Abstract
Purpose
This study aimed to investigate the relationship between cognitive impairment and homocysteine (Hcy) and S100B protein in patients with progressive ischemic stroke (PIS).
Patients and Methods
A total of 158 patients with PIS hospitalized in the Department of Neurology in Taixing People’s Hospital from January 2020 to March 2022 were enrolled in the study. After 90 days of follow-up, the patients were divided into two groups according to the MoCA score—99 cases with cognitive impairment group (observation group) and 59 cases with cognitive normal group (control group). Causal diagram was drawn to assess the association between risk factors and PIS with observation group. The risk factors indicators of cognitive impairment in patients with PIS were screened. The related predictive indicators were screened by multivariate logistic regression analysis, and Pearson correlation analysis. The predictive value was analyzed by Receiver Operating Characteristic (ROC) curve.
Results
Multivariate logistic regression analysis showed that age, hypertension, lesion position, Hcy and S100B protein were related risk factors for cognitive impairment in patients with PIS. Pearson correlation analysis was conducted between Hcy and S100 B protein and MoCA score, and revealed that Hcy and S100 B protein were negatively correlated with MoCA score. ROC curve analysis showed that the Area Under the Curve (AUC) of S100 B protein and Hcy in identifying cognitive impairment after PIS was 0.709 and 0.673, respectively, and the combined AUC of Hcy and S100B protein in predicting cognitive impairment after PIS was 0.739.
Conclusion
Hcy and S100B protein are related risk factors for cognitive impairment in patients with PIS, and may be used as in a prediction model to predict cognitive impairment after PIS in the future.
Keywords: progressive ischemic stroke, cognitive impairment, homocysteine, S100B protein
Introduction
The incidence of acute ischemic stroke (AIS) is increasing globally. AIS has a high disability rate and high fatality rate and poses a serious threat to people’s health and life, as well as placing a burden on families and society.1 Progression of stroke after the occurrence of AIS is not uncommon and has become a popular research topic in recent years.2 Progression of stroke is also a predictor of poor clinical prognosis in patients within the first 3 months after AIS. There are direct and clear causes such as symptomatic intracranial hemorrhage and malignant edema but there is no clear cause in most cases. Underlying mechanisms may be systemic factors of the nervous system such as infection, electrolyte disturbances, myocardial ischemia and venous thromboembolism, and systemic atherosclerosis. Neurological deterioration after AIS is often referred to as “early neurological deterioration (END)”,3 and END with an unexplained cause is known as progressive ischemic stroke (PIS). Specifically, PIS refers to AIS with increasing progression of neurological impairment after treatment within 72 hours of onset.4 PIS causes an inflammatory response in the central nervous system (CNS), leading to activation of glial cells and an immune cascade. Excessive inflammation in the CNS stimulates the release of pro-inflammatory cytokines such as interleukin, and induces apoptosis, leading to synaptic connection impairment, neurotoxicity, and cognitive impairment.5 Numerous studies have confirmed that PIS is closely associated with cognitive decline and dementia,6,7 and as these conditions are the most serious neuropsychiatric disorders among the elderly, there will be a huge increase in costs and demands for caregivers and family members in the coming years.8
Homocysteine (Hcy) is a reactive vascular injury amino acid, and an elevated concentration of Hcy is closely related to stroke and cognitive impairment.9–11 S100B protein is a calcium-binding protein that predominantly exists in astrocytes and Schwann cells.12,13 Damage to the CNS activates glial cells, and S100B protein can be released into the bloodstream when the blood-brain barrier (BBB) is damaged.14 An increased serum concentration of S100B protein may reflect glial cell injury or reactive astrocyte proliferation, suggesting that S100B protein has potential as a serum biochemical marker of CNS injury. Neutralization of S100B protein was recently reported to affect both long-term cognitive impairment and neuroinflammation in animal models of sepsis, similar to the long-term cognitive impairment observed in sepsis survivors, and experiments confirmed that anti-S100B protein treatment has a positive effect on cognitive function.15 S100B protein is known to play an important role in cognition. Meanwhile, Hcy and S100B may play a synergistic role in the PIS with cognitive impairment patients.16 Increased Hcy can accelerate the damage of endothelial cells and the destruction of BBB, promote the release of S100B into blood, and aggravate cognitive impairment. Combining Hcy and S100B may be a new direction to reveal the cognitive impairment after the PIS. In this study, the risk factors for cognitive impairment of PIS were investigated with the aim of providing a clinical reference for the cognitive decline of patients after PIS and conducting early identification, prevention, and active intervention.
Materials and Methods
Patients
A total of 158 patients with PIS hospitalized in the Department of Neurology in Taixing People’s Hospital from January 2020 to March 2022 were enrolled in the study. Inclusion criteria: 1) first-episode patients who met the diagnostic criteria of guidelines for early management of AIS 2019,17 2) patients confirmed by cranial magnetic resonance imaging (MRI) including diffusion weighted imaging (DWI); 3) National Institute of Health Stroke Scale (NIHSS) score increased by ≥4 points (out of 42) (ΔNIHSS≥4) within 72 hours of admission; 4) agree to participate in this study. Exclusion criteria: 1) complications including severe cardiovascular and cerebrovascular diseases, liver and kidney diseases, hematopoietic system, inflammation, tumor, and other serious primary diseases; 2) recent use of drugs that affect blood Hcy and S100B protein levels (folic acid, mecobalamin, Vitamin B6, levodopa, etc) or alcohol dependence; 3) cognitive impairment with other known clear cause, such as Alzheimer’s disease, Parkinson’s disease, depression, anxiety, and other mental disorders; 4) patients with previous history of ischemic stroke and receiving intravenous thrombolysis and arterial thrombectomy; 5) do not agree to participate in the study. A written signed consent was obtained from all the participants. The study was approved by the Ethics Committee of Taixing People’s Hospital and conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Cognitive Function Assessment
Patients were followed up 90 days after stroke and were clinically evaluated by two neurologists with expertise in neuropsychiatry using the Montreal Cognitive Assessment Scale (MoCA) score. The MoCA score was <26 for cognitive impairment; the score of those with schooling ≤12 years was increased by 1 point to correct the bias of schooling years. All patients did not have aphasia, dysarthria or other symptoms that affected the scale assessment; no obvious visual and hearing impairment; and were able to complete the relevant neuropsychological examination. Patients were divided into two groups according to the MoCA score—the PIS with cognitive impairment group (observation group) and the PIS with cognitive normal group (control group).
General Data Collection
General data of both groups were collected, including age, gender, education, smoking, drinking, hypertension, coronary heart disease, diabetes, lesion position, body mass index (BMI), MoCA score, NIHSS score, etc.
Blood Collection
A 3 mL fasting peripheral venous blood sample was collected from all selected patients within 24 h after admission, and all samples were centrifuged for 10 min at 3000 rpm within 6 h of collection. Blood test indicators included Hcy and S100B protein, fasting blood glucose (FPG), glycosylated hemoglobin (HbA1c), and blood lipids such as high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides (TG), total cholesterol (TC), etc. The upper layer of serum was separated and stored at −20°C. The contents of S100B protein and Hcy were determined by enzyme-linked immunosorbent assay (ELISA) (Shanghai Hengyuan Biotechnology Co., Ltd).
Statistical Analysis
All statistical analysis was performed by SPSS 23.0 software (IBM) and GraphPad Prism 5.01 software (GraphPad Software Inc). The measurement data conforming to normal distribution were expressed as (x±s), and the comparison between the two groups was performed by t-test. X2 test was used for comparison between groups, and geometric mean and 95% CI were reported. Multivariate logistic regression analysis was performed for the factors with influencing cognitive impairment in causal diagram analysis. Pearson partial correlation was used to analyze the relationship between each index and cognitive score. Hcy and S100B protein were used for diagnostic analysis of the cognitive impairment group and the cognitive normal group, and a receiver operating characteristic (ROC) curve of the subjects was plotted. With α = 0.05 as the test level, P < 0.05 was considered statistically significant.
Results
Demographic Characteristics of Study Population
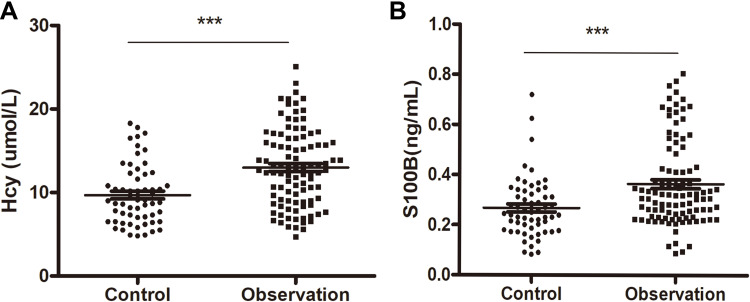
There were a total of 158 patients with PIS included in the study: 99 cases with cognitive impairment group (observation group) and 59 cases with cognitive normal group (control group) according to the NIHSS score increased by ≥4 points (out of 42) (ΔNIHSS≥4) within 72 hours of admission. Table 1 summarizes the demographic characteristics of two groups. Compared with the control group, the observation group was mainly male, older, more hypertensive patients, mainly cerebral infarction, and had lower education level and MoCA score. Meanwhile, the observation group had a significantly higher content of Hcy and S100B protein compared with the control group (Figure 1).
Table 1.
Demographic Characteristics of Study Population, n = 158
| Characteristics | Cognitive Normal(n=59) (Mean ± SD)/(n,%) 95% CI | Cognitive Impairment(n=99) (Mean ± SD)/(n,%) 95% CI | ||
|---|---|---|---|---|
| Age (years) | 70.63±5.51 | 69.05–71.83 | 73.29±6.59 | 71.98–74.61 |
| Male (%) | 33 (55.93%) | 42.9–69 | 52 (52.53%) | |
| Education (years) | 8.95±2.94 | 8.14–9.62 | 7.49±2.84 | 6.93–8.06 |
| Smoking (%) | 18 (30.51%) | 18.4–42.6 | 29 (29.29%) | 20.2–38.4 |
| Drinking (%) | 31 (52.54%) | 39.4--65.7 | 51 (51.52%) | 41.5–61.5 |
| Medical histories | ||||
| Hypertension (%) | 25 (42.37%) | 29.4–55.4 | 67 (67.68%) | 58.3–77.1 |
| Coronary heart disease (%) | 6 (10.17%) | 2.2–18.1 | 12 (12.12%) | 5.6–18.7 |
| Diabetes (%) | 19 (32.20%) | 33 (33.33%) | 23.9–42.8 | |
| Lesion position | ||||
| (Cerebral hemisphere, %) | 25 (42.37%) | 29.4–55.4 | 65 (65.66%) | 56.1–75.2 |
| BMI (kg/m2) | 24.34±3.10 | 23.53–25.15 | 24.10±2.79 | 23.54–24.66 |
| MoCA score | 28.36±1.11 | 28.07–28.65 | 14.76±5.26 | 13.71–15.81 |
| NIHSS score | 6.42±1.82 | 5.95–6.9 | 7.05±2.69 | 6.51–7.59 |
| FPG (mmol/L) | 6.40±1.50 | 6.01–6.79 | 6.71±2.04 | 6.30–7.12 |
| HbA1c (%) | 6.09±1.62 | 5.67–6.52 | 6.39±1.88 | 6.01–6.76 |
| HDL (mmol/L) | 1.21±0.48 | 1.09–1.34 | 1.10±0.38 | 1.02–1.18 |
| LDL (mmol/L) | 2.75±1.26 | 2.42–3.07 | 3.06±1.67 | 2.73–3.4 |
| TG (mmol/L) | 1.77±1.03 | 1.50–2.04 | 1.95±1.22 | 1.71–2.2 |
| TC (mmol/L) | 4.24±0.97 | 3.99–4.49 | 4.45±0.75 | 4.3–4.6 |
| Hcy | 9.7±3.58 | 8.77–10.63 | 12.99±4.65 | 12.07–13.92 |
| S100B | 0.27±0.12 | 0.24–0.3 | 0.36±1.72 | 0.33–0.4 |
Abbreviations: BMI, body mass index; MoCA, Montreal Cognitive Assessment Scale; NIHSS, National Institute of Health Stroke Scale; FPG, fasting blood glucose; HbA1c, glycosylated hemoglobin; HDL, high density lipoprotein; LDL, low density lipoprotein; TG, triglycerides; TC, total cholesterol.
Figure 1.
Serum concentration of Hcy (A) and S100B protein (B) in patients with PIS from the observation (cognitive impaired) group and control (cognitive normal) group. ***P < 0.05.
Risk Factor Analysis of the Two Groups
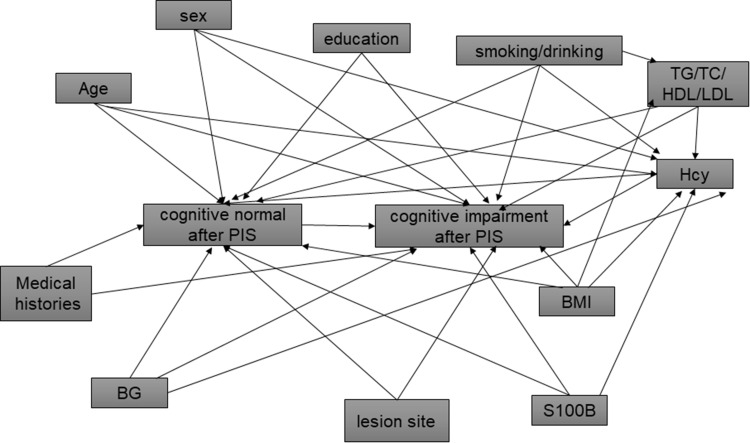
Confounding variables were identified based on a priori causal assumptions of their associations with Hcy, S100B and cognitive impairment and each other; these assumptions were based on previously published literature.18 A causal diagram (Figure 2) was constructed to present these causal assumptions, using methods described by Hernán et al.19 The minimally sufficient set of confounders needed to be adjusted for in regression models identified using the causal diagram contained age, sex, education, hypertension, lesion position, Hcy, and S100B. Multivariate logistic regression was performed on Hcy and S100B together with other risk factors, and the results showed that hypertension, lesion position, Hcy, and S100B protein were found to be independently associated with the occurrence of cognitive impairment in patients with PIS (Tables 2 and 3).
Figure 2.
Causal diagram presenting the causal assumptions among study variables in assessing the association between cognitive impairment and Hcy and S100B.
Table 2.
Logistic Regression Analysis Including Hcy Risk Factors
| B | Wald χ2 | OR (95% CI) | P | |
|---|---|---|---|---|
| Age | 0.838 | 4.019 | 2.312 (1.019–5.246) | 0.045 |
| Hypertension | 0.909 | 4.019 | 2.482 (1.134–5.433) | 0.023 |
| Lesion position (cerebral hemisphere) | 0.894 | 4.966 | 2.445 (1.114–5.368) | 0.026 |
| Hcy | 0.174 | 12.455 | 1.190 (1.081–1.311) | 0.000 |
Table 3.
Logistic Regression Analysis Including S100B Risk Factors
| B | Wald χ2 | OR (95% CI) | P | |
|---|---|---|---|---|
| Age | 0.867 | 4.41 | 2.380 (1.059–5.345) | 0.036 |
| Hypertension | 0.998 | 6.499 | 2.713 (1.260–5.844) | 0.023 |
| Lesion position (cerebral hemisphere) | 0.993 | 6.41 | 2.445 (1.114–5.368) | 0.011 |
| S100B | 1.132 | 8.93 | 3.101 (1.476–6.513) | 0.003 |
Correlation Analysis of Risk Factors for Cognitive Impairment of Patients with PIS
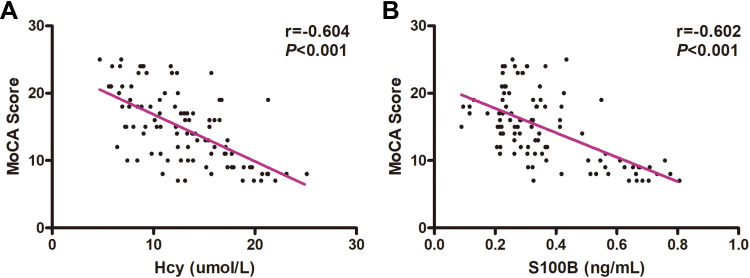
Pearson correlation analysis was conducted between Hcy and S100B protein and MoCA score. Hcy content and S100B protein were negatively correlated with MoCA score, and the differences were statistically significant (Hcy: r = −0.604, P < 0.001; S100B protein: r = −0.602, P < 0.001) (Figure 3).
Figure 3.
Correlation analysis of total MoCA score, and concentration of Hcy and S100B protein in patients with PIS. Negative correlation between MoCA score and Hcy (r = −0.604, P < 0.001) (A) and between MoCA score and S100B protein. (r = −0.602, P < 0.001) (B), as determined by Pearson analysis.
Predictive Ability of S100B Protein and Hcy for Cognitive Impairment of Patients with PIS
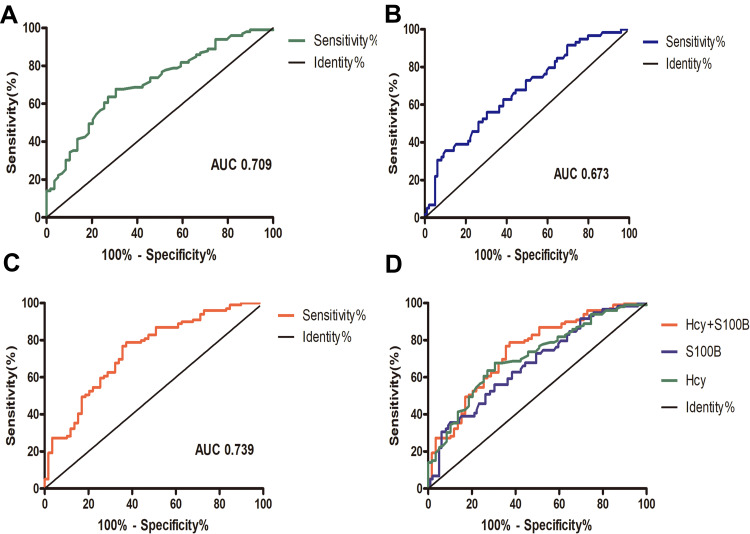
ROC curve analysis showed that the AUC of Hcy and S100B protein in recognizing cognitive impairment after PIS was 0.673 and 0.709, respectively, while the combined AUC of Hcy and S100B protein in predicting cognitive impairment after PIS was 0.739 (Figure 4).
Figure 4.
Receiver operating characteristic (ROC) curves for predicting cognitive impairment after PIS. ROC curve of Hcy (AUC = 0.709, sensitivity = 67.7%, specificity = 66.1%) (A), S100B protein (AUC = 0.673, sensitivity = 69.7%, specificity = 55.9%) (B), and combined Hcy and S100B protein (AUC = 0.739, sensitivity = 78.8%, specificity = 62.7%) (C). (D) Combination diagram of ROC curves.
Discussion
This study found that the observation group was mainly male, older, more hypertensive patients, mainly cerebral infarction, and had lower education level and MoCA score compared with the control group. Meanwhile, the observation group had a significantly higher content of Hcy and S100B protein compared with the control group. The levels of Hcy and S100B protein both correlated with the development of cognitive impairment in patients with PIS, and were thus related risk factors. The combination of the two could improve the predictive ability.
The observation group had lower education level, which may be due to the fact that MoCA score was affected by education level, and patients with a low education level may appear false positive, resulting in result bias.20 Cognitive reserve or capacity expands with the increase of level of education, and individuals with a strong cognitive reserve mostly have a high tolerance of cognitive impairment; therefore, the MoCA score value can be adjusted according to the level of education.21 In this study, the degree of education was adjusted as far as possible to reduce bias, the result indicated that a low degree of education is associated with cognitive impairment after stroke. Low education level was previously confirmed as a distinct risk factor for dementia,22 suggesting that education level may be a protective factor for cognitive function after PIS. In the current study, the observation group was older. With increasing age, the degree of brain atrophy and neurodegeneration increases, and the degree of atherosclerosis also increases with increasing age, thus the progression after ischemic stroke is liable to occur. Age is the biggest risk factor for Alzheimer’s disease,23 and education level is also associated with it.24 However, logistic regression in the current study showed that age and education level are not related risk factors for cognitive impairment after PIS, indicating that the pathogenesis of cognitive impairment after stroke is different from that of Alzheimer’s disease. Hypertension is a known risk factor for cognitive impairment.25 A review confirmed the relationship between hypertension and stroke, cognitive impairment, and dementia.26 Hypertension, as a related risk factor, adversely affects cognitive function after initial AIS.27 Furthermore, hypertension is associated with cognitive impairment caused by small cerebral vascular disease and is the most common risk factor.28 Additional evidence is emerging from observational studies and clinical trials that hypertension plays a central role in the development of cognitive impairment. The lesion position is considered as a risk factor for cognitive impairment after stroke,29,30 and the present study also suggested that the lesion position is one of the predictors of cognitive impairment after PIS, which is consistent with previous experimental conclusions.
Hcy is a sulfur-containing nonessential amino acid that powerfully oxidizes endothelial cells and has a crucial role in the transfer of methyl groups during cell metabolism. Deficiency of the vitamin B group leads to inadequate methylation of Hcy, thus reducing the synthesis of methionine and S-adenosyl methionine. This subsequently leads to a deficiency of methyl groups—these groups are essential for the metabolism of nucleic acids, proteins, neurotransmitters, membrane phospholipids, and myelin—which may lead to reduced cognitive function.31 In systematic reviews and meta-analyses of related epidemiology, high plasma concentrations of Hcy are a modifiable risk factor for cognitive impairment.32,33 There is increasing evidence that Hcy is significantly correlated with cognitive impairment after stroke.34 High concentrations of Hcy may be involved in the occurrence of post-stroke cognitive impairment through oxidative stress,35 vascular inflammation, endothelial impairment,36 and accelerated accumulation of amyloid and tau proteins.37 Hcy is affected by many factors, including age, blood sugar, blood lipid, alcohol consumption, etc, but the level is higher in patients with PIS cognitive impairment after adjustment for potential confounders, suggesting that Hcy may be involved in the pathogenesis of cognitive impairment after progressive stroke.
Serum S100B protein, an acidic, inflammatory protein derived from astrocytes, is an important factor in the feedback loop of brain injury leading to neurotoxicity. The concentration of S100B protein may be negatively correlated with ischemic stroke,38 suggesting that the higher the concentration of serum S100B protein, the more severe the condition of ischemic stroke patients, and the worse the prognosis for the patients. S100B protein is also a widely verified biomarker of the severity of brain injury, and the concentration of S100B has been proven to impact on the neurological function of AIS.39 Following BBB damage due to AIS, S100B protein is released into the bloodstream and this increase in protein content in the blood is clinically measurable. In the current study, the index of the group with cognitive impairment was higher compared with that of the group with normal cognition, indicating that the nerve cells and BBB (which might contribute to cognitive impairment of patients with PIS) were severely damaged, leading to a significant increase in clinical indicators.
Hcy and S100B increased at the same time, suggesting that there may be a close correlation between them. High Hcv expression will trigger the body’s inflammatory response and oxidative stress, resulting in inflammatory cascade reaction. In addition to causing vascular endothelial inflammatory damage, it can also cause BBB damage, inducing S100B release into the blood. At the same time, S100B in serum aggravates oxidative damage, causing brain nerve damage and cognitive impairment.40 This study then evaluated the predictive value of cognitive impairment after PIS by using ROC curve analysis. Hcy and S100B protein prediction AUC of cognitive impairment in the aftermath of the PIS was higher compared with the single index prediction ability of the two proteins. This may be related to there being multiple influencing factors of cognitive impairment after stroke, thus a single biochemical indicator cannot fully reflect the overall situation and it takes two or more indicators or imaging findings to raise the predictive value. In this study, the contents of Hcy and S100B protein were higher in the cognitive impairment group compared with the cognitive normal group, and the total MoCA score was lower compared with that in the cognitive normal group. This suggested that Hcy and S100B protein are related to cognitive impairment after stroke and deserve further study. Early detection of serum Hcy and S100B protein levels in patients and timely intervention measures to guide clinical treatment may help prevent and delay the occurrence of cognitive impairment.
This study was explored the correlation between cognitive impairment after PIS and S100B protein and Hcy. However, there are some limitations in the study. Firstly, the analyses are exploratory only, the prediction analysis needs external validation. Without any validation the predictive properties are not clear. Secondly, Hcy and S100B protein at other time periods of PIS and follow-up were not obtained in this study, and dynamic measurement may be a stronger outcome prediction tool compared with the single measurement. In future studies, Hcy and S100B protein will be continuously collected at different time periods and the influence of the combination of Hcy and S100B protein on predicting cognitive impairment and related complications after stroke will be explored. Thirdly, the findings of the current study are related to factors specific to the Taixing population, such as the prevalence of local vascular risk factors and the diet structure of the population. Consequently, these findings need to be verified by large-scale and multi-center experiments. Therefore, care should be taken when interpreting the results of the current study.
Conclusion
In summary, this study showed that the serum concentration of Hcy and S100B protein were increased in the group with cognitive impairment after PIS, and the two proteins may be used as in a prediction model to predict cognitive impairment after PIS in the future.
Acknowledgments
We would like to thank Dr. Guomei Shi for editorial help.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kefalopoulou ZM, Liossis SN, Sagona T, et al. An ischemic stroke as the presenting manifestation of rapidly progressive primary angiitis of central nervous system in a 17-year-old boy. J Neuroimmunol. 2020;341:577190. doi: 10.1016/j.jneuroim.2020.577190 [DOI] [PubMed] [Google Scholar]
- 2.Miro-Mur F, Urra X, Ruiz-Jaen F, Pedragosa J, Chamorro A, Planas AM. Antigen-dependent T cell response to neural peptides after human ischemic stroke. Front Cell Neurosci. 2020;14:206. doi: 10.3389/fncel.2020.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JM, Moon J, Ahn SW, Shin HW, Jung KH, Park KY. The etiologies of early neurological deterioration after thrombolysis and risk factors of ischemia progression. J Stroke Cerebrovasc Dis. 2016;25(2):383–388. doi: 10.1016/j.jstrokecerebrovasdis.2015.10.010 [DOI] [PubMed] [Google Scholar]
- 4.Hatakeyama J, Yanagisawa T, Kudo E, Togashi S, Shimizu H. [Emergency bypass surgery for progressive cerebral infarction following hemorrhagic onset of moyamoya disease: a case report]. No Shinkei Geka. 2016;44(10):843–849. Japanese. doi: 10.11477/mf.1436203388 [DOI] [PubMed] [Google Scholar]
- 5.Bruggemans EF. Cognitive dysfunction after cardiac surgery: pathophysiological mechanisms and preventive strategies. Neth Heart J. 2013;21(2):70–73. doi: 10.1007/s12471-012-0347-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delavaran H, Jonsson AC, Lovkvist H, et al. Cognitive function in stroke survivors: a 10-year follow-up study. Acta Neurol Scand. 2017;136(3):187–194. doi: 10.1111/ane.12709 [DOI] [PubMed] [Google Scholar]
- 7.Teng Z, Dong Y, Zhang D, An J, Lv P. Cerebral small vessel disease and post-stroke cognitive impairment. Int J Neurosci. 2017;127(9):824–830. doi: 10.1080/00207454.2016.1261291 [DOI] [PubMed] [Google Scholar]
- 8.Ramos-Estebanez C, Moral-Arce I, Rojo F, Gonzalez-Macias J, Hernandez JL. Vascular cognitive impairment and dementia expenditures: 7-year inpatient cost description in community dwellers. Postgrad Med. 2012;124(5):91–100. doi: 10.3810/pgm.2012.09.2597 [DOI] [PubMed] [Google Scholar]
- 9.He Y, Li Y, Chen Y, Feng L, Nie Z. Homocysteine level and risk of different stroke types: a meta-analysis of prospective observational studies. Nutr Metab Cardiovasc Dis. 2014;24(11):1158–1165. doi: 10.1016/j.numecd.2014.05.011 [DOI] [PubMed] [Google Scholar]
- 10.Wang CY, Chen ZW, Zhang T, et al. Elevated plasma homocysteine level is associated with ischemic stroke in Chinese hypertensive patients. Eur J Intern Med. 2014;25(6):538–544. doi: 10.1016/j.ejim.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 11.Ji Y, Li X, Teng Z, Li X, Jin W, Lv PY. Homocysteine is associated with the development of cerebral small vessel disease: retrospective analyses from neuroimaging and cognitive outcomes. J Stroke Cerebrovasc Dis. 2020;29(12):105393. doi: 10.1016/j.jstrokecerebrovasdis.2020.105393 [DOI] [PubMed] [Google Scholar]
- 12.Arrais AC, Melo L, Norrara B, et al. S100B protein: general characteristics and pathophysiological implications in the Central Nervous System. Int J Neurosci. 2022;132(3):313–321. doi: 10.1080/00207454.2020.1807979 [DOI] [PubMed] [Google Scholar]
- 13.Michetti F, D’Ambrosi N, Toesca A, et al. The S100B story: from biomarker to active factor in neural injury. J Neurochem. 2019;148(2):168–187. doi: 10.1111/jnc.14574 [DOI] [PubMed] [Google Scholar]
- 14.Wallin A, Kapaki E, Boban M, et al. Biochemical markers in vascular cognitive impairment associated with subcortical small vessel disease - a consensus report. BMC Neurol. 2017;17(1):102. doi: 10.1186/s12883-017-0877-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocha M, Vieira A, Michels M, et al. Effects of S100B neutralization on the long-term cognitive impairment and neuroinflammatory response in an animal model of sepsis. Neurochem Int. 2021;142:104906. doi: 10.1016/j.neuint.2020.104906 [DOI] [PubMed] [Google Scholar]
- 16.Wedekind D, Neumann K, Falkai P, et al. S100B and homocysteine in the acute alcohol withdrawal syndrome. Eur Arch Psychiatry Clin Neurosci. 2011;261(2):133–138. doi: 10.1007/s00406-010-0121-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 18.Bao F, Cui M, Shi X, Ju S, Cong H. Distribution characteristics and influencing factors of homocyteine in an apparently healthy examined population. BMC Cardiovasc Disord. 2021;21(1):429. doi: 10.1186/s12872-021-02238-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernan MA, Hernandez-Diaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155(2):176–184. doi: 10.1093/aje/155.2.176 [DOI] [PubMed] [Google Scholar]
- 20.Gomez F, Zunzunegui M, Lord C, Alvarado B, Garcia A. Applicability of the MoCA-S test in populations with little education in Colombia. Int J Geriatr Psychiatry. 2013;28(8):813–820. doi: 10.1002/gps.3885 [DOI] [PubMed] [Google Scholar]
- 21.Wu Y, Wang M, Ren M, Xu W. The effects of educational background on Montreal Cognitive Assessment screening for vascular cognitive impairment, no dementia, caused by ischemic stroke. J Clin Neurosci. 2013;20(10):1406–1410. doi: 10.1016/j.jocn.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 22.Sahathevan R, Brodtmann A, Donnan GA. Dementia, stroke, and vascular risk factors; a review. Int J Stroke. 2012;7(1):61–73. doi: 10.1111/j.1747-4949.2011.00731.x [DOI] [PubMed] [Google Scholar]
- 23.Litke R, Garcharna LC, Jiwani S, Neugroschl J. Modifiable risk factors in Alzheimer disease and related dementias: a review. Clin Ther. 2021;43(6):953–965. doi: 10.1016/j.clinthera.2021.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrews SJ, Fulton-Howard B, O’Reilly P, Marcora E, Goate AM; Collaborators of the Alzheimer’s Disease Genetics Committee. Causal associations between modifiable risk factors and the Alzheimer’s phenome. Ann Neurol. 2021;89(1):54–65. doi: 10.1002/ana.25918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg A, Ngandu T, Rusanen M, et al. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: the FINGER trial. Alzheimer's Dement. 2018;14(3):263–270. doi: 10.1016/j.jalz.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 26.Perrotta M, Lembo G, Carnevale D. Hypertension and dementia: epidemiological and experimental evidence revealing a detrimental relationship. Int J Mol Sci. 2016;17(3):347. doi: 10.3390/ijms17030347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matz K, Teuschl Y, Firlinger B, et al. Multidomain lifestyle interventions for the prevention of cognitive decline after ischemic stroke: randomized trial. Stroke. 2015;46(10):2874–2880. doi: 10.1161/STROKEAHA.115.009992 [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Li S, Pan Y, et al. The effects of blood pressure on post stroke cognitive impairment: BP and PSCI. J Clin Hypertens. 2021;23(12):2100–2105. doi: 10.1111/jch.14373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Dong YH, Lyu PY, Chen WH, Li R. Hypertension-induced cerebral small vessel disease leading to cognitive impairment. Chin Med J. 2018;131(5):615–619. doi: 10.4103/0366-6999.226069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weaver NA, Kuijf HJ, Aben HP, et al. Strategic infarct locations for post-stroke cognitive impairment: a pooled analysis of individual patient data from 12 acute ischaemic stroke cohorts. Lancet Neurol. 2021;20(6):448–459. doi: 10.1016/S1474-4422(21)00060-0 [DOI] [PubMed] [Google Scholar]
- 31.Porter K, Hoey L, Hughes CF, Ward M, McNulty H. Causes, consequences and public health implications of low B-vitamin status in ageing. Nutrients. 2016;8(11):725. doi: 10.3390/nu8110725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith AD, Refsum H. Homocysteine, B vitamins, and cognitive impairment. Annu Rev Nutr. 2016;36:211–239. doi: 10.1146/annurev-nutr-071715-050947 [DOI] [PubMed] [Google Scholar]
- 33.Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health. 2014;14:643. doi: 10.1186/1471-2458-14-643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine DA, Wadley VG, Langa KM, et al. Risk factors for poststroke cognitive decline: the REGARDS study (reasons for geographic and racial differences in stroke). Stroke. 2018;49(4):987–994. doi: 10.1161/STROKEAHA.117.018529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papatheodorou L, Weiss N. Vascular oxidant stress and inflammation in hyperhomocysteinemia. Antioxid Redox Signal. 2007;9(11):1941–1958. doi: 10.1089/ars.2007.1750 [DOI] [PubMed] [Google Scholar]
- 36.Gurda D, Handschuh L, Kotkowiak W, Jakubowski H. Homocysteine thiolactone and N-homocysteinylated protein induce pro-atherogenic changes in gene expression in human vascular endothelial cells. Amino Acids. 2015;47(7):1319–1339. doi: 10.1007/s00726-015-1956-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irizarry MC, Gurol ME, Raju S, et al. Association of homocysteine with plasma amyloid beta protein in aging and neurodegenerative disease. Neurology. 2005;65(9):1402–1408. doi: 10.1212/01.wnl.0000183063.99107.5c [DOI] [PubMed] [Google Scholar]
- 38.Ye H, Wang L, Yang XK, Fan LP, Wang YG, Guo L. Serum S100B levels may be associated with cerebral infarction: a meta-analysis. J Neurol Sci. 2015;348(1–2):81–88. doi: 10.1016/j.jns.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 39.Lasek-Bal A, Jedrzejowska-Szypulka H, Student S, et al. The importance of selected markers of inflammation and blood-brain barrier damage for short-term ischemic stroke prognosis. J Physiol Pharmacol. 2019;70(2). doi: 10.26402/jpp.2019.2.04 [DOI] [PubMed] [Google Scholar]
- 40.Oldreive CE, Doherty GH. Neurotoxic effects of homocysteine on cerebellar Purkinje neurons in vitro. Neurosci Lett. 2007;413(1):52–57. doi: 10.1016/j.neulet.2006.11.031 [DOI] [PubMed] [Google Scholar]