Abstract
Ovarian cancer (OC) is one of the most common gynecological malignancies with high morbidity and mortality. The peritoneum is one of the most common metastatic sites in ovarian cancer, involving large amounts of ascites. However, its mechanism is unclear. The peritoneal microenvironment composed of peritoneal effusion and peritoneum creates favorable conditions for ovarian cancer progression and metastasis. Here, we reviewed the peritoneal metastasis patterns and molecular mechanisms of ovarian cancer, as well as major components of the peritoneal microenvironment, peritoneal effusion, and immune microenvironment, and investigated the relationship between the peritoneal microenvironment and ovarian cancer metastasis.
Keywords: Ovarian cancer, Tumor microenvironment, Metastasis
Highlights
Ovarian cancer (OC) is one of the most common gynecological tumors. It commonly develops peritoneal metastasis and is associated with poor prognosis. However, the molecular biology and pathophysiology of ovarian cancer are not known.
Emerging evidence highlight the role of tumor microenvironment as a crucial factor in driving peritoneal metastatic progression.
Background
Ovarian cancer (OC) is a significant cause of female cancer mortality and morbidity, being the second new diagnosed case and the first leading death in gynecologic cancers [1]. Epithelial ovarian cancer (EOC) is the most common pathological type of OC, accounting for about 90% [2]. Despite having diverse pathological types, peritoneal metastasis is a common feature in patients with EOC and signals dismal [3], contributing to malignant ascites accumulation composed by the complex tumor-promoting microenvironmental network in the peritoneal cavity [4]. Although the peritoneal cavity provides a stage for OC peritoneal metastasis, numerous challenges of intra-abdominal metastasis in OC remain presented due to the underlying mechanism is not clearly defined, involving recurrence and morbidity. Exploring the mechanisms of intraperitoneal metastasis of ovarian cancer could improve molecular diagnosis and treatment.
The tumor microenvironment (TME) within the peritoneal cavity is a major component determining peritoneal metastasis in OC [5]. The OC TME consists of diverse cell types including tumor cells and host stromal cells, blood vessels, and the extracellular matrix (ECM) such as collagen [6]. While TME of OC intra-abdominal metastasis including malignant ascites and peritoneal metastasis is poorly understood, studies indicate that the cooperation of tumor and stromal cells support the dissemination of OC cancer cells within the peritoneal cavity [7], as the main reason for the poor prognosis and adverse outcomes [8, 9]. Clinically, OC patients with metastasis within the peritoneal cavity are related to poor prognosis [10]. Here, we review how OC formulate peritoneal metastasis and how the TME within the peritoneal cavity supports the OC peritoneal metastasis.
Models of OC in the colonization of the peritoneal cavity
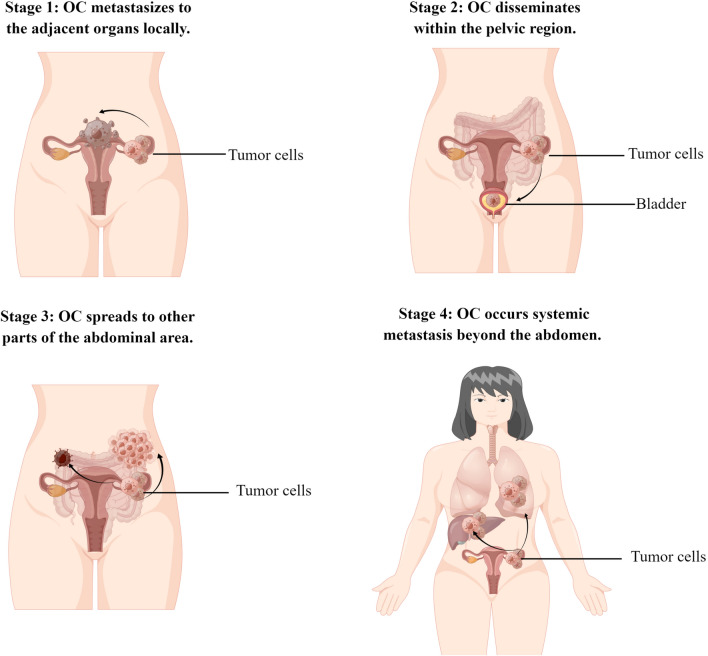
Lymphatic metastasis usually occurs in pelvic and para-aortic lymph nodes. Hematogenous spread is the least common metastatic path [11]. Currently, it is well accepted that OC metastasis proceeds through a series of stages (Fig. 1). Consistent with most tumors, the common initial stage of OC metastasis spreads to the adjacent organs such as the surrounding connective tissues, fallopian tubes, and uterus [12]. Then, the OC disseminates within the pelvic and abdominal area through transcoelomic, hematogenous, and lymphatic routes [4]. Systemic metastasis including liver and lung is relatively less frequent and usually appears in the late-stage of OC progression [7, 13].
Fig. 1.
Different stages of ovarian cancer
Compared to other tumors, ovarian cancer metastasis occurs most frequently in the omentum or peritoneum. Studies reported that almost 70% of patients with ovarian cancer presented peritoneal cavity metastasis at the time of diagnosis [4, 14]. As shown in Fig. 2, OC tumor cells are preferentially home to the omentum and develop metastatic lesions, while micro-metastases are often presented on the peritoneal surfaces. Immunohistochemical analysis of metastatic omentum and peritoneal tissues shows poor microvasculature perfusion [15]. In addition, the metastatic omentum and peritoneal tissues lose the normal collagen network.
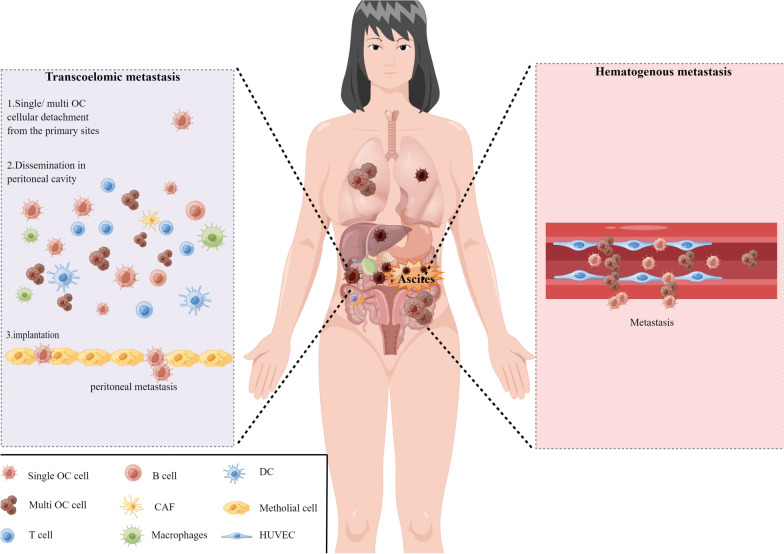
Fig. 2.
Two main pathways of peritoneal metastasis in ovarian cancer
The hypothesis of the OC peritoneal metastasis model
Currently, two hypotheses have been proposed for the peritoneal metastasis model in ovarian cancer. The first hypothesis, related to the “seed and soil” hypothesis [16] is that the peritoneal metastasis of OC originates from circulating tumor cells within the peritoneal cavity, which preferentially metastasize to the peritoneum through transcoelomic, hematogenous, or lymphatic route. The second hypothesis, referred to as the metaplasia hypothesis, is that the OC metastatic omental sites are the synchronized malignant transformation of the peritoneum or omentum due to the similar lineage between ovarian epithelium and omentum [4]. Although the “seed and soil” hypothesis has been commonly accepted historically, some studies have indicated that de-novo malignant transformation occurs in multiple lesions of the OC peritoneal metastasis [17]. However, the “seed and soil” hypothesis or the “metaplasia hypothesis” cannot completely clarify the full picture of OC peritoneal metastasis, and small studies currently explore this issue. Thus, how to form peritoneal metastasis needs further study.
Transcoelomic metastatic route of EOC
There are several reasons why OC tumor cells typically metastasize to the abdominal peritoneum or omentum through the transcoelomic pathway. First, the ovaries are close to the abdominal cavity in terms of anatomical location. Besides, there is no peritoneal coverage of the ovary, resulting in no physical barrier separating the tumor from the abdominal cavity. Therefore, the location and features of the ovaries provided an ideal place for transcoelomic metastasis in the peritoneal cavity. Second, given the common linage between the omental surface epithelium and the ovarian epithelium [4], the omentum and peritoneum surfaces provide the soil of tumor-supportive microenvironment for OC metastasis according to the seed-and-soil hypothesis [16]. Lastly, the larger area of the cavity and the existence of peritoneal fluid assist the metastasis of OC tumor cells in the peritoneal cavity.
Detachment from the primary tumor, dissemination within the peritoneal cavity, and implantation in the peritoneum of OC tumor cells are significant in successful transcoelomic metastasis. This is a complex process that requires the adaptation of OC tumor cells within the circulating ascites (Fig. 2).
Step 1: peeled from the primary tumor
The initial step of the transcoelomic journey is the relative passive exfoliation of EOC tumor cells from the primary sites into the peritoneal cavity, which could be further promoted by the peritoneal fluids [4, 18, 19]. Although the underlying mechanism of tumor cell detachment from its primary lesion is not well defined, it is commonly accepted that the loss of cell–cell contact involved in the disruption of OC cell adhesion contributes to the process. Integrins are transmembrane glycoproteins that can regulate cell functions such as adhesion, invasion, and survival [20, 21], involved in the intraperitoneal metastatic cascade of OC. Aberrant expression and functions of integrins impair cell adhesion between cells. For instance, cleaving α3-integrin contributes to the detachment of OC cells from the primary ovarian mass [22]. Up-regulation of α-integrin promotes OC metastasis by assisting OC cells to attach more efficiently [23]. Another potential factor related to the detachment of OC cells is E-cadherin. Downregulation of E-cadherin regulated by Snail promoted the initial tumor cell detachment and migration during metastatic progression in OC [24]. Another controversial event in OC metastasis is an epithelial-to-mesenchymal transition (EMT). Unlike other epithelial cancer, the role of EMT in OC progression is unclear. Similar to other epithelial tumors, some investigators consider that detachment of OC cells from the primary mass related to peritoneal metastasis experiences EMT [25]. However, others argue that separation of OC cells from the primary sites involved in peritoneal metastasis without undergoing EMT, as the metastatic tumor cells still express E-cadherin [26]. Ovarian epithelial cells occur EMT process in response to culture rather than acquire a real invasive phenotype [24, 27]. Thus, most of the EMT phenomenon in OC has only been revealed in vitro.
Step 2: dissemination in the peritoneal cavity
Following cell department, ovarian cancer cells could survive and disseminate within the peritoneal cavity. Malignant ascites provides a metastatic milieu for OC. On the one hand, the mechanical forces and natural flow resulting from the physiological movement of ascites provide aspects for the intraperitoneal implantation metastasis of the detached OC cells [28]. On the other hand, ascites facilitate OC transcolomic metastasis by generating a unique TME consisting of tumor cells, stromal cells, and an acellular compartment [29]. The ascites microenvironment supports the dissemination and seeding of OC cells within the peritoneal cavity [7]. The detailed role of ascites in OC metastasis is specified in Part III (Peritoneal Microenvironment and OC peritoneal metastasis). Although malignant ascites is crucial for OC intraperitoneal metastasis, detached cancer cells survive in ascites after overcoming many obstacles such as spheroid formation, anoikis resistance, immunological surveillance, and so on.
Aggregation is a hallmark of the metastatic OC cells within the peritoneal cavity. Tumor cells of ascites could be present as separate cells or aggregate cells such as spheroids, facilitating implantation on the surface of metastatic organs. Aggregated ovarian tumor cells survive and disseminate within the peritoneal cavity through increased complement resistance due to the ability of antibodies and complement to penetrate the OC spheroids becoming insufficient [30]. Interaction between integrin α5β1 and fibronectin promotes ovarian cancer cell aggregation in vitro [31]. UBR5 promotes the spheroid formation of OC cells through regulating p53 protein expression [32]. In addition, the ascitic tumor cells could format spheroids containing stromal cells or immune cells, which contribute to peritoneal dissemination [33]. For example, upregulating integrin α5 in ascitic tumor cells facilitates spheroids formation between tumors and fibroblasts, involved in early peritoneal metastasis [34]. However, the precise mechanisms of the role of spheroid formation related to OC peritoneal metastasis remain unclear.
Anoikis refers to a specific process of cellular apoptosis when cells lose cell–matrix interactions. Several studies have revealed that after leaving the primary lesions, OC cells could survive within the peritoneal cavity by overcoming anoikis [35]. Targeting anoikis of OC cells remises OC metastasis [36–38]. Several molecules including RAB25 small GTPase [39], integrin members [40, 41], LRRC15 [38] regulate the anoikis resistance of OC cells. Furthermore, resistance to anoikis promotes OC metastasis by activating yes-associated protein 1 (YAP1) pathway [42]. Thus, anoikis is a hallmark of normal endothelial cells while OC cells develop resistance to anoikis during dissemination within the peritoneal cavity.
Immune escape is another hallmark of the metastatic OC cells within the peritoneal cavity. High levels of immunomodulators such as IL-2, TNF exist in OC malignant ascites [43]. Besides, OC cells in ascites induce apoptosis of CD95 positive immune cells through exosomal CD95 ligand [44], impairing normal immune response. OC cells also inhibiting proliferation of immune cells through releasing metabolites [45, 46]. Ascites could also recruit regular T(Treg) cells and inhibit specific anti-tumor immunity, promoting tumor progression [47]. Malignant ascites fluid impairs metabolism and normal function of T cells through perturbing glucose uptake, which leads to immune evasion in OC TME [48]. Thus, the detached OC cells presenting as single cells or spheroids survive within the intraperitoneal cavity by overcoming many obstacles and metastasize further.
Step 3: implantation
Similar to the process of OC cells leaving from the primary sites, the underlying mechanisms involving the peritoneal metastases establishment of circulating OC cells are undefined, but seem to be associated with the dynamic interaction between the OC cells and mesothelium. It is accepted that transcoelmic metastasis is likely the predominant pathway of OC spread, while distant metastasis via hematogenous route is less important. The process includes adhesion of the metastatic OC cells to mesothelial cells, infiltration of the metastatic OC cells to the sub-mesothelial matrix and contributes to peritoneal implantation finally.
The metastatic OC cells within the peritoneal cavity adhere to the first barrier of the peritoneum which consisted of human peritoneal mesothelial cells (HPMCs), damaging the mesothelium and infiltrating into the sub-mesenchymal matrix and destructing the peritoneal barrier. Aberrant molecular expression of OC cells is related to the attachment of detached tumor cells to peritoneal mesothelium. Cancer antigen 125 (CA125), a glycoprotein, is overexpressed on OC cells and promotes adhesion and erodibility of OC cells into the peritoneal mesothelial layer through binding to mesothelin (MSLN) [49, 50]. Additionally, matrix-degrading enzymes including matrix metalloproteinases (MMP) are vital to OC metastasis to peritoneum because the basement membrane of peritoneal tissues is rich in collagen and fibronectin. Over-expression of MMP2 on the disseminated OC cells facilitates connecting to peritoneal mesothelium through cleaving fibronectin and vitronectin [51]. Upregulation of MMP14 on the OC cellular surface facilitates tumor metastasis through degrading collagen IV of the basement membrane and fibrillar collagens of the sub-mesothelial stromal matrix [22, 52]. Besides, chemokine CXCL12-CCR4 mediates the attachment of the OC cells to peritoneal mesothelial cells [53]. In addition, the disseminated OC cells attach to the peritoneal mesothelium by regulating hyaluronan expression on mesothelial cells. Angiogenesis is another significant process in metastatic lesions because tumor growth needs neo-blood vessel formation to supply enough nutrients. Stromal cells in the peritoneum supports the OC cell growth through regulating angiogenesis related molecules. Malignant ascites also facilitates the implantation of OC cells in the peritoneum. The mechanical force resulting from elevated intra-peritoneal pressure assists OC cells adhere to the peritoneum and promoting metastasis [28]. Besides, malignant ascites promotes the mesothelial-to-mesenchymal transition of mesothelial cells located at the peritoneal surface, which provides the opportunity for OC cells to erode the sub-mesothelial matrix [54, 55]. Then, metastatic OC cells that disrupt the peritoneal barrier preferentially invade specific structures of the peritoneum such as the “milky spots” in the omentum, initiating an accelerated invasive growth process. The metastatic OC cells interact with mesenchymal, immune, and endothelial cells of the peritoneal tumor microenvironment in a specific peritoneal structure such as “milky spots” to form a pro-tumor microenvironment. OC is easily transferred to the papillary structure of the omentum, which consists mainly of immune cells such as macrophages, lymphocytes, and natural killer cells [10, 56, 57]. According to the “seed and soil” theory, “milky spots” is suitable soil for ovarian cancer cells to survive and grow. Thirdly, the metastatic OC cells infiltrating into the specific peritoneal structures such as “milky spots” facilitate peritoneal interstitial fibrosis and destroy peritoneal structures. Lastly, TME of the metastatic lesions formats neovascularization and supports the metastatic tumor cells’ survival and growth. Angiogenesis is another important process in metastatic lesions, as tumor growth requires the formation of new blood vessels to provide adequate nutrition. Interstitial and tumor cells in the peritoneum support OC cell growth by regulating angiogenesis-associated molecules.
Based on the above studies, we speculate that the dynamic crosstalk between peritoneal stromal cells and disseminated OC cells is vital to fostering the complicated metastatic process. Thus, a better understanding of the OC metastatic tumor microenvironment within the intra-peritoneal cavity will aid in elucidating tumor progression.
Hematogenous metastatic route of EOC
Recently, a growing number of studies have shown that the hematogenous pathway also plays important roles in tumor metastasis of OC [58, 59], which is initiated by lympho-vascular space invasion. Clinically, inferior vena cava filters increase the risk of hematogenous spread in OC by inducing platelet activation and proinflammatory reaction [60]. Besides, circulating tumor cells detached from the primary lesions could be detected in OC patients at the time of early diagnosis [61–63]. The molecular mechanisms of hematogenous metastasis in OC are also complex. In this process, the metastatic tumor cells need to penetrate the endothelial surface and enter the circulation. Upregulation of genes such as LUM in the OC primary sites is more prone to hematogenous metastasis through promoting extracellular matrix deposition [64]. Down-regulation of CCR4 inhibits hematogenous metastasis by suppressing CTCs of OC cells [65]. Besides, OC cells can also metastasize to the omentum through the hematogenous route via ErbB3/NRG1 axis [59]. Therefore, although hematogenous route in OC metastases is relatively rare, this uncommon pathway should not be ignored, requiring further exploration.
Peritoneal microenvironment and OC peritoneal metastasis
According to the theory of “seed soil”, the peritoneal tumor microenvironment is composed of diverse cell types including peritoneal mesothelial cells (PMs), fibroblasts, adipocytes, immune cells, endothelial cells, mesenchymal stem cells, etc. and extracellular matrix provides a suitable soil for the formation of peritoneal metastasis in OC. Interaction between tumor cells and the TME can be mainly mediated through direct physical contact, soluble molecules released by the paracrine pathway, bioactive molecules transmission by exosomes, and malignant peritoneal effusion.
The role of main cell types within the peritoneal cavity in OC peritoneal metastasis
The OC TME consisting of both ascites in a liquid-state and metastatic niches in the solid-state microenvironment is complex. OC displays easy metastasis to the omentum, which is a large visceral peritoneum, located between the stomach and transverse colon. Based on the “seed and soil” theory, the omental microenvironment provides congenial conditions for the metastatic OC cells. Interestingly, omental milky spots, specific vascularized immune cell structures containing immune cells, are significant for OC omental metastasis [57]. The cell types of both the liquid and solid TME are mostly similar while the proportion of each cell type is a discrepancy. Consistent with the large heterogeneity among OC in different patients, the composition of both tumor cells and non-malignant cells within TME including ascites, primary and metastatic lesions is diverse [66–68]. However, the underlying mechanisms related to the strong tropism of OC dissemination associated with a complex dialogue between tumor cells and stromal cells within the TME remain unclear.
Tumor cells
The interaction between tumor cells and stromal cells formats the pre-metastatic niche in the omentum, which may be a prevalent precondition for OC metastasis [69]. For instance, OC cells could create pre-metastatic niches of the omentum by regulating the phenotype of cancer-associated fibroblasts via TGF-β1, thereby facilitating cancer progression [70]. Tumor cells could also induce mesothelial-to-mesenchymal transition (MMT) of peritoneal mesothelial cells, contributing to a pre-metastatic niche, which promotes OC peritoneal metastasis [71]. Of note, extensive ECM deposition and modification are the hallmark of metastatic peritoneal cancer in OC, contributing to poor prognosis [72, 73]. Studies have indicated that OC cells provide optimal tumor-supportive microenvironment through regulating the ECM in the TME [74]. Besides, OC cells facilitate tumor survival and invasion through modulating a desmoplastic reaction [73, 75]. TGF-β, a vital regulator contributing to tumor metastasis process related to regulating epithelial–mesenchymal transition (EMT) [76], cancer stem cell niche formation [77] and et al., can be released from tumor cells. Studies indicated that aberrant TGF-β signal pathway correlated with poor outcomes and metastasis in patients of ovarian cancer [78].
Mesothelial cells
Mesothelial cells are a single layer of epithelioid cells covering the abdominal organs which originate from fibroblasts of the mesoderm and are characterized by both mesenchymal and/or epithelial cells. Normal mesothelial cells resist further metastasis of tumor cells by inhibiting adhesion of tumor cells to the peritoneum. However, once MMT occurs, mesothelial cells promote tumor cell invasion by promoting tumor cell adhesion to the peritoneum [79] and accumulation of CAFs [80]. Besides, mesothelial cells have less proliferative potential and are prone to senescence. Senescent mesothelial cells help OC establish peritoneal metastases by promoting tumor cell adhesion to the peritoneum [81].
Fibroblasts
Fibroblasts and the secreted structural proteins including collagen, fibronectin, elastin, vitronectin, etc. comprise the intraperitoneal sub-mesothelial matrix. Fibroblasts are the main regulator of ECM in physiological situations [74]. Transformation of peritoneal fibroblasts into cancer-associated fibroblasts (CAFs) is one of the important causes of peritoneal metastasis of ovarian cancer. Currently, the source of intra-abdominal CAFs is not clear. The traditional belief is that peritoneal CAFs are derived from peritoneal resident cells, that is, the transformation of peritoneal resident cells into CAFs upon stimulation by tumor cells [82] while the new theory proposes that the CAFs are originated from mesothelial cells, that is, mesothelial cells undergo MMT with spindle changes, decreased expression of epithelial markers such as E-cadherin and increased expression of mesenchymal markers such as α-smooth muscle actin(αSMA) [83, 84]. Cytokines including IL-6, TGF-β could drive tumor-associated CAF phenotype and promote tumor metastasis [85, 86]. CAFs facilitate ovarian cancer progression through direct or indirect effects. As previously described, ECM deposition including collagen crosslinking plays a significant role in OC progression [73]. Studies demonstrate that CAFs assist OC progression through regulating ECM and a desmoplastic reaction [74]. Besides, CAFs secrete cytokines and promote peritoneal metastasis of OC [87, 88]. For example, fibroblasts could secrete TGF-β2, which can promote levels of CXCL12, IL-6 and VEGF-A, inducing immune evasion of cancer cells and angiogenesis [89]. TGF-β signal pathway associated molecules such as versican (VCAN) could also promote CAFs phenotypic transformation by activating the TGF-β pathway, resulting in tumor metastasis [90]. In addition, CAFs promote peritoneal metastasis by metabolic reprogramming of tumor cells. For instance, the nutrient-deficient hypoxic peritoneal microenvironment activates mitophagy and autophagy of CAFs, which provides substrates such as lactate for mitochondrial oxidative phosphorylation metabolic pathways in adjacent tumor cells, contributing to OC progression [91]. Moreover, CAFs secrete VEGF and promote tumor angiogenesis, contributing tumor progression [92].
Adipocytes
Recent studies have demonstrated that adipocytes from peritoneal metastases provide rich nutrition for the seeding of tumor cells [93]. Thus, adipose tissue promotes tumor progression. The fatty acid receptor CD36 is highly expressed in peritoneal metastatic foci of ovarian cancer patients compared with orthotopic tumor sites. Moreover, adipocytes promote fatty acid uptake and energy metabolism in ovarian cancer cells by upregulating CD36 expression on the surface of ovarian cancer cells, resulting in peritoneal metastasis of the tumor [94]. Cysteine-rich acidic secretory proteins block interaction between adipocytes and tumor cells through inhibiting adipocyte differentiation, which alleviate OC peritoneal metastasis [95]. In addition, adipose-derived stem cells from OC patients enhance the adhesion and invasion ability of tumor cells through remodeling ECM by upregulating metastasis associated proteins such as MMPs, endothelial-specific molecule 1 [96]. Hence, adipocytes play an important role in tumor peritoneal metastasis, and elucidating the role of adipocytes on OC peritoneal metastasis is vital to improve the diagnosis and treatment of the disease.
Neutrophils
Neutrophils belong to the myeloid lineage. The neutrophil to lymphocyte ratio (NLR) is a measure of systemic inflammation and in ovarian cancer, preoperative high NLR (> 3) serves as a predictive factor for poor survival [97, 98]. Mechanistically, neutrophils within milky spots facilitate the formation of a pre metastatic omental niches, thereby facilitating ovarian cancer cell implantation and colonization of the omentum [69]. Currently, the origin of neutrophils and their role in peritoneal metastasis of ovarian cancer are unclear. In other tumors, TGF-β signaling activation promotes neutrophils infiltration within the tumor microenvironment and supports metastasis [99]. For example, triple-negative breast cancer cells could recruit neutrophils to the local microenvironment by secreting TGF-β, involving in tumor progression [100].
Macrophages
Macrophages participate in inflammation and immune response under physiological and pathological conditions, which are present in the peritoneal cavity. Intraperitoneal macrophages originate from 2 sources. One type is osmotic macrophages formed by the recruitment of monocytes originating from the bone marrow. The other type is tissue-colonizing macrophages formed during embryonic development. Most macrophages infiltrated in milky spots, the most frequently metastasized omental sites in OC, originate from tissue-resident macrophages [10]. However, the proportion of tumor-associated macrophages from different sources in the abdominal cavity and their function need to be further explored. Macrophages are highly plastic in response to TME. Recently, it is proposed that macrophages are classified into M1 (classically activated type) and M2 (alternatively activated type) compared with the peritoneum of patients with benign diseases, the proportion of M2 macrophage infiltration is significantly increased in peritoneal metastases of OC patients [101, 102]. Tumor-associated macrophages (TAMs) promotes OC metastatic spread through facilitating the formation of pre-metastatic niches [10]. Besides, TAMs lead to aggressive phenotype of OC cells through facilitating spheroid formation, which is associated with OC transcoelomic metastasis [32, 102]. TAMs promote anoikis of OC cells through releasing the related soluble factors, contributing the growth and peritoneal metastasis in OC [103, 104]. TAMs facilitate angiogenesis, supporting peritoneal metastasis of OC. For instance, TAMs induce angiogenesis in TME through modulating endothelial cells via affecting angiogenic pathway [105]. TAMs enhance OC metastasis by impairing T cell function [106].
T cells
As the significant cellular types in the adaptive immune system, T lymphocytes are significant in the process of eliminating tumor cells from the host immune system. There are three main cellular subtypes of T cells in OC TME, referring to CD8+ effector cells, CD4+ helper cells and Treg cells [107]. A high CD8/CD4 ratio in TME correlates with improved outcome in OC [108], while higher infiltration of Tregs is related to worse outcome [109] due to impairing antitumor response. Tregs could release TGFβ, leading to format the tumor-promoting microenvironment and EMT of tumor cells [110]. Moreover, VEGF secreted from ascites derived T cells of ovarian cancer patients could activated VEGFR-2, which conversely inhibited T cell function [111]. Targeting anti-VEGF could augment anticancer function of CD8+ T cells [112].
Endothelial cells
After tumor metastasis to the corresponding site, new blood vessels need to be formed at this site to supply nutrients for tumor cell survival and metastasis at the site of metastasis. Cells within the TME including macrophages, tumor cells, and mesothelial cells recruit peritoneal endothelial cells to the vicinity of peritoneal metastases and form tubular structures by secreting chemokines, TGF-β, and IL-6 to provide nutrients for OC progression [113, 114].
Mesenchymal stem cells
At present, there are few studies have reported the role of mesenchymal stem cells (MSCs) in OC peritoneal metastasis. Resident omental MSCs acquire a CAF-like phenotype in reaction to TGF-β1 released from tumor cells [115], increasing OC metastasis through the creation of pre-metastatic niches. In addition, ovarian cancer ascites derived primary stem cells promote tumor progression by interacting with the cancer cells and macrophages to produce pro-angiogenic factors such as vascular endothelial growth factor (VEGF) and IL6 [116]. Moreover, intraperitoneal stem cells induce immunosuppressive effects through inhibiting T cell proliferation and function [117].
The role of peritoneal fluids in OC peritoneal metastasis
Ascites is not only the result of peritoneal metastasis of tumors, but also takes the role of “porter” in implantable metastasis of ovarian cancer and provides a medium for the metastatic growth of tumors. The malignant ascites, a highly pro-metastatic environment, provides a supportive milieu for OC metastasis and thriving.
EOC metastasis is usually confined to the peritoneal cavity and distant metastasis is less frequent [11]. Malignant ascites, a clinical hallmark of poor prognosis in OC, promotes intraperitoneal transcoelomic dissemination. More than 90% of OC patients present ascites accumulation within the peritoneal cavity at diagnosis [118]. Studies indicate that the occurrence of ascites closely correlates with disease progression [29, 119, 120]. Thus, ascites microenvironment plays an important role in tumor progression.
Unlike the tumor microenvironment of solid tumors, malignant ascites presents a microenvironment of neutral PH [121], relatively mild hypoxia [122], low glucose [48], and high levels of free fatty acids [123]. It is widely accepted that the OC cells relative passively detached from the primary tumor surface could be carried to the colonization lesions through ascitic fluids [124]. Thus, except for tumor cells, the unique microenvironment within the malignant ascites contains a variety of benign cell types including mesothelial cells, fibroblasts, immune effector cells, endothelial cells, as well as a plethora of soluble factors including cytokines, chemokines, growth factors and, matrix-degrading enzymes [29, 125], supporting OC development and metastasis. Currently, studies have demonstrated that the malignant ascites microenvironment promotes peritoneal metastasis of OC by the following mechanisms.
Arterial vasodilation and venous obstruction are physiological mechanisms for ascites formation [126]. OC tumor cells cause blockage of lymphatic drainage and formation of ascites by increasing vascular permeability and/or lymphatic obstruction in the abdominal cavity [127]. In addition, stromal cells within the TME also result in ascites accumulation of OC. For example, macrophages facilitate malignant ascites development through modulating vascular permeability [128].
Mechanism of ascites promoting OC progression
The formation of an immunosuppressive microenvironment
Most of the immune effector cells in ascites present an immunosuppressive phenotype. Studies demonstrate that malignant ascites induces an immune-suppressed phenotype of immune cells through cytokines and metabolites [129], leading to tumor metastasis via metabolic reprogramming. Clinically, high CD4+/CD8 tumor-infiltrating T lymphocytes ratio in ascites have been related to poor outcome [108]. Moreover, compared to the primary sites and peritoneal metastases, increased CD4+ T cells has been revealed in the ascites of OC patients [108, 130]. Tregs inhibit anti-tumor immune responses, thereby the accumulation of Tregs in OC ascites is associated with advanced stage [131]. γδ T cells are an unconventional subset of T cells, involving in adaptive and innate immunity. In the clinic, compared to the primary or metastatic lesions, the proportion of γδ T cells increases preferentially in the OC malignant ascites [130]. In situations of the ascites, γδ T cells impair the normal immune response of CD8+ T cells [130, 132]. Natural killer T (NKT) cells are another peculiar subset of T cells, which are characterized by both innate NK cells and adaptive conventional T cells. Studies demonstrate that ganglioside GD3, which is overexpressed in the ascites of advanced OC patients, inhibits activation of NKT cells, leading to tumor immune evasion [133]. However, the role of γδT and NKT cells in OC metastasis remains unclear. The levels of IL6, IL10 and macrophage colony stimulating factor1 (CSF-1) are abundant in OC malignant ascites, which induce polarization of tumor-associated macrophages [134]. In OC malignant ascites, the proportions of myeloid-derived suppressor cells (MDSCs) are abundant, which correlate with poor prognosis and advanced disease stage [135]. TME induces immune-suppressive functions of NK cells. For example, NK cells existing in the OC ascites show reduced NKp30 receptor activation [136].
Promotion of malignant biological behavior in tumor cells
The proliferation and migration of OC cells could be promoted by Lysophosphatidic acid (LPA) [137], a growth factor which is overexpressed in OC ascitic fluids. Exosomes are phospholipid-containing bilayer-enclosed extracellular vesicles secreted by cells, existing in OC ascites. They facilities OC peritoneal dissemination through mediating cell–cell communication. Ascites-derived exosomes could promote formation of the premetastatic niches within the peritoneal cavity or/and epithelial–mesenchymal transition (EMT) of tumor cells [138], playing a significant role in OC progression.
Establishment of angiogenesis
Tumor cells and stromal cells such as CD163+ macrophages in ascites can release VEGF, TGFβ, IL-6, IL-8 [139], promoting neovascularization and supporting OC metastasis. In ovarian cancer, blocking TGF-β downregulates VEGF expression and reduces ascites formation [140]. High levels of VEGF, TGFβ, and IL-6 in ascites are inversely correlated with progression-free survival of the disease [141, 142]. Anti-VEGF therapies such as bevacizumab reduce ascites, suggesting that VEGF plays an important role in OC ascites accumulation [103].
Conclusion
Ovarian cancer is a heterogeneous and complex disease with insidious onset, lack of effective early screening methods, and most patients are in the advanced stage at the time of presentation. The peritoneum is the most common site of metastasis of ovarian cancer, which closely corelates with the poor prognosis in patients. According to seed-pedology, the interaction between tumor cells and the peritoneal microenvironment plays an important role in the peritoneal metastasis of ovarian cancer. Ovarian cancer cells engineer metastatic sites into a soil suitable for their own survival and metastasis by remodeling the extracellular matrix within the tumor microenvironment or inducing stromal cells to undergo tumor-promoting phenotypic transformation. In addition, stromal cells promote the dissemination and growth of ovarian cancer cells in the peritoneal cavity by promoting neovascularization, helping tumor cells to immune escape, and promoting tumor cell invasion.
Acknowledgements
Figures were created with Figdraw https://www.figdraw.com/.
Abbreviations
- OC
Ovarian cancer
- EOC
Epithelial ovarian cancer
- TME
Tumor microenvironment
- EMT
Epithelial-to-mesenchymal transition
- YAP1
Yes-associated protein 1
- Treg
Regular T
- CA125
Cancer antigen 125
- MSLN
Mesothelin
- MMP
Matrix metalloproteinases
- ECM
Extracellular matrix
- MMT
Mesothelial-to-mesenchymal transition
- CAFs
Cancer-associated fibroblasts
- αSMA
α-Smooth muscle actin
- NLR
Neutrophil to lymphocyte ratio
- TAMs
Tumor-associated macrophages
- MSCs
Mesenchymal stem cells
- VEGF
Vascular endothelial growth factor
- NKT
Natural killer T
- CSF-1
Macrophage colony stimulating factor1
- MDSCs
Myeloid-derived suppressor cells
- LPA
Lysophosphatidic acid
Author contributions
SSM conceived and wrote the manuscript. XC and KW contributed to the figures. YXC revised the manuscript. All authors edited the manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors have approved the final manuscript for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Lisio MA, Fu L, Goyeneche A, Gao ZH, Telleria C. High-grade serous ovarian cancer: basic sciences, clinical and therapeutic standpoints. Int J Mol Sci. 2019;20(4):952. doi: 10.3390/ijms20040952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szender JB, Emmons T, Belliotti S, et al. Impact of ascites volume on clinical outcomes in ovarian cancer: a cohort study. Gynecol Oncol. 2017;146(3):491–497. doi: 10.1016/j.ygyno.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan DS, Agarwal R, Kaye SB. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol. 2006;7(11):925–934. doi: 10.1016/S1470-2045(06)70939-1. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Y, Wang C, Zhou S. Targeting tumor microenvironment in ovarian cancer: premise and promise. Biochim Biophys Acta Rev Cancer. 2020;1873(2):188361. doi: 10.1016/j.bbcan.2020.188361. [DOI] [PubMed] [Google Scholar]
- 7.Schoutrop E, Moyano-Galceran L, Lheureux S, et al. Seminars in cancer biology. London: Academic Press; 2022. Molecular, cellular and systemic aspects of epithelial ovarian cancer and its tumor microenvironment. [DOI] [PubMed] [Google Scholar]
- 8.Yokoi A, Yoshioka Y, Yamamoto Y, et al. Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancer. Nat Commun. 2017;8:14470. doi: 10.1038/ncomms14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fucikova J, Coosemans A, Orsulic S, et al. Immunological configuration of ovarian carcinoma: features and impact on disease outcome. J Immunother Cancer. 2021;9(10):e002873. doi: 10.1136/jitc-2021-002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etzerodt A, Moulin M, Doktor TK, et al. Tissue-resident macrophages in omentum promote metastatic spread of ovarian cancer. J Exp Med. 2020;217(4):e20191869. doi: 10.1084/jem.20191869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomakos N, Diakosavvas M, Machairiotis N, Fasoulakis Z, Zarogoulidis P, Rodolakis A. Rare distant metastatic disease of ovarian and peritoneal carcinomatosis: a review of the literature. Cancers. 2019;11(8):1044. doi: 10.3390/cancers11081044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amadori D, Sansoni E, Amadori A. Ovarian cancer: natural history and metastatic pattern. Front Biosci. 1997;2:g8-10. [PubMed] [Google Scholar]
- 13.Tanaka K, Shimada Y, Nishino K, et al. Clinical significance of mesenteric lymph node involvement in the pattern of liver metastasis in patients with ovarian cancer. Ann Surg Oncol. 2021;28(12):7606–7613. doi: 10.1245/s10434-021-09899-8. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer EA. Real-world evidence in the treatment of ovarian cancer. Ann Oncol. 2017;28(suppl_8):viii61–viii65. doi: 10.1093/annonc/mdx443. [DOI] [PubMed] [Google Scholar]
- 15.Kastelein AW, Vos L, van Baal J, et al. Poor perfusion of the microvasculature in peritoneal metastases of ovarian cancer. Clin Exp Metastasis. 2020;37(2):293–304. doi: 10.1007/s10585-020-10024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 17.Fishman A, Shalom-Paz E, Fejgin M, Gaber E, Altaras M, Amiel A. Comparing the genetic changes detected in the primary and secondary tumor sites of ovarian cancer using comparative genomic hybridization. Int J Gynecol Cancer. 2005;15(2):261–266. doi: 10.1136/ijgc-00009577-200503000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177(3):1053–1064. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prat J. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124(1):1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Dhaliwal D, Shepherd TG. Molecular and cellular mechanisms controlling integrin-mediated cell adhesion and tumor progression in ovarian cancer metastasis: a review. Clin Exp Metastasis. 2022;39(2):291–301. doi: 10.1007/s10585-021-10136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buczek-Thomas JA, Chen N, Hasan T. Integrin-mediated adhesion and signalling in ovarian cancer cells. Cell Signal. 1998;10(1):55–63. doi: 10.1016/S0898-6568(97)00074-0. [DOI] [PubMed] [Google Scholar]
- 22.Moss NM, Barbolina MV, Liu Y, Sun L, Munshi HG, Stack MS. Ovarian cancer cell detachment and multicellular aggregate formation are regulated by membrane type 1 matrix metalloproteinase: a potential role in Ip metastatic dissemination. Cancer Res. 2009;69(17):7121–7129. doi: 10.1158/0008-5472.CAN-08-4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawada K, Mitra AK, Radjabi AR, et al. Loss of E-cadherin promotes ovarian cancer metastasis via alpha 5-integrin, which is a therapeutic target. Cancer Res. 2008;68(7):2329–2339. doi: 10.1158/0008-5472.CAN-07-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22(2):255–288. doi: 10.1210/edrv.22.2.0422. [DOI] [PubMed] [Google Scholar]
- 25.Faleiro-Rodrigues C, Macedo-Pinto I, Pereira D, Lopes CS. Prognostic value of E-cadherin immunoexpression in patients with primary ovarian carcinomas. Ann Oncol. 2004;15(10):1535–1542. doi: 10.1093/annonc/mdh387. [DOI] [PubMed] [Google Scholar]
- 26.Han Q, Huang B, Huang Z, et al. Tumor cell-fibroblast heterotypic aggregates in malignant ascites of patients with ovarian cancer. Int J Mol Med. 2019;44(6):2245–2255. doi: 10.3892/ijmm.2019.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosanò L, Spinella F, Di Castro V, et al. Endothelin-1 promotes epithelial-to-mesenchymal transition in human ovarian cancer cells. Cancer Res. 2005;65(24):11649–11657. doi: 10.1158/0008-5472.CAN-05-2123. [DOI] [PubMed] [Google Scholar]
- 28.Asem M, Young A, Oyama C, et al. Ascites-induced compression alters the peritoneal microenvironment and promotes metastatic success in ovarian cancer. Sci Rep. 2020;10(1):11913. doi: 10.1038/s41598-020-68639-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kipps E, Tan DS, Kaye SB. Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nat Rev Cancer. 2013;13(4):273–282. doi: 10.1038/nrc3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjørge L, Junnikkala S, Kristoffersen EK, Hakulinen J, Matre R, Meri S. Resistance of ovarian teratocarcinoma cell spheroids to complement-mediated lysis. Br J Cancer. 1997;75(9):1247–1255. doi: 10.1038/bjc.1997.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burleson KM, Casey RC, Skubitz KM, Pambuccian SE, Oegema TR, Jr, Skubitz AP. Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecol Oncol. 2004;93(1):170–181. doi: 10.1016/j.ygyno.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 32.Song M, Yeku OO, Rafiq S, et al. Tumor derived UBR5 promotes ovarian cancer growth and metastasis through inducing immunosuppressive macrophages. Nat Commun. 2020;11(1):6298. doi: 10.1038/s41467-020-20140-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ford CE, Werner B, Hacker NF, Warton K. The untapped potential of ascites in ovarian cancer research and treatment. Br J Cancer. 2020;123(1):9–16. doi: 10.1038/s41416-020-0875-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao Q, Yang Z, Xu S, et al. Heterotypic CAF-tumor spheroids promote early peritoneal metastatis of ovarian cancer. J Exp Med. 2019;216(3):688–703. doi: 10.1084/jem.20180765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunay G, Kirit HA, Kamatar A, Baghdasaryan O, Hamsici S, Acar H. The effects of size and shape of the ovarian cancer spheroids on the drug resistance and migration. Gynecol Oncol. 2020;159(2):563–572. doi: 10.1016/j.ygyno.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Cai Q, Yan L, Xu Y. Anoikis resistance is a critical feature of highly aggressive ovarian cancer cells. Oncogene. 2015;34(25):3315–3324. doi: 10.1038/onc.2014.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zillhardt M, Park SM, Romero IL, et al. Foretinib (GSK1363089), an orally available multikinase inhibitor of c-Met and VEGFR-2, blocks proliferation, induces anoikis, and impairs ovarian cancer metastasis. Clin Cancer Res. 2011;17(12):4042–4051. doi: 10.1158/1078-0432.CCR-10-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray U, Jung DB, Jin L, et al. Targeting LRRC15 inhibits metastatic dissemination of ovarian cancer. Cancer Res. 2022;82(6):1038–1054. doi: 10.1158/0008-5472.CAN-21-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng KW, Lahad JP, Kuo WL, et al. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat Med. 2004;10(11):1251–1256. doi: 10.1038/nm1125. [DOI] [PubMed] [Google Scholar]
- 40.Takeshita Y, Motohara T, Kadomatsu T, et al. Angiopoietin-like protein 2 decreases peritoneal metastasis of ovarian cancer cells by suppressing anoikis resistance. Biochem Biophys Res Commun. 2021;561:26–32. doi: 10.1016/j.bbrc.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 41.Dolinschek R, Hingerl J, Benge A, et al. Constitutive activation of integrin αvβ3 contributes to anoikis resistance of ovarian cancer cells. Mol Oncol. 2021;15(2):503–522. doi: 10.1002/1878-0261.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haemmerle M, Taylor ML, Gutschner T, et al. Platelets reduce anoikis and promote metastasis by activating YAP1 signaling. Nat Commun. 2017;8(1):310. doi: 10.1038/s41467-017-00411-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aslam N, Marino CR. Malignant ascites: new concepts in pathophysiology, diagnosis, and management. Arch Intern Med. 2001;161(22):2733–2737. doi: 10.1001/archinte.161.22.2733. [DOI] [PubMed] [Google Scholar]
- 44.Abrahams VM, Straszewski SL, Kamsteeg M, et al. Epithelial ovarian cancer cells secrete functional Fas ligand. Cancer Res. 2003;63(17):5573–5581. [PubMed] [Google Scholar]
- 45.Wefers C, Duiveman-de Boer T, Zusterzeel P, et al. Different lipid regulation in ovarian cancer: inhibition of the immune system. Int J Mol Sci. 2018;19(1):273. doi: 10.3390/ijms19010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tran E, Nielsen JS, Wick DA, et al. Polyfunctional T-cell responses are disrupted by the ovarian cancer ascites environment and only partially restored by clinically relevant cytokines. PLoS ONE. 2010;5(12):e15625. doi: 10.1371/journal.pone.0015625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato S, Matsushita H, Shintani D, et al. Association between effector-type regulatory T cells and immune checkpoint expression on CD8(+) T cells in malignant ascites from epithelial ovarian cancer. BMC Cancer. 2022;22(1):437. doi: 10.1186/s12885-022-09534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song M, Sandoval TA, Chae CS, et al. IRE1α-XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature. 2018;562(7727):423–428. doi: 10.1038/s41586-018-0597-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coelho R, Ricardo S, Amaral AL, et al. Regulation of invasion and peritoneal dissemination of ovarian cancer by mesothelin manipulation. Oncogenesis. 2020;9(6):61. doi: 10.1038/s41389-020-00246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hilliard TS, Kowalski B, Iwamoto K, et al. Host mesothelin expression increases ovarian cancer metastasis in the peritoneal microenvironment. Int J Mol Sci. 2021;22(22):12443. doi: 10.3390/ijms222212443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kenny HA, Kaur S, Coussens LM, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest. 2008;118(4):1367–1379. doi: 10.1172/JCI33775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vos MC, van der Wurff A, van Kuppevelt TH, Massuger L. The role of MMP-14 in ovarian cancer: a systematic review. J Ovarian Res. 2021;14(1):101. doi: 10.1186/s13048-021-00852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scotton CJ, Wilson JL, Scott K, et al. Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Res. 2002;62(20):5930–5938. [PubMed] [Google Scholar]
- 54.Carduner L, Leroy-Dudal J, Picot CR, Gallet O, Carreiras F, Kellouche S. Ascites-induced shift along epithelial–mesenchymal spectrum in ovarian cancer cells: enhancement of their invasive behavior partly dependant on αv integrins. Clin Exp Metastasis. 2014;31(6):675–688. doi: 10.1007/s10585-014-9658-1. [DOI] [PubMed] [Google Scholar]
- 55.Rynne-Vidal A, Au-Yeung CL, Jiménez-Heffernan JA, et al. Mesothelial-to-mesenchymal transition as a possible therapeutic target in peritoneal metastasis of ovarian cancer. J Pathol. 2017;242(2):140–151. doi: 10.1002/path.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sorensen EW, Gerber SA, Sedlacek AL, Rybalko VY, Chan WM, Lord EM. Omental immune aggregates and tumor metastasis within the peritoneal cavity. Immunol Res. 2009;45(2–3):185–194. doi: 10.1007/s12026-009-8100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hagiwara A, Takahashi T, Sawai K, et al. Milky spots as the implantation site for malignant cells in peritoneal dissemination in mice. Cancer Res. 1993;53(3):687–692. [PubMed] [Google Scholar]
- 58.Coffman LG, Burgos-Ojeda D, Wu R, Cho K, Bai S, Buckanovich RJ. New models of hematogenous ovarian cancer metastasis demonstrate preferential spread to the ovary and a requirement for the ovary for abdominal dissemination. Transl Res. 2016;175:92–102.e2. doi: 10.1016/j.trsl.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pradeep S, Kim SW, Wu SY, et al. Hematogenous metastasis of ovarian cancer: rethinking mode of spread. Cancer Cell. 2014;26(1):77–91. doi: 10.1016/j.ccr.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsuo K, Carter CM, Ahn EH, et al. Inferior vena cava filter placement and risk of hematogenous distant metastasis in ovarian cancer. Am J Clin Oncol. 2013;36(4):362–367. doi: 10.1097/COC.0b013e318248da32. [DOI] [PubMed] [Google Scholar]
- 61.Gasparri ML, Savone D, Besharat RA, et al. Circulating tumor cells as trigger to hematogenous spreads and potential biomarkers to predict the prognosis in ovarian cancer. Tumour Biol. 2016;37(1):71–75. doi: 10.1007/s13277-015-4299-9. [DOI] [PubMed] [Google Scholar]
- 62.Yang J, Cheng S, Zhang N, Jin Y, Wang Y. Liquid biopsy for ovarian cancer using circulating tumor cells: recent advances on the path to precision medicine. Biochim Biophys Acta Rev Cancer. 2022;1877(1):188660. doi: 10.1016/j.bbcan.2021.188660. [DOI] [PubMed] [Google Scholar]
- 63.Zhu JW, Charkhchi P, Akbari MR. Potential clinical utility of liquid biopsies in ovarian cancer. Mol Cancer. 2022;21(1):114. doi: 10.1186/s12943-022-01588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yue H, Wang J, Chen R, Hou X, Li J, Lu X. Gene signature characteristic of elevated stromal infiltration and activation is associated with increased risk of hematogenous and lymphatic metastasis in serous ovarian cancer. BMC Cancer. 2019;19(1):1266. doi: 10.1186/s12885-019-6470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Figueras A, Alsina-Sanchís E, Lahiguera Á, et al. A role for CXCR4 in peritoneal and hematogenous ovarian cancer dissemination. Mol Cancer Ther. 2018;17(2):532–543. doi: 10.1158/1535-7163.MCT-17-0643. [DOI] [PubMed] [Google Scholar]
- 66.Izar B, Tirosh I, Stover EH, et al. A single-cell landscape of high-grade serous ovarian cancer. Nat Med. 2020;26(8):1271–1279. doi: 10.1038/s41591-020-0926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hao Q, Li J, Zhang Q, et al. Single-cell transcriptomes reveal heterogeneity of high-grade serous ovarian carcinoma. Clin Transl Med. 2021;11(8):e500. doi: 10.1002/ctm2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang AW, McPherson A, Milne K, et al. Interfaces of malignant and immunologic clonal dynamics in ovarian cancer. Cell. 2018;173(7):1755–1769.e22. doi: 10.1016/j.cell.2018.03.073. [DOI] [PubMed] [Google Scholar]
- 69.Lee W, Ko SY, Mohamed MS, Kenny HA, Lengyel E, Naora H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J Exp Med. 2019;216(1):176–194. doi: 10.1084/jem.20181170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cai J, Tang H, Xu L, et al. Fibroblasts in omentum activated by tumor cells promote ovarian cancer growth, adhesion and invasiveness. Carcinogenesis. 2012;33(1):20–29. doi: 10.1093/carcin/bgr230. [DOI] [PubMed] [Google Scholar]
- 71.Kenny HA, Chiang CY, White EA, et al. Mesothelial cells promote early ovarian cancer metastasis through fibronectin secretion. J Clin Invest. 2014;124(10):4614–4628. doi: 10.1172/JCI74778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pearce O, Delaine-Smith RM, Maniati E, et al. Deconstruction of a metastatic tumor microenvironment reveals a common matrix response in human cancers. Cancer Discov. 2018;8(3):304–319. doi: 10.1158/2159-8290.CD-17-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Natarajan S, Foreman KM, Soriano MI, et al. Collagen remodeling in the hypoxic tumor-mesothelial niche promotes ovarian cancer metastasis. Cancer Res. 2019;79(9):2271–2284. doi: 10.1158/0008-5472.CAN-18-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sahai E, Astsaturov I, Cukierman E, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20(3):174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Attané C, Muller C. Drilling for oil: tumor-surrounding adipocytes fueling cancer. Trends Cancer. 2020;6(7):593–604. doi: 10.1016/j.trecan.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 76.Li S, Zhang J, Qian S, et al. S100A8 promotes epithelial–mesenchymal transition and metastasis under TGF-β/USF2 axis in colorectal cancer. Cancer Commun. 2021;41(2):154–170. doi: 10.1002/cac2.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fujishita T, Kojima Y, Kajino-Sakamoto R, et al. The cAMP/PKA/CREB and TGFβ/SMAD4 pathways regulate stemness and metastatic potential in colorectal cancer cells. Cancer Res. 2022;82(22):4179–4190. doi: 10.1158/0008-5472.CAN-22-1369. [DOI] [PubMed] [Google Scholar]
- 78.Wang C, Armasu SM, Kalli KR, et al. Pooled clustering of high-grade serous ovarian cancer gene expression leads to novel consensus subtypes associated with survival and surgical outcomes. Clin Cancer Res. 2017;23(15):4077–4085. doi: 10.1158/1078-0432.CCR-17-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang C, Wang J, Shen X, et al. LncRNA SPOCD1-AS from ovarian cancer extracellular vesicles remodels mesothelial cells to promote peritoneal metastasis via interacting with G3BP1. J Exp Clin Cancer Res. 2021;40(1):101. doi: 10.1186/s13046-021-01899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pascual-Antón L, Cardeñes B, Sainz de la Cuesta R, et al. Mesothelial-to-mesenchymal transition and exosomes in peritoneal metastasis of ovarian cancer. Int J Mol Sci. 2021;22(21):11496. doi: 10.3390/ijms222111496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Książek K. Where does cellular senescence belong in the pathophysiology of ovarian cancer. Semin Cancer Biol. 2022;81:14–23. doi: 10.1016/j.semcancer.2020.11.021. [DOI] [PubMed] [Google Scholar]
- 82.Marsh T, Pietras K, McAllister SS. Fibroblasts as architects of cancer pathogenesis. Biochim Biophys Acta. 2013;1832(7):1070–1078. doi: 10.1016/j.bbadis.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Del Rio D, Masi I, Caprara V, et al. Ovarian cancer-driven mesothelial-to-mesenchymal transition is triggered by the endothelin-1/β-arr1 axis. Front Cell Dev Biol. 2021;9:764375. doi: 10.3389/fcell.2021.764375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kitami K, Yoshihara M, Tamauchi S, et al. Peritoneal restoration by repurposing vitamin D inhibits ovarian cancer dissemination via blockade of the TGF-β1/thrombospondin-1 axis. Matrix Biol. 2022;109:70–90. doi: 10.1016/j.matbio.2022.03.003. [DOI] [PubMed] [Google Scholar]
- 85.Albrengues J, Bertero T, Grasset E, et al. Epigenetic switch drives the conversion of fibroblasts into proinvasive cancer-associated fibroblasts. Nat Commun. 2015;6:10204. doi: 10.1038/ncomms10204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kojima Y, Acar A, Eaton EN, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci USA. 2010;107(46):20009–20014. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ji Z, Tian W, Gao W, Zang R, Wang H, Yang G. Cancer-associated fibroblast-derived interleukin-8 promotes ovarian cancer cell stemness and malignancy through the notch3-mediated signaling. Front Cell Dev Biol. 2021;9:684505. doi: 10.3389/fcell.2021.684505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Feng W, Dean DC, Hornicek FJ, Shi H, Duan Z. Exosomes promote pre-metastatic niche formation in ovarian cancer. Mol Cancer. 2019;18(1):124. doi: 10.1186/s12943-019-1049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ko SY, Barengo N, Ladanyi A, et al. HOXA9 promotes ovarian cancer growth by stimulating cancer-associated fibroblasts. J Clin Invest. 2012;122(10):3603–3617. doi: 10.1172/JCI62229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yeung TL, Leung CS, Wong KK, et al. TGF-β modulates ovarian cancer invasion by upregulating CAF-derived versican in the tumor microenvironment. Cancer Res. 2013;73(16):5016–5028. doi: 10.1158/0008-5472.CAN-13-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wilson RB, Solass W, Archid R, Weinreich FJ, Königsrainer A, Reymond MA. Resistance to anoikis in transcoelomic shedding: the role of glycolytic enzymes. Pleura Peritoneum. 2019;4(1):20190003. doi: 10.1515/pp-2019-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li J, Liu X, Zang S, et al. Small extracellular vesicle-bound vascular endothelial growth factor secreted by carcinoma-associated fibroblasts promotes angiogenesis in a bevacizumab-resistant manner. Cancer Lett. 2020;492:71–83. doi: 10.1016/j.canlet.2020.08.030. [DOI] [PubMed] [Google Scholar]
- 93.Xiang F, Wu K, Liu Y, et al. Omental adipocytes enhance the invasiveness of gastric cancer cells by oleic acid-induced activation of the PI3K-Akt signaling pathway. Int J Biochem Cell Biol. 2017;84:14–21. doi: 10.1016/j.biocel.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 94.Ladanyi A, Mukherjee A, Kenny HA, et al. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene. 2018;37(17):2285–2301. doi: 10.1038/s41388-017-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.John B, Naczki C, Patel C, et al. Regulation of the bi-directional cross-talk between ovarian cancer cells and adipocytes by SPARC. Oncogene. 2019;38(22):4366–4383. doi: 10.1038/s41388-019-0728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nowicka A, Marini FC, Solley TN, et al. Human omental-derived adipose stem cells increase ovarian cancer proliferation, migration, and chemoresistance. PLoS ONE. 2013;8(12):e81859. doi: 10.1371/journal.pone.0081859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Salman L, Sabah G, Jakobson-Setton A, Raban O, Yeoshoua E, Eitan R. Neutrophil-to-lymphocyte ratio as a prognostic factor in advanced stage ovarian carcinoma treated with neoadjuvant chemotherapy. Int J Gynaecol Obstet. 2020;148(1):102–106. doi: 10.1002/ijgo.12986. [DOI] [PubMed] [Google Scholar]
- 98.Jeerakornpassawat D, Suprasert P. Potential predictors for chemotherapeutic response and prognosis in epithelial ovarian, fallopian tube and primary peritoneal cancer patients treated with platinum-based chemotherapy. Obstet Gynecol Sci. 2020;63(1):55–63. doi: 10.5468/ogs.2020.63.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jackstadt R, van Hooff SR, Leach JD, et al. Epithelial NOTCH signaling rewires the tumor microenvironment of colorectal cancer to drive poor-prognosis subtypes and metastasis. Cancer Cell. 2019;36(3):319–336.e7. doi: 10.1016/j.ccell.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.SenGupta S, Hein LE, Xu Y, et al. Triple-negative breast cancer cells recruit neutrophils by secreting TGF-β and CXCR2 ligands. Front Immunol. 2021;12:659996. doi: 10.3389/fimmu.2021.659996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yin M, Li X, Tan S, et al. Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J Clin Invest. 2016;126(11):4157–4173. doi: 10.1172/JCI87252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Long L, Hu Y, Long T, et al. Tumor-associated macrophages induced spheroid formation by CCL18-ZEB1-M-CSF feedback loop to promote transcoelomic metastasis of ovarian cancer. J Immunother Cancer. 2021;9(12):e003973. doi: 10.1136/jitc-2021-003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Smolle E, Taucher V, Haybaeck J. Malignant ascites in ovarian cancer and the role of targeted therapeutics. Anticancer Res. 2014;34(4):1553–1561. [PubMed] [Google Scholar]
- 104.Yin M, Shen J, Yu S, et al. Tumor-associated macrophages (TAMs): a critical activator in ovarian cancer metastasis. Onco Targets Ther. 2019;12:8687–8699. doi: 10.2147/OTT.S216355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Larionova I, Kazakova E, Gerashchenko T, Kzhyshkowska J. New angiogenic regulators produced by TAMs: perspective for targeting tumor angiogenesis. Cancers. 2021;13(13):3253. doi: 10.3390/cancers13133253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lieber S, Reinartz S, Raifer H, et al. Prognosis of ovarian cancer is associated with effector memory CD8(+) T cell accumulation in ascites, CXCL9 levels and activation-triggered signal transduction in T cells. Oncoimmunology. 2018;7(5):e1424672. doi: 10.1080/2162402X.2018.1424672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jang M, Yew PY, Hasegawa K, et al. Characterization of T cell repertoire of blood, tumor, and ascites in ovarian cancer patients using next generation sequencing. Oncoimmunology. 2015;4(11):e1030561. doi: 10.1080/2162402X.2015.1030561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Giuntoli RL, 2nd, Webb TJ, Zoso A, et al. Ovarian cancer-associated ascites demonstrates altered immune environment: implications for antitumor immunity. Anticancer Res. 2009;29(8):2875–2884. [PubMed] [Google Scholar]
- 109.Dutsch-Wicherek MM, Szubert S, Dziobek K, et al. Analysis of the treg cell population in the peripheral blood of ovarian cancer patients in relation to the long-term outcomes. Ginekol Pol. 2019;90(4):179–184. doi: 10.5603/GP.2019.0032. [DOI] [PubMed] [Google Scholar]
- 110.Metelli A, Wu BX, Fugle CW, et al. Surface expression of TGFβ docking receptor GARP promotes oncogenesis and immune tolerance in breast cancer. Cancer Res. 2016;76(24):7106–7117. doi: 10.1158/0008-5472.CAN-16-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gavalas NG, Tsiatas M, Tsitsilonis O, et al. VEGF directly suppresses activation of T cells from ascites secondary to ovarian cancer via VEGF receptor type 2. Br J Cancer. 2012;107(11):1869–1875. doi: 10.1038/bjc.2012.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.de Almeida PE, Mak J, Hernandez G, et al. Anti-VEGF treatment enhances CD8(+) T-cell antitumor activity by amplifying hypoxia. Cancer Immunol Res. 2020;8(6):806–818. doi: 10.1158/2326-6066.CIR-19-0360. [DOI] [PubMed] [Google Scholar]
- 113.Mikuła-Pietrasik J, Sosińska P, Naumowicz E, et al. Senescent peritoneal mesothelium induces a pro-angiogenic phenotype in ovarian cancer cells in vitro and in a mouse xenograft model in vivo. Clin Exp Metastasis. 2016;33(1):15–27. doi: 10.1007/s10585-015-9753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sako A, Kitayama J, Yamaguchi H, et al. Vascular endothelial growth factor synthesis by human omental mesothelial cells is augmented by fibroblast growth factor-2: possible role of mesothelial cell on the development of peritoneal metastasis. J Surg Res. 2003;115(1):113–120. doi: 10.1016/S0022-4804(03)00307-X. [DOI] [PubMed] [Google Scholar]
- 115.Tang H, Chu Y, Huang Z, Cai J, Wang Z. The metastatic phenotype shift toward myofibroblast of adipose-derived mesenchymal stem cells promotes ovarian cancer progression. Carcinogenesis. 2020;41(2):182–193. doi: 10.1093/carcin/bgz083. [DOI] [PubMed] [Google Scholar]
- 116.Castells M, Thibault B, Mery E, et al. Ovarian ascites-derived hospicells promote angiogenesis via activation of macrophages. Cancer Lett. 2012;326(1):59–68. doi: 10.1016/j.canlet.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 117.Martinet L, Poupot R, Mirshahi P, et al. Hospicells derived from ovarian cancer stroma inhibit T-cell immune responses. Int J Cancer. 2010;126(9):2143–2152. doi: 10.1002/ijc.24881. [DOI] [PubMed] [Google Scholar]
- 118.Krugmann J, Schwarz CL, Melcher B, et al. Malignant ascites occurs most often in patients with high-grade serous papillary ovarian cancer at initial diagnosis: a retrospective analysis of 191 women treated at Bayreuth Hospital, 2006–2015. Arch Gynecol Obstet. 2019;299(2):515–523. doi: 10.1007/s00404-018-4952-9. [DOI] [PubMed] [Google Scholar]
- 119.Nasioudis D, Byrne M, Ko EM, et al. Ascites volume at the time of primary debulking and overall survival of patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2021;31(12):1579–1583. doi: 10.1136/ijgc-2021-002978. [DOI] [PubMed] [Google Scholar]
- 120.Quan Q, Zhou S, Liu Y, et al. Relationship between ascites volume and clinical outcomes in epithelial ovarian cancer. J Obstet Gynaecol Res. 2021;47(4):1527–1535. doi: 10.1111/jog.14682. [DOI] [PubMed] [Google Scholar]
- 121.Kim KS, Sengupta S, Berk M, et al. Hypoxia enhances lysophosphatidic acid responsiveness in ovarian cancer cells and lysophosphatidic acid induces ovarian tumor metastasis in vivo. Cancer Res. 2006;66(16):7983–7990. doi: 10.1158/0008-5472.CAN-05-4381. [DOI] [PubMed] [Google Scholar]
- 122.McKeown SR. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br J Radiol. 2014;87(1035):20130676. doi: 10.1259/bjr.20130676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Greco AV, Mingrone G, Gasbarrini G. Free fatty acid analysis in ascitic fluid improves diagnosis in malignant abdominal tumors. Clin Chim Acta. 1995;239(1):13–22. doi: 10.1016/0009-8981(95)06093-S. [DOI] [PubMed] [Google Scholar]
- 124.Al Habyan S, Kalos C, Szymborski J, McCaffrey L. Multicellular detachment generates metastatic spheroids during intra-abdominal dissemination in epithelial ovarian cancer. Oncogene. 2018;37(37):5127–5135. doi: 10.1038/s41388-018-0317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cho A, Howell VM, Colvin EK. The extracellular matrix in epithelial ovarian cancer—a piece of a puzzle. Front Oncol. 2015;5:245. doi: 10.3389/fonc.2015.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Garrison RN, Galloway RH, Heuser LS. Mechanisms of malignant ascites production. J Surg Res. 1987;42(2):126–132. doi: 10.1016/0022-4804(87)90109-0. [DOI] [PubMed] [Google Scholar]
- 127.Nagy JA, Herzberg KT, Dvorak JM, Dvorak HF. Pathogenesis of malignant ascites formation: initiating events that lead to fluid accumulation. Cancer Res. 1993;53(11):2631–2643. [PubMed] [Google Scholar]
- 128.Zhang S, Xie B, Wang L, et al. Macrophage-mediated vascular permeability via VLA4/VCAM1 pathway dictates ascites development in ovarian cancer. J Clin Invest. 2021;131(3):e140315. doi: 10.1172/JCI140315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gong Y, Yang J, Wang Y, Xue L, Wang J. Metabolic factors contribute to T-cell inhibition in the ovarian cancer ascites. Int J Cancer. 2020;147(7):1768–1777. doi: 10.1002/ijc.32990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rådestad E, Klynning C, Stikvoort A, et al. Immune profiling and identification of prognostic immune-related risk factors in human ovarian cancer. Oncoimmunology. 2019;8(2):e1535730. doi: 10.1080/2162402X.2018.1535730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Landskron J, Helland Ø, Torgersen KM, et al. Activated regulatory and memory T-cells accumulate in malignant ascites from ovarian carcinoma patients. Cancer Immunol Immunother. 2015;64(3):337–347. doi: 10.1007/s00262-014-1636-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Foord E, Arruda L, Gaballa A, Klynning C, Uhlin M. Characterization of ascites- and tumor-infiltrating γδ T cells reveals distinct repertoires and a beneficial role in ovarian cancer. Sci Transl Med. 2021;13(577):eabb0192. doi: 10.1126/scitranslmed.abb0192. [DOI] [PubMed] [Google Scholar]
- 133.Webb TJ, Li X, Giuntoli RL, 2nd, et al. Molecular identification of GD3 as a suppressor of the innate immune response in ovarian cancer. Cancer Res. 2012;72(15):3744–3752. doi: 10.1158/0008-5472.CAN-11-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang H, Yung M, Ngan H, Chan K, Chan DW. The impact of the tumor microenvironment on macrophage polarization in cancer metastatic progression. Int J Mol Sci. 2021;22(12):6560. doi: 10.3390/ijms22126560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Okła K, Czerwonka A, Wawruszak A, et al. Clinical relevance and immunosuppressive pattern of circulating and infiltrating subsets of myeloid-derived suppressor cells (MDSCs) in epithelial ovarian cancer. Front Immunol. 2019;10:691. doi: 10.3389/fimmu.2019.00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pesce S, Tabellini G, Cantoni C, et al. B7-H6-mediated downregulation of NKp30 in NK cells contributes to ovarian carcinoma immune escape. Oncoimmunology. 2015;4(4):e1001224. doi: 10.1080/2162402X.2014.1001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fang X, Gaudette D, Furui T, et al. Lysophospholipid growth factors in the initiation, progression, metastases, and management of ovarian cancer. Ann N Y Acad Sci. 2000;905:188–208. doi: 10.1111/j.1749-6632.2000.tb06550.x. [DOI] [PubMed] [Google Scholar]
- 138.Cai J, Gong L, Li G, Guo J, Yi X, Wang Z. Exosomes in ovarian cancer ascites promote epithelial–mesenchymal transition of ovarian cancer cells by delivery of miR-6780b-5p. Cell Death Dis. 2021;12(2):210. doi: 10.1038/s41419-021-03490-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Baci D, Bosi A, Gallazzi M, et al. The ovarian cancer tumor immune microenvironment (TIME) as target for therapy: a focus on innate immunity cells as therapeutic effectors. Int J Mol Sci. 2020;21(9):3125. doi: 10.3390/ijms21093125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liao S, Liu J, Lin P, Shi T, Jain RK, Xu L. TGF-beta blockade controls ascites by preventing abnormalization of lymphatic vessels in orthotopic human ovarian carcinoma models. Clin Cancer Res. 2011;17(6):1415–1424. doi: 10.1158/1078-0432.CCR-10-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kumari A, Shonibare Z, Monavarian M, et al. TGFβ signaling networks in ovarian cancer progression and plasticity. Clin Exp Metastasis. 2021;38(2):139–161. doi: 10.1007/s10585-021-10077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dalal V, Kumar R, Kumar S, et al. Biomarker potential of IL-6 and VEGF-A in ascitic fluid of epithelial ovarian cancer patients. Clin Chim Acta. 2018;482:27–32. doi: 10.1016/j.cca.2018.03.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.