Abstract
For three decades, the US Public Health Service has recommended that all persons capable of becoming pregnant consume 400 μg/day of folic acid (FA) to prevent neural tube defects (NTDs). The neural tube forms by 28 days after conception. Fortification can be an effective NTD prevention strategy in populations with limited access to folic acid foods and/or supplements. This review describes the status of mandatory FA fortification among countries that fortify (n = 71) and the research describing the impact of those programs on NTD rates (up to 78% reduction), blood folate concentrations [red blood cell folate concentrations increased ∼1.47-fold (95% CI, 1.27, 1.70) following fortification], and other health outcomes. Across settings, high-quality studies such as those with randomized exposures (e.g., randomized controlled trials, Mendelian randomization studies) are needed to elucidate interactions of FA with vitamin B12 as well as expanded biomarker testing.
Keywords: folic acid, vB12, neural tube defects, fortification, meta-analysis
1. INTRODUCTION
The fall of 2022 marks 30 years since the US Public Health Service [through the efforts of the Centers for Disease Control and Prevention (CDC), the Food and Drug Administration (FDA), the Health Resources and Services Administration, and the National Institutes of Health] recommended consumption of 400 μg/day of folic acid (FA) for the prevention of neural tube defects (NTDs) (19). The first trial examining FA for the prevention of NTDs took place in 1991 among women with a previous NTD-affected pregnancy (n = 1,817) who were randomized to receive a 4,000 μg/day supplement containing only FA; this study showed a 72% reduction (RR, 0.28; 95% CI, 0.12, 0.71) in subsequent pregnancies (116). In 1992, a second trial was conducted among women without a history of an NTD-affected pregnancy (n = 4,753) who were randomized to receive a daily multivitamin supplement containing 800 μg/day FA. In this trial, there were six NTD-affected pregnancies, with no cases among women who received the FA-containing supplement (p = 0.029) (32). This high-quality evidence (Figure 1) set the stage for the March of Dimes to petition the FDA to mandate FA fortification of products labeled as enriched cereal grain products (authorized in 1996 and fully implemented in 1998) (45).
Figure 1.
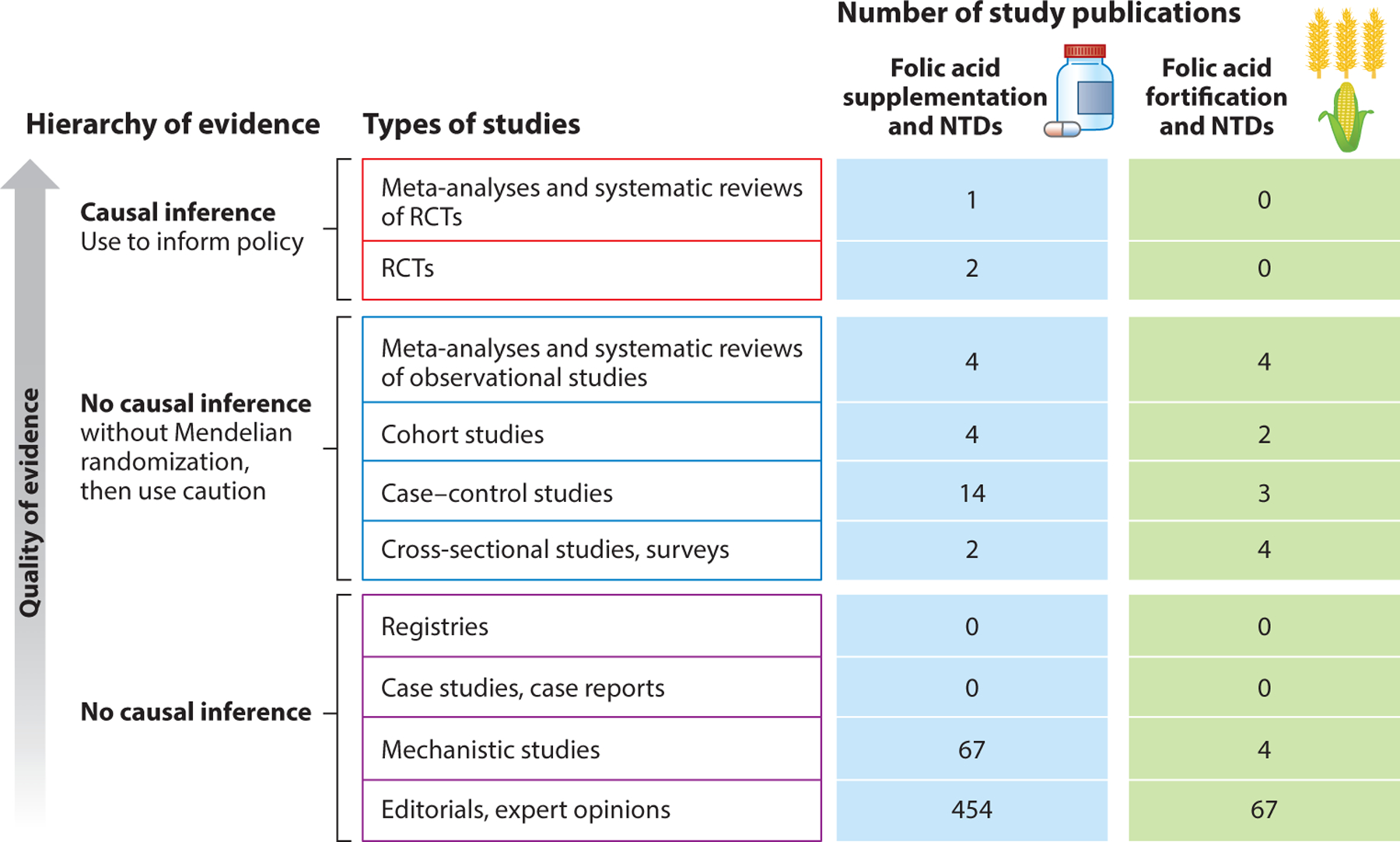
Hierarchy of evidence for folic acid and neural tube defects (NTDs). It is crucial to keep the basics of the hierarchy of evidence in mind when reviewing any new study. Policy changes generally require randomized controlled trials (RCTs) and preferably meta-analyses of multiples, given the lack of causal inference without randomization. The level of evidence is a critical issue in nutrition because both supplementation and nutritional patterns are heavily confounded by innumerable health conditions and demographic characteristics. Folic acid is one of the best-studied molecules in the published literature, with more than 93,000 peer-reviewed publications; however, no fortification studies of NTDs (only initial supplementation trials) have been conducted because of the ethical and logistical issues surrounding withdrawal of an established, successful intervention. Additional trials are generally limited to intermediate biomarkers. In contrast to other nutritional interventions, there are large RCTs that show the prevention of NTDs by periconceptional folic acid supplementation. New studies are weighted not only against these critical metrics but also against the totality of the evidence in the field.
FA fortification has been heralded as one of the top 10 public health achievements in the USA during the first 10 years of the twenty-first century (20). More than 1,300 NTDs are prevented annually in the USA, and mandatory FA fortification is estimated to provide savings of more than $600 million in direct costs each year (61). Fortification of staple grains is one of the best ways to increase health equity by reaching at-risk populations with an intervention that requires no behavioral change and has nominal costs. While many families and communities have benefited from the success of the program, concerns around safety, interactions with other nutrients, and challenges surrounding fortification implementation both domestically and abroad have arisen and will shape the future of FA fortification.
This review focuses on two areas of exploration: (a) monitoring the impact of FA fortification and (b) folate interaction with vitamin B12 (vB12). First, while FA monitoring and dosages may seem like settled science, the operationalization of the long-standing intake recommendations is remarkably complex, and information on the totality of the homeostatic process between intake and outcomes is still missing some critical steps, including summary estimates of the impact of fortification on biomarkers. Second, the interaction between FA and vB12 on megaloblastic anemia has been a multifaceted story since early clinicians first mistreated it as a fatal blood disorder that had not one but two nutritional etiologies. This review does not cover the safety literature surrounding FA supplementation and other health outcomes (such as cancers), as these conditions are routinely reviewed by multiple expert groups around the globe. To date, these reviews have produced no findings that alter recommendations for FA fortification at the current dosages (44, 50, 51, 67, 145).
2. THE BASICS
2.1. Folate Metabolism
Humans are generally reliant on external sources of one-carbon methyl groups that are necessary for basic cellular processes. Folate is a general term used to describe naturally occurring and synthetic forms of vitamin B9. Folate is essential for cell growth as well as DNA synthesis and methylation, and, if depleted, results in double-stranded breaks in the DNA, triggering apoptosis (30). Folate is particularly important for preventing megaloblastic anemia due to the high folate requirements needed to maintain adequate blood counts. In addition to the causal link with NTDs, epidemiological studies have shown that low folate status is associated with heart diseases and an increased risk of most cancers (5).
Naturally occurring dietary folate exists in both monoglutamate and polyglutamate forms. To be absorbed through the intestinal mucosa, polyglutamate dietary folate must be converted into 5-methyltetrahydrofolate (5-MTHF), a monoglutamate form of folate that is the primary form taken up by cells in peripheral tissue (5). Once in the cells, 5-MTHF must be polyglutamated to be retained and to function as a one-carbon-cycle coenzyme. To accomplish this task, methionine synthase (MS) converts 5-MTHF to tetrahydrofolate (THF). Synthetic FA is a monoglutamate form of folate that is readily transported across the intestinal epithelium. The enzyme dihydrofolate reductase reduces FA into dihydrofolate and THF (30) (Figure 2).
Figure 2.
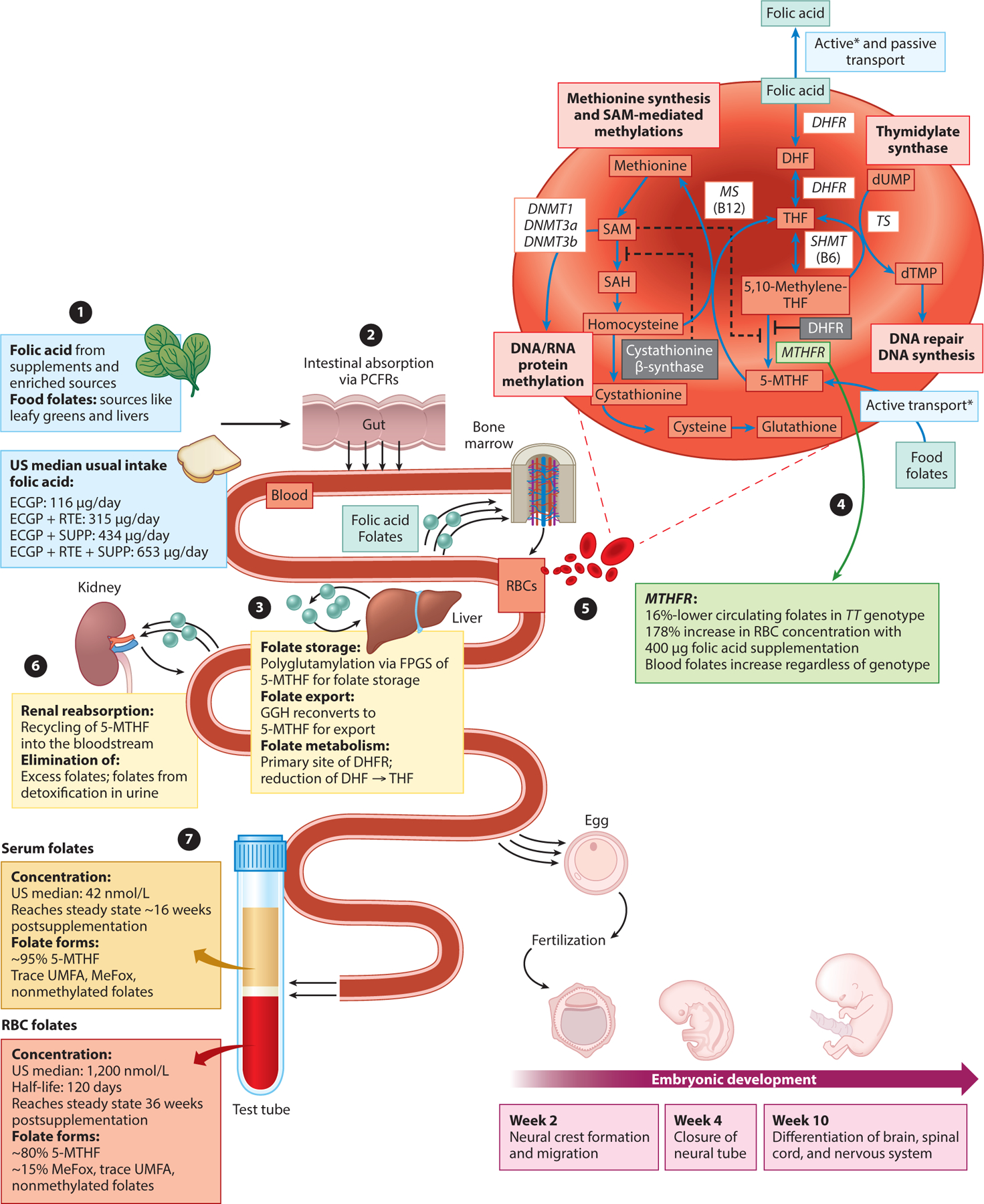
Folic acid and food folates: metabolic pathway, one-carbon pathway, and biomarker measures. (❶). In addition to naturally occurring food folates from diet (available primarily from leafy greens and liver), it is recommended that all women of reproductive age consume 400 μg/day of folic acid, a synthetic isomer of folate. In the USA, there are three primary sources of folic acid: cereal grain products labeled as enriched (ECGP) containing 140 μg of folic acid per 100 g of flour, ready-to-eat cereals (RTE) containing up to 400 μg of folic acid per serving, and supplements (SUPP) (45). These three sources generate four mutually exclusive folic acid consumption groups with varying median usual intakes of folic acid in adults over 16 years of age: ECGP, ECGP+RTE, ECGP+SUPP, and ECGP+RTE+SUPP (169). (❷) Food folates and folic acid are absorbed primarily from the small intestine via proton-coupled folate receptors (PCFRs), which have a high affinity to folic acid, and reduced folate carriers (RFCs), which have a lower affinity to folic acid (167). Once absorbed, folates reach systemic circulation and are further processed by peripheral tissue. (❸) Absorbed folates are partially removed by the liver. In the liver, folic acid may undergo biotransformation to 5-methyltetrahydrofolate (5-MTHF) via dihydrofolate reductase (DHFR), where it is highly expressed; however, the final biotransformation to 5-MTHF may be limited by MTHFR genotype (❹), leading to ~16%-lower circulating folates in TT genotypes (31, 164). 5-MTHF may be partially released into bile, allowing for further reabsorption in the small intestine. Folylpolyglutamate synthetase (FPGS) in the liver enables long-term storage of folates within the liver. γ-Glutamyl hydrolase (GGH) in the liver enables hydrolysis of stored folate polyglutamates back into bioavailable monoglutamate folate forms, which then reenter the systemic circulation via the hepatic vein (183). (❺) Circulating folates are transported from serum into newly created red blood cells (RBCs) in the bone marrow via membrane-associated folate-binding proteins with a half-life of approximately 120 days (5). Serum folates that are not bound to proteins are filtered by the kidney. (❻) Folate receptor α (FRα), which has high affinities to both folic acid and 5-MTHF, is highly expressed along tubule epithelial cells, allowing for efficient reabsorption of folates (79). Excess folates not reabsorbed and reduced folate forms with lower affinities to FRα are then eliminated into the urine by the kidney (79). In pregnant women, FRα is also highly expressed in the placenta, enabling nutritional transfer of folates to the fetus throughout pregnancy (158). (❼) RBC and serum folate measurements reflect the biological processing of dietary food folates and folic acids. The median serum folate concentration following fortification is ~42 nmol/L, and the median RBC folate concentration is ~1,200 nmol/L. Both comprise primarily 5-MTHF (41, 131). Asterisks indicate active transport via membrane-associated folate-binding proteins. Abbreviations: DHF, dihydrofolate; DHFR, dihydrofolate reductase; DNMT, DNA methyltransferase; dTMP, deoxythymidine monophosphate; dUMP, deoxyuridine monophosphate; MS, methionine synthase; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; SHMT, serine hydroxymethyltransferase; THF, tetrahydrofolate; TS, thymidylate synthase; UMFA, unmetabolized folic acid.
Once formed from either dietary folate or FA, THF can be converted to 5,10-methylene-THF by serine hydroxymethyltransferase, which is a vitamin B6–dependent reaction. Importantly for the one-carbon pathway, methylenetetrahydrofolate reductase (MTHFR) can catalyze the conversion of 5,10-methylene-THF to 5-MTHF, which serves as a carbon donor in the vB12-dependent conversion of homocysteine to methionine by MS. Methionine is an essential amino acid used for protein synthesis and as a substrate for S-adenosylmethionine (SAM), which is required for many methylation reactions for DNA, RNA, lipids, neurotransmitters, and hormones. Additionally, 5,10-methylene-THF is a cofactor for the methylation of deoxyuridylate to thymidylate, which is catalyzed by thymidylate synthase (156). Thymidylate is an essential metabolite necessary for DNA synthesis and repair (53). The folate one-carbon cycle is tightly regulated by several cofactors and metabolites, including SAM, vitamin B2, vitamin B6, and vB12 (53).
2.2. Folate Vitamers and Neural Tube Defect Prevention
Folate occurs in various forms, known as vitamers. All folate vitamers consist of a common biochemical structure but differ in the number of glutamic acids, oxidation state, and presence of one-carbon groups (e.g., FA, 5-MTHF, THF, 5,10-methylene-THF, dihydrofolate). Although most FA is converted to 5-MTHF during intestinal absorption and first-pass metabolism in the liver, concerns about low levels of circulating unmetabolized FA have led to discussions about the potential advantages of using 5-MTHF instead of FA for food fortification and periconceptional supplementation for reducing NTD risk. This concept is problematic for several reasons. No randomized controlled trials (RCTs), or even less-rigorous studies, have examined any other folate form for the prevention of NTDs. Arguments in favor of 5-MTHF supplements in the form of l-MTHF or (6S)-5-methyltetrahydro-FA claim that 5-MTHF is more bioavailable and more “natural” than FA and not as affected by polymorphisms in the MTHFR gene (47, 144). Studies have found that supplementation with l-MTHF or (6S)-5-methyltetrahydro-FA increases red blood cell (RBC) and serum folate concentrations (6, 68, 85). However, RCTs would have to be conducted to determine effectiveness, timing, dosage, stability, and safety in order for 5-MTHF (or a synthetic equivalent) to be recommended. Given its position in the folate metabolism pathway, it is unlikely that 5-MTHF in the presence of lower concentrations of vB12 would be as effective as FA. Studies have found that to reach ideal RBC folate concentrations, much higher serum/plasma folate concentrations (25.6 versus 34.6 nmol) are necessary in the presence of vB12 deficiency (22). Lastly, despite years of study, the unmetabolized FA that is in circulation has had no confirmed adverse effects, thus it is not necessary to replace a known effective intervention with one that does not have established effectiveness.
FA has a very similar dose–response increase in RBC folate concentrations across MTHFR genotypes when supplementing with 400 μg/day (27, 31). A meta-analysis of FA supplementation trials has shown that FA increases RBC folate concentrations by 178% and serum/plasma folate concentrations by 200% at the recommended dosage of 350–500 μg/day (28). Another meta-analysis showed only moderate absolute differences across MTHFR genotypes in folate concentrations, with an 18% reduction in CC versus TT (164). This finding suggests that people with MTHFR variants increase their folate status adequately to prevent NTDs with the recommended FA intake. Supporting evidence comes from studies in areas with very high rates of MTHFR C677T variants, such as northern China (12, 31) and Mexico (100), where FA reduces NTDs even when the variant is the major allele. Although efforts have been made to modify 5-MTHF to increase stability for commercial production (6, 68, 85), few studies have compared FA with these 5-MTHF isomers, and the impact of these modifications on birth defects has not been investigated.
2.3. Folate in Food and Neural Tube Defect Prevention
The Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine (73) has established intake recommendations for nutrients, including folate. To prevent anemia, the current recommended dietary allowance for folate is 400 μg/day of dietary folate equivalents (DFEs) for adults older than 19 years (73). DFEs are units of measurement that account for differences in bioavailability between naturally occurring food folate and synthetic FA (73). One DFE is equal to 0.6 μg of FA from fortified food or a supplement taken with food, or 0.5 μg of FA taken on an empty stomach, highlighting the lower bioavailability of food folate (73). Using DFEs, an individual can consume 400 μg of food folate or 240 μg of synthetic FA to meet the daily requirement of 400 DFEs. While many foods are folate rich, cooking and processing greatly reduce the available folate content in final products (142). To our knowledge, no intervention studies have shown that increasing natural food folate to target a specific intake [e.g., 667 DFEs (400 μg FA equivalent)] reduces NTDs or have elucidated interactions with other vitamins, so it is not possible to recommend an intake of natural food folate that is certain to prevent NTDs.
A meta-analysis of natural folate intake from food suggests a substantial variance in blood folate response as DFE intake increases (99). The modeled dose–response relationship between natural folate and RBC folate concentration showed that an estimated median intake of 350–800 DFEs would be needed to achieve RBC folate concentrations within the optimal range (906–1,500 nmol/L) for NTD reduction (99). Few studies have natural folate intakes in this range (99). Adequate vB12 is needed to effectively use natural folate for DNA replication (Figure 2). The high variance in the meta-analysis of the natural folate intake versus blood folate concentrations might be explained by the variation in vB12 status among populations. Thus, it is reasonable to assume that adequate vB12 status might be needed for NTD prevention among persons consuming only food folate.
3. EPIDEMIOLOGICAL RISK FACTORS FOR NEURAL TUBE DEFECTS
The etiologic heterogeneity of NTDs encompasses both modifiable and nonmodifiable risk factors, including maternal conditions, behaviors, genetics, and environmental exposures. Most risk factors for NTDs identified through epidemiological studies are folate sensitive, meaning that the risk for an NTD can be attenuated by FA before and during organogenesis. These include diabetes, obesity, MTHFR C677T variant, Hispanic ethnicity, vB12 deficiency, and hyperthermia/fever, as described in detail below.
3.1. Diabetes
Epidemiological studies have identified pregestational diabetes (types 1 and 2) as an important risk factor for numerous congenital anomalies, including NTDs. Hyperglycemia acts as a teratogen during organogenesis through the induction of oxidative stress, leading to excessive apoptosis in the neural tube and gene expression dysregulation (57). A case–control study found significantly elevated odds of anencephaly, holoprosencephaly, and encephalocele (OR, 3.5–13.1) in offspring of women with pregestational diabetes; the association with spina bifida was not significant (OR, 1.4; 95% CI, 0.8, 2.7) (162). Other studies have found an elevated risk of spina bifida in infants of mothers with pregestational diabetes (1, 115). FA supplementation may weaken the association between diabetes and risk of spina bifida (125).
3.2. Obesity
Prepregnancy obesity, defined as a body mass index (BMI) of 30 or higher, is associated with an increased risk for NTDs. Obesity affects approximately one-third of women of reproductive age (WRA) in the USA and contributes to the occurrence of NTDs through altered glucose metabolism, inadequate dietary intake, and increased FA requirements (18, 170). A recent meta-analysis of 22 case–control studies observed a maternal BMI dose–response relationship with risk of NTDs, with an odds ratio of 1.04 (95% CI, 1.03, 1.05) for every BMI increase of 1 kg/m2 in the mother (72). FA supplementation reduces the risk of NTDs among persons with obesity, but the effect might be lower than among those with a normal BMI (170). The population attributable fraction for anencephaly and spina bifida due to obesity was estimated as 2.2% and 9.9%, respectively (1). Elimination of prepregnancy obesity could result in the prevention of 405 spina bifida cases and 95 anencephaly cases per year in the USA (71).
3.3. MTHFR C677T Variant
As shown in Figure 2, MTHFR C677T polymorphism reduces the activity of the MTHFR enzyme, resulting in a decrease in plasma/serum and RBC folate concentrations across studies using an appropriate folate assay (microbiologic assay for RBC folate concentrations, where CC>CT>TT) for CC versus TT [plasma/serum, 13%; 95% creditable interval (CrI), 7%, 18%; RBC, 16%; 95% CrI, 12%, 20%] (164). Both maternal and fetal MTHFR C677T polymorphisms have been implicated as significant risk factors for NTDs (164). Depending on the genotype (homozygous versus heterozygous) for MTHFR C677T, the odds of an NTD increase by 30% to 80% (OR, 1.3, 1.8) (157, 181). In an analysis of the impact of MTHFR C677T polymorphism on RBC folate concentrations, predicted models were elevated for the MTHFR TT genotype compared with the CC genotype [RR, 1.49; 95% uncertainty interval (UI), 1.33, 1.70] and for the TT genotype compared with the CT genotype (RR, 1.28; 95% UI, 1.17, 1.39) (27). These data are very similar to those from a meta-analysis of NTD risk from MTHFR genotypes, suggesting that the impact of MTHFR C677T on NTDs arises mainly from its impact on folate concentrations at lower folate intakes as NTD risk increases dramatically (27).
3.4. Hispanic Ethnicity
In the USA, racial/ethnic disparities in NTDs exist, with a greater burden of NTDs occurring among Hispanic populations (172). The underlying reasons for this disparity might be attributable to less FA supplementation use, reduced consumption of FA-fortified flour, and a greater prevalence of the MTHFR C677T polymorphism in Hispanic populations compared with non-Hispanic populations (17, 117, 150, 160). Public health interventions specifically targeted to the Hispanic population, such as fortification of corn masa flour (CMF), may decrease NTDs in this population (161).
3.5. Vitamin B12 Deficiency
Maternal vB12 deficiency increases susceptibility to NTDs in offspring (110, 118, 126, 148). vB12 is a key micronutrient that is directly involved as a coenzyme in folate metabolism. Low vB12 status is correlated with lower serum or RBC folate concentrations (22, 118). Additionally, an association between low maternal vB12 levels and NTD risk has been established independently of folate levels (126), but additional studies are needed to elucidate these interactions. FA supplementation prevents NTDs in populations with substantial vB12 deficiency (12, 64, 65).
3.6. Hyperthermia and Fever
A meta-analysis consisting of 15 studies evaluating the association between maternal hyperthermia and the risk of NTDs observed an OR of 1.92 (95% CI, 1.61, 2.29) (111). There is evidence that, in addition to fever, external heat sources that raise core body temperature, including weather-related exposures and hot tubs, can increase the risk of NTDs (4, 38). Similarly, a recent case–control study reported elevated odds of NTDs in infants of mothers who experienced fever in the periconceptional period (OR, 2.4; 95% CI, 1.5, 4.0). This association was modified by consumption of the recommended intake of 400 μg/day of FA. Mothers who consumed 400 μg/day of FA had significantly lower odds of having a child with an NTD (OR, 1.8; 95% CI, 0.8, 4.0) than mothers with FA intake below 400 μg/day (OR, 4.2; 95% CI, 2.2, 8.2) (82).
4. APPROACHES FOR INCREASING FOLATE STATUS
4.1. Introduction
Three approaches for improving folate status among WRA exist: (a) eating a diet rich in natural food folate, (b) taking a FA supplement, and (c) consuming foods fortified with FA. The first two approaches require more active personal-level action, while the third approach hinges on a population-level intervention.
High consumption of foods rich in natural folate is a beneficial part of a healthy diet, yet the daily personal action needed to obtain sufficient folate levels for NTD prevention requires behavioral change, sustained public education, and accessible and affordable folate-rich food sources (10). Obtaining the equivalent intake of 400 μg/day of FA with natural food folate alone requires consumption of a large quantity of foods not typically consumed. For example, a person would need to consume approximately 12 cups of raw spinach daily to reach the recommended level (119). Therefore, it is extremely difficult to achieve improved folate status at a population level via dietary changes alone (10, 88), and, as previously discussed, achieving improved folate status may require adequate vB12 status.
FA supplements provide excellent bioavailability, but intake of supplements requires a daily behavioral activity during the critical periconceptional period as well as sustained public education. The early closure of the neural tube by 28 days after conception presents a major barrier to the use of FA supplements for NTD prevention. Ideally, intake should occur prior to and during early pregnancy before the closure of the neural tube, which occurs before many women know they are pregnant. Currently, only 23% of WRA and 38% of those planning a pregnancy consume a daily multivitamin in the USA (173). Given that almost half of all pregnancies in the USA are unplanned (48), FA supplementation for NTD prevention requires awareness of FA through sustained public education to reach targeted audiences. However, studies have shown that educational campaigns encouraging women to increase their use of supplements have limited effectiveness at reaching high-risk populations (134). Finally, the cost of purchasing supplements could deter some women from daily intake (92).
Food fortification with FA is a low-cost public health intervention that aims to reach populations at the country or regional level and is often delivered through national programs (33). Food fortification can be either voluntary or mandatory. In the USA, voluntary fortification allows food manufacturers to add FA to foods, as long as they abide by the FDA’s food additive regulations (46). Examples of voluntary fortified foods include ready-to-eat breakfast cereals and CMF (42). Alternatively, mandatory fortification requires food manufacturers to add FA to specified foods. The FDA requires FA to be added to products labeled “enriched cereal grain products” as part of these foods’ standard of identity (45). This population-level intervention enables WRA to obtain additional FA without requiring active behavior changes or sustained education campaigns. Women consume fortified food regardless of their pregnancy intention (10). As a result, FA food fortification is a cost-effective population-level strategy for improving folate status among WRA to prevent birth defects (62).
4.2. Mandatory Folic Acid Fortification
World Health Organization (WHO) guidelines on food fortification with micronutrients provide a procedure for estimating and selecting feasible FA levels for fortification programs (174). Globally, 71 countries have passed legislation or regulations to mandate fortification of food sources with FA on the basis of information from the Global Fortification Data Exchange, UN Food and Agriculture Organization, and WHO (see https://fortificationdata.org; see also Supplemental Appendix 1 for specific country data).
4.2.1. Food vehicles.
The primary vehicles for FA fortification are wheat flour, maize (corn) flour, and rice. Wheat flour fortification is used predominantly in countries with mandatory FA fortification. The fortified FA dosage level for wheat flour varies from 100 to 511 μg per 100 g of flour (Supplemental Appendix 1). In the USA, enriched cereal grain products are fortified at 140 μg per 100 g, contributing to a total median daily intake of 220 μg of FA and natural food folate in nonpregnant WRA (29).
Maize flour or cornmeal is the second most common food vehicle (used in 16 countries) for mandatory FA fortification. The fortified FA dosage level in countries with mandatory maize flour fortification varies from 100 to 260 μg per 100 g of flour. Mandatory rice fortification with FA has been used in five countries, with FA dosage levels varying from 100 to 231 μg per 100 g of rice. FA retention in fortified rice ranges between 76% and 96%, depending on the preparation and cooking method, and limited data show that fortified rice may slightly increase serum folate concentrations (175).
In regions where grains are not industrially milled or widely consumed, fortification of alternative nongrain food vehicles with FA might help reduce the prevalence of NTDs. Beverages, condiments, and seasonings have been or are being considered for FA fortification. Costa Rica mandated that all milk products be fortified with 40 μg FA per 250 mL of milk (7) and is the only country to have done so. Seasonings and condiments including salt, sugar, margarine, oil, and fish sauce are being explored for FA fortification. While studies on FA bioavailability in seasonings and condiments are limited (34), fortification of salt with FA might be a promising strategy. Salt is widely consumed and has succeeded as a fortification vehicle through iodization, which led to a significant global reduction in iodine deficiency (103). The efficacy of adding FA to salt has been demonstrated as a double-fortified vehicle with iodine (90, 102); a triple-fortified vehicle with iodine and iron (109); and a quadruple-fortified vehicle with iodine, iron, and vB12 (98). McGee et al. (102) reported stable double-fortified salt with iodine and FA using a single-spray solution that does not require any change in existing salt iodization equipment or procedures. After 12 months, the amount of FA and iodine retained in the salt was >80% and >90%, respectively (102). Additionally, a recent study estimated that 65% of FA-preventable NTD cases could be prevented annually through salt fortification (80).
4.2.2. Fortification of multiple food vehicles.
Safe and effective fortification requires establishing a balance between addressing inadequate nutritional intake and avoiding excessive intake, particularly in populations not at risk for nutritional inadequacies. While fortification of multiple food vehicles with the same micronutrient (e.g., FA) increases the likelihood that the benefits of a fortification program will extend to a broader range of the population, consumption of multiple fortified foods might result in a higher likelihood of nutrient intakes near or above tolerable upper levels. Estimating potential dietary contributions of fortified foods within populations can help determine which food vehicle to fortify and how much of a nutrient should be added to the fortified food.
Implementing fortification of food products not commonly consumed together might extend fortification coverage to diverse populations without overfortifying. For example, the USA allows for FA fortification of both wheat flour (mandatory) and CMF (voluntary). It is unlikely that individuals are “doubling up” on FA intake in a particular meal, given that typical dietary patterns include consumption of either CMF or wheat flour, but not both. Two recent studies (168, 169) that evaluated the usual intake of FA by WRA in the USA using data from the National Health and Nutrition Examination Survey (NHANES) suggest that the current fortification programs have not led to usual intakes above the tolerable upper intake level for FA. The usual intake from mandatory fortification alone for women aged 12–49 is only 115 μg/day [interquartile range (IQR), 79–156]. This moderate intake has very low variance and is ideal for a mandatory fortification program. Among women who consumed FA from all three potential sources of FA (i.e., mandatory enriched cereal grain products, voluntary fortified ready-to-eat cereals, and supplements), the median FA intake was 661 μg/day (IQR, 519–830), which is well within the daily intake recommendations of 400 μg/day by the CDC and 400–800 μg/day by the US Preventive Services Task Force (29). The CMF program was implemented in 2016, and Hispanic women had similar usual intakes pre-CMF (177 μg/day; IQR, 85–299) versus post-CMF (161 μg/day, IQR 71–277) (168), consistent with the general population (169).
5. MONITORING THE IMPACT OF FOLIC ACID FORTIFICATION
5.1. Monitoring the Impact of Folic Acid Fortification Using Biomarkers on Neural Tube Defects
As shown in Figure 1, no RCTs have assessed the impact of FA fortification, because it is not possible to conduct an RCT after the implementation of a fortification program due to universal exposure across the population. The literature is therefore limited to pre- and postfortification implementation studies, which are ecological and subject to the inherent biases of this type of design.
This primary method of monitoring FA fortification’s impact on NTD prevalence both pre- and postfortification (Figure 3a) (Supplemental Appendix 2) depends on many factors, including baseline NTD rates, folate concentrations, population characteristics, fortification levels, program implementation, and population folate consumption. Since FA fortification was implemented in the USA, analyses of pre- and postfortification data (1995–2011) from 19 population-based birth defect surveillance programs have shown that the prevalence of NTDs decreased by 21% to 35% (172). Several meta-analyses have reported similar reductions in NTD prevalence following mandatory FA fortification in other countries. A meta-analysis of eight population-based, large-scale studies from five countries (Canada, USA, Chile, Argentina, and South Africa) assessed the effect of mandatory FA fortification on the prevalence of NTDs and estimated a 46% risk reduction (RR, 0.54; 95% CI, 0.46, 0.63) (14). Another meta-analysis of eight studies, most of which were conducted in Central or South America, showed a significant postfortification reduction in total NTDs (OR, 0.59; 95% CI, 0.49, 0.73) as well as NTD subtypes, including both anencephaly (OR, 0.49; 95% CI, 0.4, 0.6) and spina bifida (OR, 0.66; 95% CI, 0.53, 0.82) (81). Furthermore, a study of FA fortification status in 123 countries showed that the countries with mandatory FA fortification had a lower prevalence of spina bifida (3.5 per 10,000 live births) compared with countries without mandatory FA fortification (5.3 per 10,000 live births) (3).
Figure 3.
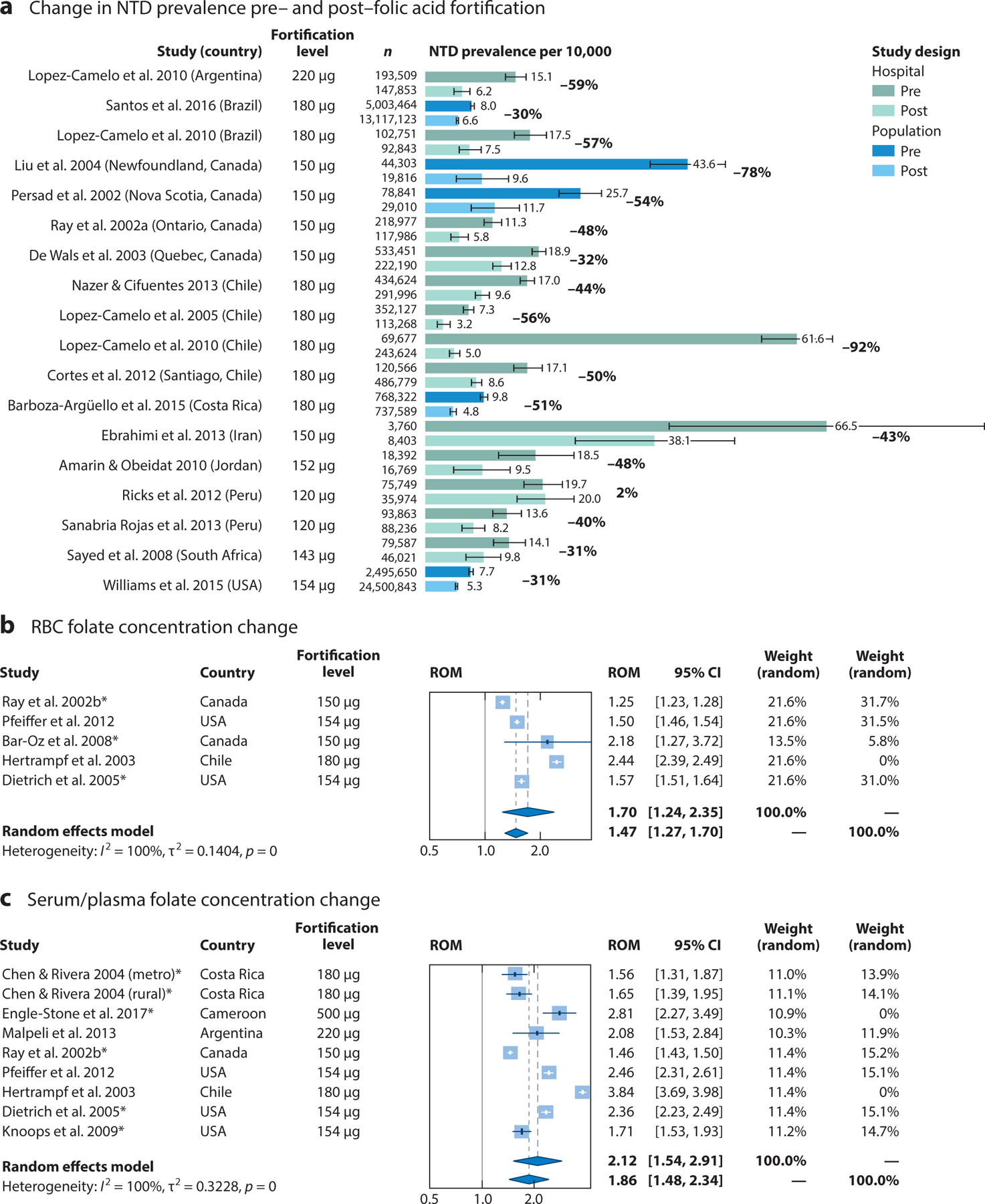
Impact of folic acid fortification on neural tube defect (NTD) prevalence, red blood cell (RBC) folate concentrations, and serum/plasma folate concentrations. (a) NTD rates per 10,000 live births before and after the implementation of mandatory folic acid fortification from population and hospital surveillance programs. Results for studies that monitored all NTDs in either population or hospital surveillance programs are compared during pre- and postfortification periods. For these studies, the postfortification period lasted at least 2 years following the implementation of mandatory folic acid fortification. The standard error was calculated using a Poisson distribution for rare events. Results for studies comparing pre- and postfortification (b) RBC folate concentrations and (c) serum folate concentrations are summarized as a ratio of means (ROM) corresponding to the fold change in post-versus prefortification folate concentration levels. Asterisks indicate concentrations measured via radioimmunological assays, as opposed to microbiological assays. Despite the high level of heterogeneity across studies, the overall effect using a random effects model demonstrated a significant fold change in folate concentration following the introduction of folic acid fortification (P < 0.001 in both cases). RBC folate concentrations increased approximately 1.70-fold (95% CI, 1.24, 2.35), and serum folate concentrations increased approximately 2.12-fold (95% CI, 1.54, 2.91). A subanalysis excluding countries that had fortification levels >400 μg per 100 g of product was also conducted; RBC folate concentrations increased approximately 1.47-fold (95% CI, 1.27, 1.70) and serum folate concentrations increased approximately 1.86-fold (95% CI, 1.48, 2.34) following folic acid fortification. For the search strategy and references, see Supplemental Appendix 3.
Changes in NTD prevalence from pre- to postfortification show that the percent decrease in NTD rates varies from country to country and that the countries with the highest baseline rates have the largest percent decrease (Figure 3a). However, using changes in NTD rates alone as an outcome makes it difficult to attribute the changes to fortification or determine which populations are being missed by a fortification program and is dependent on having an effective surveillance system that assesses birth defects not only among live-born infants but also among pregnancies ending in stillbirths or elective terminations.
In 2015, the WHO established the RBC folate concentration for NTD prevention as a tool to assess the need for or success of a fortification program (25, 163). This five-step process assesses, monitors, and adjusts programs on the basis of RBC folate concentrations among WRA. Several countries and regions, including the USA and Guatemala, have assessed RBC folate concentrations after fortification (29, 138, 163). Data on the USA show that while most WRA have RBC folate concentrations consistent with optimal NTD prevention, more than 22% remain at risk due to folate insufficiency (163). Median FA intake is only 115 μg/day (the predicted NTD prevalence in this group is 8.5 per 10,000 births); with additional sources of FA, it is estimated that up to 700 additional NTDs could be prevented annually in the USA (29). Utilizing the WHO guidelines, the USA also evaluated the addition of a new voluntary FA source (CMF) that was authorized in 2016 to address the higher rate of NTDs among Hispanic populations (168). In Guatemala, researchers demonstrated the success of fortification by showing increased RBC folate concentrations in metropolitan areas, where fortified products were readily available, compared with other regions of the country, where there was poor penetration with fortified products and different food consumption patterns (138). Countries without mandatory FA fortification, such as India, have also used the guidelines to examine recent regional population–based data. Results showed that the prevalence of RBC folate deficiency and insufficiency among WRA was 7.6% and 79.3%, respectively, and the NTD prevalence predicted on the basis of RBC folate concentrations was 20.6 per 10,000 births (95% UI, 16.5, 25.5) (49), suggesting that an FA fortification program would probably be beneficial.
The laboratory gold standard for assessing RBC folate concentrations is a microbiologic assay, as most other assays have established inaccuracies and/or need adjustments (130, 164). Few studies (Figure 3b) (Supplemental Appendix 3) have compared pre- and postfortification RBC folate concentrations using microbiological assays. To address this severe limitation, we conducted a systematic search of the literature and modeled the change for studies that used the same assay in both pre- and postfortification periods (for details, see Supplemental Appendix 3); however, the results should be interpreted with caution. Despite the limitations of the available data, changes in RBC folate concentrations are consistent with expected changes observed in supplementation trials. RBC folate concentrations increased approximately 1.47-fold (95% CI, 1.27, 1.70). Likewise, serum folate concentrations, which are not as susceptible to the assay bias, increased approximately 1.89-fold (95% CI, 1.49, 2.45) following FA fortification, also consistent with supplementation data (Figure 3c). These findings are reassuring, given their consistency with the anticipated folate concentration increases of one-and-a-half- to twofold over baseline found in supplementation studies. Pre- and postfortification biomarkers of populations in countries that are implementing new FA fortification programs (e.g., United Kingdom, New Zealand) will be of great interest.
5.2. Impact of Folic Acid Fortification on Other Health Outcomes
While folic acid fortification was implemented to target persons capable of becoming pregnant for the prevention of NTDs, this intervention affects the entire population and multiple health outcomes. Studies have examined the effects of fortification programs on other birth defects, prevention of anemia, cardiovascular disease, and cancer.
5.2.1. Orofacial clefts and congenital heart defects.
In addition to NTDs, there might be an association between FA fortification and reduction of orofacial clefts (OFCs). A meta-analysis of studies comparing the prevalence of OFCs before and after mandatory FA fortification in multiple countries, including Argentina, Brazil, Chile, and the USA, demonstrated that the prevalence of nonsyndromic cleft lip and palate significantly decreased (RR, 0.88; 95% CI, 0.81, 0.96) following fortification, but there were no significant effects of fortification on the prevalence of total OFCs or cleft palate only (105). These results are similar to those from another meta-analysis, which demonstrated a nonstatistically significant decrease in both cleft lip with or without palate (RR, 0.87; 95% CI, 0.76, 1.00) and cleft palate only (RR, 0.90; 95% CI, 0.71, 1.15) (184).
Certain types of congenital heart defects (CHDs) might also be prevented through FA fortification. A Canadian time-trend analysis that compared the prevalence of severe CHDs prefortification (1990–1998) with the prevalence postfortification (1998–2005) demonstrated a decrease from 1.64 cases (95% CI, 1.55, 1.73) per 1,000 births prefortification to 1.47 (95% CI, 1.37, 1.58) per 1,000 live births postfortification, or a 6% decrease per year in the 7 years following the initiation of mandatory FA fortification (74). Another Canadian population-based study of all live births and stillbirths from 1990 to 2011 demonstrated lower rates of conotruncal defects (RR, 0.73; 95% CI, 0.62, 0.85), coarctation of the aorta (RR, 0.77; 95% CI, 0.61, 0.96), ventricular septal defects (RR, 0.85; 95% CI, 0.75, 0.96), and atrial septal defects (RR, 0.82; 95% CI, 0.69, 0.95) following FA fortification (93). Although FA fortification was associated with an overall 11% decrease in the prevalence of CHDs, it was not associated with a significant reduction in severe nonconotruncal heart defects, suggesting a differential effect across subtypes of CHDs (93).
5.2.2. Prevention of anemia.
Folate deficiency megaloblastic anemia is a consequence of inadequate folate intake (73). In the USA, the usual intake of folate from food is only ∼170 DFEs, less than half the recommended dietary allowance of 400 DFEs for the prevention of anemia (29, 168). Studies comparing data prefortification (1988–1994) and postfortification (1999–2004) among WRA found a 27.9% reduction in the prevalence of anemia (60), a 50% increase in RBC folate concentrations, and a subsequent decrease in folate deficiency from 38% to 5% (129). The benefits were not limited to WRA. Meta-analyses of four studies and eight studies suggest that FA fortification is associated with significant declines of folate deficiency in broader populations (RR, 0.20; 95% CI, 0.15, 0.25) and with significant increases in plasma folate (standard mean difference, 1.25 nmol/L; 95% CI, 0.5, 1.99), respectively (81). In older adults in the USA, folate deficiency anemia has nearly been eliminated after mandatory FA fortification, consistent with findings in other countries such as Canada and Australia (123).
5.2.3. Cardiovascular disease.
Elevated homocysteine concentrations are consistently associated with cardiovascular disease, and FA fortification has a significant effect on plasma levels (75, 179). Following FA fortification in the USA, there were markedly lower homocysteine levels among middle-aged and older adults enrolled in the Framingham Heart Study, a large epidemiological study of heart disease (75). Data on homocysteine levels from NHANES were used to predict that fortification alone would decrease cardiovascular events by 8% in women and 13% in men (159). A study that evaluated age-adjusted stroke mortality rates in the USA, Canada, England, and Wales from 1990 to 2002 (179) found that the annual decline in stroke mortality was between 0.7% and 1.4% in each of these countries in the prefortification years but following fortification shifted to a 2.7–2.9% annual decline in the USA and a 5.4–5.6% annual decline in Canada (179). In contrast, there was no change in decline of age-adjusted stroke mortality in England and Wales after 1998, suggesting that FA fortification decreases stroke mortality. These types of ecological comparisons need to be interpreted with great caution. However, strengthening the case for this possibility, a large, randomized trial of FA supplementation from China found that intake of FA, in addition to the usual medicine taken for stroke prevention, decreased stroke risk, with greater benefit for individuals with lower initial folate concentrations (89).
5.2.4. Cancer.
Results from studies evaluating the incidence of cancer following FA fortification have been largely reassuring. While an increase in colorectal cancer (CRC) diagnoses in the USA and Canada was observed in 1996 (coinciding with the implementation of voluntary FA fortification), a decline in both CRC diagnoses and mortality has been observed since 1998, after the implementation of mandatory FA fortification (83). Data from the Australian Cancer Database also show a decrease in CRC since 1999, yet this decline became much more pronounced in 2010, when Australia mandated FA fortification (166).
Additionally, two studies used the Pediatric Oncology Group of Ontario’s database, which identifies approximately 95% of all pediatric cancers in Ontario, to evaluate the effect of FA fortification on the incidence of childhood cancer (54, 63). FA fortification was associated with a 62% reduction (95% CI, 38–77%) in the incidence of neuroblastoma (i.e., from 1.57 per 10,000 children prefortification to 0.62 cases per 10,000 children postfortification) (54) and a 26% reduction (95% CI, 5–43%) in the incidence of Wilms tumor (i.e., from 1.94 per 100,000 children to 1.43 per 100,000 children) (63). Data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results program demonstrated a similar reduction in the incidence of Wilms tumor in the USA (91). These ecological studies should be interpreted with caution.
5.3. Is There a Need for Continued Supplementation Postfortification?
The current CDC recommendation for 400 μg/day of FA from supplements or fortified foods, or a combination of both, has stood for 30 years (19). Regardless of MTHFR genotype, women will reach optimal blood folate concentrations if they consume 400 μg/day for 3–6 months or more; however, this effect is not observed at consumption levels of 100 μg/day (31). Fortification programs add a modest amount of FA to a vehicle (100–260 μg FA per 100 g of product), targeting a usual intake of approximately 200 μg/day. With current US consumption patterns, the median overall intake from all sources (natural food folates, supplements, and mandatory and voluntary fortification) is 237 μg/day (IQR, 146–370); of this amount, 115 μg/day (IQR, 79–156) comes from mandatory fortification alone (29). This intake results in 22.8% of US women having insufficient RBC folate concentrations for optimal NTD prevention (163). Therefore, the US Preventive Services Task Force continues to recommend supplementation of 400–800 μg/day for women planning pregnancy (165).
Higher intakes of FA might be needed in specific situations, such as exposure to agents that require detoxification with one-carbon sources, including heavy metals (e.g., arsenic, lead), or intake of folate agonists. Chronic exposure to inorganic arsenic affects 140 million people worldwide. FA supplementation facilitates arsenic methylation and excretion and is associated with lower levels of serum arsenic (15, 58, 59, 86). A case–control study showed that the protective effect of FA supplementation for NTDs declined as inorganic arsenic concentrations in drinking water increased from 1 μg/L to 25 μg/L (OR from 0.22 to 1.03; 95% CI from 0.13, 0.37 to 0.55, 0.91) (101). Additional research is needed.
5.4. Cost–Benefit of Folic Acid Fortification
NTDs impose a significant economic burden on health care systems, as lifetime direct and indirect costs are substantial. In addition to costs accrued shortly after birth from hospitalizations and surgeries, persons with NTDs require ongoing care; have high rates of comorbidities; and often need other services, such as special education, developmental services, or mobility-related assistive devices. Caregivers of children with NTDs are affected by reductions in paid work time. Lifetime costs of NTDs vary across countries, likely due to differences in health care prices and availability of treatments and services. In the USA, estimated lifetime direct costs for a person with spina bifida were $791,000 in 2014 (61).
Food fortification is a relatively inexpensive public health intervention to reduce micronutrient deficiencies. The cost of FA fortification is estimated to be $0.20–0.30 per metric ton, or approximately $0.06 per person per year, which equates to $607 million in costs averted each year (61). Chile has reported similar results, with an estimated savings of $11.80 for every $1.00 spent on fortification equating to $89 per disability-adjusted life year averted (95). The Chilean study considered life expenses only up to the age of 22 years, so these cost savings are probably underestimated (95). A study from South Africa reported a cost–benefit ratio of 30 to 1 of FA for the prevention of NTDs (143). More evidence is available from Europe. Using 2009 birth surveillance data from EUROCAT and assuming a 50% reduction in NTDs, researchers estimated that a total of €79.6 million (US$94.2 million) of direct lifetime medical costs could have been prevented annually if Germany had adopted FA fortification policies (122).
6. FOLIC ACID SAFETY AND INTERRELATIONSHIP BETWEEN FOLATE AND VITAMIN B12
The effectiveness and safety of FA fortification programs have withstood the test of time. However, theoretical concerns persist, including the effects of interactions between high folate and low vB12 status on anemia (especially among older adults), cognition, and metabolism. There is also heightened interest in the potential effects of antenatal maternal FA exposure on cardiometabolic risk and the development of diabetes in children from populations where vB12 deficiencies are prevalent. It is critical to evaluate all evidence for each of these concerns, keeping in mind the impact of study designs and examining each aspect of folate and vB12 metabolism (intake, absorption, processing, utilization, and excretion) to consider ways to mitigate these concerns.
6.1. Masking of Vitamin B12 Deficiency Anemia
Vitamin B12 deficiency can cause macrocytic anemias and neurological complications, which, if left untreated, can be irreversible. Neurologic complications can be present with or without a macrocytic anemia. However, early case reports describing the occurrence or progression of neurological complications among vB12-deficient patients treated with FA led to concerns that increased FA exposure through fortification might lead to the masking of vB12 deficiency by correcting macrocytic anemia while allowing neurologic complications to progress. This hypothetical situation is particularly concerning for older adults, who are more likely to have vB12 deficiency (73).
Standard clinical practice includes testing any individual with unexplained neurological complications for vB12 deficiency regardless of anemia status. A Canadian study reported that the prevalence of serum vB12 insufficiency (<150 pmol/L) with high serum folate status (>45 nmol/L) among older Canadian women increased postfortification compared with prefortification (0.09% versus 0.61%) (136). Although this study did not examine folate and vB12 in regard to hematological indicators, results raised concerns about masking of vB12 deficiency by FA. Therefore, some public health and nutrition experts have suggested adding vB12 and FA to supplements and fortified foods, respectively, to reduce this risk.
If increased FA intake truly masks anemia in individuals with vB12 deficiency, then we would expect a drop in vB12 deficiency anemia following FA fortification. Several studies have compared the prevalence of vB12 deficiency without anemia before and after FA fortification (94, 104, 108, 132); only one study from an academic hospital reported an increase in low serum vB12 without macrocytosis among US adults (≥19 years) after fortification (176). Because macrocytosis is not a specific indicator of vB12 status and could also be a result of folate deficiency, an overall improvement in population folate status may explain the lower prevalence of macrocytosis postfortification. As a whole, the available epidemiological evidence suggests that there is little risk of FA fortification masking vB12 deficiency macrocytosis and adversely affecting the clinical presentation of vB12 deficiency, in particular among older adults. This view agrees with assessments conducted by multiple governmental and independent advisory agencies that support the safety of mandatory fortification with FA (43, 45, 50–52, 67, 145).
As noted in a recent review (9), blood assays for folate and vB12 had not yet been developed in early case reports of vB12-deficient patients being treated with folic acid (37). Routine diagnostic tests used at the time (e.g., hemoglobin, hematocrit, stained blood smears) were not specific for vB12 deficiency and thus would not have been able to distinguish among macrocytic anemias from vB12, folate, or some other nutritional deficiency. Therefore, neurological complications from early reports likely arose from incorrect treatment of vB12 deficiency anemia with FA and should not be used as evidence of FA’s toxicity (9, 37). Now that clinical and laboratory tools for diagnosing vB12 deficiency are readily available, incorrect treatment of vB12 deficiency with FA is a medical error.
6.2. Folate and Vitamin B12 Interactions
Another concern about the potential health risks associated with increased FA intake is the effect of the interaction between folate and vB12 status on cognition and vB12 metabolism. Three cross-sectional studies (106, 147, 152) reported that abnormalities in total homocysteine and/or methylmalonic acid (MMA, a metabolic indicator of vB12 status) were associated with concurrent higher folate and low vB12 status (defined through different cutoffs and summary measures). Several other studies also assessed the association between high folate and low vB12. For anemia, three cross-sectional studies (two in voluntary fortification settings in the United Kingdom and Ireland and one after mandatory fortification in the USA) also found that higher serum folate concentrations did not affect the association between low vB12 status and anemia or hemoglobin concentrations (24, 66, 107).
6.2.1. Cognition and folate/vitamin B12 status.
The US Framingham Heart Study examined older adults’ pre- and postfortification serum/plasma folate levels, vB12 status, and cognition. The study found that, among older adults with low levels of plasma vB12 (<258 pmol/L), faster cognitive decline (e.g., annual change in Mini–Mental State Examination scores) was associated with plasma folate in the highest quintile (≥21.75 nmol/L) in comparison to the lowest quintile (<5 nmol/L) (114). Two cross-sectional analyses of NHANES 1999–2002 (after mandatory fortification) reported an interaction between folate and vB12 status among older adults. In older persons with low vB12 status, poorer cognitive function was associated with higher serum folate concentrations (OR, 2.6; 95% CI, 1.1, 6.1) (112). However, in the same study, higher serum folate protected older adults with normal vB12 status from cognitive impairment (OR, 0.5; 95% CI, 0.2, 0.96). In the second NHANES study of the same population, circulating unmetabolized FA was associated with lower cognitive function among seniors with low vB12 status but not among those with normal vB12 status (pinteraction = 0.007) (113).
Other studies could not corroborate these observations of higher folate status affecting the association between low vB12 status and impaired cognition. One cohort study of older adults in the United Kingdom found that while cognitive decline was not associated with either serum folate or vB12, the rate of decline slowed with indicators of active vB12 status (e.g., holotranscobalamin, MMA) (23). This study found no interaction between folate and vB12 status, as higher folate status was not associated with faster cognitive decline among persons with low vB12 status (e.g., low holotranscobalamin concentrations). Four additional cross-sectional studies found no evidence that higher folate status increased the association between low vB12 status and poor cognitive performance. Under the voluntary fortification setting of the United Kingdom, an analysis of combined data from two cross-sectional studies found that low vB12 status (holotranscobalamin <45 pmol/L) was associated with cognitive impairment among older British adults with normal folate status (OR, 1.45; 95% CI, 1.19, 1.76) but that higher serum folate status (>30 nmol/L) did not increase the association between low vB12 status and cognitive impairment (OR, 1.50; 95% CI, 0.91, 2.46) (24). These associations remained similar even at a higher serum folate cutoff point (>60 nmol/L). The association between low vB12 status and poor cognition (OR, 1.56; 95% CI, 1.30, 1.88) did not worsen in the presence of higher serum folate (OR, 2.46; 95% CI, 0.90, 6.71).
Among a population of older Latinos with low vB12 status in California after mandatory fortification, higher folate concentrations were not associated with poorer cognitive performance (106). Likewise, combined higher plasma folate and low vB12 status were not associated with cognitive impairment among a small group of octogenarian and centenarian African Americans and Caucasians living in the US state of Georgia (66). A more recent analysis using data from NHANES 2011–2014 reported that among older adults (≥60 years) with low serum vB12 concentrations (≤258 pmol/L), higher folate status (defined as serum folate ≥59 nmol/L or RBC folate ≥1,609 nmol/L) was protective against poor cognition (OR, 0.61; 95% CI, 0.45, 0.83) (139). However, individuals with concurrent higher folate and low vB12 status were not more likely to have poor cognitive performance (OR, 0.89; 95% CI, 0.36, 2.22) (139). In addition, 34% of persons with high folate and low vB12 levels were supplemented with vB12 (139). A moderate association between high MMA/high folate and poor cognition was attenuated after the authors adjusted for kidney function (139). Without increased folic acid intake, poor kidney function has been associated with increased folate concentrations (169). Taken together, these results suggest that both vB12 intake (indicator of possible malabsorption) and kidney function are important confounders of the reported association of high folate and low vB12 (139). Therefore, studies of high folate and low vB12 should be interpreted with caution.
Regardless of the limited evidence, several hypotheses have been suggested for associations between concurrent high folate and low vB12 status and cognition, anemia, and vB12 metabolism. One theory is that excess FA and folate metabolites might directly affect or worsen vB12 deficiency by inducing oxidation of vB12 and reducing the amount of available vB12 for important reactions [i.e., conversion of homocysteine to methionine via MS or the conversion of methylmalonyl–coenzyme A (CoA) to succinyl-CoA in the mitochondria via methylmalonyl-CoA mutase], which might lead to neurologic, hematologic, or abnormal metabolic consequences (147). Whether FA has any oxidative effect on vB12 is unknown.
A second theory is based on the methylfolate trap hypothesis, which centers on vB12 deficiency and the disruption in MS activity (69, 146). Without vB12, MS cannot convert homocysteine to methionine, resulting in a decrease in cellular SAM. This decrease in turn disrupts critical methylation reactions that produce and maintain myelin, the insulating layer surrounding neurons. Disturbances in myelin formation can lead to neurological complications, including cognitive impairment (171). The disruption in the function of MS would also block the conversion of 5-MTHF to THF, resulting in an accumulation of intracellular 5-MTHF. 5-MTHF can easily move from inside the cell into the blood circulation, so increases in serum folate concentrations would be observed. Thus, elevated serum folate concentrations can be observed among persons with vB12 deficiency (87, 155).
A third hypothesis to explain the outcomes observed among individuals with concurrent high folate and low vB12 status is that this group has an underlying undetected condition that affects the absorption of vB12 (e.g., pernicious anemia, other types of malabsorption) (11). According to this theory, in the absence of underlying disease, supplement users metabolize FA and vB12 normally and, consequently, have higher blood concentrations of both vitamins. However, for individuals with pernicious anemia, the inability to absorb supplemental vB12 combined with the slow progressive nature of the disease eventually depletes the body’s vB12 stores. Because these individuals are still able to absorb and metabolize FA normally, an apparent association would be observed between higher blood folate concentrations and clinical and metabolic abnormalities. Consistent with this theory are findings from studies that observed a significantly higher proportion of individuals who took supplements containing vB12 among persons with concurrent higher folate and low vB12 status than among those with concurrent normal folate and low vB12 status. Data from NHANES showed that the majority (>90%) of seniors with serum folate concentrations in the highest quintile were multivitamin supplement users who took not only FA but also enough vB12 (median intake, 16.6 μg) to correct any vB12 insufficiency under normal circumstances (11). Additional analyses from NHANES indicate that adults with higher FA intake and higher serum folate concentrations had higher vB12 intakes and were significantly less likely to have vB12 depletion (180).
Among studies examining the interaction between folate and vB12 status on health outcomes, an important methodological limitation is the inability to establish a causal link between an individual’s folate and vB12 status and their risk for anemia, cognitive impairment, and abnormal vB12 metabolism. In our review, most of the evidence comes from cross-sectional studies that are weaker in epidemiologic study design (Figure 1); findings from epidemiologically stronger cohort studies failed to provide consistent results. Cross-sectional studies supporting an association between concurrent high folate/low vB12 status and adverse clinical and metabolic outcomes might be susceptible to reverse causality, making higher folate and low vB12 status the effect rather than the cause of clinical and metabolic abnormalities. This theory is plausible if persons with low vB12 status seeking medical care for their symptoms (e.g., fatigue, cognitive changes, tingling, numbness of extremities) were advised by their providers to take vitamin supplements, thus raising their blood folate concentration and creating a spurious association between higher folate status and anemia, cognitive impairment, and elevated total homocysteine and MMA concentrations. Carefully designed prospective studies, Mendelian randomization, and systematic and ongoing monitoring of populations with greater exposure to FA could enable us to better understand any potential interactions between higher folate status and vB12 deficiency. Furthermore, interpretation of studies examining the effects of fortification may differ from that of studies examining the effects of supplementation, as amounts of FA consumption vary greatly depending on the source (180, 182). Because the contribution of fortification to higher blood concentrations is minor compared with the contribution of supplements, conclusions drawn from supplementation studies may not apply to fortification.
6.2.2. Antenatal maternal folic acid exposure and risk of diabetes and metabolic disease.
Evidence from epidemiological and animal studies suggests that unbalanced maternal nutrition might lead to changes in the intrauterine environment, thereby affecting the risk of health outcomes in the offspring later in life (78, 121, 124). Studies have suggested that shifts in the availability of FA and vB12 during critical developmental periods in utero can induce long-term modifications in gene expression via epigenetic mechanisms and alter fetal development and metabolic programming (30, 121, 151, 177). A recent topic of interest is the longer-term effect of antenatal FA exposure (i.e., prenatal FA supplementation or higher maternal folate concentration during pregnancy) on diabetes and metabolic disease in children among populations with prevalent vB12 deficiency (13, 16).
6.2.3. Diabetes and metabolic disease.
A population-based cohort study in India reported that mothers with higher maternal RBC folate status during pregnancy (at 28 weeks gestation) were more likely to have children with higher insulin resistance [as measured by homeostasis model assessment of insulin resistance (HOMA-IR)] and greater adiposity, as measured by fat mass and percent body fat at age 6 years (178). Approximately two-thirds of this population had vB12 deficiency. Insulin resistance was highest among children born to mothers with a combination of plasma vB12–deficient status and higher RBC folate concentrations (178). These findings raised concerns that higher folate or FA intake during pregnancy among women who are vB12 deficient might lead to an increased risk of type 2 diabetes in children.
In contrast to these findings, two analyses from a community-based RCT in Nepal reported that antenatal supplementation with FA, either alone or in combination with other micronutrients, did not increase the risk for metabolic disease in children (153, 154). This RCT under the Nepal Nutrition Intervention Project–Sarlahi examined the effects of antenatal micronutrient supplementation on health outcomes in offspring. A total of 4,926 pregnant women were enrolled and randomized into five different supplement groups: vitamin A alone (control); vitamin A and FA; vitamin A, FA, and iron; vitamin A, FA, iron, and zinc; and vitamin A, FA, iron, zinc, and 11 other micronutrients. Apart from those in the control group, the women were given daily supplements containing 400 μg of FA from the time of enrollment in early pregnancy (mean gestation at enrollment, 11 ± 5 weeks) to 3 months postpartum. Approximately 28% of the women had vB12 deficiency (serum vB12 <150 pmol/L) in the first trimester prior to supplementation, and 12% had folate deficiency (serum folate <6.7 nmol/L) (77). Children were followed from birth through age 6–8 years and were assessed for cardiometabolic risk factors, insulin resistance, and peripheral adiposity.
While no differences in insulin, glucose, HOMA-IR, cholesterol, triglycerides, or blood pressure were observed across the supplement groups in comparison to children from mothers in the control group, the risk for metabolic syndrome was significantly lower among children from mothers in the FA-only group (OR, 0.63; 95% CI, 0.41, 0.97; P = 0.03) (154). In comparison to controls, children from mothers in the FA-only group and the FA, iron, and zinc group had a lower risk for microalbuminuria, a well-known marker for kidney and cardiovascular disease. These results suggest that antenatal maternal FA supplementation does not increase the potential for cardiovascular disease and diabetes in children within this population with prevalent vB12 deficiency.
In a substudy of this RCT among women who provided blood samples early (at 10.2 + 4.1 weeks gestation) and late (at 32.6 + 2.9 weeks gestation) in pregnancy, HOMA-IR was 26.7% higher among children whose mothers had vB12 deficiency during early pregnancy compared with children whose mothers did not have vB12 deficiency during early pregnancy (p = 0.02) (153). In this analysis, neither early nor late pregnancy maternal folate status was associated with insulin resistance in children, and no interaction was observed between maternal folate and vB12 status relative to childhood insulin resistance. Among children whose mothers had vB12 deficiency early in pregnancy, maternal supplementation with FA alone or in combination with other micronutrients did not change the risk for insulin resistance in children. Furthermore, no interaction was observed between maternal vB12 status and vitamin supplementation for HOMA-IR in children (p > 0.1 for all supplementation groups). Taken together, results from these RCT analyses suggest that maternal vB12 deficiency in early pregnancy is an important predictor of childhood insulin resistance and that antenatal supplementation with FA (either alone or in combination with other micronutrients) does not lead to (and may even lower the risk for) diabetes and metabolic disease among their offspring.
The mechanisms by which maternal folate and vB12 status might affect metabolic status and neurodevelopment in children are not well understood. Because both folate and vB12 are important methyl donors in the one-carbon metabolism pathway, they could play a part in epigenetic regulation through DNA and/or histone methylation, controlling when and where genes are expressed and generating significant effects on disease development (30, 55, 56, 76, 121, 149, 151, 177). Further research is required to clarify the role of maternal folate and vB12 nutrition during pregnancy on health outcomes in children.
Supplementary Material
SUMMARY POINTS.
Mandatory FA fortification reduces NTD prevalence, increases blood folate concentration less than twofold, and has not resulted in any known increased risk for adverse outcomes at the recommended dosages.
To fully establish the effectiveness of FA programs, additional RBC folate concentration population-based studies can be conducted to monitor program reach, as specified in WHO guidelines.
Only a few programs have used the WHO tool to identify populations that have not been reached by fortified products in quantities sufficient for NTD prevention.
High-quality studies of the interactions of FA and vB12 that carefully measure and control for intake, metabolism, and excretion may help maximize benefit and alleviate concerns about fortification.
ACKNOWLEDGMENTS
Each of the authors contributed to the writing and reviewing of the article and approved its final content. Data extraction and analysis were done in replicate by K.D. and A.W.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might affect the objectivity of this review. The writing of this review was supported by the Centers for Disease Control and Prevention through salaries alone. Findings and conclusions in this report are those of the authors and do not necessarily represent the official positions of the Centers for Disease Control and Prevention.
LITERATURE CITED
- 1.Agopian AJ, Tinker SC, Lupo PJ, Canfield MA, Mitchell LE. 2013. Proportion of neural tube defects attributable to known risk factors. Birth Defects Res. A 97:42–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amarin ZO, Obeidat AZ. 2010. Effect of folic acid fortification on the incidence of neural tube defects. Paediatr. Perinat. Epidemiol 24:349–51 [DOI] [PubMed] [Google Scholar]
- 3.Atta CA, Fiest KM, Frolkis AD, Jette N, Pringsheim T, et al. 2016. Global birth prevalence of spina bifida by folic acid fortification status: a systematic review and meta-analysis. Am. J. Public Health 106:e24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auger N, Fraser WD, Arbour L, Bilodeau-Bertrand M, Kosatsky T. 2017. Elevated ambient temperatures and risk of neural tube defects. Occup. Environ. Med 74:315–20 [DOI] [PubMed] [Google Scholar]
- 5.Bailey LB, Stover PJ, McNulty H, Fenech MF, Gregory JF 3rd, et al. 2015. Biomarkers of nutrition for development—folate review. J. Nutr 145(Suppl.):1636–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey SWA, June E. 2018. The pharmacokinetic advantage of 5-methyltetrahydrofolate for minimization of the risk for birth defects. Sci. Rep 8:4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barboza-Argüello M, Umaña-Solís LM, Azofeifa A, Valencia D, Flores AL, et al. 2015. Neural tube defects in Costa Rica, 1987–2012: origins and development of birth defect surveillance and folic acid fortification. Matern. Child Health J 19:583–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bar-Oz B, Koren G, Nguyen P, Kapur BM. 2008. Folate fortification and supplementation—are we there yet? Reprod. Toxicol 25:408–12 [DOI] [PubMed] [Google Scholar]
- 9.Berry RJ. 2019. Lack of historical evidence to support folic acid exacerbation of the neuropathy caused by vitamin B12 deficiency. Am. J. Clin. Nutr 110:554–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berry RJ, Bailey L, Mulinare J, Bower C. 2010. Fortification of flour with folic acid. Food Nutr. Bull 31(Suppl. 1):22–35 [DOI] [PubMed] [Google Scholar]
- 11.Berry RJ, Carter HK, Yang Q. 2007. Cognitive impairment in older Americans in the age of folic acid fortification. Am. J. Clin. Nutr 86:265–69 [DOI] [PubMed] [Google Scholar]
- 12.Berry RJ, Li Z, Erickson JD, Li S, Moore CA, et al. 1999. Prevention of neural-tube defects with folic acid in China. China–U.S. Collaborative Project for Neural Tube Defect Prevention. N. Engl. J. Med 341:1485–90. Erratum. 1999. N. Engl. J. Med. 341:1864 [DOI] [PubMed] [Google Scholar]
- 13.Black MM. 2008. Effects of vitamin B12 and folate deficiency on brain development in children. Food Nutr. Bull 29(Suppl. 2):126–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blencowe H, Cousens S, Modell B, Lawn J. 2010. Folic acid to reduce neonatal mortality from neural tube disorders. Int. J. Epidemiol 39(Suppl. 1):110–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bozack AK, Hall MN, Liu X, Ilievski V, Lomax-Luu AM, et al. 2018. Folic acid supplementation enhances arsenic methylation: results from a folic acid and creatine supplementation randomized controlled trial in Bangladesh. Am. J. Clin. Nutr 109:380–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burdge GC, Lillycrop KA. 2012. Folic acid supplementation in pregnancy: Are there devils in the detail? Br. J. Nutr 108:1924–30 [DOI] [PubMed] [Google Scholar]
- 17.Canfield MA, Ramadhani TA, Shaw GM, Carmichael SL, Waller DK, et al. 2009. Anencephaly and spina bifida among Hispanics: maternal, sociodemographic, and acculturation factors in the National Birth Defects Prevention Study. Birth Defects Res. A 85:637–46 [DOI] [PubMed] [Google Scholar]
- 18.Carmichael SL, Rasmussen SA, Shaw GM. 2010. Prepregnancy obesity: a complex risk factor for selected birth defects. Birth Defects Res. A 88:804–10 [DOI] [PubMed] [Google Scholar]
- 19.CDC (Cent. Dis. Control Prev.). 1992. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. Morb. Mortal. Wkly. Rep. Recomm. Rep 41:1–7 [PubMed] [Google Scholar]
- 20.CDC (Cent. Dis. Control Prev.). 2010. CDC Grand Rounds: additional opportunities to prevent neural tube defects with folic acid fortification. Morb. Mortal. Wkly. Rep 59:980–84 [PubMed] [Google Scholar]
- 21.Chen LT, Rivera MA. 2004. The Costa Rican experience: reduction of neural tube defects following food fortification programs. Nutr. Rev 62:S40–43 [DOI] [PubMed] [Google Scholar]
- 22.Chen MY, Rose CE, Qi YP, Williams JL, Yeung LF, et al. 2019. Defining the plasma folate concentration associated with the red blood cell folate concentration threshold for optimal neural tube defects prevention: a population-based, randomized trial of folic acid supplementation. Am. J. Clin. Nutr 109:1452–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clarke R, Birks J, Nexo E, Ueland PM, Schneede J, et al. 2007. Low vitamin B-12 status and risk of cognitive decline in older adults. Am. J. Clin. Nutr 86:1384–91 [DOI] [PubMed] [Google Scholar]
- 24.Clarke R, Sherliker P, Hin H, Molloy AM, Nexo E, et al. 2008. Folate and vitamin B12 status in relation to cognitive impairment and anaemia in the setting of voluntary fortification in the UK. Br. J. Nutr 100:1054–59 [DOI] [PubMed] [Google Scholar]
- 25.Cordero AM, Crider KS, Rogers LM, Cannon MJ, Berry RJ. 2015. Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects: World Health Organization guidelines. Morb. Mortal. Wkly. Rep 64:421–23 [PMC free article] [PubMed] [Google Scholar]
- 26.Cortes F, Mellado C, Pardo RA, Villarroel LA, Hertrampf E. 2012. Wheat flour fortification with folic acid: changes in neural tube defects rates in Chile. Am. J. Med. Genet. A 158A:1885–90 [DOI] [PubMed] [Google Scholar]
- 27.Crider KS, Devine O, Hao L, Dowling NF, Li S, et al. 2014. Population red blood cell folate concentrations for prevention of neural tube defects: Bayesian model. BMJ 349:g4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crider KS, Devine O, Qi YP, Yeung LF, Sekkarie A, et al. 2019. Systematic review and Bayesian meta-analysis of the dose–response relationship between folic acid intake and changes in blood folate concentrations. Nutrients 11:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crider KS, Qi YP, Devine O, Tinker SC, Berry RJ. 2018. Modeling the impact of folic acid fortification and supplementation on red blood cell folate concentrations and predicted neural tube defect risk in the United States: Have we reached optimal prevention? Am. J. Clin. Nutr 107:1027–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crider KS, Yang TP, Berry RJ, Bailey LB. 2012. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv. Nutr 3:21–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crider KS, Zhu JH, Hao L, Yang QH, Yang TP, et al. 2011. MTHFR 677C→T genotype is associated with folate and homocysteine concentrations in a large, population-based, double-blind trial of folic acid supplementation. Am. J. Clin. Nutr 93:1365–72 [DOI] [PubMed] [Google Scholar]
- 32.Czeizel AE, Dudás I. 1992. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N. Engl. J. Med 327:1832–35 [DOI] [PubMed] [Google Scholar]
- 33.Das JK, Salam RA, Mahmood SB, Moin A, Kumar R, et al. 2019. Food fortification with multiple micronutrients: impact on health outcomes in general population. Cochrane Database Syst. Rev 12:CD011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Degerud EM, Manger MS, Strand TA, Dierkes J. 2015. Bioavailability of iron, vitamin A, zinc, and folic acid when added to condiments and seasonings. Ann. N. Y. Acad. Sci 1357:29–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Wals P, Rusen ID, Lee NS, Morin P, Niyonsenga T. 2003. Trend in prevalence of neural tube defects in Quebec. Birth Defects Res. A 67:919–23 [DOI] [PubMed] [Google Scholar]
- 36.Dietrich M, Brown CJ, Block G. 2005. The effect of folate fortification of cereal-grain products on blood folate status, dietary folate intake, and dietary folate sources among adult non-supplement users in the United States. J. Am. Coll. Nutr 24:266–74 [DOI] [PubMed] [Google Scholar]
- 37.Dickinson CJ. 1995. Does folic acid harm people with vitamin B12 deficiency? QJM 88:357–64 [PubMed] [Google Scholar]
- 38.Duong HT, Hashmi SS, Ramadhani T, Canfield MA, Scheuerle A, Waller DK. 2011. Maternal use of hot tub and major structural birth defects. Birth Defects Res. A 91:836–41 [DOI] [PubMed] [Google Scholar]
- 39.Ebrahimi S, Ashkani-Esfahani S, Bagheri F. 2013. Prevalence of neural tube defects in Yasuj, Southwest Iran. Shiraz E Med. J 14:54–62 [Google Scholar]
- 40.Engle-Stone R, Nankap M, Ndjebayi AO, Allen LH, Shahab-Ferdows S, et al. 2017. Iron, zinc, folate, and vitamin B-12 status increased among women and children in Yaounde and Douala, Cameroon, 1 year after introducing fortified wheat flour. J. Nutr 147:1426–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fazili Z, Sternberg MR, Potischman N, Wang CY, Storandt RJ, et al. 2020. Demographic, physiologic, and lifestyle characteristics observed with serum total folate differ among folate forms: cross-sectional data from fasting samples in the NHANES 2011–2016. J. Nutr 150:851–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.FDA (US Food Drug Adm.). Food additives permitted for direct addition to food for human consumption; folic acid. 81 Fed. Reg 22176–83 (April 15, 2016) [PubMed] [Google Scholar]
- 43.FDA (US Food Drug Adm.). Food labeling: health claims and label statements; folate and neural tube defects; proposed rules. 61 Fed. Reg 8752–81 (March 5, 1996) [Google Scholar]
- 44.FDA (US Food Drug Adm.). Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid; correction. 61 Fed. Reg 40513 (Aug. 5, 1996) [Google Scholar]
- 45.FDA (US Food Drug Adm.). Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid; final rule. 61 Fed. Reg 8781–97 (March 5, 1996) [Google Scholar]
- 46.FDA (US Food Drug Adm.). 2018. Determining the regulatory status of a food ingredient Fact Sheet, FDA, Washington, DC. https://www.fda.gov/food/food-ingredients-packaging/determining-regulatory-status-food-ingredient [Google Scholar]
- 47.Ferrazzi E, Tiso G, Di Martino D. 2020. Folic acid versus 5-methyl tetrahydrofolate supplementation in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol 253:312–19 [DOI] [PubMed] [Google Scholar]
- 48.Finer LB, Zolna MR. 2016. Declines in unintended pregnancy in the United States, 2008–2011. N. Engl. J. Med 374:843–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finkelstein JL, Fothergill A, Johnson CB, Guetterman HM, Bose B, et al. 2021. Anemia and vitamin B-12 and folate status in women of reproductive age in southern India: estimating population-based risk of neural tube defects. Curr. Dev. Nutr 5:nzab069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Food Saf. Auth. Irel. 2009. Currently no need for mandatory fortification—increased folate status negates mandatory folic acid fortification at this time Press Release, Food Saf. Auth. Irel., Dublin, March 11. https://www.fsai.ie/details.aspx?id=7706 [Google Scholar]
- 51.Food Stand. Aust. N. Z. 2006. Mandatory folic acid fortification Rep., Food Stand. Aust. N. Z., Kingston, Aust. https://www.foodstandards.gov.au/code/proposals/documents/Short%20Guide%20_mandatory%20folic%20acid%20fortification_FINAL_%20_4_.pdf [Google Scholar]
- 52.Food Stand. Aust. N. Z. 2007. Proposal P295: consideration of mandatory fortification with folic acid. Final Assess. Rep 7–06, Food Stand. Aust. N. Z., Kingston, Aust. [Google Scholar]
- 53.Fox JT, Stover PJ. 2008. Folate-mediated one-carbon metabolism. Vitam. Horm 79:1–44 [DOI] [PubMed] [Google Scholar]
- 54.French AE, Grant R, Weitzman S, Ray JG, Vermeulen MJ, et al. 2003. Folic acid food fortification is associated with a decline in neuroblastoma. Clin. Pharmacol. Ther 74:288–94 [DOI] [PubMed] [Google Scholar]
- 55.Friso S, Choi SW. 2002. Gene–nutrient interactions and DNA methylation. J. Nutr 132(Suppl. 8):2382–87 [DOI] [PubMed] [Google Scholar]
- 56.Fuks F. 2005. DNA methylation and histone modifications: teaming up to silence genes. Curr. Opin. Genet. Dev 15:490–95 [DOI] [PubMed] [Google Scholar]
- 57.Gabbay-Benziv R, Reece EA, Wang F, Yang P. 2015. Birth defects in pregestational diabetes: defect range, glycemic threshold and pathogenesis. World J. Diabetes 6:481–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gamble MV, Liu X, Ahsan H, Pilsner JR, Ilievski V, et al. 2006. Folate and arsenic metabolism: a double-blind, placebo-controlled folic acid-supplementation trial in Bangladesh. Am. J. Clin. Nutr 84:1093–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gamble MV, Liu X, Slavkovich V, Pilsner JR, Ilievski V, et al. 2007. Folic acid supplementation lowers blood arsenic. Am. J. Clin. Nutr 86:1202–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ganji V, Kafai MR. 2009. Hemoglobin and hematocrit values are higher and prevalence of anemia is lower in the post–folic acid fortification period than in the pre–folic acid fortification period in US adults. Am. J. Clin. Nutr 89:363–71 [DOI] [PubMed] [Google Scholar]
- 61.Grosse SD, Berry RJ, Tilford JM, Kucik JE, Waitzman NJ. 2016. Retrospective assessment of cost savings from prevention: folic acid fortification and spina bifida in the U.S. Am. J. Prev. Med 50(Suppl. 5):74–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grosse SD, Waitzman NJ, Romano PS, Mulinare J. 2005. Reevaluating the benefits of folic acid fortification in the United States: economic analysis, regulation, and public health. Am. J. Public Health 95:1917–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grupp SG, Greenberg ML, Ray JG, Busto U, Lanctôt KL, et al. 2011. Pediatric cancer rates after universal folic acid flour fortification in Ontario. J. Clin. Pharmacol 51:60–65 [DOI] [PubMed] [Google Scholar]
- 64.Hao LM, Jing, Stampfer MJ, Ren A, Tian Y, et al. 2003. Geographical, seasonal and gender differences in folate status among Chinese adults. J. Nutr 133:3630–35 [DOI] [PubMed] [Google Scholar]
- 65.Hao L, Yang QH, Li Z, Bailey LB, Zhu J-H, et al. 2008. Folate status and homocysteine response to folic acid doses and withdrawal among young Chinese women in a large-scale randomized double-blind trial. Am. J. Clin. Nutr 88:448–57 [DOI] [PubMed] [Google Scholar]
- 66.Hausman DB, Johnson MA, Davey A, Woodard JL, Poon LW, et al. 2011. The oldest old: red blood cell and plasma folate in African American and white octogenarians and centenarians in Georgia. J. Nutr. Health Aging 15:744–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Health Counc. Neth. 2008. Towards an optimal use of folic acid. Rep. 2008/02E, Health Counc. Neth., The Hague [Google Scholar]
- 68.Henderson AM, Aleiunas RE, Loh SP, Khor GL, Harvey-Leeson S, et al. 2018. l-5-methyltetrahydrofolate supplementation increases blood folate concentrations to a greater extent than folic acid supplementation in Malaysian women. J. Nutr 148:885–90 [DOI] [PubMed] [Google Scholar]
- 69.Herbert V, Zalusky R. 1962. Interrelations of vitamin B12 and folic acid metabolism: folic acid clearance studies. J. Clin. Investig 41:1263–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hertrampf E, Cortes F, Erickson JD, Cayazzo M, Freire W, et al. 2003. Consumption of folic acid-fortified bread improves folate status in women of reproductive age in Chile. J. Nutr 133:3166–69 [DOI] [PubMed] [Google Scholar]
- 71.Honein MA, Devine O, Sharma AJ, Rasmussen SA, Park S, et al. 2013. Modeling the potential public health impact of prepregnancy obesity on adverse fetal and infant outcomes. Obesity 21:1276–83 [DOI] [PubMed] [Google Scholar]
- 72.Huang HY, Chen HL, Feng LP. 2017. Maternal obesity and the risk of neural tube defects in offspring: a meta-analysis. Obes. Res. Clin. Pract 11:188–97 [DOI] [PubMed] [Google Scholar]
- 73.Inst. Med. 1998. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Panthothenic Acid, Biotin, and Choline. Washington, DC: Natl. Acad. [PubMed] [Google Scholar]
- 74.Ionescu-Ittu R, Marelli AJ, Mackie AS, Pilote L. 2009. Prevalence of severe congenital heart disease after folic acid fortification of grain products: time trend analysis in Quebec, Canada. BMJ 338:b1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. 1999. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N. Engl. J. Med 340:1449–54 [DOI] [PubMed] [Google Scholar]
- 76.Jhaveri MS, Wagner C, Trepel JB. 2001. Impact of extracellular folate levels on global gene expression. Mol. Pharmacol 60:1288–95 [DOI] [PubMed] [Google Scholar]
- 77.Jiang T, Christian P, Khatry SK, Wu L, West KP Jr. 2005. Micronutrient deficiencies in early pregnancy are common, concurrent, and vary by season among rural Nepali pregnant women. J. Nutr 135:1106–12 [DOI] [PubMed] [Google Scholar]
- 78.Joseph KS, Kramer MS. 1996. Review of the evidence on fetal and early childhood antecedents of adult chronic disease. Epidemiol. Rev 18:158–74 [DOI] [PubMed] [Google Scholar]
- 79.Kamen BA, Smith AK. 2004. A review of folate receptor α cycling and 5-methyltetrahydrofolate accumulation with an emphasis on cell models in vitro. Adv. Drug Deliv. Rev 56:1085–97 [DOI] [PubMed] [Google Scholar]
- 80.Kancherla V, Tsang B, Wagh K, Dixon M, Oakley GP Jr. 2020. Modeling shows high potential of folic acid–fortified salt to accelerate global prevention of major neural tube defects. Birth Defects Res 112:1461–74 [DOI] [PubMed] [Google Scholar]
- 81.Keats EC, Neufeld LM, Garrett GS, Mbuya MNN, Bhutta ZA. 2019. Improved micronutrient status and health outcomes in low- and middle-income countries following large-scale fortification: evidence from a systematic review and meta-analysis. Am. J. Clin. Nutr 109:1696–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kerr SM, Parker SE, Mitchell AA, Tinker SC, Werler MM. 2017. Periconceptional maternal fever, folic acid intake, and the risk for neural tube defects. Ann. Epidemiol 27:777–82.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Keum N, Giovannucci EL. 2014. Folic acid fortification and colorectal cancer risk. Am. J. Prev. Med 46(Suppl. 1):65–72 [DOI] [PubMed] [Google Scholar]
- 84.Knoops KT, Spiro A 3rd, de Groot LC, Kromhout D, van Staveren WA, Tucker KL. 2009. Do dietary patterns in older men influence change in homocysteine through folate fortification? The Normative Aging Study. Public Health Nutr 12:1760–66 [DOI] [PubMed] [Google Scholar]
- 85.Kubo Y, Fukuoka H, Kawabata T, Shoji K, Mori C, et al. 2020. Distribution of 5-methyltetrahydrofolate and folic acid levels in maternal and cord blood serum: longitudinal evaluation of Japanese pregnant women. Nutrients 12:1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kurzius-Spencer M, da Silva V, Thomson CA, Hartz V, Hsu CH, et al. 2017. Nutrients in one-carbon metabolism and urinary arsenic methylation in the National Health and Nutrition Examination Survey (NHANES) 2003–2004. Sci. Total Environ 607/608:381–90 [DOI] [PubMed] [Google Scholar]
- 87.Lavoie A, Tripp E, Hoffbrand AV. 1974. The effect of vitamin B12 deficiency on methylfolate metabolism and pteroylpolyglutamate synthesis in human cells. Clin. Sci. Mol. Med 47:617–30 [DOI] [PubMed] [Google Scholar]
- 88.Lee-Kwan SH, Moore LV, Blanck HM, Harris DM, Galuska D. 2017. Disparities in state-specific adult fruit and vegetable consumption—United States, 2015. Morb. Mortal. Wkly. Rep 66:1241–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Y, Huang T, Zheng Y, Muka T, Troup J, Hu FB. 2016. Folic acid supplementation and the risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. J. Am. Heart Assoc 5:e003768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li YO, Diosade LL, Wesley AS. 2011. Folic acid fortification through existing fortified foods: iodized salt and vitamin A–fortified sugar. Food Nutr. Bull 32:35–41 [DOI] [PubMed] [Google Scholar]
- 91.Linabery AM, Johnson KJ, Ross JA. 2012. Childhood cancer incidence trends in association with US folic acid fortification (1986–2008). Pediatrics 129:1125–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lindsey LLM, Hamner HC, Prue CE, Flores AL, Valencia D, et al. 2007. Understanding optimal nutrition among women of childbearing age in the United States and Puerto Rico: employing formative research to lay the foundation for national birth defects prevention campaigns. J. Health Commun 12:733–57 [DOI] [PubMed] [Google Scholar]
- 93.Liu S, Joseph KS, Luo W, León JA, Lisonkova S, et al. 2016. Effect of folic acid food fortification in Canada on congenital heart disease subtypes. Circulation 134:647–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu S, West R, Randell E, Longerich L, O’Connor KS, et al. 2004. A comprehensive evaluation of food fortification with folic acid for the primary prevention of neural tube defects. BMC Pregnancy Childbirth 4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Llanos AH, Hertrampf E, Cortes F, Pardo A, Grosse SD, Uauy R. 2007. Cost-effectiveness of a folic acid fortification program in Chile. Health Policy 83:295–303 [DOI] [PubMed] [Google Scholar]
- 96.Lopez-Camelo JS, Castilla EE, Orioli IM. 2010. Folic acid flour fortification: impact on the frequencies of 52 congenital anomaly types in three South American countries. Am. J. Med. Genet. A 152A:2444–58 [DOI] [PubMed] [Google Scholar]; 96a. Lopez-Camelo JS, Orioli IM, da Graça Dutra M, Nazer-Herrera J, Rivera N, et al. 2005. Reduction of birth prevalence rates of neural tube defects after folic acid fortification in Chile. Am. J. Med. Genet. A 135(2):120–25 [DOI] [PubMed] [Google Scholar]
- 97.Malpeli A, Ferrari MG, Varea A, Falivene M, Etchegoyen G, et al. 2013. Short-term evaluation of the impact of a fortified food aid program on the micronutrient nutritional status of Argentinian pregnant women. Biol. Trace Elem. Res 155:176–83 [DOI] [PubMed] [Google Scholar]
- 98.Mannar VD, Diosady L. 2019. Quadruple fortification of salt with iodine, iron, vitamins B9 and B12 to reduce maternal and neonatal mortality by reducing anemia and nutritional deficiency prevalence. Curr. Dev. Nutr 3(Suppl. 1):P24–041-19 [Google Scholar]
- 99.Marchetta CM, Devine OJ, Crider KS, Tsang BL, Cordero AM, et al. 2015. Assessing the association between natural food folate intake and blood folate concentrations: a systematic review and Bayesian meta-analysis of trials and observational studies. Nutrients 7:2663–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martínez de Villarreal L, Villareal Pérez JZ, Arredondo Vázquez P, Hernández Herrera R, Velazco Campos MDR, et al. 2002. Decline of neural tube defects cases after a folic acid campaign in Nuevo Leon, Mexico. Teratology 66:249–56 [DOI] [PubMed] [Google Scholar]
- 101.Mazumdar M, Hasan MOSI, Hamid R, Valeri L, Paul L, et al. 2015. Arsenic is associated with reduced effect of folic acid in myelomeningocele prevention: a case control study in Bangladesh. Environ. Health 14:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McGee EJT, Sangakkara AR, Diosady LL. 2017. Double fortification of salt with folic acid and iodine. J. Food Eng 198:72–80 [Google Scholar]
- 103.Mejia LA, Bower AM. 2015. The global regulatory landscape regarding micronutrient fortification of condiments and seasonings. Ann. N. Y. Acad. Sci 1357:1–7 [DOI] [PubMed] [Google Scholar]
- 104.Metz JM, McNeil AR, Levin M. 2004. The relationship between serum cobalamin concentration and mean red cell volume at varying concentrations of serum folate. Clin. Lab. Haematol 26:323–25 [DOI] [PubMed] [Google Scholar]
- 105.Millacura N, Pardo R, Cifuentes L, Suazo J. 2017. Effects of folic acid fortification on orofacial clefts prevalence: a meta-analysis. Public Health Nutr 20:2260–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miller JW, Garrod MG, Allen LH, Haan MN, Green R. 2009. Metabolic evidence of vitamin B-12 deficiency, including high homocysteine and methylmalonic acid and low holotranscobalamin, is more pronounced in older adults with elevated plasma folate. Am. J. Clin. Nutr 90:1586–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mills JL, Carter TC, Scott JM, Troendle JF, Gibney ER, et al. 2011. Do high blood folate concentrations exacerbate metabolic abnormalities in people with low vitamin B-12 status? Am. J. Clin. Nutr 94:495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mills JL, Von Kohorn I, Conley MR, Zeller JA, Cox C, et al. 2003. Low vitamin B-12 concentrations in patients without anemia: the effect of folic acid fortification of grain. Am. J. Clin. Nutr 77:1474–77 [DOI] [PubMed] [Google Scholar]
- 109.Modupe O, Krishnaswamy K, Diosady LL. 2019. Technology for triple fortification of salt with folic acid, iron, and iodine. J. Food Sci 84:2499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Molloy AM. 2018. Should vitamin B12 status be considered in assessing risk of neural tube defects? Ann. N. Y. Acad. Sci 1414:109–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moretti ME, Bar-Oz B, Fried S, Koren G. 2005. Maternal hyperthermia and the risk for neural tube defects in offspring: systematic review and meta-analysis. Epidemiology 16:216–19 [DOI] [PubMed] [Google Scholar]
- 112.Morris MS, Jacques PF, Rosenberg IH, Selhub J. 2007. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am. J. Clin. Nutr 85:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morris MS, Jacques PF, Rosenberg IH, Selhub J. 2010. Circulating unmetabolized folic acid and 5-methyltetrahydrofolate in relation to anemia, macrocytosis, and cognitive test performance in American seniors. Am. J. Clin. Nutr 91:1733–44 [DOI] [PubMed] [Google Scholar]
- 114.Morris MS, Selhub J, Jacques PF. 2012. Vitamin B-12 and folate status in relation to decline in scores on the Mini–Mental State Examination in the Framingham Heart Study. J. Am. Geriatr. Soc 60:1457–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mowla SG, Gissler M, Räisänen S, Kancherla V. 2020. Association between maternal pregestational diabetes mellitus and spina bifida: a population-based case-control study, Finland, 2000–2014. Birth Defects Res 112:186–95 [DOI] [PubMed] [Google Scholar]
- 116.MRC Vitam. Study Res. Group. 1991. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 338:131–37 [PubMed] [Google Scholar]
- 117.Mukhtar A, Kramer MR, Oakley GP Jr., Kancherla V. 2017. Race and ethnicity and preconception folic acid supplement use among pregnant women in Georgia, PRAMS 2009 to 2011. Birth Defects Res 109:38–48 [DOI] [PubMed] [Google Scholar]
- 118.Nasri KBF, Ben Fradj MK, Touati A, Aloui M, Ben Jemaa N, et al. 2015. Association of maternal homocysteine and vitamins status with the risk of neural tube defects in Tunisia: a case-control study. Birth Defects Res. A 103:1011–20 [DOI] [PubMed] [Google Scholar]
- 119.Natl. Inst. Health. 2020. Folate: fact sheet for health professionals Fact Sheet, Natl. Inst. Health, Washington, DC. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/#en12 [Google Scholar]
- 120.Nazer HJ, Cifuentes OL. 2013. Resultados del Programa de Prevención de Defectos de Tubo Neural en Chile mediante la fortificación de la harina con ácido fólico [Effects of wheat flour fortification with folic acid on the prevalence of neural tube defects in Chile]. Rev. Med. Chil 141:751–57 [DOI] [PubMed] [Google Scholar]
- 121.Newnham JP, Ross MG, eds. 2009. Early Life Origins of Human Health and Disease Basel, Switz.: Karger [Google Scholar]
- 122.Obeid R, Pietrzik K, Oakley GP Jr., Kancherla V, Holzgreve W, Wieser S. 2015. Preventable spina bifida and anencephaly in Europe. Birth Defects Res. A 103:763–71 [DOI] [PubMed] [Google Scholar]
- 123.Odewole OA, Williamson RS, Zakai NA, Berry RJ, Judd SE, et al. 2013. Near-elimination of folate-deficiency anemia by mandatory folic acid fortification in older US adults: Reasons for Geographic and Racial Differences in Stroke study 2003–2007. Am. J. Clin. Nutr 98:1042–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Paneth N, Susser M. 1995. Early origin of coronary heart disease (the “Barker hypothesis”). BMJ 310:411–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Parker SE, Yazdy MM, Tinker SC, Mitchell AA, Werler MM. 2013. The impact of folic acid intake on the association among diabetes mellitus, obesity, and spina bifida. Am. J. Obstet. Gynecol 209:239.e1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Peker E, Demir N, Tuncer O, Üstyol L, Balahoroğlu R, et al. 2016. The levels of vitamın B12, folate and homocysteine in mothers and their babies with neural tube defects. J. Matern. Fetal Neonatal Med 29:2944–48 [DOI] [PubMed] [Google Scholar]
- 127.Persad VL, Van den Hof MC, Dube JM, Zimmer P. 2002. Incidence of open neural tube defects in Nova Scotia after folic acid fortification. CMAJ 167:241–45 [PMC free article] [PubMed] [Google Scholar]
- 128.Pfeiffer CM, Hughes JP, Lacher DA, Bailey RL, Berry RJ, et al. 2012. Estimation of trends in serum and RBC folate in the U.S. population from pre- to postfortification using assay-adjusted data from the NHANES 1988–2010. J. Nutr 142:886–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pfeiffer CM, Johnson CL, Jain RB, Yetley EA, Picciano MF, et al. 2007. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988–2004. Am. J. Clin. Nutr 86:718–27 [DOI] [PubMed] [Google Scholar]
- 130.Pfeiffer CM, Sternberg MR, Hamner HC, Crider KS, Lacher DA, et al. 2016. Applying inappropriate cutoffs leads to misinterpretation of folate status in the US population. Am. J. Clin. Nutr 104:1607–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pfeiffer CM, Sternberg MR, Zhang M, Fazili Z, Storandt RJ, et al. 2019. Folate status in the US population 20 y after the introduction of folic acid fortification. Am. J. Clin. Nutr 110:1088–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qi YP, Do AN, Hamner HC, Pfeiffer CM, Berry RJ. 2014. The prevalence of low serum vitamin B-12 status in the absence of anemia or macrocytosis did not increase among older U.S. adults after mandatory folic acid fortification. J. Nutr 144:170–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ray JG, Meier C, Vermeulen MJ, Boss S, Wyatt PR, Cole DE. 2002a. Association of neural tube defects and folic acid food fortification in Canada. Lancet 360:2047–48 [DOI] [PubMed] [Google Scholar]
- 134.Ray JG, Singh G, Burrows RF. 2004. Evidence for suboptimal use of periconceptional folic acid supplements globally. BJOG 111:399–408 [DOI] [PubMed] [Google Scholar]
- 135.Ray JG, Vermeulen MJ, Boss SC, Cole DE. 2002b. Declining rate of folate insufficiency among adults following increased folic acid food fortification in Canada. Can. J. Public Health 93:249–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ray JG, Vermuelen MJ, Langman LJ, Boss SC, Cole DE. 2003. Persistence of vitamin B12 insufficiency among elderly women after folic acid food fortification. Clin. Biochem 36:387–91 [DOI] [PubMed] [Google Scholar]
- 137.Ricks DJ, Rees CA, Osborn KA, Crookston BT, Leaver K, et al. 2012. Peru’s national folic acid fortification program and its effect on neural tube defects in Lima. Rev. Panam. Salud Publica 32:391–98 [PubMed] [Google Scholar]
- 138.Rosenthal J, Lopez-Pazos E, Dowling NF, Pfeiffer CM, Mulinare J, et al. 2015. Folate and vitamin B12 deficiency among non-pregnant women of childbearing age in Guatemala 2009–2010: prevalence and identification of vulnerable populations. Matern. Child Health J 19:2272–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Samson ME, Yeung LF, Rose CE, Qi YP, Taylor CA, Crider KS. 2022. Vitamin B-12 malabsorption and renal function are critical considerations in studies of folate and vitamin B-12 interactions in cognitive performance: NHANES 2011–2014. Am. J. Clin. Nutr 116:74–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sanabria Rojas H, Tarqui-Mamani C, Arias Pachas J, Lam Figueroa N. 2013. Impact of fortifying wheat flour with folic acid on neural tube defects in Lima, Peru. An. Fac. Med 75:175–80 [Google Scholar]
- 141.Santos LM, Lecca RC, Cortez-Escalante JJ, Sanchez MN, Rodrigues HG. 2016. Prevention of neural tube defects by the fortification of flour with folic acid: a population-based retrospective study in Brazil. Bull. World Health Organ 94:22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Saubade F, Hemery YM, Rochette I, Guyot JP, Humblot C. 2018. Influence of fermentation and other processing steps on the folate content of a traditional African cereal-based fermented food. Int. J. Food Microbiol 266:79–86 [DOI] [PubMed] [Google Scholar]
- 143.Sayed AR, Bourne D, Pattinson R, Nixon J, Henderson B. 2008. Decline in the prevalence of neural tube defects following folic acid fortification and its cost–benefit in South Africa. Birth Defects Res. A 82:211–16 [DOI] [PubMed] [Google Scholar]
- 144.Scaglione F, Panzavolta G. 2014. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica 44:480–88 [DOI] [PubMed] [Google Scholar]
- 145.Sci. Advis. Comm. Nutr. 2006. Folate and Disease Prevention Norwich, UK: H. M. Station. Off. [Google Scholar]
- 146.Scott JM, Weir DG. 1981. The methyl folate trap: a physiological response in man to prevent methyl group deficiency in kwashiorkor (methionine deficiency) and an explanation for folic-acid induced exacerbation of subacute combined degeneration in pernicious anaemia. Lancet 2:337–40 [DOI] [PubMed] [Google Scholar]
- 147.Selhub J, Morris MS, Jacques PF. 2007. In vitamin B12 deficiency, higher serum folate is associated with increased total homocysteine and methylmalonic acid concentrations. PNAS 104:19995–20000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Senousy SM, Farag MK, Gouda AS, El Noury MA, Dabbous OA, Gaber KR. 2018. Association between biomarkers of vitamin B12 status and the risk of neural tube defects. J. Obstet. Gynaecol. Res 44:1902–8 [DOI] [PubMed] [Google Scholar]
- 149.Shilatifard A. 2006. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu. Rev. Biochem 75:243–69 [DOI] [PubMed] [Google Scholar]
- 150.Shumate C, Hoyt A, Liu C, Kleinert A, Canfield M. 2019. Understanding how the concentration of neighborhood advantage and disadvantage affects spina bifida risk among births to non-Hispanic white and Hispanic women, Texas, 1999–2014. Birth Defects Res 111:982–90 [DOI] [PubMed] [Google Scholar]
- 151.Smith AD, Kim YI, Refsum H. 2008. Is folic acid good for everyone? Am. J. Clin. Nutr 87:517–33 [DOI] [PubMed] [Google Scholar]
- 152.Solomon LR. 2013. Vitamin B12 and peripheral nerve function in elderly adults: “functional” B12 deficiency as a confounding variable. J. Am. Geriatr. Soc 61:310–11 [DOI] [PubMed] [Google Scholar]
- 153.Stewart CP, Christian P, Schulze KJ, Arguello M, Leclerq SC, et al. 2011. Low maternal vitamin B-12 status is associated with offspring insulin resistance regardless of antenatal micronutrient supplementation in rural Nepal. J. Nutr 141:1912–17 [DOI] [PubMed] [Google Scholar]
- 154.Stewart CP, Christian P, Schulze KJ, Leclerq SC, West KP Jr., Khatry SK. 2009. Antenatal micronutrient supplementation reduces metabolic syndrome in 6- to 8-year-old children in rural Nepal. J. Nutr 139:1575–81 [DOI] [PubMed] [Google Scholar]
- 155.Stokstad EL, Reisenauer A, Kusano G, Keating JN. 1988. Effect of high levels of dietary folic acid on folate metabolism in vitamin B12 deficiency. Arch. Biochem. Biophys 265:407–14 [DOI] [PubMed] [Google Scholar]
- 156.Stover PJ. 2009. One-carbon metabolism–genome interactions in folate-associated pathologies. J. Nutr 139:2402–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tabatabaei RS, Fatahi-Meibodi N, Meibodi B, Javaheri A, Abbasi H, et al. 2022. Association of fetal MTHFR C677T polymorphism with susceptibility to neural tube defects: a systematic review and update meta-analysis. Fetal Pediatr. Pathol 41:225–41 [DOI] [PubMed] [Google Scholar]
- 158.Tamura T, Picciano MF. 2006. Folate and human reproduction. Am. J. Clin. Nutr 83:993–1016 [DOI] [PubMed] [Google Scholar]
- 159.Tice JA, Ross E, Coxson PG, Rosenberg I, Weinstein MC, et al. 2001. Cost-effectiveness of vitamin therapy to lower plasma homocysteine levels for the prevention of coronary heart disease: effect of grain fortification and beyond. JAMA 286:936–43 [DOI] [PubMed] [Google Scholar]
- 160.Tinker SC, Cogswell ME, Devine O, Berry RJ. 2010. Folic acid intake among U.S. women aged 15–44 years, National Health and Nutrition Examination Survey, 2003–2006. Am. J. Prev. Med 38:534–42 [DOI] [PubMed] [Google Scholar]
- 161.Tinker SC, Devine O, Mai C, Hamner HC, Reefhuis J, et al. 2013. Estimate of the potential impact of folic acid fortification of corn masa flour on the prevention of neural tube defects. Birth Defects Res. A 97:649–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tinker SC, Gilboa SM, Moore CA, Waller DK, Simeone RM, et al. 2020. Specific birth defects in pregnancies of women with diabetes: National Birth Defects Prevention Study, 1997–2011. Am. J. Obstet. Gynecol 222:176.e1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tinker SC, Hamner HC, Qi YP, Crider KS. 2015. U.S. women of childbearing age who are at possible increased risk of a neural tube defect–affected pregnancy due to suboptimal red blood cell folate concentrations, National Health and Nutrition Examination Survey 2007 to 2012. Birth Defects Res. A 103:517–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tsang BL, Devine OJ, Cordero AM, Marchetta CM, Mulinare J, et al. 2015. Assessing the association between the methylenetetrahydrofolate reductase (MTHFR) 677C>T polymorphism and blood folate concentrations: a systematic review and meta-analysis of trials and observational studies. Am. J. Clin. Nutr 101:1286–94 [DOI] [PubMed] [Google Scholar]
- 165.US Prev. Serv. Task Force. 2017. Folic acid supplementation for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. JAMA 317:183–89 [DOI] [PubMed] [Google Scholar]
- 166.van der Pols JC, Baade P, Spencer LB. 2021. Colorectal cancer incidence in Australia before and after mandatory fortification of bread flour with folic acid. Public Health Nutr 24:1989–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Visentin M, Diop-Bove N, Zhao R, Goldman ID. 2014. The intestinal absorption of folates. Annu. Rev. Physiol 76:251–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang A, Rose CE, Qi YP, Williams JL, Pfeiffer CM, Crider KS. 2021. Impact of voluntary folic acid fortification of corn masa flour on RBC folate concentrations in the U.S. (NHANES 2011–2018). Nutrients 13:1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang A, Yeung L, Burrows NR, Rose CE, Fazili Z, et al. 2022. Reduced kidney function is associated with increasing red blood cell folate concentration and changes in folate form distributions (NHANES 2011–2018). Nutrients 14:1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang M, Wang ZP, Gao LJ, Gong R, Sun XH, Zhao ZT. 2013. Maternal body mass index and the association between folic acid supplements and neural tube defects. Acta Paediatr 102:908–13 [DOI] [PubMed] [Google Scholar]
- 171.Weir DG, Scott JM. 1999. Brain function in the elderly: role of vitamin B12 and folate. Br. Med. Bull 55:669–82 [DOI] [PubMed] [Google Scholar]
- 172.Williams J, Mai CT, Mulinare J, Isenburg J, Flood TJ, et al. 2015. Updated estimates of neural tube defects prevented by mandatory folic acid fortification—United States, 1995–2011. Morb. Mortal. Wkly. Rep 64:1–5 [PMC free article] [PubMed] [Google Scholar]
- 173.Wong EC, Rose CE, Flores AL, Yeung LF. 2019. Trends in multivitamin use among women of reproductive age: United States, 2006–2016. J. Women’s Health 28:37–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.World Health Organ. 2006. Guidelines on food fortification with micronutrients. Rep. 9241594012, World Health Organ., Geneva [Google Scholar]
- 175.World Health Organ. 2018. Fortification of rice with vitamins and minerals as a public health strategy. Rep. 9789241550291, World Health Organ, Geneva: [PubMed] [Google Scholar]
- 176.Wyckoff KF, Ganji V. 2007. Proportion of individuals with low serum vitamin B-12 concentrations without macrocytosis is higher in the post–folic acid fortification period than in the pre–folic acid fortification period. Am. J. Clin. Nutr 86:1187–92 [DOI] [PubMed] [Google Scholar]
- 177.Yajnik CS, Deshmukh US. 2008. Maternal nutrition, intrauterine programming and consequential risks in the offspring. Rev. Endocr. Metab. Disord 9:203–11 [DOI] [PubMed] [Google Scholar]
- 178.Yajnik CS, Deshpande SS, Jackson AA, Refsum H, Rao S, et al. 2008. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia 51:29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yang Q, Botto LD, Erickson JD, Berry RJ, Sambell C, et al. 2006. Improvement in stroke mortality in Canada and the United States, 1990 to 2002. Circulation 113:1335–43 [DOI] [PubMed] [Google Scholar]
- 180.Yang Q, Cogswell ME, Hamner HC, Carriquiry A, Bailey LB, et al. 2010. Folic acid source, usual intake, and folate and vitamin B-12 status in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2006. Am. J. Clin. Nutr 91:64–72 [DOI] [PubMed] [Google Scholar]
- 181.Yang Y, Chen J, Wang B, Ding C, Liu H. 2015. Association between MTHFR C677T polymorphism and neural tube defect risks: a comprehensive evaluation in three groups of NTD patients, mothers, and fathers. Birth Defects Res. A 103:488–500 [DOI] [PubMed] [Google Scholar]
- 182.Yeung L, Yang Q, Berry RJ. 2008. Contributions of total daily intake of folic acid to serum folate concentrations. JAMA 300:2486–87 [DOI] [PubMed] [Google Scholar]
- 183.Zhao R, Matherly LH, Goldman ID. 2009. Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert Rev. Mol. Med 11:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhou Y, Sinnathamby V, Yu Y, Sikora L, Johnson CY, et al. 2020. Folate intake, markers of folate status and oral clefts: an updated set of systematic reviews and meta-analyses. Birth Defects Res 112:1699–719 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


