for reproducible and reliable data. Recent data suggest that diurnal effects may be small,21,22 but standardizing time of day is still recommended to limit even subtle confounding effects. The original recommendation to restrict assessments until at least 12 h following acute exercise has been eased in response to recent evidence involving both moderate and high intensity exercise protocols. 23 Furthermore, TFA is reliant on the assumption that recorded signals are approximately stationary, i.e., with relatively consistent behaviour over time. 24
Physiological measurements and equipment
RECOMMENDATION #2 (Revised)
Continuous recordings of cerebral blood flow, arterial blood pressure, and arterial (or end-tidal) partial pressure of carbon dioxide form the minimum acceptable data assemblage to permit robust reporting of dynamic cerebral autoregulation findings. Monitoring of both hemispheres is preferred. Intracranial pressure should be reported, if available.
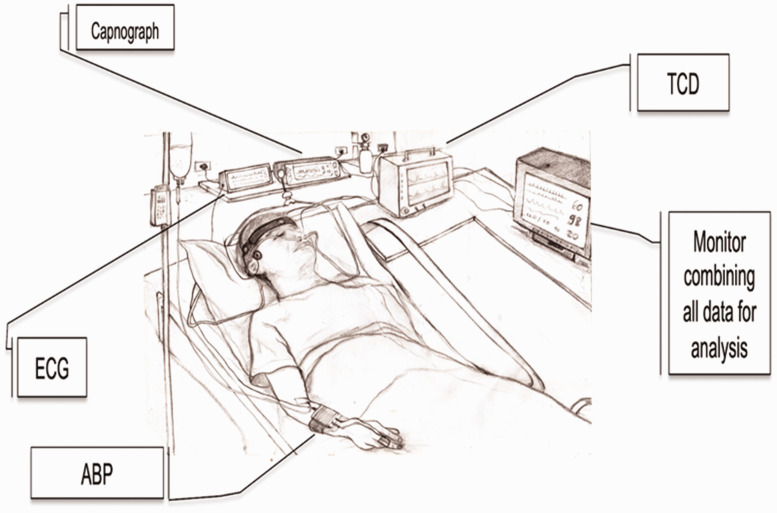
As stated, dCA informs about the intrinsic characteristics of the cerebral vasculature that modulate the dynamic association between BP and CBF. As such, a base set of continuous physiological measurements is essential.13,25 Figure 1 shows a typical data acquisition arrangement for dCA assessment using spontaneous fluctuations. The most common approach to obtain estimates of CBF is the measurement of cerebral blood velocity, in both middle cerebral arteries, using transcranial Doppler ultrasound (TCD).1,7,26,27 Historically, the envelope of the TCD output has been referred to as cerebral blood flow velocity (CBFV) in the wider literature, but there is a growing consensus that using the term ‘flow’ is not appropriate, since the physical quantity that is being measured is the average speed of blood across the insonated vessel diameter, and not absolute flow. 28 For this reason, we recommend the use of CBv as the general abbreviation for cerebral blood velocity. Specifying the artery that was insonated, with the abbreviations MCAv, PCAv, or ACAv, for example, is also highly recommended. 28 With respect to BP, the majority of dCA studies use arterial volume clamping of a finger artery to obtain continuous non-invasive measurements. Importantly, good agreement exists between non-invasive arterial volume clamping and invasive (intra-arterial catheter) techniques, with the former not known to distort time-varying estimates of dCA. 29 Several alternatives are available for measuring CBF surrogates (Recommendation #22), but one must appreciate that estimates of gain, phase, and coherence can vary considerably, depending on the adopted modality.
Figure 1.
Typical set of recordings in a clinical setting. See new Recommendation 20 for details about particular aspects affecting studies of dCA in critically ill patients. TCD: transcranial Doppler ultrasound device; ECG: electrocardiograph; ABP: either non-invasive continuous arterial blood pressure (finger photoplethysmograph sensor combined with arterial volume clamping), or invasive intra-radial catheter sampling in critical care settings.
Importantly, delays which are secondary to equipment processing steps, such as the fixed 1-s output delay of the Finometer® reconstituted brachial artery BP signal, need to be considered and corrected. It is essential to take these delays into account when synchronizing BP and CBF (and other signals) prior to analysis. In addition, inaccuracies need to be considered. The accuracy of the signal output from devices providing continuous non-invasive BP measurements is often unreliable. 30 More consistent results can be obtained by recalibrating signals using systolic and diastolic readings from brachial sphygmomanometry. Non-invasive BP may also be subject to signal drift over extended-duration acquisitions, which can be minimized using a servo-controlled finger-clamp calibration at the cost of a periodic loss of data.
Cerebral and systemic haemodynamic variables, as well as markers of dCA, are strongly dependent on partial pressure of arterial carbon dioxide (PaCO2).1,31–34 Non-invasive estimates of PaCO2 are usually derived from measurements of end-tidal CO2 (EtCO2) with infra-red capnography or mass spectroscopy. Scrutiny of fluctuations in PaCO2 (or EtCO2) during continuous measurements should be exercised in all studies to account for the confounding effects of hypo- or hypercapnia, which are particularly apparent in some protocols.31,34,35 As with CBF and BP, as noted above, consideration for delays and/or inaccuracies in the capnograph output, dependent on the nature and complexity of the gas sampling system, is necessary (e.g., mouthpiece/facemask, connectors, tubing length/calibre, pump speed, processing delay). While donning a facemask tends to amplify EtCO2, as compared to nasal cannula CO2 sampling for example, this was not seen to influence TFA measures of dCA. 36 Recordings with large fluctuations or unwanted step changes in CO2 should be discarded to avoid adverse interference in TFA results (see Supplementary Material). The stability of CO2 during normo-, hyper-, or hypocapnia can be improved with the use of end-tidal forcing, but it is important to discard transient changes until a steady-state is achieved.31,37,38 Moreover, body position is also relevant, since ventilation increases in the upright position and EtCO2 will show a reduction compared to PaCO2. 39 A need remains for evidence to inform standardization of protocols and analytical methods for the study of dCA in both hypercapnic and hypocapnic states.
In certain clinical conditions (e.g., subarachnoid hemorrhage and traumatic brain injury), raised intracranial pressure (ICP) can disrupt the relationship between BP and CBF.40,41 If measured, mean ICP should be reported. While agreement between TFA parameters derived from either CPP (calculated directly as mean ICP subtracted from mean BP) or BP alone exists, 42 further investigation is warranted.
Representative recordings of CBv, BP and airway CO2 partial pressure are shown as Supplementary Material, illustrating what is considered ‘good quality data’, as well as recordings showing typical artefacts, noise or signal loss in situations where further editing can lead to acceptable data. Examples are also provided of data that cannot be accepted for further analyses.
Duration
RECOMMENDATION #3 (Revised)
Recordings of spontaneous fluctuations of arterial blood pressure and cerebral blood flow for transfer function analysis should last a minimum of 5 min, assuming stationary physiological conditions and uninterrupted good quality data.
A recording length of at least 5 min is required to stabilise estimates of the transfer function phase in the low-frequency band 43 and provide adequate frequency resolution. Longer recordings may not lead to further improvement. For example, dCA metrics are not altered when extending a recording to 10 min. 44 While this recommendation is a corollary of the technical specifications described in Recommendations #11 and #12 (below), the duration may be adjusted for some patient groups and/or experimental conditions (Recommendation #20). For example, in the neuro-intensive care unit, a longer duration (7 min) has been recommended, 45 whereas in patients with acute ischemic stroke (AIS), a reduction from 5 to 3 min has been shown to be acceptable when longer recordings are not achievable. 46 Consideration should be given to the stability of recordings in specific patient groups, and any reduction in duration should be accommodated by the use of windows of less than 100 s in the Welch method (Recommendation #11). 47 Recordings with a duration of <5 min have also been considered with protocols that induce dynamic changes in BP rather than relying on spontaneous fluctuations (Recommendation #19).48,49
Sampling frequency
RECOMMENDATION #4
For analog-to-digital conversion of the continuous physiological signals, a minimum sampling frequency of 50 Hz is recommended, following appropriate anti-aliasing filtering, typically with a low-pass frequency cut-off at 20 Hz.
Procedures for analog-to-digital conversion of acquired signals and treatment of cardiovascular data are well established24,50 and remain the recommended approach.
Data pre-processing
Hemodynamic signal format
RECOMMENDATION #5
Input to transfer function analysis models should be beat-to-beat data rather than raw waveforms.
The use of beat-to-beat data is less sensitive to short segments of missing data,24,50 and remains the recommended approach, subject to the considerations in the Recommendation #6.
Visual inspection and replacement
RECOMMENDATION #6 (Revised)
Both raw and beat-to-beat signals should always be visually inspected prior to analysis to ensure they are free from excessive noise and artefacts. Short periods (up to three beats) of large artefact, where the physiological waveforms are distorted, should be removed and replaced by interpolated values; linear interpolation is recommended. It is advisable to reject recordings with large trends that could suggest drifts in the blood pressure recording (either invasive or not), or fluctuating physiological conditions, such as transient hypercapnia or hypocapnia.
Examples of excessive noise and artefacts are provided as Supplementary Material and have also been published in the literature (Figure 2 in and Figure 4 in 51 ). Simultaneous visual inspection of BP and CBF signals is often helpful in distinguishing artefacts from true physiological signals. Interpolation through short segments of noisy data (or missing data, for example due to physical artefacts in finger arterial volume clamping BP signals) does not affect TFA parameters. 50 Ectopic beats, however, can be included in the analysis. 52 The maximum number of contaminated beats that can be reliably replaced by interpolation is not yet known. The loss of <10 s in the recording for analysis within the 0.03–0.07 Hz frequency band appears to be acceptable, whereas loss of 5 s every 50 s in the 0.07–0.5 Hz frequency band leads to unreliable results. 50 Different TFA parameters (gain, phase, coherence – see below) may exhibit different degrees of sensitivity to missing data. 50 Large artefacts in BP have been shown to lead to bias in autoregulation index (ARI) estimates; 53 comparable bias may be expected in TFA parameter estimation, though this has not yet been systematically assessed. When excessive artefacts persist for longer periods, the data segment should be excluded from analysis (see Supplementary Material).
Figure 2.
Critical values for coherence estimates at the 99%, 95% and 90% significance level for 3–25 windows. Solid lines: Monte Carlo simulation from 10,000 pairs of independent white Gaussian noise using Hanning windows with 50% overlap and spectral smoothing (see Table 2). The dotted lines give the critical values without spectral smoothing and with non-overlapping windows, calculated from theory. The line-thickness denotes the significance level.
Figure 4.
On the left side of the figure are the typical trace for blood pressure (BP), middle cerebral artery blood velocity (MCAv) and end-tidal CO2 (PETCO2) during: spontaneous upright (a), 0.05 Hz (b) and 0.10 Hz (c) repeated squat-stand maneuvers (rSSM), spontaneous supine (d), 0.05 Hz (e) and 0.10 Hz (f) oscillatory lower body negative pressure (OLBNP) maneuvers in a young healthy adult male. On the right side of the figure are the absolute values of the power spectrum densities (PSD) for the mean arterial pressure (MAP) and cerebral blood velocity (CBv) under spontaneous (grey), 0.05 Hz (dashed) and 0.10 Hz (black) conditions. The PSD during the OLBNP pressure maneuvers are represented on the left side (g–j) and rSSM are on the right side (k–n). Note the substantial increase in PSD power during the forced oscillations, with the greatest augmentation (approximately 20x higher peak PSD than OLBNP) clearly delineated for both MAP and CBv occurring during the rSSM maneuvers presented in the far-right panel. Adapted from Smirl et al. 51
Resampling and interpolation
RECOMMENDATION #7 (Revised)
Linear interpolation through beat-to-beat data should be used to create equidistant time intervals for implementation within TFA, though cubic spline interpolation is also feasible. Visual inspection of interpolated signals is strongly recommended, particularly in the case of spline interpolation which can lead to the insertion of large overshoots. For mean beat-to-beat physiological signals, the minimum re-sampling frequency should be 4 Hz.
TFA supposes equidistant data points. No differences in TFA parameters were observed when comparing different types of beat-to-beat interpolation. 13 This recommendation aims to produce greater standardization and to prevent potentially distorted results at higher heart rates.
Detrending
RECOMMENDATION #8
Detrending or high-pass filtering of signals prior to TFA should be avoided. The removal of mean values, however, is recommended to minimize spectral leakage.
This advice aims to produce greater standardization and remains the recommended approach. Detrending reduces the amount of low-frequency power by removing any linear or non-linear trends from the data. These trends may have prognostic and/or diagnostic value in clinical applications that has yet to be elucidated. While no difference in TFA outcomes was observed following multiple detrending procedures (i.e., no detrending, linear trend removal, and third-order polynomial detrending), 13 these procedures were tested using computer-generated (synthetic) data in which the input-output relationship was purely linear and no noise was present. This may explain why neither benefits nor disadvantages of detrending were observed, as may be the case for signals recorded in human subjects. Furthermore, detrending will remove very low frequencies that will primarily lie below 0.01 Hz. As it is recommended to exclude frequencies below that range for TFA (see Recommendation #13), this could explain why detrending has no clear effects on the outcome. Additional studies using synthesized data with added noise, including signal drifts, should be performed to confirm these observations.
Filtering
RECOMMENDATION #9
Low- or high-pass filtering of signals prior to TFA should be avoided.
This advice aims to produce greater standardization and remains the recommended approach.
TFA methodology
The dominant technique in the literature for calculation of the auto- and cross-spectra used for TFA is the fast Fourier transform, combined with Welch’s method 54 to improve statistical reliability. This involves averaging spectral estimates from separate data segments, which can be overlapped by variable degrees of superposition to adjust the degree of smoothing. The need for standardization of parameter settings and their accurate reporting remains paramount for reproducibility and reliability amongst TFA studies. These settings are described in Recommendations #10-16 and are summarized in Table 1.
Table 1.
Standardized TFA parameter settings.
| TFA setting | Parameter values | Recommendation number |
|---|---|---|
| Minimum duration of recordings | 5 min | 3 |
| Sample frequency (raw signals) | ≥50 Hz | 4 |
| Anti-aliasing low-pass filter | 20 Hz | 4 |
| Re-sample frequency (beat-to-beat) | ≥4 Hz | 7 |
| Interpolation | Lineara | 7 |
| Detrending | None | 8 |
| Normalization | None | 8 |
| Filtering (re-sampled data) | None | 9 |
| Anti-leakage window | Hanning | 10 |
| Window length | 100 s | 11 |
| Superposition | 50% | 11,12 |
| Spectral smoothing | Triangular window (¼, ½, ¼) | 13 |
| Coherence threshold | 95% confidence limit (Table 1) | 14 |
| Reporting results | Plot of mean gain, phase and coherence over complete frequency range (0.02–0.5 Hz), showing mean and SD curves | 15,17 |
| Other outcome measures | MAP | 8 |
| Mean CBF/CBv | 8 | |
| Mean EtCO2 | 2 | |
| EtCO2 variability | 2 | |
| Heart rate variability | ||
| MAP spectral power | 17 | |
| Mean CBF/CBv spectral power | 17 |
Suggested parameter settings for transfer function analysis (TFA) of dCA using mean arterial blood pressure (MAP) as input and cerebral blood flow (CBF) or cerebral blood velocity (CBv) as output. As described in revised Recommendation 11, the number of data segments (‘windows’) adopted with Welch’s method depends on the total duration of the recordings and should be chosen as a compromise between spectral frequency resolution and the confidence limit of the coherence function (Table 2).
aThird order polynomial interpolation also acceptable, but linear is simpler and provides similar results.
Windowing
RECOMMENDATION #10
The Hanning window procedure should be implemented to minimize spectral leakage and provide robust spectral estimates.
Overlapped, tapered windows (Welch method) and spectral smoothing are used to provide robust spectral estimates, while controlling spectral distortion.13,24,54 The standardized use of the Hanning window remains the recommended approach.
Window length
RECOMMENDATION #11 (Revised)
To allow sufficient frequency resolution, use window segments with a minimum length of 100 s. When recordings longer than 5 min are available, it is preferable to increase the number of windows, rather than the length of individual windows when using the Welch algorithm.
The frequency resolution of TFA estimates ( ) is given by , where the window length, T (in seconds), is equal to N/fs, with N being the number of samples in the window and fs being the sampling frequency (in Hz). Higher frequency resolution will provide greater flexibility regarding the choice of frequency bands for averaging coherence, gain, and phase. 24 This decision also has relevance for Recommendation #14, which describes the effect of the number of windows on the coherence threshold for acceptance of estimates of gain and phase. Coherence thresholds, corresponding to the 95% confidence limit have been provided for window durations of 25, 50, and 100 s. 47 When using segments of data shorter than 5 min (Recommendation #3), it is important to identify a compromise between the frequency resolution of different window durations (for example 25 s or 50 s), and the statistical robustness of TFA estimates, which depends on the number of windows. In clinical studies, where good quality measurements lasting 5 min are difficult to obtain, the use of shorter recordings combined with shorter window durations has been shown not to alter estimates of phase and ARI in patients with AIS.46,47
Number and superposition of data segments
RECOMMENDATION #12
A superposition of approximately 50% for overlapping data segments in conjunction with Welch’s method should be used.
Varying the degree of superposition, using percentages of 25, 50, and 75%, does not affect the outcomes of TFA, 13 with 50% being the most common degree of superposition reported in the literature. The use of 50% overlap remains the recommended approach.
Spectral smoothing
RECOMMENDATION #13
Both auto- and cross-spectra should be smoothed using a triangular window with coefficients [¼, ½, ¼].
This remains the recommended approach. Spectral smoothing improves the reliability of TFA-estimated parameters and reduces the threshold for statistical significance of coherence (Table 2 and Recommendation #14).
Table 2.
Calculated cut-off values (critical values) for coherence as a function of the number of windows adopted for data segmentation with Welch’s method at the 99%, 95% and 90% significance levels. All other settings for the Welch method followed the values suggested in revised Table 1.
| Number of windows | Critical values of coherence |
||
|---|---|---|---|
| 99% | 95% | 90% | |
| 3 | 0.65 | 0.51 | 0.43 |
| 4 | 0.54 | 0.41 | 0.33 |
| 5 | 0.46 | 0.34 | 0.27 |
| 6 | 0.40 | 0.29 | 0.23 |
| 7 | 0.35 | 0.25 | 0.20 |
| 8 | 0.31 | 0.22 | 0.18 |
| 9 | 0.29 | 0.20 | 0.16 |
| 10 | 0.26 | 0.18 | 0.14 |
| 11 | 0.24 | 0.17 | 0.13 |
| 12 | 0.22 | 0.15 | 0.12 |
| 13 | 0.21 | 0.14 | 0.11 |
| 14 | 0.19 | 0.13 | 0.10 |
| 15 | 0.18 | 0.12 | 0.10 |
| 20 | 0.14 | 0.09 | 0.07 |
| 25 | 0.11 | 0.08 | 0.06 |
TFA reporting
Coherence function
RECOMMENDATION #14 (Revised)
When calculating average phase and gain within different frequency bands (Recommendation #17), those frequencies in which coherence does not exceed the critical values should be excluded from averaging. Defining a threshold value for coherence to decide on subsequent rejection of data must be guided by calculation of the statistical significance of the coherence function. Table 2 provides the confidence limits (or more strictly the critical values) for coherence as a function of the number of windows. Due to the influence of the degrees of freedom, a fixed threshold for acceptance of coherence, such as 0.5 as is often seen in the literature, can lead to some frequencies being erroneously included or rejected in the analysis and should not be used. If coherence is persistently low (i.e., not significant) across all frequency bands, the recording should be excluded from analysis, as poor data quality and thus unreliable results are expected.
At each frequency, coherence expresses the fraction of output variance (usually mean CBv) that can be explained by the input variance (usually mean BP) using a linear model, similarly to the concept of a correlation coefficient. Readers who are new to TFA in studies of dCA are referred to the original white paper, 7 or more specialised literature.24,55–57 The key points to consider are:
Statistically significant coherence should be present when considering estimates of gain, phase, or other dCA parameters, such as the ARI. Significance is usually based on constructing a null-hypothesis (absence of a linear input-output relationship), with confidence limits set at the 95% level.
Both theoretical analysis and simulations using surrogate data demonstrate that the distribution of coherence depends on the degrees of freedom of spectral estimates (e.g., number of window segments, amount of spectral smoothing, window overlap).12,24,56 This will subsequently be reflected in the confidence limits adopted for their acceptance. Figure 2 and Table 2 both demonstrate the effect of data segmentation for Welch’s method on critical values for coherence and provide the reader with quantitative thresholds that can be incorporated into their study. These values apply to single harmonics (individual frequency points within a frequency band).
-
Coherence is reduced in the presence of:
noise (low signal-to-noise ratio),
a non-linear input-output relationship. In the very low frequency range (<0.07 Hz), the BP-CBF relationship becomes non-linear (due to large time-varying changes in arterial diameter, which dynamically modify hemodynamic resistance). Consequently, coherence values will tend to be lower, despite a strong response of CBF to changes in BP. 58
other variables influencing the output variable (e.g., changing PaCO2).
Units of measurement
RECOMMENDATION #15 (Revised)
The phase of TFA should be reported in either degrees or radians. Estimates of gain obtained by TFA, when included, should be expressed in both absolute units (cm · s−1 · mmHg−1) as well as percentage changes (% · mmHg−1 or %/%) to aid comparison with other published studies.
The coherence function is dimensionless and assumes values between 0 and 1. The literature shows an almost equal divide between the use of radians or degrees for phase estimates. 9 Changing from one to the other is straightforward:
| (1) |
where refers to the angle in degrees, and to the angle in radians. Regarding units of gain, the choice of which units to adopt is less obvious. With measurements at rest, spontaneous fluctuations in mean BP and CBF will normally be <10% of their baseline value. For calculation of the fast Fourier transform, it is good practice to first remove mean values. In addition, for some techniques, such as TCD, comparison of absolute values can be misleading due to measurement uncertainties (e.g., insonation angle, inter-subject differences in vessel diameter). Expressing CBv as a percentage change with respect to its mean value is expected to reduce inter-subject variability in CBv fluctuations and hence also in gain. An example of how different baseline CBv may have a confounding impact on dCA measurements is shown in. 59 Expressing CBv as percentage changes can also facilitate comparisons with results from studies using different modalities to measure CBF (see Recommendation #22). BP variability can be normalized in a similar way, but there are some arguments against this. Firstly, a 10% change for example, would be physiologically very distinct for an individual with a baseline mean BP of 90 mmHg, compared to an individual with a baseline of 150 mmHg. Secondly, the BP value may be subject to drift, especially when using the ubiquitous finger arterial volume clamping method with the intermittent self-adjustment (‘physiocal’) switched off. Though these non-invasive BP devices have been deemed to be robust for assessing changes in BP29,60 and newer versions appear to be more robust, 61 any drift in mean value (e.g., due to a change in finger temperature) would affect the normalization (see Supplementary Material). Thirdly, CPP rather than BP would ideally be applied in assessing dCA, and therefore normalization should be by mean CPP; using mean BP instead would artificially decrease the normalized change and hence inflate the estimated gain. The advantage of normalizing both CBv and BP (i.e., measuring gain in % · %–1) is that one might expect a gain of approximately 1% · %–1 when autoregulation is absent (under ideal conditions). The existing options are thus to express both variables in absolute units or as relative values, which corresponds to cm · s−1 · mmHg−1, % · mmHg−1, % · %,–1 or cm · s−1 · %.–1 The literature shows that the first three options have all been adopted by different investigators, but the last option is rather unusual. 20 Ideally, the units adopted for gain should be those that would maximise sensitivity and specificity for various conditions, but, unfortunately, studies to answer this question are lacking.
One of the major benefits of using phase or ARI to express CA efficacy (Recommendation #18), rather than gain from TFA, is that they are determined from the time-delay between BP and CBF, and thus insensitive to any amplitude scaling. This removes the risk of change in autoregulation being confounded by a change in scale of either physiological variable. A recent meta-analysis of dCA following stroke 17 showed that phase displayed stronger changes than gain, providing further evidence for this recommendation. Furthermore, several studies have calculated confidence intervals on phase estimates55,62 from individual recordings. This can aid in drawing inferences on any changes in dCA, especially when monitoring changes over time in individual patients. Phase is also less sensitive to missing data compared to gain. 50 Phase appears to be a more reliable measure of dCA than gain in clinical studies,17,27,62 although gain is extensively used. In the absence of studies comparing the clinical interpretation of the two different units of gain usually adopted, inter-centre comparisons will be facilitated by reporting gain both in units of cm · s−1 · mmHg−1 and % · mmHg−1.
Phase wrap-around
RECOMMENDATION #16
Inclusion of phase ‘wrap around’ values into averages of multiple harmonics will introduce considerable distortion and should be avoided.
This remains the recommended approach. When negative values of phase are detected for frequencies <0.1 Hz, these should be removed from any averages over the VLF or LF frequency bands. Smaller negative values of phase are to be expected for frequencies above 0.1 Hz and do not need to be removed when producing averages for the HF band. When negative values of phase <0.1 Hz are present, we advise to check the data for errors (e.g. in signal synchronisation).
Frequency bands
RECOMMENDATION #17 (Revised)
Presenting the complete frequency dependence of coherence, gain and phase in the range 0.02–0.5 Hz, as mean and standard deviation values, is important when reporting TFA results. Until further evidence is available, statistical analyses should be based on averaged values within the very-low-frequency (0.02–0.07 Hz), low-frequency (0.07–0.2 Hz) and high-frequency (0.2–0.5 Hz) bands.10 Ideally, the population spectral power density of BP and CBF should also be given at each frequency. Specific averaging methods such as weighting of individual values within bands should be presented separately, and the method(s) used must be reported clearly. In addition, mean values of BP and CBF and their intra-recording variability should be reported. When using forced oscillations (Recommendation #19), averaging over frequency bands should be replaced by estimates at the frequency of oscillation.
While insufficient evidence is available to justify the choice of specific frequency bands for averaging values of coherence, gain and phase, this recommendation aims to improve standardization. The recommended bands are widely adopted for the respective cut-off frequencies; however, alternative choices should not be discouraged if evidence is provided. 56 Indeed, other choices of frequency band limits exist, e.g., the upper frequency limits of dCA can range between 0.094 ± 0.040 Hz during hypercapnia, and 0.167 ± 0.036 Hz during hypocapnia. 63 Moreover, individual harmonics, which behave as independent samples, can be combined in different ways for statistical testing, but note that statistical independence will be affected by the degree of spectral smoothing.11,34 The lower cut-off frequency of 0.02 Hz was chosen because, in most cases, no reliable estimates of phase and gain between BP and CBF are possible below this frequency as the BP-CBF relationship becomes increasingly non-linear.58,64,65 If authors have data with high spectral resolution from data segments longer than 5 min, 6 and are confident that they can provide estimates for lower frequencies, these can be provided in addition.
Note: Standardization of TFA procedures for analysis of dCA in physiological and clinical studies is important to facilitate comparison of results between studies and research centres. However, rigid adoption of specific frequency bands may stifle further developments in TFA of dCA, and also result in sub-optimal sensitivity to detect pathological changes in CA.17,66 We need to remain open to the multiple possibilities to extract optimal information from TFA studies of dCA. 67
Alternative metrics
Step response
RECOMMENDATION #18 (New)
Checking the temporal pattern of the CBF step response is recommended to improve the reliability of TFA in studies of dCA.
In addition to the use of gain and phase for individual harmonics, or averaged over selected frequency bands (Recommendation #17), the information contained in the frequency spectrum can be integrated in the form of a CBF response to a hypothetical step change in BP. Estimation of the CBF step response is usually performed with the use of the inverse fast Fourier transform, to obtain the impulse response, followed by numerical integration. 24 One advantage of the CBF step response is the ability to represent the physiological response that would be expected if BP could be changed nearly instantaneously (Figure 3); something that can only be approximated with maneuvers such as the sudden release of inflated thigh cuffs. 1 From the expected temporal pattern of the step response, it is possible to reject estimates that are not physiologically plausible, either by visual inspection, or subject to using objective criteria. 57 Examples of non-plausible responses to a positive step in BP would be cases where the step response does not show a sharp initial rise, has very large negative values, shows a continuous rise with time, or presents large oscillations in the first 10 seconds of the response. 57
Figure 3.
Representative spontaneous fluctuations in CBv, BP and ICP with corresponding CBv step responses from two patients with severe head injury. Patient A did not show a recovery of the step response following the sudden change in BP, with a corresponding value of ARI = 0 (absent dCA). Patient B had a working dCA, with ARI = 6. Reproduced with permission from. 108
Metrics that reflect the speed of recovery can be derived from the CBF step response, thereby expressing the dCA efficiency. Two metrics based on a derived step response include ARI and the rate of recovery (RoRc). The ARI was initially proposed to quantify dCA during a thigh cuff maneuver, 2 but it can also be derived from recordings with spontaneous BP fluctuations 68 or during other maneuvers, such as repeated squat-stands 69 or deep breathing. 70 The RoRc is an index obtained from the first three seconds of the CBF step response, 71 and several studies have demonstrated a close association between estimates of TFA phase, ARI, and RoRc.17,69,72–76 See Recommendation #20 for use of ARI and RoRc in clinical studies.
Alternative protocols
Forced oscillations
RECOMMENDATION #19 (New)
When feasible, we recommend forced oscillations in BP as the TFA input to increase the signal-to-noise ratio, compared with spontaneous fluctuations in BP. As with spontaneous recordings, forced BP oscillations should be monitored for a period of at least 5 min, discarding the first 10–30 seconds for stable oscillations to be achieved. When forcing BP oscillations at a fixed frequency, TFA coherence, gain, and phase of the forced oscillations should be sampled at the point estimate of the forced frequency (e.g. 0.05 or 0.10 Hz), instead of choosing the standard frequency bands. In such circumstances, this would supersede Recommendation #17.
In recent years, a number of protocols have been developed to increase the signal-to-noise ratio and coherence between BP and CBF for assessment of TFA phase and gain, such as repeated squat-stands (usually at 0.05 and 0.10 Hz),21,77–83 repeated sit-stands,84–86 oscillatory lower body negative pressure (OLBNP),51,87,88 respiratory-induced oscillations89,90 and passive leg raising. 91 Current evidence suggests that the optimal protocol for inducing mean BP oscillations to increase the input power and enhance the linear interpretability of the TFA parameters is the repeated squat-stand model.21,51 For studies in older adults, or participants unable to perform 5 min of squat-stands, the repeated sit-stand protocol is a good alternative.84,86 By inducing larger swings in mean BP, the OLBNP protocol does improve TFA coherence compared to spontaneous monitoring 51 and the passive leg raise maneuver, 91 and it does not alter PaCO2 if lower magnitudes of LBNP are used. 51 However, OLBNP yields lower TFA coherence values and within-day/between-day reproducibility when compared to repeated squat-stands. 51 Passive methods to force oscillations in BP (e.g. OLBNP) are particularly appealing protocols, however, in situations where repeated squat-stands or sit-stands are not feasible due to mobility impairment, disabilities, or for participant safety (dCA quantification following sympathetic nervous activity blockades). Other protocols exist to force BP in order to characterize the BP-CBF relationship (e.g. random duration squat-stands, 69 random inflation/deflation of thigh cuffs, 92 single thigh cuff deflation, 1 single sit-to-stand,81,82 head-up tilt,93,94 cold pressor test,95–97 hand grip96,98 and the Valsalva maneuver96,99,100). Sympathetic activation caused by some of these maneuvers may affect dCA and confound comparisons between results from different protocols.23,88,96 However, their inherent non-stationarity and short duration (in many cases restricted to one or two oscillations), prevent the utilization of TFA to capture the gain and phase parameters. Other methodological strategies are preferred within the broader dCA literature to quantify the outcomes of these protocols, such as the rate of regulation (RoR) 1 and ARI.2,48,92
Recordings of forced oscillations of mean BP for TFA should last for a period of at least 5 min,48,49 assuming consistent physiological conditions and continuous good quality signals, to ensure that recordings can yield robust estimates of TFA parameters, and to improve frequency resolution (Recommendation #3). Valid and reliable TFA parameters may be drawn from recordings of a shorter length (minimum 3 min), if physiological covariates (i.e., respiratory rate, PaCO2, BP, heart rate, and CBF) are controlled.48,49 Since the peak input power spectral density of BP and CBF occurs in alignment with the forced fixed frequency, the utilization of point-estimates, rather than frequency bands, will increase coherence values and improve the interpretability of the associated phase and gain metrics as shown in Fig. 4 51 . For researchers employing random durations squat-stands, 69 or selecting to alternate squat-stands of 0.05 Hz with 0.10 Hz, it may be justified to avoid the point estimates and perform narrow bands around these frequencies. However, insufficient evidence exists to recommend this to the broader community and more research is warranted. Furthermore, performing TFA across the cardiac cycle (e.g., systole and diastole) may be important for future research investigations, as dCA appears to be more effective in dampening systolic BP oscillations, compared with diastolic and mean BP oscillations (see Table 4 (mean) and Supplementary Table S.1 (systolic and diastolic)).21,23,101,102 Future research is needed to resolve questions around directional differences (responses to increases versus decreases in BP) during augmented oscillations and potential non-linearities driven by the magnitude of forced oscillations in relation to baseline BP.103–105
Table 4.
Normative transfer function analysis of the mean BP-CBv relationship obtained via squat-stand maneuvers from the pooled data of 157 healthy individuals (non-athletic, average fitness levels, and across the ages of 19-55 years) with 194 measures in the middle cerebral artery (MCAv) and 99 in the posterior cerebral artery (PCAv).21,23,51,101,102
| Parameter | 0.05 Hz | 0.10 Hz |
|---|---|---|
| BP power (mmHg2 s−2) | 21812 (16081) | 18991 (10906) |
| MCAv power (cm2 s−2) | 10210 (8350) | 14091 (13155) |
| MCAv Coherence | 0.98 (0.02) | 0.99 (0.06) |
| MCAv Gain (cm s−1 · mmHg−1) | 0.68 (0.23) | 0.83 (0.25) |
| MCAv Gain (% · mmHg−1) | 1.10 (0.31) | 1.32 (0.33) |
| MCAv Phase (radians) | 0.82 (0.34) | 0.74 (0.34) |
| MCAv Phase (degrees) | 47 (19) | 42 (19) |
| PCAv Power (cm2 · s−2) | 3393 (3299) | 6126 (5860) |
| PCAv Coherence | 0.98 (0.03) | 0.99 (0.01) |
| PCAv Gain (cm s−1 · mmHg−1) | 0.38 (0.13) | 0.56 (0.20) |
| PCAv Gain (% · mmHg−1) | 1.06 (0.42) | 1.44 (0.42) |
| PCAv Phase (radians) | 1.05 (0.28) | 0.98 (0.26) |
| PCAv Phase (degrees) | 60 (16) | 56 (15) |
All values are mean (SD). Normative TFA of the systolic BP-CBv and diastolic BP-CBv relationship are given in Supplementary Table S.1. BP: arterial blood pressure; CBv: cerebral blood flow velocity.
Clinical studies/trials
RECOMMENDATION #20 (New)
Multicenter clinical trials are warranted to demonstrate the importance of dCA monitoring in clinical practice.
Dynamic CA has been associated with the outcome of many disorders, namely acute ischemic stroke,17,26,106 subarachnoid hemorrhage 26 and trauma tic brain injury,107,108 and has been shown to be intact or depressed in non-neurological diseases, such as hypertension27,88,116 and heart failure. 109 ARI has been shown to be reduced during hypercapnia 32 and hypoxia, 75 and in multiple cerebrovascular conditions such as intra- and extra-cranial disease, 68 vasovagal syncope, 110 acute ischemic stroke,17,106 intra-cerebral hemorrhage,41,76 small vessel disease, 72 severe head injury, 108 pre-eclampsia and eclampsia,73,109 delirium, 112 circulatory shock, 113 and right-to-left shunt.74,76,114 Thus, dCA could be helpful for evaluating individual therapeutic strategies during acute and sub-acute stages of care (e.g., during recanalization therapies 115 and active BP lowering 26 ) but more research is needed. Finally, other non-acute neurological diseases that have already been included in dCA studies are dementia3,117 and Parkinson’s disease. 118
The recommendations in this paper provide general information that should be followed including in clinical settings to guide research in this field; however, fulfilling all requisites in studies on patients with acute disease is obviously challenging. Nevertheless, attempts should be taken to optimally control the environment and minimize the occurrence of undesired stimuli (e.g., avoiding contact with personnel, use of privacy curtains, silence signs). The time of the day should be noted, as well as any conditions departing from Recommendation #1. Furthermore, recordings should be avoided when BP, heart rate and PaCO2 stability is not guaranteed. We emphasize that it is essential to have simultaneous measurements of PaCO2 for correct interpretation of dCA (Recommendation #2) and also report the ventilation status of the patient during the collection (e.g., intubated, O2 supply, pathological respiratory cycle, etc). We highly recommend the reporting of other variables of interest such as ICP (see Recommendation #2), the use of vasoactive drugs, and the existence of hypermetabolic states (e.g., infection/fever, seizures) for a correct interpretation of TFA results. In a critical care setting, it is difficult to acquire good quality measurements for long durations. When this is not possible, for example in agitated patients, shorter segments of data could be used (Recommendation #3). Also, in such populations, data will be more likely to have noise and artifacts, so special attention should be given to data pre-processing (Recommendation #6 and Supplementary Material) and to using appropriate coherence cut-off values according to the recording length (Recommendation #14). If spontaneous BP fluctuations are less pronounced, and induced BP oscillations (Recommendation #19) cannot be adopted, longer duration recordings should be collected. 16 In the presence of cardiac arrhythmia, for example atrial fibrillation, analysis of such data should be performed separately, with comparisons drawn to data collected in sinus rhythm during the same protocol.52,119 In addition, whenever possible, critical carotid stenosis should be acknowledged and included as a co-factor in the statistical analysis. When reporting the results, in addition to all the factors mentioned above, the possibility of narrowing or dilatation of insonated arteries should be noted in the discussion of results, addressing the possibility of confounding.
Alternative models
Time-domain methods
RECOMMENDATION #21 (New)
Time-domain methods should be considered as an alternative to TFA when multiple inputs are needed to model changes in CBF. In particular, adding PaCO2 as an additional input is recommended if such measurements are available.
While the use of frequency-domain methods (i.e., TFA) has been more prevalent in the context of quantifying dCA, time-domain methods have been implemented in several studies.70,120–124 Time-domain methods allow for quantification of the effect of additional physiological signals (e.g., PaCO2 or SaO2 when using NIRS)125–127 and non-stationarities. 125 Time-domain methods typically aim at estimating the parameters of a difference equation model that relates the present value of CBF to present and past values of BP using experimental time-series. The corresponding system impulse response and transfer function can be readily obtained from these coefficients. The reader is referred to18,19 for a detailed comparison of time-domain and frequency-domain methods with regards to the reproducibility of measures typically used to quantify dCA, using both simulated 18 and experimental 19 data.
Time-domain analyses have been presented using both finite impulse response (FIR) (or moving average, MA)121,124,125 models and autoregressive (AR) models with exogenous inputs (ARX).70,120,122–124 The former aim at explaining CBF fluctuations using past values of BP only, while the latter also include past CBF values. According to time-series modelling and systems identification literature, it is strictly, however, more correct to refer to models that include past CBF and BP terms as ARX, instead of ARMA. The main reason for using ARX rather than FIR models is that it typically reduces the number of the required free parameters, which can be rather high in the case of FIR (or MA) models with a long memory. On the other hand, expanding the unknown impulse response function in terms of a suitable set of basis functions (e.g., Laguerre functions 125 or principal dynamic modes 128 ) also reduces the number of free parameters in the case of FIR models and, due to this reduction, may yield more robust results in low SNR environments, as well as in the case of models with two or more inputs. Note that basis expansion models can be readily extended to non-linear models, though in this case the minimum duration of the required experimental data would likely increase, compared to what is recommended for performing linear analyses.
Selecting an appropriate model order is crucial and should be done using a statistical criterion (e.g., Akaike or Bayesian Information Criterion) or cross-validation. Previous studies that used ARX models have reported orders ranging between 1 and 2 for the AR component (number of past CBF values),70,120,123 as well as orders between 3 and 5 for the exogenous input component (number of past and present BP values).70,120,127 A wider range for both the AR (between 2 and 9) and exogenous input (between 4 and 9) orders was reported in the recent CARNet study on dCA measure reproducibility. 19 The same study reported that ARX-derived measures were the most reproducible among all examined time-domain methods, yielding similar reproducibility to TFA, even though all methods exhibited relatively low reproducibility, which was likely due to physiological variability and time-varying behavior. 19
Alternative measurement techniques
RECOMMENDATION #22 (New)
Research to further validate the use of alternative imaging approaches to that of TCD will benefit our understanding of dCA and is warranted.
When intracranial arteries cannot be insonated with TCD, mainly due to poor temporal windows, often associated with age or ethnicity,129–132 alternative methods for CBF estimation should be considered. Alternative options to TCD that have been used to date for dCA assessment have included both near infrared spectroscopy (NIRS) and magnetic resonance imaging (MRI), while longer term monitoring of dCA in patients with severe head injury has been done via the invasive monitoring of ICP and MAP.108,133 However, readers should be aware of the indirect nature of CBF outcome measures that some alternatives utilise (e.g., blood oxygenation-level dependent-MRI as an index of local blood flow 134 ), differences in waveform morphology between measured vasculature sites (i.e., TCD vs. NIRS, see 135 ), and the strengths and weaknesses of the different CBF signal acquisition modalities. 136 A new optical modality, diffuse correlation spectroscopy (DCS), is also showing considerable promise. 137
NIRS-based approaches have provided the most extensive literature currently available with respect to potential alternatives to TCD. Several studies have utilised NIRS to assess dCA and applied the TFA approach.4,135,138 However, while a recent validation study, 116 comparing NIRS- and TCD-derived markers of dCA, highlighted the potential utility of NIRS, the correlation between the two modalities was relatively low and the NIRS-derived data exhibited limited ability to discern differences between the cohorts used in this study (younger and older controls, hypertensive patients, and cognitively impaired patients). When using this modality, considerations around the effect of differences in waveform morphology and temporal characteristics between the macro- and microcirculation on TFA phase metrics in the very-low-frequency and low-frequency bands are paramount. NIRS-derived oxyhemoglobin-deoxyhemoglobin (oxyHb-HHb) estimates of dCA become more similar to TCD-derived estimates if corrected for transit time and the balance between blood flow and blood volume oscillations. 135 These correction parameters can be estimated from the oxyHb/HHb phase difference spectrum in the high-frequency band. 135 Perhaps unsurprisingly, repeated sit-to-stand transitions appear to improve the reliability of NIRS-derived estimates of dCA, with their correlation to TCD-derived dCA also becoming stronger. 116
In contrast to NIRS, the assessment of dCA using MRI remains a developing area of research. MRI-based studies of dCA based on spontaneous fluctuations of BP and CBF are problematic due to the difficulty of performing simultaneous measurements near the magnet. Instead, alternative BP perturbation protocols have been tested, with thigh cuff release the most common to date, combined with the use of the rate of return as a marker of dCA efficiency.139,140 More recently, commercial devices, e.g., the CareTaker (Biopac) 141 or a modified and shielded Finometer NOVA, 5 have been tested for the non-invasive continuous measurement of BP inside the magnet that can be assessed alongside real-time MRI-derived measures of CBF, but further studies are needed to demonstrate agreement of dCA estimates with those obtained using TCD, and to validate the accuracy of the BP measurements, for absolute BP and for detecting BP oscillations. Consequently, there are limited studies available to support any recommendations at the present time, particularly with respect to the use of TFA on spontaneous measurements. For MRI studies, the recommendation for concurrent measurement of PaCO2 (or EtCO2) (Recommendation #2) is particularly relevant, as participants may experience anxiety due to the enclosed nature of the head coil and bore of the magnet, causing spontaneous hyperventilation or irregular breathing that leads to a hypo- or hypercapnic state.
Normative data and thresholds
RECOMMENDATION #23 (New)
Studies should include a properly matched control group to compare results. In addition to variables described in Recommendations #1 and #2, the interpretation of dCA metrics should consider age, sex, cardiorespiratory fitness, and other phenotypical characteristics until more detailed population data are available.
There are insufficient data to establish reference and normative values for TFA parameters. Normative data on healthy subjects have been published,21,23,51,101,102,142,143 but these have come from only a few centers which limits the external validity of the results. Despite this limitation, the mean and standard deviation of pooled data for each of the TFA parameters and ARI are presented in Tables 3 and 4, respectively, and could be used as a preliminary reference for dCA studies.
Table 3.
Preliminary normative values of the mean BP-CBv relationship, obtained from the pooled middle cerebral artery data of 187 healthy individuals from two studies.142,143
| Parameter | VLF (0.02–0.07 Hz) | LF (0.07–0.2 Hz) | HF (0.2–0.5 Hz) |
|---|---|---|---|
| BP power (mmHg2) | 21.6 (18.4) | 2.9 (3.8) | 0.5 (0.7) |
| CBv power (cm2 · s−2) | 25.6 (19.2) | 2.8 (4.6) | 0.3 (0.4) |
| Coherence | 0.5 (0.1) | 0.6 (0.1) | 0.6 (0.1) |
| Gain (cm s−1 · mmHg−1) | 0.7 (0.3) | 1 (0.5) | 1.1 (0.4) |
| Gain (% · mmHg−1) | 1.4 (0.5) | 2.1 (0.7) | 2.0 (0.6) |
| Phase (radians) | 0.8 (0.4) | 0.5 (0.2) | 0.0 (0.3) |
| Phase (degrees) | 46 (23) | 29 (11) | 0.0 (17) |
| ARI | 5.3 (1.5) | ||
All values are mean (SD). ARI: autoregulatory index; BP: arterial blood pressure, CBv: cerebral blood velocity; VLF: very-low-frequency; LF: low-frequency; HF: high-frequency.
The influence of age and sex on dCA metrics is equivocal. While most studies show no influence of age and sex, 144 for example using TFA phase 142 or ARI, 143 other recent evidence hints at the possible interaction of these two variables such that dCA may be impacted by increasing age in men but not women. 145 Moreover, phase and gain were shown to be reduced in older subjects when protocols that increase coherence between CBv and BP were adopted. 146 Coherence, itself, can also be affected by age. 143 In summary, datasets from cohorts with different age and sex proportions, and determined through different protocols, have detected some effect of age and sex on dCA indices but further work is necessary to quantify these effects. Additionally, cardiorespiratory fitness may confound this relationship further.80,81 Clarity of the age and sex effects on dCA within the healthy population is essential before thoughts on clinical thresholds for impaired autoregulation can be entertained.
Hitherto, difficulty to precisely delineate reference and cut-off values remains. Based on statistical criteria, an ARI < 4 57 and a very-low-frequency phase < 0.5 radians (<29 degrees) 143 have been suggested as the thresholds for abnormality, because they correspond to the 5% lower confidence limit. Some studies partly support their clinical significance109,112,115,147 but the high variability of these indices, particularly phase, precludes their widespread application. Gain is also highly variable and no clear criteria for abnormal values has been proposed. 17 Currently, no defined thresholds are recommended, and thus we should be careful in interpreting the reduced values of CA indices as impairment. Future studies on the prognostic information of these measures could reveal clinically relevant parameters and delineate their optimal cut-off values more closely.
Calibration database
Readers developing their own software to estimate TFA parameters, or using dedicated software packages for this purpose,148,149 are advised to become familiar with key steps involved in obtaining reliable results (see Supplementary Material). To confirm the accuracy of bespoke TFA software, developers and users are encouraged to engage with the procedure described in the original white paper, 7 which has not been altered.
Discussion
This updated, CARNet-endorsed, TFA white paper has enhanced many of the previous recommendations and introduced six novel recommendations that extend the application of TFA and other methods for dCA assessment into both physiological and clinical studies. In the original consensus document, a 10-point program of research was outlined, mainly focusing on areas where more evidence was needed to strengthen recommendations and improve standardization. 7 When examining the contents of this revision, it is reassuring to note the confluence of the recent research output with the original priorities that were established. Despite this progress, however, many topics are far from being concluded. Above all, the need for more clinical studies, specifically of a multicentre nature, remains a top priority. With this in mind, we expect that the novel Recommendations (notably #18, #19, #20, and #23), utilized alongside the updated recommendations (notably #3, #11 and #17), will lead to improvements in reliability, as well as the sensitivity and specificity, of TFA-derived parameters to detect pathological alterations in dCA. Indeed, the challenge that lies ahead is to bring to the fore the performance of different dCA metrics, either derived by TCD or other methods (Recommendation #22), for diagnostic and prognostic purposes. This goal will be greatly facilitated by the incorporation of the recommendations from this updated white paper into clinical studies, with the expectation that future revisions will have stronger evidence to base recommendations on the classification accuracy of different indices.
We hope that new evidence will surface in the coming years to allow more forceful recommendations, such as the potential influence of circadian rhythms on dCA. Although initial reports suggest that dCA is not affected during the daytime,21,22,150 to err on the side of caution, our recommendation is that all recordings in a study be performed the same time of the day whenever possible, particularly for a repeated measures design. More evidence is also needed on refrainment from different substances and exercise prior to measurements (Recommendation #1). A review of the literature shows considerable diversity between centres, and further evidence is needed to support more rigorous recommendations leading to further standardization. Finally, regional differences in dCA efficacy and how these are affected by physiological interventions and clinical conditions deserve greater attention. By far, the middle cerebral artery (MCA) has been the source of CBv(MCAv) measurements in studies of dCA, with a much smaller number of studies reporting results from the posterior (PCAv) or anterior cerebral arteries (ACAv). Most of the recommendations in this white paper apply equally to the MCA, PCA or ACA, but regional differences are obviously highly relevant when performing clinical studies (recommendation #20) or establishing reference values (recommendation #23). For the PCA, initial results in healthy subjects suggest that dCA metrics do not show differences from corresponding values from the MCA (Table 4)21,81 but further work is needed to improve the evidence base.
To conclude, it is important to note that the focus of this updated white paper on TFA is due to its popularity in the assessment of dCA in physiological and clinical studies. It should not be seen as a pronouncement that TFA is superior to other techniques for this purpose. More comparative, inter-method studies are needed, mainly in different clinical conditions, as stated above, to allow identification of optimal approaches for dCA assessment.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221119760 for Transfer function analysis of dynamic cerebral autoregulation: a CARNet white paper 2022 update by Ronney B Panerai, Patrice Brassard, Joel S Burma, Pedro Castro, Jurgen AHR Claassen, Johannes J van Lieshout, Jia Liu, Samuel JE Lucas, Jatinder S Minhas, Georgios D Mitsis, Ricardo C Nogueira, Shigehiko Ogoh, Stephen J Payne, Caroline A Rickards, Andrew D Robertson, Gabriel D Rodrigues, Jonathan D Smirl David M Simpson in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
The authors would like to thank the following members of CARNet for revising the final version of the white paper and providing comments that led to further improvements of the manuscript: Marcel Aries, Tony Birch, Sergio Brasil, Igor Braz, David Busch, Alexander Caicedo, Max Chacon, Heather Edgell, Kun Hu, Rehan Junejo, John Karemaker, Osian Llwyd, Vasilis Marmarelis, Vera Novak, Laura Parkes, Aaron Phillips, co-author Nhu Tran, and Rong Zhang.
Glossary
Abbreviations
- ACA
anterior cerebral artery
- ACAv
ACA cerebral blood velocity
- AIS
acute ischemic stroke
- AR
autoregressive
- ARI
autoregulation index
- ARX
AR with exogenous inputs
- BP
arterial blood pressure
- CA
cerebral autoregulation
- CBF
cerebral blood flow
- CBv
Cerebral blood velocity
- CO2
carbon dioxide
- CPP
cerebral perfusion pressure
- dCA
dynamic CA
- DCS
diffuse correlation spectroscopy
- DF
degrees of freedom
- EtCO2
end-tidal CO2
- FIR
finite impulse response
- HHb
deoxyhaemoglobin
- ICP
intracranial pressure
- LBNP
lower body negative pressure
- MA
moving average
- MAP
mean arterial blood pressure
- MCA
middle cerebral artery
- MCAv
MCA cerebral blood velocity
- MRI
magnetic resonance imaging
- NIRS
near infrared spectroscopy
- OLBNP
oscillatory LBNP
- OxyHb
oxyhemoglobin
- PaCO2
partial pressure of arterial CO2
- PCA
posterior cerebral artery
- PCAv
PCA cerebral blood velocity
- RoR
rate of regulation
- RoRc
rate of recovery
- SNR
signal-to-noise ratio
- SSM
squat-stand maneuver
- TCD
transcranial Doppler ultrasound
- TFA
transfer function analysis
Funding: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Jatinder S. Minhas is funded by an NIHR Clinical Lectureship in Older People and Complex Health Needs. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health or the authors’ respective organisations.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs
Ronney B Panerai https://orcid.org/0000-0001-6983-8707
Patrice Brassard https://orcid.org/0000-0002-6254-5044
Joel S Burma https://orcid.org/0000-0001-9756-5793
Pedro Castro https://orcid.org/0000-0003-1401-2398
Jurgen AHR Claassen https://orcid.org/0000-0002-1778-8151
Georgios D Mitsis https://orcid.org/0000-0001-9975-5128
Ricardo C Nogueira https://orcid.org/0000-0003-3309-3760
Shigehiko Ogoh https://orcid.org/0000-0001-5297-6468
Stephen J Payne https://orcid.org/0000-0003-1156-2810
Andrew D Robertson https://orcid.org/0000-0002-9095-9877
Jonathan D Smirl https://orcid.org/0000-0003-1054-0038
David M Simpson https://orcid.org/0000-0001-9072-5088
Appendix
List of scientists who endorse the white paper 2022 update and have provided comments and suggestions
The CARNet group was formed in 2011 to foster collaboration within the diverse research base of CA. The network has approximately 150 members, involving scientists with expertise in physiology, neurology, neurosurgery, medical physics, mathematics, and engineering. The following scientists have provided comments and suggestions to earlier versions of the paper and endorse its publication.
Belgium
Fabio Taccone (Leuven)
Brazil
Sergio Brasil (Sao Paulo), Igor Braz (Volta Redonda), Juliana Caldas (Salvador), Angela Macedo (São Paulo), Pedro Paulo Soares (Niteroi), Lauro Vianna (Brasilia)
Canada
Phillip Ainslie (British Columbia), Heather Edgell (York), Lawrence Labrecque (Québec), Aaron Phillips (Calgary), Frederick Zeller (Manitoba)
Chile
Max Chacon (Santiago), Jose Luis Jara (Santiago)
Colombia
Alexander Caicedo Dorado (Bogota)
France
Julien Brugniaux (Grenoble), Nathalie Nasr (Grenoble)
Japan
Kin-ichi Iwasaki (Tokyo)
New Zealand
Fiona McBryde (Auckland)
Portugal
Elsa Azevedo (Porto)
Switzerland
Nicolai Goettel (Basel)
Taiwan
Men-tzung Lo (Taoyuom)
Thailand
Kannakorn Intharakham (Thammasat)
The Netherlands
Marcel Aries (Maastricht), Nick Eleveld (Groningen), Jan Willem Elting (Groningen), John Karemaker (Amsterdam), Teelkien van Veen (Groningen), Ralf Weijs (Nijmegen)
United Kingdom
Damien Bailey (Pontypridd), Lucy Beishon (Leicester), Tony Birch (Southampton), Victoria Haunton (Plymouth), Rehan Junejo (Manchester), Osian Llywd (Oxford), Sam Oliver (Bangor), Laura Parkes (Manchester), Tom Robinson (Leicester)
United States
R. Matthew Brothers (Texas, David Busch (Texas), Igor Fernandes (Florida), Kun Hu (Massachusetts), Blair Johnson (Indiana), Caroline Maciel (Florida), Vasilis Marmarelis (California), Joseph Miller (Michigan), Vera Novak (Massachusetts), Takashi Tarumi (Texas), Nhu Tran (South Carolina), Rong Zhang (Texas)
Uruguay
Corina Puppo (Montevideo).
References
- 1.Aaslid R, Lindegaard KF, Sorteberg W, et al. Cerebral autoregulation dynamics in humans. Stroke 1989; 20: 45–52. [DOI] [PubMed] [Google Scholar]
- 2.Tiecks FP, Lam AM, Aaslid R, et al. Comparison of static and dynamic cerebral autoregulation measurements. Stroke 1995; 26: 1014–1019. [DOI] [PubMed] [Google Scholar]
- 3.Claassen JAHR, Thijssen DHJ, Panerai RB, et al. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev 2021; 101: 1487–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinhard M, Wehrle-Wieland E, Grabiak D, et al. Oscillatory cerebral hemodynamics – the macro- vs. microvascular level. J Neurol Sci 2006; 250: 103–109. [DOI] [PubMed] [Google Scholar]
- 5.Whittaker JR, Driver ID, Venzi M, et al. Cerebral autoregulation evidenced by synchronized low frequency oscillations in blood pressure and resting-state fMRI. Front Neurosci 2019; 13: 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang R, Zuckerman JH, Levine BD. Spontaneous fluctuations in cerebral blood flow: insights from extended- duration recordings in humans. Am J Physiol Heart Circ Physiol 2000; 278: H1848–H1855. [DOI] [PubMed] [Google Scholar]
- 7.Claassen JAHR, Meel-van den Abeelen ASS, Simpson DM, et al. Transfer function analysis of dynamic cerebral autoregulation: a white paper from the international autoregulation research network (CARNet). J Cereb Blood Flow Metab 2016; 36: 665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giller CA. The frequency-dependent behavior of cerebral autoregulation. Neurosurgery 1990; 27: 362–368. [DOI] [PubMed] [Google Scholar]
- 9.Meel-van den Abeelen ASS, Van Beek AHEA, Slump CH, et al. Transfer function analysis for the assessment of cerebral autoregulation using spontaneous oscillations in blood pressure and cerebral blood flow. Med Eng Phys 2014; 36: 563–575. [DOI] [PubMed] [Google Scholar]
- 10.Zhang R, Zuckerman JH, Giller CA, et al. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol Heart Circ Physiol 1998; 274: H233–H241. [DOI] [PubMed] [Google Scholar]
- 11.Fraser IIC, Brady KM, Rhee CJ, et al. The frequency response of cerebral autoregulation. J Appl Physiol 2013; 115: 52–56. [DOI] [PubMed] [Google Scholar]
- 12.Gommer ED, Shijaku E, Mess WH, et al. Dynamic cerebral autoregulation: different signal processing methods without influence on results and reproducibility. Med Biol Eng Comput 2010; 48: 1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meel-van den Abeelen ASS, Simpson DM, Wang LJY, et al. Between-centre variability in transfer function analysis, a widely used method for linear quantification of the dynamic pressure-flow relation: the CARNet study. Med Eng Phys 2014; 36: 620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beishon LC, Minhas JS, Nogueira RC, et al. INFOMATAS multi-center systematic review and meta-analysis individual patient data of dynamic cerebral autoregulation in ischemic stroke. Int J Stroke 2020; 24: 1747493020907003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castro P, Azevedo E, Sorond FA. Cerebral autoregulation in stroke. Curr Atherosc Rep 2018; 20: 1–12. [DOI] [PubMed] [Google Scholar]
- 16.Elting JW, Sanders ML, Panerai RB, et al. Assessment of dynamic cerebral autoregulation in humans: is reproducibility dependent on blood pressure variability? PLOS One 2020; 15: e0227651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Intharakham K, Beishon LC, Panerai RB, et al. Assessment of cerebral autoregulation in stroke: a systematic review and meta-analysis of studies at rest. J Cereb Blood Flow Metab 2019; 39: 2105–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanders ML, Claassen JAHR, Aries M, et al. Reproducibility of dynamic cerebral autoregulation parameters: a multi-centre, multi-method study. Physiol Meas 2018; 39: 125002. [DOI] [PubMed] [Google Scholar]
- 19.Sanders ML, Elting JW, Panerai RB, et al. Dynamic cerebral autoregulation reproducibility is affected by physiological variability. Front Physiol 2019; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ainslie PN, Barach A, Murrell C, et al. Alterations in cerebral autoregulation and cerebral blood flow velocity during acute hypoxia: rest and exercise. Am J Physiol Heart Circ Physiol 2007; 292: H976–H983. [DOI] [PubMed] [Google Scholar]
- 21.Burma JS, Copeland P, Macaulay A, et al. Comparison of diurnal variation, anatomical location, and biological sex within spontaneous and driven dynamic cerebral autoregulation measures. Physiol Rep 2020; 8: e14458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labrecque L, Burma JS, Roy MA, et al. Reproducibility and diurnal variation of the directional sensitivity of the cerebral pressure-flow relationship in men and women. J Appl Physiol 2022; 132: 154–166. [DOI] [PubMed] [Google Scholar]
- 23.Burma JS, Copeland P, Macaulay A, et al. Dynamic cerebral autoregulation across the cardiac cycle during 8 hr of recovery from acute exercise. Physiol Rep 2020; 8: e14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bendat JS., Piersol AG. Random data analysis and measurement procedures. New York: John Wiley & Sons, 1986. [Google Scholar]
- 25.Mehagnoul-Schipper DJ, Kraaij DJW, Jansen RWMM. Achieving haemodynamic baseline values with finapres in elderly subjects during supine rest. Clin Physiol 2000; 20: 466–473. [DOI] [PubMed] [Google Scholar]
- 26.Nogueira RC, Aries M, Minhas JS, et al. Review of studies on dynamic cerebral autoregulation in the acute phase of stroke and the relationship with clinical outcome. J Cereb Blood Flow Metab 2022; 42: 430–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Beek AHEA, Claassen JAHR, Rikkert O, et al. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab 2008; 28: 1071–1085. [DOI] [PubMed] [Google Scholar]
- 28.Skow RJ, Brothers RM, Claassen JAHR, et al. On the use and misuse of cerebral hemodynamics terminology using transcranial Doppler ultrasound: a call for standardization. Am J Physiol Heart Circ Physiol 2022; 323: H350–H357. [DOI] [PubMed] [Google Scholar]
- 29.Sammons EL, Samani NJ, Smith SM, et al. Influence of noninvasive peripheral arterial blood pressure measurements on assessment of dynamic cerebral autoregulation. J Appl Physiol 2007; 103: 369–375. [DOI] [PubMed] [Google Scholar]
- 30.Imholz BPM, Wieling W, van Montfrans GA, et al. Fifteen years experience with finger arterial pressure monitoring: assessment of the technology. Cardiovasc Res 1998; 38: 605–616. [DOI] [PubMed] [Google Scholar]
- 31.Hoiland RL, Fisher JA, Ainslie PN. Regulation of the cerebral circulation by arterial carbon dioxide. Compr Physiol 2019; 9: 1101–1154. [DOI] [PubMed] [Google Scholar]
- 32.Minhas JS, Panerai RB, Robinson TG. Modelling the cerebral haemodynamic response in the physiological range of PaCO2. Physiol Meas 2018; 39: 1–11. [DOI] [PubMed] [Google Scholar]
- 33.Minhas JS, Panerai RB, Robinson TG. Sex differences in cerebral haemodynamics across the physiological range of PaCO2. Physiol Meas 2018; 39: 105009. [DOI] [PubMed] [Google Scholar]
- 34.Panerai RB, Deverson ST, Mahony P, et al. Effect of CO2 on dynamic cerebral autoregulation measurement. Physiol Meas 1999; 20: 265–275. [DOI] [PubMed] [Google Scholar]
- 35.Claassen JAHR, Zhang R, Fu Q, et al. Transcranial doppler estimation of cerebral blood flow and cerebrovascular conductance during modified rebreathing. J Appl Physiol 2007; 102: 870–877. [DOI] [PubMed] [Google Scholar]
- 36.Minhas JS, Robinson TG, Panerai RB. PaCO2 measurement in cerebral haemodynamics: face mask or nasal cannulae? Physiol Meas 2017; 38: N101–N106. [DOI] [PubMed] [Google Scholar]
- 37.Burley CV, Lucas RAI, Whittaker AC, et al. The CO2 stimulus duration and steady-state time point used for data extraction alters the CO2 reactivity outcome measure. Exp Physiol 2020; 105: 893–903. [DOI] [PubMed] [Google Scholar]
- 38.Minhas JS, Kennedy C, Robinson TG, et al. Different strategies to initiate and maintain hyperventilation: their effect on continuous estimates of dynamic cerebral autoregulation. Physiol Meas 2019; 40: 015003. [DOI] [PubMed] [Google Scholar]
- 39.Immink RV, Secher NH, Roos CM, et al. The postural reduction in middle cerebral artery blood velocity is not explained by PaCO2. Eur J Appl Physiol 2006; 96: 609–614. [DOI] [PubMed] [Google Scholar]
- 40.Lewis PM, Smielewski P, Rosenfeld JV, et al. A continuous correlation between intracranial pressure and cerebral blood flow velocity reflects cerebral autoregulation impairment during intracranial pressure Plateau waves. Neurocrit Care 2014; 21: 514–525. [DOI] [PubMed] [Google Scholar]
- 41.Minhas JS, Panerai RB, Swienton D, et al. Feasibility of improving cerebral autoregulation in acute intracerebral hemorrhage (BREATHE-ICH) study: Results from an experimental interventional study. Int J Stroke 2020; 15: 627–637. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Czosnyka M, Donnelly J, et al. Comparison of frequency and time domain methods of assessment of cerebral autoregulation in traumatic brain injury. J Cereb Blood Flow Metab 2015; 35: 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahdi A, Nikolic D, Birch AA, et al. At what data length do cerebral autoregulation measures stabilise? Physiol Meas 2017; 38: 1396–1404. [DOI] [PubMed] [Google Scholar]
- 44.Chi NF, Wang CY, Chan L, et al. Comparing different recording lenghts of dynamic cerebral autoregulation: 5 versus 10 minutes. BioMed Res Int 2018; 7803426: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Guo ZN, Simpson DM, et al. A data-driven approach to transfer function analysis for superior discriminative power: optimized assessment of dynamic cerebral autoregulation. IEEE J Biomed Health Inform 2021; 25: 909–921. [DOI] [PubMed] [Google Scholar]
- 46.Intharakham K, Panerai RB, Katsogridakis E, et al. Can we use short recordings for assessment of dynamic cerebral autoregulation? A sensitivity analysis study in acute ischaemic stroke and healthy subjects. Physiol Meas 2019; 40: 085002. [DOI] [PubMed] [Google Scholar]
- 47.Panerai RB, Intharakham K, Haunton VJ, et al. Chasing the evidence: the influence of data segmentation on estimates of dynamic cerebral autoregulation. Physiol Meas 2020; 41: 035006. [DOI] [PubMed] [Google Scholar]
- 48.Barnes SC, Ball N, Haunton VJ, et al. How many squat-stand manoeuvres to assess dynamic cerebral autoregulation? Eur J Appl Physiol 2018; 118: 2377–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burma JS, Miutz LN, Newel KT, et al. What recording duration is required to provide physiologically valid and reliable dynamic autoregulation transfer function analysis estimates? Physiol Meas 2021; 42; doi:10.1088/1361-6579/abf1af. [DOI] [PubMed] [Google Scholar]
- 50.Deegan BM, Serrador JM, Nakagawa K, et al. The effect of blood pressure calibrations and transcranial doppler signal loss on transfer function estimates of cerebral autoregulation. Med Eng Phys 2011; 33: 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smirl JD, Hoffman K, Tzeng YC, et al. Methodological comparison of active- and passive-driven oscillations in blood pressure: implications for the assessment of cerebral pressure-flow relationships. J Appl Physiol 2015; 119: 487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Llwyd O, Haunton VJ, Salinet ASM, et al. Can we assess dynamic cerebral autoregulation in stroke patients with high rates of cardiac ectopicity? Med Biol Eng Comput 2019; 57: 2731–2739. [DOI] [PubMed] [Google Scholar]
- 53.Mahdi A, Rutter EM, Payne SJ. Effects of non-physiological blood pressure artefacts on cerebral autoregulation. Med Eng Phys 2017; 47: 218–221. [DOI] [PubMed] [Google Scholar]
- 54.Welch PD. The use of the fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans Audio Electroacoust 1967; 15 : 70–73. [Google Scholar]
- 55.Bryant JED, Birch AA, Panerai RB, et al. Estimating confidence intervals for cerebral autoregulation: a parametric bootstrap approach. Physiol Meas 2021; 4210.1088/1361-6579/ac27b8. [DOI] [PubMed] [Google Scholar]
- 56.Panerai RB, Haunton VJ, Minhas JS, et al. Inter-subject analysis of transfer function coherence in studies of dynamic cerebral autoregulation. Physiol Meas 2018; 39: 125006. [DOI] [PubMed] [Google Scholar]
- 57.Panerai RB, Haunton VJ, Hanby MF, et al. Statistical criteria for estimation of the cerebral autoregulation index (ARI) at rest. Physiol Meas 2016; 37: 661–680. [DOI] [PubMed] [Google Scholar]
- 58.Panerai RB, Eames PJ, Potter JF. Multiple coherence of cerebral blood flow velocity in humans. Am J Physiol Heart Circ Physiol 2006; 291: H251–H259. [DOI] [PubMed] [Google Scholar]
- 59.Reehal N, Cummings S, Mullen MT, et al. Differentiating dynamic cerebral autoregulation across vascular territories. Front Neurol 2021; 12: 653167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Imholz BPM, Wieling W, Langewouters GJ, et al. Continuous finger arterial pressure: utility in the cardiovascular laboratory. Clin Auton Res 1991; 1: 43–53. [DOI] [PubMed] [Google Scholar]
- 61.Martina JR, Westerhof BE, van Goudoever J, et al. Noninvasive continuous arterial blood pressure monitoring with nexfin(R). Anesthesiology 2012; 116: 1092–1103. [DOI] [PubMed] [Google Scholar]
- 62.Sheriff F, Castro P, Kozberg M, et al. Dynamic cerebral autoregulation post endovascular thrombectomy in acute ischemic stroke. Brain Sci 2020; 10: 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Panerai RB, Robinson TG, Minhas JS. The upper frequency limit of dynamic cerebral autoregulation. J Physiol 2019; 597: 5821–5833. [DOI] [PubMed] [Google Scholar]
- 64.Katsogridakis E, Simpson DM, Bush G, et al. Revisiting the frequency domain: the multiple and partial coherence of cerebral blood flow velocity. Physiol Meas 2016; 37: 1056–1073. [DOI] [PubMed] [Google Scholar]
- 65.Peng T, Rowley AB, Ainslie PN, et al. Multivariate system identification for cerebral autoregulation. Ann Biomed Eng 2008; 36: 308–320. [DOI] [PubMed] [Google Scholar]
- 66.Tzeng YC, Ainslie PN, Cooke WH, et al. Assessment of cerebral autoregulation: the quandary of quantification. Am J Physiol Heart Circ Physiol 2012; 303: H658–H671. [DOI] [PubMed] [Google Scholar]
- 67.Liu J, Simpson DM, Panerai RB. Point-Counterpoint: Transfer function analysis of dynamic cerebral autoregulation: to band or not to band? J Cereb Blood Flow Metab 2022; : 0271678X2210984. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Panerai RB, White RP, Markus HS, et al. Grading of cerebral dynamic autoregulation from spontaneous fluctuations in arterial blood pressure. Stroke 1998; 29: 2341–2346. [DOI] [PubMed] [Google Scholar]
- 69.Barnes SC, Ball N, Haunton VJ, et al. Randon squat-stand maneuvers: a novel approach for assessment of dynamic cerebral autoregulation? J Appl Physiol 2017; 123: 558–566. [DOI] [PubMed] [Google Scholar]
- 70.Panerai RB, Eames PJ, Potter JF. Variability of time-domain indices of dynamic cerebral autoregulation. Physiol Meas 2003; 24: 367–381. [DOI] [PubMed] [Google Scholar]
- 71.Angarita-Jaimes N, Kouchakpour H, Liu J, et al. Optimising the assessment of cerebral autoregulation from black box models. Med Eng Phys 2014; 36: 607–612. [DOI] [PubMed] [Google Scholar]
- 72.Xiong L, Tian G, Lin W, et al. Is dynamic cerebral autoregulation bilaterally impaired after unilateral acute ischemic stroke? J Stroke Cerebrovasc Dis 2017; 26: 1081–1087. [DOI] [PubMed] [Google Scholar]
- 73.Bergman L, Cluver C, Carlberg N, et al. Cerebral perfusion pressure and autoregulation in eclampsia – a case control study. Am J Obstet Gynecol 2021; 225: 185.e1-185–e9. [DOI] [PubMed] [Google Scholar]
- 74.Guo ZN, Xing Y, Liu J, et al. Compromised dynamic cerebral autoregulation with a right-to-left shunt: a potential mechanism of migraine and criptogenic stroke. PLOS One 2014; 9: e104849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Subudhi AW, Panerai RB, Roach RC. Effects of hypobaric hypoxia on cerebral autoregulation. Stroke 2010; 41: 641–646. [DOI] [PubMed] [Google Scholar]
- 76.Ma H, Guo ZN, Liu J, et al. Temporal course of dynamic cerebral autoregulation in patients with intracerebral hemorrhage. Stroke 2016; 47: 674–681. [DOI] [PubMed] [Google Scholar]
- 77.Birch AA, Dirnhuber MJ, Hartley-Davies R, et al. Assessment of autoregulation by means of periodic changes in blood pressure. Stroke 1995; 26: 834–837. [DOI] [PubMed] [Google Scholar]
- 78.Claassen JAHR, Levine BD, Zhang R. Dynamic cerebral autoregulation during repeated squat-stand maneuvers. J Appl Physiol 2009; 106: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Drapeau A, Labrecque L, Imhoff S, et al. Six weeks of high-intensity interval training to exhaustion attenuates dynamic cerebral autoregulation without influencing resting cerebral blood velocity in young fit men. Physiol Rep 2019; 7: e14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Labrecque L, Rahimaly K, Imhoff S, et al. Dynamic cerebral autoregulation is attenuated in young fit women. Physiol Rep 2019; 7: e13984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Labrecque L, Drapeau A, Rahimaly K, et al. Dynamic cerebral autoregulation and cerebrovascular carbon dioxide reactivity in middle and posterior cerebral arteries in young endurance-trained women. J Appl Physiol 2021; 130: 1724–1735. [DOI] [PubMed] [Google Scholar]
- 82.Labrecque L, Rahimaly K, Imhoff S, Paquette M, Le Blanc O, Malenfant S, et al. Diminished dynamic cerebral autoregulatory capacity with forced oscillations in mean arterial pressure with elevated cardiorespiratory fitness. Physiol Rep 2017; 5: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Malenfant S, Brassard P, Paquette M, et al. Compromised cerebrovascular regulation and cerebral oxygenation in pulmonary arterial hypertension. J Am Heart Assoc 2017; 6: e006126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aengevaeren VL, Claassen JA, Levine BD, et al. Cardiac baroreflex function and dynamic cerebral autoregulation in elderly masters athletes. J Appl Physiol (1985) 2013; 114: 195–202. [DOI] [PubMed] [Google Scholar]
- 85.Klein T, Bailey TG, Wollseiffen P, et al. The effect of age on cerebral blood flow responses during repeated and sustained stand to sit transitions. Physiol Rep 2020; 8: 314421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Beek AHEA, Rikkert O, Pasman JW, et al. Dynamic cerebral autoregulation in the old using a repeated sit-stand maneuver. Ultrasound Med Biol 2010; 36: 192–201. [DOI] [PubMed] [Google Scholar]
- 87.Brothers RM, Zhang R, Wingo JE, et al. Effects of heat stress on dynamic autoregulation during large fluctuations in arterial blood pressure. J Appl Physiol 2009; 107: 1722–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tzeng YC, Chan GS, Willie CK, et al. Determinants of human cerebral pressure-flow velocity relationships: new insights from vascular modelling and Ca(2)(+) channel blockade. J Physiol 2011; 589: 3263–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Diehl RR, Linden D, LüCke D, et al. Phase relationship between cerebral blood flow velocity and blood pressure. A clinical test of autoregulation. Stroke 1995; 26: 1801–1804. [DOI] [PubMed] [Google Scholar]
- 90.Reinhard M, Muller T, Guschlbauer B, et al. Transfer function analysis for clinical evaluation of dynamic cerebral autoregulation – a comparison between spontaneous and respiratory-induced oscillations. Physiol Meas 2003; 24: 27–43. [DOI] [PubMed] [Google Scholar]
- 91.Elting JW, Aries MJH, van der Hoeven JH, et al. Reproducibility and variability of dynamic cerebral autoregulation during passive cycling leg raising. Med Eng Phys 2014; 36: 585–591. [DOI] [PubMed] [Google Scholar]
- 92.Katsogridakis E, Bush G, Fan L, et al. Detection of impaired cerebral autoregulation improves by increasing arterial blood pressure variability. J Cereb Blood Flow Metab 2013; 33: 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carey BJ, Panerai RB, Potter JF. Effect of aging on dynamic cerebral autoregulation during head-up tilt. Stroke 2003; 34: 1871–1875. [DOI] [PubMed] [Google Scholar]
- 94.Castro P, Freitas J, Santos R, et al. Indexes of cerebral autoregulation do not reflect impairment in syncope: insights from head-up tilt test of vasovagal an autonomic failure subjects. Eur J Appl Physiol 2017; 117: 1817–1831. [DOI] [PubMed] [Google Scholar]
- 95.Herrington BA, Thrall SF, Mann LM, et al. The effect of steady-state CO2 on regional brain blood flow responses to increases in blood pressure via the cold pressor test. Auton Neurosci 2019; 222: 102581. [DOI] [PubMed] [Google Scholar]
- 96.Panerai RB, Dawson SL, Eames PJ, et al. Cerebral blood flow velocity response to induced and spontaneous sudden changes in arterial blood pressure. Am J Physiol Heart Circ Physiol 2001; 280: H2162–H2174. [DOI] [PubMed] [Google Scholar]
- 97.Washio T, Watanabe H, Ogoh S. Dynamic cerebral autoregulation in anterior and posterior cerebral circulation during cold pressor test. J Physiol Sci 2020; 70: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nogueira RC, Bor-Seng-Shu E, Santos MR, et al. Dynamic cerebral autoregulation changes during Sub-maximal handgrip maneuver. PLoS ONE 2013; 8: e70821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Castro P, Santos R, Freitas J, et al. Autonomic dysfunction affects dynamic cerebral autoregulation during valsalva maneuver: comparison between healthy and autonomic dysfunction subjects. J Appl Physiol (1985) 2014; 117: 205–213. [DOI] [PubMed] [Google Scholar]
- 100.Perry BG, Cotter JD, Mejuto G, et al. Cerebral hemodynamics during graded Valsalva maneuvers. Front Physiol 2014; 5: 1–7. article 349: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wright AD, Smirl JD, Bryk K, et al. Systolic and diastolic regulation of the cerebral pressure-flow relationship differentially affected by acute sport-related concussion. Acta Neurochir Suppl 2018; 126: 303–307. [DOI] [PubMed] [Google Scholar]
- 102.Smirl JD, Wright AD, Ainslie PN, et al. Differential systolic and diastolic regulation of the cerebral pressure-flow relationship during squat-stand manoevres. Acta Neurochir 2018; 126: 263–268. [DOI] [PubMed] [Google Scholar]
- 103.Brassard P, Ferland-Dutil H, Smirl JD, et al. Evidence for hysteresis in the cerebral pressure-flow relationship in healthy men. Am J Physiol Heart Circ Physiol 2017; 312: H701–H704. [DOI] [PubMed] [Google Scholar]
- 104.Panerai RB, Barnes SC, Nath M, et al. Directional sensitivity of dynamic cerebral autoregulation in squat-stand maneuvers. Am J Physiol Regul Integ Comp Physiol 2018; 315: R730–R740. [DOI] [PubMed] [Google Scholar]
- 105.Tzeng YC, Willie CK, Atkinson G, et al. Cerebrovascular regulation during transient hypotension and hypertension in humans. Hypertension 2010; 56: 268–273. [DOI] [PubMed] [Google Scholar]
- 106.Salinet AS, Silva NC, Caldas J, et al. Impaired cerebral autoregulation and neurovascular coupling in Middle cerebral artery stroke: Influence of severity? J Cereb Blood Flow Metab 2019; 39: 2277–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Depreitere B, Citerio G, Smith M, et al. Cerebrovascular autoregulation monitoring in the management of adult severe traumatic brain injury: a delphi consensus of clinicians. Neurocrit Care 2021; 34: 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Panerai RB, Kerins V, Fan L, et al. Association between dynamic cerebral autoregulation and mortality in severe head injury. Br J Neurosurg 2004; 18: 471–479. [DOI] [PubMed] [Google Scholar]
- 109.Caldas JR, Panerai RB, Haunton VJ, et al. Cerebral blood flow autoregulation in ischemic heart failure. Am J Physiol Regul Integr Comp Physiol 2017; 312: R108–R113. [DOI] [PubMed] [Google Scholar]
- 110.Carey BJ, Manktelow BN, Panerai RB, et al. Cerebral autoregulatory responses to head-up tilt in normal subjects and patients with recurrent vasovagal syncope. Circulation 2001; 104: 898–902. [DOI] [PubMed] [Google Scholar]
- 111.van Veen TR, Panerai RB, Haeri S, et al. Cerebral autoregulation in normal pregnancy and preeclampsia. Obstet Gynecol 2013; 122: 1064–1069. [DOI] [PubMed] [Google Scholar]
- 112.Caldas JR, Panerai RB, Bor-Seng-Shu E, et al. Dynamic cerebral autoregulation: a marker of post-operative delirium? Clin Neurophysiol 2019; 130: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Caldas JR, Passos RH, Ramos JGR, et al. Dynamic autoregulation is impaired in circulatory shock. Shock 2020; 54: 183–189. [DOI] [PubMed] [Google Scholar]
- 114.Chen J, Liu J, Duan L, et al. Impaired dynamic cerebral autoregulation in moyamoya disease. CNS Neurosci Ther 2013; 19: 638–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nogueira RC, Lam MY, Llwyd O, et al. Cerebral autoregulation and response to intravenous thrombolysis for acute ischemic stroke. Sci Rep 2020; 10: 10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mol A, Meskers CGM, Sanders ML, et al. Cerebral autoregulation assessed by near-infrared spectroscopy: validation using transcranial doppler in patients with controlled hypertension, cognitive impairment and controls. Eur J Appl Physiol 2021; 121: 2165–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.de Heus RAA, de Jong DLK, Sanders ML, et al. Dynamic regulation of cerebral blood flow in patients with Alzheimer disease. Hypertension 2018; 72: 139–100. [DOI] [PubMed] [Google Scholar]
- 118.Haubrich C, Pies K, Dafotakis M, et al. Transcranial doppler monitoring in Parkinson's disease: cerebrovascular compensation of orthostatric hypotension. Ultrasound Med Biol 2010; 36: 1581–1587. [DOI] [PubMed] [Google Scholar]
- 119.Junejo RT, Braz ID, Lucas SJE, et al. Neurovascular coupling and cerebral autoregulation in atrial fibrillation. J Cereb Blood Flow Metab 2020; 40: 1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu Y, Birch AA, Allen R. Dynamic cerebral autoregulation assessment using an ARX model: comparative study using step response and phase shift analysis. Med Eng Phys 2003; 25: 647–653. [DOI] [PubMed] [Google Scholar]
- 121.Simpson DM, Panerai RB, Evans DH, et al. A parametric approach to measuring cerebral blood flow autoregulation from spontaneous variations in blood pressure. Ann Biomed Eng 2001; 29: 18–25. [DOI] [PubMed] [Google Scholar]
- 122.Giller CA, Mueller M. Linearity and non-linearity in cerebral hemodynamics. Med Eng Phys 2003; 25: 633–646. [DOI] [PubMed] [Google Scholar]
- 123.Mahdi A, Nikolic D, Birch AA, et al. Increased blood pressure variability upon standing improves reproducibility of cerebral autoregulation indices. Med Eng Phys 2017; 47: 151–158. [DOI] [PubMed] [Google Scholar]
- 124.Kostoglou K, Wright AD, Smirl JD, et al. Dynamic cerebral autoregulation in young athletes following concussion. 38th ann int conf. IEEE Eng Med Biol Soc 2016; Aug: 696–699. [DOI] [PubMed] [Google Scholar]
- 125.Kostoglou K, Debert CT, Poulin MJ, et al. Nonstationary multivariate modeling of cerebral autoregulation during hypercapnia. Med Eng Phys 2014; 36: 592–600. [DOI] [PubMed] [Google Scholar]
- 126.Panerai RB, Eyre M, Potter JF. Multivariate modeling of cognitive-motor stimulation on neurovascular coupling: transcranial doppler used to characterize myogenic and metabolic influences. Am J Physiol Regul Integr Comp Physiol 2012; 303: R395–R407. [DOI] [PubMed] [Google Scholar]
- 127.Panerai RB, Salinet ASM, Robinson TG. Contribution of arterial blood pressure and PaCO2 to the cerebrovascular responses to motor stimulation. Am J Physiol Heart Circ Physiol 2012; 302: H459–H466. [DOI] [PubMed] [Google Scholar]
- 128.Marmarelis VZ, Shin D, Zhang R. Linear and nonlinear modeling of cerebral flow autoregulation using principal dynamic modes. Open Biomed Eng J 2012; 6: 42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brunser AM, Silva C, Cárcamo D, et al. Transcranial doppler in a Hispanic-Mestizo population with neurological diseases: a study of sonographic window and its determinants. Brain Behav 2012; 2: 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kwon JH, Kim JS, Kang DW, et al. The thickness and texture of temporal bone in brain CT predict acoustic window failure of transcranial doppler. J Neuroimag 2006; 16: 347–352. [DOI] [PubMed] [Google Scholar]
- 131.Nader JA, Andrade ML, Espinosa V, et al. Technical difficulties due to poor acoustic insonation during transcranial doppler recordings in Amerindians and individuals of European origin. A comparative study. Eur Neurol 2015; 73: 230–232. [DOI] [PubMed] [Google Scholar]
- 132.Wijnhoud AD, Franckena M, van der Lugt A, et al. Inadequate acoustical temporal bone window in patients with a transient ischemic attack or minor stroke: role of skull thickness and bone density. Ultrasound Med Biol 2008; 34: 923–929. [DOI] [PubMed] [Google Scholar]
- 133.Czosnyka M, Smielewski P, Piechnik SK, et al. Clinical significance of cerebral autoregulation. Acta Neurochir Suppl 2002; 81: 117–119. [DOI] [PubMed] [Google Scholar]
- 134.Bright MG, Croal PL, Blockley NP, et al. Multiparametric measurement of cerebral physiology using calibrated fMRI. Neuroimage 2019; 187: 128–144. [DOI] [PubMed] [Google Scholar]
- 135.Elting JW, Tas J, Aries MJH, et al. Dynamic cerebral autoregulation estimates derived from near infrared spectroscopy and transcranial doppler are similar after correction for transit time and blood flow and blood volume oscillations. J Cereb Blood Flow Metab 2020; 40: 135–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Burley CV, Mullinger KJ, Thomas KN, et al. Imaging cerebral blood flow for brain health measurement. In: Della Sala S (ed.) Encyclopedia of behavioral Neuroscience, 2nd Edition. Oxford: Elsevier Science, 2021, pp.126–135.
- 137.Selb J, Boas DA, Chan ST, et al. Sensitivity of near-infrared spectroscopy and diffuse correlation spectroscopy to brain hemodynamics: simulations and experimental findings during hypercapnia. Neurophoton 2014; 1: 015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kainerstorfer JM, Sassaroli A, Tgavalekos KT, et al. Cerebral autoregulation in the microvasculature measured with near-infrared spectroscopy. J Cereb Blood Flow Metab 2015; 35: 959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Saeed NP, Horsfield MA, Panerai RB, et al. Measurement of cerebral blood flow responses to the thigh cuff maneuver: a comparison of TCD with a novel MRI method. J Cereb Blood Flow Metab 2011; 31: 1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Panerai RB, Jara JL, Saeed NP, et al. Dynamic cerebral autoregulation following acute ischaemic stroke: Comparison of transcranial doppler and magnetic resonance imaging techniques. J Cereb Blood Flow Metab 2016; 36: 2194–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gerlach DA, Manuel J, Hoff A, et al. Novel approach to elucidate human baroreflex regulation at the brainstem level: pharmacological testing during fMRI. Front Neurosci 2019; 13: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Madureira JB, Castro P, Azevedo E. Demographic and systemic hemodynamic influences in mechanisms of cerebrovascular regulation in healthy adults. J Stroke Cerebrovasc Dis 2017; 26: 500–508. [DOI] [PubMed] [Google Scholar]
- 143.Patel N, Panerai RB, Haunton VJ, Katsogridakis E, et al. The Leicester cerebral haemodynamics database: normative values and the influence of age and sex. Physiol Meas 2016; 37: 1485–1498. [DOI] [PubMed] [Google Scholar]
- 144.Beishon L, Clough RH, Kadicheeni M, et al. Vascular and haemodynamic issues of brain ageing. Pflugers Arch 2021; 473: 735–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Panerai RB, Haunton VJ, Llwyd O, et al. Cerebral critical closing pressure and resistance-area product: the influence of dynamic cerebral autoregulation, age and sex. J Cereb Blood Flow Metab 2021; 41: 2456–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Smirl JD, Hoffman K, Tzeng YC, et al. Relationship between blood pressure and cerebral blood flow during supine cycling: influence of aging. J Appl Physiol 2016; 120: 552–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Castro P, Serrador JM, Rocha I, et al. Efficacy of cerebral autoregulation in early ischemic stroke predicts smaller infarcts and better outcomes. Front Neurol 2017; 8: 1–5. article 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Salinet J, Moura FS, Romaneli R, et al. CAAos platform: an integrated platform for analysis of cerebral hemodynamics data. Physiol Meas 2021; 42; 10.1088/1361-6579/ac0c0b. [DOI] [PubMed] [Google Scholar]
- 149.Simpson DM. Matlab TFA function, http://car-net/content/resources 2017; Elucimed: Ensemble-R 1st Edition, Wellington New Zealand, http://elucimed.com/ensemble-r/; Olsen MH: R TFA package, http://car-net/content/resources 2021. (last accessed 26 July 2022).
- 150.Guo W, Ma H, Liu J, et al. Dynamic cerebral autoregulation remains stable during the daytime (8 a.m. to 8 p.m.) in healthy adults. Front Physiol 2018; 9: 1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221119760 for Transfer function analysis of dynamic cerebral autoregulation: a CARNet white paper 2022 update by Ronney B Panerai, Patrice Brassard, Joel S Burma, Pedro Castro, Jurgen AHR Claassen, Johannes J van Lieshout, Jia Liu, Samuel JE Lucas, Jatinder S Minhas, Georgios D Mitsis, Ricardo C Nogueira, Shigehiko Ogoh, Stephen J Payne, Caroline A Rickards, Andrew D Robertson, Gabriel D Rodrigues, Jonathan D Smirl David M Simpson in Journal of Cerebral Blood Flow & Metabolism