Abstract
Background
Chronic obstructive pulmonary disease (COPD) varies significantly in symptomatic and physiologic presentation. Identifying disease subtypes from molecular data, collected from easily accessible blood samples, can help stratify patients and guide disease management and treatment.
Methods
Blood gene expression measured by RNA-sequencing in the COPDGene Study was analyzed using a network perturbation analysis method. Each COPD sample was compared against a learned reference gene network to determine the part that is deregulated. Gene deregulation values were used to cluster the disease samples.
Results
The discovery set included 617 former smokers from COPDGene. Four distinct gene network subtypes are identified with significant differences in symptoms, exercise capacity and mortality. These clusters do not necessarily correspond with the levels of lung function impairment and are independently validated in two external cohorts: 769 former smokers from COPDGene and 431 former smokers in the Multi-Ethnic Study of Atherosclerosis (MESA). Additionally, we identify several genes that are significantly deregulated across these subtypes, including DSP and GSTM1, which have been previously associated with COPD through genome-wide association study (GWAS).
Conclusions
The identified subtypes differ in mortality and in their clinical and functional characteristics, underlining the need for multi-dimensional assessment potentially supplemented by selected markers of gene expression. The subtypes were consistent across cohorts and could be used for new patient stratification and disease prognosis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-023-02316-6.
Keywords: COPD, Graphical models, Gene expression, Disease subtypes
Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous disease, including emphysema and small and large airways disease [1, 2]. COPD diagnosis [3] is based on spirometric measures reflecting reduced airflow obstruction, specifically a reduced ratio of forced expiratory volume in 1-s (FEV1) to forced vital capacity (FVC) less than 0.70 [4]. But this definition does not account for the vast heterogeneity observed in COPD cases in terms of the rate of progression of the disease [5], response to treatment [6–8], symptom burden [9], inflammatory response [10], and lung physiology [11]. Therefore, there has been tremendous interest in identifying COPD subtypes that reflect differences in these disease aspects [12, 13]. Well-characterized subtypes with readily assessable biomarkers would allow for the selection of high-risk COPD populations for therapeutic intervention and patient stratification leading to more highly-powered clinical trials. Molecular subtyping could also help to identify rare genetic variants and individuals at elevated risk for development of the disease [14].
Disease subtyping has been relatively successful in asthma [15], but efforts in COPD have proven more difficult. Previous attempts to subtype COPD have been limited due to lack of reproducibility and constraints in study design. Another limitation to COPD subtyping efforts is the barrier to validating and interpreting subtypes that are based on clinical characteristics (e.g., spirometry, body mass index). Some studies have tried to circumvent this problem by withholding a pre-defined subset of clinical characteristics at the clustering step and then using those to assess the resulting clusters [16]; however, this raises the question of whether the holdout set is representative of the population. While it is possible to find distinct groups of subjects regarding these clinical variables, these classifications are unlikely to identify novel disease mechanisms.
Incorporation of genomic information can greatly enhance the relevance of COPD subtypes. Peripheral blood gene expression is an attractive candidate for potential biomarkers because it is easily accessible. One previous study identified four COPD clusters based on blood gene expression with a non-negative matrix factorization approach [17]. These clusters of subjects promisingly varied in the severity of their disease, but, because the study relied on microarray gene expression data, discovery was limited to the genes included on those platforms.
We recently developed a new method for evaluating gene network perturbations in single samples (single sample Network Perturbation Assessment, ssNPA) [18]. ssNPA uses probabilistic graphs [19–22] to estimate the gene network from a set of reference (control) samples and assesses perturbations in each individual disease sample. ssNPA outperformed existing algorithms in identifying subgroups of samples based on these gene expression perturbation features and had superior clustering performance compared to gene expression itself [18] and other methods [23, 24]. In this paper, we apply ssNPA to the Genetic Epidemiology of COPD Study (COPDGene) and the Multi-ethnic Study of Atherosclerosis (MESA) data in order to identify and validate new COPD phenotypes solely from gene expression measured in peripheral blood samples.
Methods
COPD subtyping: discovery and validation cohorts
The COPDGene Study is a longitudinal study that aims to investigate the genetic basis of COPD susceptibility and progression. Our subtype discovery dataset consisted of 1211 COPDGene subjects for whom whole blood RNA-seq data were collected at the 5 year follow-up visit [25]. The first validation dataset included 1444 COPDGene participants that were sequenced later. These samples were not included in the training dataset and were processed independently. The second validation dataset consisted of 821 unrelated MESA participants. MESA is an ongoing prospective cohort study that recruited over 6000 participants in six communities throughout the United States between 2000 and 2002 [26]. Peripheral blood mononuclear cell (PBMC) gene expression was measured by RNA-seq at Exam 5 between 2010 and 2012. Detailed phenotype data (including spirometry and CT scan) were also collected at this exam.
Reference subject selection
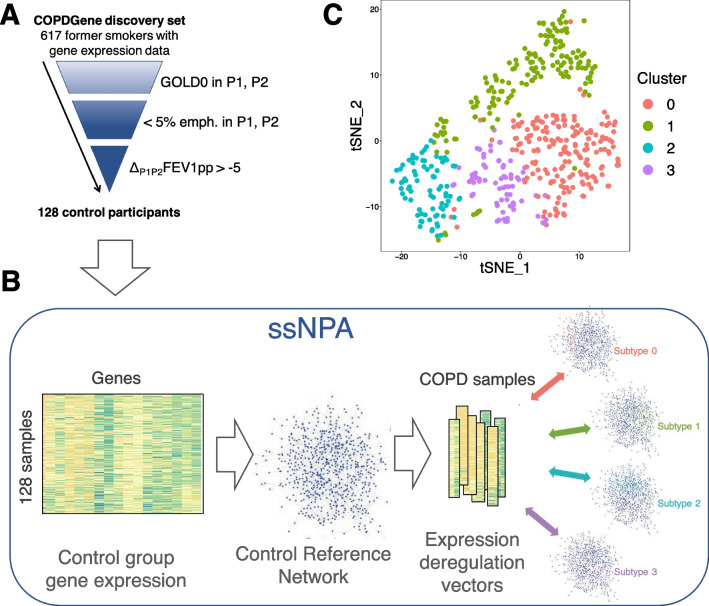
The COPDGene RNA-seq samples were preprocessed as in the Additional file 1: Methods. The reference gene network was built on a group of former smokers, selected conservatively based on the following criteria (Fig. 1A): participant had (a) both Phase 1 (baseline) and Phase 2 (5-yr) visits; (b) normal spirometry both visits; (c) less than 5% percent emphysema in both visits (LAA-950); (d) less than 5% decrease in percent predicted FEV1 between the two visits. This filtering resulted in 128 reference samples (training dataset). To increase power, all remaining samples from participants who formerly smoked cigarettes and who did not meet the criteria for the reference group were included in the disease group, leaving 489 samples for subtype discovery.
Fig. 1.
Overview of the subtyping procedure. A Selection of reference (control) samples from the COPDGene discovery cohort (617 former smokers). B Network deregulation procedure to identify COPD subtypes. C t-SNE plot of the four COPD sample clusters identified by ssNPA. Clusters 0 and 1 have similar clinical characteristics, as do clusters 2 and 3
For the COPDGene validation dataset, the same filtering criteria identified 149 control and 614 COPD former smoker samples. The MESA reference group was selected with similar criteria, except the threshold for FEV1 decline between Exam 3/4 and Exam 5 (< 3% predicted). Participants with no spirometry data were included in the non-reference group. This resulted in 104 reference and 327 case MESA samples. Table 1 summarizes the characteristics of these three study groups.
Table 1.
Characteristics of the three study groups
| COPDGene discovery group | COPDGene validation group | MESA validation group | ||||
|---|---|---|---|---|---|---|
| Reference group | Case group | Reference group | Case group | Reference group | Case group | |
| Participants, n | 128 | 489 | 149 | 614 | 104 | 327 |
| Age, yr, mean (SD) | 66.7 (8.8) | 70.1 (7.7) | 67.7 (8.6) | 69.5 (7.6) | 67.5 (8.3) | 70.1 (9.3) |
| Sex, F, n (%) | 78 (60.9) | 216 (44.1) | 91 (61.1) | 290 (47.2) | 53 (51.0) | 137 (41.9) |
| Race, Non-Hispanic White, n (%) | 111 (86.7) | 457 (93.5) | 138 (92.6) | 562 (91.5) | 45 (43.3) | 177 (54.1) |
| Race, African American, n (%) | 17 (13.3) | 32 (6.5) | 11 (7.4) | 52 (8.5) | 18 (17.3) | 68 (20.8) |
| Race, Hispanic, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 41 (39.4) | 82 (25.1) |
| GOLD stage 0, n (%) | 128 (100) | 145 (29.7) | 149 (100) | 210 (34.2) | 104 (100) | 109 (33.3) |
| PRISm, n (%) | NA | 62 (12.7) | NA | 84 (13.7) | NA | 0 |
| GOLD stage 1, n (%) | NA | 57 (11.7) | NA | 74 (12.1) | NA | 98 (30.0) |
| GOLD stage 2, n (%) | NA | 130 (26.6) | NA | 137 (22.3) | NA | 0 |
| GOLD stage 3, n (%) | NA | 76 (15.6) | NA | 85 (13.9) | NA | 0 |
| GOLD stage 4, n (%) | NA | 18 (3.7) | NA | 21 (3.4) | NA | 0 |
| Smoking history, pack-year, mean (SD) | 37.2 (21.9) | 45.9 (25.6) | 35.5 (21.0) | 43.6 (23.4) | 12.7 (14.1) | 16.8 (20.2) |
NB: There were 120 participants in the MESA group for whom spirometry data was not available. These were all included in the case group, but GOLD stage could not be calculated
GOLD Global Initiative for Chronic Obstructive Lung Disease, MESA Multi-Ethnic Study of Atherosclerosis, PRISm Preserved Ratio Impaired Spirometry
COPD subtyping from blood RNA-seq data
We used ssNPA, a network-based disease subtyping method, to learn COPD subtypes in the discovery cohort (Fig. 1B). The details of the process are presented in Additional file 1: Methods. To investigate the clinically relevant differences among the clusters of COPD samples we identified, we compared the values of 105 clinical variables across the clusters (Additional file 2: Table S1). These included spirometry, chest CT scan, symptom questionnaires, and white blood cell differentials as well as medical history, medication, and comorbidity information. The p-values were calculated using Kruskal–Wallis (continuous, ordinal) and Chi-squared test (binary). A false discovery rate (FDR) threshold of q-value < 0.05 was used for multiple test correction. Pairwise comparisons between cluster means were assessed by Wilcoxon test. Survival analysis was performed by the Kaplan–Meier method with the survfit function from the survival R package (v. 3.1-8).
To better understand how the clusters were separated, we considered the magnitude of the PCA loading for each feature. Gene features with the highest loading values in the top principal components correspond to the genes whose deregulation relative to the controls contributes the most to separating the clusters.
Finally, MESA and COPDGene validation cohort samples were assigned to one of the four subtypes using the following procedure described in Additional file 1: Methods.
Results
COPD clusters exhibit different clinical phenotypes
ssNPA separated the 489 COPD PBMC samples (all former smokers) into four clusters (Fig. 1C, Additional file 1: Fig. S8). The first two clusters were of roughly equal size (cluster 0 and cluster 1 have 37.0% and 31.9% of the samples, respectively). Cluster 2 and cluster 3 were smaller (with 16.2% and 14.9% of the samples). Mean age was similar in all clusters. Cluster 2 had a higher proportion of women and its subjects had a higher neutrophil and lower eosinophil percent, which are indicative of inflammation.
Statistical analyses identified clinical characteristics that are significantly associated with these clusters (Table 2). Overall, cluster 2 represented the most impaired subjects, with lower 6-min walk distance, more symptoms (measured by COPD Assessment Test, CAT) and dyspnea (measured by the Modified Medical Research Council Dyspnea Scale, MMRC), and the worst disease-related quality of life on the St. George’s Respiratory Questionnaire (SGRQ). However, spirometry (FEV1 percent predicted and FEV1/FVC) was similar in clusters 2 and 3, but worse than cluster 0 and 1. Clusters 0 and 1 have the best spirometric lung function, but ~ 4 times more subjects in cluster 0 are using inhaled corticosteroids (5.08% vs 1.3%; Table 2). Regardless, the percentage of corticosteroid usage in subjects of cluster 2 and cluster 3 is even higher (11.54%, 13.7%), making this feature significantly different between clusters. Diffusing capacity of carbon monoxide (DLCO) percent predicted was highest in cluster 0 (76.7%), but similar in the other 3 clusters (70.8, 69.1, 70.5% predicted, respectively). Cluster 3 had the greatest percent emphysema (12.03%), followed by cluster 2 (10.84%), while cluster 0 and cluster 1 had the least emphysema (8.59%, 8.47%) (Additional file 2: Table S1). However, these differences are not significant at the 5% FDR level (q-value = 0.068). Similarly, observed differences in Pi10 and Perc15 are not significant (q-value = 0.072–0.076).
Table 2.
Clinical characteristics of COPD participants vary across clusters. The variables are sorted by descending significance
| Variable | q-value | Cluster 0 | Cluster 1 | Cluster 2 | Cluster 3 | All reference participants |
|---|---|---|---|---|---|---|
| Participants, n | 181 | 156 | 79 | 73 | 128 | |
| Sex, F, n (%) | 80 (44.2) | 63 (40.4) | 42 (53.2) | 31 (42.5) | 78 (60.9) | |
| Age, yr, mean (SD) | 69.5 (7.8) | 70.8 (7.3) | 70.3 (7.9) | 70.0 (8.1) | 66.7 (8.8) | |
| Cell types | ||||||
| Lymphocyte percentage, mean (SD) | 4.30E−04 | 28.07 (7.91) | 26.97 (7.3) | 23.13 (11.24) | 24.84 (8.07) | 30.91 (8.58) |
| Neutrophil percentage, mean (SD) | 5.09E−04 | 60.3 (8.42) | 60.76 (7.95) | 65.94 (12.95) | 62.78 (8.59) | 57.73 (9.56) |
| Lymphocytes, K/µL, mean (SD) | 4.74E−03 | 1.95 (0.65) | 1.91 (0.61) | 1.7 (0.79) | 1.78 (0.6) | 2.03 (0.7) |
| Neutrophils, K/µL, mean (SD) | 3.11E−03 | 4.32 (1.43) | 4.4 (1.41) | 5.36 (2.23) | 4.7 (1.69) | 3.93 (1.47) |
| Medications | ||||||
| Oral corticosteroids, n (%) | 4.30E−04 | 3 (1.67) | 1 (0.65) | 8 (10.3) | 0 (0) | 1 (0.78) |
| Inhaled corticosteroids, n (%), mean (SD) | 3.03E−03 | 9 (5.08) | 2 (1.30) | 9 (11.54) | 10 (13.70) | 3 (2.34) |
| Symptoms | ||||||
| Distance walked, mean (SD) | 2.73E−03 | 1384.88 (414) | 1321.76 (403.63) | 1142.14 (428.41) | 1238.29 (407.99) | 1520.71 (358.38) |
| Change in distance walked, mean (SD) | 3.03E−03 | − 53.9 (276.36) | − 134.48 (289.36) | − 208.35 (321.96) | − 172.2 (299.63) | − 87.64 (323.56) |
| SGRQ score: active, mean (SD) | 3.11E−03 | 32.23 (27.42) | 34.13 (29.9) | 48.42 (31.68) | 36.66 (25.38) | 16.93 (20.25) |
| SGRQ score: total, mean (SD) | 1.27E−02 | 19.99 (18.17) | 21.87 (19.37) | 30.47 (22.96) | 22.04 (17.54) | 9.24 (12.51) |
| MMRC dyspnea score, mean (SD) | 1.27E−02 | 1.05 (1.29) | 1.14 (1.28) | 1.7 (1.6) | 1.4 (1.29) | 0.40 (0.86) |
| CAT score, mean (SD) | 0.017 | 9.65 (6.95) | 10.69 (7.47) | 13.43 (8.84) | 9.56 (6.82) | 6.61 (5.73) |
| Spirometry | ||||||
| FRC/TLC ratio, Thirona, mean (SD) | 1.25E−03 | 0.56 (0.11) | 0.6 (0.11) | 0.63 (0.12) | 0.61 (0.12) | 0.51 (0.08) |
| FEV1, pre-BD, mean (SD) | 0.018 | 1.95 (0.85) | 1.88 (0.75) | 1.62 (0.86) | 1.77 (0.89) | 2.53 (0.61) |
| FEV1/FVC ratio, mean (SD) | 0.020 | 0.64 (0.15) | 0.63 (0.16) | 0.59 (0.17) | 0.58 (0.16) | 0.78 (0.06) |
| FEV1 percent predicted, mean (SD) | 0.027 | 73.79 (24.65) | 73.16 (24.49) | 65.38 (26.96) | 65.44 (27.12) | 99.05 (11.33) |
| FEV1/FVC ratio, pre-BD, mean (SD) | 0.034 | 0.62 (0.15) | 0.62 (0.15) | 0.58 (0.16) | 0.58 (0.16) | 0.75 (0.06) |
| DLCO percent predicted, adjusted, mean (SD) | 0.042 | 76.69 (22.77) | 70.84 (21.52) | 69.09 (24.01) | 70.52 (21.71) | 92.08 (18.25) |
| BODE, mean (SD) | 4.95E−03 | 1.11 (1.58) | 1.26 (1.6) | 1.92 (2.05) | 1.82 (1.85) | 0.22 (0.64) |
P-values were calculated with a Kruskal-Wallis test for continuous and ordinal variables and or a Chi-squared test for discrete and binary variables and asses if there are differences in variable distribution among clusters. Variable means (standard deviations) are also reported for each COPD cluster and all control subjects for comparison. Variables of interest are included with a < 5% FDR cut-off. A full table of variables tested is provided in Additional file 2: Table S1
BD bronchodilator, BODE body mass index, airflow obstruction, dyspnea, and exercise capacity, CAT COPD Assessment Test, DLCO diffusing capacity for carbon monoxide, FEV1 forced expiratory volume in 1-s, FRC functional residual capacity, FVC forced vital capacity, MMRC Modified Medical Research Council Dyspnea Scale, SGRQ St. George’s Respiratory Questionnaire, TLC total lung capacity
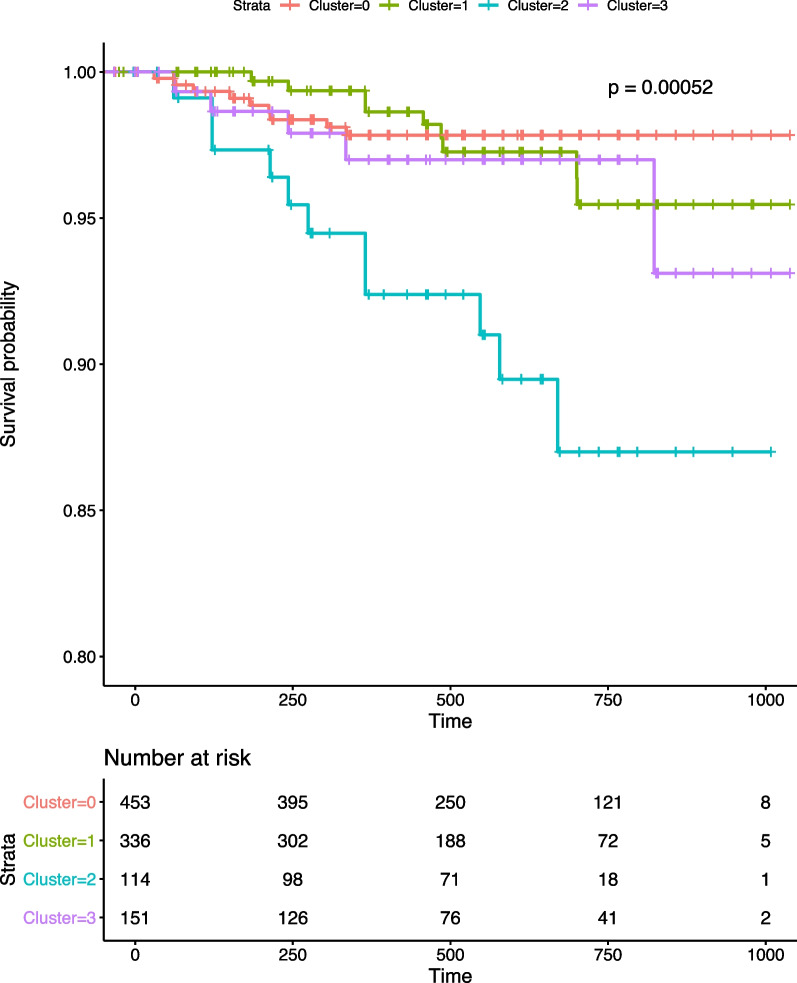
Survival analysis shows significant differences across the four clusters of COPDGene participants (Fig. 2; p-value < 0.001). This significance was driven by cluster 2, which has the worst outcome with the steepest decline in survival probability. Despite a lower FEV1 in cluster 3, survival probabilities in this cluster and cluster 1 began to decline similarly after approximately 2 years following study visit 2 to an intermediate level. Cluster 0 maintained the highest survival probability after this time. The observed survival differences between clusters 0, 1, and 3 are not significant. We also note that clustering based on spirometry characteristics alone (e.g., FEV1 percent predicted) did not produce differences in mortality (Additional file 1: Fig. S9).
Fig. 2.
COPDGene discovery set participant survival varies by cluster. Survival analysis was performed with the Kaplan–Meier method, and the log-rank p-value from the score test is reported. Time measures days elapsed since Phase 2 visit
We are aware that comorbidities may drive mortality either directly or through medications that people take because of them. Although our sample subtyping was based on blood gene expression and any comorbidities or medications that may influence our subtyping had to exert their effect through gene expression, we tested to see if our subtypes differ in terms of comorbidities. We found that none of the comorbidities recorded in COPDGene was significantly different between clusters (Additional file 1: Table S3.)
We further look in the variables that significantly differ between cluster 0 and cluster 1 (the two clusters with the highest FEV1). Both DLCO and the change in 6-min walk distance between the two visits are significantly worse in cluster 1 compared to cluster 0 (p-value = 0.011 and 0.017, respectively). DLCO differences are interesting given that percent emphysema was similar in the two clusters (Additional file 3: Table S2), which may indicate another process such as pulmonary vascular disease. A marker of pulmonary hyperinflation (FRC/TLC ratio) and a fatigue symptom also were found to be significantly different between these clusters (p-value = 0.005 and 0.036, respectively). However, the correlation between FRC/TLC ratio and adjusted DLCO percent predicted in each of these two clusters was quite low (− 0.41 and − 0.53 for cluster-0 and cluster-1, respectively), which is in line with previous studies [27, 28]. One explanation is that DLCO captures more than just emphysema, including pulmonary vascular disease and small airway disease [29].
Subtype validation in independent datasets from COPDGene and MESA
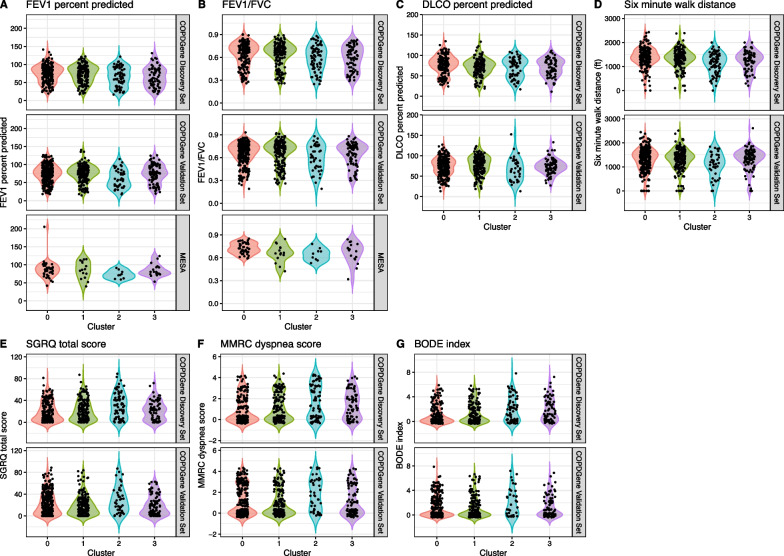
We validated the ssNPA-generated subtypes by scoring new COPD samples from the validation groups using the model we learned from the COPDGene training cohort. We chose this type of validation because it demonstrates the ability to correctly assign new samples to these four subtypes, which is an attractive feature for future clinical application of these subtypes. Out of remaining 614 COPD samples in the COPDGene validation set, 288 were assigned to cluster 0, 193 to cluster 1, 42 to cluster 2, and 91 to cluster 3 (Additional file 1: Fig. S10). We emphasize that this cluster assignment was based solely on gene expression data. Therefore, we next checked to see if the clinical features that differed across clusters in the primary analysis followed the same trends across the clusters of these new samples. In general, we observed the same trends for the significant features when we looked at the discovery set of samples compared to the validation set of samples (Fig. 3), and many pairwise relationships between the cluster means of these features were replicated in the validation set (Additional file 1: Fig. S11). In general, we see higher concordance between the two cohorts when it comes to symptoms (CAT, MMRC Dyspnea and SGRQ Total scores) as well as forced expiratory flow (FEF 25–75).
Fig. 3.
Clinical measures of disease severity and symptoms including (A) FEV1 percent predicted, (B) FEV1/FVC, (C) DLCO percent predicted (D) distance walked in six minutes, (E) SGRQ total score, (F) MMRC dyspnea score, and (G) BODE index, varying by cluster in the COPDGene discovery sample set as well as in the COPDGene and MESA validation sets. Some measures were not present in the MESA cohort. BODE body mass index, airflow obstruction, dyspnea, and exercise capacity, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, MMRC Modified Medical Research Council Dyspnea Scale, SGRQ St. George’s Respiratory Questionnaire
The validation in the MESA cohort produced a similar result. 150 samples were assigned to cluster 0, 64 were assigned to cluster 1, 41 to cluster 2, and 72 to cluster 3 (Additional file 1: Fig. S12). Cluster 2 contained the most severe COPD phenotypes and contained the fewest MESA samples, and, conversely, cluster 0 exhibited the mildest disease and contains the most MESA samples. Again, we observed the same general trends for the clinical features in the MESA validation analysis as we did in the COPDGene discovery analysis (Fig. 3). Fewer of the pairwise comparisons of the means from the cluster of the clinical features were replicated in MESA, likely due to the smaller number of participants for whom these phenotypes were measured (Additional file 1: Fig. S13).
ssNPA identifies a list of candidate genes deregulated in COPD
We further analyzed the identified COPD subtypes to investigate the molecular differences among them, which lead to different clinical phenotypes. We examined the gene deregulation features that had the largest PCA loadings to identify the genes that make the largest contribution to the clustering of the COPDGene discovery dataset (Additional file 1: Table S4). We focused on the top five loadings for each of the first six principal components (PCs) that were used to cluster the subjects and found that many of the genes came up more than once, including DSP and GSTM1, which have been previously associated with COPD. For many of these genes, we observe large differences across clusters in the distributions of deregulation magnitude (Additional file 1: Fig. S14), even between clusters whose subjects have similar lung function.
Discussion
COPD subtyping is essential for not only understanding the diversity of molecular mechanisms of the disease, but also to aid in the development of new intervention strategies. Here we present a new clustering of COPD former smokers based on PBMC gene expression. The focus of this work was restricted to former smokers because we wanted to eliminate biases, since current smoking status has a large impact in gene expression. This clustering was the result of a novel network deregulation-based approach (ssNPA), which has been shown to outperform many standard methods in sample clustering [18]. We identify four COPD subtypes, which exhibit different degrees of symptom presentation, exercise capacity and mortality. Two of the clusters (cluster 0 and cluster 1) have similar (milder) impairment in spirometry, but show differences in DLCO, disease progression and mortality. The other two clusters (cluster 2 and cluster 3) have similar levels of lung function impairment, which is significantly worse than clusters 0 and 1. Compared to cluster 3, subjects in cluster 2 have more symptoms, lower 6-min walk distance, higher neutrophil count and worse survival despite similar reductions in FEV1 percent predicted and FEV1/FVC. Cluster 3 subjects have the most emphysema, although the differences are not significant.
We show that these clusters are stable by validating them using (1) additional COPDGene samples and (2) the MESA study cohort. To demonstrate the utility of our subtyping method for future patient classification, the samples from the two validation cohorts were assigned to one of these four clusters based on their own gene network deregulation vectors (instead of re-clustering these cohorts). We find that the clinical differences of the new sets of samples remained largely the same, which not only validates our findings but also demonstrates the ability of accurately assigning new samples to these four clusters. Unsurprisingly, the distribution of subtypes in MESA is skewed to include more in cluster 0 (mildest disease phenotype), since MESA enrolled subjects representing the general population. By contrast, COPDGene is a case–control study of COPD, so this distribution of MESA samples is consistent with our expectations.
Previous results in COPD subtype identification have proven difficult to replicate. For example, the number of identified subtypes generally varies from 2 to 5, and women and participants with mild disease are generally underrepresented [30]. One study applied a consistent clustering analysis to 10 independent cohorts and found only modest reproducibility across cohorts, but had more success with a continuous PCA-based projection of the individuals [31]. The authors suggest that the disease is best represented as a COPD continuum instead of separate and mutually exclusive subtypes. However, this interpretation does not account for the suspected varied genetic basis of COPD and, without clear cut-off points along the continuum, the practical utility is limited. Another study also applied a network-based clustering approach to blood microarray data and identified four clusters [17]. These clusters differed in spirometry and emphysema, but the network component in that study was coming from existing knowledge (STRING database), which has its own biases and limitations.
Next, we investigate the underlying molecular changes and how they may be implicated in the mechanism of the disease. Several of the genes whose deregulation drive the clustering to subtypes have previously been noted as having a role in COPD. Desmoplakin (DSP, 6p24.3) was identified in a genome-wide association study (GWAS) of COPD as one of 22 genes containing a top coding variant (rs2076295) [32]. DSP is a desmosomal protein that plays an essential role in cell–cell linkages, especially in epidermis and cardiac muscle [33, 34]. DSP variants have also been associated with idiopathic pulmonary fibrosis [35], although these variants may be protective against COPD [32]. This GWAS was included 15,256 COPD cases and 47,936 controls. This locus also colocalized with an expression quantitative trait locus (eQTL) from another lung tissue dataset that included subjects with COPD [36]. In another study, the locus was associated with change two quantitative measures of emphysema, percentage of low-attenuation area less than -950 Hounsfield units (%LAA-950) and adjusted lung density [37]. Recently, the variant was shown to regulate DSP expression in airway epithelial cells, and loss of DSP expression led to increased expression of extracellular matrix-related genes and cell migration [38].
Another gene we identified, GSTM1 (gluthathione S-transferase μ 1, 1p13.3), belongs to a family of enzymes that are relevant for lung disease, likely through their roles in detoxifying electrophilic compounds, such as cigarette smoke and environmental toxins [39]. A homozygous GSTM1-null genotype has been associated with lung cancer pathogenesis [40, 41], emphysema [42, 43], and COPD susceptibility [44, 45]. However, GSTM1 has not been previously identified by COPD GWAS, although the presumed functional variation is a gene deletion and not a single nucleotide polymorphism that would be included in GWAS chips.
Even though cluster 0 and cluster 1 had similar lung function, we identify a number of genes whose deregulation is different between these clusters. For example, the deregulation of CTNNA2 and SLC44A5 is higher in cluster 0 compared to cluster 1, and the deregulation of MRGPRE was lower in cluster 0 compared to cluster 1. Similarly, in cluster 2 and cluster 3 (also similar lung function), the deregulation of several genes, including MUC16, ZMAT4, GSTM1, MRGPRE, and ADAM29, differed between these two clusters. These observations indicate the presence of different underlying molecular mechanisms despite similar lung function.
The list of genes we have identified provides important insights into the molecular mechanism of susceptibility, such as the role of environmental toxin processing, and progression, including pathways involved in extracellular matrix organization. Several of the genes on the list such as Fibroblast Growth Factor 9 (FGF9) have not been specifically cited for an association with COPD, but they code for important signaling proteins and may play a role in lung development or airway remodeling.
As this is study is not meant to investigate the detailed molecular mechanisms of the four subtypes, we mention these genes as a proof-of-principle of our method. Future studies could investigate the role of the molecular mechanisms based on our results.
Conclusions
Using the ssNPA method on blood gene expression data, we identify and validate four clusters of former smokers with COPD, which correspond to clinically relevant disease subtypes, reflecting differences in severity, symptoms and mortality. These differences are not fully reflected by lung function impairment alone. Furthermore, the focus on differential regulation at the gene level provides insight into the disease mechanisms that differentiate COPD cases from the control group of subjects without COPD. We identify a set of genes whose deregulation drives the subtype separation. Several of these genes have previously described connections to COPD, although some new genes emerged as well. The network learning and gene selection were completely unbiased, using no prior knowledge of clinical characteristics, disease mechanism or biology pathways. Finally, we show that ssNPA is a flexible general framework for disease subtyping. As more omics data become available through COPDGene and other studies, future work could incorporate genetic variant, epigenetic, proteomic, or metabolomic variables into the network learning and feature calculations that would provide a multi-layered, more complete picture of the molecular pathology and heterogeneity of COPD.
Supplementary Information
Additional file 1: Figure S1. PCA of COPDGene primary analysis RNA-seq data colored according to batch (A) before and (B) after batch correction. Batch detection with guided principal component analysis showed strong batch effects before batch correction (p < 0.001) that were removed after batch correction (p = 0.538). Figure S2. PCA of COPDGene validation RNA-seq dataset colored according to batch (A) before and (B) after batch correction. Batch detection with guided principal component analysis showed strong batch effects before batch correction (p = 0.001) that were removed after batch correction (p = 0.937). Figure S3. PCA of MESA validation RNA-seq dataset colored according to batch (A) before and (B) after batch correction. Batch detection with guided principal component analysis showed strong batch effects before batch correction (p = 0.003) that were removed after batch correction (p = 1). Figure S4. Clustering tree illustrates the stability of clusters over a range of values for clustering resolution (res). We chose 4 as optimal number of clusters, because cluster number and content (samples) remains constant for res=0.6 to 0.9. When res>0.9 produced some subclusters of these four, but samples did not move across the four branches extending from these clusters. Figure S5. PC elbow plot of the COPDGene discovery set ssNPA features. We heuristically chose 6 principal components for clustering because they captured a large percentage of the variance in the data. Figure S6. Clustering tree illustrates the stability of clusters over a range of values for k in the kNN classification for the COPDGene validation analysis. We chose k=3 because the clusters were stable by this value. Figure S7. Clustering tree illustrates the stability of clusters over a range of values for k in the kNN classification for the MESA validation analysis. We chose k=3 because the clusters were stable by this value. Figure S8. Participant GOLD stage composition according to cluster. The reference group was composed of only GOLD 0 participants by design. Figure S9. Clustering based on FEV1 percent predicted does not sufficiently separate COPD individuals with different mortalities. Figure S10. COPDGene validation samples were projected into the same PCA space as the discovery analysis and assigned to clusters with kNN. Density clouds show the distribution of samples in each cluster from the discovery analysis. Individual points represent validation COPDGene samples and are colored according to the cluster to which they were assigned. Figure S11. Heatmaps display the p-value bins for the inter-cohort pairwise comparisons of cluster means by Wilcoxon test for: physiology (A) FEV1 percent predicted, (B) FEV1/FVC, (C) DLCO, (D) FRC/DLC ratio, (E) distance walked in 6 minutes; symptoms (F) SGRQ total score, (G) MMRC dyspnea score, and (H) CAT score. The upper right triangle shows the pairwise comparisons between cluster in the COPDGene discovery set, and the lower left triangle shows the comparisons between clusters in the COPDGene validation set. Blue (red) arrows indicate concordance in the significance (non-significance) of the comparisons between the two cohorts. Figure S12. MESA validation samples were projected into the same PCA space as the discovery analysis and assigned to clusters with kNN. Density clouds show the distribution of samples in each cluster from the discovery analysis. Individual points represent validation MESA samples and are colored according to the cluster to which they were assigned. Figure S13. Heatmaps display the p-value bins for the pairwise comparisons of cluster means by Wilcoxon test for (A) FEV1 percent predicted, (B) FEV1/FVC, (C) FEF 25-75%, and (D) percent emphysema. The upper right triangle shows the pairwise comparisons between cluster in the COPDGene discovery set, and the lower left triangle shows the comparisons between clusters in the COPDGene validation set. Blue (red) arrows indicate concordance in the significance (non-significance) of the comparisons between the two cohorts. Figure S14. ssNPA feature values show a difference in the degree of deregulation of (A) MUC16, (B) ZMAT4, (C), GSTM1, (D) CTNNA2, (E) MRGPRE, (F) SLC44A5, (G) ADARB2, and (H) ADAM29 across clusters. Wilcoxon test p-values highlight where there are differences in the distributions between clusters 0 and 1 and between clusters 2 and 3. Table S3. Analysis of various COPDGene comorbidities did not show any significant difference between the four identified subtypes. All comorbidities recorded at the time blood samples were collected. p-val: chi-square p-value. Table S4. The genes with the top 5 loadings for each of the first 6 PCs used for clustering the COPD samples in the training COPDGene dataset. Genes are sorted by decreasing contribution to the clustering (sum of the absolute values of the loadings across the first 6 PCs). Loading value is not provided if gene did not rank among the top 5 loadings for a given PC. The sample clustering is driven by differences in the regulation of these genes.
Additional file 2: Table S1. Excel file containing this table is attached. Clinical characteristics of COPD participants vary across clusters. The variables are sorted by descending significance. P-values were calculated with a Kruskal-Wallis test for continuous and ordinal variables and or a Chi-squared test for discrete and binary variables and asses if there are differences in variable distribution among clusters. Variable means (standard deviations) are also reported for all COPD participants overall, each COPD cluster, and all control subjects for comparison.
Additional file 3: Table S2. Excel file containing this table is attached. Differences in clinical characteristics between clusters 0 and 1. The variables are sorted by descending significance. P-values were calculated with a Wilcoxon rank sum test for continuous and ordinal variables and or a Chi-squared test for discrete and binary variables and asses if there are differences in variable distribution between these clusters.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the COPDGene Study and the MESA Study for their valuable contributions. A full list of participating COPDGene investigators and institutions can be found at http://www.copdgene.org. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Abbreviations
- ADAM29
ADAM metallopeptidase domain 29
- BD
Bronchodilator
- BODE
Body mass index, airflow obstruction, dyspnea, and exercise capacity
- CAT
COPD assessment test
- COPD
Chronic obstructive pulmonary disease
- COPDGene
Genetic epidemiology of COPD study
- CT
Computed tomography
- CTNNA2
Catenin Alpha 2
- DLCO
Diffusing capacity for carbon monoxide
- DSP
Desmoplakin
- eQTL
Expression quantitative trait locus
- FEF 25–75
Forced expiratory flow over the middle one half of FVC
- FEV1
Forced expiratory volume in 1 s
- FGF9
Fibroblast growth factor 9
- FRC
Functional residual capacity
- FVC
Forced vital capacity
- GOLD
Global initiative for chronic obstructive lung disease
- GSTM1
Gluthathione S-transferase μ 1
- GWAS
Genome-wide association study
- HU
Hounsfield units
- LAA-950
Low-attenuation area less than -950 Hounsfield units
- MESA
Multi-ethnic study of atherosclerosis
- MMRC
Modified Medical Research Council Dyspnea Scale
- MRGPRE
MAS related GPR family member E
- MUC16
Mucin 16, cell surface associated
- PBMC
Peripheral blood mononuclear cell
- PCA
Principal component analysis
- Perc15
The 15th percentile
- PRISm
Preserved ratio impaired spirometry
- SGRQ
St. George’s Respiratory Questionnaire
- SLC44A5
Solute Carrier Family 44 Member 5
- ssNPA
Single sample Network Perturbation Assessment
- TLC
Total lung capacity
- ZMAT4
Zinc Finger Matrin-Type 4
Author contributions
Study Design: KLB, CR, AS, FS, CPH, PVB. Acquisition, analysis, or interpretation of the data: KLB, CR, AS, PC, GZ, FA, KGA, PD, WCJ, SK, YL, AM, SSR, JIR, JS, KDT, RPT, TL, RGB, FS, CPH, PVB. Critical revision of the manuscript for important intellectual content: KLB, CR, AS, PC, GZ, FA, KGA, PD, WCJ, SK, YL, AM, SSR, JIR, JS, KDT, RPT, TL, RGB, FS, CPH, PVB. Statistical analysis: KLB, CPH, PVB. Obtained funding: YL, SSR, JIR, KDT, RPT, TL, RGB, FS, CPH, PVB. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the National Institutes of Health to PVB (R01HL159805, R01HL157879, U01HL137159, R01HL140963), CPH (R01HL125583, R01HL130512), RGB (R01HL121270), TL (R01HL142028). KLB was supported by T32HL144442. COPDGene data collection was supported by Award Number U01HL089897 and Award Number U01HL089856 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. COPDGene is also supported by the COPD Foundation through contributions made to an Industry Advisory Board comprised of AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, Siemens, and Sunovion. The Trans-Omics for Precision Medicine (TOPMed) program was supported by the National Heart, Lung, and Blood Institute (NHLBI). Whole genome sequencing (WGS) for “NHLBI TOPMed: Multi-Ethnic Study of Atherosclerosis (MESA)” (phs001416.v1.p1) was performed at the Broad Institute of MIT and Harvard (3U54HG003067-13S1); RNA-seq was conducted by the Broad Institute of MIT and Harvard (3R01HL092577-06S1) and the Northwest Genomics Center at the University of Washington (3R01HL098433-05S1). Centralized read mapping and genotype calling, along with variant quality metrics and filtering, were provided by the TOPMed Informatics Research Center (3R01HL117626-02S1, contract HHSN268201800002I) (Broad RNA Seq, Proteomics HHSN268201600034I, UW RNA Seq HHSN268201600032I). Phenotype harmonization, data management, sample-identity quality control, and general study coordination, were provided by the TOPMed Data Coordinating Center (3R01HL120393; contract HHSN268180001I). The MESA project is supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with MESA investigators. Support for MESA is provided by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420. Also supported in part by the National Center for Advancing Translational Sciences, CTSI grant UL1TR001881, and the National Institute of Diabetes and Digestive and Kidney Disease Diabetes Research Center (DRC) grant DK063491 to the Southern California Diabetes Endocrinology Research Center”.
Declarations
Ethics approval and consent to participate
All COPDGene and MESA study participants provided written informed consent. Each study center obtained institutional review board (IRB) approval.
Consent for publication
Not applicable.
Competing interests
Peter Castaldi reports receiving grant support from GSK and Bayer and personal fees from GSK and Novartis. Tuuli Lappalainen is an advisor to Goldfinch Bio, Variant Bio, and GSK, and has equity in Variant Bio. Craig Hersh reports grant support from Bayer, Boehringer-Ingelheim, Novartis, and Vertex, and personal fees from Astra-Zeneca and Takeda.
Availability of data and materials
Information about how to access the human cohort data can be found at http://www.copdgene.org and http://www.mesa-nhlbi.org.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Craig P. Hersh and Panayiotis V. Benos: Co-senior authors
References
- 1.Burrows B, Niden A, Fletcher CM, et al. Clinical types of chronic obstructive lung disease in London and in Chicago: a study of one hundred patients. Am Rev Respir Dis. 1964;90(1):14–27. doi: 10.1164/arrd.1964.90.1.14. [DOI] [PubMed] [Google Scholar]
- 2.Burrows B, Fletcher C, Heard B, et al. The emphysematous and bronchial types of chronic airways obstruction: a clinicopathological study of patients in London and Chicago. The Lancet. 1966;287(7442):830–835. doi: 10.1016/S0140-6736(66)90181-4. [DOI] [PubMed] [Google Scholar]
- 3.Lowe KE, Regan EA, Anzueto A, et al. COPDGene((R)) 2019: redefining the diagnosis of chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis. 2019;6(5):384–399. doi: 10.15326/jcopdf.6.5.2019.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 5.Casanova C, de Torres JP, Aguirre-Jaíme A, et al. The progression of chronic obstructive pulmonary disease is heterogeneous: the experience of the BODE cohort. Am J Respir Crit Care Med. 2011;184(9):1015–1021. doi: 10.1164/rccm.201105-0831OC. [DOI] [PubMed] [Google Scholar]
- 6.Renkema TE, Schouten JP, Koëter GH, et al. Effects of long-term treatment with corticosteroids in COPD. Chest. 1996;109(5):1156–1162. doi: 10.1378/chest.109.5.1156. [DOI] [PubMed] [Google Scholar]
- 7.Antus B, Barta I, Horvath I, et al. Relationship between exhaled nitric oxide and treatment response in COPD patients with exacerbations. Respirology. 2010;15(3):472–477. doi: 10.1111/j.1440-1843.2010.01711.x. [DOI] [PubMed] [Google Scholar]
- 8.Cushen B, Greene G, Mac Hale E, et al. Clinical and exacerbation characteristics may predict treatment response in acute exacerbations of COPD. Eur Respir Soc. 2018.
- 9.Johnson KM, Safari A, Tan WC, et al. Heterogeneity in the respiratory symptoms of patients with mild-to-moderate COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3983. doi: 10.2147/COPD.S184424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes PJ, Shapiro S, Pauwels R. Chronic obstructive pulmonary disease: molecular and cellularmechanisms. Eur Respir J. 2003;22(4):672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 11.O’donnell DE, Neder JA, Elbehairy AF. Physiological impairment in mild COPD. Respirology. 2016;21(2):211–223. doi: 10.1111/resp.12619. [DOI] [PubMed] [Google Scholar]
- 12.Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castaldi PJ, Boueiz A, Yun J, et al. Machine learning characterization of COPD subtypes: insights from the COPDGene study. Chest. 2020;157(5):1147–1157. doi: 10.1016/j.chest.2019.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rennard SI, Vestbo J. The many “small COPDs”: COPD should be an orphan disease. Chest. 2008;134(3):623–627. doi: 10.1378/chest.07-3059. [DOI] [PubMed] [Google Scholar]
- 15.Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castaldi PJ, Dy J, Ross J, et al. Cluster analysis in the COPDGene study identifies subtypes of smokers with distinct patterns of airway disease and emphysema. Thorax. 2014;69(5):416–423. doi: 10.1136/thoraxjnl-2013-203601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang Y, Glass K, Liu Y-Y, et al. COPD subtypes identified by network-based clustering of blood gene expression. Genomics. 2016;107(2–3):51–58. doi: 10.1016/j.ygeno.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buschur KL, Chikina M, Benos PV. Causal network perturbations for instance-specific analysis of single cell and disease samples. Bioinformatics. 2019 doi: 10.1093/bioinformatics/btz949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sedgewick AJ, Buschur K, Shi I, et al. Mixed graphical models for integrative causal analysis with application to chronic lung disease diagnosis and prognosis. Bioinformatics. 2019;35(7):1204–1212. doi: 10.1093/bioinformatics/bty769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sedgewick AJ, Shi I, Donovan RM, et al. Learning mixed graphical models with separate sparsity parameters and stability-based model selection. BMC Bioinformatics. 2016;17(Suppl 5):175. doi: 10.1186/s12859-016-1039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manatakis DV, Raghu VK, Benos PV. piMGM: incorporating multi-source priors in mixed graphical models for learning disease networks. Bioinformatics. 2018;34(17):i848–i856. doi: 10.1093/bioinformatics/bty591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raghu VK, Ramsey JD, Morris A, et al. Comparison of strategies for scalable causal discovery of latent variable models from mixed data. Int J Data Sci Anal. 2018;6(1):33–45. doi: 10.1007/s41060-018-0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drier Y, Sheffer M, Domany E. Pathway-based personalized analysis of cancer. Proc Natl Acad Sci USA. 2013;110(16):6388–6393. doi: 10.1073/pnas.1219651110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbie DA, Tamayo P, Boehm JS, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462(7269):108–112. doi: 10.1038/nature08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker MM, Chase RP, Lamb A, et al. RNA sequencing identifies novel non-coding RNA and exon-specific effects associated with cigarette smoking. BMC Med Genomics. 2017;10(1):58. doi: 10.1186/s12920-017-0295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 27.Nambu A, Zach J, Schroeder J, et al. Relationships between diffusing capacity for carbon monoxide (DLCO), and quantitative computed tomography measurements and visual assessment for chronic obstructive pulmonary disease. Eur J Radiol. 2015;84(5):980–985. doi: 10.1016/j.ejrad.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Balasubramanian A, MacIntyre NR, Henderson RJ, et al. Diffusing capacity of carbon monoxide in assessment of COPD. Chest. 2019;156(6):1111–1119. doi: 10.1016/j.chest.2019.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Criner RN, Hatt CR, Galban CJ, et al. Relationship between diffusion capacity and small airway abnormality in COPDGene. Respir Res. 2019;20(1):269. doi: 10.1186/s12931-019-1237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinto LM, Alghamdi M, Benedetti A, et al. Derivation and validation of clinical phenotypes for COPD: a systematic review. Respir Res. 2015;16(1):50. doi: 10.1186/s12931-015-0208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castaldi PJ, Benet M, Petersen H, et al. Do COPD subtypes really exist? COPD heterogeneity and clustering in 10 independent cohorts. Thorax. 2017;72(11):998–1006. doi: 10.1136/thoraxjnl-2016-209846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hobbs BD, de Jong K, Lamontagne M, et al. Genetic loci associated with chronic obstructive pulmonary disease overlap with loci for lung function and pulmonary fibrosis. Nat Genet. 2017;49(3):426–432. doi: 10.1038/ng.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vasioukhin V, Bowers E, Bauer C, et al. Desmoplakin is essential in epidermal sheet formation. Nat Cell Biol. 2001;3(12):1076. doi: 10.1038/ncb1201-1076. [DOI] [PubMed] [Google Scholar]
- 34.Norman M, Simpson M, Mogensen J, et al. Novel mutation in desmoplakin causes arrhythmogenic left ventricular cardiomyopathy. Circulation. 2005;112(5):636–642. doi: 10.1161/CIRCULATIONAHA.104.532234. [DOI] [PubMed] [Google Scholar]
- 35.Mathai SK, Pedersen BS, Smith K, et al. Desmoplakin variants are associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;193(10):1151–1160. doi: 10.1164/rccm.201509-1863OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hao K, Bosse Y, Nickle DC, et al. Lung eQTLs to help reveal the molecular underpinnings of asthma. PLoS Genet. 2012;8(11):e1003029. doi: 10.1371/journal.pgen.1003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim W, Cho MH, Sakornsakolpat P, et al. DSP variants may be associated with longitudinal change in quantitative emphysema. Respir Res. 2019;20(1):160. doi: 10.1186/s12931-019-1097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hao Y, Bates S, Mou H, et al. Genome-wide association study: functional variant rs2076295 regulates desmoplakin expression in airway epithelial cells. Am J Respir Crit Care Med. 2020;202(9):1225–1236. doi: 10.1164/rccm.201910-1958OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strange RC, Spiteri MA, Ramachandran S, et al. Glutathione-S-transferase family of enzymes. Mutation Res/Fundamental Mol Mech Mutagen. 2001;482(1–2):21–26. doi: 10.1016/S0027-5107(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 40.Seidegard J, Pero RW, Miller DG, et al. A glutathione transferase in human leukocytes as a marker for the susceptibility to lung cancer. Carcinogenesis. 1986;7(5):751–753. doi: 10.1093/carcin/7.5.751. [DOI] [PubMed] [Google Scholar]
- 41.Hirvonen A, Husgafvel-Pursiainen K, Anttila S, et al. The GSTM1 null genotype as a potential risk modifier for squamous cell carcinoma of the lung. Carcinogenesis. 1993;14(7):1479–1481. doi: 10.1093/carcin/14.7.1479. [DOI] [PubMed] [Google Scholar]
- 42.Harrison D, Cantlay A, Rae F, et al. Frequency of glutathione S-transferase M1 deletion in smokers with emphysema and lung cancer. Hum Exp Toxicol. 1997;16(7):356–360. doi: 10.1177/096032719701600703. [DOI] [PubMed] [Google Scholar]
- 43.Lakhdar R, Denden S, Knani J, et al. Association of GSTM1 and GSTT1 polymorphisms with chronic obstructive pulmonary disease in a Tunisian population. Biochem Genet. 2010;48(7–8):647–657. doi: 10.1007/s10528-010-9346-z. [DOI] [PubMed] [Google Scholar]
- 44.Cheng SL, Yu CJ, Chen CJ, et al. Genetic polymorphism of epoxide hydrolase and glutathione S-transferase in COPD. Eur Respir J. 2004;23(6):818–824. doi: 10.1183/09031936.04.00104904. [DOI] [PubMed] [Google Scholar]
- 45.Young RP, Hopkins RJ, Hay BA, et al. GSTM1 null genotype in COPD and lung cancer: evidence of a modifier or confounding effect? Appl Clin Genet. 2011;4:137. doi: 10.2147/TACG.S21517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. PCA of COPDGene primary analysis RNA-seq data colored according to batch (A) before and (B) after batch correction. Batch detection with guided principal component analysis showed strong batch effects before batch correction (p < 0.001) that were removed after batch correction (p = 0.538). Figure S2. PCA of COPDGene validation RNA-seq dataset colored according to batch (A) before and (B) after batch correction. Batch detection with guided principal component analysis showed strong batch effects before batch correction (p = 0.001) that were removed after batch correction (p = 0.937). Figure S3. PCA of MESA validation RNA-seq dataset colored according to batch (A) before and (B) after batch correction. Batch detection with guided principal component analysis showed strong batch effects before batch correction (p = 0.003) that were removed after batch correction (p = 1). Figure S4. Clustering tree illustrates the stability of clusters over a range of values for clustering resolution (res). We chose 4 as optimal number of clusters, because cluster number and content (samples) remains constant for res=0.6 to 0.9. When res>0.9 produced some subclusters of these four, but samples did not move across the four branches extending from these clusters. Figure S5. PC elbow plot of the COPDGene discovery set ssNPA features. We heuristically chose 6 principal components for clustering because they captured a large percentage of the variance in the data. Figure S6. Clustering tree illustrates the stability of clusters over a range of values for k in the kNN classification for the COPDGene validation analysis. We chose k=3 because the clusters were stable by this value. Figure S7. Clustering tree illustrates the stability of clusters over a range of values for k in the kNN classification for the MESA validation analysis. We chose k=3 because the clusters were stable by this value. Figure S8. Participant GOLD stage composition according to cluster. The reference group was composed of only GOLD 0 participants by design. Figure S9. Clustering based on FEV1 percent predicted does not sufficiently separate COPD individuals with different mortalities. Figure S10. COPDGene validation samples were projected into the same PCA space as the discovery analysis and assigned to clusters with kNN. Density clouds show the distribution of samples in each cluster from the discovery analysis. Individual points represent validation COPDGene samples and are colored according to the cluster to which they were assigned. Figure S11. Heatmaps display the p-value bins for the inter-cohort pairwise comparisons of cluster means by Wilcoxon test for: physiology (A) FEV1 percent predicted, (B) FEV1/FVC, (C) DLCO, (D) FRC/DLC ratio, (E) distance walked in 6 minutes; symptoms (F) SGRQ total score, (G) MMRC dyspnea score, and (H) CAT score. The upper right triangle shows the pairwise comparisons between cluster in the COPDGene discovery set, and the lower left triangle shows the comparisons between clusters in the COPDGene validation set. Blue (red) arrows indicate concordance in the significance (non-significance) of the comparisons between the two cohorts. Figure S12. MESA validation samples were projected into the same PCA space as the discovery analysis and assigned to clusters with kNN. Density clouds show the distribution of samples in each cluster from the discovery analysis. Individual points represent validation MESA samples and are colored according to the cluster to which they were assigned. Figure S13. Heatmaps display the p-value bins for the pairwise comparisons of cluster means by Wilcoxon test for (A) FEV1 percent predicted, (B) FEV1/FVC, (C) FEF 25-75%, and (D) percent emphysema. The upper right triangle shows the pairwise comparisons between cluster in the COPDGene discovery set, and the lower left triangle shows the comparisons between clusters in the COPDGene validation set. Blue (red) arrows indicate concordance in the significance (non-significance) of the comparisons between the two cohorts. Figure S14. ssNPA feature values show a difference in the degree of deregulation of (A) MUC16, (B) ZMAT4, (C), GSTM1, (D) CTNNA2, (E) MRGPRE, (F) SLC44A5, (G) ADARB2, and (H) ADAM29 across clusters. Wilcoxon test p-values highlight where there are differences in the distributions between clusters 0 and 1 and between clusters 2 and 3. Table S3. Analysis of various COPDGene comorbidities did not show any significant difference between the four identified subtypes. All comorbidities recorded at the time blood samples were collected. p-val: chi-square p-value. Table S4. The genes with the top 5 loadings for each of the first 6 PCs used for clustering the COPD samples in the training COPDGene dataset. Genes are sorted by decreasing contribution to the clustering (sum of the absolute values of the loadings across the first 6 PCs). Loading value is not provided if gene did not rank among the top 5 loadings for a given PC. The sample clustering is driven by differences in the regulation of these genes.
Additional file 2: Table S1. Excel file containing this table is attached. Clinical characteristics of COPD participants vary across clusters. The variables are sorted by descending significance. P-values were calculated with a Kruskal-Wallis test for continuous and ordinal variables and or a Chi-squared test for discrete and binary variables and asses if there are differences in variable distribution among clusters. Variable means (standard deviations) are also reported for all COPD participants overall, each COPD cluster, and all control subjects for comparison.
Additional file 3: Table S2. Excel file containing this table is attached. Differences in clinical characteristics between clusters 0 and 1. The variables are sorted by descending significance. P-values were calculated with a Wilcoxon rank sum test for continuous and ordinal variables and or a Chi-squared test for discrete and binary variables and asses if there are differences in variable distribution between these clusters.
Data Availability Statement
Information about how to access the human cohort data can be found at http://www.copdgene.org and http://www.mesa-nhlbi.org.