Abstract
BACKGROUND:
The prevalence of familial hypercholesterolemia is 1 in 250, but <10% of patients are diagnosed. Cascade testing enables early detection of cases through systematic family tracing. Establishment of familial hypercholesterolemia cascade testing programs in the US could be informed by approaches used elsewhere.
METHODS:
We conducted a systematic review of published studies in the English language of cascade testing for familial hypercholesterolemia, which reported the number of index cases and number of relatives tested and specified methods of contacting relatives and testing modalities methods utilized. For each study, we calculated yield (proportion of relatives who test positive) and new cases per index case, to facilitate comparison.
RESULTS:
We identified 10 studies from the literature that met inclusion criteria; the mean number of probands and relatives per study was 242 and 826, respectively. The average yield was 44.76% with a range of 30% to 60.5%, and the mean new cases per index case was 1.65 with a range of 0.22 to 8.0. New cases per index case tended to be greater in studies that used direct contact versus indirect contact (2.06 versus 0.86), tested beyond first-degree relatives versus only first-degree relatives (3.65 versus 0.80), used active sample collection versus collection at clinic (4.11 versus 1.06), and utilized genetic testing versus biochemical testing (2.47 versus 0.42).
CONCLUSIONS:
New case detection in familial hypercholesterolemia cascade testing programs tended to be higher with direct contact of relatives, testing beyond first-degree relatives, in-home–based sample collection, and genetic testing. These findings should be helpful for establishing cascade testing programs in the United States.
Keywords: cholesterol, humans, hyperlipoproteinemia type II, prevalence, systematic review
Familial hypercholesterolemia (FH) is an autosomal dominant disorder that results in lifelong elevation of serum cholesterol levels and is associated with significantly increased risk of coronary heart disease.1,2 The prevalence of FH is 1 in 250, making it the most common serious genetic disorder.3,4 It is estimated that there are ≈1.3 million patients with FH in the United States of whom <10% have been identified despite established clinical scoring systems including the Dutch Lipid Clinic Network Diagnostic Criteria,5 Simon Broome Diagnostic Criteria,6 and the Making Early Diagnosis to Prevent Early Death diagnostic criteria.7
The molecular basis for autosomal dominant FH is the presence of pathogenic/likely pathogenic (P/LP) variants in LDLR, APOB, or PCSK9. P/LP variants in LDLR account for 85% to 90% of cases of autosomal dominant FH.8 P/LP APOB variants account for 5% to 10% of FH cases in northern European populations but a lower proportion in other populations.8 PCSK9 P/LP variants are the least common monogenic cause of FH, implicated in <5% of cases.8 A rare autosomal recessive form of FH is attributed to the reduced expression of LDLRAP1.9 The genetic basis of FH also includes elevations in lipoprotein (a) and polygenic influences10 and potentially monogenic causes that have yet to be identified.
Cascade testing, by early detection and treatment of family members, can reduce mortality and morbidity from FH. Based on the SAFEHEART Registry (Spanish Familial Hypercholesterolemia Cohort) data, over 10 years, the detection of 9000 new FH cases could prevent 847 coronary events including 203 coronary deaths.11 Cascade testing is considered an effective method for identifying new cases of FH by a process of systematic family tracing and is recommended by UK National Institute for Health and Clinical Excellence.12 A number of countries have assessed the efficacy of cascade testing for FH including the United Kingdom,13–15 the Netherlands,16 Australia,17 Latvia,18 South Africa,19 and Brazil.20 These studies vary in methodology making comparison difficult. Some studies utilized genetic testing, some relied on lipid testing and clinical criteria, and others used both. Additionally, the studies differed in data collection, participant interaction, and the degree of relatedness with family members who were tested subsequently.
In the United States, a nationwide cascade testing program is yet to be established, likely because of several reasons—the US healthcare system comprises many providers and payers across a large geographic region.21 The Health Insurance Portability and Accountability Act prohibits direct notification of at-risk relatives by healthcare providers unless prior authorization has been obtained from probands.22 We conducted a systematic review of the literature to compare diagnostic yield and new case detection between published studies of cascade testing and to describe these values in the context of individual study methodology. Our goal was to generate comparative data from FH cascade testing studies to inform establishment of cascade testing programs in the United States.21
METHODS
Institutional review board approval was not required for this study per local institutional guidelines. All supporting data used in the study along with a detailed study methodology are available in the Data Supplement. These data, methods used in the analysis, and materials used to conduct the research are available to any researcher for purposes of reproducing the results or replicating the procedures of this study. A flowchart depicting selection of FH cascade testing studies for this systematic review (based on preferred reporting items for systematic reviews and meta-analyses for protocols) is depicted in the Figure. The research studies are listed in the Data Supplement.
Figure.
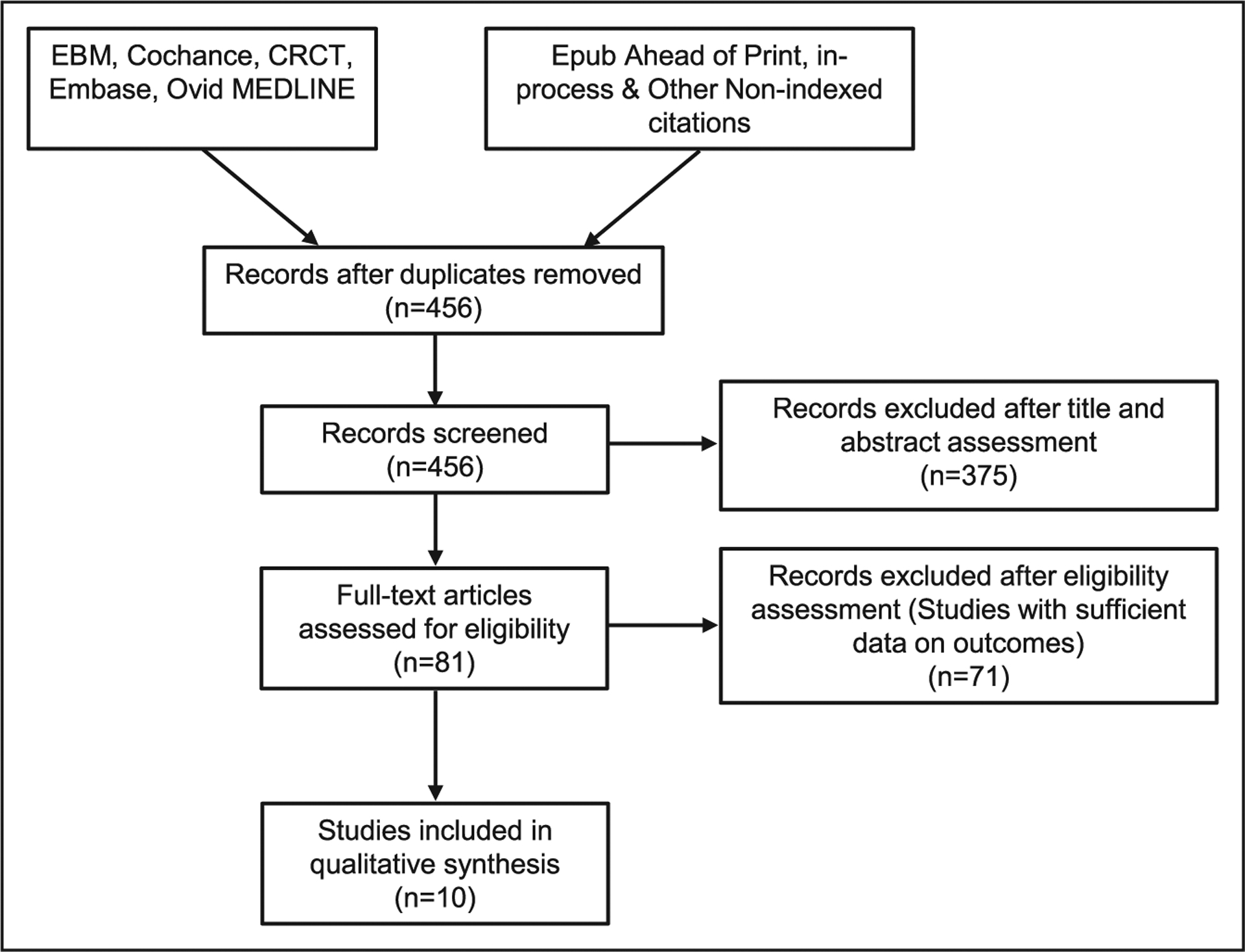
Flowchart depicting selection of familial hypercholesterolemia cascade testing studies for this systematic review (based on preferred reporting items for systematic reviews and meta-analyses for protocols). CRCT indicates Cochrane Central Register of Controlled Trials; and EBM, evidence based medicine.
RESULTS
Study Characteristics
Ten studies were examined as part of this review, with the earliest published in 2001 and the most recent in 2018 (Table 1). Countries represented United Kingdom,13–15 the Netherlands,16 Australia,17 Latvia,18 South Africa,19 and Brazil20; no studies from the United States were available. The total number of index cases per study ranged from 21 to 733 with a mean of 242 and a median of 232. Family members included in the cascade were selected from either first-degree relatives (FDRs) or both FDRs and second-degree relatives (SDRs) and third-degree relatives (TDRs). The number of relatives in each study population ranged from 6423 to 5442.16 The mean diagnostic yield was 44.76% with a range of 30% to 60.50%.
Table 1.
Study Characteristics
| Study | Country | Probands, n | Detection of Index Cases | Family Members, n | Contact and Collection | Testing |
|---|---|---|---|---|---|---|
| Bhatnagar et al14 | United Kingdom | 259 | SBR | 200 | Letter advising an appointment with PCP | Biochemical |
| Umans-Eckenhausen et al16 | The Netherlands | 237 | Diagnostic protocol followed by GT | 5442 | Nurse home visit | LDLR+Biochemical |
| Vergotine et al19 | South Africa | 379 | GT | 790 | … | LDLR |
| Marks et al15 | United Kingdom | 227 | SBR | 165 | Letter with choice of home visit or an SAC visit | Biochemical |
| Hadfield et al13 | United Kingdom | 733 | SBR | 769 | Letter advising an appointment with GP or to attend SAC | Biochemical |
| Muir et al24 | New Zealand | 76 | GT | 353 | Letter advising to attend SAC | LDLR, APOB |
| Bell et al17 | Australia | 100 | GT | 366 | Letter with telephone counseling and advice to attend SAC | LDLR, APOB, PCSK9 |
| Jannes et al20 | Brazil | 248 | GT | 394 | Referral to SAC | LDLR, APOB, PCSK9 |
| Latkovskis et al18 | Latvia | 140 | DLCN score | 68 | … | Biochemical |
| Alver et al23 | Estonia | 21 | GT | 64 | Family member directed invite to SAC | LDLR, APOB, PCSK9 |
DLCN indicates Dutch Lipid Clinic Network; GP, general practitioner; GT, genetic testing; PCP, primary care provider; SAC, study-associated clinic; and SBR, Simon Broome Register.
CONTACTING RELATIVES
The design of each contact protocol met local privacy and confidentially requirements. The method of contact was either direct or indirect, with indirect being more common (Table 2). Indirect contact involved the index case being provided with a letter to distribute to family members; in some cases, this letter was labeled specifically for the attention of individual family members who were considered at risk. Direct contact involved the mailing of letters by study staff to at-risk family members with the consent of the index case. The letter to relatives typically outlined that a family member had been diagnosed with FH and that they may be at risk, as well as explaining the benefits of screening and how to enroll in the study. In the study by Marks et al,15 the diagnosis of FH was initially withheld in an attempt to minimize any undue stress on the contacted relative; however, at-risk family members were informed of the potential risks and the nature of the disease if they did not respond. Nine of the 10 studies provided information on family member contact with 4 being indirect, 4 direct, and 1 being a combination of direct and indirect. The mean new cases per index case (NCIC) with direct contact was 2.06 and with indirect contact was 0.86.
Table 2.
Yield and NCIC: Contact Method, Degree of Relatedness, Sample Acquisition, and Testing Method
| Study | Initial Contact Method | Relatives Tested | Site for Obtaining Sample | New Case Ascertainment | Yield, % | NCIC |
|---|---|---|---|---|---|---|
| Bhatnagar et al14 | Direct | FDR | SAC or PCP | SBR criteria | 60.50 | 0.47 |
| Umans-Eckenhausen et al16 | Direct | FDR, SDR, TDR | Nurse home visit | Diagnostic protocol followed by GT | 37 | 8 |
| Vergotine et al19 | Unclear | FDR (close relatives) | Unclear | GT | 42 | 0.89 |
| Marks et al15 | Indirect (response rate 35%) | FDR | Home visit or SAC | SBR criteria | 30 | 0.22 |
| Hadfield et al13 | Direct and indirect | FDR | SAC or PCP | SBR criteria | 30 | 0.7 |
| Muir et al24 | Direct (letter, laboratory form, consent) | FDR | Local laboratory | GT | 45 | 2.09 |
| Bell et al17 | Indirect | FDR, SDR, TDR | SAC | GT | 51.40 | 2 |
| Jannes et al20 | Direct | FDR* | SAC | GT | 59.40 | 0.94 |
| Latkovskis et al18 | Indirect | FDR | SAC | DLCN score | 60.30 | 0.29 |
| Alver et al23 | Indirect | FDR, SDR | SAC | GT | 31 | 0.95 |
DLCN indicates Dutch Lipid Clinic Network; FDR, first-degree relative; FH, familial hypercholesterolemia; GT, genetic testing; NCIC, new cases per index case; PCP, primary care provider; SAC, study-associated clinic; SBR, Simon-Broom Registry; SDR, second-degree relative; and TDR, third-degree relative.
Cascade testing extended to SDRs of the proband upon detection of FH in FDRs.
Degrees of Relatedness of Proband With Tested Family Members
Each study set out the degree of relatedness to which the cascade would progress either explicitly or implicitly (Table 2). Seven of the studies were limited to FDRs. In the 2001 study by Vergotine et al,19 testing was described as confined to close relatives, which was inferred as referring to FDRs. One study included both FDRs and SDRs,23 whereas 2 studies included FDRs, SDRs, and TDRs.16,17 The mean NCIC for studies that advanced beyond FDRs was 3.65 versus 0.80 for studies that tested FDRs only.
COLLECTION OF SAMPLES FROM RELATIVES
The method of collection of samples ranged from passive approaches including attending a primary care clinic or an associated study clinic to the more proactive strategy of in-home testing by study staff (Table 3). The most common approach was an invitation to relatives either by the proband or directly by the study team to attend a primary care clinic or a study-associated clinic, which was typically located in an academic center associated with the study investigators.13,14,17,18,20,23 These clinic visits included blood draws and in some cases outpatient assessment including physical exam and the completion of a study questionnaire. In 2 studies, relatives were given the option of either visiting a centralized clinic or their primary care clinic.13,14 The most active approach was taken by Umans-Eckenhausen et al16 in the Netherlands with relatives visited in their home by specialist nursing staff who performed blood draws and collected family and personal history. In the United Kingdom, Marks et al15 offered both primary care clinic–based testing and in-home blood draws by a nurse. Muir et al,24 in New Zealand, included in their mail-out packets a laboratory requisition form, allowing participants to attend a local health center for blood draw. The mean NCIC with home-based testing (considered active) versus clinic-based testing was 4.11 versus 1.06.
Table 3.
Mean Yield and NCIC Compared Between Various Study Methodologies
| Yield, % | NCIC | |
|---|---|---|
| Direct contact | 46.38 | 2.06 |
| Indirect contact | 43.17 | 0.86 |
| Beyond FDR | 39.8 | 3.65 |
| FDR only | 54.5 | 0.80 |
| Active sample collection | 33.5 | 4.11 |
| Centralized collection | 47.45 | 1.06 |
| Genetic testing | 44.3 | 2.47 |
| Biochemical testing | 45.2 | 0.42 |
FDR indicates first-degree relative; and NCIC, new cases per index case.
MODE OF TESTING: BIOCHEMICAL VERSUS GENETIC
Testing of relatives was either primarily genetic (n=6 studies) or biochemical (n=4 studies). The study by Umans-Eckenhausen et al was unique in that it used a combination of biochemical and LDLR testing but for the purpose of this review was considered to represent a genetic testing study. Of the studies utilizing genetic testing, 2 studies tested only LDLR, 1 study tested LDLR and APOB, and 3 studies tested LDLR, APOB, and PCSK9 (Table 2). The diagnostic yield for each individual study is summarized in Table 2. The mean yield in biochemical and genetic testing cohorts was similar at 45% and 44%, respectively. The mean NCIC (Table 2) for genetic testing–based studies was 2.47 (range, 0.89–8.0; SD, 2.8), whereas the mean NCIC for nongenetic-based studies was 0.42 (range, 0.22–0.70; SD, 0.21). Even when excluding the prodigious study from the Netherlands that had an NCIC of 8,16 the mean for studies using genetic testing was higher than for biochemical studies at 1.37.
DISCUSSION
FH poses a significant public health burden by increasing the risk of early-onset coronary heart disease, including myocardial infarction and sudden cardiac death.25 Yet, awareness of FH in the United States continues to be poor, recommended screening approaches are limited by several barriers, and the uptake and yield of current methods of cascade testing is low.26 Given this reality, there is a need for cascade testing programs in the United States to enable early detection and treatment and thereby help reduce the morbidity and mortality from FH. In this systematic review, we compared diagnostic yield and NCIC in 10 previous FH cascade testing studies in the context of each study’s methodology. Based on our review, the number of new cases detected in a FH cascade testing program was higher with direct contact of relatives, inclusion of more distant (second and third degree) relatives, in-home sample collection, and the use of genetic testing.
The 2 studies with the highest NCIC pursued only direct participant contact.7,21 Direct contact may relieve probands of the burden and anxiety of contacting relatives and overcome barriers related to proband communication with relatives.15,27 Probands are generally welcoming of assistance from the healthcare team to contact relatives,28 and relatives are more likely to follow recommendations from healthcare providers.29 Direct contact may be particularly effective for more distant (second or third degree) relatives.30,31
The Dutch study by Umans-Eckenhausen et al and the Australian study by Bell et al17 both tested beyond FDRs to include SDRs and TDRs, whereas the Brazilian study by Alver et al23 included SDRs. Overall, the average NCIC for studies that advanced beyond FDRs was 3.65 versus 0.80 for studies that did not test beyond FDRs. It is logical that studies that include SDRs and TDRs would have a higher NCIC given the increased number of tested individuals. Thus, to maximize new case detection, a cascade testing program should extend to SDRs and TDRs.
Utilizing in-home testing directed by dedicated nursing staff is likely to have had an impact on the studies’ success. The Dutch study by Umans-Eckenhausen et al—a clear outlier in terms of NCIC—can be considered the most active in its design, utilized home-based testing and extending the cascade through SDR and TDR. Such an approach could impose a significant cost and logistical burden in the United States. However, approaches used by genetic testing companies might reduce such burden—saliva kits to obtain DNA can be mailed to relatives, and some companies also offer in-home blood draws.
Four of the studies used LDL (low-density lipoprotein) cholesterol measurement and either Dutch Lipid Clinic Network or SBR criteria to diagnose FH in relatives. A limitation of this approach is that LDL cholesterol levels of members with and without an FH variant can overlap.32 Measuring lipid levels of family members of an FH patient in the absence of genetic testing could potentially miss up to 20% of new FH cases.16 Also, detailed family history and additional FH clinical criteria at the time of case ascertainment may not be easily available. An advantage of genetic testing is that it provides unambiguous diagnosis in relatives of a proband with a defined P/LP variant. Nonetheless, biochemical testing is inexpensive, convenient and potentially the only feasible method in low-resource setting and when FH is ascertained in the proband based on clinical criteria.
At the time of writing this systematic review, no FH cascade testing studies in the US setting have been published. A majority of family members do not under-go cascade testing once a proband sends out letters recommending such testing.27 The proportion is likely to increase with direct contact but remains low.27 In an ongoing study of cascade testing in the United States, where personnel obtained consent from a proband to contact relatives, the NCIC was low; 0.8 cases were identified per proband (unpublished data).33 These data indicate a need to develop and implement the best practices for cascade testing in different settings in the United States34 and increase awareness and knowledge of FH among healthcare providers, probands, and their relatives, to promote cascade testing.
The findings of this systematic review can inform the establishment of future FH cascade testing programs in the United States. The cost-effectiveness and feasibility of measures such as direct contact of relatives, home visits by staff, extension of cascade testing beyond close relatives, and use of genetic testing need further study. Overall, higher case detection is likely to have positive impact on cost and practical implementation of a screening program.
Limitations
It is important to note that none of the included studies were completed in the United States, and as result, they did not have to conform to Health Insurance Portability and Accountability Act. The included European studies were completed before the implementation of the European General Data Protection and Regulation (2018), which is broadly comparable to Health Insurance Portability and Accountability Act but arguably more restrictive to large-scale medical research. Moreover, nearly all of the prior studies were based in jurisdictions with established single-payer healthcare models, which negated concerns related to effects on providers, payers, and participants. The number of available studies was small, which limited our ability to establish statistical significance in the association of NCIC with degrees of relatives tested, sample collection, testing, or contact methods. We did not include studies using reverse cascade testing such as the one reported by Wald et al12 because the methodology was fundamentally different from conventional cascade testing studies. Such wider screening, however, may be a useful complementary strategy by identifying new FH cases which can feed into a cascade testing program.
Conclusions
Cascade testing is considered a cost-effective method for detecting new cases of FH.11,35 Active approaches including direct relative contact and in-home visits had a higher new case detection rate than passive participant engagement. Studies that include SDRs and TDRs are likely to detect more cases of FH than those limited to FDRs only, and genetic-based testing appeared more successful than biochemical screening. Based on our systematic review of the literature, the ideal FH cascade screening program would involve direct contact of relatives, progress beyond FDRs through a family tree, utilize in-home sample collection, and would use genetic testing.
Supplementary Material
Sources of Funding
This study was supported by National Human Genome Research Institute-supported Electronic Medical Records and Genomics Network (U01HG006379) and National Heart, Lung, and Blood Institute grant K24 HL137010 (Dr Kullo).
Footnotes
Disclosures
None.
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCGEN.119.002723.
REFERENCES
- 1.Goldberg AC, et al. ; National Lipid Association Expert Panel on Familial Hypercholesterolemia. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association expert panel on familial hypercholesterolemia. J Clin Lipidol. 2011;5(suppl 3):S1–S8. doi: 10.1016/j.jacl.2011.04.003 [DOI] [PubMed] [Google Scholar]
- 2.Watts GF, et al. Familial hypercholesterolemia: a missed opportunity in preventive medicine. Nat Clin Pract Cardiovasc Med. 2007;4:404–405. doi: 10.1038/ncpcardio0941 [DOI] [PubMed] [Google Scholar]
- 3.Hopkins PN, et al. ; National Lipid Association Expert Panel on Familial Hypercholesterolemia. Familial hypercholesterolemias: prevalence, genetics, diagnosis and screening recommendations from the National Lipid Association expert panel on familial hypercholesterolemia. J Clin Lipidol. 2011;5(3 suppl):S9–17. doi: 10.1016/j.jacl.2011.03.452 [DOI] [PubMed] [Google Scholar]
- 4.de Ferranti SD, et al. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES). Circulation. 2016;133:1067–1072. doi: 10.1161/CIRCULATIONAHA.115.018791 [DOI] [PubMed] [Google Scholar]
- 5.Fouchier SW, et al. The molecular basis of familial hypercholesterolemia in the Netherlands. Hum Genet. 2001;109:602–615. doi: 10.1007/s00439-001-0628-8 [DOI] [PubMed] [Google Scholar]
- 6.Heath KE, et al. A molecular genetic service for diagnosing individuals with familial hypercholesterolaemia (FH) in the United Kingdom. Eur J Hum Genet. 2001;9:244–252. doi: 10.1038/sj.ejhg.5200633 [DOI] [PubMed] [Google Scholar]
- 7.Williams RR, et al. Diagnosing heterozygous familial hypercholesterolemia using new practical criteria validated by molecular genetics. Am J Cardiol. 1993;72:171–176. doi: 10.1016/0002-9149(93)90155-6 [DOI] [PubMed] [Google Scholar]
- 8.Varret M, et al. Genetic heterogeneity of autosomal dominant hypercholesterolemia. Clin Genet. 2008;73:1–13. doi: 10.1111/j.1399-0004.2007.00915.x [DOI] [PubMed] [Google Scholar]
- 9.Garcia CK, et al. Autosomal recessive hypercholesterolemia caused by mutations in a putative LDL receptor adaptor protein. Science. 2001;292:1394–1398. doi: 10.1126/science.1060458 [DOI] [PubMed] [Google Scholar]
- 10.Paquette M, et al. Polygenic risk score predicts prevalence of cardiovascular disease in patients with familial hypercholesterolemia. J Clin Lipidol. 2017;11:725–732 e725. [DOI] [PubMed] [Google Scholar]
- 11.Lázaro P, et al. Cost-effectiveness of a cascade screening program for the early detection of familial hypercholesterolemia. J Clin Lipidol. 2017;11:260–271. doi: 10.1016/j.jacl.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 12.Wald DS, et al. Child-parent familial hypercholesterolemia screening in primary care. N Engl J Med. 2016;375:1628–1637. doi: 10.1056/NEJMoa1602777 [DOI] [PubMed] [Google Scholar]
- 13.Hadfield SG, et al. ; Steering Group for the Department of Health Familial Hypercholesterolaemia Cascade Testing Audit Project. Family tracing to identify patients with familial hypercholesterolaemia: the second audit of the department of health familial hypercholesterolaemia cascade testing project. Ann Clin Biochem. 2009;46(pt 1):24–32. doi: 10.1258/acb.2008.008094 [DOI] [PubMed] [Google Scholar]
- 14.Bhatnagar D, et al. Outcome of case finding among relatives of patients with known heterozygous familial hypercholesterolaemia. BMJ. 2000;321:1497–1500. doi: 10.1136/bmj.321.7275.1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marks D, et al. Cascade screening for familial hypercholesterolaemia: implications of a pilot study for national screening programmes. J Med Screen. 2006;13:156–159. doi: 10.1258/096914106778440617 [DOI] [PubMed] [Google Scholar]
- 16.Umans-Eckenhausen MA, et al. Review of first 5 years of screening for familial hypercholesterolaemia in the Netherlands. Lancet. 2001;357:165–168. doi: 10.1016/S0140-6736(00)03587-X [DOI] [PubMed] [Google Scholar]
- 17.Bell DA, et al. Effectiveness of genetic cascade screening for familial hypercholesterolaemia using a centrally co-ordinated clinical service: an Australian experience. Atherosclerosis. 2015;239:93–100. doi: 10.1016/j.atherosclerosis.2014.12.036 [DOI] [PubMed] [Google Scholar]
- 18.Latkovskis G, et al. Latvian registry of familial hypercholesterolemia: the first report of three-year results. Atherosclerosis. 2018;277:347–354. doi: 10.1016/j.atherosclerosis.2018.06.011 [DOI] [PubMed] [Google Scholar]
- 19.Vergotine J, et al. Clinical versus molecular diagnosis of heterozygous familial hypercholesterolaemia in the diverse South African population. S Afr Med J. 2001;91:1053–1059. [PubMed] [Google Scholar]
- 20.Jannes CE, et al. Familial hypercholesterolemia in Brazil: cascade screening program, clinical and genetic aspects. Atherosclerosis. 2015;238:101–107. doi: 10.1016/j.atherosclerosis.2014.11.009 [DOI] [PubMed] [Google Scholar]
- 21.Roberts MC, et al. Delivery Of cascade screening for hereditary conditions: a scoping review of the literature. Health Aff (Millwood). 2018;37:801–808. doi: 10.1377/hlthaff.2017.1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Health Insurance Portability and Accountability Act. In. 104th Congress ed; 1996:1–18. [Google Scholar]
- 23.Alver M, et al. Recall by genotype and cascade screening for familial hypercholesterolemia in a population-based biobank from Estonia. Genet Med. 2019;21:1173–1180. doi: 10.1038/s41436-018-0311-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muir LA, et al. Preventing cardiovascular disease: a review of the effectiveness of identifying the people with familial hypercholesterolaemia in New Zealand. N Z Med J. 2010;123:97–102. [PubMed] [Google Scholar]
- 25.Safarova MS, et al. My approach to the patient with familial hypercholesterolemia. Mayo Clin Proc. 2016;91:770–786. doi: 10.1016/j.mayocp.2016.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Safarova MS, et al. Lessening the burden of familial hypercholesterolemia using health information technology. Circ Res. 2018;122:26–27. doi: 10.1161/CIRCRESAHA.117.312319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suthers GK, et al. Letting the family know: balancing ethics and effectiveness when notifying relatives about genetic testing for a familial disorder. J Med Genet. 2006;43:665–670. doi: 10.1136/jmg.2005.039172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardcastle SJ, et al. Patients’ perceptions and experiences of familial hypercholesterolemia, cascade genetic screening and treatment. Int J Behav Med. 2015;22:92–100. doi: 10.1007/s12529-014-9402-x [DOI] [PubMed] [Google Scholar]
- 29.Agård A, et al. Familial hypercholesterolemia: ethical, practical and psychological problems from the perspective of patients. Patient Educ Couns. 2005;57:162–167. doi: 10.1016/j.pec.2004.05.010 [DOI] [PubMed] [Google Scholar]
- 30.Finlay E, et al. Factors determining dissemination of results and uptake of genetic testing in families with known BRCA1/2 mutations. Genet Test. 2008;12:81–91. doi: 10.1089/gte.2007.0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maxwell SJ, et al. Communicating familial hypercholesterolemia genetic information within families. Genet Test Mol Biomarkers. 2009;13:301–306. doi: 10.1089/gtmb.2008.0138 [DOI] [PubMed] [Google Scholar]
- 32.Knowles JW, et al. Cascade screening for familial hypercholesterolemia and the use of genetic testing. JAMA. 2017;318:381–382. doi: 10.1001/jama.2017.8543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kullo IJ, et al. Design of a controlled trial of cascade screening for hypercholesterolemia: the (CASH) Study. J Pers Med. 2018;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Academies of Sciences, Engineering, and Medicine. Action collaboratives: Genomics and Population Health Action Collaborative [Internet]. Washington (DC): National Academies; [cited 2018 Mar 26]. Available at: http://www.nationalacademies.org/hmd/Activities/Research/GenomicBasedResearch/Innovation-Collaboratives/Genomics-and-Population-Health.aspx. [Google Scholar]
- 35.Kerr M, et al. Cost effectiveness of cascade testing for familial hypercholesterolaemia, based on data from familial hypercholesterolaemia services in the UK. Eur Heart J. 2017;38:1832–1839. doi: 10.1093/eurheartj/ehx111 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


