Abstract
Drought is a major abiotic stress to rice (Oryza sativa) during growth. Ideal Plant Architecture (IPA1), the first cloned gene controlling the ideal plant type in rice, has been reported to function in both ideal rice plant architecture and biotic resistance. Here, we report that the IPA1/OsSPL14, encoding a transcriptional factor, positively regulates drought tolerance in rice. The IPA1 is constitutively expressed and regulated by H2O2, abscisic acid, NaCl and polyethylene glycol 6000 treatments in rice. Furthermore, the IPA1-knockout plants showed much greater accumulation of H2O2 as measured by 3,3′-diaminobenzidine staining in leaves compared with WT plants. Yeast one-hybrid, dual-luciferase and electrophoretic mobility shift assays indicated that the IPA1 directly activates the promoter of SNAC1. Expression of SNAC1 is significantly down-regulated in IPA1 knockout plants. Further investigation indicated that the IPA1 plays a positive role in drought-stress tolerance by inducing reactive oxygen species scavenging in rice. Together, these findings indicated that the IPA1 played important roles in drought tolerance by regulating SNAC1, thus activating the antioxidant system in rice.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-023-04062-9.
Keywords: IPA1, Rice (Oryza sativa L.), Drought stress, SNAC1, Reactive oxygen species (ROS)
Introduction
Drought is one of the most important factors affecting crop production and has severe effects on food security [1]. Breeding varieties to improve their agronomic traits, such as ideal plant structure, is a key factor in increasing food production [2]. Several key genes controlling agronomic traits have been cloned, including those encoding several transcription factors of the SQUAMOSA-PROMOTER BINDING PROTEIN-LIKE (SPL) gene family [3]. The SPL gene family regulates the structure of rice plants [4] and controls flowering time [5]. In Arabidopsis, 11 of the 17 SPL genes are targets of the microRNA (miRNA) miR156 [5], and in rice 11 of 19 SPL genes are targets of miR156 [3, 6].
Previous research of miR156 shows that it is an important regulatory element in a variety of plant biological processes, including plant growth, development, environmental stress response and defense [7]. MiR156 and its target gene SBP/SPL have major roles in plant development [8, 9]. Plants coordinate plant development and abiotic stress tolerance through the miR156-SPL9 pathway [10]. MiRNA expression decreases, and SPL expression increases throughout development [11]. Previous studies have shown that MIR156 expression is induced, and SPL gene expression is suppressed, when plants are exposed to drought stress, and that MIR156 expression decreases, and SPL gene expression begins to increase, when normal growth conditions are restored; this MIR156-SPL mediated abiotic stress response is functionally conserved in rice [12].
The SPL genes have been studied primarily for their functions in growth and development. In rice, overexpression of OsSPL16 promotes cell division and seed filling [13]. Overexpression of OsSPL17 decreases tillering in rice plants [14]. OsSPL14 is an IDEAL PLANT ARCHITECTURE1 (IPA1) gene controlling tillering in rice. The concept of ideal plant architecture was first identified through a mutation in a recognition site on miRNA that alters the transcription of IPA1 gene, thus decreasing tiller number, and resulting in taller plants and more panicle branches [15]. Full gene expression profiling has indicated that IPA1 directly binds GTAC, the core motif of the SBP-box, and influences plant growth and development by regulating OsTB1 and DEP1 [16]. Since then, IPA1 has been identified to play a role in abiotic stresses in rice [17, 18]. Phosphorylated IPA1 binds the promoter of WKRY45 and enhances resistance to disease [17]. Overexpression of IPA1 increases disease resistance to bacterial blight in rice [18]. Recent studies have shown that IPA1 increases drought tolerance in rice seedlings through the abscisic acid (ABA) pathway [19]. In conclusion, IPA1 plays a role not only in regulating plant structure but also in plant resistance to abiotic stresses and diseases.
Under stressful conditions, the balance between intracellular ROS production and clearance is disrupted [20, 21]. Thus, exposure of plants to various environmental stresses can lead to excessive production of ROS [22]. Drought is a limiting factor for rice production and can lead to overproduction of reactive oxygen species. However, when ROS accumulates in excess, it triggers progressive oxidative damage, leading to retarded rice growth and eventually cell death [23]. Several studies have shown that increasing the expression of ROS scavenging-related genes can increase tolerance to drought stress. For example, overexpression of SNAC3 regulates ROS homeostasis by regulating the expression of ROS scavenging genes, thereby increasing drought tolerance in rice [24]. Overexpression of OSLG3 improves drought tolerance in rice by enhancing ROS scavenging enzyme activity and significantly reducing ROS accumulation [25]. Therefore, an effective way to improve tolerance to drought-induced oxidative damage is to increase the efficiency of antioxidant activity in rice [26]. Therefore, the relationship between drought stress and ROS homeostasis is critical. In the present study, we generated IPA1 knockout transgenic rice and analyzed its function related to drought stress in rice. Research on IPA1 currently focuses on rice plant structure and biotic stresses, whereas studies of abiotic stresses are relatively scarce in rice. Here, IPA1 is demonstrated to positively regulate drought tolerance by affecting reactive oxygen species (ROS) content in rice. Previously, we found that IPA1 regulates many drought-related transcription factors [16, 19], and through screening and validation we determined that IPA1 directly regulates SNAC1 and might regulate drought resistance in rice,and suggest a new role for IPA1 in the involvement of drought stress in rice.
Materials and methods
Plant material and growth conditions
The rice varieties FH7185 and MH86 (Oryza sativa L.) from Rice Research Institute, Fujian Academy of Agricultural Sciences, China was used in this study. The germinated seeds were planted in soil, and the seedlings were grown under standard greenhouse conditions (16-h light at 28 °C/8-h dark at 26 °C).
Vector construction and rice transformation
The full-length cDNA of IPA1 was amplified from rice cultivar FH7185 by RT-PCR, and the sequence-confirmed PCR fragment was inserted into the pHUE411 vector under the control of. The vectors were constructed by insertion of synthesized oligonucleotides into the BsaI site of the vector pYLCRISPR/Cas9, which contains a codon-optimized Cas9 driven by a maize ubiquitin promoter for knockout, which was introduced into the A. tumefaciens strain EHA105. Agrobacterium-mediated transformation of rice (Oryza sativa L. subsp. indica FH7185) was performed according to (References) [27]. The plasmid pCAMBIA1300-OsSPL14 was introduced into mature rice (O. sativa L. indica cultivar‘MH86’) embryos with Agrobacterium-mediated transformation. Stably inherited transgenic plants possessing single copy insertions of the transgene were selected and used in this study.
Expression pattern of IPA1 under abiotic stress
Selecting seedlings at the three-leaf stage for treatment. Polyethylene glycol (PEG) 6000 concentration of 20%, ABA and GA at a concentration of 100 μM, NaCl at a concentration of 100 mM. PEG treatment times of 0 h, 1 h, 2 h, 4 h, 8 h, 12 h, 24 h and 36 h. ABA, GA and NaCl treatment times of 0 h, 1 h, 3 h, 6 h, 12 h, 24 h and 36 h. Sampling by time point, each group with clear water as blank control, three biological replicates per group, three plants per replicate.
Drought treatment of plants at the seedling stage for rice
T4 generation rice plants were used in the experiment. The drought stress experiment was conducted in plastic buckets with soil. For assays of drought treatment, 12 plants of each line were used in each replicate, with three replicates for each line. The plants grew in buckets until the five-leaf stage, after which irrigation stopped for 14 days. After recovery with water for 7 days, the survival rate was measured. Drought stress was simulated in a plastic bucket with 0.5 m soil depth.
In dehydration treatment, In the dehydration treatment, WT (MH86) and IPA1-OE plants of uniform growth were transplanted onto 96-well PCR plates and hydroponically grown with nutrient solution. The plants grew until the five-leaf stage, after which polyethylene glycol (PEG) 6000 concentration of 25% treatment for 10 days.
RNA extraction, quantification and RT-qPCR analysis
Rice leaf RNA was extracted from transgenic rice plants and wild-type FH7185 rice plants. Total RNA was extracted with a TriZol Up kit (TransGen Biotech, China) according to the manufacturer’s protocol. An RNA reverse-transcription kit with gDNA Remover (Toyobo, Japan) was used to generate cDNA for 10 min at 25 °C, 120 min at 37 °C and 5 min at 85 °C. qRT-PCR was performed with ChamQ Universal SYBR qPCR Master Mix (Vazyme, China) on an ABI Prism 7500 real-time PCR system.
Yeast one-hybrid assays
The coding region of IPA1 was amplified and cloned into the PJG4–5 prey vector. The promoters of SNAC1 were amplified and cloned into pLaczi-2u bait vectors. The prey vectors were co-transformed with bait vector into EYG48 strain. The transformed yeast cells were grown on SD/−Trp/−Ura medium and then applied to yeast plates containing 5-bromo-4-chloro-3-indolyl β-D-galactoside. Interactions were screened on the basis of presence of blue pigment. The empty vector pJG4–5 and recombinant pLacZi vectors were co-transformed as negative controls.
Electrophoretic mobility shift assay (EMSA)
The full length IPA1 coding sequence was fused into the pMAL-C5X vector, and the fusion protein was expressed in Escherichia coli and purified. The double-stranded Cy5.5-labeled probes used in this assay were synthesized by Biosun (China). The EMSA was performed with an EMSA/Gel-Shift Kit (Beyotime, China) according to the manufacturer’s instructions. Briefly, 2 mg of purified MBP or MBP-IPA1 protein was added to the binding reaction and incubated for 20 min at 25 °C in a thermal cycler (Bio-Rad, United States). The mixture was separated on a 4% polyacrylamide gel in 0.5× Tris-Borate-EDTA buffer, and the gel images were taken with an Odyssey R Infrared Imaging System (LI-COR, United States).
Dual-luciferase reporter assays
We used a dual LUC reporter assay system to analyze transcriptional activity in rice protoplasts. First, the promoter of SNAC1 was inserted into the LUC reporter vector pGreen II 0800, which includes a Renilla LUC (REN) gene driven by CaMV35S as an internal control. Then the pRTVcIPA1-HA vector was used as an effector. The reporter and effector plasmids were co-transformed into the protoplasts through the method described above. The transformed protoplasts were incubated in the dark for 36 h at 28 ° C. LUC and REN activity was measured according to the instructions of the Dual Luciferase Reporter Assay Kit (Vazyme, China). The min35s promoter was used as a negative control. The binding of IPA1 to the candidate gene promoters was expressed as the LUC/REN ratio. All experiments were repeated in three biological replicates.
Measurement of physiological characteristics
The hydrogen peroxide (H2O2) was stained using DAB, malondialdehyde (MDA), catalase (CAT) and peroxidase (POD) activity were measured according to the manufacturer’s protocol (Beijing Solarbio Science Technology Co., Ltd., Beijing, China). Rice leaves were taken at 10 days of drought treatment to measure CAT and POD activity, and at 14 days of drought treatment to measure MDA content.
Results
Expression patterns of IPA1
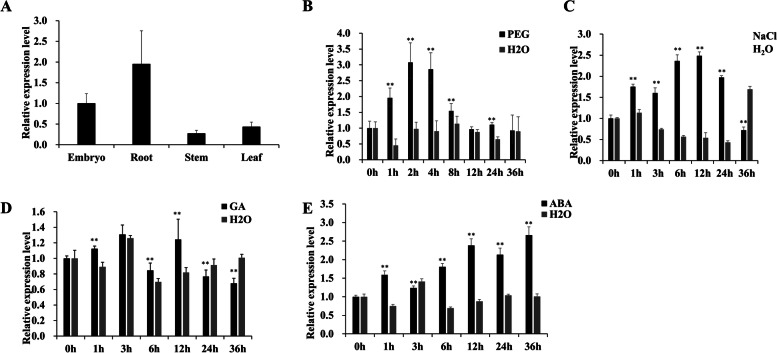
We analyzed the expression profiles of four representative tissues (root, stem, leaf and embryo from seedlings). RNA was extracted from different tissues, and RT-qPCR was performed to determine the expression pattern of IPA1. IPA1 was expressed in seedlings in all tissues, with the highest levels in the roots (Fig. 1a).
Fig. 1.
Expression patterns of IPA1. A Tissue-specific expression of IPA1 in rice. B IPA1 expression under simulated drought with 20% PEG treament. C IPA1 expression under 100 mM NaCl solution. D, E Expression of IPA1 under GA and ABA. Data are means ± se (n = 3). Statistical significance was determined by Student’s t-test. *, P < 0.05; **, P < 0.01
We also assessed whether and how IPA1 contributes to the responses to abiotic stress and hormone treatment. The IPA1 transcript levels increased significantly after polyethylene glycol (PEG) 6000 and NaCl treatments (Fig. 1b,c), after hormone treatment, no significant improvement in IPA1 expression levels was observed after GA treatment, and the expression of IPA1 peaked at 36 h after ABA treatment (Fig. 1d, e), thus indicating that the expression of IPA1 varied in response to different abiotic stresses.
Phenotype of IPA1 knockout plants in the mature stage
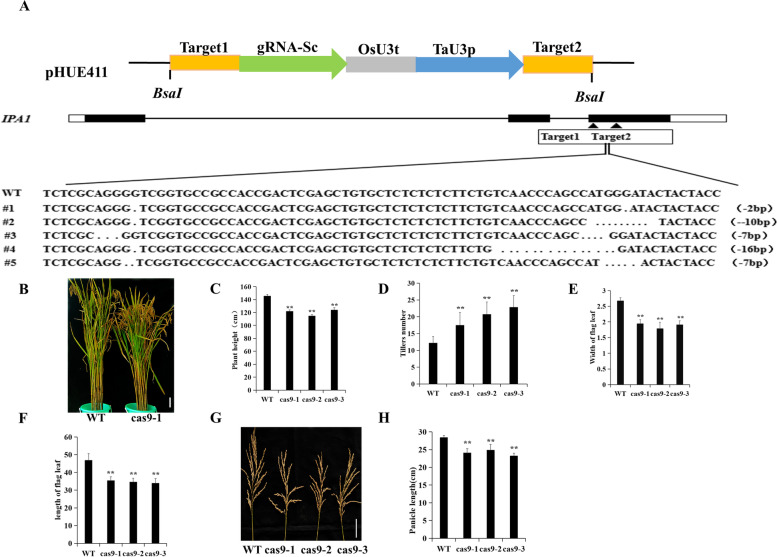
To investigate whether IPA1 is associated with drought, we constructed a knockout vector with the pHUE411-CRISPR/Cas9 system and obtained positive transgenic plants, as mediated by Agrobacterium transformation (Fig. 2a), which were denoted Cas9–1, Cas9–2 and Cas9–3. Compared with the WT mature plants, the IPA1 Cas9 plants showed dwarfism and more tillers (Fig. 2b, c, d), shorter and narrower flag leaves (Fig. 2e, f), and shorter panicles (Fig. 2g, h). The change in tiller number was opposite from that in plants with IPA1 overexpression [28].
Fig. 2.
Phenotype of mature IPA1 knockout plants. A Construction of CRISPR/Cas9 vector with pHUE411. Sequence analysis of mutation sites in IPA1 knockout plants. Note:… are deleted bases; #1–#5 are knockout transgenic plants; and Target1 and Target2 are the targets for the design. Scale bar is 18 cm. B Phenotype of FH7185 and knockout transgenic plants. C Heights of FH7185 and knockout transgenic plants. D Numbers of tillers of FH7185 and knockout transgenic plants. E Flag leaf widths of FH 7185 and knockout transgenic plants. F Flag leaf lengths of FH7185 and knockout transgenic plants. G Ear lengths of FH7185 and knockout transgenic plants. Scale bar is 5 cm (H) Panicle lengths of FH7185 and knockout transgenic plants. Data are means ± se (n = 3). Statistical significance was determined by Student’s t-test. *, P < 0.05; **, P < 0.01
Knockout of IPA1 is sensitive to drought stress
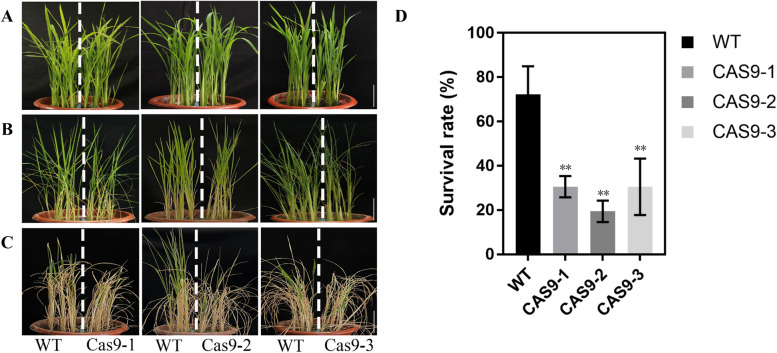
The effects of drought stress on the performance of IPA1 Cas9 plants were investigated and compared. Five-leaf stage plants were used for analysis. Under normal conditions, all lines grew normally, and no significant differences were observed (Fig. 3a). When water was withheld from these plants for 8 days, phenotypic changes were observed. Leaf rolling was substantially in IPA1 Cas9 plants compared with WT plants (FH7185) (Fig. 3b). After 14 days of drought stress treatment, all knockout lines exhibited severe symptoms of drought stress and almost complete wilting, in contrast to their corresponding wild-type lines. After drought treatment, the plants were watered for recovery. Four days after watering resumed, the IPA1 knockout became withered, as compared with the corresponding wild-type lines (Fig. 3c). After 14 days of watering, more than 72.2% of the WT (FH7185) lines recovered and grew normally. However, only 30.56, 19.44 and 30.56% of the IPA1 knockout lines recovered (Fig. 3d), indicated that knockout of IPA1 decreases drought tolerance at the seedling stage in rice.
Fig. 3.
Drought tolerance of IPA1 knockout seedlings. A, B, C Phenotypes of IPA1 seedlings in dry soil conditions. Scale bar is 5 cm (D) Survival of IPA1 under soil drought treatment. Data are means ± se (n = 3). Statistical significance was determined by Student’s t-test. *, P < 0.05; **, P < 0.01
IPA1 directly activates the expression of SNAC1
NAC, basic leucine zipper (bZIP) and WRKY transcription factors are relatively well described families of transcription factors associated with resistance to abiotic stresses in plants [29–31]. To investigate how IPA1 regulates drought tolerance, we selected transcription factors associated with drought resistance and other drought-associated genes from these three transcription factor families and analyzed the relative expression of these genes by RT-qPCR (Fig. S1a-e). The expression of NAC transcription factors was more variable and consistent with the trend in IPA1 function-deficient transgenic plants and wild-type plants with significant differences. The expression of OsWKRY13, OsWRKY47, DMS2 and OsbZIP71 significantly differed between the transgenic and wild type plants. We furtherly analyzed genes with high expression variation between IPA1 overexpressing transgenic plants and MH86 plants. Only the SNAC1, OsNAC10 and OsNAC52 indicated the same trends and significant differences in expression between the transgenic plants with IPA1 overexpression and MH86 plants (Fig. S1f, g). The yeast one hybrid results indicated that the OsNAC10 and OsNAC52 did not bind specifically to IPA1 (Fig. S1h, i).
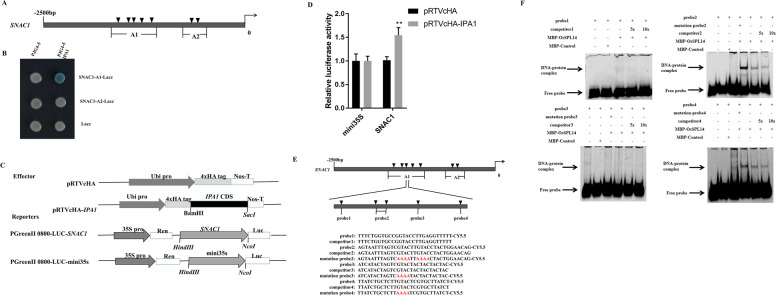
SNAC1, a transcriptional factor belonging to the NAC family, regulates ROS homeostasis and confers drought tolerance in transgenic plants [32]. In addition, as a transcriptional activator, IPA1 regulates its target genes by binding directly to the core motif GTAC or indirectly to the core motif TGGGCC/T of target gene promoters [16]. Bioinformatics analysis has identified seven GTAC motifs in the promoter of SNAC1 (Fig. 4a). We searched previously published ChIP-seq data for IPA1 [16] and found that SNAC1 is a potential target of IPA1, suggesting that the IPA1 may directly activate the expression of SNAC1.
Fig. 4.
IPA1 directly binds the promoter of SNAC1. A Segmented simplified map of the target genes; sequence is GTAC. B Interactions between IPA1 and the promoter fragments of SNAC1, shown with a yeast one-hybrid assay. C The vector used in dual luciferase assays. D Detection of the luciferase activity of reporter genes. E Segmented simplified map of the target genes; sequence represents the GTAC motif. Sequences of segments: A1 and A2; probe sequence: probe-probe4; probe competitor: competitor; mutation probe: mutation probe; red bases: mutant bases. F Results of EMSA detection of IPA1 and SNAC1 promoters
Therefore, we used yeast one-hybrid assays to investigate direct binding between IPA1 and the promoter of SNAC1. The IPA1 directly binds the A1 promoter region of SNAC1 (Fig. 4b). Both fragments of the SNAC1 promoter contain GTAC motifs. In co-transfected into rice protoplasts, we used an IPA1-HA fusion protein driven by the ubiquitin promoter and a firefly LUC driven by the target gene promoter as the effector and reporter, respectively. The IPA1 directly bound the GTAC motifs in promoter fragments of SNAC1. The transcriptional activity of LUC driven by the target gene promoter was significantly up-regulated in cells. The results suggested that IPA1 interacts with the promoters of these two target genes rather than with the min35S promoter (Fig. 4c, d).
To further determine whether IPA1 specifically binds the GTAC motif, we performed EMSA. The double-stranded Cy5.5 labeled probes used in this experiment were synthesized according to the GTAC motif region, and unlabeled probes were used as competitors. IPA1 protein was successfully expressed in E. coli (Rosetta) after fusion with an N-terminal MBP tag. MBP-IPA1 bound GTAC motif labeled probes and formed DNA-protein complexes. Migration then decreased under increasing doses of competitive probes. The fusion protein could bind to the probe2 and probe4.Moreover, the specific binding of the fusion protein toprobe2 and probe4 was eliminated by addition of unlabeled competitors (Fig. 4e, f). Our results suggested that the IPA1 directly binds GTAC in the SNAC1 promoter region.
IPA1 knockout rice plants are susceptible to drought stress
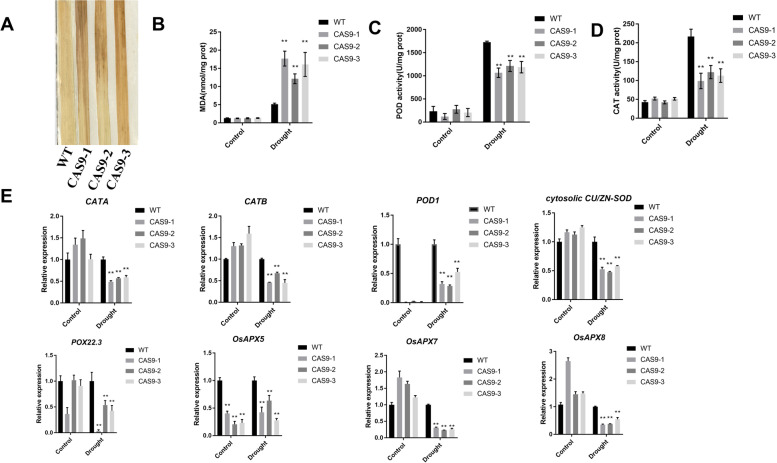
To investigate whether the IPA1 promotes drought tolerance by regulating ROS homeostasis in rice, we treated WT and IPA1-Cas9 plants with drought stress and then used 3,3′-diaminobenzidine (DAB) staining to qualitatively detect H2O2 accumulation. The IPA1-Cas9 strain exhibited more H2O2 accumulation than the WT strain (Fig. 5a). Under normal growth conditions, MDA levels was similar between WT and all transgenic plants under drought stress, but was significantly higher IPA1-Cas9 than WT plants (Fig. 5b). These results suggested that knockout of IPA1 significantly increased the excessive accumulation of ROS caused by drought stress.
Fig. 5.
IPA1 plants susceptible to drought stress in rice. A DAB staining for H2O2 in leaves from oxidative stressed WT and IPA1 mutant plants. B, C, D MDA, POD and CAT content of the WT and IPA1 mutants during drought stress treatment. E Expression levels of ROS-related genes in WT (FH7185) and IPA1-CAS9 in response to drought stress. The relative expression levels of ROS-related genes were determined by qRT-PCR using gene-specific primer sets (Supplementary Table S1) and normalized to that of Actin150. Data are means ± se (n = 3). Statistical significance was determined by Student’s t-test. *, P < 0.05; **, P < 0.01
The activity of CAT and POD was measured in 5-week-old seedlings under drought stress for 10 days. Under normal growth conditions, the POD and CAT activity did not appear to be significantly affected in IPA1-Cas9. Under drought stress, the POD and CAT activity of Cas9 plants significantly decreased (Fig. 5c, d). These results indicated that the role of IPA1 in drought resistance may be associated with the antioxidant response to resist oxidative stress under drought. We further examined the transcript levels of a dozen genes associated with ROS scavenging. A total of eight genes, including two CAT-family genes (CATA and CATB), three APX-family genes (OsAPX5, OsAPX7 and OsAPX8), a POD1, POX22.3and Cu/Zn-SOD, were expressed less in IPA1 knockout plants than in WT plants under drought conditions (Fig. 5e). Enzymatic antioxidants, including catalase (CAT), and ascorbate peroxidase (APX), peroxidase (POD) and superoxide dismutase (SOD) are key ROS scavenging enzymes [33–35]. Altogether, these data provide a clear demonstration that knockout of IPA1 might have reduced activity of ROS scavenging enzymes and thereby leads to lower expression of such important genes for reduced efficient ROS scavenging, may significantly contribute to excessive H2O2 accumulation and greater oxidative damage in the IPA1 knockout plants, which is associated with the increased sensitivity of the plants to drought stress.
Overexpression of IPA1 increases the ROS-scavenging ability and decreases oxidative damage that are associated with increased drought tolerance
To elucidate the mechanisms of rice drought tolerance of IPA1 in rice, the IPA1-OE vector was constructed and transformed into rice MH86. Three individual transgenic lines with single copy insertions, named as OE line 1, OE line 2 and OE line 3, were randomly selected for further study. qRT-PCR was carried out to detect the IPA1 transcript in transgenic plants. The expression level of IPA1 was significantly higher in the transgenic plants than in the WT (Fig. S2).
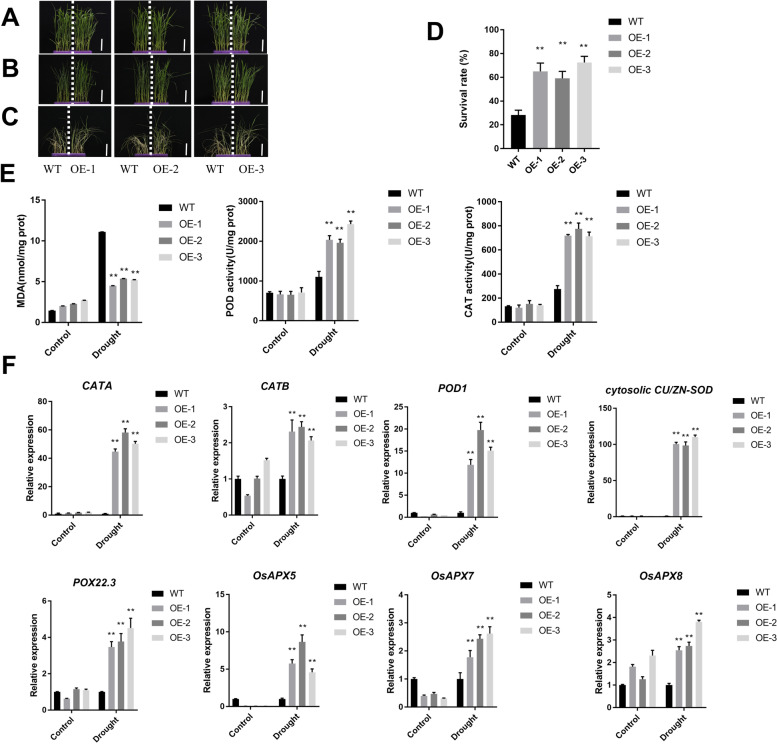
Five-leaf stage plants were used for analysis. Twenty-five percentage of PEG treatments simulate drought stress. Under normal conditions, all lines grew normally, and no significant differences were observed (Fig. 6a). When water was withheld from these plants for 48 hours, the phenotypic changes were observed. The leaf rolling was substantially in WT plants compared with IPA1 OE plants (MH86) (Fig. 6b). After 10 days of drought stress treatment, all WT lines exhibited severe symptoms of drought stress and almost complete wilting, in contrast to their corresponding the IPA1-OE lines. After drought treatment, the plants were recovery watered for four days, the wild-type lines became withered compared with the corresponding IPA1-OE lines (Fig. 6c). The results indicated that the ratio of the IPA1-OE plants survived was from 59.17 to 72.50%, while only 28.33% of the WT(MH86) plants survived under drought treatment (Fig. 6d).
Fig. 6.
Overexpression of IPA1 increases the ROS-scavenging ability and decreases oxidative damage that are associated with increased drought tolerance. A, B, C Phenotypes of IPA1 seedlings in PEG treatment. Scale bar is 5 cm. D Survival of IPA1 under soil drought treatment. E MDA levels, POD and CAT activity of the WT (MH86) and IPA1 overexpression during drought stress treatment. F Expression levels of ROS-related genes in WT (MH86) and IPA1-OE in response to drought stress. The relative expression levels of ROS-related genes were determined by qRT-PCR using gene-specific primer sets (Supplementary Table S1) and normalized to that of Actin150. Data are the means ± SD (*P < 0.05, **P < 0.01, Student’s t-test) of three technically independent experiments
To verify the important role of IPA1 in drought resistance. We measured the MDA levels, POD and CAT activity of IPA1-OE plants and WT plants before and after drought treatment. Under normal growth conditions, the MDA levels, POD and CAT activity was not appeared to be significant difference compared to WT plants. Under drought stress, the MDA levels of IPA1-OE plants significantly decreased, and the POD and CAT activity of IPA1-OE plants significantly higher than those of WT plants (Fig. 6e). We furtherly examined the transcript levels of a dozen genes associated with ROS scavenging. A total of eight genes, including two CAT-family genes (CATA and CATB), three APX-family genes (OsAPX5, OsAPX7 and OsAPX8), a POD1 and Cu/Zn-SOD, were highly expressed in IPA1-OE plants than in WT plants under drought conditions (Fig. 6f). These results implied that the function of IPA1 in drought tolerance may be associated with the enhanced antioxidant response to counteract oxidative stress under drought.
Discussion
Drought stress is an important factor affecting crop production [1]. Drought stress causes leaf senescence, and affects root water uptake and various physiological processes [1, 36]. Previous studies have shown that the transcriptional factors not only regulate plant growth and development, but also play important roles in plant resistance to abiotic stresses [37, 38]. The SPL gene encodes a plant-specific transcription factor that plays a critical role in plant growth and development [4, 39]. Previous studies have shown that the OsSPL genes, such as OsSPL3, OsSPL7, OsSPL14 and OsSPL18, increase tiller numbers [40]. IPA1/OsSPL14 is a transcriptional factor associated with the ideal plant architecture. IPA1 expression confers an ideal plant architecture in rice, including fewer tillers, stronger culms (stalks), larger panicles and higher grain weight [15]. In this study, we used the CRISPR/Cas9 system to generate IPA1 knockout mutants to characterize the role of IPA1 in drought stress, and found that knockout of ipa1 significantly decreased rice drought tolerance at the seedling stage. The mature IPA1-Cas9 plants demonstrated dwarfism and more tillers, shorter and narrower flag leaves, and shorter panicles compared with WT plants. Previous studies have shown that overexpression of IPA1 decreases tiller number and yields a strong stalk [41, 42]. IPA1 is most highly expressed in the roots of seedlings, and knocking down IPA1 may affect root development and thus decrease the soil water uptake capacity and tolerance to drought stress.
Under drought stress, plants have evolved many stress-associated defense mechanisms, among which transcription factors are responsible for regulating the expression of a variety of stress-associated genes. The bZIPs are important transcriptional factors that regulate ABA signaling and drought response in plants [43]. OsbZIP23 plays a key role in drought tolerance [44]. OsbZIP16 and OsbZIP71 positively regulate drought tolerance and the ABA signaling pathway [45, 46]. NAC transcriptional factors play important roles in regulating plant growth and the abiotic stress response [47]. Overexpression of SNAC1 increases drought tolerance in rice [48]. OsNAC2 regulates the drought stress response and ABA-mediated response [29]. Overexpression of OsNAC52 is highly sensitive to ABA and increases drought tolerance in rice [49]. WRKY transcriptional factors play important roles in regulating the abiotic response in plants [31]. OsWRKY47 mutants are more sensitive to drought than wild type [50]. Overexpression of OsWRKY30 in rice significantly increases drought tolerance [51]. We previously selected many drought-tolerant genes for expression analysis in WT and IPA1-Cas9 plants (Fig. S1a-e). The eight genes, including CATA, CATB, POD1, CU/ZN-SOD, POX22.3, OsAPX5, OsAPX7, OsAPX8 with significantly lower expression were used to expression analysis in those plants than the wild type. We analyze the expression of the above eight genes and found that only SNAC1, OsNAC10 and OsNAC52 showed significantly up-regulated expression with IPA1 overexpressing and WT plants (Fig. S1f, g). Furtherly, we searched for previously Chip-seq data for IPA1 published [16] and found that SNAC1, OsNAC10 and OsNAC52 were potential targets of IPA1. The yeast one hybrid results indicated that the OsNAC10 and OsNAC52 did not specifically bind IPA1 (Fig. S1h, i).
SNAC1 is a NAC transcription factor involved in the ROS regulatory pathway in rice [52]. Several stress-associated cis-acting elements are present in the NAC gene promoter [53]. Our results indicated that IPA1 directly activated the promoter of SNAC1 and significantly increased the promoter activity with the yeast one-hybrid and dual-luciferase assays (Fig. 4b, d). Further verification indicated that IPA1 directly influenced the promoter activity of SNAC1 by EMSA (Fig. 4f). SNAC1 has been reported to be a key regulator in rice [54]. SNAC1 regulates OsPP18, thus modulating ROS homeostasis through an ABA-independent pathway [52]. Meanwhile, our study demonstrated that IPA1 regulates SNAC1 and enhances drought resistance in rice, thus positively influencing drought and oxidative stress homeostasis by regulating ROS homeostasis.
Plant growth and development, and abiotic and biotic stress adaptation, are regulated by endogenous small signaling molecules. Among them, plant hormones such as ROS play important roles in the growth or death responses of many specific cells [55]. As a toxic byproduct of aerobic metabolism, ROS can damage plant cells through their high activity and toxicity. Drought stress causes excessive accumulation of ROS in plants [56]. ROS have a dual function in abiotic stress. In the presence of abiotic stress, intracellular ROS levels are low and when plants are exposed to abiotic stress, ROS levels increase activating stress pathways in the plant cell, when ROS act as a signaling sensor to activate defence mechanisms in the plant system. In severe abiotic high levels of ROS accumulation, the excessive production of ROS can be toxic to plant cells and eventually lead to plant death [57]. Some studies have shown that plants increase drought stress tolerance by regulating ROS homeostasis [24]. For instance, OsEBP89 knockout increases the ability of rice to scavenge ROS and enhances tolerance to drought stress [58]. OsRbohB exerts drought resistance in rice by mediating ROS production [59]. In this study, IPA1-Cas9 seedlings exhibited more withering under soil drought stress (Fig. 2a-c). Significantly greater H2O2 and MDA accumulation was observed in the leaves of IPA1-Cas9 plants than WT plants (Fig. 5b). The diminished POD and CAT enzyme activity (Fig. 5c, d) in IPA1-Cas9 plants suggested that the decreased drought tolerance might have been due to excessive accumulation of ROS and higher levels of MDA, decreased accumulation of POD and CAT enzymes, and increased accumulation of H2O2. We obtained the opposite results in the IPA1-OE plants. Therefore, the function of IPA1 in drought tolerance may be associated with the regulation of antioxidant capacity, thus suggesting that IPA1 gene may act in response to drought stress by regulating the production of ROS.
To control ROS levels and protect cells under stressful conditions, plant tissues contain several enzymes to scavenge ROS (SOD, CAT, peroxidases and glutathione peroxidase and so on) [60]. However, excessive accumulation of ROS can lead to oxidative stress resulting in cellular damage or even death. Scavenging excess ROS can avoid or mitigate the damage to plant metabolism caused by stress and thus improve tolerance to drought [56]. Increased expression levels of genes encoding ROS detoxification enzymes successfully improve drought tolerance in plant [61]. In this study, after the drought treatment the expression levels of ROS detoxification-related genes CATA, CATB, POD1, CU/ZN-SOD, POX22.3, OsAPX5, OsAPX7 and OsAPX8 were significantly increased in IPA1-OE plants (Fig. 6f). In addition, the POD and CAT activities were significantly higher in IPA1-OE plants compared to WT plants (Fig. 6e).
In summary, our results indicated that the knockout of IPA1 decreases drought resistance in rice seedlings and overexpression of IPA1 increases drought tolerance in rice. The IPA1 regulate the drought tolerance by affecting reactive oxygen species (ROS) content in rice. The Yeast one-hybrid, dual-luciferase and EMSA analyses indicated that IPA1 directly activates SNAC1 expression and directly affects tolerance to drought and oxidative stress through the regulation of ROS homeostasis. Our findings may have application value in increasing the drought resistance of rice.
Statement
The study protocol complies with relevant institutional, national, and international guidelines and legislation. We have permission to collect “Oryza sativa” materials in this study.
Supplementary Information
Additional file 1: Fig. S1. (A-G) Gene expression associated with the drought tolerance pathway. (H,I) Interactions between IPA1 and the promoter fragments of OsNAC10 and OsNAC52, shown with yeast one-hybrid assays. Data are means ± se (n = 3). Statistical significance was determined by Student’s t-test. *, P < 0.05; **, P < 0.01. Fig. S2. Gene expression analysis of IPA1 in WT and OE lines. Data are means ± se (n = 3). Statistical significance was determined by Student’s t-test. *, P < 0.05; **, P < 0.01. Table S1. Primers used in this study.
Acknowledgements
We thank Professor Jiayang Li from the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, for providing the plasmid pCAMBIA1300-OsSPL14.
Authors’ contributions
J.Z., conceived and designed the project. F.C., H.L., H.Z., L.L., Y.W. and Y.L., performed the experiments. L.W., W.H., Q.C. H.X., J.F. and M.Y., created the rice materials. J.Z., F.C., H.L. and H.Z. wrote the manuscript. J.Z revised and edited the manuscript. The authors read and approved the final manuscript.
Funding
The work was supported by the National Major Projects of Cultivated Transgenic New Crop Varieties Foundation of China (grant No. 2016ZX08001–004), National Rice Industry Technology System of Modern Agriculture for China (grant no. CARS-01-20), “5511” Collaborative Innovation Project for High-quality Development and Surpasses of Agriculture between the Government of Fujian and Chinese Academy of Agricultural Sciences (grant no. XTCXGC2021001), Key Program of Science and Technology in Fujian Province, China (No. 2020NZ08016) and Special Foundation of Non-Profit Research Institutes of Fujian Province (grant no. 2018R1021–5).
Availability of data and materials
The mutant and wild type plant used in the study are from Rice Research Institute, Fujian Academy of Agricultural Sciences, China are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Feihe Chen, Haomin Zhang and Hong Li contributed equally to this work.
References
- 1.Zhang J, Zhang S, Cheng M, Jiang H, Zhang X, Peng C, et al. Effect of drought on agronomic traits of rice and wheat: a meta-analysis. Int J Environ Res Public Health. 2018;15(5):839. [DOI] [PMC free article] [PubMed]
- 2.Wang Y, Li J. Molecular basis of plant architecture. Annu Rev Plant Biol. 2008;59:253–279. doi: 10.1146/annurev.arplant.59.032607.092902. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Wang H. The miR156/SPL module, a regulatory hub and versatile toolbox, gears up crops for enhanced agronomic traits. Mol Plant. 2015;8(5):677–688. doi: 10.1016/j.molp.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Zhang Q. Boosting rice yield by fine-tuning SPL gene expression. Trends Plant Sci. 2017;22(8):643–646. doi: 10.1016/j.tplants.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Wang JW, Czech B, Weigel D. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell. 2009;138(4):738–749. doi: 10.1016/j.cell.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Xie K, Wu C, Xiong L. Genomic organization, differential expression, and interaction of SQUAMOSA promoter-binding-like transcription factors and microRNA156 in rice. Plant Physiol. 2006;142(1):280–293. doi: 10.1104/pp.106.084475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jerome Jeyakumar JM, Ali A, Wang W-M, Thiruvengadam M. Characterizing the role of the miR156-SPL network in plant development and stress response. Plants. 2020;9(9):1206. doi: 10.3390/plants9091206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu S, Lian H, Wang JW. Plant developmental transitions: the role of microRNAs and sugars. Curr Opin Plant Biol. 2015;27:1–7. doi: 10.1016/j.pbi.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Shikata M, Yamaguchi H, Sasaki K, Ohtsubo N. Overexpression of Arabidopsis miR157b induces bushy architecture and delayed phase transition in Torenia fournieri. Planta. 2012;236(4):1027–1035. doi: 10.1007/s00425-012-1649-3. [DOI] [PubMed] [Google Scholar]
- 10.Cui LG, Shan JX, Shi M, Gao JP, Lin HX. The miR156-SPL9-DFR pathway coordinates the relationship between development and abiotic stress tolerance in plants. Plant J. 2014;80(6):1108–1117. doi: 10.1111/tpj.12712. [DOI] [PubMed] [Google Scholar]
- 11.Wu G, Poethig RS. Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development. 2006;133(18):3539–3547. doi: 10.1242/dev.02521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng C, Ye M, Sang M, Wu R. A regulatory network for miR156-SPL Module in Arabidopsis thaliana. Int J Mol Sci. 2019;20(24):6166. [DOI] [PMC free article] [PubMed]
- 13.Wang S, Wu K, Yuan Q, Liu X, Liu Z, Lin X, et al. Control of grain size, shape and quality by OsSPL16 in rice. Nat Genet. 2012;44(8):950–954. doi: 10.1038/ng.2327. [DOI] [PubMed] [Google Scholar]
- 14.Dai Z, Wang J, Yang X, Lu H, Miao X, Shi Z. Modulation of plant architecture by the miR156f-OsSPL7-OsGH3.8 pathway in rice. J Exp Bot. 2018;69(21):5117–5130. doi: 10.1093/jxb/ery273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiao Y, Wang Y, Xue D, Wang J, Yan M, Liu G, et al. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat Genet. 2010;42(6):541–544. doi: 10.1038/ng.591. [DOI] [PubMed] [Google Scholar]
- 16.Lu Z, Yu H, Xiong G, Wang J, Jiao Y, Liu G, et al. Genome-wide binding analysis of the transcription activator ideal plant architecture1 reveals a complex network regulating rice plant architecture. Plant Cell. 2013;25(10):3743–3759. doi: 10.1105/tpc.113.113639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Zhou L, Shi H, Chern M, Yu H, Yi H, et al. A single transcription factor promotes both yield and immunity in rice. Science. 2018;361(6406):1026–1028. doi: 10.1126/science.aat7675. [DOI] [PubMed] [Google Scholar]
- 18.Liu M, Shi Z, Zhang X, Wang M, Zhang L, Zheng K, et al. Inducible overexpression of Ideal Plant Architecture1 improves both yield and disease resistance in rice. Nat Plants. 2019;5(4):389–400. doi: 10.1038/s41477-019-0383-2. [DOI] [PubMed] [Google Scholar]
- 19.Zhu M, He Y, Zhu M, Ahmad A, Xu S, He Z, et al. ipa1 improves rice drought tolerance at seedling stage mainly through activating abscisic acid pathway. Plant Cell Rep. 2022;41(1):221–232. doi: 10.1007/s00299-021-02804-3. [DOI] [PubMed] [Google Scholar]
- 20.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signaling transduction. Annu Rev Plant Biol. 2004;55:373. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 21.Vellosillo T, Vicente J, Kulasekaran S, Hamberg M, Castresana C. Emerging complexity in reactive oxygen species production and signaling during the response of plants to pathogens. Plant Physiol. 2010;154(2):444–448. doi: 10.1104/pp.110.161273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9(10):490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Hou X, Xie K, Yao J, Qi Z, Xiong L. A homolog of human ski-interacting protein in rice positively regulates cell viability and stress tolerance. Proc Natl Acad Sci. 2009;106(15):6410–6415. doi: 10.1073/pnas.0901940106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang Y, Liao K, Du H, Xu Y, Song H, Li X, et al. A stress-responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice. J Exp Bot. 2015;66(21):6803–6817. doi: 10.1093/jxb/erv386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong H, Yu J, Miao J, Li J, Zhang H, Wang X, et al. Natural variation in OsLG3 increases drought tolerance in rice by inducing ROS scavenging. Plant Physiol. 2018;178(1):451–467. doi: 10.1104/pp.17.01492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin X, Huang L, Wang M, Cui Y, Xia X. OsDSR-1, a calmodulin-like gene, improves drought tolerance through scavenging of reactive oxygen species in rice (Oryza sativa L.) Mol Breed. 2017;37(6):1–13. doi: 10.1007/s11032-017-0668-y. [DOI] [Google Scholar]
- 27.Nishimura A, Aichi I, Matsuoka M. A protocol for Agrobacterium-mediated transformation in rice. Nat Protoc. 2006;1(6):2796–2802. doi: 10.1038/nprot.2006.469. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Yu H, Xiong G, Lu Z, Jiao Y, Meng X, et al. Tissue-specific ubiquitination by IPA1 INTERACTING PROTEIN1 modulates IPA1 protein levels to regulate plant architecture in rice. Plant Cell. 2017;29(4):697–707. doi: 10.1105/tpc.16.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen J, Lv B, Luo L, He J, Mao C, Xi D, et al. The NAC-type transcription factor OsNAC2 regulates ABA-dependent genes and abiotic stress tolerance in rice. Sci Rep. 2017;7:40641. doi: 10.1038/srep40641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh KB, Foley RC, Oñate-Sánchez L. Transcription factors in plant defense and stress responses. Curr Opin Plant Biol. 2002;5(5):430–436. doi: 10.1016/S1369-5266(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 31.Rushton PJ, Somssich IE, Ringler P, Shen QJ. WRKY transcription factors. Trends Plant Sci. 2010;15(5):247–258. doi: 10.1016/j.tplants.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 32.You J, Zong W, Li X, Ning J, Hu H, Li X, et al. The SNAC1-targeted gene OsSRO1c modulates stomatal closure and oxidative stress tolerance by regulating hydrogen peroxide in rice. J Exp Bot. 2013;64(2):569–583. doi: 10.1093/jxb/ers349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Q, Zhou L, Liu J, Du X, Huang F, Pan G, et al. Relationship of ROS accumulation and superoxide dismutase isozymes in developing anther with floret fertility of rice under heat stress. Plant Physiol Biochem. 2018;122:90–101. doi: 10.1016/j.plaphy.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Hu L, Liang W, Yin C, Cui X, Zong J, Wang X, et al. Rice MADS3 regulates ROS homeostasis during late anther development. Plant Cell. 2011;23(2):515–533. doi: 10.1105/tpc.110.074369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu M-D, Zhang M, Gao D-J, Zhou K, Tang S-J, Zhou B, et al. Rice OsHSFA3 gene improves drought tolerance by modulating polyamine biosynthesis depending on abscisic acid and ROS levels. Int J Mol Sci. 2020;21(5):1857. doi: 10.3390/ijms21051857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qureshi MK. Role of reactive oxygen species and contribution of new players in defense mechanism under drought stress in rice. Int J Agric Biol. 2018;20(6):1339-1352. 10.17957/IJAB/15.0640.
- 37.Hussain SS, Kayani MA, Amjad M. Transcription factors as tools to engineer enhanced drought stress tolerance in plants. Biotechnol Prog. 2011;27(2):297–306. doi: 10.1002/btpr.514. [DOI] [PubMed] [Google Scholar]
- 38.Wang B, Zhong Z, Wang X, Han X, Yu D, Wang C, et al. Knockout of the OsNAC006 transcription factor causes drought and heat sensitivity in rice. Int J Mol Sci. 2020;21(7):2288. [DOI] [PMC free article] [PubMed]
- 39.Liu Q, Harberd NP, Fu X. SQUAMOSA promoter binding protein-like transcription factors: targets for improving cereal grain yield. Mol Plant. 2016;9(6):765–767. doi: 10.1016/j.molp.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Yuan H, Qin P, Hu L, Zhan S, Wang S, Gao P, et al. OsSPL18 controls grain weight and grain number in rice. J Genet Genom. 2019;46(1):41–51. doi: 10.1016/j.jgg.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Lian L, Xu H, Zhang H, He W, Cai Q, Lin Y, et al. Overexpression of OsSPL14 results in transcriptome and physiology changes in indica rice ‘MH86’. Plant Growth Regul. 2020;90(2):265–278. doi: 10.1007/s10725-019-00569-0. [DOI] [Google Scholar]
- 42.Miura K, Ikeda M, Matsubara A, Song XJ, Ito M, Asano K, et al. OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat Genet. 2010;42(6):545–549. doi: 10.1038/ng.592. [DOI] [PubMed] [Google Scholar]
- 43.Joo H, Baek W, Lim CW, Lee SC. Post-translational modifications of bZIP transcription factors in Abscisic acid signaling and drought responses. Curr Genom. 2021;22(1):4–15. doi: 10.2174/18755488MTEx6OTQj0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiang Y, Tang N, Du H, Ye H, Xiong L. Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice. Plant Physiol. 2008;148(4):1938–1952. doi: 10.1104/pp.108.128199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen H, Chen W, Zhou J, He H, Chen L, Chen H, et al. Basic leucine zipper transcription factor OsbZIP16 positively regulates drought resistance in rice. Plant Sci. 2012;193-194:8–17. doi: 10.1016/j.plantsci.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Liu C, Mao B, Ou S, Wang W, Liu L, Wu Y, et al. OsbZIP71, a bZIP transcription factor, confers salinity and drought tolerance in rice. Plant Mol Biol. 2014;84(1–2):19–36. doi: 10.1007/s11103-013-0115-3. [DOI] [PubMed] [Google Scholar]
- 47.Shao H, Wang H, Tang X. NAC transcription factors in plant multiple abiotic stress responses: progress and prospects. Front Plant Sci. 2015;6:902. doi: 10.3389/fpls.2015.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu G, Li X, Jin S, Liu X, Zhu L, Nie Y, et al. Overexpression of rice NAC gene SNAC1 improves drought and salt tolerance by enhancing root development and reducing transpiration rate in transgenic cotton. PLoS One. 2014;9(1):e86895. doi: 10.1371/journal.pone.0086895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao F, Xiong A, Peng R, Jin X, Xu J, Zhu B, et al. OsNAC52, a rice NAC transcription factor, potentially responds to ABA and confers drought tolerance in transgenic plants. Plant Cell Tissue Organ Cult (PCTOC) 2009;100(3):255–262. doi: 10.1007/s11240-009-9640-9. [DOI] [Google Scholar]
- 50.Raineri J, Wang S, Peleg Z, Blumwald E, Chan RL. The rice transcription factor OsWRKY47 is a positive regulator of the response to water deficit stress. Plant Mol Biol. 2015;88(4–5):401–413. doi: 10.1007/s11103-015-0329-7. [DOI] [PubMed] [Google Scholar]
- 51.Shen H, Liu C, Zhang Y, Meng X, Zhou X, Chu C, et al. OsWRKY30 is activated by MAP kinases to confer drought tolerance in rice. Plant Mol Biol. 2012;80(3):241–253. doi: 10.1007/s11103-012-9941-y. [DOI] [PubMed] [Google Scholar]
- 52.Xiong L, Schumaker KS, Zhu JK. Cell signaling during cold, drought, and salt stress. Plant Cell. 2002;14(Suppl):S165–S183. doi: 10.1105/tpc.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakashima K, Takasaki H, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K. NAC transcription factors in plant abiotic stress responses. Biochim Biophys Acta. 2012;1819(2):97–103. doi: 10.1016/j.bbagrm.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Hu H, Dai M, Yao J, Xiao B, Li X, Zhang Q, et al. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc Natl Acad Sci. 2006;103(35):12987–12992. doi: 10.1073/pnas.0604882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steffens B. The role of ethylene and ROS in salinity, heavy metal, and flooding responses in rice. Front Plant Sci. 2014;5:685. doi: 10.3389/fpls.2014.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33(4):453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- 57.Nadarajah KK. ROS homeostasis in abiotic stress tolerance in plants. Int J Mol Sci. 2020;21(15):5208. doi: 10.3390/ijms21155208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Li J, Chen S, Ma X, Wei H, Chen C, et al. An APETALA2/ethylene responsive factor, OsEBP89 knockout enhances adaptation to direct-seeding on wet land and tolerance to drought stress in rice. Mol Gen Genomics. 2020;295(4):941–956. doi: 10.1007/s00438-020-01669-7. [DOI] [PubMed] [Google Scholar]
- 59.Shi Y, Chang YL, Wu HT, Shalmani A, Liu WT, Li WQ, et al. OsRbohB-mediated ROS production plays a crucial role in drought stress tolerance of rice. Plant Cell Rep. 2020;39(12):1767–1784. doi: 10.1007/s00299-020-02603-2. [DOI] [PubMed] [Google Scholar]
- 60.Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. 2003;91(2):179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ning J, Li X, Hicks LM, Xiong L. A Raf-like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol. 2010;152(2):876–890. doi: 10.1104/pp.109.149856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. (A-G) Gene expression associated with the drought tolerance pathway. (H,I) Interactions between IPA1 and the promoter fragments of OsNAC10 and OsNAC52, shown with yeast one-hybrid assays. Data are means ± se (n = 3). Statistical significance was determined by Student’s t-test. *, P < 0.05; **, P < 0.01. Fig. S2. Gene expression analysis of IPA1 in WT and OE lines. Data are means ± se (n = 3). Statistical significance was determined by Student’s t-test. *, P < 0.05; **, P < 0.01. Table S1. Primers used in this study.
Data Availability Statement
The mutant and wild type plant used in the study are from Rice Research Institute, Fujian Academy of Agricultural Sciences, China are available from the corresponding author on reasonable request.