Abstract
Neurodegeneration refers to the selective and progressive loss-of-function and atrophy of neurons, and is present in disorders such as Alzheimer’s, Huntington’s, and Parkinson’s disease. Although each disease presents with a unique pattern of neurodegeneration, and subsequent disease phenotype, increasing evidence implicates alterations in energy usage as a shared and core feature in the onset and progression of these disorders. Indeed, disturbances in energy metabolism may contribute to the vulnerability of neurons to apoptosis. In this review we will outline these disturbances in glucose metabolism, and how fatty acids are able to compensate for this impairment in energy production in neurodegenerative disorders. We will also highlight underlying mechanisms that could contribute to these alterations in energy metabolism. A greater understanding of these metabolism-neurodegeneration processes could lead to improved treatment options for neurodegenerative disease patients.
Keywords: Energy metabolism, Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, insulin resistance
Introduction
Neurodegenerative disorders, including Alzheimer’s disease (AD), Huntington’s disease (HD) and Parkinson’s disease (PD) are characterised by a progressive loss of specific neurons that results in both functional and cognitive deficits. 1 All three diseases display a unique pattern of neuronal loss, which is associated with different symptoms and pathological features. In AD, pyramidal neurons in the Ammon’s horn of the hippocampus are highly vulnerable to damage, and their loss leads to gradual deterioration of cognitive function and memory. 2 This degeneration is associated with abnormal extra-neuronal deposition of amyloid-β peptides (Aβ) in senile plaques and the intraneuronal accumulation of hyper-phosphorylated tau in neurofibrillary tangles (NFTs). 2 HD is an autosomal dominant genetic disorder caused by a longer than usual CAG expansion in exon 1 of the HTT gene, encoding an expanded polyglutamine (polyQ) tract near the N-terminus of the huntingtin protein. 3 Neuronal loss in HD is observed in the medium-sized projection neurons of the dorsal striatum, with symptoms including involuntary movements (chorea), cognitive decline and psychiatric symptoms. 3 PD is characterised by motor abnormalities such as tremors, muscular rigidity and bradykinesia, caused by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and the degeneration of projecting nerve fibres to the striatum. 4 The exact pathophysiological mechanisms underlying neuronal loss in these neurodegenerative disorders are poorly understood, although perturbations in glucose metabolism appear to be a common feature in all of these diseases. 1 The degenerating neurons in all three diseases are reported to be particularly vulnerable to metabolic stress for a variety of reasons, as previously reviewed by Muddapu et al. 1 In this review, we provide an overview of the common disturbances in both glucose uptake and metabolism in the aforementioned neurodegenerative disorders, and how increased fatty acid metabolism can restore energy supply. We also discuss the long-term disadvantages for the brain of relying on fatty acids as an alternate fuel source, and present hypotheses for other pathways that could be therapeutically targeted to restore energy homeostasis in these neurodegenerative disorders.
Glucose uptake and metabolism in neurodegeneration
Initially it was believed that the reduction of glucose uptake and metabolism was a secondary response to the atrophy and neuronal loss that occurs in these neurodegenerative disorders. However, increasing evidence suggests that changes in energy metabolism may occur prior to significant brain atrophy and the onset of clinical symptoms. This suggest that changes in glucose metabolism may increase the vulnerability of these cells and consequently contribute to neuronal loss. 5 The brain is comprised of several different cell types, all with distinctive metabolic phenotypes. Neurons are the highest energy demanding cell in the brain, consuming up to 80% of oxygen in the brain. 6 This alone suggests that neurons are highly sensitive to disruptions in glucose metabolism and mitochondrial dysfunction. Multiple neurodegenerative disorders including AD, HD and PD are commonly associated with a loss of glucose uptake and, perturbations in several glucose-related pathways including glycolysis, pentose phosphate pathway (PPP), and tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS). 6 As neurons have little regenerative capacity, it is of importance to reduce the local environment stressors to neurons that render them more vulnerable to death. 7 Understanding the mechanisms of metabolic disturbances during disease progression has become a major focus of recent research, and interventions in these processes may relieve the neurons from these metabolic stressors and reduced degeneration.
Alzheimer’s disease
Accumulation of Aβ and phosphorylated tau are believed to contribute to early cognitive decline, however the findings from one recent study in 551 AD patients suggests that the aggregation of these proteins alone may not be sufficient to develop clinical symptoms. 8 Interestingly, glucose hypometabolism appears to be crucial to further the cognitive decline between mild cognitive impaired and clinically diagnosed AD patients. 8 Using [18F]-fluoro-deoxyglucose (18FDG) positron emission tomography (PET) scans, various studies have revealed a correlation between cortical hypometabolism and cognitive decline in AD patients, even at early stages of disease.9–11 Glucose uptake is reduced by up to 45% in the hippocampus, parieto-temporal lobe, posterior cingulate cortex and frontal cortex of AD patients. 9 Young cognitively normal individuals with ApoE4 mutations showed similar patterns of reduced glucose uptake decades before the expected onset of clinical symptoms of early onset familial AD. 10 Furthermore, a 2-year longitudinal study indicated that ApoE4 carriers at risk for familial AD showed the greatest decline in glucose uptake in the parietal and temporal cortices, where there is extensive early neuropathological legions in patients with AD. 11 However, the results are more conflicting during the symptomatic stages of disease, with some studies indicating that ApoE4 is associated with decreased glucose metabolism, whilst others found no association. 12 As disturbed glucose metabolism can impair the functional capacity of neurons and subsequently contribute to neuronal death, this loss of glucose uptake in these memory-related cortical regions has been associated with cognitive impairments. 13 In a more recent longitudinal study, a reduction in glucose metabolism measured by FDG-PET imaging was strongly associated with cognitive decline in patients presenting with both Aβ and phosphorylated tau pathology. 14 Although these findings show that glucose hypometabolism in the central nervous system (CNS) may contribute to the functional and structural changes observed in AD, there are also conflicting results. A recent study of 232 Aβ-positive patients with mild cognitive impairment or AD, concluded that decreased FDG-PET correlated with local atrophy, suggesting it is a consequence rather than a contributing factor to neurodegeneration. 15 Furthermore, it must be noted that these studies were not sufficiently sensitive to detect glucose uptake at a cellular level. Thus, it cannot be determined which cells are contributing to the change in glucose utilisation.
Along with a decrease in glucose uptake, the expression of glucose transporters was also altered in autopsied AD brains. The expression of both GLUT1 and GLUT3 were decreased in the cerebral cortex of AD patients and animal models, with a loss of GLUT3 also found in the dentate gyrus of the hippocampus of patients.16,17 As GLUT3 is primarily expressed on neurons it is important to note that this loss in expression was still apparent after correcting for neuronal loss. 17 In contrast, GLUT2, which is exclusively expressed in astrocytes, was elevated over 2-fold in the frontal cortex, thus these changes may be more representative of the altered brain cellular composition at the late stages of disease. 16 The activities of several enzymes involved in glucose metabolism, downstream of uptake have been noted to be impaired in individuals with AD. Microarray analysis of post-mortem brains identified that 15 of the 51 genes associated with glycolytic, TCA cycle and oxidative phosphorylation were reduced in AD. 18 Specifically two regulatory enzymes in the glycolytic pathway, hexokinase and phosphofructokinase are reduced in the post-mortem brain.19,20 Thus, together with the finding of increased glucose accumulation in the brains of AD patients, 21 this supports the hypothesis that deficits in glycolytic activity may contribute to a loss of glucose usage and energy production in AD. However, as these studies were conducted in post-mortem brain samples, it is possible that these decreases in both the glucose transporters and enzyme activity could be due to either neuronal loss or a change in cellular composition within the brain, rather than an early pathological feature.
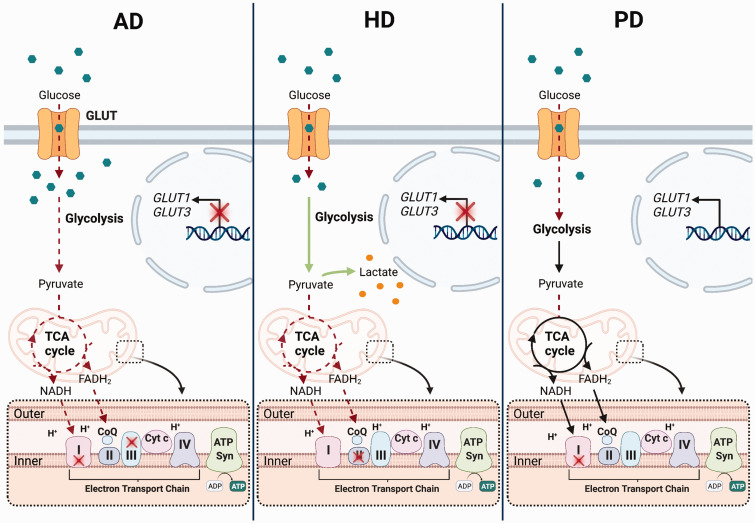
Additionally, mitochondrial dysfunction has been shown to contribute to metabolic deficits in AD.22–24 Both bioinformatic and protein expression studies have identified the oxidative phosphorylation pathway as one of the most affected processes in AD.22,24 Several studies have investigated the activities of the enzymes involved in oxidative phosphorylation. Collectively these studies have reported between a 10–50% loss in the activities of Complexes I–IV in different cortical regions, with most significant reductions observed in complex II, III and IV activity.23,24 However at the mRNA level, the results are more controversial, with some studies reporting a reduction in complex I, IV and V expression, whilst others have shown increases in complex III and IV.25,26 These changes have been found in both early and late-stage AD patients. 26 The activities of complexes I and III were also reduced by 45% in the brains of TgCRND8 mice, a model of AD which overexpresses the human mutant amyloid precursor protein (APP). 27 Moreover, the mitochondrial membrane potential was 40–60% lower in two cell lines (7WD4 and 7PA2) that are used as cellular models of AD, compared to Chinese hamster ovary cell controls. 28 In addition to the loss in ETC activity, a 41% and 57% reduction in the activities of key enzymes in the TCA cycle, pyruvate dehydrogenase complex (PDHc) and oxoglutarate dehydrogenase complex (OGDHc) were found in the post-mortem brain from AD patients. 29 Interestingly, the activity of transketolase, an enzyme involved in non-oxidative branch of the pentose phosphate pathway, was also lower in AD brains. 30 All three enzymes are multi-enzyme complexes, that include thiamine diphosphate as one of the co-enzymes. Thiamine diphosphate levels were reduced by up to 21% in autopsied brain samples from AD patients compared to controls, suggesting that this loss in thiamine diphosphate contributes to the loss of these enzyme complexes. 31 PDH E1α expression was also reduced by 19% in mitochondria isolated from the brains of 9-month-old 3xTg mice. 32 Overall, this suggests that glucose metabolism is reduced in AD due to dysfunction in glucose uptake, as well as reductions in glycolytic and TCA cycle activity (Figure 1). This impairment of glucose metabolism, and thus ATP production, increases the vulnerability of cells to functional and structural changes, and may contribute to neuronal apoptosis.
Figure 1.
Disturbances in cellular glucose metabolism in neurodegenerative disorders. In Alzheimer’s Disease (AD) the transcription of the glucose receptors, GLUT1 and GLUT3, are reduced in the brain which leads to a loss in glucose uptake. Despite this loss in uptake, glucose (green hexagons) accumulates in cells due to a decrease in metabolism via glycolysis and the Tricarboxylic Acid (TCA) cycle. This suggests that the production of the high energy metabolites, nicotinamide dinucleotide (NADH) and flavin adenine dinucleotide (FADH2), that feed into the electron transport chain (ETC) is reduced, ultimately lowering ATP production. In addition, the activities of Complex I and III of the electron transport chain are reduced in AD, further contributing to a loss in ATP synthesis. Similarly, in Huntington’s Disease (HD) glucose uptake is reduced in the brain, at least in part due to the loss of GLUT1/3 transcription. However, in contrast to AD, glycolytic flux is reported to be elevated in HD, with lactate (orange circles) accumulation also reported in the striatum. Elevated lactate concentrations may also be a consequence of the reduced entry and metabolism of pyruvate via the TCA cycle, due to impairments in the activities of several enzymes in this pathway. This, along with the loss of Complex II activity in the ETC, likely results in the loss of ATP synthesis in HD. Less is known about the changes in brain glucose metabolism in Parkinson’s Disease (PD) as compared to AD and HD. Glucose uptake is reported to be reduced in several regions of the brain in PD. However, the mechanisms that drive this loss in uptake are currently unknown. Downstream of uptake, there are no reports of specific changes to the rate of the glycolytic and TCA cycle, however pyruvate entry into the TCA cycle is reduced. Furthermore, Complex I activity is reduced in the brain, suggesting that the high energy substrate cannot feed into the ETC, and produce the proton gradient required for ATP synthesis. Red dotted arrows or red crosses indicate pathways that are reduced in each disease, whereas green arrows indicate pathways that are increased.
Huntington’s disease
Similar to AD, cellular glucose metabolism is also perturbed in the CNS in HD. Striatal glucose uptake, quantified by functional imaging studies using 18F-FDG PET scans, was reduced in symptomatic HD patients. 33 Reduced glucose uptake in caudate, putamen and thalamic regions were positively correlated with disease severity. 34 In newly diagnosed patients, this loss in glucose uptake was restricted to the frontal and inferior parietal cortex; however, in patients who had displayed symptoms for longer than 5 years, a global loss of glucose uptake was observed. 33 Both early symptomatic patients and asymptomatic mutant huntingtin (mHTT) protein carriers displayed reduced glucose uptake in the caudate nucleus and putamen, with the loss of glucose uptake correlating with the number of CAG repeats. 35 In addition, reduced striatal 18F-FDG uptake was found in 30-50% of mHTT carriers and chorea-free high-risk individuals, suggesting that the abnormalities in CNS glucose metabolism precedes the clinical or structural alterations in HD patients. 36 Although these studies are unable to determine what cells are responsible for this loss in glucose uptake, a study utilising primary cortical neuronal cultures from the HD140Q/140Q mouse model showed a reduction in glucose uptake. 37 Consistent with the results using in vivo imaging, glucose transporters were also reduced in post-mortem samples of HD patients. 38 The expression of GLUT1 and GLUT3 were reduced by three- and four-fold, respectively, in the post-mortem samples of caudate and cortex from patients with HD. 38 However, the expression of these transporters were unchanged in post-mortem samples from patients in the earlier stages of disease. Thus, this loss, in particularly of GLUT3 may be more indicative of the significant neuronal degeneration in these brain regions. 38 Furthermore, overexpression of human GLUT3 (hGLUT3) in drosophila HD models rescued the neurological dysfunction and increased life expectancy. 39 Overall this demonstrates that glucose uptake is reduced in the HD cortex, in part due to the loss of glucose transporter expression.
Alongside a loss in glucose uptake, glycolytic activity is impaired in HD. 40 Lactate accumulation, which suggests an increase in glycolytic flux, was also demonstrated in the striatum and occipital cortex of HD patients using localized 1H spectroscopy. Interestingly, lactate concentrations positively correlated with CAG repeat numbers. 41 This increased flux through glycolysis is not necessarily due to changes in the striatal neurons themselves. In fact, activated microglia and reactive astrocytes are both present in the HD striatum, and their levels correlate with disease severity in HD patients. 42 Both these cells are known to increase their glycolytic rates in these activated states, which suggests that these cell populations may be contributing to the increased glycolytic flux observed in HD.
Similarly to AD, mitochondrial metabolism has also been reported to be impaired in both patients and animal models of HD. Complex II activity and expression levels were shown to be reduced in HD mouse models and patients.43,44 These reductions were limited to the striatum, suggesting complex II activity could directly contribute to the neuronal vulnerability in HD. Further, inhibition of complex II via chronic administration of 3-NP in rats lead to the development of striatal lesions, along with HD-associated motor deficits such as bradykinesia and wobbling gait. 45 However, iPSC-derived striatal neurons and astrocytes from unaffected individuals showed no differences in mitochondrial mass, mitochondrial membrane potential or the activities of mitochondrial enzyme activities, suggesting that mitochondria may not be affected in the early stages of the disease process. 46 Along with deficits in the complex activity, a loss of the TCA cycle enzymes PDHc and OGDHc was also reported in post-mortem samples from HD patients, indicating that mitochondrial dysfunction is evident in HD similar to AD, at least in the later stages of disease (Figure 1).47,48
Parkinson’s disease
An early study found that a global loss in cerebral glucose uptake was observed in PD patients using 18F-FDG PET scans in comparison to healthy controls, with no selective metabolic changes in the striatum. 49 This loss in uptake was even more severe in PD patients with dementia. Both blood flow and glucose uptake were reduced on the contralateral side of the frontal cortex to the side of the body with muscle deficits in patients with unilateral disease. 49 Likewise, a more recent study has revealed widespread glucose hypometabolism in PD patients with mild to severe cognitive impairments in the parietal, occipital, temporal and frontal lobes. 50 Furthermore, parieto-occipital and temporal glucose hypometabolism was found to be correlated with poor performance in cognitive assessment. 51 However, this pattern of reduced glucose uptake could be detected even in patients without significant cognitive impairment. This suggests that altered cortical glucose metabolism can occur independently of cognitive decline and is further exacerbated as cognitive states worsen. Thus, like AD and HD, PD is associated with reduced brain glucose uptake in regions that are vulnerable to neuronal death. Interestingly, although a decline in glucose uptake is observed in PD and considered to be detrimental, pre-treatment with 2-deoxyglucose protected human dopaminergic neurons against rotenone-induced death. Moreover, 2-deoxyglucose treatment decreased the vulnerability of SNpc dopaminergic neurons to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced damage. 52 This response only seemed to occur when 2-deoxyglucose was administered prior to the toxic insult, therefore it is believed that the protection may be due to the conditioning of cells, which induces upregulation of alternative metabolic pathways, such as the pentose phosphate pathway. 52
To the best of our knowledge no data has been collected on the expression of glucose transporters or the activities of enzymes involved in glycolysis in PD. However, several studies have shown that enhancing glycolysis is beneficial in animal models of PD.53–55 Exogenous glucose 6-phosphate isomerase, a glycolytic enzyme, protects drosophila and primary mouse neurons from proteotoxicity induced by α-synuclein. 53 Furthermore, the anti-histamine drug meclizine, which increases glycolytic flux, protected against the neurotoxicity of 6-hydroxydopamine (6-OHDA) in SH-SY5Y cells and rat primary cortical neurons. 54 Most recently, the α1-adrenergic receptor blocker terazosin, which binds to and enhances the activity of phosphoglycerate kinase 1, and subsequently glycolysis, attenuated neurodegeneration and improved behavioural phenotypes in both the MPTP mouse, and 6-OHDA rat models of PD. 55 Terazosin or similar drugs were also shown to reduce the risk of developing PD by 12 to 37% in men, compared to those who received an α1-adrenergic receptor blockers that did not enhance glycolysis. 56 Overall, this shows that modifying and enhancing glycolytic flux may be neuroprotective in both in vitro and in vivo models of PD.
Mitochondrial dysfunction is also recognised as a disease modifier of PD. Complex I activity is reduced in the post-mortem brain of parkinsonian patients. The maximal respiration was also reduced by up to 25% in mitochondria isolated from the ventral midbrain and striatum of mice overexpressing human wild-type α-synuclein. 57 In the same study, Complex I activity was also reduced by 40% in mitochondria isolated from the ventral midbrain. 57 This loss of Complex I activity was specific to regions containing nigrostriatal dopaminergic neurons. 57 A significant decline in complex I activity was also observed when wild-type α-synuclein accumulated in mitochondria of cultured human dopaminergic neurons. This decline in activity was further exacerbated in cells that were incubated with α-synuclein carrying the A53T mutation, which is known to be present in familial PD patients, suggesting that complex I function is particularly vulnerable in PD. 58 The key role of complex I activity in the progression of PD was further supported by a study investigating MPTP-induced PD symptoms in patients that took illicit drugs. A metabolite of MPTP, 1-methyl-4-phenylpyridinium (MPP+) accumulates in the mitochondria of dopaminergic neurons and inhibits complex I activity, leading to the loss of ATP production and increased reactive oxygen species, resulting in dopaminergic neuron loss. 59 Genetically deleting the core subunit of Complex I, NADH – ubiquinone oxidoreductase core subunit S2 in the dopaminergic neurons of mice was sufficient to drive neuronal loss and the development of parkinsonian-like symptoms. 60 Despite this, patients with genetic complex I deficiencies rarely develop a parkinsonian phenotype, suggesting that dysfunction in oxidative phosphorylation alone is not sufficient to develop PD. 61 Like what is observed in AD, the activities of both PDHc and OGDHc are affected in PD. In the lateral third of the SNpc, OGDHc immunostaining was reduced, and correlated with the level of degeneration in post-mortem brain samples. 62 OGDHc activity was also reduced by approximately 50% in the cerebellum of PD patients, suggesting this loss in activity is not limited to the affected brain regions in PD. 63 Furthermore, pyruvate dehydrogenase alpha 1 (PDHA1) protein, which forms the core of the PDHc, is relocated from the mitochondrial matrix to Lewy bodies in the PD brain, which is likely to result in the loss of activity. 64 Thus, mitochondrial dysfunction is a common feature of neurodegenerative disorders, potentially reducing mitochondrial ATP production and consequently contributing to neuronal vulnerability in these diseases (Summarised in Figure 1).
Are the changes in glucose metabolism a cause or consequence of disease
AD, HD, and PD all show similar profiles in metabolic dysfunction, including reduced glucose uptake and mitochondrial dysfunction. If these disturbances are contributing to neuronal degeneration, it begs the question as to why such similar metabolic phenotypes results in different patterns of neurodegeneration, and consequently different clinical symptoms. Several reports suggest that protein aggregation, which is another common feature of these neurodegenerative diseases occurs prior to, and contributes to, the disturbances in energy metabolism, in particular mitochondrial dysfunction. In AD, Aβ deposition has been reported as the triggering event that induces impairments in glucose metabolism, brain atrophy and cognitive impairment. Several studies have shown that glucose uptake is reduced in neurons exposed to Aβ. In primary hippocampal cultures, Aβ25–35 reduced glucose uptake by inhibiting the docking and fusion of GLUT3 to the plasma membrane. 65 Another study also reported that this could be mediated through Aβ-induced oxidative stress. 66 The HTT protein which has been implicated in HD, has also been shown to regulate energy metabolism. Knocking out HTT in mouse embryonic stem cells severely limited ATP production via oxidative phosphorylation whilst increasing glycolytic activity, potentially as a compensatory mechanism, suggesting that wild-type HTT protein plays a role in regulating energy metabolism pathways. 67 The mHTT was also shown to negatively corelate with the ATP/ADP ratio in both mouse embryonic stem cells and human lymphoblasts, indicating that mHTT in HD can promote loss of energy metabolism. 68 However, another study found no change in ATP levels in knock-in embryonic stem cells containing one copy of humanised exon 1 with an extended polyglutamine tract (HTT-Q140/Q7). 67 HTT has also been shown to be localised to the mitochondrial outer membrane, with mHTT reportedly lowering mitochondrial membrane potential via defects in calcium handling. 69 Similarly, evidence indicates that α-synuclein also preferentially accumulates in mitochondria, and this accumulation leads to mitochondrial defects in PD. 70 Aggregated α-synuclein inhibited activity of both Complex I, which leads to reduced respiration and ATP production. 71 Furthermore, α-synuclein accumulation reduced oxygen consumption and reduced cell viability in neurons, astrocytes and skin-derived fibroblasts. 72 Overall, this suggests that protein aggregation is driving at least some of the metabolic disturbances observed in these neurodegenerative disorders.
The degenerating neurons in all three disorders are all uniquely vulnerable to metabolic stress as they typically require more energy. In AD, hippocampal neurons located in the CA1 region are the most vulnerable to degeneration as they have higher firing rates, which means they have a higher energy demand. 47 In HD, GABAergic striatal projection neurons are the initial cell type affected. These neurons are termed medium spiny neurons due to their long axons and spiny dendrites. These long axons may increase their vulnerability. Furthermore, these neurons usually maintain a hyperpolarised state, which requires high amounts of energy. 73 Finally, dopaminergic neurons in the SNpc characteristically have long unmyelinated axons with a large amount of synapses. This neuronal architecture would require large amounts of energy to maintain membrane potential and propagate action potentials. 74 Therefore, regardless of whether impaired glucose metabolism is causative or a consequence of other features of these neurodegenerative disorders, there is specific evidence that upregulating glucose metabolism can delay clinical symptoms. Therefore, it would be beneficial to further understand the complexities in the regulation of glucose metabolism and oxidative phosphorylation during neurodegeneration.
Lipids can replace glucose as a fuel in neurodegenerative disorders
It is evident that glucose metabolism is impaired in neurodegenerative disorders, via a multitude of mechanisms, including reduced glucose uptake, along with a loss in glycolytic flux and mitochondrial dysfunction. As the brain requires constant energy supply to maintain its functions, evidence suggests that the brain can adapt to using different fuel sources such as fatty acids and ketone bodies to support ATP production. 75 Fatty acids and ketones usually contribute to less than 5% of the brain’s metabolism. But during a prolonged fast, ketone bodies are elevated in the plasma and can contribute up to 60% of the brain’s energy requirements. 75 Therefore, approaches which support brain energetics, such as ketone treatments, could potentially slow disease progression that is due to energy deficiency.
Alzheimer’s disease
Many studies have investigated whether elevating ketone levels in the blood could improve symptoms in both AD patients and rodent models. A single dose of MCFAs, which are readily metabolised to ketone bodies in the liver, improved scores for the Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) and paragraph recall in ApoE4-negative AD patients. 76 These acute effects are assumed to be caused by the use of ketone bodies as an energy source. Another acute study found no cognitive improvement in 20 Japanese patients with mild-to-moderate AD following the ingestion of MCFAs. 77 However, in these same patients, treatment with 1 g/kg/day of MCFAs for 8 weeks improved some cognitive functions compared to their baseline scores, despite no increases in plasma βHB levels. 77 It must be noted that this study did not identify the participants according to ApoE4 genotype. Several other long term trials that did stratify patients based on their ApoE4 genotype determined that MCFA or βHB treatment was only beneficial for patients who were ApoE4-negative.78,79 A 90-day placebo-controlled trial of caprylic acid, a MCFA, in mild to moderate AD patients improved ADAS-Cog scores in ApoE4-negative patients. 78 Another cross-over study showed that 30-day MCFA supplementation in ApoE4 negative AD patients led to a greater improvement in ADAS-Cog scores than the placebo treatment. 79 It is suggested this genotype-dependent effect of MCFA treatment is due to the differences in pathophysiology between ApoE4 positive and negative patients. Mitochondrial function has been shown to be lower in ApoE4 positive individuals which may limit use of ketones. However, further research is required to elucidate the mechanisms that underlie this phenotype.
Huntington’s disease
Ketogenic diets and βHB supplementation have been trialled in rodent models of HD, with several studies showing promising responses. R6/2 mice fed a ketogenic diet from 6 weeks of life showed a 13-fold increase in plasma βHB concentrations, delay in weight loss and complete reversal of impairments in working memory in female R6/2 mice at 12 weeks of age. 80 Interestingly, the ketogenic diet had no effect on working memory in R6/2 males, however it must be noted that unlike in females, working memory was already impaired in males prior to the implementation of the diet. 80 So, it seems that the diet can only delay the onset of cognitive deficits in this model. A ketogenic diet rescued some motor deficits in R6/2 mice when fed from birth. 81 The mice fed a ketogenic diet travelled further and faster in the open field test, but the ketogenic diet had no effect on the latency to fall on the accelerating rotarod test. 81 Despite an increase in brain concentration of βHB, the ketogenic diet did not prevent brain atrophy in the R6/2 model. 81 In comparison, βHB infusion significantly delayed the onset of motor deficits in the R6/2 mouse model and extended lifespan by approximately 30% on average. 82 In the 3-NP model of HD, βHB infusion completely ameliorated striatal neuronal loss and partially improved locomotor activity. 82 Together this suggests that fatty acid supplementation is neuroprotective and able to partially slow disease progression in HD.
Parkinson’s disease
MCFA and βHB treatment improved symptoms in both patients and rodent models of PD. βHB increased survival by up to 60% in cultured neurons that were incubated with both MPP+ and rotenone.83,84 βHB also improved mitochondrial membrane potential and reduced cytochrome C release in a rotenone induced in vitro model of PD. 84 Twenty-four hour infusion of βHB using mini osmotic pumps also protected SNpc dopaminergic neurons against MPTP toxicity, and reduced motor abnormalities in mice. 85 OXPHOS is enhanced in these neurons via a mechanism independent of complex I activity, which circumvents the known deficits in complex I activity in PD. 85 This study also reported βHB treatment improved neuronal cellular respiration and ATP production, improved motor skills and elevated dopamine concentrations in the mesencephalon of mice following MPTP injections. 85 Octanoic acid, a MCFA, also reduced the MPTP-induced depletion of dopamine in the striatum. 86 Similar to βHB, octanoic acid is reported to be neuroprotective by increasing mitochondrial metabolic activity in the striatum. 86 In a recent study, PD patients underwent a 8-week dietary intervention, where they were administered either a low fat or ketogenic diet. 87 Interestingly, both diets led to improvements in non-motor and motor scores, assessed using the Movement Disorder Society – Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS). 87 However, the ketogenic diet improved non-motor scores significantly more than the low-fat diet, with the biggest differences found in fatigue, pain, daytime sleepiness and cognitive impairments. 87 This could possibly result from supplying neurons with an alternative energy source and thereby increasing ATP production, but further studies would be required to clarify the underlying causes of this improvement.
Harmful side effects of fatty acid metabolism in the brain
Even though increasing fatty acid metabolism has shown some positive outcomes in treating neurodegenerative disorders, there are two main reasons why relying on β-oxidation to fuel neurons long term may not be beneficial. Firstly, to generate the same amount of ATP, fatty acids require approximately 15% more oxygen than when using glucose as a fuel. 88 Thus, primarily using fatty acids as a fuel source increases the risk of hypoxia. Secondly, β-oxidation increases mitochondrial electron leakage, consequently increasing superoxide generation. 89 Fatty acids can also bind to the ETC complexes, which impairs their activity. This not only reduces ATP generation, but also increases the production of ROS. 88 Elevations in oxidative stress are known to induce cellular damage, impairment in the DNA repair system and mitochondrial dysfunction, all of which have been known as key factors in the development of neurodegenerative disorders. 90 Therefore, long-term reliance on fatty acid β-oxidation to produce ATP could be harmful, by increasing oxidative stress. Instead of elevating fatty acid and ketone body metabolism as a therapy for neurodegenerative disorders, it may be more beneficial to understand the mechanisms that are driving the loss in glucose uptake and metabolism and try to develop therapies that can directly restore glucose metabolism.
Does a loss of insulin signalling reduce glucose uptake in neurodegenerative disorders?
One potential mechanism that contributes to a loss of glucose uptake in neurodegenerative disorders is insulin resistance. Insulin is produced in the β-cells of pancreatic islets and is secreted in response to elevated blood glucose. 91 By binding to the insulin receptor, insulin facilitates glucose disposal in tissues such as adipose tissue and skeletal muscle. Insulin resistance occurs when there is a breakdown along this signalling pathway. 91 This loss in insulin signalling is not limited to the periphery. Although the brain was once considered to be an insulin-insensitive organ, more recent reports have found both insulin and its receptors localised to neurons in the brain. 92 This suggests that insulin signalling could play an important role in regulating glucose uptake and metabolism in these neurodegenerative disorders.
Recently, the role of phospholipids in insulin resistance has been receiving increased attention. It has been proposed that insulin resistance may be a consequence of lipid disorders such as abnormalities in cell membrane phospholipid composition. 93 Modifications of membrane composition, in particular phosphatidylcholine (PC), phosphatidylethanolamine (PE) and ceramides, alter membrane fluidity and as a consequence perturb the insulin signalling and the translocation of glucose transporters to the cell membrane. A change in the PC/PE ratios in cell membranes of various tissues can influence energy metabolism. Although it is yet to be determined the role that phospholipid composition plays in managing insulin resistance and glucose uptake in neurodegenerative disorder, studies have shown an accumulation of certain phospholipids can inhibit glucose uptake.
Alzheimer’s disease
Diabetes mellitus (specifically type II diabetes) has been proposed as a risk factor for AD and decline in cognitive function. 94 A longitudinal cohort study of 824 individuals identified a 65% increase in the risk of developing AD in individuals with diabetes mellitus when adjusted for age, sex, and educational level. Using a random effects model they found that diabetes mellitus was associated with lower levels of cognition, memory, and visuospatial ability. 95 However, others failed to show a correlation between type II diabetes and AD pathology.96,97 Furthermore, in several diabetic mouse models of AD, increased tau phosphorylation is observed. 98 Indeed, defects in insulin receptor function, induced in rats by intracerebroventricular injection of streptozotocin, led to impaired learning, memory and cognition, along with decreased glucose uptake and oxidative glucose metabolism. 99 Similarly, 3xTg-AD mice with a diabetic phenotype induced by streptozotocin had exacerbated memory impairments with greater accumulation of toxic Aβ and phosphorylated tau in the cortices and hippocampus. 100 Thus, it is evident that there is a connection between underlying mechanisms that regulate the diabetic and AD phenotypes.
An expanding body of evidence also suggests that insulin abnormalities and insulin resistance contribute to AD pathophysiology. 101 Higher plasma insulin concentrations and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) scores in healthy middle-aged people were shown to predict cognitive decline and increase the risk of developing AD later in life. 102 Furthermore, AD patients were shown to be glucose intolerant, despite increased plasma insulin concentrations in both fasting states and following an oral glucose tolerance test (OGTT). 103 Insulin resistance and glucose intolerance also promoted AD-like neuropathology in mouse models of AD. 104 Diet-induced insulin resistant Tg2576 mice were found to have a 2-fold increase in Aβ plaques in the hippocampus. This increase in Aβ plaques was correlated with an earlier decline in cognitive ability. 104 Peripheral insulin resistance is also reported to correlate with Aβ deposition in the frontal and temporal cortex. 105 However, it must be noted that some studies contradict these results, thus it is clear that the role that peripheral insulin resistance plays in the development and progression of AD is not completely understood.
There also appears to be a disruption in the relationship between central and peripheral insulin levels in AD patients. The ratio of CSF/serum insulin was reduced in AD patients compared to that of healthy controls, and this reduction negatively correlated with the severity of disease. 106 The expression of the insulin receptor (IR), insulin-like growth factor 1 (IGF-1), IGF-1 receptor (IGF-1R) were also reduced in post-mortem brain samples, suggesting that CNS insulin signalling is compromised in AD. 107 Along with this decline in the levels of expression of these receptors, their ability to bind to cell membranes may be impaired in AD. An increase in saturated fatty acid content in phospholipid species decreases membrane fluidity and reduces membrane bound insulin receptors. 108 In contrast, the presence of polyunsaturated fatty acids increases fluidity and has been associated with improved insulin sensitivity. 108 This shift from unsaturated towards saturated fatty acids, is also proposed to affect glucose transporters translocation to the cell membrane. 108 Polyunsaturated fatty acids were significantly reduced in both PC and PE species in the CSF of patients with AD, suggesting that cell membranes in AD have less fluidity. 109
Downstream signalling of the insulin receptor is also impaired in AD due to inactivation of insulin receptor substrate 1 (IRS-1), by serine phosphorylation. IRS-1 acts as an intermediary between IR/IGF-1R and the downstream phosphatidylinositol 3-kinase (PI3K)-protein kinase B (AKT) signalling cascade. 91 Thus, the loss of IR and IGF-1R expression, along with inactivation of IRS-1 suggests that neuronal insulin signalling is impaired in AD. Indeed, PI3K and AKT phosphorylation was reduced in post-mortem brain samples from AD patients. 110 Furthermore, the levels and activation of the insulin-PI3K-Akt signalling components correlated negatively with the level of tau phosphorylation and positively with tau O-GlcNAcylation. 110 This suggests that impaired insulin-PI3K-Akt signalling might contribute to neurodegeneration in AD through decreased O-GlcNAcylation and consequent tau hyperphosphorylation. Changes in cell membrane composition may also contribute to this loss in the insulin-PI3K-Akt signalling pathway. Ceramides, members of the sphingolipid family, have also been proposed to stabilise the cell membrane structure and modulate the distribution of receptors. Increased proportions of ceramides in the cell membrane antagonise insulin signalling by disrupting PI3K/Akt signalling, thus impairing glucose uptake. 111 Ceramide levels were shown to be elevated in autopsied brain tissue from AD patients, along with several enzymes involved in ceramide formation. Higher serum ceramides levels were associated with an increased risk of AD later in life. 112 Furthermore, inhibiting ceramide production protected neurons from Aβ-induced cell death. 112 Collectively, these results indicate that insulin resistance, due to a loss of insulin signalling, could play a role in the pathogenesis of AD (Figure 2).
Figure 2.
Mechanisms of insulin resistance in neurodegenerative disorders. Diabetes mellitus and insulin resistance are common features of the neurodegenerative disorders, AD, HD, and PD, however the underlying mechanisms that drive a loss in both insulin and glucose tolerance are unique for each disease. In AD, glucose-stimulated insulin secretion is elevated, leading to increased circulating insulin (green triangle) concentrations. Despite this increase in insulin secretion, blood glucose (blue hexagons) concentrations are elevated during an oral glucose tolerance test (OGTT, indicated by the glucometer), suggesting that glucose clearance is reduced in AD. Impairments in cellular insulin signalling contributes to this loss of glucose clearance, with the transcription of the insulin receptor (IR), insulin-like growth factor-1 (IGF-1) and IGF-1 receptor (IGF-1R) reduced in the brain, and likely leads to lower membrane-bound IR. Furthermore, serine phosphorylation of the insulin receptor substrate 1 (IRS-1) proteins reduces their ability to attract phosphatidylinositol 3-kinase (PI3K), thereby minimizing its activation and lowering the PI3K-protein kinase B (AKT) signalling cascade. This ultimately leads to a reduction in GLUT4 translocation to the cell membrane. Conversely, in HD insulin secretion rather than cellular insulin signalling is the primary contributor to the development of the diabetes-related phenotype. Both pancreatic β-cell mass and insulin production are reduced in HD. This leads to a reduction in glucose-stimulated insulin secretion, and lower circulating insulin concentrations. It is proposed that this loss in insulin secretion drives the higher blood glucose levels during an OGTT. As there is less insulin to bind to the IR and initiate the PI3K-AKT signalling pathway, this ultimately lowers GLUT4 translocation to the cell membrane, thereby reducing cellular glucose uptake. Glucose clearance is also reduced in PD, with an increase in blood glucose measured following an OGTT, despite no changes in insulin secretion. Therefore, it is proposed that a reduction in cellular insulin signalling drives this glucose intolerance. IR immunoreactivity is reduced in the substantia nigra, which likely contributes to a loss of insulin signalling. Furthermore, similar to AD, higher levels of IRS-2 serine phosphorylation are found in PD, which would reduce the downstream PI3K-AKT phosphorylation cascade. Thus, like in AD and HD, GLUT4 translocation to the cell membrane is reduced, consequently leading to a loss in glucose uptake. Red dotted arrows or red crosses indicate pathways that are reduced in each disease, whereas green arrows indicate pathways that are increased.
Huntington’s disease
Diabetic-like symptoms have also been identified in HD patients as well as in rodent models. 113 The correlation between diabetes and HD has been documented in two early studies that found a higher prevalence of diabetes mellitus in HD patients than in control population.114,115 It was also suggested that affected relatives of a HD proband with diabetes were 7 times more likely to develop diabetes than the non-HD affected relatives. 115 However, there are also studies that show contradictory conclusions associated with glucose homeostasis in HD patients. Two separate studies involving 21 HD and 21 age-matched control subjects demonstrated no difference in glucose and insulin responses to an OGTT.116,117
Unlike in AD, insulin secretion rather than insulin signalling seemed to be the primary contributor to the development of the diabetes-related phenotype in HD. Several studies have shown that at least some patients with HD display abnormal glucose tolerance during an OGTT.118,119 In one study, although patients showed similar glucose tolerance, they were shown to be less insulin sensitive using two different indexes, the HOMA-IR and the insulin sensitivity index. 120 Early-phase insulin secretion was also reduced in these HD patients, and it suggested that this loss of insulin secretion underlies the loss in insulin sensitivity. 120 Boesgaard et al. (2009) demonstrated that the capacity of β-cells to release insulin in response to glucose was reduced in HD patients, and this was negatively correlated with the length of the polyQ region in mHTT. 118 End-stage R6/2 mice were hyperglycaemic and hypo-insulinemic, and both basal and glucose-stimulated insulin secretion were reduced in isolated islets. 121 Both β-cell mass and insulin content within the pancreatic islet cells were significantly reduced in R6/2 mice at end-stage compared to wild-type mice. 121 Insulin mRNA levels in the pancreas were also shown to be approximately 50% and 25% of that in wild-types at 8 weeks and 12 weeks of age, respectively. 122 This loss in insulin production and secretion is further supported by the outcomes of various diabetic drug treatments in mouse models of HD. R6/2 mice responded to glibenclamide, which stimulates insulin release. However, no response was observed when mice were treated with rosiglitazone, which increased peripheral insulin sensitivity. 123 Exendin-4 treatment, another anti-diabetic drug that indirectly stimulates insulin release was also shown to be beneficial in the N171-82Q mouse model of HD. Exendin-4 alleviated the disturbed peripheral glucose metabolism and impaired pancreatic function, as well as delayed the onset of mortality in these mice, most likely due to a combination of peripheral and central effects of exendin-4. 124 Together, these findings suggest that glucose uptake might be impaired due to the loss of pancreatic β-cell mass and insulin secretion in HD (Figure 2).
Although the impairments in insulin signalling in HD seem to be due to a loss of insulin synthesis and release rather than insulin resistance, a change in phospholipid membrane composition could still impact the translocation of glucose transporters to the cell membrane, and ultimately reduce the glucose transporters in the membrane. In plasma from HD patients, both PC and PE levels were decreased in correlation to clinical progression. 125 Whereas, in the CSF of these same patients PC levels were elevated and negatively correlated with some functional assessments. 125 PE levels were unchanged in the CSF, suggesting that in the CNS the PC/PE ratio is increased and may contribute to a loss of function in HD. 126 Interestingly, Htt14A2.6 PC12 cells, which express the HTT exon 1 containing either 25Q or 97Q repeats have increased in membrane fluidity. 127 However, similar to AD, ceramide accumulation has been shown to occur early in disease progression in the R6/2 mouse model of HD. 128 Thus, it appears further investigations are needed to fully understand how membrane fluidity is affected in HD, and what role phospholipid composition plays in regulating that fluidity, and in turn glucose uptake.
Parkinson’s disease
A large body of evidence has shown that type II diabetes increases the risk of developing PD by up to 40-50%. 129 Indeed, the presence of diabetic phenotype in PD has been shown to be associated with more severe impairments in parkinsonian features, specifically cognitive decline, postural instability and gait difficulty. 130 However, a meta-analysis investigating the relationship between diabetes and PD, found that the association was not significant. 131 Between 50–80% of patients with PD display an abnormal response to OGTT, and increased insulin resistance. 132 Furthermore, in response to an OGTT, glucose clearance was reduced in PD patients, with no change in plasma insulin concentrations compared to healthy controls. 133 In this study reduced glucose clearance was associated with a longer disease duration and an increase in the severity of symptoms. 133 Thus, glucose intolerance in PD patients is associated with a more severe phenotype, accelerated disease progression and an increased risk of developing dementia.
Several lines of evidence suggest that the insulin response in the brain may play a role in the development of PD. For example, insulin receptor immunoreactivity was shown to be reduced in the SNpc in post-mortem brain samples of PD patients. 134 Furthermore, in the rat 6-OHDA model of PD, serine phosphorylation of IRS-2 was elevated, consequently leading to a loss of downstream AKT phosphorylation. 135 Moreover, intranasal insulin treatment in rats ameliorated decline of locomotor activity and motor performance induced by 6-OHDA in comparison to non-treated 6-OHDA-lesion rats. 136 In the same study, insulin treatment was shown to alleviate 6-OHDA-induced injury in the SNpc, further supporting neuroprotective role of insulin. 136 Moreover, post-treatment with exendin-4 reduced methamphetamine-induced rotational behaviour, and increased dopamine production in the SN of mice injected with both 6-OHDA and MPTP.137,138 In mesencephalic and SH-SY5Y neuronal cell cultures, known to be rich in dopaminergic neurons, exendin-4 protected dopaminergic cells from 6-OHDA damage. 138 Thus, as in AD, it seems that insulin resistance contributes to disease progression and neuronal loss in PD (Figure 2).
Unlike in AD and HD, little is known about the mechanisms that drive insulin resistance in PD. However, similar changes in membrane lipid composition have been observed in PD, when compared to these other neurodegenerative disorders, suggesting that a change in membrane fluidity could contribute to a loss of insulin signalling in PD. The incorporation of polyunsaturated and saturated fatty acids into cell membranes is altered in PD, with the unsaturation index of lipid rafts from the frontal cortex reduced by 38% and 52% in PD and incidental PD patients, respectively. 139 This is caused by both a dramatic decrease in both n-3 and n-6 long chain poly unsaturated fatty acids, along with an increased incorporation of saturated fatty acids in phospholipids. 139 This results in a more viscous membrane, and potentially reduced brain insulin signalling in PD. Furthermore, ceramide levels are elevated in the plasma and post-mortem brain samples from PD patients. 140 Higher plasma ceramide levels were also associated with a disease severity. 140 Overall, these results show that cell membrane fluidity is reduced in PD due to changes in phospholipid composition. To date it is unknown how these changes alter insulin signalling in PD or other neurodegenerative disorders, but it would be on interest to investigate these links in the future.
Is insulin resistance a consequence of protein aggregation in neurodegenerative disorders?
Similarly, to the deficits in energy metabolism, insulin resistance appears to be a common feature of these neurodegenerative disorders. There is evidence that protein aggregation is also driving this insulin resistance both in the periphery and CNS. Tau hyperphosphorylation drives insulin accumulation in SH-SY5Y neuroblastoma cells, which consequently decreases insulin receptor and GLUT4 expression. 141 Aβ oligomers also reduced phosphorylation of insulin receptor in response to insulin in mature hippocampal neurons. 142 Alternatively, ApoE4 expression in mice impaired insulin signalling in the brain by binding to the insulin receptor and inhibiting translocation to the cell membrane. 143 This may explain why some studies found altered glucose metabolism is associated with ApoE4 expression. In relation to HD and PD, both mHTT and α-synuclein aggregation have been shown to alter the activity of pancreatic islets. Glucose-stimulated insulin secretion was reduced in NIT-1 cells, a pancreatic β-cell line, that was transfected with HTT protein with 150 CAG repeats. In R6/2 mice, pancreatic β-cell dysfunction occurs after mHTT aggregates are observed in the nuclei of β-cells. Pathological phosphorylated α-synuclein deposits have also been found in pancreatic β-cells in over 90% of PD patients. 144 One study found that α-synuclein inhibits insulin secretion in mice pancreatic islets, through direct or indirect interactions between α-synuclein and the insulin granule KATP channel. 145 Ablation of α-synuclein in mice also induced glucose intolerance and impaired glucose-stimulated insulin secretion, where insulin secretion was reduced by 25% in the isolated islets. 146 In contrast, mice overexpressing human α-synuclein and acute peripheral administration of α-synuclein had a positive effect on glucose homeostasis and insulin secretion. 146 Thus, it appears that α-synuclein is important in regulating insulin secretion on pancreatic β-cells. More importantly, there are also studies that suggest insulin and insulin resistance can influence protein aggregation, suggesting these interactions are complex in these neurodegenerative disorders. 147 Indeed these processes become even more complicated if we consider, glycation, another mechanism that may link several pathways that are associated with neurodegeneration, including insulin resistance, mitochondrial dysfunction and protein aggregation. Glycation is the non-enzymatic reaction of reducing sugars or carbonyl compounds with proteins or lipids, wherein reactive carbonyl groups link to free amino groups. 148 This process can lead to the formation of advanced glycation end products (AGEs) which are known to disrupt the structure and function of proteins. 148 Indeed, glycation and AGE formation are reported to promote protein aggregation and disease progression in AD, HD and PD. 149 Glycation is also influenced by glucose metabolism, as methylglyoxal, an active carbonyl compound can be produced as a by-product of glycolysis, via the degradation of the triose phosphates, glyceraldehyde-3-phosphate and dihydroxyacetone-phosphate. 148 This would indicate that higher glycolytic rates would increase methylglyoxal levels. However, it is suggested that impairments in downstream pathways, including the glyceraldehyde-3-phosphate dehydrogenase activity and TCA cycle may contribute to methylglyoxal synthesis, due to the accumulation of glyceraldehyde-3-phosphate. 148 It must be noted that although there is some evidence to suggest that methylglyoxal levels are elevated in both diabetic patients and patients with these neurodegenerative disorders, the mechanisms underlying this elevation are not fully understood. 150 Furthermore, methylglyoxal is also reported to interfere with insulin secretion and signalling, however this is beyond the scope of this review, and is more thoroughly examined by Schalkwijk and Stehouwer. 150 Ultimately, it appears that it is not a single insult that underlie the selective vulnerability of specific neurons in each of these diseases. Rather it is a complex array of molecular interactions, whereby, disturbances in energy metabolism may be one aspect that contributes to this neuronal vulnerability that is observed in these neurodegenerative disorders. Clearly, more research into the complex interactions between protein aggregation, impaired glucose metabolism, mitochondrial dysfunction, oxidative stress, and inflammation is required to understand how they all contribute to neuronal degeneration.
Conclusion
This review has summarized the evidence that demonstrates impairments in glucose metabolism, in particular glucose uptake and mitochondrial dysfunction are a common feature of multiple neurodegenerative disorders. Although it is not yet understood whether these changes are a cause or consequence, glucose metabolism appears to play an important role in the progression of neurodegenerative disorders, likely due to the reduction in ATP synthesis which increases neuronal vulnerability to toxic and damaging stimuli. We have highlighted that although increasing fatty acid metabolism appears to be an effective strategy in overcoming this loss of glucose metabolism, long-term treatments have their drawbacks. Increased fatty acid metabolism is likely to increase ROS production, which is known to contribute to neurodegeneration. Therefore, we identified other common features of these neurodegenerative disorders that could be contributing to these deficits in glucose metabolism, particularly the glucose uptake. Future studies should address whether modifying the actions of insulin can increase brain glucose uptake and metabolism. In AD and PD, this target would need to address elevating tissue insulin signalling. Whereas, in HD, insulin secretion would need to be addressed. Moreover, future investigations need to identify if changes in cell membrane composition or protein aggregation is affecting insulin signalling and glucose uptake in neurodegenerative disorders and contribute to mitochondrial dysfunction. In conjunction, future research needs to further investigate the complex relationship between several molecular disturbances that occur in neurons which all interact and contribute to the vulnerability of neurons in these diseases. Although a significant amount of research has been conducted on this topic, due to the complexities of metabolism and many cellular processes that these pathways interact with, we are far from a complete understanding of the role that metabolism plays in these neurodegenerative disorders. Further investigation into these complex interactions may present new therapeutic approaches for treating neurodegenerative diseases.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs
Titaya Lerskiatiphanich https://orcid.org/0000-0002-6153-313X
John D Lee https://orcid.org/0000-0002-9976-7396
References
- 1.Muddapu VR, Dharshini SAP, Chakravarthy VS, et al. Neurodegenerative diseases – is metabolic deficiency the root cause? Front Neurosci 2020; 14: 213. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knopman DS, Amieva H, Petersen RC, et al. Alzheimer disease. Nat Rev Dis Primers 2021; 7: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabrizi SJ, Flower MD, Ross CA, et al. Huntington disease: new insights into molecular pathogenesis and therapeutic opportunities. Nat Rev Neurol 2020; 16: 529–546. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease: a review. JAMA 2020; 323: 548–560. [DOI] [PubMed] [Google Scholar]
- 5.Błaszczyk JW. Energy metabolism decline in the aging Brain-Pathogenesis of neurodegenerative disorders. Metabolites 2020; 10: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watts ME, Pocock R, Claudianos C. Brain energy and oxygen metabolism: Emerging role in normal function and disease. Front Mol Neurosci 2018; 11: 216. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fricker M, Tolkovsky AM, Borutaite V, et al. Neuronal cell death. Physiol Rev 2018; 98: 813–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammond TC, Xing X, Wang C, et al. β-amyloid and tau drive early Alzheimer's disease decline while glucose hypometabolism drives late decline. Commun Biol 2020; 3: 352–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith GS, de Leon MJ, George AE, et al. Topography of cross-sectional and longitudinal glucose metabolic deficits in Alzheimer's disease: pathophysiologic implications. Arch Neurol 1992; 49: 1142–1150. [DOI] [PubMed] [Google Scholar]
- 10.Reiman EM, Chen K, Alexander GE, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci U S A 2004; 101: 284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Small GW, Ercoli LM, Silverman DH, et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer's disease. Proc Natl Acad Sci U S A 2000; 97: 6037–6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ossenkoppele R, van der Flier WM, Zwan MD, et al. Differential effect of APOE genotype on amyloid load and glucose metabolism in AD dementia. Neurology 2013; 80: 359–365. [DOI] [PubMed] [Google Scholar]
- 13.Mosconi L, Mistur R, Switalski R, et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer's disease. Eur J Nucl Med Mol Imaging 2009; 36: 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ou Y-N, Xu W, Li J-Q, et al. FDG-PET as an independent biomarker for Alzheimer’s biological diagnosis: a longitudinal study. Alzheimers Res Ther 2019; 11: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strom A, Iaccarino L, Edwards L, et al. Cortical hypometabolism reflects local atrophy and tau pathology in symptomatic Alzheimer’s disease. Brain 2022; 145: 713–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kyrtata N, Emsley HCA, Sparasci O, et al. A systematic review of glucose transport alterations in Alzheimer's disease. Front Neurosci 2021; 15: 626636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson IA, Chundu KR, Davies-Hill T, et al. Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer's disease. Ann Neurol 1994; 35: 546–551. [DOI] [PubMed] [Google Scholar]
- 18.Brooks WM, Lynch PJ, Ingle CC, et al. Gene expression profiles of metabolic enzyme transcripts in Alzheimer's disease. Brain Research 2007; 1127: 127–135. [DOI] [PubMed] [Google Scholar]
- 19.Sims NR, Blass JP, Murphy C, et al. Phosphofructokinase activity in the brain in Alzheimer's disease. Ann Neurol 1987; 21: 509–510. [DOI] [PubMed] [Google Scholar]
- 20.Marcus DL, de Leon MJ, Goldman J, et al. Altered glucose metabolism in microvessels from patients with Alzheimer's disease. Ann Neurol 1989; 26: 91–94. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Alshakhshir N, Zhao L. Glycolytic metabolism, brain resilience, and Alzheimer’s disease. Frontiers in Neuroscience 2021; 15: 662242. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minjarez B, Calderón-González KG, Rustarazo MLV, et al. Identification of proteins that are differentially expressed in brains with Alzheimer's disease using iTRAQ labeling and tandem mass spectrometry. J Proteomics 2016; 139: 103–121. [DOI] [PubMed] [Google Scholar]
- 23.Reichmann H, Flörke S, Hebenstreit G, et al. Analyses of energy metabolism and mitochondrial genome in post-mortem brain from patients with Alzheimer's disease. J Neurol 1993; 240: 377–380. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Guo XQ, Chu JF, et al. Potential hippocampal genes and pathways involved in Alzheimer's disease: a bioinformatic analysis. Genet Mol Res 2015; 14: 7218–7232. [DOI] [PubMed] [Google Scholar]
- 25.Hirai K, Aliev G, Nunomura A, et al. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci 2001; 21: 3017–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manczak M, Park BS, Jung Y, et al. Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease. NeuroMolecular Med 2004; 5: 147–162. [DOI] [PubMed] [Google Scholar]
- 27.Francis BM, Yang J, Song BJ, et al. Reduced levels of mitochondrial complex I subunit NDUFB8 and linked complex I + III oxidoreductase activity in the TgCRND8 mouse model of Alzheimer's disease. J Alzheimers Dis 2014; 39: 347–355. [DOI] [PubMed] [Google Scholar]
- 28.Krako N, Magnifico MC, Arese M, et al. Characterization of mitochondrial dysfunction in the 7PA2 cell model of Alzheimer's disease. J Alzheimers Dis 2013; 37: 747–758. [DOI] [PubMed] [Google Scholar]
- 29.Bubber P, Haroutunian V, Fisch G, et al. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann Neurol 2005; 57: 695–703. [DOI] [PubMed] [Google Scholar]
- 30.Gibson GE, Sheu KF, Blass JP, et al. Reduced activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer's disease. Arch Neurol 1988; 45: 836–840. [DOI] [PubMed] [Google Scholar]
- 31.Mastrogiacoma F, Bettendorff L, Grisar T, et al. Brain thiamine, its phosphate esters, and its metabolizing enzymes in Alzheimer's disease. Ann Neurol 1996; 39: 585–591. [DOI] [PubMed] [Google Scholar]
- 32.Yao J, Irwin RW, Zhao L, et al. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A 2009; 106: 14670–14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin WR, Clark C, Ammann W, et al. Cortical glucose metabolism in Huntington's disease. Neurology 1992; 42: 223–229. [DOI] [PubMed] [Google Scholar]
- 34.Berent S, Giordani B, Lehtinen S, et al. Positron emission tomographic scan investigations of Huntington's disease: cerebral metabolic correlates of cognitive function. Ann Neurol 1988; 23: 541–546. [DOI] [PubMed] [Google Scholar]
- 35.Antonini A, Leenders KL, Eidelberg D. [11C]Raclopride-PET studies of the Huntington's disease rate of progression: relevance of the trinucleotide repeat length. Ann Neurol 1998; 43: 253–255. [DOI] [PubMed] [Google Scholar]
- 36.Kuwert T, Lange HW, Boecker H, et al. Striatal glucose consumption in chorea-free subjects at risk of Huntington's disease. J Neurol 1993; 241: 31–36. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Valencia A, McClory H, et al. Deficient Rab11 activity underlies glucose hypometabolism in primary neurons of Huntington's disease mice. Biochem Biophys Res Commun 2012; 421: 727–730. [DOI] [PubMed] [Google Scholar]
- 38.Gamberino WC, Brennan WA., Jr. Glucose transporter isoform expression in Huntington's disease brain. J Neurochem 1994; 63: 1392–1397. [DOI] [PubMed] [Google Scholar]
- 39.Besson MT, Alegria K, Garrido-Gerter P, et al. Enhanced neuronal glucose transporter expression reveals metabolic choice in a HD drosophila model. PLoS One 2015; 10: e0118765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powers WJ, Videen TO, Markham J, et al. Selective defect of in vivo glycolysis in early Huntington's disease striatum. Proc Natl Acad Sci U S A 2007; 104: 2945–2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenkins BG, Rosas HD, Chen YCI, et al. H-1 NMR spectroscopy studies of Huntington's disease – correlations with CAG repeat numbers. Neurology 1998; 50: 1357–1365. [DOI] [PubMed] [Google Scholar]
- 42.Pavese N, Gerhard A, Tai Y, et al. Microglial activation correlates with severity in Huntington disease: a clinical and PET study. Neurology 2006; 66: 1638–1643. [DOI] [PubMed] [Google Scholar]
- 43.Damiano M, Diguet E, Malgorn C, et al. A role of mitochondrial complex II defects in genetic models of Huntington's disease expressing N-terminal fragments of mutant huntingtin. Hum Mol Genet 2013; 22: 3869–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benchoua A, Trioulier Y, Zala D, et al. Involvement of mitochondrial complex II defects in neuronal death produced by N-terminus fragment of mutated huntingtin. Mol Biol Cell 2006; 17: 1652–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guyot MC, Hantraye P, Dolan R, et al. Quantifiable bradykinesia, gait abnormalities and Huntington's disease-like striatal lesions in rats chronically treated with 3-nitropropionic acid. Neuroscience 1997; 79: 45–56. [DOI] [PubMed] [Google Scholar]
- 46.Hamilton J, Brustovetsky T, Sridhar A, et al. Energy metabolism and mitochondrial superoxide anion production in pre-symptomatic striatal neurons derived from human-induced pluripotent stem cells expressing mutant huntingtin. Mol Neurobiol 2020; 57: 668–684. [DOI] [PubMed] [Google Scholar]
- 47.Dubinsky JM. Towards an understanding of energy impairment in Huntington's disease brain. J Huntingtons Dis 2017; 6: 267–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sorbi S, Bird ED, Blass JP. Decreased pyruvate dehydrogenase complex activity in Huntington and Alzheimer brain. Ann Neurol 1983; 13: 72–78. [DOI] [PubMed] [Google Scholar]
- 49.Kuhl DE, Metter EJ, Riege WH. Patterns of local cerebral glucose utilization determined in Parkinson's disease by the [18F]fluorodeoxyglucose method. Ann Neurol 1984; 15: 419–424. [DOI] [PubMed] [Google Scholar]
- 50.Blum D, la Fougère C, Pilotto A, et al. Hypermetabolism in the cerebellum and brainstem and cortical hypometabolism are independently associated with cognitive impairment in Parkinson's disease. Eur J Nucl Med Mol Imaging 2018; 45: 2387–2395. [DOI] [PubMed] [Google Scholar]
- 51.Edison P, Ahmed I, Fan Z, et al. Microglia, amyloid, and glucose metabolism in Parkinson’s disease with and without dementia. Neuropsychopharmacology 2013; 38: 938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson's disease. J Neurosci Res 1999; 57: 195–206. [DOI] [PubMed] [Google Scholar]
- 53.Knight Adam L, Yan X, Hamamichi S, et al. The glycolytic enzyme, GPI, is a functionally conserved modifier of dopaminergic neurodegeneration in Parkinson’s models. Cell Metab 2014; 20: 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong CT, Chau KY, Schapira AH. Meclizine-induced enhanced glycolysis is neuroprotective in Parkinson disease cell models. Sci Rep 2016; 6: 25344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cai R, Zhang Y, Simmering JE, et al. Enhancing glycolysis attenuates Parkinson's disease progression in models and clinical databases. J Clin Invest 2019; 129: 4539–4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simmering JE, Welsh MJ, Liu L, et al. Association of glycolysis-enhancing α-1 blockers with risk of developing Parkinson disease. JAMA Neurol 2021; 78: 407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Subramaniam SR, Vergnes L, Franich NR, et al. Region specific mitochondrial impairment in mice with widespread overexpression of alpha-synuclein. Neurobiol Dis 2014; 70: 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Devi L, Raghavendran V, Prabhu BM, et al. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem 2008; 283: 9089–9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Przedborski S, Tieu K, Perier C, et al. MPTP as a mitochondrial neurotoxic model of Parkinson's disease. J Bioenerg Biomembr 2004; 36: 375–379. [DOI] [PubMed] [Google Scholar]
- 60.González-Rodríguez P, Zampese E, Stout KA, et al. Disruption of mitochondrial complex I induces progressive parkinsonism. Nature 2021; 599: 650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Langston JW. The MPTP story. J Parkinsons Dis 2017; 7: S11–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mizuno Y, Matuda S, Yoshino H, et al. An immunohistochemical study on alpha-ketoglutarate dehydrogenase complex in Parkinson's disease. Ann Neurol 1994; 35: 204–210. [DOI] [PubMed] [Google Scholar]
- 63.Gibson GE, Kingsbury AE, Xu H, et al. Deficits in a tricarboxylic acid cycle enzyme in brains from patients with Parkinson's disease. Neurochem Int 2003; 43: 129–135. [DOI] [PubMed] [Google Scholar]
- 64.Miki Y, Tanji K, Mori F, et al. Alteration of mitochondrial protein PDHA1 in lewy body disease and PARK14. Biochem Biophys Res Commun 2017; 489: 439–444. [DOI] [PubMed] [Google Scholar]
- 65.Prapong T, Buss J, Hsu WH, et al. Amyloid beta-peptide decreases neuronal glucose uptake despite causing increase in GLUT3 mRNA transcription and GLUT3 translocation to the plasma membrane. Exp Neurol 2002; 174: 253–258. [DOI] [PubMed] [Google Scholar]
- 66.Mark RJ, Pang Z, Geddes JW, et al. Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation. J Neurosci 1997; 17: 1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ismailoglu I, Chen Q, Popowski M, et al. Huntingtin protein is essential for mitochondrial metabolism, bioenergetics and structure in murine embryonic stem cells. Dev Biol 2014; 391: 230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jacobsen JC, Gregory GC, Woda JM, et al. HD CAG-correlated gene expression changes support a simple dominant gain of function. Hum Mol Genet 2011; 20: 2846–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jin YN, Johnson GV. The interrelationship between mitochondrial dysfunction and transcriptional dysregulation in Huntington disease. J Bioenerg Biomembr 2010; 42: 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang X, Becker K, Levine N, et al. Pathogenic alpha-synuclein aggregates preferentially bind to mitochondria and affect cellular respiration. Acta Neuropathol Commun 2019; 7: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reeve AK, Ludtmann MH, Angelova PR, et al. Aggregated α-synuclein and complex I deficiency: exploration of their relationship in differentiated neurons. Cell Death Dis 2015; 6: e1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Braidy N, Gai W-P, Xu YH, et al. Uptake and mitochondrial dysfunction of alpha-synuclein in human astrocytes, cortical neurons and fibroblasts. Transl Neurodegener 2013; 2: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Her L-S, Goldstein LS. Enhanced sensitivity of striatal neurons to axonal transport defects induced by mutant huntingtin. J Neurosci 2008; 28: 13662–13672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bolam JP, Pissadaki EK. Living on the edge with too many mouths to feed: Why dopamine neurons die. Mov Disord 2012; 27: 1478–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jensen NJ, Wodschow HZ, Nilsson M, et al. Effects of ketone bodies on brain metabolism and function in neurodegenerative diseases. IJMS 2020; 21: 8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reger MA, Henderson ST, Hale C, et al. Effects of β-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging 2004; 25: 311–314. [DOI] [PubMed] [Google Scholar]
- 77.Ota M, Matsuo J, Ishida I, et al. Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer's disease. Neurosci Lett 2019; 690: 232–236. [DOI] [PubMed] [Google Scholar]
- 78.Henderson ST, Vogel JL, Barr LJ, et al. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer's disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond) 2009; 6: 31–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu Q, Zhang Y, Zhang X, et al. Medium-chain triglycerides improved cognition and lipid metabolomics in mild to moderate Alzheimer's disease patients with APOE4−/−: a double-blind, randomized, placebo-controlled crossover trial. Clin Nutr 2020; 39: 2092–2105. [DOI] [PubMed] [Google Scholar]
- 80.Ruskin DN, Ross JL, Kawamura M, et al. A ketogenic diet delays weight loss and does not impair working memory or motor function in the R6/2 1J mouse model of Huntington's disease. Physiol Behav 2011; 103: 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen JY, Tran C, Hwang L, et al. Partial amelioration of peripheral and central symptoms of Huntington's disease via modulation of lipid metabolism. J Huntingtons Dis 2016; 5: 65–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lim S, Chesser AS, Grima JC, et al. D-β-hydroxybutyrate is protective in mouse models of Huntington's disease. PloS One 2011; 6: e24620-e24620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kashiwaya Y, Takeshima T, Mori N, et al. D-beta-hydroxybutyrate protects neurons in models of Alzheimer's and Parkinson's disease. Proc Natl Acad Sci U S A 2000; 97: 5440–5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Imamura K, Takeshima T, Kashiwaya Y, et al. D-beta-hydroxybutyrate protects dopaminergic SH-SY5Y cells in a rotenone model of Parkinson's disease. J Neurosci Res 2006; 84: 1376–1384. [DOI] [PubMed] [Google Scholar]
- 85.Tieu K, Perier C, Caspersen C, et al. D-β-Hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest 2003; 112: 892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Joniec-Maciejak I, Wawer A, Turzyńska D, et al. Octanoic acid prevents reduction of striatal dopamine in the MPTP mouse model of Parkinson's disease. Pharmacol Rep 2018; 70: 988–992. [DOI] [PubMed] [Google Scholar]
- 87.Phillips MCL, Murtagh DKJ, Gilbertson LJ, et al. Low-fat versus ketogenic diet in Parkinson's disease: a pilot randomized controlled trial. Mov Disord 2018; 33: 1306–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schönfeld P, Reiser G. Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain. J Cereb Blood Flow Metab 2013; 33: 1493–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schönfeld P, Wojtczak L. Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic Biol Med 2008; 45: 231–241. [DOI] [PubMed] [Google Scholar]
- 90.Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 2004; 3: 205–214. [DOI] [PubMed] [Google Scholar]
- 91.Wilcox G. Insulin and insulin resistance. Clin Biochem Rev 2005; 26: 19–39. [PMC free article] [PubMed] [Google Scholar]
- 92.Pomytkin I, Costa-Nunes JP, Kasatkin V, et al. Insulin receptor in the brain: Mechanisms of activation and the role in the CNS pathology and treatment. CNS Neurosci Ther 2018; 24: 763–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van der Veen JN, Kennelly JP, Wan S, et al. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim Biophys Acta Biomembr 2017; 1859: 1558–1572. [DOI] [PubMed] [Google Scholar]
- 94.Huang C-C, Chung C-M, Leu H-B, et al. Diabetes mellitus and the risk of Alzheimer’s disease: a nationwide population-based study. Plos One 2014; 9: e87095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arvanitakis Z, Wilson RS, Bienias JL, et al. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol 2004; 61: 661–666. [DOI] [PubMed] [Google Scholar]
- 96.Sadrolashrafi K, Craft S, Decourt B, et al. Is diabetes associated with increased pathological burden in Alzheimer's disease? Alzheimer's & dementia: Diagnosis. Alz & Dem Diag Ass & Dis Mo 2021; 13: e12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.dos Santos Matioli MNP, Suemoto CK, Rodriguez RD, et al. Diabetes is not associated with Alzheimer’s disease neuropathology. J Alzheimers Dis 2017; 60: 1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chatterjee S, Mudher A. Alzheimer's disease and type 2 diabetes: a critical assessment of the shared pathological traits. Front Neurosci 2018; 12: 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sharma M, Gupta YK. Intracerebroventricular injection of streptozotocin in rats produces both oxidative stress in the brain and cognitive impairment. Life Sci 2001; 68: 1021–1029. [DOI] [PubMed] [Google Scholar]
- 100.Imamura T, Yanagihara YT, Ohyagi Y, et al. Insulin deficiency promotes formation of toxic amyloid-β42 conformer co-aggregating with hyper-phosphorylated tau oligomer in an Alzheimer's disease model. Neurobiol Dis 2020; 137: 104739. [DOI] [PubMed] [Google Scholar]
- 101.Ferreira LSS, Fernandes CS, Vieira MNN, et al. Insulin resistance in Alzheimer's disease. Front Neurosci 2018; 12: 830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ekblad LL, Rinne JO, Puukka P, et al. Insulin resistance predicts cognitive decline: an 11-year follow-up of a nationally representative adult population sample. Diabetes Care 2017; 40: 751–758. [DOI] [PubMed] [Google Scholar]
- 103.Fujisawa Y, Sasaki K, Akiyama K. Increased insulin levels after OGTT load in peripheral blood and cerebrospinal fluid of patients with dementia of Alzheimer type. Biol Psychiatry 1991; 30: 1219–1228. [DOI] [PubMed] [Google Scholar]
- 104.Kohjima M, Sun Y, Chan L. Increased food intake leads to obesity and insulin resistance in the Tg2576 Alzheimer’s disease mouse model. Endocrinology 2010; 151: 1532–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Willette AA, Johnson SC, Birdsill AC, et al. Insulin resistance predicts brain amyloid deposition in late middle-aged adults. Alzheimers Dement 2015; 11: 504–510.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Craft S, Peskind E, Schwartz MW, et al. Cerebrospinal fluid and plasma insulin levels in Alzheimer's disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology 1998; 50: 164–168. [DOI] [PubMed] [Google Scholar]
- 107.Steen E, Terry BJ, Rivera E, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease – is this type 3 diabetes? J Alzheimers Dis 2005; 7: 63–80. [DOI] [PubMed] [Google Scholar]
- 108.Perona JS. Membrane lipid alterations in the metabolic syndrome and the role of dietary oils. Biochim Biophys Acta Biomembr 2017; 1859: 1690–1703. [DOI] [PubMed] [Google Scholar]
- 109.Söderberg M, Edlund C, Kristensson K, et al. Fatty acid composition of brain phospholipids in aging and in Alzheimer's disease. Lipids 1991; 26: 421–425. [DOI] [PubMed] [Google Scholar]
- 110.Liu Y, Liu F, Grundke-Iqbal I, et al. Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J Pathol 2011; 225: 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sokolowska E, Blachnio-Zabielska A. The role of ceramides in insulin resistance. Front Endocrinol 2019; 10: 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mielke MM, Bandaru VV, Haughey NJ, et al. Serum ceramides increase the risk of Alzheimer disease: the women's health and aging study II. Neurology 2012; 79: 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Montojo MT, Aganzo M, González N. Huntington's disease and diabetes: chronological sequence of its association. J Huntingtons Dis 2017; 6: 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Podolsky S, Leopold NA, Sax DS. Increased frequency of diabetes mellitus in patients with Huntington's chorea. Lancet 1972; 1: 1356–1358. [DOI] [PubMed] [Google Scholar]
- 115.Farrer LA. Diabetes mellitus in Huntington disease. Clin Genet 1985; 27: 62–67. [DOI] [PubMed] [Google Scholar]
- 116.Davidson MB, Green S, Menkes JH. Normal glucose, insulin, and growth-hormone responses to oral glucose in Huntingtons disease. Journal of Laboratory and Clinical Medicine 1974; 84: 807–812. [Google Scholar]
- 117.Kremer HP, Roos RA, Frolich M, et al. Endocrine functions in Huntington's disease. A two-and-a-half years follow-up study. J Neurol Sci 1989; 90: 335–344. [DOI] [PubMed] [Google Scholar]
- 118.Boesgaard TW, Nielsen TT, Josefsen K, et al. Huntington's disease does not appear to increase the risk of diabetes mellitus. J Neuroendocrinol 2009; 21: 770–776. [DOI] [PubMed] [Google Scholar]
- 119.Podolsky S, Leopold NA. Abnormal glucose tolerance and arginine tolerance tests in Huntington's disease. Gerontology 1977; 23: 55–63. [DOI] [PubMed] [Google Scholar]
- 120.Lalic NM, Maric J, Svetel M, et al. Glucose homeostasis in Huntington disease: abnormalities in insulin sensitivity and early-phase insulin secretion. Arch Neurol 2008; 65: 476–480. [DOI] [PubMed] [Google Scholar]
- 121.Björkqvist M, Fex M, Renström E, et al. The R6/2 transgenic mouse model of Huntington's disease develops diabetes due to deficient beta-cell mass and exocytosis. Hum Mol Genet 2005; 14: 565–574. [DOI] [PubMed] [Google Scholar]
- 122.Andreassen OA, Dedeoglu A, Stanojevic V, et al. Huntington's disease of the endocrine pancreas: insulin deficiency and diabetes mellitus due to impaired insulin gene expression. Neurobiol Dis 2002; 11: 410–424. [DOI] [PubMed] [Google Scholar]
- 123.Hunt MJ, Morton AJ. Atypical diabetes associated with inclusion formation in the R6/2 mouse model of Huntington's disease is not improved by treatment with hypoglycaemic agents. Exp Brain Res 2005; 166: 220–229. [DOI] [PubMed] [Google Scholar]
- 124.Martin B, Golden E, Carlson OD, et al. Exendin-4 improves glycemic control, ameliorates brain and pancreatic pathologies, and extends survival in a mouse model of Huntington's disease. Diabetes 2009; 58: 318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McGarry A, Gaughan J, Hackmyer C, et al. Cross-sectional analysis of plasma and CSF metabolomic markers in Huntington’s disease for participants of varying functional disability: a pilot study. Sci Rep 2020; 10: 20490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mehrotra A, Sood A, Sandhir R. Mitochondrial modulators improve lipid composition and attenuate memory deficits in experimental model of Huntington's disease. Mol Cell Biochem 2015; 410: 281–292. [DOI] [PubMed] [Google Scholar]
- 127.Sameni S, Malacrida L, Tan Z, et al. Alteration in fluidity of cell plasma membrane in Huntington disease revealed by spectral phasor analysis. Sci Rep 2018; 8: 734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhao L, Spassieva SD, Jucius TJ, et al. A deficiency of ceramide biosynthesis causes cerebellar purkinje cell neurodegeneration and lipofuscin accumulation. PLOS Genet 2011; 7: e1002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Santiago JA, Potashkin JA. Shared dysregulated pathways lead to Parkinson's disease and diabetes. Trends Mol Med 2013; 19: 176–186. [DOI] [PubMed] [Google Scholar]
- 130.Giuntini M, Baldacci F, Del Prete E, et al. Diabetes is associated with postural and cognitive domains in Parkinson's disease. Results from a single-center study. Parkinsonism Relat Disord 2014; 20: 671–672. [DOI] [PubMed] [Google Scholar]
- 131.Cereda E, Barichella M, Pedrolli C, et al. Diabetes and risk of Parkinson’s disease: a systematic review and meta-analysis. Diabetes Care 2011; 34: 2614–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sandyk R. The relationship between diabetes mellitus and Parkinson's disease. Int J Neurosci 1993; 69: 125–130. [DOI] [PubMed] [Google Scholar]
- 133.Marques A, Dutheil F, Durand E, et al. Glucose dysregulation in Parkinson's disease: too much glucose or not enough insulin? Parkinsonism Relat Disord 2018; 55: 122–127. [DOI] [PubMed] [Google Scholar]
- 134.Moroo I, Yamada T, Makino H, et al. Loss of insulin receptor immunoreactivity from the substantia nigra pars compacta neurons in Parkinson's disease. Acta Neuropathol 1994; 87: 343–348. [DOI] [PubMed] [Google Scholar]
- 135.Morris JK, Zhang H, Gupte AA, et al. Measures of striatal insulin resistance in a 6-hydroxydopamine model of Parkinson's disease. Brain Res 2008; 1240: 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pang Y, Lin S, Wright C, et al. Intranasal insulin protects against substantia nigra dopaminergic neuronal loss and alleviates motor deficits induced by 6-OHDA in rats. Neuroscience 2016; 318: 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen S, Yu SJ, Li Y, et al. Post-treatment with PT302, a long-acting exendin-4 sustained release formulation, reduces dopaminergic neurodegeneration in a 6-Hydroxydopamine rat model of Parkinson's disease. Sci Rep 2018; 8: 10722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li Y, Perry T, Kindy MS, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and parkinsonism. Proc Natl Acad Sci U S A 2009; 106: 1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fabelo N, Martín V, Santpere G, et al. Severe alterations in lipid composition of frontal cortex lipid rafts from Parkinson's disease and incidental Parkinson's disease. Mol Med 2011; 17: 1107–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mielke MM, Maetzler W, Haughey NJ, et al. Plasma ceramide and glucosylceramide metabolism is altered in sporadic Parkinson's disease and associated with cognitive impairment: a pilot study. PLoS One 2013; 8: e73094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rodriguez-Rodriguez P, Sandebring-Matton A, Merino-Serrais P, et al. Tau hyperphosphorylation induces oligomeric insulin accumulation and insulin resistance in neurons. Brain 2017; 140: 3269–3285. [DOI] [PubMed] [Google Scholar]
- 142.Zhao W-Q, De Felice FG, Fernandez S, et al. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J 2008; 22: 246–260. [DOI] [PubMed] [Google Scholar]
- 143.Zhao N, Liu C-C, Van Ingelgom AJ, et al. Apolipoprotein E4 impairs neuronal insulin signaling by trapping insulin receptor in the endosomes. Neuron 2017; 96: 115–129.e5. e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Martinez-Valbuena I, Amat-Villegas I, Valenti-Azcarate R, et al. Interaction of amyloidogenic proteins in pancreatic β cells from subjects with synucleinopathies. Acta Neuropathol 2018; 135: 877–886. [DOI] [PubMed] [Google Scholar]
- 145.Geng X, Lou H, Wang J, et al. α-Synuclein binds the KATP channel at insulin-secretory granules and inhibits insulin secretion. Am J Physiol Endocrinol Metab 2011; 300: E276–E286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wijesekara N, Ahrens R, Wu L, et al. α-Synuclein regulates peripheral insulin secretion and glucose transport. Front Aging Neurosci 2021; 13:▪. Brief Research Report. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wei Z, Koya J, Reznik SE. Insulin resistance exacerbates Alzheimer disease via multiple mechanisms. Front Neurosci 2021; 15: 687157. Mini Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Muronetz VI, Melnikova AK, Seferbekova ZN, et al. Glycation, glycolysis, and neurodegenerative diseases: is there any connection? Biochemistry (Mosc) 2017; 82: 874–886. [DOI] [PubMed] [Google Scholar]
- 149.Li J, Liu D, Sun L, et al. Advanced glycation end products and neurodegenerative diseases: mechanisms and perspective. J Neurol Sci 2012; 317: 1–5. [DOI] [PubMed] [Google Scholar]
- 150.Schalkwijk CG, Stehouwer CDA. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol Rev 2020; 100: 407–461. [DOI] [PubMed] [Google Scholar]