Abstract
Enteropathogenic Escherichia coli (EPEC) is an extracellular bacterial pathogen that infects the human intestinal epithelium and is a major cause of infantile diarrhea in developing countries. EPEC belongs to the group of attaching and effacing (A/E) pathogens. It uses a type III secretion system to deliver proteins into the host cell that mediate signal transduction events in host cells. We used gene array technology to study epithelial cell responses to EPEC infection at the level of gene expression. We found that EPEC induces the expression of several genes in infected HeLa cells by a lipopolysaccharide (LPS)-independent mechanism, including cytokines and early growth response factor 1 (Egr-1). The transcription factor Egr-1 is an immediate-early-induced gene that is activated in most cell types in response to stress. EPEC-induced upregulation of egr-1 is mediated by the activation of the MEK/extracellular signal-regulated kinase signal transduction pathway and is dependent on the type III secretion system. egr-1 is also induced during infection of mice by the A/E pathogen Citrobacter rodentium, suggesting that both Egr-1 and the activation of this mitogen-activated protein kinase signal transduction pathway may play a role in disease.
Intestinal epithelial cells are the first physical barrier that pathogens encounter in the gastrointestinal tract. As a consequence of constant exposure to such pathogens, the epithelium has evolved mechanisms to discriminate between pathogenic and nonpathogenic bacteria. In the case of infection, epithelial cells become activated to express and secrete proinflammatory and chemoattractant cytokines, including interleukin 8 (IL-8), GROα, GROβ, monocyte chemoattractant protein 1, tumor necrosis factor alpha, granulocyte-macrophage colony-stimulating factor, or IL-1 (reviewed in reference 15). Moreover, activated epithelial cells play an important role in the initiation of inflammatory and immune responses by transmitting signals to underlying cells of the reticuloendothelial system. Bacterial lipopolysaccharide (LPS) induces the production of inflammatory cytokines by immune cells such as macrophages and monocytes. However, intestinal epithelial cells do not normally respond to LPS from extracellular pathogens to prevent exaggerated responses to the LPS of normal flora.
One of the best-studied extracellular intestinal pathogens is enteropathogenic Escherichia coli (EPEC). EPEC infects the human small intestinal epithelium and is a prominent cause of diarrhea in infants in developing countries. EPEC uses a type III secretion system to deliver bacterial effectors into host cells. Among the EPEC-secreted proteins (Esp), EspA, EspB, and EspD are constituents of the translocation machinery, with EspB and EspD being inserted in the host cell membrane. EPEC attaches to intestinal epithelial cells by the interaction of an outer membrane protein, intimin, and a type III secreted protein that is translocated into the host cell membrane, Tir. This interaction induces the polymerization of actin into characteristic pedestal-like structures (reviewed in reference 31). Two other type III secreted proteins have been described: EspF, a proline-rich protein recently shown to be translocated into the host cell cytoplasm and involved in disrupting epithelial barrier function (20), and open reading frame 19, which has recently been shown to be translocated to the host mitochondria (17). EPEC's secreted proteins induce signal transduction events within the host cell, including inositol phosphate fluxes and protein kinase C and phospholipase Cγ activation (2, 10, 16). EPEC also triggers IL-8 secretion through NF-κB activation in T84 epithelial cells and recruitment of polymorphonuclear cells in a coculture system (28, 29). These changes in signaling may contribute to disease. However, the molecular mechanisms by which EPEC causes diarrhea or induces pedestal formation are still unknown.
Recent studies have shown that some bacterial pathogens are able to activate mitogen-activated protein (MAP) kinase pathways in the host cell (13, 23, 32, 33) to alter processes such as cell differentiation, growth, and death. MAP kinase signaling pathways described thus far include the extracellular signal-regulated kinases (ERK), c-Jun N-terminal kinases (JNK) (also known as stress-activated protein kinases) and p38 MAP kinases. The activation of these pathways proceeds through a cascade of phosphorylation events leading to the phosphorylation of downstream kinases and to the transcriptional activation of several genes. MEK1 (MAPKK1) kinase activation leads to phosphorylation of ERK1 and ERK2, resulting in their translocation to the nucleus and phosphorylation of other proteins, including the transcription factor Elk-1. This event induces the transcription of genes like fos and egr-1. The early growth response factor 1 (Egr-1) is an 80- to 82-kDa zinc finger transcription factor that belongs to the early growth response gene family that participates in several cellular processes such as differentiation and proliferation. egr-1 is an immediate-early gene induced in response to changes in the local cell environment, including exposure to growth factors and cytokines, hypoxia, tissue damage, UV, or LPS (18, 19). egr-1 can also be induced by the p38 MAP kinase pathway.
The aims of this study were to use genomic arrays to determine if epithelial cells respond to EPEC infection by altering global gene expression and to identify novel factors and pathways involved in this process. Our findings show that EPEC infection of epithelial cells causes upregulation of expression of cytokines. We also demonstrate that EPEC activates the MEK/ERK signal transduction cascade that leads to the expression of the transcription factor Egr-1. In vivo studies of Citrobacter rodentium-infected mice confirmed egr-1 induction and suggest that the induction of egr-1 may play a role in disease.
MATERIALS AND METHODS
Bacterial strains, mammalian cell lines, and culture conditions.
EPEC strain E2348/69 (6) and the mutants escC (see below) and espB (8) were grown overnight in Luria-Bertani (LB) broth at 37°C without shaking prior to infection. “Preactivated” bacteria were prepared by diluting LB overnight cultures 1:50 in Dulbecco's modified Eagle medium (DMEM) without serum and incubating at 37°C with 5% CO2 for 3 h. C. rodentium (formerly Citrobacter freundii biotype 4280) strain DBS100 was grown overnight in LB prior to mouse infection.
HeLa cells (CCL2; American Type Culture Collection) were cultured in DMEM containing 10% heat-inactivated fetal calf serum (FCS) and grown at 37°C with 5% CO2.
Animal experiments.
C57BL/6J mice (Jackson Laboratory, Bar Harbor, Maine) were infected by oral gavage with 0.1 ml of standing LB overnight cultures of C. rodentium (2.5 × 108 CFU). Mice were killed at days 6 and 10 postinfection, and colons were dissected for RNA isolation.
Construction of a nonpolar escC deletion mutant.
The oligonucleotides ESCC-01F (5′-GTTAACCTCGAGGCGGTTCCGATAG-3′) (XhoI restriction site) and ESCC-02R (5′-GATGCGAGCTCTGTTGCTATCCAATG-5′) (SacI restriction site) were used to amplify escC from chromosomal DNA from EPEC E2348/69. The amplification product was cloned into pCR2.1 TOPO (Invitrogen), generating pCR-escC. Primers ESCC-03R (5′-GGCGACGCGTGTATACCGCTGTTAAGCGACATTCC-3′) and ESCC-04F (5′-GGCGACGCGTCATTACACAATTCGTCCTATATCAG-3′) were used to create an in-frame deletion of 1,408 bp between bp 2 and bp 1410 of the escC gene in pCR-escC using inverse PCR amplification. Both oligonucleotides ESCC-03R and ESCC-04F introduced an MluI restriction site. The 2,049-bp SacI-XhoI escC deletion fragment was cloned into the positive-selection suicide vector pCVD442 (7), digested with SacI-SalI. The resulting plasmid, pCVD442-ΔescC, was used to construct the escC deletion mutant in EPEC E2348/69 (streptomycin resistant) by allelic exchange as described (7), generating the EPEC ΔescC strain.
RNA isolation.
HeLa cells (1.5 × 106) were seeded in 10-cm-diameter tissue culture plates in 10 ml of DMEM plus 10% FCS and cultured overnight. Culture medium was changed to DMEM without FCS, and cells were infected for 3 h with overnight bacterial cultures: EPEC wild type (wt) (30 μl), the ΔespB mutant (100 μl), the ΔescC mutant (30 μl), or EPEC exposed to LPS from E. coli O111:B4 (10 μg/ml; Sigma, St. Louis, Mo.). Cells were washed five times with diethyl pyrocarbonate-treated phosphate-buffered saline and scraped in 1 ml of diethyl pyrocarbonate–phosphate-buffered saline. Cells were pelleted and resuspended in 1 ml of Trizol (Life Technologies), and RNA was purified following the manufacturer's instructions. RNA was treated with DNase I (Clontech, Palo Alto, Calif.) to remove contaminant genomic DNA for 1.5 h in the presence of RNase inhibitor (Ambion, Austin, Tex.), and the reaction was stopped using 10× termination mix (0.1 M EDTA, pH 8; glycogen, 1 mg/ml). The enzyme was removed by phenol-chloroform extraction, and RNA was precipitated with 2 volumes of ethanol and a 1/10 volume of sodium acetate, pH 5.2. RNA was resuspended in 15 μl of H2O containing the RNase inhibitor and stored at −70°C. RNA was tested for the presence of remaining DNA contamination by 35 cycles of PCR amplification using GAPDH (glyceraldehyde-3-phosphate dehydrogenase)-specific primers (Table 1).
TABLE 1.
Oligonucleotides and conditions used for RT-PCR
| Gene | Oligonucleotide | Oligonucleotide sequence | Product (bp) | No. of cycles |
|---|---|---|---|---|
| GAPDH | hgap+ | GGCTCTCCAGAACATCATCC | 265 | 23 |
| hgap− | GTCGCTGTTGAAGTCAGAGG | |||
| egr-1 | egr+ | TCACCTATACTGGCCGCTTT | 502 | 32 |
| egr− | TGAGTGGCAAAGGCCTTAAT | |||
| MIP-2α | mip2a+ | AGCAGGAGCGCCCCTGGC | 172 | 35 |
| mip2a− | GATTTTCTTAACCATGGGC | |||
| IL-8 | LK055 | ATGACTTCCAAGCTGGCCGTGGCT | 289 | 30 |
| LK056 | TCTCAGCCCTCTTCAAAAACTTCTC | |||
| zyxin 1 + zyxin 2 | zyx+ | CGAGGGCTGTTACACTGACA | 351 | 35 |
| zyx− | TCATCTGCCTCAATCGACAG | |||
| ETR101 | etr+ | GAAGTGCAGAAAGAGGCACA | 457 | 35 |
| etr− | ATGACGCTCCCTCCTCTTCT | |||
| IEX-1L | IEX+ | GTGGTGAGTATCGCCGAAGT | 360 | 35 |
| IEX− | GGTGTTGCTGGAGGAAAGTG | |||
| Mouse GAPDH | CR127 | AGAACATCATCCCTGCATCC | 499 | 23 |
| CR128 | CTGGGATGGAAATTGTGAGG | |||
| Mouse egr-1 | msegr+ | TGAGCACCTGACCACAGAGTCC | 502 | 35 |
| msegr− | TCAGGTCTCCCTGTTGTTGTGG |
Colonic tissues from mice were transferred immediately after dissection to 1 ml of Trizol reagent and frozen in liquid N2. Each colon (200 to 400 mg) was homogenized in 2 ml of Trizol, and RNA was purified as described above. RNA from three mice was pooled for each infection time. After DNase I treatment the RNA was used in array and reverse transcription (RT)-PCR experiments.
Human cDNA expression arrays and image analysis.
Atlas Human cDNA expression arrays 1.2 contain 1,176 partial human cDNAs (Clontech). 32P-labeled cDNA probes were synthesized by RT (according to the manufacturer's instructions) using 5 μg of total RNA, [32P]dATP (Amersham), and gene-specific primers. Array membranes were hybridized with 3 × 106 to 5 × 106 cpm of cDNA probe. Atlas Image 1.0 (Clontech) and Excel 5.0 (Microsoft) software was used for quantification and comparison of the hybridization signals. The intensity of the signals was corrected for background and normalized to the nonvariable genes coding for GAPDH, tubulin, and ubiquitin that are spotted on the membranes. Genes were considered to be induced when they gave a detectable signal and were induced by more than twofold in EPEC-infected cells in two independent experiments (9).
Northern blots.
RNA (10 μg/lane) was resolved by electrophoresis on 1.5% agarose–formaldehyde gels and transferred to positively charged nylon membranes (Ambion), cross-linked with long-wave UV light, and baked at 80°C for 30 min. cDNA was synthesized with Superscript II reverse transcriptase (Life Technologies) using total RNA purified from EPEC-infected HeLa cells as the template, with oligo(dT) for the GAPDH probe and with the oligonucleotide egr− for the egr-1 probe. Specific double-stranded DNA (dsDNA) fragments of GAPDH and egr-1 were PCR amplified from the cDNA using the primers listed in Table 1. Antisense cDNA was synthesized by PCR using the specific dsDNA fragments as templates, the appropriate 3′ primer and modified nucleotides (Strip-EZ PCR; Ambion). The single-stranded DNA PCR products were column purified (Qiagen, Mississauga, Ontario, Canada) and labeled with biotin using psoralen-biotin (Ambion) and cross-linking with 365-nm UV light. Northern blotting was performed with the NorthernMax-Gly kit (Ambion). Hybridizations were performed in 5 ml of UltraHyb solution (Ambion)and incubated overnight at 45°C. The BrightStar nonisotopic detection kit (Ambion) was used for probe detection.
RT-PCR analysis of mRNA levels.
RT was performed with Superscript II reverse transcriptase following the manufacturer's instructions. cDNA was synthesized in 20-μl reaction mixtures using oligo(dT) or specific reverse primers for Egr-1 and MIP-2α and 3 μg of total RNA as the template. PCR amplification was performed in 0.5 μl of cDNA using gene-specific primers and the number of cycles as listed in Table 1. For all PCRs, the following conditions were used: a 10-min denaturing step at 94°C; cycles of 40 s at 94°C, 40 s at 61°C (68°C for mouse Egr-1), and 50 s at 72°C; and 10 min at 72°C. The PCR cycle number was optimized for each gene to prevent saturation of the reaction. PCR products were analyzed by 1.5% ethidium bromide-agarose gel electrophoresis.
Preparation of protein extracts and Western blots.
HeLa cells (3 × 105) were seeded in 60-mm-diameter tissue culture plates and incubated overnight. The medium was changed to DMEM without serum 2 h prior to infection. Overnight standing LB bacterial cultures were diluted 1:50 in prewarmed DMEM without serum and incubated for 3 h in a 37°C, 5% CO2 incubator. Aliquots (10 μl) of these cultures were used for infections. Infected cells were scraped in 100 μl of 1× boiling sodium dodecyl sulfate-polyacrylamide gel electrophoresis loading buffer. Total protein lysates were resolved by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis, electrotransferred to a nitrocellulose membrane, and blocked with 5% skim milk in Tris-buffered saline–0.1% Tween 20. The following antibodies were used at the indicated concentrations: rabbit anti-ERK1 (New England Biolabs, Beverly, Mass.), 1:2,000; monoclonal phosphospecific anti-p44/p42 (ERK1/2; New England Biolabs), 1:1,000; rabbit anti-Egr-1 (Santa Cruz Biotechnology, Santa Cruz, Calif.), 1:300; monoclonal anti-TirA2, 1:500 (4). Primary antibodies were incubated on blots overnight at 4°C.
Cells were pretreated with MAP kinase inhibitors for 30 min prior to infection with the following at the indicated concentrations: PD 98059 (Calbiochem) at 50 μM and SB 203580 (Calbiochem) at 20 μM.
RESULTS
EPEC induces changes in gene expression in epithelial cells.
It is believed that EPEC infection of epithelial cells initiates a complex chain of events that ultimately result in disease. It is likely that many of these changes originate at the gene expression level. Therefore, we used DNA array technology to investigate transcriptional responses of epithelial cells to EPEC infection.
HeLa cells provide a well-established model to study EPEC interactions with human epithelial cells (26). Monolayers were infected with wt EPEC for 3 h, and total RNA was purified. This time was chosen because after 3 h of infection effectors have been delivered and actin has been rearranged into pedestals. Radiolabeled cDNA was synthesized from infected and uninfected cells as described in Materials and Methods and used to hybridize two array membranes in parallel, containing spotted cDNAs for 1,176 human genes. The data were analyzed by phosphorimaging, and the intensities of the signals were normalized to each other using the housekeeping genes coding for GAPDH, ubiquitin, and tubulin that were also on the membranes. The majority of the genes showed no significant variation in mRNA levels following infection. Genes that had detectable signal and were induced more than twofold in EPEC-infected cells in two independent experiments are listed in Table 2. They include the transcription factors Egr-1 and ETR101, the neutrophil chemoattractants MIP-2α (GROβ) and IL-8, the antideath factor IEX-1L, and the cytoskeleton protein zyxin.
TABLE 2.
Genes upregulated in EPEC-infected HeLa cells by cDNA array analysis
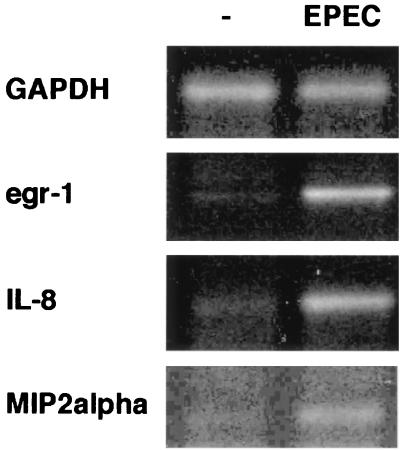
To confirm the results obtained from the array hybridization, mRNA levels of the genes listed in Table 2 were analyzed by RT-PCR. Increased transcription of Egr-1, MIP-2α, and IL-8 was confirmed by this method (Fig. 1). Our array hybridization data, supported by RT-PCR analysis, also confirm an earlier report that EPEC induced IL-8 secretion (28). These results also identified egr-1 as a previously unrecognized gene upregulated by EPEC in vitro.
FIG. 1.
egr-1, IL-8, and MIP-2α gene expression in HeLa cells is increased by EPEC infection. Total RNA was purified from HeLa cells infected with wt EPEC for 3 h. Transcriptional levels of the indicated genes were analyzed by RT-PCR using the primers and cycle number shown in Table 1. GAPDH was used as a control for total RNA.
Confirmation of egr-1 induction by Northern blotting and RT-PCR.
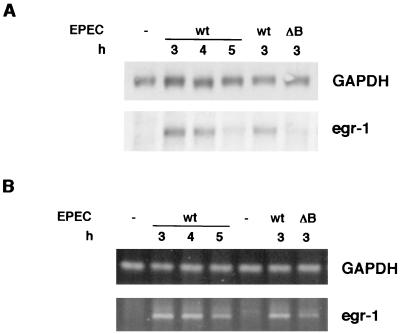
The early growth response factor egr-1 is an immediate-early-induced gene that is activated in most cell types in response to stress. The induction of egr-1 was assessed by Northern blot analysis and RT-PCR in two independent experiments: a time course infection in which HeLa cells were infected with wt EPEC for 3, 4 and 5 h and for 3 h with wt EPEC and the espB mutant. mRNA levels were normalized to GAPDH expression, which is unaffected by EPEC infection. EspB is a type III secreted protein that is inserted into the host cell membrane during infection. A mutant in this protein is unable to deliver type III effectors into the host cell (34). Similar results were obtained with both techniques (Fig. 2), showing that egr-1 mRNA increases after 3 h of infection with wt EPEC and is still induced after 4 h but decreases after 5 h. While egr-1 is induced after a 3-h infection with wt EPEC, the espB mutant stimulated a reduced transcription of this gene. These results show that egr-1 expression is induced over time, with high levels observed at 3 to 4 h after infection, and then decreases. EspB contributes to this induction, suggesting that the type III secretion system may be involved in this event. Since both Northern blotting and RT-PCR yielded similar results, only RT-PCR was used for further gene expression studies.
FIG. 2.
egr-1 induction in EPEC-infected HeLa cells follows a time course, and full induction depends on EspB. Total RNA was isolated from HeLa cells infected with wt EPEC or espB mutant for the indicated time. Levels of egr-1 mRNA were analyzed by Northern blotting (A) and RT-PCR (B) and compared to GAPDH as a control.
LPS does not trigger egr-1 or IL-8 gene expression in epithelial cells, but the type III secretion system is necessary for full induction.
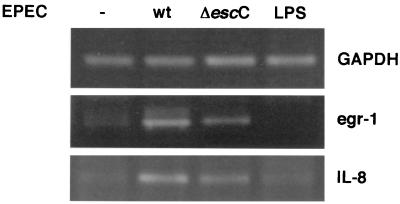
egr-1 is induced by several stimuli in a variety of cell lines, including bacterial LPS in macrophages. In order to investigate whether the induction of egr-1 and IL-8 genes is triggered by LPS or by some EPEC-specific factor, transcriptional levels of these genes were analyzed in cells exposed to LPS from E. coli (10 μg/ml). To test if the reduced transcription observed with the espB mutant is EspB or type III dependent, cells were infected with the type III secretion mutant ΔescC. EscC is a structural component of the type III secretion system believed to be the outer membrane secretin, and a mutant in this gene is unable to secrete or translocate any of the Esp, while complementation of this mutant with escC on a plasmid restores translocation (data not shown). As shown in Fig. 3, egr-1 and IL-8 gene expression is not induced by exposure to E. coli LPS. Densitometric quantification of the RT-PCR products and normalization relative to GAPDH expression indicated a (4.2 ± 0.3)-fold induction of egr-1 and (2.5 ± 0.4)-fold induction of IL-8 by wt EPEC relative to uninfected cells (n = 3). By the same analysis, the type III secretion mutant caused no significant change in expression of egr-1 or IL-8 genes ([1.1 ± 0.4]- and [0.9 ± 0.2]-fold induction relative to uninfected cells, respectively). Therefore, in cells infected with the escC mutant, expression of these genes is significantly decreased compared to wt EPEC. The same result was obtained for MIP-2α expression (data not shown). These experiments show that a functional type III secretion system is needed for full induction of egr-1, IL-8, and MIP-2α genes.
FIG. 3.
Induction of egr-1 and IL-8 gene expression is LPS independent but requires the type III secretion system for full induction. Total RNA was extracted from HeLa cells infected with wt EPEC or the escC mutant or was treated with E. coli LPS (10 μg/ml) for 3 h. mRNA levels for egr-1 and IL-8 were analyzed by RT-PCR and compared to GAPDH levels.
EPEC infection induces egr-1 expression by activation of the MEK signal transduction pathway in HeLa cells.
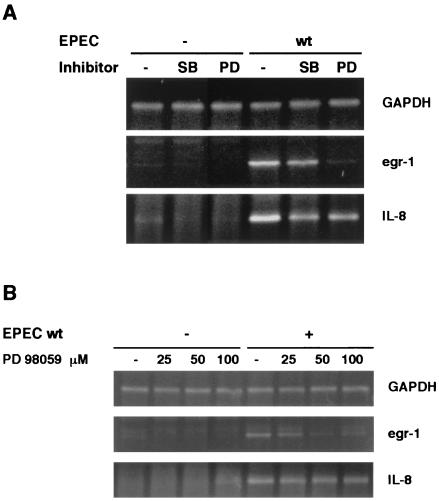
We focused our research on egr-1 expression because it is a previously undescribed host response to EPEC infection. Activation of MAP kinase signal transduction pathways mediates egr-1 induction in response to extracellular stimuli. Of the three MAP kinase cascades described (MEK/ERK, p38, and stress-activated protein kinase–Jun-N-terminal protein kinase), both MEK/ERK and p38 have been shown to upregulate egr-1 expression (5, 24). Therefore, we investigated which of these two signal transduction pathways is involved in EPEC-induced transcription of egr-1. HeLa cells were pretreated with specific inhibitors of these pathways prior to infection with EPEC. PD 98059 inhibits MEK activation by blocking its phosphorylation, and SB 203580 selectively inhibits p38–stress-activated protein kinase 2. The presence of PD 98059 abolished EPEC-mediated egr-1 induction, while SB 203580 only slightly decreased egr-1 mRNA levels (Fig. 4A). Both inhibitors decreased IL-8 expression partially, but neither abrogated it.
FIG. 4.
Effect of inhibition of MEK or p38 MAP kinases on egr-1- and IL-8 EPEC-mediated induction. (A) HeLa cells were treated with the MAP kinase inhibitors SB 203580 (SB) (20 μM) or PD 98059 (PD) (50 μM) for 30 min prior to infection for 3 h with wt EPEC. egr-1 and IL-8 mRNA levels were analyzed by RT-PCR. (B) HeLa cells pretreated for 30 min with the indicated concentrations of PD 98059 were infected with wt EPEC for 3 h. egr-1 and IL-8 gene expression was analyzed by RT-PCR.
To provide additional evidence that activation of the MEK pathway is involved in egr-1 upregulation, HeLa cells were infected with EPEC in the presence of increasing concentrations of PD 98059, and egr-1 transcription was assayed by RT-PCR. As shown in Fig. 4B, PD 98059 treatment resulted in a dose-dependent inhibition of egr-1. However, only a modest decrease in IL-8 mRNA was observed when the inhibitor concentration was increased.
These results show that EPEC-infected epithelial cells induce egr-1 transcription by activating the MEK/ERK kinase signaling pathway. The p38 kinase cascade might also contribute to this induction, although to a lesser extent. Although both pathways may participate in the regulation of IL-8 transcription, they are not the main activators of this event. These data suggest that EPEC induces IL-8 and egr-1 gene expression in the host cell by different mechanisms.
EPEC-mediated ERK1/2 phosphorylation occurs before Egr-1 synthesis and is type III dependent.
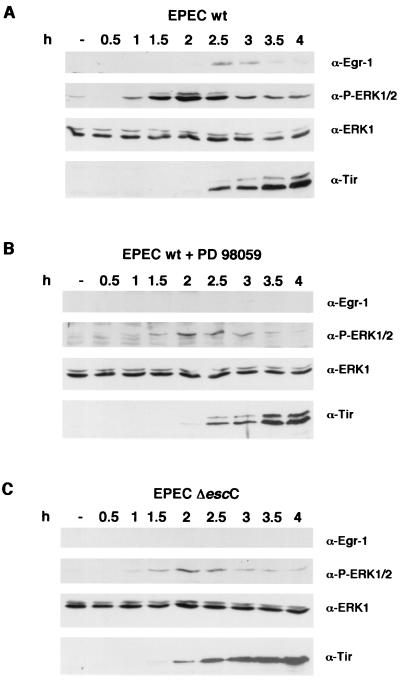
MEK mediates phosphorylation of ERK1/2 (p44/p42) kinases, which then phosphorylate other factors such as Elk-1, which ultimately activates egr-1 transcription. HeLa cells were infected with EPEC in a time course experiment in the presence or absence of 50 μM PD 98059. The role of the type III secretion system in this induction was also examined by infecting with the escC mutant. Because PD 98059 degrades over time, and to minimize the time that cells are exposed to this inhibitor, infection conditions were changed to the preactivation method (27). Overnight EPEC cultures were subcultured for 3 h at 37°C with 5% CO2 in cell culture medium without shaking before being used to infect epithelial cells. Under these culture conditions EPEC becomes activated and attachment to cultured cells occurs faster. Total protein extracts prepared from infected cells at different time points were assayed by Western blotting and probed with antibodies recognizing phosphorylated ERK1/2. As shown in Fig. 5A, EPEC induces phosphorylation of ERK1/2 after 1 h of infection, peaking at 2 h and decreasing afterwards. Anti-Egr-1 antibodies detected this protein at 2.5 h after infection. When cells were pretreated with the inhibitor PD 98059, only a small amount of ERK1/2 was phosphorylated and no Egr-1 protein was detected (Fig. 5B). The type III mutant escC was also unable to induce ERK1/2 phosphorylation to wt levels, and Egr-1 was undetectable (Fig. 5C). To prove that the inhibitor does not impair type III secretion, the same protein extracts were probed with anti-Tir antibodies. The presence of PD 98059 did not interfere with Tir delivery into the host cell membrane as the phosphorylated 90-kDa form of Tir, which is only observed after Tir delivery into host cells, was present in both infections and follows the same time course. The 78-kDa form of Tir, which corresponds to the bacterial form of the protein before being translocated into the host cell membrane, is observed due to the bacteria attached to infected cells. The phosphorylated 90-kDa form of Tir is absent in cells infected with the ΔescC mutant, as a result of the inability of this mutant to translocate Tir into the host cell, where it undergoes phosphorylation. Furthermore, the amount of total Tir protein appeared to be the same, indicating that PD 98059 does not affect attachment or bacterial growth. Samples were also probed with anti-ERK1 antibodies which cross-react with ERK2 to show that similar amounts of ERK1/2 were present in the different samples, suggesting that neither EPEC infection nor PD 98059 affects ERK expression. When the same experiment was performed with the normal infection method (where bacteria were not preactivated), ERK1/2 phosphorylation peaked at 3 h and Egr-1 synthesis was observed after 4 h (data not shown).
FIG. 5.
EPEC induces ERK1/2 phosphorylation prior to Egr-1 synthesis. HeLa cells were infected with preactivated wt EPEC (A and B) or the escC mutant (C) during the indicated times, in the presence of 50 μM PD 98059 (B). Total protein lysates were analyzed by Western blotting. Duplicate samples were probed with anti-Egr-1, anti-phospho-p44/p42 (α-P-ERK1/2), anti-ERK1, and anti-Tir antibodies.
These experiments indicate that a functional type III secretion system contributes to ERK activation. As well, they corroborate the previous findings that the observed increase in egr-1 mRNA leads to an increase in the level of Egr-1 protein and further demonstrate the involvement of the MEK pathway in EPEC induced egr-1 expression.
Egr-1 is induced in C. rodentium-infected mice.
C. rodentium is an enteric bacterial pathogen of mice that causes attaching and effacing (A/E) lesions on the mouse intestinal epithelium, similar to those caused by EPEC on human cells. Although infected mice do not get diarrhea, they develop hyperplasia and inflammation in the colon. C. rodentium also uses a type III secretion system to deliver effectors into epithelial cells that share high homology with EPEC's virulence factors. C. rodentium-infected mice have been previously used as an in vivo model to study EPEC effectors (11).
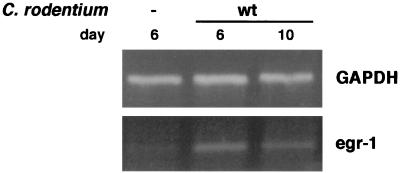
We investigated whether the equivalent mouse egr-1 gene is induced during C. rodentium infection. C57BL/6J mice were orally infected with C. rodentium, and gene expression was examined in colons dissected after 6 and 10 days. While at day 6 little, if any, sign of inflammation was observed, after 10 days the infected mice had developed hyperplasia and an immune and inflammatory cell infiltrate (12). Total RNA was isolated from these colons, and egr-1 expression was analyzed by RT-PCR. As shown in Fig. 6, egr-1 mRNA was increased at both time points, indicating that in vivo the colon responds to Citrobacter infection by increasing egr-1 expression even at very early time points, resembling the in vitro response of human epithelial cells to EPEC infection.
FIG. 6.
C. rodentium induces egr-1 transcription in vivo. C57BL/6J mice were infected orally with 0.1 ml of LB overnight cultures of C. rodentium. At days 6 and 10 postinfection, total RNA was purified from dissected colons and mRNA levels for egr-1 were analyzed by RT-PCR. RNA isolated from three mice was pooled for each condition prior to RT-PCR analysis.
DISCUSSION
Gene array technology is increasingly being used to study host cell responses at the gene expression level to different kinds of stimuli, including bacterial infection (1, 9, 25). In this study, we have used array analysis to gain new insights into how epithelial cells respond to EPEC infection at the molecular level. Although EPEC infection does not induce as dramatic changes in gene expression in infected cells as have been described for the invasive pathogen Salmonella (9, 25), several genes showed increased transcription that could be confirmed by RT-PCR, including IL-8, MIP-2α, and egr-1 genes. While LPS is a potent inducer of macrophages' transcriptional responses to gram-negative bacteria, it is not the principal inducer of the upregulation of these genes in epithelial cells, as shown by exposing cells to a high concentration (10 μg/ml) of purified E. coli LPS. Other groups have demonstrated that intestinal epithelial cells are not responsive to LPS from pathogenic and nonpathogenic bacteria under normal conditions (14), presumably to prevent chronic inflammation within the intestine.
IL-8 and MIP-2α participate in the initiation of immune responses by attracting neutrophils to the sites of infection. Both are induced by other pathogens such as Salmonella in epithelial cells, indicating that the initiation of an inflammatory process is a general response of epithelial cells to bacterial infections. Savkovic et al. have shown that EPEC infection of the intestinal epithelial cell line T84 induces NF-κB activation, leading to IL-8 secretion and transmigration of polymorphonuclear leukocytes in a coculture system by an EspB-dependent mechanism (28, 29). The data presented here confirm those observations by showing that EPEC's type III secreted virulence factors contribute to the increase in IL-8 mRNA levels in infected HeLa cells, presumably through NF-κB activation.
We have identified the transcription factor egr-1 as a novel gene that is highly induced by EPEC in epithelial cells. Increased egr-1 expression was observed in two independent array experiments and confirmed by Northern blot and RT-PCR. Immediate-early genes like egr-1 act as connectors between membrane-linked signal transduction pathways and downstream effectors, expanding and diversifying the response by inducing different genes. We hypothesize that in vivo egr-1 expression leads to the induction of other genes that participate in host defense. Egr-1 can regulate the expression of several genes in different cell lines, including those encoding platelet-derived growth factor, tumor necrosis factor alpha, transforming growth factor β, intracellular adhesion molecule 1, CD44, macrophage colony-stimulating factor, C-ets2, tissue factor, urokinase-type plasminogen activator, and metalloproteinases (18). Among these genes, we have analyzed the expression of intracellular adhesion molecule 1, CD44, C-ets2, and tissue factor in HeLa cells after 5 h of infection. No significant changes were observed in their levels of transcription (data not shown). However, this result does not exclude these genes from being upregulated in vivo during infection or in vitro at other time points. Furthermore, promoters of many Egr-1-controlled genes have additional binding sites for other factors such as Sp1 that contribute to their transcription. The role of Egr-1 in the host response to infection is unclear. One must be cautious in directly translating in vitro findings into the in vivo situation as the increased expression of egr-1 seen in the infected colon may involve other cell types beyond just epithelial cells. However, the demonstration that EPEC infection in tissue culture and C. rodentium infection in mice both lead to increased egr-1 expression does suggest a potential role for this gene in the host response to infection by A/E pathogens.
The fact that these genes were expressed 3 h after infection suggests that IL-8 and MIP-2α may be involved in an early host response aimed at attracting neutrophils to the site of infection, while genes regulated by Egr-1 may be responsible for later events such as secretion of other cytokines or upregulation of adhesion molecules. Gene expression was analyzed at 3 h after infection, because at this time point bacteria are intimately attached to the host cell through the Tir-intimin interaction and type III effectors that could have some role in altering cellular processes have been delivered.
The use of MAP kinase cascade inhibitors revealed that the MEK/ERK pathway is involved in EPEC-mediated egr-1 induction. The inhibitor PD 98059 prevented egr-1 expression in a dose-dependent fashion, and Western blot analysis showed that Egr-1 is produced after ERK1/2 phosphorylation. However, it cannot be excluded that other signaling pathways, including the p38 kinase pathway, contribute to egr-1 activation. MAP kinases transmit signals from the cell surface to the nucleus to regulate cell survival, cytokine production, and cell responses to stress and growth factors. MAP kinases represent a conserved target for a variety of bacterial pathogens: ERK2 activation is required for Listeria monocytogenes invasion into HeLa cells (30); the Yersinia pseudotuberculosis effector YopJ inhibits the three MAP kinase pathways by targeting the superfamily of MAP kinase kinases and blocking their activation (22); Helicobacter pylori activates the ERK/MAP kinase cascade, which induces c-fos transcription, leading to epithelial hyperproliferation (21); and p38 MAP kinase is involved in IL-8 activation in Salmonella enterica serovar Typhimurium-infected intestinal cells (13) and Clostridium difficile toxin A-treated monocytes (32).
It is likely that EPEC activation of the MEK pathway leads to other cellular responses in addition to egr-1 induction that may also be relevant in infection. Blocking of the MEK or p38 kinase cascades did not abrogate EPEC-induced IL-8 expression, although it was slightly reduced. Furthermore, when cells were treated with increasing concentrations of the MEK inhibitor PD 98059, IL-8 expression was not significantly altered. Our results suggest that activation of NF-κB leading to IL-8 induction occurs through alternate signal transduction pathways to egr-1 upregulation. A recently published report by Czerucka et al. (3) showed that EPEC infection of T84 cells induces activation of the MAP kinase pathways MEK/ERK, p38, and Jun N-terminal protein kinase in a type III-dependent fashion. The results presented here further characterize those observations using a different cell system by examining MEK activity at later time points. While Czerucka et al. show that ERK phosphorylation is completely type III dependent after 1 h of infection with preactivated bacteria, we see an attenuated yet reproducible phosphorylation of ERK1/2 that is maximal at 2 h in cells infected by the type III bacterial mutant. Furthermore, we show how the MEK/ERK and p38 kinase pathways activated by EPEC infection (3) diverge in their downstream targets, by providing evidence that MEK/ERK activation results in egr-1 induction.
The type III secretion system is required for many EPEC-induced signal transduction events. We present data to show that this system is also needed for MEK/ERK activation. At the gene expression level, ΔescC- and ΔespB-infected HeLa cells show markedly decreased egr-1 expression compared to the increased expression caused by wt EPEC infection. The fact that egr-1 expression was not abolished suggests that other bacterial factors may also participate in this process. When protein levels were analyzed, very low phosphorylation levels of ERK1/2 were detected in cells infected with the escC mutant, which could account for the lower egr-1 mRNA levels found. We also investigated whether the type III secreted proteins EspF and Tir are responsible for MEK activation, because, unlike the type III secretion mutants ΔespB and ΔescC, they are not involved in the translocation of other effectors. HeLa cells were infected with the mutants in espF and tir, and ERK1/2 phosphorylation was analyzed by Western blotting at different infection times. Both of these mutants were able to activate the MEK/ERK cascade (data not shown), suggesting that they are not crucial for the activation of this pathway and that another type III effector may be involved.
In conclusion, our observations provide evidence that EPEC activates MAP kinase signaling pathways in epithelial cells, which then leads to the upregulation of egr-1. Further studies are needed to address the functional consequences of egr-1 induction in infected cells, as well as in C. rodentium-infected mice.
ACKNOWLEDGMENTS
We thank the entire Finlay laboratory and José Luis Puente for their encouragement and support.
This work was supported by operating grants to B.B.F. from the Medical Research Council of Canada (MRC) and the Canadian Bacterial Disease Network. C.M.R. is supported by a Natural Sciences and Engineering Research Council of Canada postgraduate scholarship, A.G. is supported by an MRC doctoral research award, and B.A.V. is funded by an MRC postdoctoral fellowship. B.B.F. is a Howard Hughes International Scholar and is an MRC Scientist.
REFERENCES
- 1.Belcher C E, Drenkow J, Kehoe B, Gingeras T R, McNamara N, Lemjabbar H, Basbaum C, Relman D A. From the cover: the transcriptional responses of respiratory epithelial cells to Bordetella pertussis reveal host defensive and pathogen counter-defensive strategies. Proc Natl Acad Sci USA. 2000;97:13847–13852. doi: 10.1073/pnas.230262797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crane J K, Oh J S. Activation of host cell protein kinase C by enteropathogenic Escherichia coli. Infect Immun. 1997;65:3277–3285. doi: 10.1128/iai.65.8.3277-3285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czerucka D, Dahan S, Mograbi B, Rossi B, Rampal P. Implication of mitogen-activated protein kinases in T84 cell responses to enteropathogenic Escherichia coli infection. Infect Immun. 2001;69:1298–1305. doi: 10.1128/IAI.69.3.1298-1305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Grado M, Abe A, Gauthier A, Steele-Mortimer O, DeVinney R, Finlay B B. Identification of the intimin-binding domain of Tir of enteropathogenic Escherichia coli. Cell Microbiol. 1999;1:7–17. doi: 10.1046/j.1462-5822.1999.00001.x. [DOI] [PubMed] [Google Scholar]
- 5.Dieckgraefe B K, Weems D M. Epithelial injury induces egr-1 and fos expression by a pathway involving protein kinase C and ERK. Am J Physiol. 1999;276:G322–G330. doi: 10.1152/ajpgi.1999.276.2.G322. [DOI] [PubMed] [Google Scholar]
- 6.Donnenberg M S, Donohue-Rolfe A, Keusch G T. A comparison of HEp-2 cell invasion by enteropathogenic and enteroinvasive Escherichia coli. FEMS Microbiol Lett. 1990;57:83–86. doi: 10.1016/0378-1097(90)90417-o. [DOI] [PubMed] [Google Scholar]
- 7.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnenberg M S, Yu J, Kaper J B. A second chromosomal gene necessary for intimate attachment of enteropathogenic Escherichia coli to epithelial cells. J Bacteriol. 1993;175:4670–4680. doi: 10.1128/jb.175.15.4670-4680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckmann L, Smith J R, Housley M P, Dwinell M B, Kagnoff M F. Analysis by high density cDNA arrays of altered gene expression in human intestinal epithelial cells in response to infection with the invasive enteric bacteria Salmonella. J Biol Chem. 2000;275:14084–14094. doi: 10.1074/jbc.275.19.14084. [DOI] [PubMed] [Google Scholar]
- 10.Foubister V, Rosenshine I, Finlay B B. A diarrheal pathogen, enteropathogenic Escherichia coli (EPEC), triggers a flux of inositol phosphates in infected epithelial cells. J Exp Med. 1994;179:993–998. doi: 10.1084/jem.179.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frankel G, Phillips A D, Novakova M, Field H, Candy D C, Schauer D B, Douce G, Dougan G. Intimin from enteropathogenic Escherichia coli restores murine virulence to a Citrobacter rodentium eaeA mutant: induction of an immunoglobulin A response to intimin and EspB. Infect Immun. 1996;64:5315–5325. doi: 10.1128/iai.64.12.5315-5325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins L M, Frankel G, Douce G, Dougan G, MacDonald T T. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect Immun. 1999;67:3031–3039. doi: 10.1128/iai.67.6.3031-3039.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hobbie S, Chen L M, Davis R J, Galan J E. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J Immunol. 1997;159:5550–5559. [PubMed] [Google Scholar]
- 14.Jung H C, Eckmann L, Yang S K, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Investig. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kagnoff M F, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Investig. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenny B, Finlay B B. Intimin-dependent binding of enteropathogenic Escherichia coli to host cells triggers novel signaling events, including tyrosine phosphorylation of phospholipase C-γ1. Infect Immun. 1997;65:2528–2536. doi: 10.1128/iai.65.7.2528-2536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenny B, Jepson M. Targeting of an enteropathogenic Escherichia coli (EPEC) effector protein to host mitochondria. Cell Microbiol. 1999;2:579–590. doi: 10.1046/j.1462-5822.2000.00082.x. [DOI] [PubMed] [Google Scholar]
- 18.Khachigian L M, Collins T. Early growth response factor 1: a pleiotropic mediator of inducible gene expression. J Mol Med. 1998;76:613–616. doi: 10.1007/s001090050258. [DOI] [PubMed] [Google Scholar]
- 19.McMahon S B, Monroe J G. The role of early growth response gene 1 (egr-1) in regulation of the immune response. J Leukoc Biol. 1996;60:159–166. doi: 10.1002/jlb.60.2.159. [DOI] [PubMed] [Google Scholar]
- 20.McNamara B P, Koutsouris A, O'Connell C B, Nougayrede J P, Donnenberg M S, Hecht G. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J Clin Investig. 2001;107:621–629. doi: 10.1172/JCI11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer-ter-Vehn T, Covacci A, Kist M, Pahl H L. Helicobacter pylori activates mitogen-activated protein kinase cascades and induces expression of the proto-oncogenes c-fos and c-jun. J Biol Chem. 2000;275:16064–16072. doi: 10.1074/jbc.M000959200. [DOI] [PubMed] [Google Scholar]
- 22.Orth K, Palmer L E, Bao Z Q, Stewart S, Rudolph A E, Bliska J B, Dixon J E. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science. 1999;285:1920–1923. doi: 10.1126/science.285.5435.1920. [DOI] [PubMed] [Google Scholar]
- 23.Reimann T, Buscher D, Hipskind R A, Krautwald S, Lohmann-Matthes M L, Baccarini M. Lipopolysaccharide induces activation of the Raf-1/MAP kinase pathway. A putative role for Raf-1 in the induction of the IL-1 beta and the TNF-alpha genes. J Immunol. 1994;153:5740–5749. [PubMed] [Google Scholar]
- 24.Rolli M, Kotlyarov A, Sakamoto K M, Gaestel M, Neininger A. Stress-induced stimulation of early growth response gene-1 by p38/stress-activated protein kinase 2 is mediated by a cAMP-responsive promoter element in a MAPKAP kinase 2-independent manner. J Biol Chem. 1999;274:19559–19564. doi: 10.1074/jbc.274.28.19559. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberger C M, Scott M G, Gold M R, Hancock R E, Finlay B B. Salmonella typhimurium infection and lipopolysaccharide stimulation induce similar changes in macrophage gene expression. J Immunol. 2000;164:5894–5904. doi: 10.4049/jimmunol.164.11.5894. [DOI] [PubMed] [Google Scholar]
- 26.Rosenshine I, Donnenberg M S, Kaper J B, Finlay B B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenshine I, Ruschkowski S, Finlay B B. Expression of attaching/effacing activity by enteropathogenic Escherichia coli depends on growth phase, temperature, and protein synthesis upon contact with epithelial cells. Infect Immun. 1996;64:966–973. doi: 10.1128/iai.64.3.966-973.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savkovic S D, Koutsouris A, Hecht G. Activation of NF-kappaB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am J Physiol. 1997;273:C1160–C1167. doi: 10.1152/ajpcell.1997.273.4.C1160. [DOI] [PubMed] [Google Scholar]
- 29.Savkovic S D, Koutsouris A, Hecht G. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect Immun. 1996;64:4480–4487. doi: 10.1128/iai.64.11.4480-4487.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang P, Sutherland C L, Gold M R, Finlay B B. Listeria monocytogenes invasion of epithelial cells requires the MEK-1/ERK-2 mitogen-activated protein kinase pathway. Infect Immun. 1998;66:1106–1112. doi: 10.1128/iai.66.3.1106-1112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vallance B A, Finlay B B. Exploitation of host cells by enteropathogenic Escherichia coli. Proc Natl Acad Sci USA. 2000;97:8799–8806. doi: 10.1073/pnas.97.16.8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warny M, Keates A C, Keates S, Castagliuolo I, Zacks J K, Aboudola S, Qamar A, Pothoulakis C, LaMont J T, Kelly C P. p38 MAP kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J Clin Investig. 2000;105:1147–1156. doi: 10.1172/JCI7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wessler S, Hocker M, Fischer W, Wang T C, Rosewicz S, Haas R, Wiedenmann B, Meyer T F, Naumann M. Helicobacter pylori activates the histidine decarboxylase promoter through a mitogen-activated protein kinase pathway independent of pathogenicity island-encoded virulence factors. J Biol Chem. 2000;275:3629–3636. doi: 10.1074/jbc.275.5.3629. [DOI] [PubMed] [Google Scholar]
- 34.Wolff C, Nisan I, Hanski E, Frankel G, Rosenshine I. Protein translocation into host epithelial cells by infecting enteropathogenic Escherichia coli. Mol Microbiol. 1998;28:143–155. doi: 10.1046/j.1365-2958.1998.00782.x. [DOI] [PubMed] [Google Scholar]