Abstract
Background:
Men of African ancestry (AA) with prostate cancer suffer from worse outcomes. However, a recent analysis of patients treated with the dendritic cell vaccine sipuleucel-T for prostate cancer suggested that AA patients could have improved outcomes relative to whites.
Methods:
We conducted a focused literature review of Medline-indexed articles and clinical trials listed on clinicaltrials.gov.
Results:
We identify several studies pointing to enrichment of inflammatory cellular infiltrates and cytokine signaling among AA patients with prostate cancer. We outline potential genomic and transcriptomic alterations that may contribute to immunogenicity. Last, we investigate differences in host immunity and vaccine responsiveness that may be enhanced in AA patients.
Conclusions:
AA patients with prostate cancer may be enriched for an immunogenic phenotype. Dedicated studies are needed to better understand the immune mechanisms that contribute to existing cancer disparities and test immune-based therapies in this population.
Keywords: African ancestry, cancer disparities, cancer vaccines, immunology, prostate cancer
1 |. INTRODUCTION
Prostate cancer remains the most incident and prevalent non-cutaneous malignancy in US men, accounting for up to 20% of new cancer diagnoses; it is also the second leading cause of cancer death among US men.1 However, men of African ancestry (AA) with prostate cancer have higher incidence (1.8×) and mortality (2.2×) compared to white patients.2 Potential contributing factors include tumor-specific biology, medical comorbidities, access to and receipt of care, and other social determinants of health. Several studies have shown that in equal access to care settings (e.g. the VA system), the differences in prostate cancer mortality were comparable across AA and other populations.3–5 However, there is also growing evidence that AA men may have unique biological phenotypes of prostate cancer.6–9 In addition to tumor biology, host immunity may also be different in AA men and shape the antitumor immune response.10,11 Characterizing these unique phenotypes could have significant implications for personalized therapeutic approaches, including immunotherapies. Recently, data has emerged on an observed survival advantage in AA patients with metastatic castration-resistant prostate cancer (mCRPC) who had received sipuleucel-T, a cell-based form of immunotherapy.12,13 This latest finding, along with others in the literature, raises the question whether prostate cancer patients of AA can particularly benefit from immunotherapy.
At this point, it is crucial to emphasize that race is a social construct that has deep historical and cultural connotations. There is ongoing debate in the medical literature about whether using race as a proxy for biological differences in patient populations further perpetuates health inequities,14 or rather the resulting “color blindness” exacerbates existing disparities.15 Importantly, reported race often mixes together multiple populations, limiting the generalizability of findings in studies (e.g., patients with ancestors from sub-Saharan Africa can have different genomics than those with Caribbean predecessors).16 In addition, the concept of race dichotomizes a range of ancestry. Recently, the concept of “genetic ancestry” has emerged as a more appropriate surrogate to tease apart biological traits within populations.17,18 Keeping these clarifications in mind, there is a need for dedicated studies in AA patients to better inform treatment decisions and close the gap on existing inequities. This review aims to identify distinct and recurrent phenotypes in immune pathways found in AA prostate cancer, with the hope of promoting studies with AA representation that can help reduce prostate cancer disparities.
2 |. METHODS
Peer reviewed articles included in this review were obtained from searches in publicly available journal databases—PubMed, Embase, Cochrane, Web of Science—for the period up to 04/2021, containing combination of the keywords “prostate cancer,” “African ancestry,” “African American,” “Black,” “cancer disparities,” and the specific topic of interest (e.g., “genetics”). Additional targeted searches were carried out for articles on “cancer vaccines” and “immunotherapy” that were not necessarily limited to prostate cancer, as well as other noncancer pertaining topics like “immunology” and “vaccines.”
3 |. RESULTS
3.1 |. Clinical observation: Increased effectiveness of sipuleucel-T in AA with mCRPC
Sipuleucel-T is a therapeutic immune stimulating cancer vaccine approved for treating mCRPC after the pivotal phase III IMPACT trial showed an overall survival benefit.19 The vaccine contains autologous dendritic cells (DCs) and other antigen presenting cells (APCs) activated ex vivo to a fusion protein of prostatic acid phosphatase (PAP) with granulocyte-macrophage colony-stimulating factor. Building on the findings of prior studies,20,21 the IMPACT trial showed a prolonged median survival of 4.1 months in mCRPC patients who received sipuleucel-T compared to placebo (25.8 months vs. 21.7).19 The survival effect observed was as long as 13 months in patients with low baseline PSA in a subgroup exploratory analysis.22 The effect on OS was not matched by PSA responses or progression-free survival benefits, suggestive of possible delayed immune effects. Indeed, sipuleucel-T was shown to induce a robust, durable humoral and cellular immune response—in particular, the activation of APCs that led to production of antibody titers to the fusion protein was associated with a survival benefit.23 While sipuleucel-T also induced T-cell proliferation (although particular T-cell subsets were not specified), there was no associated change in survival.19,23 A deeper analysis on the antibody production after sipuleucel-T administration identified “antigenic spread,” or off-target immune response to tumor-associated antigens (TAAs) such as PSA or LGALS3, which was additionally associated with improved survival.24 Last, to complete the understanding of immune mechanisms derived from sipuleucel-T, neoadjuvant use of the vaccine in localized prostate cancer found T-cell recruitment and activation, predominantly of CD4 helper T-cells, at the tumor microenvironment (TME).25 Despite its recommendation as an option in the treatment of mCRPC, uptake of sipuleucel-T has remained relatively low. In a recent large retrospective study, sipuleucel-T was selected as treatment in approximately 10% of mCRPC patients; importantly, administration of sipuleucel-T to AA patients was not significantly different than to other patient populations.26
Participants in the IMPACT trial included 6.7% (n = 23/341) AA patients, but no AA-specific data was reported. However, a subsequent subgroup analysis of AA participants in several sipuleucel-T phase III trials identified a signal of a significantly improved OS of 30.7 months in AA patients receiving sipuleucel-T (compared to an overall OS of 3.9 months in all participants), although AA comprised ~6% (n = 43/737) of the sample size.27 In another study, AA patients appeared to have greater rates of PSA stabilization in response to sipuleucel-T.28 It is possible that differential tumor PAP expression is partly behind this increased response to sipuleucel-T given its mechanism of action. Indeed, elevated serum levels of PAP had previously been observed in a higher percentage (85% vs. 27%) of AA men with locally advanced prostate cancer, compared to whites; notably “race” was designated by the investigators and not self-reported.29 Recently, an analysis of the observational multicenter registry PROCEED included roughly twice the proportion of AA patients than in IMPACT (11.6%, n = 221/1902). The observed median survival was 30.7 months (there was no placebo comparison group to determine survival benefit), but importantly, this study identified AA as an independent predictor of prolonged survival. Furthermore, the median survival was 9.5 months longer in AA patients than in whites when controlling for PSA (35.3 vs. 25.8) and 5.3 months longer without PSA matching (35.2 vs. 29.9).13 After sipuleucel-T, both populations received similar antineoplastic treatment and had similar rates of adverse events. The reasons behind these observed differences in AA patients remain largely unexplored. In a subsequent analysis of biomarkers obtained from PROCEED participants, higher baseline inflammatory cytokines, such as interleukin (IL)-4 and IL-10, have been observed in mCRPC AA patients compared to whites, which were significantly more augmented after receiving sipuleucel-T. Interestingly, while the AA cohort had higher OS, there were no race-dependent differences in humoral response or antigenic spread.30 These findings raise the hypothesis that AA men may have a uniquely immunogenic phenotype of prostate cancer. Below, we highlight the research performed thus far that could explain these differences.
3.2 |. Established differences in tumor immune microenvironment
The TME of prostate tumors is composed of immune cells, cancer-associated fibroblasts, blood vessels, and secreted molecules such as cytokines and growth factors.31 Traditionally, prostate cancer has been viewed as a “cold” and poorly immunogenic tumor.32 However, there is growing evidence that an immunogenic TME may be over-represented among AA patients, with significantly higher expression of inflammatory cytokines and lymphocytic infiltrates,33–36 raising the potential for responsiveness to immune therapies.
Examining cytokine profiles, higher levels of IL-6, IL-8, IL-1B, and CXCR4 have been observed in AA tumors compared to whites and were linked with increased tumor grade.37 Interleukin expression is particularly interesting as a biomarker of inflammatory AA tumors. IL-6 is a proinflammatory cytokine, promoting cell migration and evasion of apoptosis through interactions with STAT and PI3K pathways,10 however, its overall effects can be considered immunosuppressive as it attracts myeloid derived suppressor cells to the TME.38 Additionally, IL-6 was one of several inflammatory paracrine mediators enriched in tumor stromal fibroblasts from AA patients relative to white patients.39 IL-8 acts as a neutrophil activator with a role in cell proliferation and invasion. The evidence behind IL-8 appears mixed, as a study demonstrated a link between higher levels of IL-8 expression from prostate epithelium and higher tumor grade but the effect was comparable across AA and white tumors.40
Furthermore, tumor-infiltrating immune cells and expression of programmed death-ligand 1 (PD-L1) in the TME may indicate increased inflammatory signaling. Presence of intratumoral T-cells (particularly CD8+) and PD-L1 expression are associated with higher risk of biochemical recurrence.41–43 One study of prostatectomy samples demonstrated increased PD-L1 expression in AA vs non-AA tumors; interestingly, these PD-L1 positive tumors were linked with CD8+ infiltration.44 Moreover, a recent study identified enriched populations of lymphocytes, particularly plasma cells, in the TME of AA patients, independent of age, PSA level, or genomic alterations. Increased B-cell infiltrates in the TME were associated with prolonged disease-free survival in this localized prostate cancer cohort.36 This was further corroborated in a different study where AA tumors had higher expression of CD4+ and CD8+ T-cell markers at the TME, but there was no difference in PD-1/PD-L1 or CTLA-4 expression.33 In contrast, another study found no difference in the density of T-cell infiltration in prostate cancers in AA men versus whites, but did find that among AAs, increased regulatory T-cells (but not CD3+ or CD8+ cells overall) correlated with disease recurrence.45 Additionally, the presence of PTEN loss and ERG fusion (both less common in AA patients, as discussed below) were linked with T-cell infiltration regardless of ethnicity. Notably, confidence intervals were wide, requiring validation in other cohorts.
The poor prognosis associated with lymphocytic TME infiltrates may be because they are associated with more aggressive tumors, or that these T-cells are dysfunctional or actively immunesuppressive.46 Indeed, immunosuppressive regulatory T-lymphocytes are frequently found in the TME of prostate cancer, as noted above.47–49 In contrast, stem-like progenitor T-cells marked by the transcriptional factor TCF-1 may be responsible for expansion in response to vaccines and checkpoint inhibitors,50,51 and their presence in antigen-presenting cell niches may correlate with decreased risk of relapse in other tumor types.52 Further studies are required to better describe the role of T-cell infiltrates in AA prostate cancer, particularly with an eye towards predicting the response to PD-1/PD-L1 inhibitors. Other types of immune cells in the TME, for example macrophages and neutrophils, are generally regarded as inflammatory and protumorigenic, while natural killer cells (NKs) and DCs are responsible for innate antitumor effect and increased antigen presentation, respectively, and are associated with improved outcomes.48,53,54 However, there have not been dedicated studies evaluating these immune cell populations in AA patients.
Based on the current evidence, there appears to be higher expression of cytokines and the interferon-gamma-triggered PD-L1 in AA men, reflecting more immunogenic tumors. Data on T-cell subsets and other immune cells remains incomplete. A better understanding of the immune cell subpopulations present in the TME will be needed for better patient selection and co-targeting of unique mechanisms of immunotherapy resistance.
3.3 |. Potential genomic contributors to tumor immunogenicity
The current understanding of prostate cancer genetics and tumor biology is largely derived from studies performed in homogeneous, largely white, populations, limiting external validity.55,56 However, over the past two decades, dedicated research on AA patients has shed some light on tumor-specific differences. These findings are highlighted in several review articles.6,9,31,57,58 Here, we specifically explore the growing literature on genomic ancestral differences seen in AA prostate cancer, and whether these might or might not be related to observed differences in tumor immunogenicity.
While a large body of work has evaluated the role of single nucleotide polymorphisms (SNPs) in increasing the susceptibility of prostate cancer in AA men,58–62 none found a direct link between polymorphisms and higher risk through immune-mediated mechanisms. In turn, studies evaluating germline alterations have been generally underpowered to identify differences in AA compared to other populations.58 For instance, a study of early-onset AA prostate cancer identified germline alterations in DNA damage repair (DDR) pathways like BRCA1/2, BRIP1, ATM, and PMS2, although their relatively low frequency (<10%) was insufficient to determine whether these alterations were more or less prevalent than in an unselected population.63
As noted below, DDR alterations have been associated with response to immune checkpoint inhibitors as well as potential immune-mediated interactions with PARP inhibitors (PARPi), making this question of particular relevance for immunotherapy in AAs. A recent study revealed an increased number of actionable somatic mutations in metastatic AA patients compared with other cohorts, particularly in DDR genes such as BRCA1 or MSH6.64 Furthermore, another recent study identified a high (>35%) prevalence of driver somatic mutations in AA tumors affecting DDR genes, such as ATM and BRCA2, compared to whites. It also identified a higher prevalence of mutations in ZMYM3, a protein that links BRCA1 with damaged chromatin and thus indirectly affects DDR mechanisms65 These studies contrast with another study where neither alterations in DDR genes like BRCA2 or ATM, nor proportion of microsatellite instability, appeared to be more frequent in AA cohorts.66
Loss of key tumor suppressor genes and activation of oncogenes may impact not only tumorigenesis but also the tumor immune microenvironment. TMPRSS2-ERG fusion and PTEN deletion are prevalent somatic alterations in prostate cancer in predominantly white cohorts.53,67 Interestingly, AA patients consistently have less frequent ERG fusion68–71 and higher rates of intact PTEN.66,69–71 No relationship was found among white patients between tumors featuring ERG fusion and higher expression levels of immune-related genes (such as interleukins and CTLA-4).72 There is a signal that there may be increased macrophage and T-lymphocyte infiltration in tumors with higher ERG fusion, but further studies, specifically in AA patients, are needed.73 PTEN activity has been shown to regulate adaptive immunity in in vitro studies, such as participating in T-cell development and proliferation.74 In breast cancer and melanoma, PTEN loss has been linked with decreased T-cell infiltration and low efficacy of PD-1 inhibitors.75,76 In prostate cancer, PTEN loss is associated with higher grade tumors and increased metastatic potential.46,53 This has also been observed in AA patients, where PTEN-deleted tumors were linked with a higher rate of biochemical recurrence in AA patients, irrespective of ERG fusion.69,77 Since AA prostate cancer appears to have intact PTEN more often, this might correlate with increased T-cell infiltration. Notably, ERG fusion and/or PTEN loss actually correlated with decreased T-cell infiltration in one study described above,45 but it is possible that this T-cell population was primarily immunosuppressive.
3.4 |. Potential transcriptomic contributors to tumor immunogenicity
Several pathways involved in immune response appear to have enhanced expression in AA with prostate cancer. A landmark microarray study found enrichment of genesets associated with immune response, antigen presentation, lymphocyte function, and cytokine signaling in AA tumors.35 Among several upregulated chemokine ligands and receptors, CXCR4, a chemokine receptor, was specifically linked with higher metastatic potential and was specifically upregulated in tumors versus surrounding benign tissue in AAs but not in whites. CXCR4 has been shown in other malignancies to promote tumor metastasis by activating proliferation pathways through the MEK/ERK signaling cascade and activation of nuclear factor kappa B (NF-κB).78,79
These findings were corroborated by a more recent study using transcriptomic data from a much larger set of microarray gene probes.33 Geneset enrichment analysis revealed enrichment of several immune-related pathways, similar to the prior study, including IFN alpha and gamma response and TNF alpha signaling via NF-κB. Expression of IFN alpha and gamma, as well as markers of CD4 and CD8 T-cells, were enriched in AAs, with lower level of hallmark genomic DNA repair scores. Six immune-related genes were validated to have differential expression by race in The Cancer Genome Atlas RNA-sequencing data, including interferon-induced transmembrane protein 3 (IFITM3), interferon alpha inducible protein 6 (IFI6), interferon induced protein 44 like (IF44L), and CD38, and also correlated with increased risk of biochemical recurrence in AAs. The interferon-stimulated genes are involved in regulation of viral response and may have immunosuppressive effects; similarly, CD38 is involved in generation of immunosuppressive adenosine and is a potentially actionable target with therapies inhibiting adenosine signaling.
The link between these potentially immunosuppressive immune pathways and aggressive biological behavior suggests that immunogenic tumors may be forced to harness immunosuppressive pathways as a survival mechanism. In that vein, a different study revealed that MICA, an MHC-associated protein, had decreased expression in AA tumor cells, which had a borderline correlation with lower survival.80 MICA is a stress-induced ligand for the NKG2D receptor on NKs and some T-cells but can be cleaved by tumors, potentially resulting in decreased innate immune recognition and activation in AA tumors. Antibodies targeting MICA are currently being developed to decrease its proteolytic shedding and increase NK-cell activation.81
Last, a study evaluating chromosomal copy number alterations in AA tumors identified that, compared to whites, there was a higher prevalence of altered chromosomal regions that corresponded to genes involved in immune function, such as T-cell proliferation and activation, which in turn affected gene expression of immunoglobulin-related genes.82 This mirrors findings of increased immunoglobulin gene expression35 and increased plasma cells36 in AA tumors, pointing to adaptive immunity as a potentially upregulated immune response correlated with improved outcomes.
Clearly, there may be multiple pathways up- and downregulated in a complex balance of immune responses, and it is unlikely that one single genomic or transcriptomic difference will fully explain the differential effectiveness of sipuleucel-T in AA patients. However, multiple studies highlight distinct alterations seen in AA tumors that affect immunogenicity, such as a possible increase in DDR deficiency and lower prevalence of PTEN loss, that could have downstream effects in immune mediated pathways. Similarly, upregulation of genes involved in cytokine signaling and lymphocyte activity could reflect existing immune response. Combined, these findings raise the possibility that these tumors could show enhanced immunotherapy responsiveness, but also identify potential key immunosuppressive barriers. Finally, we also need to understand the context in which tumors arise: the host immune response.
3.5 |. Potential host immunity differences contributing to enhanced immunotherapy response
Priming the immune system to fight cancer is a focus of active esearch given the success of immunotherapies in treating a variety of tumors. As such, it is critical to detect and understand population-specific differences in host immunity. Variations in immune development in AA patients can be traced to selective genetic drift, generally to infectious pathogens (e.g., malaria).16 Here we list the potential differences in innate and adaptive immunity in AA that could play a role in tumor oncogenesis and response to treatment.
The innate immune system is the rapid, nonspecific arm of immunity. It is most susceptible to inherited genetic variants as it needs to quickly identify and confront pathogens.83 Together with the adaptive response, innate immune cells can provide immune surveillance against the development of new cancers.10 While the initial inflammatory environment is beneficial to eliminate tumors, uncontrolled chronic inflammation increases the risk of developing prostate cancer.11 Toll-like receptors (TLR) are the most characterized of the pattern-recognition mechanisms. TLRs are present in APCs such as DCs and macrophages, but also in tumor cells. Activation of TLRs causes a robust local inflammatory response through release of cytokines and launches adaptive immunity.84,85 Some African populations developed extensive TLR polymorphisms as means to cope with endemic pathogens,86 showing the highest haplotype diversity compared to other groups.87 There is extensive literature on the role of TLRs in prostate cancer, which has previously been reviewed.85 There is conflicting evidence on whether they combat or contribute to tumor growth, which is not surprising given the disparate roles of TLR signaling pathways in immune function. For instance, TLR-3 is associated with inhibiting tumor growth by inactivating the PI3K/AKT pathway,88 while TLR-4 promotes tumor development via VEGF and TGF-b.89 In AA cohorts, there is interest in linking TLR polymorphisms with prostate cancer risk—a good example is that SNPs in TLR-2 and TLR-6 appear to confer higher risk in AA patients compared to whites.83,84 TLR-2 and TLR-6 work as a heterodimer that recognize mostly bacterial patterns but also viral agonists and lead to the activation of the NK-kB pathway.85 It is plausible that these TLR polymorphisms produce higher affinity to tumor-associated ligands, or cause increased signaling in downstream pathways that lead to the recruitment of a potent inflammatory response at the TME. Therefore, while there is significant TLR diversity in AA patients, the mechanistic link with prostate cancer development is still unclear. In turn, cytokines are molecules that coordinate the innate and adaptive immune response.10 Cytokine gene polymorphisms also significantly differ across populations.90,91 Variations in cytokine production and release have been associated with increased prostate cancer susceptibility.92 Specifically, a study observed an increased risk of prostate cancer in AA patients who had the combination of SNPs in IL-1 and IL-10 genes; these were not observed in whites.93 As part of innate immunity, APCs recognize tumor antigens released from killed tumor cells and travel to lymphoid tissue to present these antigens to CD4 T-cells.94 One study found enhanced costimulatory function in T-lymphocytes after antigen presentation by APCs in healthy AA patients,95 which offers a potential mechanism behind the enhanced effects of sipuleucel-T in AA prostate cancer.
Adaptive immunity is a highly selective process that plays a critical role in combating cancer. Unfortunately, there is limited research examining differences in adaptive immunity in AA patients. An older study identified a slightly higher proportion of lymphocytes in the leukocyte count of AA men, but no difference in B- or T-cell subpopulations.96 Additionally, alterations in B-cell receptors and downstream signaling in B-lymphocytes were observed in healthy AA men, although the clinical significance of this finding is unknown.97 Some studies have pointed to differential antibody responses to TAAs in AA patients with prostate cancer, suggesting distinctive B-cell and CD4-T-cell responses to tumors. Antibodies to nucleophosmin 1 have been observed in association with prostate cancer, and relative to non-AAs, titers among AAs were particularly different between those patients with cancer versus benign prostatic hypertrophy.98 Among AA patients, certain immunoglobulin allele variants may be associated with reduced antibody response to cyclin B1, overexpressed in some prostate cancers; this would be expected to decrease immune surveillance and promote metastasis.99 Serum from AA patients had generally stronger immunoreactivity to cell-line antigens in another study; differential reactivity was noted between AA and non-AA patients’ antitumor-antigen autoantibodies to the same epitope in different contexts, an effect seemingly mediated by differential response to posttranslational modifications of the epitopes.100 Since autoantibodies may also be observed in healthy individuals, further work will be needed to associate these antitumor-antigen antibodies with different clinical outcomes or response to immunotherapies. It could be particularly interesting to investigate whether any antigen-spreading from sipuleucel-T treatment includes autoantibodies and/or clonally expanded T cells against these targets.101
Altogether, there seem to be differences in AA host immunity, with polymorphisms in TLRs emerging as the most consistent differences, but further research is needed to explain its role in prostate cancer.
3.6 |. Vaccine immunogenicity in patients of AA
Vaccines may be used prophylactically, to prevent infections, or therapeutically, to enhance antitumor immunity. In patients with active cancer who are receiving treatment, there may be baseline immunodeficiency.102 Therefore, development of effective cancer vaccines must critically consider the characteristics that affect vaccine immunogenicity, including host factors (such as age and comorbidities), and specifics to vaccine design like antigen selection, timing of administration, and need for boosters.103 Notably, genetic ancestry has been evaluated as a possible influence to vaccine efficacy.
A number of studies in the infectious disease literature examined the effect of genetic ancestry in children receiving childhood immunizations. The overarching conclusion from this body of work is that, compared to other cohorts, AA children appear to have higher humoral and cellular response to vaccination, whether it is against measles,104 rubella,105 or pertussis106—a notable exception being smallpox.107 The source of this augmented vaccine response among AA children seems to stem from genetic differences in both innate and adaptive immunity, such as polymorphisms in cytokines and TLRs, as discussed earlier.105
In AA adults, the enhanced immunogenic effect of vaccines is also described, however, this is somewhat dampened by immunosenescence, as both antibody production and cellular response to a vaccine can become quantitative and qualitatively affected by aging.108 The relationship between genetic ancestry and response to the influenza vaccine has been the most studied. AA adults were able to produce higher antibody titers than whites independently of prior flu vaccination,109 but there was an age-dependent decrease in cellular response.110 Moreover, a few studies focused on therapeutic HIV vaccines found mixed results regarding genetic ancestry. For instance, there was no change in immunogenicity in recipients of a DC vaccine,111 while a post-hoc analysis of several trials on a recombinant vaccine reflected higher titers of HIV-neutralizing antibody in AA patients.112 Still, this effect appeared dependent on vaccine type and clinical implications were unclear. Finally, it is worth highlighting that the ongoing vaccine literature on Covid-19 presents an unprecedented opportunity to explore the immunogenicity generated in AA populations.
In summary, there is some evidence that AA may confer increased immunogenicity to infectious vaccines. Whether this effect has a genetic basis remains to be seen; potential confounders could be prior pathogen exposure or the living environment during upbringing.97,100 Nevertheless, immunosenescence can present an important obstacle to therapeutic vaccine efficacy in elderly patients regardless of genetic background.
3.7 |. Immunotherapy in prostate cancer
Compared to other malignancies, the role of immunotherapy in prostate cancer has thus far been marginal based on lack of efficacy observed in clinical trials. To date, the only Food and Drug Administration-approved immunotherapy for prostate cancer remains the cellular vaccine sipuleucel-T.113 As we will discuss below, insufficient recruitment and lack of validation in AA cohorts remains a common theme across immunotherapy trials.
Other cancer vaccines have had less success in prostate cancer. PROSTVAC, a poxvirus vector vaccine which targets PSA as its TAA, had promising preliminary data for prolonging OS in mCRPC, however the phase III trial was stopped early for meeting criteria for futility. Of note, the study only included around 5% AA patients.114 Additional trials of PROSTVAC and other prostate cancer vaccines, like GVAC, have failed to show clinical benefit, although some are still underway.115,116 Outside of prostate cancer, the only currently approved cancer vaccine is T-VEC for melanoma, not specifically examined in AA cohorts given the rare incidence of melanoma in this population.117 There are multiple trials underway on cancer vaccines, including several in combination with checkpoint inhibitors.118–120
In turn, checkpoint inhibitors are a booming class of cancer immunotherapy that target inhibitors of T-cell regulation, including cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and programmed cell death receptor/ligand 1 (PD-1/PD-L1).118 In prostate cancer, ipilimumab monotherapy has failed to show a survival benefit in mCRPC patients. The phase III trial included 5% AA patients and suggested a trend towards improved OS in the AA cohort, but the study was underpowered for this subset analysis.121 Similarly, phase II trials of pembrolizumab in mCRPC demonstrated low response rates.122,123 Interestingly, a trial testing the addition of avelumab in AA men with mCRPC progressing on androgen signaling inhibitors was stopped early for rapid clinical progression in the initial cohort.124 This raises the hypothesis that the observed immunogenic phenotype in AA patients might not be associated with response to PD-1 inhibition, but rather might respond to other checkpoint inhibitors or other immunotherapy approaches. Alternatively, the immune microenvironment may be sufficiently tilted towards immunosuppression as a result of prior hormonal therapies to prevent response to PD-1 inhibition,125 presumably in a race-independent manner, but this approach could be effective in an earlier disease setting and in AA patients.
DDR deficiency may be associated not only with response to PARPi26 but also immune checkpoint inhibitors.127,128 There are conflicting reports as to whether DDR deficiency is more prevalent in in AA patients,64,66,67,129,130 but if true, then this could have implications for patient selection for checkpoint inhibitors and potentially combinations of checkpoint inhibitors with PARPi. Notably, PARPi may have effects beyond their role in DDR. In DDR-deficient breast cancer models, PARPi treatment activates STING signaling and downstream PD-L1 expression and lymphocyte infiltration.131 Several trials are underway to investigate PARPi in combination with checkpoint inhibitors in prostate cancer, including in nonselected populations.132,133 Enrollment of AA patients will be crucial to determine the effectiveness of these strategies in this population.
In sum, the use of immunotherapy in prostate cancer is a rapidly growing field, with multiple strategies being tested in clinical trials. Elucidating the specific immune pathways that become activated with these novel immunotherapies should become a priority to predict whether AA patients could expect greater clinical benefit. Unfortunately, these studies frequently fail to report the ethnicity of their study population, which greatly prevents subgroup analysis of the response of AA patients. This is a broader concern across oncology, where the proportion of AA cancer patients enrolled in immunotherapy trials is not reported. This “color blindness” can also negatively affect tracking of recruitment of minority patients to cancer studies.134 Attempts to find dedicated research of immunotherapy in AA patients across all cancer types yielded a single retrospective study that suggested improved response to nivolumab in non-small cell lung cancer.135 At the very least, data on self-reported race or ethnicity should be included to allow for subgroup exploratory analysis. Ultimately, the future goal would be to better stratify study populations based on their genetic ancestry as a much more precise and clinically relevant tool. Based on the evidence of improved survival in AA cohorts who received sipuleucel-T, focused research on immunotherapy in underrepresented populations is crucial and long overdue to reduce cancer disparities.
4 |. CONCLUSIONS
Prostate cancer disparities in AA patients are complex and multifactorial (see Figure 1 for a visual summary of the evidence discussed). On an immunological basis, the strongest evidence to date has been at the TME. The findings point towards a uniquely inflammatory phenotype, including higher levels of interleukin expression and tumor infiltrating lymphocytes. Variations in AA tumor genomics and transcriptomics, as well as host immunity, may additionally affect the body’s immune response to prostate cancer. Here, the most promising leads are increased expression of immune-related genes and more frequent host genetic variations that developed as a result of genetic drift. Last, AA may grant enhanced immunogenicity to vaccination, including therapeutic cancer vaccines like sipuleucel-T.
FIGURE 1.
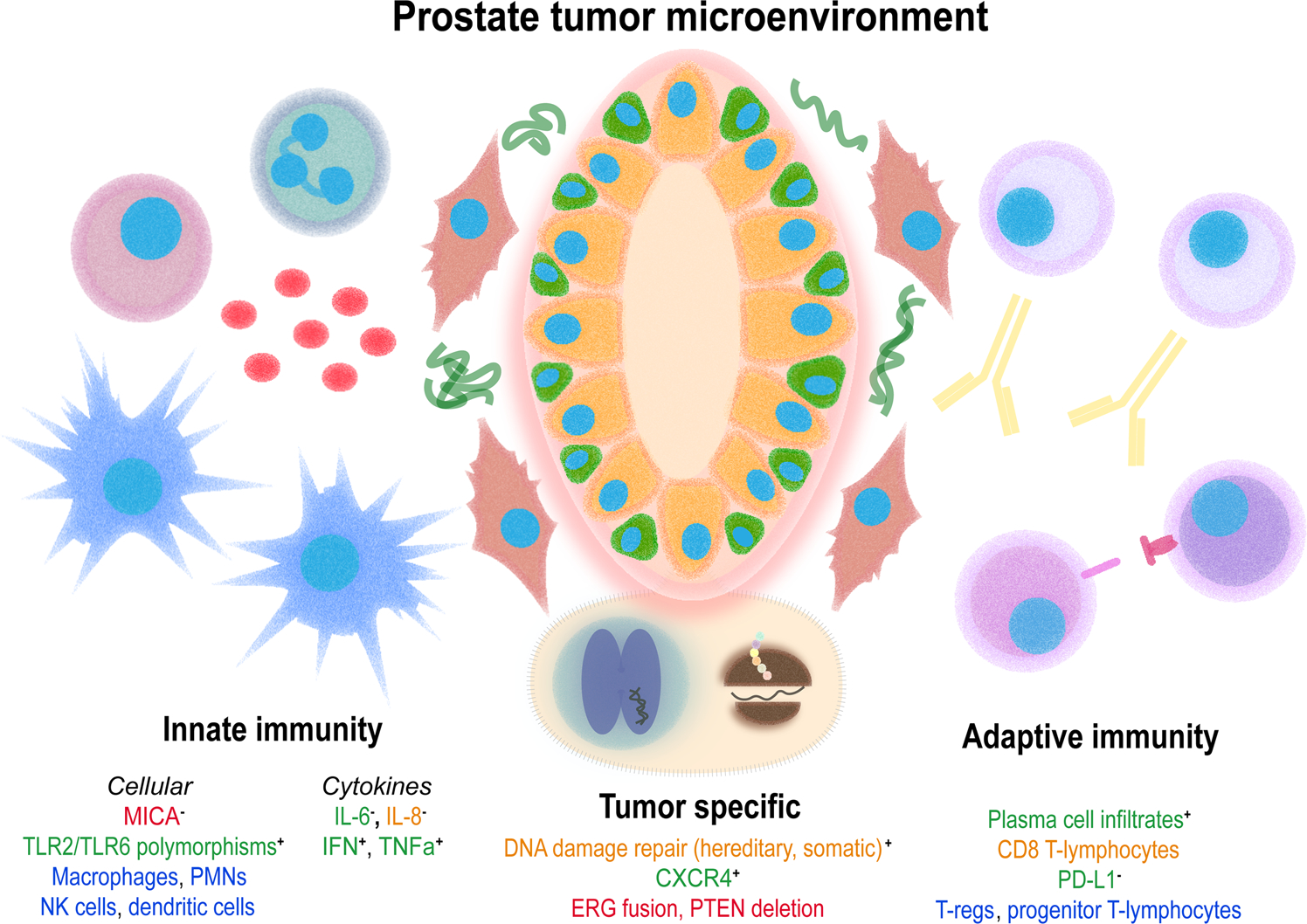
Summary of differences with an immune basis in prostate cancer in men of African origin. Font color reflects whether the relationship is more (green) or less (red) prevalent in AA, or if the data is mixed (orange) or unknown (blue). Where appropriate, + and − reflect pro- or antiinflammatory effects in the tumor microenvironment, respectively
All in all, the evidence (or lack thereof) found in the studies above underscores the urgent need to perform dedicated studies of prostate cancer in patients of AA, particularly on immunotherapy and biomarkers. In this review, we have identified multiple areas of future research to better understand prostate cancer disparities. The hope would be for further validation of PROCEED’s results in other large, AA prominent, cohorts. The highest level of evidence would naturally result from prospective studies comparing sipuleucel-T, alone or in combination to novel therapies, to current standard of care treatments in mCRPC. Adequate representation from AA cohorts could be achieved via pre-specified accrual or enrolling clinical sites that serve a racially diverse patient population. Furthermore, an in-depth analysis of TME composition and activity in AA patients, specifically those that received sipuleucel-T, could open a myriad of opportunities for researching the biology behind survival advantage in this population. Recent dedicated studies focused on AA patients, such as a trial evaluating enzalutamide vs bicalutamide use in mCRPC,136 are a promising start. However, representation of AA patients in prostate cancer and oncology clinical trials remains scarce and not reflective of the general population. Low accrual of AA patients to clinical trials has been linked with socioeconomic and cultural barriers.137 Overcoming these obstacles is an area of active work, requiring investigator as well as institution-wide initiatives.138 Still, given the differences in treatment response observed with sipuleucel-T in AA mCRPC patients, there is tremendous reward in elucidating the biology of prostate cancer and immunotherapy in the AA population to eliminate long-standing cancer disparities.
ACKNOWLEDGEMENTS
This work was supported by the Prostate Cancer Foundation Challenge 18CHAL09 (Steven P. Balk, David J. Einstein), P50 CA090381 (Steven P. Balk), Developmental Research Project P20 CA233255 (Steven P. Balk, David J. Einstein), and Department of Defense W81XWH-17-1-0350 (David J. Einstein) and W81XWH-16-1-0431 (Steven P. Balk).
Funding information
U.S. Department of Defense, Grant/Award Numbers: W81XWH-16-1-0431, W81XWH-17-1-0350; Dana-Farber/Harvard Cancer Center, Grant/Award Numbers: P20 CA233255, P50 CA090381; Prostate Cancer Foundation, Grant/Award Number: 18CHAL09
Footnotes
CONFLICTS OF INTEREST
Daniel Sentana-Lledo: None. Oliver Sartor: Consultant for Advanced Accelerator Applications (AAA), Astellas, AstraZeneca, Bavarian Nordic, Bayer, Blue Earth Diagnostics, Inc., Bristol Myers Squibb, Clarity Pharmaceuticals, Clovis, Constellation, Dendreon, EMD Serono, Fusion, Isotopen Technologien Meunchen, Janssen, Myovant, Myriad, Noria Therapeutics, Inc., Novartis, Noxopharm, Progenics, POINT Biopharma, Pfizer, Sanofi, Tenebio, Telix, and Theragnostics; receives grant or research support from Advanced Accelerator Applications, AstraZeneca, Bayer, Constellation, Dendreon, Endocyte, Invitae, Janssen, Merck, Progenics, Sanofi, and SOTIO. Steven P. Balk: None. David J. Einstein: Funding to institution from Bristol Myers Squibb, Cardiff Oncology, and Puma Biotechnology for clinical trials; discounted research sequencing from Foundation Medicine.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):211–233. [DOI] [PubMed] [Google Scholar]
- 3.Dess RT, Hartman HE, Mahal BA, et al. Association of Black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5(7):975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krimphove MJ, Cole AP, Fletcher SA, et al. Evaluation of the contribution of demographics, access to health care, treatment, and tumor characteristics to racial differences in survival of advanced prostate cancer. Prostate Cancer Prostatic Dis. 2019;22(1):125–136. [DOI] [PubMed] [Google Scholar]
- 5.Riviere P, Luterstein E, Kumar A, et al. Survival of African American and non-Hispanic white men with prostate cancer in an equal-access health care system. Cancer. 2020;126(8):1683–1690. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Singh R, Malik S, Manne U, Mishra M. Prostate cancer health disparities: an immuno-biological perspective. Cancer Lett. 2018;414:153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahal BA, Alshalalfa M, Spratt DE, et al. Prostate cancer genomic-risk differences between African-American and White men across gleason scores. Eur Urol. 2019;75(6):1038–40. [DOI] [PubMed] [Google Scholar]
- 8.Mahal BA, Berman RA, Taplin ME, Huang FW. Prostate cancer-specific mortality across Gleason scores in black vs nonblack men. JAMA. 2018;320(23):2479–2481. [DOI] [PubMed] [Google Scholar]
- 9.McGinley KF, Tay KJ, Moul JW. Prostate cancer in men of African origin. Nat Rev Urol. 2016;13(2):99–107. [DOI] [PubMed] [Google Scholar]
- 10.King Thomas J, Mir H, Kapur N, Singh S. Racial differences in immunological landscape modifiers contributing to disparity in prostate cancer. Cancers (Basel). 2019;11(12):1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Özdemir BC, Dotto G. Racial differences in cancer susceptibility and survival: more than the color of the skin? Trends Cancer. 2017;3(3):181–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higano CS, Armstrong AJ, Sartor AO, et al. Real-world outcomes of sipuleucel-T treatment in PROCEED, a prospective registry of men with metastatic castration-resistant prostate cancer. Cancer. 2019;125(23):4172–4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sartor O, Armstrong AJ, Ahaghotu C, et al. Survival of African-American and Caucasian men after sipuleucel-T immunotherapy: outcomes from the PROCEED registry. Prostate Cancer Prostatic Dis. 2020;23(3):517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight—reconsidering the use of race correction in clinical algorithms. N Engl J Med. 2020;383(9):874–882. [DOI] [PubMed] [Google Scholar]
- 15.Oni-Orisan A, Mavura Y, Banda Y, Thornton TA, Sebro R. Embracing genetic diversity to improve black health. N Engl J Med. 2021;384(12):1163–1167. [DOI] [PubMed] [Google Scholar]
- 16.Campbell MC, Tishkoff SA. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Annu Rev Genomics Hum Genet. 2008;9:403–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adeola H, Blackburn JM, Rebbeck TR, Zerbini LF. Emerging proteomics biomarkers and prostate cancer burden in Africa. Oncotarget. 2017;8(23):37991–38007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stefflova K, Dulik MC, Barnholtz-Sloan JS, Pai AA, Walker AH, Rebbeck TR. Dissecting the within-Africa ancestry of populations of african descent in the Americas. PLoS One. 2011;6(1):e14495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. [DOI] [PubMed] [Google Scholar]
- 20.Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115(16):3670–3679. [DOI] [PubMed] [Google Scholar]
- 21.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24(19):3089–3094. [DOI] [PubMed] [Google Scholar]
- 22.Schellhammer PF, Chodak G, Whitmore JB, Sims R, Frohlich MW, Kantoff PW. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology. 2013;81(6):1297–1302. [DOI] [PubMed] [Google Scholar]
- 23.Sheikh NA, Petrylak D, Kantoff PW, et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. 2013;62(1):137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.GuhaThakurta D, Sheikh NA, Fan LQ, et al. Humoral immune response against nontargeted tumor antigens after treatment with sipuleucel-T and its association with improved clinical outcome. Clin Cancer Res. 2015;21(16):3619–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fong L, Carroll P, Weinberg V, et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J Natl Cancer Inst. 2014;106(11):dju268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caram ME, Ross R, Lin P, Mukherjee B. Factors associated with use of sipuleucel-T to treat patients with advanced prostate cancer. JAMA Netw Open. 2019;2(4):e192589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLeod DG, Quinn DI, Whitmore JB, Tabesh M. Sipuleucel-T in African Americans: a subgroup analysis of three phase 3 trials of sipuleucel-T in metastatic castrate resistant prostate cancer. J Clin Oncol. 2011;29(15_suppl):e15148. [Google Scholar]
- 28.Holl EK, McNamara MA, Healy P, et al. Prolonged PSA stabilization and overall survival following sipuleucel-T monotherapy in metastatic castration-resistant prostate cancer patients. Prostate Cancer Prostatic Dis. 2019;22(4):588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fair WR, Heston WDW, Kabmon D, et al. Prostatic cancer, acid phosphatase, creatine kinase-BB and race: a prospective study. J Urol. 1982;128(4):735–738. [DOI] [PubMed] [Google Scholar]
- 30.Hawley JE, Pan S, Kandadi H, Chairmowitz MG, Sheikh N, Drake CG. Analysis of circulating immune biomarkers by race in men with CRPC treated with sipuleucel-T. abstract presented at. The 27th Annual Scientific Retreat - PCF 2020 Prostate Cancer Foundation, Oct 20 2020. [Google Scholar]
- 31.Bhardwaj A, Srivastava SK, Khan MA, et al. Racial disparities in prostate cancer: a molecular perspective. Front Biosci (Landmark Ed). 2017;22:772–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reimers M, Slane KE, Pachynski RKl. Immunotherapy in metastatic castration-resistant prostate cancer: past and future strategies for optimization. Curr Urol Rep. 2019;20(10):64. [DOI] [PubMed] [Google Scholar]
- 33.Awasthi S, Berglund A, Abraham-Miranda J, et al. Comparative genomics reveals distinct immune-oncologic pathways in African American men with prostate cancer. Clin Cancer Res. 2021;27(1):320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kinseth MA, Jia Z, Rahmatpanah F, et al. Expression differences between African American and Caucasian prostate cancer tissue reveals that stroma is the site of aggressive changes. Int J Cancer. 2014;134(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallace TA, Prueitt RL, Yi M, et al. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res. 2008;68(3):927–936. [DOI] [PubMed] [Google Scholar]
- 36.Weiner AB, Vidotto T, Liu Y, et al. Plasma cells are enriched in localized prostate cancer in Black men and are associated with improved outcomes. Nat Commun. 2021;12(1):935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powell IJ, Dyson G, Land S, Ruterbusch J, Bock CH, Lenk S. Genes associated with prostate cancer are differentially expressed in African American and European American men. Cancer Epidemiol Biomarkers Prev. 2013;22(5):891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu C, Hsieh C, Lin C, Chen W, Hong J, Chen M. Significance of IL-6 in the transition of hormone-resistant prostate cancer and the induction of myeloid-derived suppressor cells. J Mol Med (Berl). 2012;90(11):1343–1355. [DOI] [PubMed] [Google Scholar]
- 39.Gillard M, Javier R, Ji Y, et al. Elevation of stromal-derived mediators of inflammation promote prostate cancer progression in African-American men. Cancer Res. 2018;78(21):6134–45. [DOI] [PubMed] [Google Scholar]
- 40.Maynard JP, Ertunc O, Kulac I, Baena-Del Valle JA, De Marzo AM, Sfanos KS. IL8 Expression is associated with prostate cancer aggressiveness and androgen receptor loss in primary and metastatic prostate cancer. Mol Cancer Res. 2020;18(1):153–165. [DOI] [PubMed] [Google Scholar]
- 41.McArdle PA, Canna K, McMillan DC, McNicol A, Campbell R, Underwood MA. The relationship between T-lymphocyte subset infiltration and survival in patients with prostate cancer. Br J Cancer. 2004;91(3):541–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ness N, Andersen S, Valkov A, et al. Infiltration of CD8+ lymphocytes is an independent prognostic factor of biochemical failure-free survival in prostate cancer. Prostate. 2014;74(14):1452–1461. [DOI] [PubMed] [Google Scholar]
- 43.Petitprez F, Fossati N, Vano Y, et al. PD-L1 expression and CD8 + T-cell infiltrate are associated with clinical progression in patients with node-positive prostate cancer. Eur Urol Focus. 2019;5(2):192–196. [DOI] [PubMed] [Google Scholar]
- 44.Calagua C, Russo J, Sun Y, et al. Expression of PD-L1 in hormone-naïve and treated prostate cancer patients receiving neoadjuvant abiraterone acetate plus prednisone and leuprolide. Clin Cancer Res. 2017;23(22):6812–6822. [DOI] [PubMed] [Google Scholar]
- 45.Kaur HB, Guedes LB, Lu J, et al. Association of tumor-infiltrating T-cell density with molecular subtype, racial ancestry and clinical outcomes in prostate cancer. Mod Pathol. 2018;31(10):1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vitkin N, Nersesian S, Siemens DR, Koti M. The tumor immune contexture of prostate cancer. Front Immunol. 2019;10:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sfanos KS, De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology. 2012;60(1):199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shiao S, Chia-Yi Chu G, Chung LW. Regulation of prostate cancer progression by the tumor microenvironment. Cancer Lett. 2016;380(1):340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strasner A, Karin M. Immune infiltration and prostate cancer. Front Oncol. 2015;5:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Im SJ, Hashimoto M, Gerner MY, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537(7620):417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siddiqui I, Schaeuble K, Chennupati V, et al. Intratumoral Tcf1 + PD-1 + CD8 + T Cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity. 2019;50(1):195–211. [DOI] [PubMed] [Google Scholar]
- 52.Jansen CS, Prokhnevska N, Master VA, et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature. 2019;576(7787):465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jamaspishvili T, Berman DM, Ross AE, et al. Clinical implications of PTEN loss in prostate cancer. Nat Rev Urol. 2018;15(4):222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao SG, Lehrer J, Chang SL, et al. The Immune landscape of prostate cancer and nomination of PD-L2 as a potential therapeutic target. J Natl Cancer Inst. 2019;111(3):301–310. [DOI] [PubMed] [Google Scholar]
- 55.Wang G, Zhao D, Spring DJ, DePinho RA. Genetics and biology of prostate cancer. Genes Dev. 2018;32(17–18):1105–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan J, Hu Z, Mahal BA, et al. Integrated analysis of genetic ancestry and genomic alterations across cancers. Cancer Cell. 2018;34(4):549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karakas C, Wang C, Deng F, Huang H, Wang D, Lee P. Molecular mechanisms involving prostate cancer racial disparity. Am J Clin Exp Urol. 2017;5(3):34–48. [PMC free article] [PubMed] [Google Scholar]
- 58.Tan S, Petrovics G, Srivastava S. Prostate cancer genomics: recent advances and the prevailing underrepresentation from racial and ethnic minorities. Int J Mol Sci. 2018;19(4):1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conti DV, Darst BF, Moss LC, et al. Trans-ancestry genome-wide association meta-analysis of prostate cancer identifies new susceptibility loci and informs genetic risk prediction. Nat Genet. 2021;53(1):65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han Y, Rand KA, Hazelett DJ, et al. Prostate cancer susceptibility in men of african ancestry at 8q24. J Natl Cancer Inst. 2016;108(7):djv431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Troutman SM, Sissung TM, Cropp CD, et al. Racial disparities in the association between variants on 8q24 and prostate cancer: a systematic review and meta-analysis. Oncologist. 2012;17(3):312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kwon EM, Salinas CA, Kolb S, et al. Genetic polymorphisms in inflammation pathway genes and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2011;20(5):923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beebe-Dimmer JL, Zuhlke KA, Johnson AM, Liesman D, Cooney KA. Rare germline mutations in African American men diagnosed with early-onset prostate cancer. Prostate. 2018;78(5):321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mahal BA, Alshalalfa M, Kensler KH, et al. Racial differences in genomic profiling of prostate cancer. N Engl J Med. 2020;383(11):1083–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu W, Zheng SL, Na R, et al. Distinct genomic alterations in prostate tumors derived from African American men. Mol Cancer Res. 2020;18(12):1815–1824. [DOI] [PubMed] [Google Scholar]
- 66.Koga Y, Song H, Chalmers ZR, et al. Genomic profiling of prostate cancers from men with African and European Ancestry. Clin Cancer Res. 2020;26(17):4651–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu R, Zhou J, Xia S, Li T. The impact of PTEN deletion and ERG rearrangement on recurrence after treatment for prostate cancer: a systematic review and meta-analysis. Clin Transl Oncol. 2020;22(5):694–702. [DOI] [PubMed] [Google Scholar]
- 68.Jaratlerdsiri W, Chan E, Gong T, et al. Whole-genome sequencing reveals elevated tumor mutational burden and initiating driver mutations in African men with treatment-naïve, high-risk prostate cancer. Cancer Res. 2018;78(24):6736–6746. [DOI] [PubMed] [Google Scholar]
- 69.Khani F, Mosquera JM, Park K, et al. Evidence for molecular differences in prostate cancer between African American and Caucasian men. Clin Cancer Res. 2014;20(18):4925–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lindquist KJ, Paris PL, Hoffmann TJ, et al. Mutational landscape of aggressive prostate tumors in African American men. Cancer Res. 2016;76(7):1860–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yuan J, Kensler KH, Hu Z, et al. Integrative comparison of the genomic and transcriptomic landscape between prostate cancer patients of predominantly African or European genetic ancestry. PLoS Genet. 2020;16(2):e1008641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gerashchenko GV, Grygoruk OV, Kononenko OA, Gryzodub OP, Stakhovsky EO, Kashuba VI. Expression pattern of genes associated with tumor microenvironment in prostate cancer. Exp Oncol. 2018;40(4):315–322. [PubMed] [Google Scholar]
- 73.Burdova A, Rulisek P, Bouchal J, Kral M, Student V, Kolar Z. Infiltration of prostate cancer by CD204+ and CD3+ cells correlates with ERG expression and TMPRSS2-ERG gene fusion. Klin Onkol. 2018;31(6):421–428. [DOI] [PubMed] [Google Scholar]
- 74.Chen L, Guo D. The functions of tumor suppressor PTEN in innate and adaptive immunity. Cell Mol Immunol. 2017;14(7):581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barroso-Sousa R, Keenan TE, Pernas S, et al. Tumor mutational burden and PTEN alterations as molecular correlates of response to PD-1/L1 blockade in metastatic triple-negative breast cancer. Clin Cancer Res. 2020;26(11):2565–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peng W, Chen JQ, Liu C, et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 2016;6(2):202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tosoian JJ, Almutairi F, Morais CL, et al. Prevalence and prognostic significance of PTEN loss in African-American and European-American men undergoing radical prostatectomy. Eur Urol. 2017;71(5):697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun X, Cheng G, Hao M, et al. CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010;29(4):709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang L, Yeger H, Das B, Irwin MS, Baruchel S. Tissue microenvironment modulates CXCR4 expression and tumor metastasis in neuroblastoma. Neoplasia. 2007;9(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sakiyama MJ, Espinoza I, Reddy A, et al. Race-associated expression of MHC class I polypeptide-related sequence A (MICA) in prostate cancer. Exp Mol Pathol. 2019;108:173–182. [DOI] [PubMed] [Google Scholar]
- 81.Ferrari de Andrade L, Tay RE, Pan D, et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science. 2018;359(6383):1537–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rose AE, Satagopan JM, Oddoux C, et al. Copy number and gene expression differences between African American and Caucasian American prostate cancer. J Transl Med. 2010;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yeyeodu ST, Kidd LR, Kimbro KSS. Protective innate immune variants in racial/ethnic disparities of breast and prostate cancer. Cancer Immunol Res. 2019;7(9):1384–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rogers EN, Jones DZ, Kidd NC, et al. Toll-like receptor-associated sequence variants and prostate cancer risk among men of African descent. Genes Immun. 2013;14(6):347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao S, Zhang Y, Zhang Q, Wang F, Zhang D. Toll-like receptors and prostate cancer. Front Immunol. 2014;5:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nédélec Y, Sanz J, Baharian G, et al. Genetic ancestry and natural selection drive population differences in immune responses to pathogens. Cell. 2016;167(3):657–669. [DOI] [PubMed] [Google Scholar]
- 87.Barreiro LB, Ben-Ali M, Quach H, et al. Evolutionary dynamics of human toll-like receptors and their different contributions to host defense. PLoS Genet. 2009;5(7):e1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harashima N, Inao T, Imamura R, Okano S, Suda T, Harada M. Roles of the PI3K/Akt pathway and autophagy in TLR3 signaling-induced apoptosis and growth arrest of human prostate cancer cells. Cancer Immunol Immunother. 2012;61(5):667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pei Z, Lin D, Song X, Li H, Yao H. TLR4 signaling promotes the expression of VEGF and TGFbeta1 in human prostate epithelial PC3 cells induced by lipopolysaccharide. Cell Immunol. 2008;254(1):20–27. [DOI] [PubMed] [Google Scholar]
- 90.Cox ED, Hoffmann SC, Dimercurio BS, et al. Cytokine polymorphic analyses indicate ethnic differences in the allelic distribution of interleukin-2 and interleukin-6. Transplantation. 2001;72(4):720–726. [DOI] [PubMed] [Google Scholar]
- 91.Meenagh A, Williams F, Ross OA, et al. Frequency of cytokine polymorphisms in populations from Western Europe, Africa, Asia, the Middle East and South America. Hum Immunol. 2002;63(11):1055–1061. [DOI] [PubMed] [Google Scholar]
- 92.Zabaleta J, Su LJ, Lin HY, et al. Cytokine genetic polymorphisms and prostate cancer aggressiveness. Carcinogenesis. 2009;30(8):1358–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zabaleta J, Lin HY, Sierra RA, et al. Interactions of cytokine gene polymorphisms in prostate cancer risk. Carcinogenesis. 2008;29(3):573–578. [DOI] [PubMed] [Google Scholar]
- 94.Sabado RL, Balan S, Bhardwaj N. Dendritic cell-based immunotherapy. Cell Res. 2017;27(1):74–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hutchings A, Purcell WM, Benfield MR. Peripheral blood antigen-presenting cells from African-Americans exhibit increased CD80 and CD86 expression. Clin Exp Immunol. 1999;118(2):247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Freedman DS, Gates L, Flanders WD, et al. Black/white differences in leukocyte subpopulations in men. Int J Epidemiol. 1997;26(4):757–764. [DOI] [PubMed] [Google Scholar]
- 97.Longo DM, Louie B, Mathi K, et al. Racial differences in B cell receptor signaling pathway activation. J Transl Med. 2012;10:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dai L, Li J, Xing M, Sanchez TW, Casiano CA, Zhang J. Using serological proteome analysis to identify serum anti-nucleophosmin 1 autoantibody as a potential biomarker in European-American and African-American patients with prostate cancer. Prostate. 2016;76(15):1375–1386. [DOI] [PubMed] [Google Scholar]
- 99.Pandey JP, Namboodiri AM, Kistner-Griffin E. A genetic variant of FcγRIIIa is strongly associatedwith humoral immunity to cyclin B1 in African American patients with prostate cancer. Immunogenetics. 2013;65(2):91–96. [DOI] [PubMed] [Google Scholar]
- 100.Sanchez TW, Zhang G, Li J, et al. Immunoseroproteomic profiling in African American men with prostate cancer: evidence for an autoantibody response to glycolysis and plasminogen-associated proteins. Mol Cell Proteomics. 2016;15(12):3564–3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gulley JL, Madan RA, Pachynski R, et al. Role of antigen spread and distinctive characteristics of immunotherapy in cancer treatment. J Natl Cancer Inst. 2017;109(4):djw261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Santos P, Butterfield LH. Dendritic cell-based cancer vaccines. J Immunol. 2018;200(2):443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019;32(2):e00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Voigt EA, Ovsyannikova IG, Haralambieva IH, et al. Genetically defined race, but not sex, is associated with higher humoral and cellular immune responses to measles vaccination. Vaccine. 2016;34(41):4913–4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Haralambieva IH, Salk HM, Lambert ND, et al. Associations between race, sex and immune response variations to rubella vaccination in two independent cohorts. Vaccine. 2014;32(17):1946–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Christy C, Pichichero ME, Reed GF, et al. Effect of gender, race, and parental education on immunogenicity and reported reactogenicity of acellular and whole-cell pertussis vaccines. Pediatrics. 1995;96(3 Pt 2):584–587. [PubMed] [Google Scholar]
- 107.Haralambieva IH, Ovsyannikova IG, Kennedy RB, Larrabee BR, Pankratz VS, Poland GA. Race and sex-based differences in cytokine immune responses to smallpox vaccine in healthy individuals. Hum Immunol. 2013;74(10):1263–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Frasca D, Blomberg BB. Aging affects human B cell responses. J Clin Immunol. 2011;31(3):430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kurupati R, Kossenkov A, Haut L, et al. Race-related differences in antibody responses to the inactivated influenza vaccine are linked to distinct pre-vaccination gene expression profiles in blood. Oncotarget. 2016;7(39):62898–62911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gardner EM, Gonzalez EW, Nogusa S, Murasko DM. Age-related changes in the immune response to influenza vaccination in a racially diverse, healthy elderly population. Vaccine. 2006;24(10):1609–1614. [DOI] [PubMed] [Google Scholar]
- 111.Segat L, Brandão L, Guimarães RL, et al. Polymorphisms in innate immunity genes and patients’ response to dendritic cell-based HIV immuno-treatment. Vaccine. 2010;28(10):2201–2206. [DOI] [PubMed] [Google Scholar]
- 112.Montefiori DC, Metch B, McElrath MJ, et al. Demographic factors that influence the neutralizing antibody response in recipients of recombinant HIV-1 gp120 vaccines. J Infect Dis. 2004;190(11):1962–1969. [DOI] [PubMed] [Google Scholar]
- 113.NCCN Clinical Practice Guidelines in Oncology Prostate Cancer. Version 2.2021. Available at: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed April 30, 2021.
- 114.Gulley JL, Borre M, Vogelzang NJ, et al. Phase III Trial of PROSTVAC in asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer. J Clin Oncol. 2019;37(13):1051–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McNeel DG, Eickhoff JC, Johnson LE, et al. Phase II trial of a DNA vaccine encoding prostatic acid phosphatase (pTVG-HP [MVI-816]) in patients with progressive, nonmetastatic, castration-sensitive prostate cancer. J Clin Oncol. 2019;37(36):3507–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Prostvac in Patients With Biochemically Recurrent Prostate Cancer. ClinicalTrials.gov identifier: NCT02649439. Available at: https://clinicaltrials.gov/ct2/show/NCT02649439. Accessed April 30, 2021.
- 117.Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–2788. [DOI] [PubMed] [Google Scholar]
- 118.Al Harthy M, Redman J, Madan RA. Novel immunotherapy combinations for genitourinary cancers. Expert Opin Biol Ther. 2020;20(3):253–262. [DOI] [PubMed] [Google Scholar]
- 119.DeMaria PJ, Bilusic M. Cancer vaccines. Hematol Oncol Clin North Am. 2019;33(2):199–214. [DOI] [PubMed] [Google Scholar]
- 120.Jochems C, Tucker JA, Tsang KY, et al. A combination trial of vaccine plus ipilimumab in metastatic castration-resistant prostate cancer patients: immune correlates. Cancer Immunol Immunother. 2014;63(4):407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Beer TM, Kwon ED, Drake CG, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35(1):40–47. [DOI] [PubMed] [Google Scholar]
- 122.Antonarakis ES, Piulats JM, Gross-Goupil M, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol. 2020;38(5):395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hansen AR, Massard C, Ott PA, et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol. 2018;29(8):1807–1813. [DOI] [PubMed] [Google Scholar]
- 124.Avelumab Plus 2nd-generation ADT in African American Subjects With mCRPC. ClinicalTrials.gov identifier: NCT03770455. Available at: https://clinicaltrials.gov/ct2/show/NCT03770455. Accessed April 30, 2021.
- 125.Calcinotto A, Spataro C, Zagato E, et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature. 2018;559(7714):363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.DeBono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091–2102. [DOI] [PubMed] [Google Scholar]
- 127.Hsiehchen D, Hsieh A, Samstein RM, et al. DNA repair gene mutations as predictors of immune checkpoint inhibitor response beyond tumor mutation burden. Cell Rep Med. 2020;1(3):100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Teo MY, Seier K, Ostrovnaya I, et al. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J Clin Oncol. 2018;36(17):1685–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chandrasekar T, Gross L, Gomella LG, Hegarty SE, Leong JY, Giri VN. Prevalence of suspected hereditary cancer syndromes and germline mutations among a diverse cohort of probands reporting a family history of prostate cancer: toward informing cascade testing for men. Eur Urol Oncol. 2020;3(3):291–297. [DOI] [PubMed] [Google Scholar]
- 130.Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pantelidou C, Sonzogni O, De Oliveria Taveira M, et al. PARP inhibitor efficacy depends on CD8+ T-cell recruitment via intratumoral STING pathway activation in BRCA-deficient models of triple-negative breast cancer. Cancer Discov. 2019;9(6):722–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vikas P, Borcherding N, Chennamadhavuni A, Garje R. Therapeutic potential of combining PARP inhibitor and immunotherapy in solid tumors. Front Oncol. 2020;10:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Study of Pembrolizumab (MK-3475) Plus Olaparib Versus Abiraterone Acetate or Enzalutamide in Metastatic Castration-resistant Prostate Cancer (mCRPC) (MK-7339–010/KEYLYNK-010). ClinicalTrials.gov identifier: NCT03834519. Available at: https://clinicaltrials.gov/ct2/show/NCT03834519. Accessed April 30,2021.
- 134.Manchanda EC, Couillard C, Sivashanker K. Inequity in crisis standards of care. N Engl J Med. 2020;383(4):e16. [DOI] [PubMed] [Google Scholar]
- 135.Tiu AC, Potdar R, Djibo DA, Masab M, Dourado C. Clinical outcomes of African American patients with advanced or metastatic non-small cell lung cancer on Nivolumab in a single community-based cancer center. Med Oncol. 2018;35(7):109. [DOI] [PubMed] [Google Scholar]
- 136.Vaishampayan UN, Heilbrun LK, Monk P, et al. Clinical efficacy of enzalutamide vs bicalutamide combined with androgen deprivation therapy in men with metastatic hormone-sensitive prostate cancer: a randomized clinical trial. JAMA Netw Open. 2021;4(1):e2034633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Senft N, Hamel LM, Manning MA, et al. Willingness to discuss clinical trials among Black vs White men with prostate cancer. JAMA Oncol. 2020;6(11):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ahaghotu C, Tyler R, Sartor O. African American Participation in Oncology Clinical Trials—focus on prostate cancer: implications, barriers, and potential solutions. Clin Genitourin Cancer. 2016;14(2):105–116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


