Abstract
The leukotoxin of Mannheimia haemolytica is an important virulence factor that contributes to much of the pathology observed in the lungs of animals with bovine shipping fever pneumonia. We believe that identification of factors that regulate leukotoxin expression may provide insight into M. haemolytica pathogenicity. The DNA sequence upstream of the leukotoxin operon is divergently shared by PlapT, which transcribes an arginine permease gene. The intergenic region contains several elements that are potential sites for transcriptional modulation of the promoters. We have developed plasmid-borne chloramphenicol acetyltransferase (cat) operon fusions, as well as lktC::cat chromosomal fusions, to study transcription initiation in M. haemolytica. Using these genetic tools, we have identified cis-acting sequences and environmental conditions that modulate transcription of the leukotoxin and lapT promoters. By deletion analysis, promoters were shown to rely on sequences upstream of their −10 and −35 regions for full activity. Direct repeats of the sequence TGT-N(11)-ACA and a static bend region caused by phased adenine tracts were necessary for full activation of Plkt. A computer-generated model of the promoter's structure shows how DNA bending brings the repeat sequences within close proximity to the Plkt RNA polymerase, and we hypothesize that these repeats are a binding site for an activator of leukotoxin transcription. The lktC::cat operon fusion was also used to demonstrate that, like that of other RTX toxins, leukotoxin transcription is environmentally regulated. Roles for iron deprivation and temperature change were identified.
Mannheimia (Pasteurella) haemolytica serotype A1 is the major bacterial pathogen involved in a respiratory disease known as bovine shipping fever pneumonia (13). M. haemolytica is normally a commensal bacterium of the upper respiratory tracts of healthy ruminant animals (12). However, when the animals have a predisposing viral infection and/or are exposed to stress, the lungs can become infected with M. haemolytica and cause pneumonia. This disease has a significant effect on the cattle industry in terms of population morbidity and mortality and economic losses (16, 21). Characteristics of the disease include necrosis, fibrinous pleuritis, and infiltration of inflammatory cells (8).
Much of the lung pathology observed in shipping fever pneumonia is attributed to the leukotoxin of M. haemolytica. Leukotoxin lyses bovine leukocytes and platelets (5, 38), and at sublytic concentrations, leukotoxin can cause apoptosis, neutrophil activation, and inflammatory mediator release (6, 35, 40). While M. haemolytica is part of the normal flora of the upper respiratory tracts of cattle, the destructive effects of leukotoxin are not observed there. Pathogenicity of M. haemolytica is marked by rapid replication, subsequent inhalation of the organism into the lungs, and expression of leukotoxin. Therefore, investigation of the regulation of leukotoxin could help our understanding of how M. haemolytica switches from a nonpathogenic to a virulent organism.
Leukotoxin is expressed from an operon containing genes necessary for posttranslational activation (lktC) of the protoxin (lktA) and its secretion (lktBD) (18, 28). Leukotoxin is a member of the RTX (repeats in toxin) family of cytolysins, based on structural characteristics of the toxin, genetic organization, and cytotoxic activity (47). Other RTX family members include the hemolysin from Escherichia coli (HlyA), the leukotoxin of Actinobacillus actinomycetemcomitans (LtxA), the Bordetella pertussis adenylate cyclase-hemolysin (CyaA), the iron-regulated proteins FrpA and FrpC of Neisseria meningitidis, and the recently identified RtxA protein of Vibrio cholerae (11, 14, 24, 26, 41). RTX toxins are regulated at the level of transcription by a diverse array of cis- and trans-acting factors. It is understandable that expression of RTX toxins would be tightly controlled by multiple factors and perhaps regulated coordinately with other virulence genes.
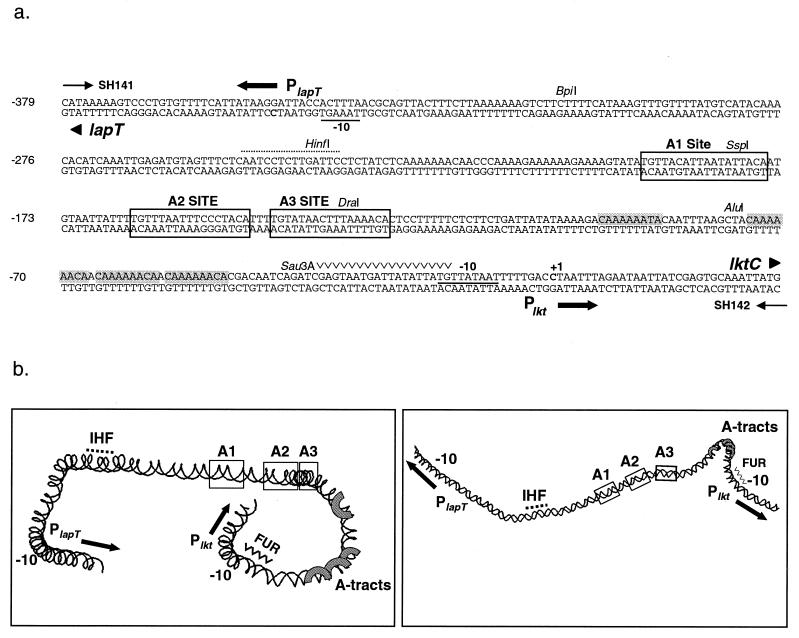
The leukotoxin of M. haemolytica appears to have a very complex promoter region. The leukotoxin operon (lktCABD) is preceded by a 406-bp intergenic region (Fig. 1A). Within this sequence, the leukotoxin operon uses a cytosine residue at position −30 relative to the first amino acid codon (ATG) as its transcriptional start site (18). The gene for an l-arginine binding protein, lapT, is transcribed in the opposite direction, starting at position −349 relative to the lkt transcriptional start site (20). lapT is the first gene of a four-gene cluster involved in arginine binding and transport (4). Sequence inspection revealed several elements that may be involved in regulation of transcription of the leukotoxin operon and the arginine permease locus (19). Plkt has an extended −10 promoter sequence, TGNTATAAT, found in E. coli promoters that lack a consensus −35 region and that rely on upstream activators for transcription initiation (25). Plkt does not have a recognizable −35 region; however, at positions −47 to −96 are four adenine tracts that cause the promoter to bend approximately 80°. This intrinsic bending of the DNA has been implicated in promoter activation (19). Upstream of the adenine tracts are three direct repeats, A1, A2, and A3, of the sequence TGT-N(11–12)-ACA that may function as binding sites for a regulatory protein (19). The E. coli DNA-bending protein integration host factor (IHF) binds to the intergenic region, and a near-consensus IHF binding site is observed at positions −237 to −251 (19). Another potential regulatory site within the promoter is a near-consensus binding site for the iron regulator protein Fur. A computer-generated model of the region, based on individual contributions of base pairs to DNA bending, shows the intrinsic curvature of the DNA. Figure 1B displays the location of the cis elements mentioned above and their predicted spatial relationship to one another.
FIG. 1.
(a) Nucleotide sequence of the lapT-lktC intergenic regulatory region of M. haemolytica SH1217. Bolded cytosine residues indicate the transcriptional start sites of PlapT and Plkt. The sequence is numbered in reference to the Plkt transcriptional start site at +1. Underlined nucleotides indicate the predicted −10 promoter regions of PlapT and Plkt. Shaded nucleotides correspond to the four phased adenine tracts. Putative upstream activator sites A1, A2, and A3 are boxed. The near-consensus IHF binding site is indicated with a dotted line above the sequence, and a potential Fur binding site is marked above the nucleotides (∨∨∨∨∨). Unique enzyme restriction sites are shown. (b) Computer-predicted DNA structure of the 0.4-kb lapT/lktC promoter region that was generated by the CURVATURE program (42). Two different views were created by rotating the image along its x axis by using RasMac v2.6 (37). The image on the right has been reduced in size. Sequence elements are noted on the model. IHF and FUR are potential IHF and FUR binding sites, and A-tracts are adenine tracts. Solid arrows show the direction of transcription.
Expression of virulence factors in response to environmental changes is a common theme in microbial pathogenesis (29). During the disease state, M. haemolytica experiences dramatic changes in its physical environment. At the onset of disease, M. haemolytica has a phase of rapid growth. The bacteria are then inhaled into the lung, where they encounter an environment different from that in the nasopharynx. It can be expected that the bacteria experience changes in temperature, oxygen concentration, and availability of nutrients and molecules such as iron. In addition, the bacteria face the host immune response and the need to elaborate defenses. Variations in the microenvironment of M. haemolytica may be a cue to modulate leukotoxin expression.
Previous studies of transcription in M. haemolytica were difficult and limited to Northern analysis (18, 39). As described here, we can now quantitate gene expression in M. haemolytica by using chloramphenicol acetyltransferase (CAT) operon fusions. In this study, we began to address M. haemolytica pathogenicity by studying virulence control at the level of transcription. Because transcription of virulence genes is often coordinately regulated, information gained about leukotoxin transcription may provide insight into the overall transition toward pathogenicity. Here we describe cis elements and environmental cues that regulate initiation at the leukotoxin promoter.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All of the bacterial strains used in this study are listed in Table 1. For standard growth of M. haemolytica cells, a culture was streaked from frozen stocks onto 5% sheep blood agar and incubated at 37°C overnight. Colonies from fresh plates were then grown at 37°C in 25 ml of brain heart infusion broth (BHI; Difco, Detroit, Mich.) in 125-ml flasks and shaken at 200 rpm. For iron depletion studies, iron was chelated by adding 10 to 250 μM 2,2-dipyridyl (Sigma, St. Louis, Mo.). Iron repletion was performed by adding 200 μM FeCl3 to BHI containing either 100 or 250 μM 2,2-dipyridyl. Temperature shift assays were performed either by growing M. haemolytica cells at 37°C continuously or by growing the bacteria at 30°C until mid-log phase and then shifting the temperature to 37°C. When antibiotics were necessary for plasmid selection or maintenance in M. haemolytica, cells were grown in ampicillin (AP) at 25 μg/ml, streptomycin (SM) at 100 μg/ml, or chloramphenicol (CM) at 5 μg/ml. E. coli strain XL1-Blue (Stratagene, La Jolla, Calif.) was used for cloning. For E. coli, antibiotics were used at the following concentrations: AP at 100 μg/ml, CM at 20 μg/ml, tetracycline at 15 μg/ml, and kanamycin at 25 μg/ml.
TABLE 1.
Strains, plasmids, and oligonucleotides used in this study
| Strain, plasmid, or oligonucleotide | Description | Reference or source |
|---|---|---|
| Strains | ||
| SH1217 | M. haemolytica A1, Sms | 10 |
| XLI-Blue | E. coli recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10] | Stratagene |
| SH2020 | SH1217 Φ(lktC::cat)2 | This study |
| SH2410 | SH1217 Φ(lktC::cat)3 SspI | This study |
| SH2405 | SH1217 Φ(lktC::cat)4 Sau3A | This study |
| Plasmids | ||
| pYFC1 | Native M. haemolytica plasmid, Smr | |
| pSH224 | pBlueScript KSII+::7-kb PstI (lapCABT lktCA′) | 19 |
| pNF2237 | pYFCI::Φ(lktC::cat)1 | 9 |
| pNF2283 | pYFC1::cat (promoterless) | 10 |
| Oligonucleotides | ||
| SH48 | 5′-cctaaaccatcacc-3′ | This study |
| SH141 | 5′-gcgcggatccgtcgacataaaaagtccctgtgttttcattataagg-3′ | This study |
| SH142 | 5′-cctaatttagaataattatcgagtgcaaattatgaatcccgggatccgccg-3′ | This study |
| SH186 | 5′-cgatgccattgggatatatcaacggtgg-3′ | This study |
DNA manipulations.
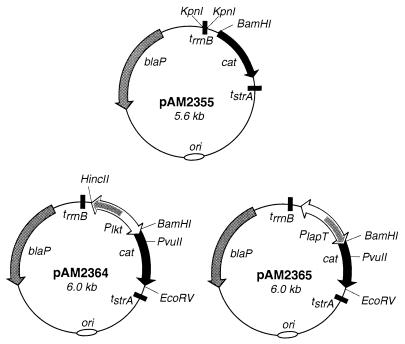
The oligonucleotides used in this study are listed in Table 1. The plasmids used in this study are listed and described in Table 1, Fig. 2, or Fig. 5A. Plasmids were created and isolated by using standard DNA manipulation techniques (36). To create cat reporter plasmid pAM2355, plasmid pTP128 (generously provided by T. Palzkill; Baylor College of Medicine, Houston, Tex.) was digested with KpnI to obtain a 0.2-kb fragment containing trrnB from E. coli. The KpnI fragment was then ligated into the KpnI site on pNF2283 upstream of the cat gene (Fig. 2) (10). To create Plkt and PlapT operon fusion plasmids pAM2364 and pAM2365, respectively, the 412-bp intergenic region from ATG to ATG was PCR amplified with oligonucleotides SH141 and SH142 by using the SH1217 chromosome as template DNA and then digested with BamHI and ligated into the BamHI site of pAM2355 (Fig. 2). To create lktC::cat chromosomal fusions, a plasmid for homologous recombination was constructed. A 1.6-kb PvuII fragment of pNF2237, containing a portion of the cat gene and downstream leukotoxin operon sequences, was ligated to pAM2364 cut with PvuII and EcoRV. The resulting plasmid, pAM2434, contained the flanking 3′ sequence for chromosomal recombination (see below) while maintaining the identical operon fusion used for plasmid studies described in this paper.
FIG. 2.
Plasmid maps of cat promoter probe vector pAM2355 and operon fusion plasmids pAM2364 (lkt::cat) and pAM2365 (lapT::cat). Plasmid pAM2355 contains transcriptional terminators on either side of the cat reporter gene to prevent readthrough from other sites on the plasmid. A PCR-amplified fragment containing the lapT/lktC promoter region was ligated upstream of the promoterless cat gene in both orientations to create pAM2364 and pAM2365. All of the plasmids contain the M. haemolytica origin of replication from pYFCI. Only relevant restriction sites are shown.
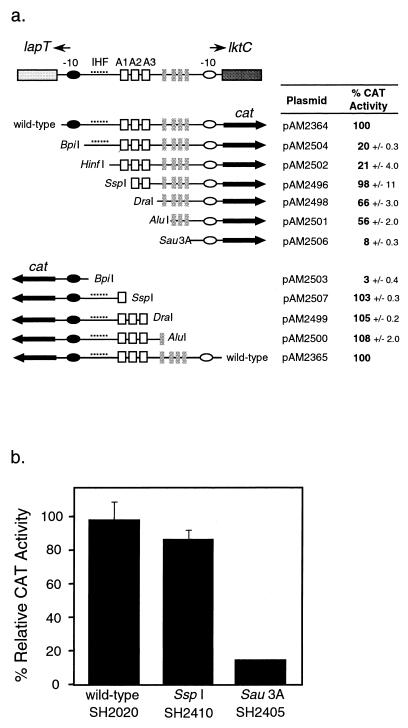
FIG. 5.
(a) CAT activities of fusion plasmids with promoter fragments deleted. The diagram shows the lapT/lktC promoter region with particular sequence elements marked. Symbols: closed and open ovals, −10 region; dotted line, near-consensus IHF binding site; open squares, direct repeats A1, A2, and A3; closed rectangles, adenine tracts. By using the restriction enzyme listed next to the fragment, sequential deletions were made in the promoter to remove the elements indicated and then the promoter was ligated to the promoterless cat gene. The CAT activity reported is relative to that of the full-length promoter (wild type) in either the Plkt or the PlapT orientation, as indicated. CAT activity is the average of duplicate samples ± 1 standard deviation. Strains containing each plasmid were grown under identical conditions in BHI at 37°C for 18 h. (b) CAT activity of chromosomal operon fusions. Operon fusions equivalent to plasmids pAM2496 and pAM2506 were recombined onto the M. haemolytica chromosome to create strains SH2410 and SH2405, respectively. CAT activities were determined for cultures grown in BHI at 37°C for 18 h. Results are presented as percentages of the CAT activity of SH2020, which was set at 100%. Results are the averages of duplicate samples, and error bars show 1 standard deviation.
To create sets of nested deletions within the leukotoxin and lapT promoters, SH1217 chromosomal DNA was used as a template to PCR amplify the promoter region using primers SH141 and SH142. Amplified DNA was digested with one of the following enzymes or enzyme pairs: BpiI/BamHI, HinfI, SspI/BamHI, DraI/BamHI, AluI/BamHI, or Sau3A. Digested fragments were ligated into the BamHI site of pAM2355 or the HincII/BamHI sites of pAM2364. Cloned DNA was sequenced to ensure that no mutations had been introduced during the amplification step.
Mutated Plkt operon fusion plasmids for chromosomal recombination were created by modifying plasmid pAM2434. First, additional sequences for recombination were added upstream of the Plkt promoter. This was done by using oligonucleotides SH48 and SH142 to PCR amplify a portion of the lapT gene and its promoter from plasmid pSH224. The 1.0-kb fragment was cloned into pAM2434 digested with HincII/BamHI to create pAM2515. Next, to create the SspI Plkt operon fusion deletion, the promoter sequences of pAM2515 were deleted by using FspI and BamHI. The 412-bp promoter was then amplified by PCR using primers SH141 and SH142 and digested with SspI/BamHI, and the 212-bp fragment containing Plkt was ligated into the FspI/BamHI site of pAM2515 to create pAM2516. Similarly, to create the Sau3A Plkt deletion operon fusion, the PCR-amplified promoter was digested with Sau3A and the 66-bp fragment containing Plkt was ligated into the FspI/BamHI site of pAM2515 to create pAM2518.
Primer extension.
Transcriptional start sites were mapped by primer extension using a primer specific for the cat gene. Total RNA was obtained by using the RNeasy Maxi Kit (Qiagen, Valencia, Calif.), and RNA was quantitated by measuring the A260. Oligonucleotide SH186 (0.01 μg) was 5′ labeled with 25 pmol of [γ-32P]ATP (6,000 Ci/mmol, 10 mCi/ml) using 10 U of T4 polynucleotide kinase (Promega, Madison, Wis.). Primer extension was performed as recommended by the supplier, by using avian myeloblastosis virus reverse transcriptase (Promega), 1 pmol of labeled primer SH186, and 10 μg of total RNA. Reverse transcription was carried out at 47°C for 30 min.
The DNA reference ladder was made by a standard dideoxy-sequencing reaction. The leukotoxin promoter and cat region of the operon fusion plasmid pAM2364 were PCR amplified by using primers SH186 and SH141. Sequencing of the PCR product was performed as described by the supplier, by using Sequenase, version 2.0 (United States Biochemical, Cleveland, Ohio). DNA sequencing and reverse transcriptase reaction mixtures were electrophoresed on a 6% denaturing polyacrylamide gel, and then the gel was autoradiographed. Densitometry analysis was performed by using a Kodak Digital Science DC40 camera and 1D Image Analysis Software (Kodak, Rochester, N.Y.).
CAT assay.
To monitor transcription, a liquid diffusion CAT assay was modified for M. haemolytica (9, 32). For studies throughout all growth phases, fresh BHI was inoculated with a 1:50 dilution of an overnight culture. At various times, cells were collected for cell density measurements and lysate preparation. All samples were normalized to an optical density at 600 nm of 0.3, and then cells were centrifuged and washed in 0.5 ml of cold buffer (0.1 M Tris-HCl, pH 7.8). After washing, the cells were resuspended in 0.1 ml of cold buffer plus 1 mM dithiothreitol and immediately stored at −20°C. After all of the samples had been collected, lysates were made and CAT activity was measured. Cell lysates were prepared by adding 200 μg of lysozyme per ml and 2% Triton X-100 to each sample, mixing the combination, and then incubating the mixture on ice for 10 min. Cell debris was removed by centrifugation, and supernatants were collected and used immediately. At precise 1-min intervals, 5 μl of cell lysate or lysate diluted in 0.1 M Tris was added to 250 μl of CAT reaction mixture (2.0 ml of 0.1 M Tris-HCl [pH 7.8], 0.5 ml of 5 mM CM [dissolved in distilled H2O], 2 μl of [3H]acetyl coenzyme A [200 mCi/mmol, 1 mCi/ml]; ICN Biomedicals, Inc., Irvine, Calif.) in 5-ml plastic scintillation vials. Three milliliters of scintillation fluid (Econofluor; Packard) was then overlaid onto the reaction mixture. Samples were incubated at room temperature for 30 min and then counted for 1 min using a scintillation counter (Beckman LS 7500). To verify response linearity, samples were counted again at 1-h intervals for up to 4 h. CAT activity is expressed as CAT units per unit of optical density at 600 nm. CAT units = [(cpm − control)/(3H counting efficiency)]/(time × 0.44 dpm/nmol).
Construction of chromosomal operon fusions.
Chromosomal lktC::cat operon fusions were created by using a method previously described (9). M. haemolytica strain SH1217 carrying Apr plasmid pAM2434, pAM2516, or pAM2518 was transformed with incompatible Smr plasmid pYFC1. Double transformants were selected on AP SM blood agar plates. Colonies were pooled together and propagated overnight in 5 ml of BHI containing SM at 100 μg/ml. Approximately 103 cells from the overnight culture were plated on blood agar plates containing SM at 100 μg/ml and CM at 5 μg/ml (except for cells carrying pAM2518, which was Cms) and incubated overnight at 37°C. Colonies were screened for loss of hemolysis, indicating chromosomal rescue of the cat gene at the leukotoxin locus. Candidate colonies were replica plated onto AP at 25 μg/ml to confirm loss of plasmid DNA. Colonies that were nonhemolytic and Smr Aps were grown for 8 h in novobiocin at 20 μg/ml to cure cells of pYFCI. Sms Cmr Aps strains SH2020 and SH2410 and Sms Cms Aps strain SH2405 were chosen for further characterization.
RESULTS
Creation of reporter plasmids and strains.
Previously, the ability to perform transcriptional analysis in M. haemolytica was limited by the lack of a manipulable reporter gene system. We have created a promoter probe vector to quantitate transcription in M. haemolytica by using the cat reporter gene. Plasmid pNF2283, which contains a promoterless cat cassette and a 3′ transcription terminator, was modified by adding a terminator, trrnB, upstream of the cat gene to prevent possible transcriptional readthrough from the vector (10). This modified promoter probe vector, pAM2355, can be used in both M. haemolytica and E. coli to monitor transcription (Fig. 2). First we cloned a 695-bp DNA fragment containing the 406-bp intergenic promoter region and portions of the lapT and lktC genes upstream of the cat cassette in pAM2355. Transformation of this plasmid into M. haemolytica and E. coli cells resulted in poor cell growth (data not shown). We had previously observed cell toxicity when the lktC gene was overexpressed, so we next created operon fusions that contained only the PlapT-Plkt intergenic region. The resulting plasmids directed transcription of the cat gene either by Plkt on pAM2364 or by PlapT on pAM2365 (Fig. 2). Creation of such operon fusions containing only the region from the PlapT ATG codon to the Plkt ATG codon allowed us to focus our studies on transcriptional initiation rather than initiation plus elongation.
We chose to use the multicopy plasmid operon fusions to study transcription because of their ease of manipulation for mutagenesis. Nevertheless, to validate our results, we also created a single-copy chromosomal leukotoxin::cat operon fusion in M. haemolytica. The lktC::cat fusion from pAM2364 was crossed to the chromosomal leukotoxin locus by a previously described method (9). Recombination was detected by loss of hemolytic activity on blood agar plates as a result of inactivation of the lktC posttranslational leukotoxin activator gene. Strain SH2020 carrying a chromosomal lktC::cat operon fusion was selected, and the fusion was confirmed by PCR and DNA sequencing (data not shown).
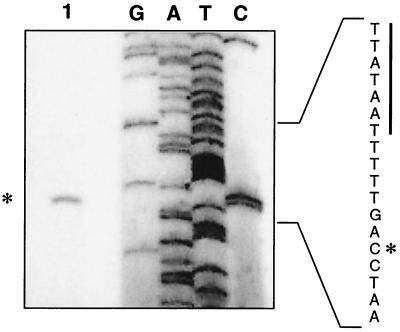
The transcriptional start site of Plkt, from both plasmid and chromosomal operon fusions, was mapped by primer extension. Previously, the leukotoxin transcriptional start site was mapped to one of two cytosine residues at position −30 or −31 relative to the transcriptional start site (18). As shown in Fig. 3, transcription of the leukotoxin promoter on pAM2364 begins at the cytosine residue at position −30. The transcriptional start site of the chromosomal operon fusion SH2020 was similarly mapped by primer extension and occurred at the same residue as in pAM2364 (data not shown). The primer extension product was quantitated by densitometry and was 10-fold higher when plasmid borne (data not shown). This was expected, since we have predicted that the copy number of pYFCI-based plasmids is approximately 8 to 10 plasmids per cell (S. K. Highlander, unpublished results). No primer extension product was observed when we used RNA from M. haemolytica cells containing the reporter vector pAM2355 (data not shown). Our results indicate that both the plasmid and chromosomal operon fusions utilize the same start site as the wild-type leukotoxin promoter. Plasmids were used for promoter mutational analysis, and activity was confirmed when necessary by using chromosomal operon fusions. Strain SH2020 was used to study the effects of environmental changes on leukotoxin transcription.
FIG. 3.
Primer extension analysis of pAM2364. Total RNA from M. haemolytica strain SH1217 harboring plasmid pAM2364 was obtained, and the transcriptional start site of the promoter was identified by primer extension. The asterisk indicates the mapped transcriptional start site of lkt::cat (lane 1). The DNA sequence on the right was generated by using the same primer as that used for the primer extension analysis. The −10 region of the lkt promoter is underlined for reference to the start site location.
Plkt and PlapT activity in M. haemolytica
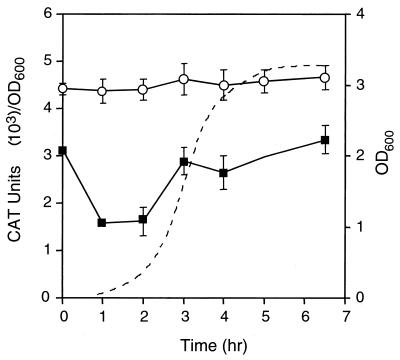
For initial characterization of Plkt and PlapT transcription, we monitored CAT activity in BHI at 37°C during the lag, log, and stationary phases of growth. M. haemolytica cells harboring either lkt reporter plasmid pAM2364 or lapT reporter plasmid pAM2365 were grown, samples were taken at various time points throughout growth, and promoter activity was determined by CAT assay (Fig. 4). Although we had expected that Plkt would be modulated by growth phase, we observed that Plkt expression was constant through all phases of growth in BHI. This could represent maximal or basal transcription in a rich, undefined medium, suggesting that additional experiments under different growth conditions might be necessary to demonstrate differential expression. Expression of the lapT promoter in BHI did, however, vary as a function of growth phase. lapT promoter activity decreased twofold during early log phase and then increased as the cells entered stationary phase. Vector plasmid pAM2355, which lacks a promoter, had no significant CAT activity in M. haemolytica (data not shown). The growth curves of all three strains were identical.
FIG. 4.
Transcription of lkt::cat and lapT::cat operon fusions in M. haemolytica. The CAT activity of SH1217 harboring pAM2364 (open circles) or pAM2365 (closed squares) was measured throughout all phases of growth in BHI at 37°C. Each point represents the average of two samples. Standard deviations are indicated with error bars. The growth curve of SH1217 harboring pAM2364 is shown as a dotted line. OD600, optical density at 600 nm.
Since plasmid supercoiling could affect transcriptional activity, we verified our results from the plasmid-borne promoter by using a chromosomal lkt operon fusion. The chromosomal operon fusion SH2020 was monitored for leukotoxin promoter activity under the same standard growth conditions (data not shown). Results were similar to those obtained with plasmid pAM2364, although the expression level was reduced about 10-fold, consistent with gene dosage effects. Chromosomal leukotoxin promoter activity was constant during the lag, log, and stationary growth phases. These data indicate that transcription of the promoter on the plasmid is initiated in a manner similar to that on the chromosome.
Deletion analysis of Plkt and PlapT.
To identify sequence elements important for regulation of transcription from Plkt and PlapT, we mutagenized the region and then examined CAT activity. We chose to create deletions within the region rather than use site-directed mutagenesis of putative regulatory motifs. Nested deletions of the promoters were created by restriction digestion at unique sites within the PCR-amplified promoter fragment. Each deletion removed sequence elements that were approximately 50-bp apart (Fig. 5A). By measuring the transcriptional activity of the deleted fragments, we identified several regions that appear to be involved in regulation. The SspI deletion fragment directed a wild-type level of activity, suggesting that it contains all of the sequences required for transcription. Surprisingly, the BpiI and HinfI deletion fragments, which include additional upstream regions, had reduced expression. Apparently, loss of the lapT initiation region had a negative effect on transcription of the downstream leukotoxin promoter. Nevertheless, as expected, deletion of the A2 and A3 protein binding sites (DraI deletion) reduced transcription by 32%. Further deletion of the static DNA bend (Sau3A deletion) reduced transcription to 8% of the wild-type level (Fig. 5A).
CAT activity of the fragments was also tested in the opposite orientation to examine effects on PlapT. Removal of 211 bp of the sequence upstream of the lapT translational start site (SspI deletion) still allowed full PlapT transcription. However, removal of 129 bp more (BpiI deletion) reduced promoter function to 3% of the wild-type level (Fig. 5A). Since this segment of DNA includes the putative IHF binding site, the results support our previous hypothesis that IHF is involved in regulation of the lapT promoter (19). Unlike the results obtained for the leukotoxin promoter, the Plkt initiation region does not appear to have an influence on PlapT transcription.
To confirm results obtained by using the plasmid-borne fragments, identical chromosomal leukotoxin operon fusions were created by using the SspI and Sau3A fragments. The relative activity of the chromosomal promoter was the same as that obtained from the plasmids (Fig. 5B): the SspI fragment maintained nearly wild-type activity, and the Sau3A deletion had only 12% of wild-type activity.
Environmental regulation of Plkt.
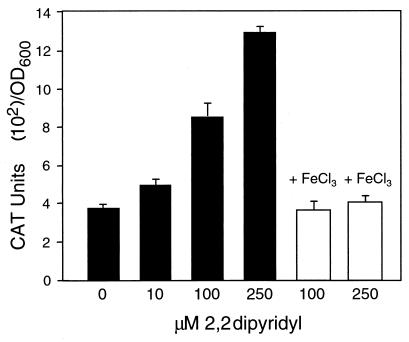
Because promoter elements observed within the intergenic region suggested some level of transcriptional control, we were interested in identifying environmental cues that might be involved. CAT assays were performed by using chromosomal operon fusion strain SH2020 grown under various conditions. The conditions tested included iron depletion, temperature variation, minimal medium, varied pH, and anaerobiosis. To test the effects of iron depletion on leukotoxin transcription, cells were grown in the presence of the iron chelator 2,2-dipyridyl. As the 2,2-dipyridyl concentration increased, so did leukotoxin promoter activity (Fig. 6). At 250 μM, promoter activity increased more than threefold. Cell density was not affected by growth in the presence of 250 μM 2,2-dipyridyl, although higher concentrations inhibited growth. When cells were grown in iron-replete medium created by adding 200 μM FeCl3 to 2,2-dipyridyl-treated medium, transcription decreased to the level seen in cells grown in iron-rich BHI (Fig. 6).
FIG. 6.
Effect of iron depletion on leukotoxin transcription. SH2020 cells were grown for 18 h in the presence of 0, 10, 100, or 250 μM 2,2-dipyridyl (filled bars), and transcription was analyzed by CAT assay. Iron repletion was carried out by adding 200 μM FeCl3 plus 100 or 250 μM 2,2-dipyridyl (open bars) to the culture medium. The data represent duplicate samples, and error bars represent 1 standard deviation. OD600, optical density at 600 nm.
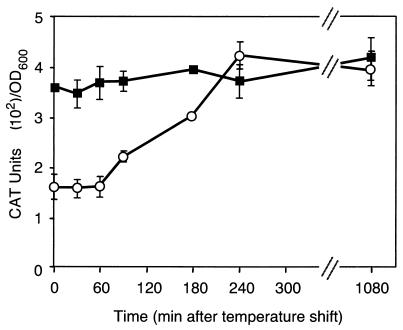
Since some virulence genes respond to temperature cues in the host, we examined the effects of temperature on leukotoxin transcription. M. haemolytica strain SH2020 was grown at 30°C to mid-log phase, and the temperature was then shifted to 42°C. Promoter activity increased within 90 min following the temperature upshift (Fig. 7). By 4 h postshift, leukotoxin transcription increased twofold and remained at a level equivalent to that observed when cells were grown at 42°C. For comparison, SH2020 cells were grown at 37 or at 30°C without a temperature change. The kinetics of expression were similar to those shown in Fig. 4, although cells grown at 30°C had twofold less promoter activity throughout all growth phases than did those grown at 37°C (data not shown).
FIG. 7.
Influence of temperature on leukotoxin transcription. SH2020 was grown at 42°C (filled squares) or at 30°C (open circles) until mid-log phase and then shifted to 42°C. CAT assays were performed on samples taken post temperature upshift. The data points represent averages of two samples, and error bars indicate 1 standard deviation. OD600, optical density at 600 nm.
Another factor tested for its effect on leukotoxin regulation was oxygen availability. Cells were grown in either high aeration by rapid shaking in dimpled flasks, with low aeration in a sealed flask without shaking, or in an anaerobic culture achieved by using the Oxyrase Enzyme System (Oxyrase, Inc., Mansfield, Ohio) (1). After the stationary phase of growth was achieved for each sample, CAT assays were performed. No difference in leukotoxin promoter activity was observed (data not shown). Alterations of the pH of the medium also had no effect on leukotoxin promoter transcription. Cells were grown in BHI buffered to various pHs ranging from 6.2 to 8.0. Samples taken from overnight cultures showed no differences in leukotoxin promoter activity (data not shown).
DISCUSSION
Until now, studies of promoter regulation in M. haemolytica were limited due to the lack of appropriate genetic tools. We have created a cat promoter probe vector that is easily manipulated in E. coli, and transcription from the promoter can be monitored in M. haemolytica by CAT assay. We have used this system to study the divergent leukotoxin and lapT promoters. Identification of the regulatory cues and components of leukotoxin transcription may provide information regarding the transcription of other M. haemolytica virulence factors and a possible master “virulence switch.”
The discovery of global regulators and coordinately controlled virulence genes has been an important step in understanding the pathogenicity of an organism. When the bacterium encounters a change in its environment, whether within or outside of a host, a subset of genes is often coregulated to ensure the survival of the organism (30). M. haemolytica may transcriptionally regulate a variety of genes when it enters the lungs in order to survive the change in its environment from that of the nasopharynx. We suspect that leukotoxin is one of many genes regulated at this stage.
Most of the RTX toxins studied are regulated at the level of transcription initiation and/or elongation by cis, trans, and environmental factors. The plasmid-borne alpha-hemolysin of E. coli is transcriptionally enhanced by the cis element hlyR and the trans activator HlyT (2, 45). A sequence determinant within the leukotoxin promoter of A. actinomycetemcomitans has a significant effect on transcription. Variations in promoter sequence are strain specific and correlate with leukotoxin expression levels and periodontal disease (23). Environmental control of RTX toxin transcription is exemplified by the frp genes of N. meningitidis, which are up-regulated under iron-limiting conditions, and the E. coli hly genes, which are regulated by temperature, osmolarity, and anaerobiosis (31, 41). The adenylate cyclase-hemolysin of B. pertussis is trans regulated by the BvgAS two-component regulatory system, which senses environmental conditions and differentially expresses appropriate genes (43).
Our study indicates that the leukotoxin of M. haemolytica is also regulated at the level of transcription. The leukotoxin promoter contains DNA sequence elements upstream of the −10 and −35 regions that are required for full promoter activity. One of these regions, the A2 and A3 direct repeats of the sequence TGT-N-ACA, has been shown in other systems to act as a binding site for trans regulator proteins. In Rhodobacter sphaeroides 2.4.1, the sequence TGT-N12-ACA is necessary for PpsR repression of the puc operon involved in photopigment biosynthesis (15). The sequence TGT-N12-ACA acts as a binding site for the P2 Org family of activators involved in transcription of the late promoters of bacteriophages P2 and P4 (17, 22). The motif is also recognized by NifA of Klebsiella pneumoniae, where it is involved in the regulation of nitrogen fixation genes (3). We know that the A2 and A3 direct repeats of the leukotoxin promoter are binding sites for an M. haemolytica-specific protein (19), and based on our deletion analysis, the site appears to be involved in positive regulation.
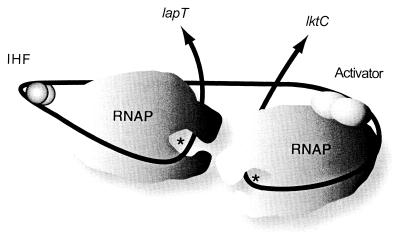
The lapT initiation region appears to have an effect on Plkt transcription. Deletion of the region containing the lapT promoter and the putative IHF binding site negatively affected leukotoxin transcription. However, a more extensive deletion (ΔSspI) restored wild-type levels of expression. It is possible that protein bound between the HinfI and SspI sites can repress Plkt transcription in the absence of the divergent lapT promoter. Removal of such a repressor binding site could restore full activity to the promoter. Alternatively, the deletion may topologically destabilize the promoter, resulting in reduced Plkt transcription. Nevertheless, wild-type levels of Plkt transcription can occur when the fragment contains the putative activator sites A2 and A3 and downstream sequences. In our model (Fig. 8), leukotoxin transcription is activated or enhanced by a protein bound to sites A2 and A3. The static DNA bend acts to facilitate the interaction of the putative activator with RNA polymerase. We hypothesize that both the bend and the activator can enhance RNA polymerase binding and may stabilize open-complex formation. It is important to note that for both the lapT and lkt promoters, the smallest fragments tested, BpiI and Sau3A, respectively, had less than 10% of the activity of the complete sequences. This implies that the −10 and −35 sequences are not sufficient for maximal promoter activity, in support of our activation hypothesis.
FIG. 8.
Model of PlapT and Plkt transcription. The black line represents lapT-lktC intergenic DNA. Arrows indicate the direction of transcription, and asterisks denote transcriptional start sites of PlapT and Plkt. We have tried to maintain appropriate scale between RNA polymerase (RNAP) and IHF and the predicted DNA-protein interactions for open-complex formation, as well as approximate angles induced by IHF or the four adenine tracts. As shown in the model, we predict that DNA bending is an important part of stable RNA polymerase open-complex formation and transcriptional activation for both PlapT and Plkt. We hypothesize that transcription of Plkt is further enhanced in the presence of an activator protein (shown here as a dimer) bound to upstream activator sites.
Environmental signals have been shown to influence virulence gene expression in several different organisms (29). For example, when bacterial cells are starved for iron, such as those within a mammalian host, they elicit several responses to acquire iron from their surroundings. In some organisms, the response is increased expression of RTX toxins and hemolysins (27) that can lyse host cells and cause them to release their internal stores of iron. Our studies show that a decrease in iron concentration causes increased transcription of the M. haemolytica leukotoxin promoter, both on the chromosome (Fig. 6) and on plasmids (data not shown). In contrast, a previous report indicated that iron depletion resulted in a decrease in leukotoxin mRNA and secreted protein (39). However, it is reasonable to expect that M. haemolytica would need to increase its ability to scavenge iron due to competition and hypoferremia induced by host cytokines during infection (46). Nucleotides −15 to −33 of the leukotoxin promoter contain a sequence similar to the consensus “iron box” that is bound by the Fur repressor (Fig. 1). Fur could be the protein responsible for the iron-dependent regulation that we observed. A role for Fur repression could not be proven by our deletion study because the iron box is contained within the Sau3A fragment and this promoter fragment would remain repressed in the presence of iron. Site-directed mutation of the potential Fur binding site would be expected to increase expression of leukotoxin under iron-rich conditions.
Neither the level of oxygenation nor the pH of the medium had an effect on leukotoxin transcription, although previous reports had suggested roles for these conditions. Anaerobic modulation was hypothesized based on a recent report linking the ferric nitrate regulator Fnr to the transcription of leukotoxin (44). When fnr was cloned in E. coli, no differential regulation was observed under anaerobic conditions, but when the M. haemolytica Fnr homologue FnrP was coexpressed in E. coli, transcription was activated about fivefold. The relevance of this observation to leukotoxin transcription in M. haemolytica remains to be determined. Likewise, we did not observe a difference in leukotoxin promoter activity when the pH of the medium was altered. This is in contrast to a study by Strathdee and Lo that showed increased leukotoxin mRNA when cultures were shifted from pH 6.5 to pH 7.3 (39). Because our experiments used steady-state cultures and not pH shifts, the results observed by Strathdee and Lo may have been transient and therefore would not be detected in our system.
In support of the results presented by Strathdee and Lo (39), leukotoxin transcription was affected by temperature. Shifts from a low-temperature to a higher-temperature environment resulted in an increase in promoter activity. Although growth rates differed between 30 and 42°C, we do not believe that the results are related to this because initiation is constant during all phases and rates of growth (Fig. 4). Furthermore, in our anaerobic studies, the growth rate was significantly reduced and leukotoxin transcription was not affected. The E. coli plasmid-borne hemolysin has also been shown to be thermoregulated (31). In E. coli, this effect is due to Hha and HNS, two temperature-dependent DNA binding proteins (33, 34). HNS does not have a sequence-specific binding site but, instead, prefers to bind to AT-rich DNA or bent DNA such as that found in the M. haemolytica leukotoxin promoter. As the temperature increases, HNS loses its ability to bind DNA, causing a release of repression. Using both chromosomal and plasmid operon fusions in an hns mutant E. coli strain, we observed an increase in leukotoxin relative to that of a wild-type host at 30°C (A. M. Marciel, L. Alvarez, and S. K. Highlander, unpublished data). Thus, like the E. coli hemolysin, HNS may repress leukotoxin transcription at low temperatures.
Information gained from our plasmid operon fusion studies and previous studies in our laboratory have also provided us with a working model for lapT transcription (19). PlapT promoter activity was minimal in early log phase and increased toward stationary phase. This pattern parallels the protein expression of IHF in E. coli (7). Deletion of the near-consensus IHF binding site within the lapT/lkt promoter region resulted in loss of PlapT activity, indicating that IHF acts as an activator of lapT transcription (Fig. 8).
We have shown by mutational analysis and environmental variation that the lapT and lkt promoters are regulated at the level of transcription initiation. These studies were possible due to the creation of both chromosomal and plasmid-borne cat operon fusions. It appears that the leukotoxin promoter is subject to complex regulation by both repression and activation. Repression by Fur and HNS is indicated, and our deletion analysis provides support for the role of an activator. Now that certain regions have been recognized as necessary for PlapT and Plkt activity, a targeted-mutagenesis approach can be taken to specifically identify the regulating sequences. An important future step in the investigation of leukotoxin transcriptional regulation is to identify trans-acting factors from M. haemolytica that interact with the promoter. Eventually, a comprehensive understanding of M. haemolytica virulence might include a comparison of all of the genes turned on during the organism's virulent phase and identification of common factors that control their regulation.
ACKNOWLEDGMENTS
We thank Richard R. Sinden, of The Institute of Biosciences and Technology, Houston, Tex., for assistance in generating the computer topology model of the lapT/lkt promoter.
This project was funded by USDA grants 96-35204-3825 and 99-35204-7875 to S.K.H., and A.M.M. was supported by the B.C.M. M.D./Ph.D. Patent Royalties Fund.
REFERENCES
- 1.Adler H I. The use of microbial membranes to achieve anaerobiosis. Crit Rev Biotechnol. 1990;10(2):119–127. doi: 10.3109/07388559009068263. [DOI] [PubMed] [Google Scholar]
- 2.Bailey M J A, Koronakis V, Schmoll T, Hughes C. Escherichia coli HlyT protein, a transcriptional activator of haemolysin synthesis and secretion, is encoded by the rfaH (sfrB) locus required for expression of sex factor and lipopolysaccharide genes. Mol Microbiol. 1992;6:1003–1012. doi: 10.1111/j.1365-2958.1992.tb02166.x. [DOI] [PubMed] [Google Scholar]
- 3.Cannon W, Charlton W, Buck M. Organization and function of binding sites for the transcriptional activator NifA in the Klebsiella pneumoniae nifE and nifU promoters. J Mol Biol. 1991;220:915–931. doi: 10.1016/0022-2836(91)90363-b. [DOI] [PubMed] [Google Scholar]
- 4.Caskey L S, Lamphear J G, Highlander S K. Binding-protein-dependent arginine transport in Pasteurella haemolytica. Microbiology. 1996;142:1739–1747. doi: 10.1099/13500872-142-7-1739. [DOI] [PubMed] [Google Scholar]
- 5.Clinkenbeard K D, Upton M L. Lysis of bovine platelets by Pasteurella haemolytica leukotoxin. Am J Vet Res. 1991;52:453–457. [PubMed] [Google Scholar]
- 6.Czuprynski C J, Noel E J, Ortiz-Carranza O, Srikumaran S. Activation of bovine neutrophils by partially purified Pasteurella haemolytica leukotoxin. Infect Immun. 1991;59:3126–3133. doi: 10.1128/iai.59.9.3126-3133.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ditto M D, Roberts D, Weisberg R A. Growth phase variation of integration host factor level in Escherichia coli. J Bacteriol. 1994;176:3738–3748. doi: 10.1128/jb.176.12.3738-3748.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dungworth D L. The respiratory system. In: Jubb K V F, Kennedy P C, Palmer N, editors. Pathology of domestic animals. 3rd ed. Vol. 2. New York, N.Y: Academic Press, Inc.; 1985. pp. 413–556. [Google Scholar]
- 9.Fedorova N D, Highlander S K. Generation of targeted nonpolar gene insertions and operon fusions in Pasteurella haemolytica and creation of a strain that produces and secretes inactive leukotoxin. Infect Immun. 1997;65:2593–2598. doi: 10.1128/iai.65.7.2593-2598.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fedorova N D, Highlander S K. Plasmids for heterologous expression in Pasteurella haemolytica. Gene. 1997;186:207–211. doi: 10.1016/s0378-1119(96)00704-4. [DOI] [PubMed] [Google Scholar]
- 11.Felmlee T, Pellett S, Welch R A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985;163:94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank G H. Pasteurellosis of cattle. In: Adlam C F, Rutter J M, editors. Pasteurella and pasteurellosis. London, United Kingdom: Academic Press Limited; 1989. pp. 197–222. [Google Scholar]
- 13.Frank G H, Smith P C. Prevalence of Pasteurella haemolytica in transported calves. Am J Vet Res. 1983;44:981–985. [PubMed] [Google Scholar]
- 14.Glaser P, Sakamoto H, Bellalou J, Ullmann A, Danchin A. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase- haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988;7:3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomelsky M, Kaplan S. Genetic evidence that PpsR from Rhodobacter sphaeroides 2.4.1 functions as a repressor of puc and bchF expression. J Bacteriol. 1995;177:1634–1637. doi: 10.1128/jb.177.6.1634-1637.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez C T, Maheswaran S K. The role of induced virulence factors produced by Pasteurella haemolytica in the pathogenesis of bovine pneumonic pasteurellosis: review and hypothesis. Br Vet J. 1993;149:183–193. doi: 10.1016/S0007-1935(05)80088-0. [DOI] [PubMed] [Google Scholar]
- 17.Grambow N J, Birkeland N K, Anders D L, Christie G E. Deletion analysis of bacteriophage P2 late promoter. Gene. 1990;95:9–15. doi: 10.1016/0378-1119(90)90407-i. [DOI] [PubMed] [Google Scholar]
- 18.Highlander S K, Engler M J, Weinstock G M. Secretion and expression of the Pasteurella haemolytica leukotoxin. J Bacteriol. 1990;172:2343–2350. doi: 10.1128/jb.172.5.2343-2350.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Highlander S K, Weinstock G M. Static DNA bending and protein interactions within the Pasteurella haemolytica leukotoxin promoter region: development of an activation model for leukotoxin transcriptional control. DNA Cell Biol. 1994;13:171–181. doi: 10.1089/dna.1994.13.171. [DOI] [PubMed] [Google Scholar]
- 20.Highlander S K, Wickersham E A, Garza O, Weinstock G M. Expression of the Pasteurella haemolytica leukotoxin is inhibited by a locus that encodes an ATP-binding cassette homolog. Infect Immun. 1993;61:3942–3951. doi: 10.1128/iai.61.9.3942-3951.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen R, Pierson R E, Braddy P M, Saari D A, Lauerman L H, England J J, Keyvanfar H, Collier J R, Horton D P, McChesney A E, Benitez A, Christie R M. Shipping fever pneumonia in yearling feedlot cattle. J Am Vet Med Assoc. 1976;169:500–506. [PubMed] [Google Scholar]
- 22.Julien B, Calendar R. Bacteriophage PSP3 and ΦR73 activator proteins: analysis of promoter specificities. J Bacteriol. 1996;178:5668–5674. doi: 10.1128/jb.178.19.5668-5675.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolodrubetz D, Spitznagel J, Jr, Wang B, Phillips L H, Jacobs C, Kraig E. cis elements and trans factors are both important in strain-specific regulation of the leukotoxin gene in Actinobacillus actinomycetemcomitans. Infect Immun. 1996;64:3451–3460. doi: 10.1128/iai.64.9.3451-3460.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraig E, Dailey T, Kolodrubetz D. Nucleotide sequence of the leukotoxin gene from Actinobacillus actinomycetemcomitans: homology to the alpha-hemolysin/leukotoxin gene family. Infect Immun. 1990;58:920–929. doi: 10.1128/iai.58.4.920-929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar A, Malloch R A, Fujita N, Smillie D A, Ishihama A, Hayward R S. The minus 35-recognition region of Escherichia coli sigma 70 is inessential for initiation of transcription at an “extended minus 10” promoter. J Mol Biol. 1993;232:406–418. doi: 10.1006/jmbi.1993.1400. [DOI] [PubMed] [Google Scholar]
- 26.Lin W, Fullner K J, Clayton R, Sexton J A, Rogers M B, Calia K E, Calderwood S B, Fraser C, Mekalanos J J. Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc Natl Acad Sci USA. 1999;96:1071–1076. doi: 10.1073/pnas.96.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Litwin C M, Calderwood S B. Role of iron in regulation of virulence genes. Clin Microbiol Rev. 1993;6:137–149. doi: 10.1128/cmr.6.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lo R Y C, Strathdee C A, Shewen P E. Nucleotide sequence of the leukotoxin genes of Pasteurella haemolytica A1. Infect Immun. 1987;55:1987–1996. doi: 10.1128/iai.55.9.1987-1996.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mekalanos J J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992;174:1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller J F, Mekalanos J J, Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989;243:916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- 31.Mourino M, Munoa F, Balsalobre C, Diaz P, Madrid C, Juarez A. Environmental regulation of α-haemolysin expression in Escherichia coli. Microb Pathog. 1994;16:249–259. doi: 10.1006/mpat.1994.1026. [DOI] [PubMed] [Google Scholar]
- 32.Neumann J R, Morency C A, Russian K O. A novel rapid assay for chloramphenicol acetyltransferase gene expression. Bio/Technology. 1987;5:444–447. [Google Scholar]
- 33.Nieto J M, Madrid C, Prenafeta A, Miquelay E, Balsalobre C, Carrascal M, Juarez A. Expression of the hemolysin operon in Escherichia coli is modulated by a nucleoid-protein complex that includes the proteins Hha and H-NS. Mol Gen Genet. 2000;263:349–358. doi: 10.1007/s004380051178. [DOI] [PubMed] [Google Scholar]
- 34.Nieto J M, Mourino M, Balsalobre C, Madrid C, Prenafeta A, Munoa F J, Juarez A. Construction of a double hha hns mutant of Escherichia coli: effect on DNA supercoiling and alpha-haemolysin production. FEMS Microbiol Lett. 1997;155:39–44. doi: 10.1111/j.1574-6968.1997.tb12683.x. [DOI] [PubMed] [Google Scholar]
- 35.Saban R, Broadstone R V, Haak-Frendscho M, Skoyen S, Fialkowski J, Maheswaran S K, Bjorling D E, Czuprynski C. Effects of Pasteurella haemolytica leukotoxin and lipopolysaccharide on histamine, prostanoid, and leukotriene release by bovine lung parenchyma in vitro. Am J Vet Res. 1997;58:1227–1231. [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 37.Sayle R A, Milner-White E J. RASMOL: biomolecular graphics for all. Trends Biochem Sci. 1995;20:374–376. doi: 10.1016/s0968-0004(00)89080-5. [DOI] [PubMed] [Google Scholar]
- 38.Shewen P E, Wilkie B N. Cytotoxin of Pasteurella haemolytica acting on bovine leukocytes. Infect Immun. 1982;35:91–94. doi: 10.1128/iai.35.1.91-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strathdee C A, Lo R Y C. Regulation of expression of the Pasteurella haemolytica leukotoxin determinant. J Bacteriol. 1989;171:5955–5962. doi: 10.1128/jb.171.11.5955-5962.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Y, Clinkenbeard K D, Clarke C, Cudd L, Highlander S K, Dabo S M. Pasteurella haemolytica leukotoxin induced apoptosis of bovine lymphocytes involves DNA fragmentation. Vet Microbiol. 1999;65:153–166. doi: 10.1016/s0378-1135(98)00286-7. [DOI] [PubMed] [Google Scholar]
- 41.Thompson S A, Wang L L, West A, Sparling P F. Neisseria meningitidis produces iron-regulated proteins related to the RTX family of exoproteins. J Bacteriol. 1993;175:811–818. doi: 10.1128/jb.175.3.811-818.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trifonov E N, Sussman J L. The pitch of the chromatin DNA is reflected in its nucleotide sequence. Proc Natl Acad Sci USA. 1980;77:3816–3820. doi: 10.1073/pnas.77.7.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uhl M A, Miller J F. Bordetella pertussis BvgAS virulence control system. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: ASM Press; 1995. pp. 333–349. [Google Scholar]
- 44.Uhlich G A, McNamara P J, Iandolo J J, Mosier D A. FnrP interactions with the Pasteurella haemolytica leukotoxin promoter. FEMS Microbiol Lett. 2000;186:73–77. doi: 10.1111/j.1574-6968.2000.tb09084.x. [DOI] [PubMed] [Google Scholar]
- 45.Vogel M, Hess J, Then I, Juarez A, Goebel W. Characterization of a sequence (hylR) which enhances synthesis and secretion of hemolysin in Escherichia coli. Mol Gen Genet. 1988;212:76–84. doi: 10.1007/BF00322447. [DOI] [PubMed] [Google Scholar]
- 46.Weinberg E D. Iron withholding: a defense against infection and neoplasia. Physiol Rev. 1984;64:65–102. doi: 10.1152/physrev.1984.64.1.65. [DOI] [PubMed] [Google Scholar]
- 47.Welch R A. Pore-forming cytolysins of gram-negative bacteria. Mol Microbiol. 1991;5:521–528. doi: 10.1111/j.1365-2958.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]