Abstract
Background:
Fine particulate matter () has been found to be detrimental to respiratory health of children, but few studies have examined the effects of prenatal oxidative potential (OP) on lung function in infants and preschool children.
Objectives:
We estimated the associations of personal exposure to and OP during pregnancy on offspring objective lung function parameters and compared the strengths of associations between both exposure metrics.
Methods:
We used data from 356 mother–child pairs from the SEPAGES cohort. PM filters collected twice during a week were analyzed for OP, using the dithiothreitol (DTT) and the ascorbic acid (AA) assays, quantifying the exposure of each pregnant woman. Lung function was assessed with tidal breathing analysis (TBFVL) and nitrogen multiple-breath washout () test, performed at 6 wk, and airwave oscillometry (AOS) performed at 3 y. Associations of prenatal mass and OP with lung function parameters were estimated using multiple linear regressions.
Results:
In neonates, an interquartile (IQR) increase in () was associated with a decrease in functional residual capacity (FRC) measured by [; 95% confidence interval (CI): , 0.15]. Associations with showed similar patterns in comparison with but of smaller magnitude. Lung clearance index (LCI) and TBFVL parameters did not show any clear association with the exposures considered. At 3 y, increased frequency-dependent resistance of the lungs () from AOS tended to be associated with higher (; 95% CI: , 0.24) and (; ; 95% CI: , 0.27) but not with (; ; 95% CI: , 0.16). Results for FRC and remained similar in OP models adjusted on .
Discussion:
Prenatal exposure to was associated with several offspring lung function parameters over time, all related to lung volumes. https://doi.org/10.1289/EHP11155
Introduction
Exposure to ambient particulate matter (PM) increases risk of chronic respiratory diseases and triggers asthma and chronic obstructive pulmonary diseases.1–3 Early life, including pregnancy, is a vulnerable time window for the health effects of air pollution.4,5 Exposure to PM during pregnancy is reported to influence fetal and infant lung development and respiratory health.6–10
Measures of children’s respiratory health, including spirometry outcomes,11–13 asthma incidence,14 or fraction of nitric oxide in exhaled air (),15 have been widely investigated in association with outdoor air pollution. Although studying lung function of children in early childhood is of great interest for the evaluation of their susceptibility to respiratory diseases later in life, most previous studies11–15 were limited to children older than 5 y of age, when spirometry becomes feasible. Very few studies8,10,16 have used noninvasive techniques that allow for the measurement of lung function in very young children, such as tidal breathing flow-volume loops analysis (TBFVL), nitrogen multiple-breath washout (), or airwave oscillometry (AOS). Yet, these techniques rely on tidal breathing, making them particularly suitable and feasible in population-based cohorts. Muttoo et al.16 and Latzin et al.10 found decreases in functional residual capacity (FRC) and tidal volume (), respectively, estimated by MBW and TBFVL, in children who had higher prenatal exposure to nitrogen oxides () or particulate matter (PM) with diameter (). Dutta et al.8 found higher airway reactance () measured by AOS in children with higher postnatal exposures to particles with diameter ().
Most epidemiological studies examining the health effects of PM used the mass concentration metric in association with health parameters.17,18 Although the biological pathways are not fully understood yet, evidence suggest that oxidative stress caused by PM is a key factor in understanding PM-associated health effects.19–22 The ability of PM to generate reactive oxygen species (ROS) and thereby induce oxidative stress is measured by the oxidative potential (OP), an integrative metric of several physical and chemical properties of PM and its health effects.23 Several recent studies have presented OP as a better predictor than concentration for assessing association with some cardiorespiratory diseases.24,25 The studies addressing the effects of OP exposure on children’s lung function, although few in number, converged to a stronger detrimental effect of OP as compared to PM mass.26–28 These latter studies used average urban ambient OP measurements or OP estimated by land-use regression (LUR) models, which could lead to measurement errors, given that most people in Western countries spend more than 80% of their time indoors.29 Thereby, personal sampling has been proposed to increase the accuracy in exposure assessment; however, to the best of our knowledge, no study has estimated personal prenatal exposure to OP in relation to respiratory function in the first years of life.
The aim of this study was to assess whether maternal personal exposure to mass concentration and to the OP of is associated with lung function in newborns and in preschool children. The effects of OP and PM were also compared, and the independency of OP effects from were tested.
Methods
Study Population
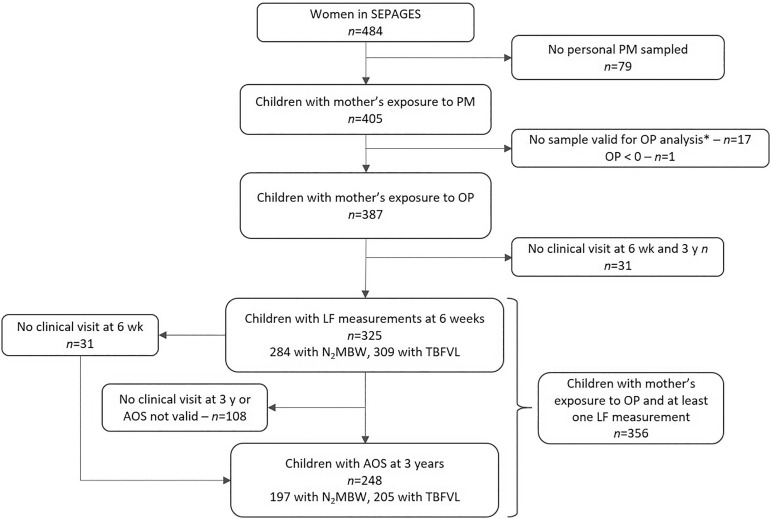
This study is based on the data from the French mother–child SEPAGES cohort that has been set up to describe maternal and child personal exposure to environmental pollutants and their effects on health. The study design and protocol have been previously described by Lyon-Caen et al.30 Briefly, pregnant women were recruited from July 2014 to July 2017 in eight obstetrical ultrasonography practices located in the Grenoble area in the French Alps. The included women had to be pregnant by gestational weeks, be older than 18 y old, to have a singleton pregnancy, to be planning to give birth in one of the four maternities clinics from Grenoble area, and to live in the study area (i.e., living 1 h driving from Grenoble city center). The volunteers were then followed during pregnancy, and their children were recruited at birth and then followed up. The mother–child pairs selected for this study had at least one period of sampling during pregnancy (), with validated and positive OP analysis () and the children had performed at least one lung function test at either 6 wk or 3 y () (Figure 1).
Figure 1.
Flow chart for the selection of the study population. Note: * net weight . AOS, airwave oscillometry; LF, lung function; , nitrogen multiple-breath washout; OP, oxidative potential; PM, particulate matter; , PM with aerodynamic diameter ; TBFVL, tidal breathing flow-volume loops.
Parents signed an informed consent for themselves and their child, and the study protocol was approved by the Comité de Protection des Personnes Sud-Est V (CPP) and the French data privacy institution (Commission Nationale de l’Informatique et des Libertés, CNIL).
Maternal Exposure
Active personal air samplers (MicroPEM™; RTI International) were used to sample onto Teflon filters. The participants were asked to carry the devices or keep them at close proximity during the entire sampling period (consecutive 7–8 d). The measurements took place at different periods of the pregnancy. The sample filters on which OP was measured consisted of 286 collected at a median gestational age (GA) of 18 wk (min: 12, Q1: 32, Q3: 19, max: 28) and 294 at 34 wk (min: 28, Q1: 32, Q3: 35, max: 38). Therefore, the median [interquartile range (IQR)] of time between the first and second measurement was 16 (14, 18) wk, with a minimum of 4 wk and a maximum of 23 wk, mainly due to the availability of the samplers or the volunteers. For each participant, personal exposure was estimated from one (132 out of 356, 37%) or 2 wk (224 out of 356, 63%) of sampling. An average exposure was calculated when two periods of measurements were available.
The net mass (micrograms) of collected was determined by gravimetric analysis (Mettler Toledo UMX2 ultra-microbalance) before and after sampling at the same hygrometric conditions (21°C, 25% relative humidity). Following gravimetric analysis, the samples filters were cold-stored () until OP analysis, for an average of 26 wk. OP analysis followed the protocol established by Calas et al.31,32 Briefly, a simulated lung fluid [SLF, mixture of Gamble and dipalmitoylphosphatidylcholine (DPPC)] was used to extract from the filters for a final concentration of , maintaining a constant amount of extracted for intercomparison. The extracts were then subjected to vortex mixing at 37°C for 1.25 h. The OP was measured using the dithiothreitol (DTT) and ascorbic acid (AA) assays.
For the DTT assay, extracts were mixed with a DTT solution using a 96-well plate (CELLSTAR, Greiner-Bio). Every 10 min, the remaining DTT was titrated by dithionitrobenzoic acid (DTNB) and the formation of 2-nitro-5-thiobenzoic acid (TNB) was measured by absorbance at (TECAN spectrophotometer Infinite M200 Pro), for a total reaction time of 30 min (e.g., 3 titrations in total). For the AA assay, a modified version of the synthetic respiratory tract lining fluid (RTLF) was used.33 AA was mixed with the extract in a 96-well plate, and the AA consumption was evaluated measuring the change in absorbance at over time. Absorbance measurements were collected at 4-min intervals for a total reaction time of 30 min. For both assays, the consumption rate (nanomoles per minute) was then normalized by the corresponding filtrated air sample volume (cubic meters) to represent human exposure through inhalation. corresponds to the consumption of DTT (nanomoles per minute per cubic meter), and corresponds to the consumption of AA (nanomoles per minute per cubic meter). All samples were subjected to triplicate analysis, and each sample result is reported as the mean of the repeated measurements. The coefficient of variation (CV) is between 0 and 10% for each assay.
To ensure accuracy of each OP measurement, positive control tests were performed for every experiment. A 1,4-naphthoquinone (1,4-NQ) solution was used for both the DTT and AA assays. Particularly, a of stock solution was used for the DTT assay and an of 1,4-NQ solution for AA assay.31,32 The measurement quality, estimated by the CV of the positive control tests, were at for both OP assays.
Lung Function at 6 Weeks
Lung function tests were performed on infants age 6–12 wk, using an infant face mask during natural sleep, in supine position and with the head midline, following guidelines of the European Respiratory Society (ERS) and American Thoracic Society (ATS).34 After stabilization of the breathing pattern (20–30 breaths rejected), 10 min of tidal breathing flow-volume loops (TBFVL) were recorded, and three measurements of nitrogen multiple-breath washout () were performed.
For TBFVL measurements, the first 30 to 50 regular breaths were used. The sighs and 10 breaths preceding and following a sigh were excluded. The following TBFVL parameters were retained in the present analysis: tidal volume () and the ratio of time to peak tidal expiratory flow () to expiratory time (). Out of the 484 mother–child pairs, 325 children performed the TBFVL test.
The technique measures lung volumes and ventilation heterogeneity. For this test, infants inhaled pure oxygen () and the concentration of exhaled was monitored employing the Exhalyzer© and Spiroware© equipment (Ecomedics). The main outcomes were a) functional residual capacity (FRC) and b) lung clearance index (LCI), defined as the number of respirations required to reduce the concentration of below 2.5%. Up to three valid measurements were obtained, guided by the following criteria: regular breathing during quiet sleep, tidal volume within target, no swallowing or sighs in the first five breaths, no sign of leak, and concentration below 2.5% for at least three consecutive breaths to end the test. A transient decrease in tidal volume may be induced by using pure oxygen during the test, which has been shown to affect FRC and LCI measures.35 Hence, the degree of hypoventilation was calculated for each test, comparing the maximum drop of tidal volume during the first 15 breaths after inhalation and the mean tidal volume before inhalation. Then, FRC and LCI values were corrected for the degree of hypoventilation using a 2-step standardization method based on regression residuals.36 First, the influence of hypoventilation was characterized using adjusted linear mixed regression models (accounting for the repeated data), and, in a second step, the model estimate was used to remove the variability in FRC (or LCI) due to hypoventilation. A total of 865 valid tests were retained, with a median (Q1; Q3) of 3 (2; 3) tests per child. Out of the 484 mother–child pairs, 350 children performed the test. For each child, both LCI and FRC corrected values were averaged.
Lung Function at 3 Years
At the age of 3 y (median: 3.1 y), the impedance of the respiratory system was assessed based on airwave oscillometry (AOS) using commercial device (TremoFlo; Thorasys Systems) complying with current European standards.37 The device was calibrated daily, using a reference resistance.
For this technique, pressure waves with frequencies varying from 7 to 41 Hz are applied during tidal breaths and lung impedance is calculated from the changes in flow and pressure. To ensure the quality and reproducibility of the measurements, they were performed at least 15 d after any respiratory infection (self-reported by the mother via a questionnaire administrated by a clinical research assistant at the clinical visit), with the child sitting, the head slightly extended, and wearing a nose clip. Children were asked to firmly close their lips around the mouthpiece while their cheeks and chins were maintained by the technician to avoid any signal damping by the mouth walls. After getting used to the device during approximately 30 s, three to five acceptable measurements were obtained and averaged. A rest interval of 1 min was respected between each 16-s-long measurement. We excluded measurements with the following artefacts: leakage, swallowing, glottis closure, vocalization, or obstruction of the mouthpiece by the tongue.
The key components of impedance are the resistance and the reactance of the respiratory system. The resistance is representative of friction forces mainly in the airways and the reactance depends on the inertive and elastic behaviors of the respiratory system.38 The parameters included in this study are raw values of resistance and reactance at a frequency of 7 Hz ( and ), the area under the reactance curve (AX), and the frequency dependence of the resistance, defined by the resistance difference between 7 and 19 Hz (). is a parameter that reflects large airway resistance, whereas AX and better characterize the peripheral airways. also evaluates the heterogeneous obstruction of the distal bronchi.39 Increased Rrs, , and AX and decreased Xrs are associated with a reduced lung function.
Among the 320 children to the 3-y follow-up who performed AOS (66% follow-up rate), measurements for 306 children (96% success rate for AOS test) were retained, complying with validity and reproducibility criteria (at least two measurements with CV for ). The mean value of the valid measurements was calculated for each parameter and used for the analyses. Out of them, 248 had personal prenatal exposure to OP, resulting in a total attrition rate of 51% for the exposure to personal prenatal OP–AOS parameters association study.
Statistical Methods
Both univariate and multiple linear regressions were used to study the associations between maternal personal exposure to and OP with each lung function parameter. The three exposure metrics used in this study (, , ) were continuous and scaled by their IQR, allowing to compare their respective effects on the outcomes. The Spearman correlation coefficient () was used to calculate correlations between the exposures. Linear regressions were used after confirming linearity by a likelihood ratio test between the adjusted model, modeling the exposure with a natural spline with 5 degrees of freedom and the adjusted main model (Figures S1 to S6 in the Supplement, all ). All analyses were performed using R software (version 4.1; R Development Core Team).
Potential confounders were selected a priori, based on previous studies,10,13 a) parental characteristics: educational level (defined as the maximum number of studying years after high school degree between the parents and expressed in two classes: above or ; self-reported through an self-administrated questionnaire), parental history of rhinitis (binary, self-reported by a questionnaire administrated by a clinical research assistant), mother’s age (calculated with the date of birth self-reported by a questionnaire administrated by a clinical research assistant) and body mass index (BMI) before pregnancy (continuous; calculated based on self-reported weight before pregnancy and height measured by a clinical research assistant during a SEPAGES clinical visit); b) infant characteristics: child sex (male/female), age (continuous, calculated with the date of birth collected in the child health booklet), height and weight (continuous, measured by a clinical research assistant at the clinical visit), passive smoking (yes/no, in utero, including maternal passive smoking or until the clinical visit; assessed by several self-administrated questionnaires during and after the pregnancy), breastfeeding (still some breastfeeding at 6 wk, yes/no, self-reported by a questionnaire administrated by a clinical research assistant); c) exposure characteristics: season of sampling [3-class variable: cold (all filters sampled between October and March), warm (all filters sampled between April and September), and (one filter sampled in the cold season and one filter sampled in the warm season)], mean temperature during pregnancy (continuous, assessed at home address by Hough’s model).40 The effects of the confounders were analyzed by looking at the effect of each confounder separately on the regression model adjusted for sex, height, and weight (Figures S9, S10). Missing data regarding covariates in the main model were imputed by multiple chained equation, using the R package mice,41 assuming that the data was missing completely at random (MCAR), which was checked by Little’s test42 (p-values of the test ). Descriptive statistics of the covariates can be found in Table S1. Ten imputed data sets were created, and results from each data set were combined using Rubin’s rule.43 We did not correct for multiple tests, but results were interpreted by looking at the consistency of association of PM and OP exposures across the different lung function parameters.
Several sensitivity analyses were conducted to address the robustness of the results from the main model by assessing the impacts of: a) data imputation, by conducting a complete case analysis; b) extreme exposure and health outcome values, by excluding the lowest (below first percentile) and highest values (above the 99th percentile) of the outcomes and exposures, resulting in the exclusion of 4%–5% of the population of each analysis; c) the number of PM and OP measurement weeks, by excluding participants with only one measurement week (); d) the independency of OP effects to PM, by adjusting OP models on ; e) LCI and FRC measurement error due to the degree of hypoventilation, by adding an analysis excluding one-fourth of the children who had the highest hypoventilation degree during the test (); f) leverage and influencing points, by excluding points that had a Cook’s distance44 higher than 4/n, where is the number of observations in the main model (exclusion of 4%–7% of the observations); g) the independency of OP and PM effects to personal concentrations during the same weeks of sampling [passive sampler (Passam AG), worn simultaneously to the active PM sampler]. Multicollinearity was assessed using the variance inflation factor in the two-pollutant models ().
Results
Description of the Population
The present study was conducted with children that had at least one prenatal measurement of OP and one lung function parameter assessed, leading to 356 mother–child couples (73% of SEPAGES cohort) (Figure 1). The included children had parents with a higher educational level, had less parental history of rhinitis, had higher exposure to , higher , and a lower in comparison with the children not included in the study (Table 1). No difference between the included and excluded population was observed for both OP and lung function at 6 wk. In the study population, 52% () of the children were boys, and the majority of children were born on term (96%, ) by vaginal delivery (85%, ) from mothers who were mainly nulliparous or primiparous (45%, and 46%, , respectively). In infancy, most children were still breastfed at 6 wk (86%, ) and () were exposed to tobacco smoke in utero (including maternal passive smoking) and after birth ( wk). The parental level of education is high because 72% () of the parents had studied 5 y or more after receiving their French high school diploma (i.e., having at least a MSc diploma). Only 15 children were born before the 37th week, with a minimum of 34 gestational weeks. Regarding lung function tests (Figure 1), 325 children performed a valid test of the lung function at 6 wk (284 had a valid analysis and 309 had valid TBFVL measurements), and 248 children had valid AOS measurements. Out of these 248 children, 197 had available results and 205 had valid TBFVL test results.
Table 1.
Characteristics of the included () and excluded () population from the cohort SEPAGES in this study. Included population corresponds to children who had at least both one prenatal oxidative potential assessment and one test of lung function.
| Characteristics | Included populationa () | Excluded populationa () | -Valueb |
|---|---|---|---|
| Sex of child | — | — | 0.2 |
| Male | 185 (52%) | 73 (59%) | — |
| Female | 171 (48%) | 51 (41 %) | — |
| Missing | 0 | 4 | — |
| Birth weight (g) | — | — | 0.13 |
| Median (IQR) | 3,295 (3,048, 3,580) | 3,220 (2,995, 3,507) | — |
| Missing | 0 | 5 | — |
| Preterm birth ( wk) | — | — | 0.2 |
| 0 (No) | 341 (96%) | 115 (93%) | — |
| 1 (Yes) | 15 (4%) | 9 (7%) | — |
| Missing | 0 | 4 | — |
| Parental educational level y | — | — | 0.048 |
| 0 (No) | 100 (28%) | 48 (38%) | — |
| 1 (Yes) | 256 (72%) | 80 (62%) | — |
| Delivery mode | — | — | 0.084 |
| Vaginal | 302 (85%) | 96 (78%) | — |
| C-section | 54 (15%) | 27 (22%) | — |
| Missing | 0 | 5 | — |
| Still breastfed at 6 wk | — | — | 0.11 |
| 0 (No) | 49 (14%) | 20 (20%) | — |
| 1 (Yes) | 306 (86%) | 78 (80%) | — |
| Missing | 1 | 30 | — |
| Parental history of rhinitis | — | — | 0.003 |
| 0 (No) | 132 (40%) | 26 (24%) | — |
| 1 (Yes) | 202 (60%) | 83 (76%) | — |
| Missing | 22 | 19 | — |
| Parity | — | — | 0.6 |
| 0 (nulliparous) | 160 (45%) | 62 (48%) | |
| 1 (primiparous) | 162 (46%) | 52 (41%) | |
| 2 or more (multiparous) | 34 (9.6%) | 14 (11%) | |
| ETS in utero and wk | — | — | 0.7 |
| 0 (No) | 259 (73%) | 84 (75%) | — |
| 1 (Yes) | 95 (27%) | 28 (25%) | — |
| Missing | 2 | 16 | — |
| ETS y | — | — | 0.7 |
| 0 (No) | 270 (79%) | 70 (77%) | — |
| 1 (Yes) | 73 (21%) | 21 (23%) | — |
| Missing | 13 | 37 | — |
| Exposure to particulate air pollutionc | |||
| () | 13.3 (10.6, 17.5) | 12.2 (8.2, 16.6) | 0.033 |
| Missing | 0 | 79 | — |
| () | 1.49 (1.11, 2.00) | 1.53 (1.05, 1.91) | 0.8 |
| Missing | 0 | 97 | — |
| () | 1.56 (1.07, 2.21) | 1.66 (0.93, 2.30) | |
| Missing | 0 | 97 | |
| Mean temperature during pregnancy (°C) | |||
| Median (IQR) | 13.0 (10.6, 14.6) | 11.6 (10.1, 13.6) | 0.001 |
| Missing | 0 | 4 | |
| parametersc (6 wk) | |||
| FRC (mL) | 105 (95, 115) | 108 (95, 115) | 0.6 |
| Missing | 72 | 62 | — |
| LCI | 7.58 (6.75, 8.47) | 7.49 (6.99, 8.12) | 0.9 |
| Missing | 72 | 62 | — |
| TBFVL parametersc (6 wk) | |||
| (mL) | 34 (29, 39) | 33 (29, 36) | 0.4 |
| Missing | 47 | 112 | — |
| (%) | 35 (29, 42) | 36 (26, 45) | 0.8 |
| Missing | 47 | 112 | — |
| AOS parametersc (3 y) | |||
| () | 11.53 (10.05, 13.04) | 12.67 (10.87, 14.17) | 0.021 |
| Missing | 108 | 70 | — |
| () | 1.02 (0.56, 1.61) | 1.18 (0.63, 1.98) | 0.2 |
| Missing | 108 | 70 | — |
| () | (, ) | (, ) | 0.037 |
| Missing | 108 | 70 | — |
| AX () | 68 (45, 92) | 70 (53, 105) | 0.3 |
| Missing | 108 | 70 | — |
Note: —, no data; AA, ascorbic acid; AOS, airwave oscillometry; DTT, dithiothreitol; AX, area under the reactance curve; ETS, environmental tobacco smoke; FRC, functional residual capacity; LCI, lung clearance index; , nitrogen multiple-breath washout; OP, oxidative potential; , volume-normalized oxidative potential measured by the AA assay; , volume-normalized oxidative potential measured by the DTT assay; PM, particulate matter; , PM with an aerodynamic diameter ; , resistance at a frequency of 7 Hz; , difference between the resistance at 7 Hz and at 19 Hz; TBFVL, tidal breathing flow-volume loops; ratio of time to peak tidal expiratory flow to expiratory time; , tidal volume, , reactance at a frequency of 7 Hz.
Expressed in (%) or Median (IQR).
-Value from Wilcoxon rank sum test and Pearson’s chi-squared test comparing included and excluded population.
Variables used for population selection (selected children had prenatal exposure to PM and OP and either or TBFVL or AOS measures).
Exposure to and Its OP
The median (Q1, Q3) of average prenatal personal exposures to , , and were , and . Personal and OP (particularly ) presented a seasonal trend, with higher levels reached during the cold season (Figure 2; Table S2). was highly correlated with both concentration and ( and , respectively; , for both), whereas the correlation between concentration and was moderate (, ) (Figure S7). For participants with two periods of sampling, there were no differences in , and levels at early vs. late pregnancy (Figure S8; Table S3).
Figure 2.
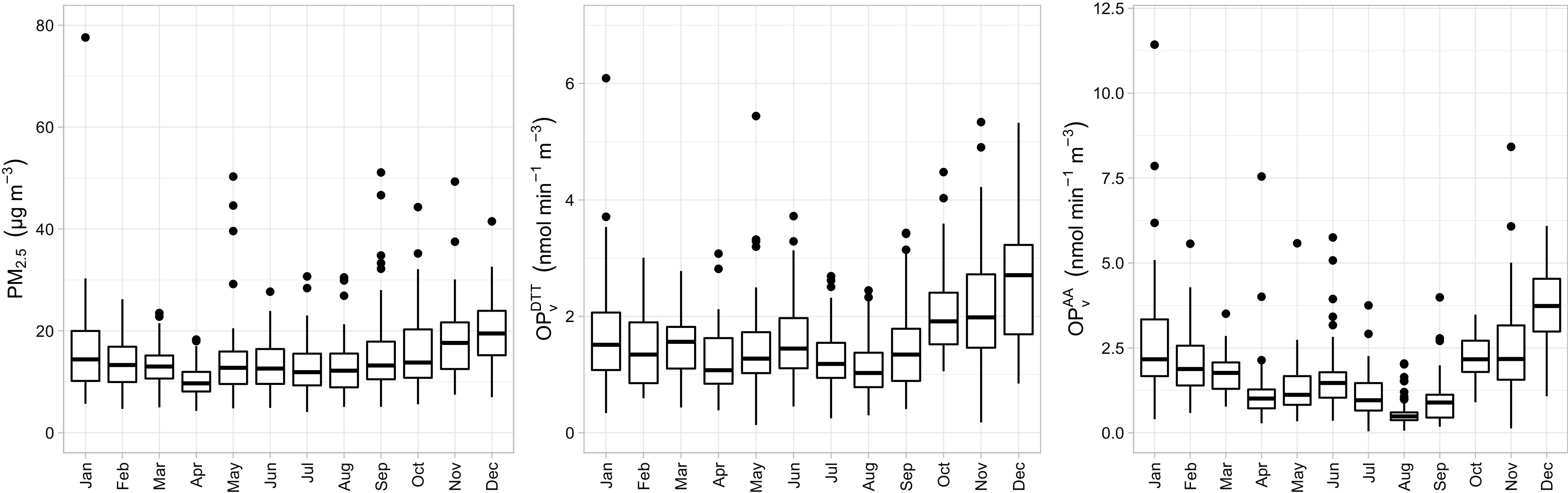
Monthly distribution of personal measurements of (left), (center), and (right). See Table S2 for corresponding numeric data. Note: Boxes represent 25th–75th percentiles; the middle horizontal line represents the median; whiskers extend to the most extreme point within 1.5 IQRs of the box and the dots outside boxes indicate outliers. Note: AA, ascorbic acid; DTT, dithiothreitol; IQR, interquartile range; , volume-normalized oxidative potential measured by the AA assay (nmol); , volume-normalized oxidative potential measured by the DTT assay (nmol); PM, particulate matter; , PM with an aerodynamic diameter ().
Association between Exposures to Prenatal and OP and Lung Function
Lung function at 6 wk.
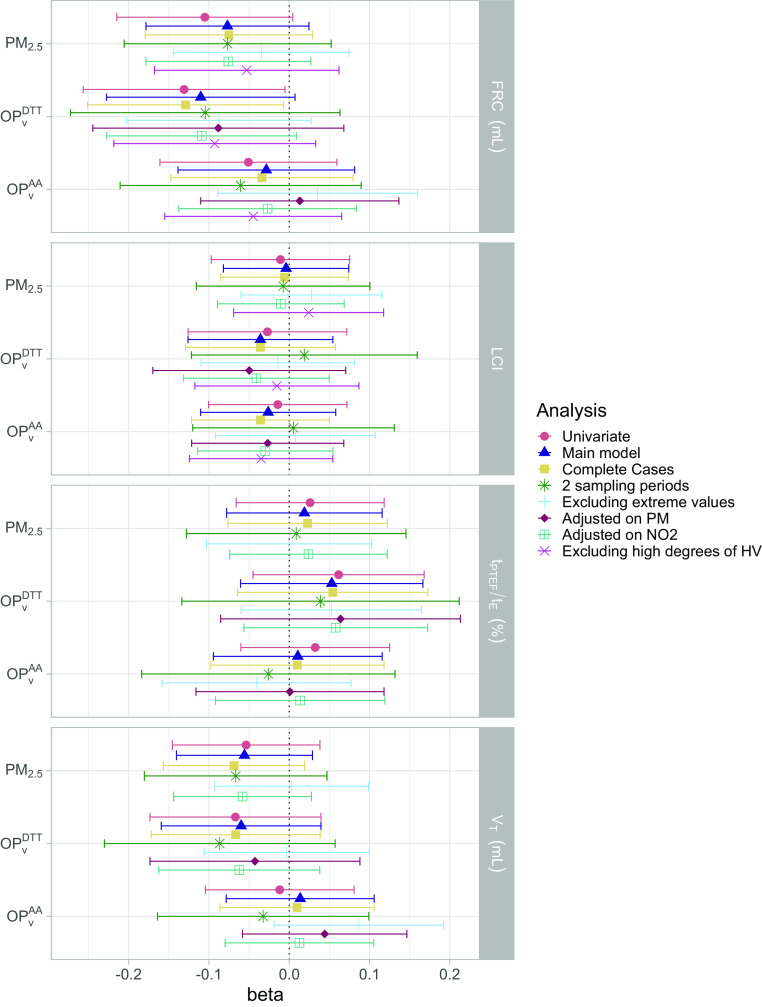
In the univariate analysis, increased personal prenatal exposure to and were associated with a lower FRC at 6 wk (; 95% CI: , 0.09 for each increase of , and ; 95% CI: , for each increase of ). After adjusting for potential confounders (Table 2; Table S4; Figure 3), in both main and complete-case analysis, the magnitude of association between and FRC slightly decreased, and associations were borderline significant (: ; 95% CI: , 0.15 for the main model and : ; 95% CI: , for the complete-case analysis). The confounders mainly driving the differences between the univariate and the main analysis were the season of sampling and the parental history of rhinitis (Figure S9). LCI and did not show any clear association trend for all exposures considered. In general, for air pollution–lung function associations showing marginal association, the sensitivity analyses showed patterns of association similar to the ones in the main model, except for the negative association that disappeared when excluding extreme values. The analyses excluding leverage and influencing points (estimated by Cook’s distance) overall led to similar results and resulted in statistically significant association for FRC and exposure to both and (Table S4; Figure S11). The analyses further adjusted on personal sampled simultaneously with showed that did not modify the estimates and 95% CI for any of the studied associations. The magnitude of the associations of for lung volumes, estimated by FRC, remained similar in models further adjusted for . The change in FRC in the two-pollutant model with and showed a stronger effect of than [ (95% CI: , 1.40) for vs. (95% CI: , 2.19) for ], although this association became nonsignificant (Figure 3; Table S6).
Table 2.
Associations between prenatal exposure to air pollution and lung function at 6 wk and 3 y. Regression coefficients are estimated from univariate and multiple linear models.
| Age | Pollutants | () | (nmol) | (nmol) | |||
|---|---|---|---|---|---|---|---|
| Regression model | Unadjusted coefficients (95% CI) | Adjusteda coefficients (95% CI) | Unadjusted coefficients (95% CI) | Adjusteda coefficients (95% CI) | Unadjusted coefficients (95% CI) | Adjusteda coefficients (95% CI) | |
| 6 wk | FRC (mL)b | (, 0.09) | (, 0.5) | (, ) | (, 0.15) | (, 1.22) | (, 1.68) |
| LCIb | (, 0.13) | (, 0.13) | (, 0.12) | (, 0.09) | (, 0.12) | (, 0.1) | |
| (mL)c | (, 0.37) | (, 0.28) | (, 0.38) | (, 0.38) | (, 0.78) | 0.13 (, 1.02) | |
| (%)c | 0.34 (, 1.54) | 0.25 (, 1.51) | 0.8 (, 2.19) | 0.69 (, 2.17) | 0.42 (, 1.63) | 0.14 (, 1.51) | |
| 3 y | ()d | (, 0.32) | (, 0.3) | 0.12 (, 0.46) | 0.05 (, 0.37) | (, 0.28) | (, 0.25) |
| ()d | (, 0.14) | 0.02 (, 0.16) | 0.08 (, 0.23) | 0.09 (, 0.24) | 0.1 (, 0.24) | 0.12 (, 0.27) | |
| ()d | (, 0.16) | 0.01 (, 0.17) | (, 0.08) | (, 0.11) | (, 0.1) | (, 0.1) | |
| AX ()d | 0.65 (, 5.74) | 0.22 (, 5.25) | 2.34 (, 7.64) | 1.07 (, 6.22) | (, 4.06) | (, 3.07) | |
Note: Coefficients are calculated for an increase of one IQR for , , and , corresponding to , , and , respectively. AA, ascorbic acid; AX, area under the reactance curve; BMI, body mass index; CI, confidence interval; FRC, functional residual capacity; IQR, interquartile range; LCI, lung clearance index; , volume-normalized oxidative potential measured by the AA assay; , volume-normalized oxidative potential measured by the DTT assay; PM, particulate matter; , PM with an aerodynamic diameter ; , resistance at a frequency of 7 Hz; , difference between the resistance at 7 Hz and at 19 Hz; ratio of time to peak tidal expiratory flow to expiratory time; , tidal volume; , reactance at a frequency of 7 Hz.
Model adjusted for child’s height, weight, sex, age, season of sampling, breastfeeding, environmental tobacco smoke, maternal age and BMI before pregnancy, parental level of education, parental history of rhinitis, and mean temperature during pregnancy.
Number of observations is 284 for FRC and LCI.
Number of observations is 309 for and .
Number of observations is 248 for , , , and AX.
Figure 3.
Association between personal exposure to , , and during pregnancy and lung function parameters measured at 6 wk in the univariate and multiple linear models and in the sensitivity analyses. Outcomes and exposures were scaled by their IQR. See Tables S4 and S6 for corresponding numeric data. Whiskers represent the 95% confidence interval around the estimate. The main model was adjusted on child’s height, weight, sex, age, season of sampling, breastfeeding, environmental tobacco smoke, maternal age and BMI before pregnancy, parental level of education, parental history of rhinitis, and mean temperature during pregnancy. In addition, “2 sampling periods” are the analyses reduced to the children that had 2 wk of prenatal measurements of air pollution (63%–66% of the population); “Excluding extreme values” are the analyses excluding the exposures and outcomes below the first percentile and above the 99th (exclusion of approximately 5% of the population); “Adjusted on PM” corresponds to adding personal exposure to in the set of confounders, “Adjusted on ” corresponds to adding personal exposure to in the set of confounders, and the last analyses were performed excluding children that had the highest hypoventilation degree during the nitrogen multiple breath washout test (excluding 25% of the population). Note: AA, ascorbic acid; BMI, body mass index; DTT, dithiothreitol; FRC, functional residual capacity; IQR, interquartile range; LCI, lung clearance index; , volume-normalized oxidative potential measured by the AA assay (); , volume-normalized oxidative potential measured by the DTT assay (nmol); PM, particulate matter; , PM with an aerodynamic diameter (); , ratio of time to peak tidal expiratory flow to expiratory time; , tidal volume.
Lung function at 3 y.
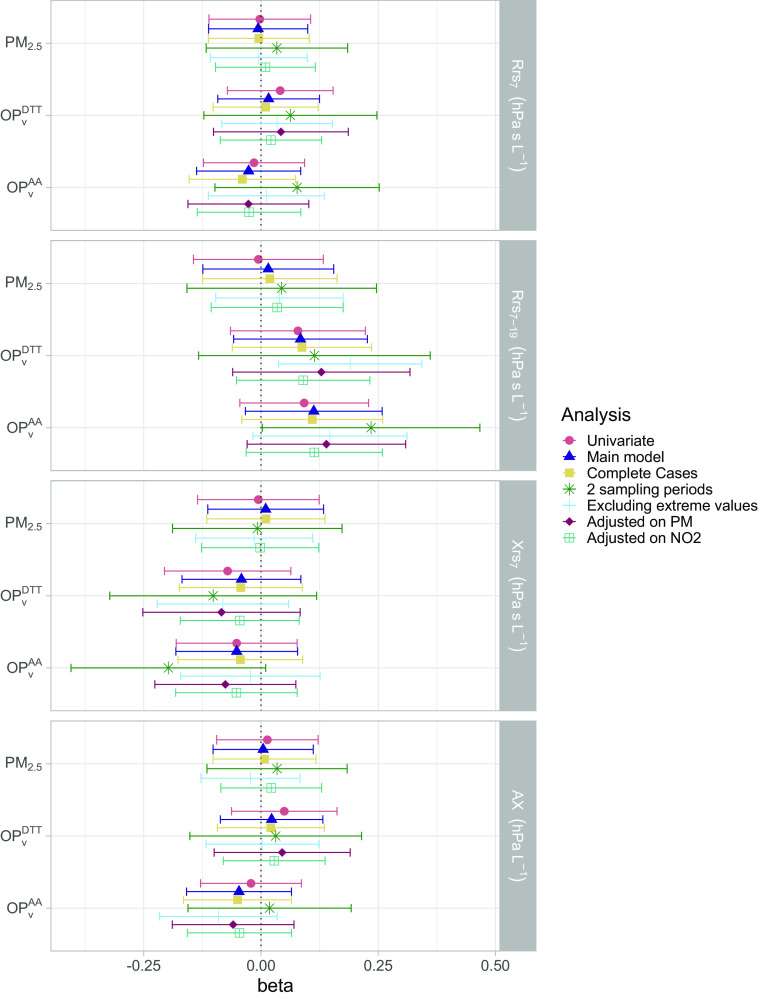
Increased personal prenatal exposures to and were associated with an increase of 0.09 (95% CI: , 0.24) and 0.12 (95% CI: , 0.27) in respectively, whereas no trend for association was found with exposure to (: ; 95% CI: , 0.16) (Table 2; Figure 4). The confounders mainly driving the differences between the univariate and the adjusted model were the season of sampling, parental history of rhinitis, and maternal age before pregnancy (Figure S10). The sensitivity analyses confirmed these trends of association. In particular, the analysis excluding extreme values resulted in a statistically significant positive association, with an IQR increase in OP being associated with an increase of 0.20 (95% CI: 0.04, 0.36) in for . Likewise, the model excluding leverage and influencing points led to statistically significant results with and exposure to both , whereas the results for other outcomes were not modified, with their 95% CI largely overlapping with that of the main model (Table S5; Figure S12). The analyses further adjusted on personal sampled simultaneously to showed that did not modify the estimates and 95% CI for any of the studied association. The two-pollutant models for showed that the effects of both were stronger than the effects of [0.14 (, 0.34) and (, 0.13) for and ; 0.15 (, 0.33) and (, 0.12) for and ], and other associations were not modified in this model (Figure 4; Table S7). No clear trends were observed for the other AOS parameters in the main model, and this was confirmed by the sensitivity analyses.
Figure 4.
Association between personal exposure to , , and during pregnancy and lung function parameters measured at 3 y in the univariate and multiple linear models and in the sensitivity analyses. Outcomes and exposures were scaled by their IQR. See Tables S5 and S7 for corresponding numeric data. Whiskers represent the 95% confidence interval around the estimate. The main model was adjusted on child’s height, weight, sex, age, season of sampling, breastfeeding, environmental tobacco smoke, maternal age and BMI before pregnancy, parental level of education, parental history of rhinitis and mean temperature during pregnancy. In addition, “2 sampling periods” are the analyses reduced to the children that had 2 wk of prenatal measurements of air pollution (61% of the population); “Excluding extreme values” are the analyses excluding the exposures and outcomes below the first percentile and above the 99th (exclusion of approx. 5% of the population); “Adjusted on PM” corresponds to adding personal exposure to in the set of confounders; “Adjusted on ” corresponds to adding personal exposure to in the set of confounders. Note: AA, ascorbic acid; AX, area under the reactance curve; BMI, body mass index; DTT, dithiothreitol; IQR, interquartile range; , volume-normalized oxidative potential measured by the AA assay (nmol); , volume-normalized oxidative potential measured by the DTT assay (nmol); PM, particulate matter; , PM with an aerodynamic diameter (); , resistance at a frequency of 7 Hz; , difference between the resistance at 7 Hz and at 19 Hz; , reactance at a frequency of 7 Hz.
Discussion
To the best of our knowledge, this study is the first one to address the associations between maternal personal exposures to and OP and children’s objective lung function parameters measured as early as 6 wk of age and at 3 y. Regarding , our findings showed consistency across some lung function parameters with higher prenatal exposure being associated with a lowered indicator of lung volumes (FRC) at 6 wk and with a trend toward reduced at 3 y, an indicator influenced by both lung volumes and ventilation heterogeneity. An interesting finding is that the effects of exposure on FRC were stronger than those of mass in the two-pollutant model.
PM and OP Exposures and Lung Function
Our results are in agreement with existing studies reporting a higher prevalence of reduced lung function in participants who are exposed to higher levels of .12,45–48 Regarding TBFVL and tests, our findings are in line with the results of the South African birth cohort, MACE,16 that investigated the effects of from LUR models and lung function of children at 1.5, 6, 12, and 24 months of age, and with the results of a Swiss birth cohort10 that examined the association between and from an ambient monitoring station and lung function measured in neonates (median age of 34 d). Both studies showed decreases in FRC and in infants prenatally exposed to higher concentrations of or and , whereas no effects were found on LCI. Our results extend their findings by confirming the pattern of decreased FRC with exposure to and OP, further supporting the importance of considering the oxidative stress caused by PM during pregnancy to predict lung growth restriction of children. In our study, none of the exposures considered were associated with LCI or with , two parameters still poorly studied in association with air pollution and with conflicting results regarding LCI.10,16 The decrease in with and is not confirmed by all sensitivity analyses, indicating limited robustness of this association. is usually used to detect the obstruction of the distal bronchi and can be modified by both lung volumes and heterogeneity of ventilation.39 The trend for an increase of this parameter in children prenatally exposed to higher OP is in accordance with the results found at 6 wk, because lower lung volume could lead to an increased resistance of the small airways. This partially confirms the results from previous studies indicating a detrimental effect of air pollution on respiratory mechanical parameters. In the BAMSE birth cohort, Schultz et al.49 investigated the effects of early-life exposure to on lung mechanic components measured by impulse oscillometry in 2,415 adolescents and found increased frequency dependent resistance () and with higher exposure, although the associations were not statistically significant. Shao et al.50 found increased AX in 84 children exposed to from a 6-wk episode of fire during infancy. In addition, regarding acute respiratory effect of OP, He et al.27 found that an increase in OP measured 2 d prior to visit was significantly associated with increased and in 43 asthmatic children age 5–13 y. Although AOS parameters have been found associated with air pollution in previous studies, the parameter varies between studies.8,27,49,50 In our case, we confirmed results with , a parameter specific of the small airways.
Comparison of the Exposure Metrics
Our study, which identified associations between OP and PM with FRC, an indicator of lung volume, and with , an indicator also accounting for lung volumes, indicates specific effects on lung growth. These observations are supported by studies showing that prenatal exposure to environmental pollutants impacts in utero growth, including organ growth,51,52 and that oxidative stress may cause placental tissue damage, which could in turn affect lung growth in utero.53,54
Only a few cohort studies tackled the associations of PM and OP exposure with lung function.26–28 The associations found with reduced lung function seemed generally clearer with OP than with mass concentration, which agrees with the existing literature.24 For example, the PIAMA birth cohort study28 found associations between at home address and increased asthma and rhinitis prevalence and decreased lung function in 12-y-old children but no association with mass. The effect magnitudes of OP models adjusted on other pollutants were similar, although more sensitive to adjustment, which was not the case in our model. In children with asthma diagnosis at age 9–18 y, Delfino et al.26 found significant positive associations between ambient and OP measured by the in vitro ROS-macrophage assay and airway inflammation, whereas no association was found for . Conclusions were not modified in their two-pollutant model. He et al.27 also used the ROS-macrophage assay and found associations with , , and for OP, whereas associations for PM were only found with . Overall our results add to the existing evidence indicating that the OP of PM has a stronger effect on various respiratory outcomes than PM mass and is thereby a relevant complementary health metric for air pollution.26–28,55–58 The different health effects found for and OP could be partially explained by the difference in sources contributing to OP and concentration in the SEPAGES study area (Grenoble). In fact, previous studies showed that biomass burning and regional transport of secondary inorganic pollutants (nitrates and sulfates) were the main sources contributing to the ambient mass concentration, whereas vehicular emissions and biomass burning were the main drivers of OP levels over the area.59,60 We acknowledge that by using active personal samplers, exposure measurements incorporate both indoor- and outdoor-generated pollution, which can have different compositions and thus different health effects.61
Our study extends the findings of others by comparing OP measured by the AA and the DTT assays. In their reviews, Bates et al.24 and Rao et al.62 showed that was a better predictor than for most health outcomes. Here, we found that had an effect comparable to that of on lung function as measured by at 3 y. However, results at 6 wk were more contrasted. The effects of on FRC seemed to be influenced by mass concentration, because the coefficients in the model adjusted on PM were pulled toward zero. Overall, although both OP assays (i.e., DTT and AA) were developed to account for the toxicity of PM components, their health impact may differ, which could be explained by their different sensitivities to chemical components (traffic-related metals, organic carbon, and inorganic species for and metals only for )63–65 and their different reactivities to specific ROS.66
Strengths and Limitations
One of the main strengths of this study is the assessment of maternal exposure by personal measurements, which was proven to be more representative of real exposure29,61,67 than assessments in studies using ambient measurement from monitoring stations or exposure models. It is also expected to be more accurate as compared to approaches modeling the personal exposure,27 combining a) self-reported time-activity patterns in different microenvironments (at home, at work, in a car, in public transport, outdoors) and b) indoor–outdoor ratios estimation for each identified microenvironment, both being at risk for errors. Additionally, the use of OP in this study is a way to consider the potential oxidative stress caused by PM, which is thought to be a better predictor of PM damages than its concentration. An interesting finding is that the similitude of the seasonality observed in personal levels of and OP in the present study with the results of a previous study that showed higher ambient and OP during winter in the Grenoble area,60 supports the external validity of our exposure data.
We acknowledge that a mixed influence of pre- and postnatal exposure cannot be totally ruled out, but such influence cannot be assessed because OP of was not measured in early childhood in SEPAGES. Nevertheless, other studies6,68 that considered both pre- and postnatal exposure to PM found an effect of prenatal exposure on reduced lung function in children. Although the design of the study enables evaluation of the effects of air pollution on child’s lung function at different stages of the pregnancy, we a priori decided not to perform this analysis in our study to avoid lowering the number of participants included (224 with two measurement weeks) and increasing the number of statistical tests.
One limitation of active personal samplers is that it cannot be used by the participants during their entire pregnancy. The compromise in this study was to perform sampling for two 1-wk periods during the pregnancy and to use the average of the two measures in the association studies. This approach tended to avoid the influence of seasonality and extreme pollution events during the sampling weeks, especially for (Figure 2; Figure S8). However, this influence could not be avoided for individuals with only 1 wk of measurement (). To account for this limitation, models were adjusted for the season of sampling, and sensitivity analysis excluding participants with only one measurement week were conducted. It is interesting to note that, in general, the associations between OP or and FRC and were stronger in this latter sensitivity analysis consisting of a restricted population with a more accurate exposure assessment, which supports our a priori hypothesis.
The novelty of this study also lies in the repeated assessment of lung function in early life, whereas most of the other studies considered children older than 5 y old, when spirometry starts to be feasible. Assessing lung function at the youngest age allows researchers to better investigate the effects related to pregnancy and early infancy time windows, which are believed to predict long-term respiratory morbidity. However, the use of pure oxygen during the test ( being forbidden in France) induced a transient decrease in tidal volume, which could affect the measurement of FRC and LCI. Although parameters were a posteriori corrected for the degree of hypoventilation, and sensitivity analysis excluding children with the highest hypoventilation degree showed similar patterns of association, residual errors in lung function assessment that would lead to underestimated effect estimates cannot be totally excluded. Because two different techniques of lung function measurement were used at 6 wk and 3 y of age, the effect of prenatal exposure of air pollution on lung function growth could not be assessed.
The amount of data collected during the follow-up of the cohort allowed us to adjust for a number of confounding factors. However, the residual confounding due to the observational design of this study remains a limitation. An interesting finding is that the analysis excluding leverage and influencing points showed that these points tended to drive some of the regression estimates toward the null hypothesis, which indicates that influencing points might be partly related to measurement errors. Although the aim of our study was based on an a priori hypothesis derived from previous association studies and from the biological specificities of OP of , the number of associations tested was still relatively high () and we did not apply any formal correction for multiple comparisons. Thus, we acknowledge part of the associations observed may result from chance findings and thus should be interpreted cautiously. The attrition rate of 51% for the associations between the personal prenatal OP and lung function at 3 y could not be a priori defined as low, but given the demanding protocol and the originality of the longitudinal data collected, both for exposures (personal prenatal exposure to OP) and health outcomes (with objective lung function measures in preschool children, which is rare in population-based cohorts), this can be considered acceptable. However, a selection bias cannot be totally ruled out; in particular, the associations for may have been underestimated because included participants tended to have both higher exposure to and better lung function on two AOS parameters (lower , higher ) at 3 y in comparison with the excluded participants. Nevertheless, with no differences in OP between included and excluded children, the associations reported with OP are probably not driven by selection bias. Although a bigger sample would lead to more statistical power and therefore clearer conclusions, the use of objective and validated respiratory health parameters in early life and novel personal prenatal air pollution exposure metrics offers important and relevant information on PM exposure and its health effects.
In summary, our study shows consistency in the associations between personal prenatal and several early-life lung function parameters related to lung growth restriction and therefore supports findings of the detrimental health effects of exposure on health through oxidative stress and the relevance of OP of as a useful health-based metric. These findings, together with identifying sources of OP of PM, could help target emission sources that are critical in decreasing health effects of atmospheric pollution.
Supplementary Material
Acknowledgments
The SEPAGES cohort study group: E. Eyriey, A. Licinia, A. Vellement (Groupe Hospitalier Mutualiste, Grenoble), I. Pin, P. Hoffmann, E. Hullo, C. Llerena (Grenoble Alpes University Hospital, La Tronche), X. Morin (Clinique des Cèdres, Echirolles), A. Morlot (Clinique Belledonne, Saint-Martin d’Hères), J. Lepeule, S. Lyon-Caen, C. Philippat, I. Pin, J. Quentin, V. Siroux, R. Slama (Grenoble Alpes University, Inserm, CNRS, IAB). The authors express their sincere thanks to all participants in the SEPAGES study.
The authors thank all the numerous people (who could not be listed exhaustively here) from the different laboratories (IGE and Air-O-Sol analytical platform) and from the Grenoble University Hospital who performed mother recruitment and child follow-ups. The authors would like to kindly thank I. Hough, I. Kloog and A. Guilbert for their help with the assessment of temperature exposure.
The authors thank A. Benlakhryfa, L. Borges, Y. Gioria, clinical research assistants; J. Giraud, M. Marceau, M-P. Martin, nurses; E. Charvet, A. Putod, midwives; M. Graca, K. Gridel, C. Pelini, M. Barbagallo, fieldworkers; A. Bossant, K. Guichardet, J.T. Iltis, A. Levanic, C. Martel, E. Quinteiro, S. Raffin, neuropsychologists; the staff from Grenoble Center for Clinical Investigation (CIC): J.-L. Cracowski, C. Cracowski, E. Hodaj, D. Abry, N. Gonnet, and A. Tournier. The authors extend a warm thank you also to M. Althuser, S. Althuser, F. Camus-Chauvet, P. Dusonchet, S. Dusonchet, L. Emery, P. Fabbrizio, P. Hoffmann, D. Marchal André, X. Morin, E. Opoix, L. Pacteau, P. Rivoire, A. Royannais, C. Tomasella, T. Tomasella, D. Tournadre, P. Viossat, E. Volpi, S. Rey, E. Warembourg, and clinicians from Grenoble University Hospital for their support in the recruitment of the study volunteers. The authors also thank A. Buchet, S.F. Caraby, J.-N. Canonica, J. Dujourdil, E. Eyriey, P. Hoffmann, M. Jeannin, A. Licina, X. Morin, A. Nicolas, and all midwives from the four maternity wards of Grenoble urban areas.
SEPAGES data are stored thanks to Inserm RE-CO-NAI platform funded by Commissariat Général à l’Investissement, with the implication of Sophie de Visme (Inserm DSI). Many thanks to M.A. Charles, RE-CO-NAI coordinator, for her support.
This work was supported in part by the Agence de la Transition Écologique (ADEME) and by the Agence Nationale de Sécurité Sanitaire de l’Alimentation, de l’Environnement et du Travail (ANSES), both supporting A.M.’s PhD grant. OP measurements were funded by a University Grenoble Alpes grant CDP IDEX UGA MOBILAIR (ANR-15-IDEX-02) and by the French Research Agency - ANR (GetOPstandOP ANR-19-CE34-0002). The postdoctoral position of L.J.S.B. is funded by the Predict’air project (grant Fondation UGA-UGA 2022-16 and grant PR-PRE-2021 FUGAFondation Air Liquide). The SEPAGES cohort was supported by the European Research Council (No. 311765-E-DOHaD), the European Community’s Seventh Framework Programme (FP7/2007-206 - No. 308333-892 HELIX), the European Union’s Horizon 2020 research and innovation program (No. 874583 ATHLETE Project, No. 825712 OBERON Project), the French Research Agency - ANR (PAPER project ANR-12-PDOC-0029-01, SHALCOH project ANR-14-CE21-0007, ANR-15-IDEX-02 and ANR-15-IDEX5, GUMME project ANR-18-CE36-005, ETAPE project ANR - EDeN project ANR-19-CE36-0003-01), the French Agency for Food, Environmental and Occupational Health & Safety - ANSES (CNAP project EST-2016-121, PENDORE project EST-2016-121, HyPAxE project EST-2019/1/039), the Plan Cancer (Canc’Air project), the French Cancer Research Foundation Association de Recherche sur le Cancer – ARC, the French Endowment Fund AGIR for chronic diseases – APMC (projects PRENAPAR and LCI-FOT), the French Endowment Fund for Respiratory Health, the French Fund – Fondation de France (CLIMATHES – 00081169, SEPAGES 5 - 00099903).
References
- 1.Janssen NAH, Brunekreef B, van Vliet P, Aarts F, Meliefste K, Harssema H, et al. . 2003. The relationship between air pollution from heavy traffic and allergic sensitization, bronchial hyperresponsiveness, and respiratory symptoms in Dutch schoolchildren. Environ Health Perspect 111(12):1512–1518, PMID: , 10.1289/ehp.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJL, Aravkin AY, Zheng P, Abbafati C, Abbas KM, Abbasi-Kangevari M, et al. . 2020. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet 396(10258):1223–1249, 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO (World Health Organization). 2016. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease. https://www.who.int/phe/publications/air-pollution-global-assessment/en/ [accessed 5 May 2021].
- 4.Capello F, Pili G. 2018. Air Pollution in Infancy, Childhood and Young Adults. In: Clinical Handbook of Air Pollution-Related Diseases. Capello F, Gaddi AV, eds. Cham, Switzerland: Springer International Publishing, 141–186, 10.1007/978-3-319-62731-1_10. [DOI] [Google Scholar]
- 5.Sly PD, Flack F. 2008. Susceptibility of children to environmental pollutants. Ann NY Acad Sci 1140(1):163–183, PMID: , 10.1196/annals.1454.017. [DOI] [PubMed] [Google Scholar]
- 6.Cai Y, Hansell AL, Granell R, Blangiardo M, Zottoli M, Fecht D, et al. . 2020. Prenatal, Early-Life, and childhood exposure to air pollution and lung function: the ALSPAC cohort. Am J Respir Crit Care Med 202(1):112–123, PMID: , 10.1164/rccm.201902-0286OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carraro S, Scheltema N, Bont L, Baraldi E. 2014. Early-life origins of chronic respiratory diseases: understanding and promoting healthy ageing. Eur Respir J 44(6):1682–1696, PMID: , 10.1183/09031936.00084114. [DOI] [PubMed] [Google Scholar]
- 8.Dutta A, Alaka M, Ibigbami T, Adepoju D, Adekunle S, Olamijulo J, et al. . 2021. Impact of prenatal and postnatal household air pollution exposure on lung function of 2-year-old Nigerian children by oscillometry. Sci Total Environ 755(pt 2):143419, PMID: , 10.1016/j.scitotenv.2020.143419. [DOI] [PubMed] [Google Scholar]
- 9.Korten I, Ramsey K, Latzin P. 2017. Air pollution during pregnancy and lung development in the child. Paediatr Respir Rev 21:38–46, PMID: , 10.1016/j.prrv.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Latzin P, Röösli M, Huss A, Kuehni CE, Frey U. 2009. Air pollution during pregnancy and lung function in newborns: a birth cohort study. Eur Respir J 33(3):594–603, PMID: , 10.1183/09031936.00084008. [DOI] [PubMed] [Google Scholar]
- 11.Bergstra AD, Brunekreef B, Burdorf A. 2018. The effect of industry-related air pollution on lung function and respiratory symptoms in school children. Environ Health 17(1):30, PMID: , 10.1186/s12940-018-0373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gehring U, Gruzieva O, Agius RM, Beelen R, Custovic A, Cyrys J, et al. . 2013. Air pollution exposure and lung function in children: the ESCAPE project. Environ Health Perspect 121(11–12):1357–1364, PMID: , 10.1289/ehp.1306770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schultz ES, Hallberg J, Bellander T, Bergström A, Bottai M, Chiesa F, et al. . 2016. Early-life exposure to traffic-related air pollution and lung function in adolescence. Am J Respir Crit Care Med 193(2):171–177, PMID: , 10.1164/rccm.201505-0928OC. [DOI] [PubMed] [Google Scholar]
- 14.He B, Huang JV, Kwok MK, Au Yeung SL, Hui LL, Li AM, et al. . 2019. The association of early-life exposure to air pollution with lung function at ∼17.5 years in the “children of 1997” Hong Kong Chinese birth cohort. Environ Int 123:444–450, PMID: , 10.1016/j.envint.2018.11.073. [DOI] [PubMed] [Google Scholar]
- 15.He L, Cui X, Li Z, Teng Y, Barkjohn KK, Norris C, et al. . 2020. Malondialdehyde in nasal fluid: a biomarker for monitoring asthma control in relation to air pollution exposure. Environ Sci Technol 54(18):11405–11413, PMID: , 10.1021/acs.est.0c02558. [DOI] [PubMed] [Google Scholar]
- 16.Muttoo N, Jeena Asharam K. 2019. Air pollution exposure and infant lung function in the MACE cohort, South Africa. Environ Epidemiol 3:280, 10.1097/01.EE9.0000609000.48206.68. [DOI] [Google Scholar]
- 17.Pope CAI. 2000. Review: epidemiological basis for particulate air pollution health standards. Aerosol Sci Technol 32(1):4–14, 10.1080/027868200303885. [DOI] [Google Scholar]
- 18.Gao D, Ripley S, Weichenthal S, Godri Pollitt KJ. 2020. Ambient particulate matter oxidative potential: chemical determinants, associated health effects, and strategies for risk management. Free Radic Biol Med 151:7–25, PMID: , 10.1016/j.freeradbiomed.2020.04.028. [DOI] [PubMed] [Google Scholar]
- 19.Crobeddu B, Aragao-Santiago L, Bui LC, Boland S, Baeza Squiban A. 2017. Oxidative potential of particulate matter 2.5 as predictive indicator of cellular stress. Environ Pollut 230:125–133, PMID: , 10.1016/j.envpol.2017.06.051. [DOI] [PubMed] [Google Scholar]
- 20.Hatzis C, Godleski JJ, González-Flecha B, Wolfson JM, Koutrakis P. 2006. Ambient particulate matter exhibits direct inhibitory effects on oxidative stress enzymes. Environ Sci Technol 40(8):2805–2811, PMID: , 10.1021/es0518732. [DOI] [PubMed] [Google Scholar]
- 21.Leni Z, Cassagnes LE, Daellenbach KR, El Haddad I, Vlachou A, Uzu G, et al. . 2020. Oxidative stress-induced inflammation in susceptible airways by anthropogenic aerosol. PLoS One 15(11):e0233425, PMID: , 10.1371/journal.pone.0233425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riva DR, Magalhães CB, Lopes AA, Lanças T, Mauad T, Malm O, et al. . 2011. Low dose of fine particulate matter (PM2.5) can induce acute oxidative stress, inflammation and pulmonary impairment in healthy mice. Inhal Toxicol 23(5):257–267, PMID: , 10.3109/08958378.2011.566290. [DOI] [PubMed] [Google Scholar]
- 23.Hellack B, Yang A, Cassee FR, Janssen NAH, Schins RPF, Kuhlbusch TAJ. 2014. Intrinsic hydroxyl radical generation measurements directly from sampled filters as a metric for the oxidative potential of ambient particulate matter. J Aerosol Sci 72:47–55, 10.1016/j.jaerosci.2014.02.003. [DOI] [Google Scholar]
- 24.Bates JT, Fang T, Verma V, Zeng L, Weber RJ, Tolbert PE, et al. . 2019. Review of acellular assays of ambient particulate matter oxidative potential: methods and relationships with composition, sources, and health effects. Environ Sci Technol 53(8):4003–4019, PMID: , 10.1021/acs.est.8b03430. [DOI] [PubMed] [Google Scholar]
- 25.Weichenthal S, Lavigne E, Traub A, Umbrio D, You H, Pollitt K, et al. . 2021. Association of sulfur, transition metals, and the oxidative potential of outdoor PM2.5 with acute cardiovascular events: a case-crossover study of Canadian adults. Environ Health Perspect 129(10):107005, PMID: , 10.1289/EHP9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delfino RJ, Staimer N, Tjoa T, Gillen DL, Schauer JJ, Shafer MM. 2013. Airway inflammation and oxidative potential of air pollutant particles in a pediatric asthma panel. J Expo Sci Environ Epidemiol 23(5):466–473, PMID: , 10.1038/jes.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He L, Norris C, Cui X, Li Z, Barkjohn KK, Brehmer C, et al. . 2021. Personal exposure to PM2.5 oxidative potential in association with pulmonary pathophysiologic outcomes in children with asthma. Environ Sci Technol 55(5):3101–3111, PMID: , 10.1021/acs.est.0c06114. [DOI] [PubMed] [Google Scholar]
- 28.Yang A, Janssen NAH, Brunekreef B, Cassee FR, Hoek G, Gehring U. 2016. Children’s respiratory health and oxidative potential of PM2.5: the PIAMA birth cohort study. Occup Environ Med 73(3):154–160, PMID: , 10.1136/oemed-2015-103175. [DOI] [PubMed] [Google Scholar]
- 29.Avery CL, Mills KT, Williams R, McGraw KA, Poole C, Smith RL, et al. . 2010. Estimating error in using residential outdoor PM2.5 concentrations as proxies for personal exposures: a meta-analysis. Environ Health Perspect 118(5):673–678, PMID: , 10.1289/ehp.0901158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyon-Caen S, Siroux V, Lepeule J, Lorimier P, Hainaut P, Mossuz P, et al. . 2019. Deciphering the impact of Early-Life exposures to highly variable environmental factors on foetal and child health: design of SEPAGES Couple–Child cohort. Int J Environ Res Public Health 16(20):3888, PMID: , 10.3390/ijerph16203888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calas A, Uzu G, Martins JMF, Voisin D, Spadini L, Lacroix T, et al. . 2017. The importance of simulated lung fluid (SLF) extractions for a more relevant evaluation of the oxidative potential of particulate matter. Sci Rep 7(1):11617, PMID: , 10.1038/s41598-017-11979-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calas A, Uzu G, Kelly FJ, Houdier S, Martins JMF, Thomas F, et al. . 2018. Comparison between five acellular oxidative potential measurement assays performed with detailed chemistry on PM10 samples from the city of Chamonix (France). Atmos Chem Phys 18(11):7863–7875, 10.5194/acp-18-7863-2018. [DOI] [Google Scholar]
- 33.Kelly FJ, Mudway IS. 2003. Protein oxidation at the air-lung interface. Amino Acids 25(3–4):375–396, PMID: , 10.1007/s00726-003-0024-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bates JH, Schmalisch G, Filbrun D, Stocks J. 2000. Tidal breath analysis for infant pulmonary function testing. ERS/ATS task force on standards for infant respiratory function testing. European Respiratory Society/American Thoracic Society. Eur Respir J 16(6):1180–1192, PMID: , 10.1034/j.1399-3003.2000.16f26.x. [DOI] [PubMed] [Google Scholar]
- 35.Gustafsson PM, Bengtsson L, Lindblad A, Robinson PD. 2017. The effect of inert gas choice on multiple breath washout in healthy infants: differences in lung function outcomes and breathing pattern. J Appl Physiol (1985) 123(6):1545–1554, PMID: , 10.1152/japplphysiol.00524.2017. [DOI] [PubMed] [Google Scholar]
- 36.Mortamais M, Chevrier C, Philippat C, Petit C, Calafat AM, Ye X, et al. . 2012. Correcting for the influence of sampling conditions on biomarkers of exposure to phenols and phthalates: a 2-step standardization method based on regression residuals. Environ Health 11(1):29, PMID: , 10.1186/1476-069X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beydon N, Davis SD, Lombardi E, Allen JL, Arets HGM, Aurora P, et al. . 2007. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med 175(12):1304–1345, PMID: , 10.1164/rccm.200605-642ST. [DOI] [PubMed] [Google Scholar]
- 38.Gosselink R, Stam H. 2005. Lung Function Testing: European Respiratory Monograph. Lausanne, Switzerland: European Respiratory Society. [Google Scholar]
- 39.Lundblad LKA, Siddiqui S, Bossé Y, Dandurand RJ. 2021. Applications of oscillometry in clinical research and practice. Can J Respir Crit Care Sleep Med 5(1):54–68, 10.1080/24745332.2019.1649607. [DOI] [Google Scholar]
- 40.Hough I, Just AC, Zhou B, Dorman M, Lepeule J, Kloog I. 2020. A multi-resolution air temperature model for France from MODIS and Landsat thermal data. Environ Res 183:109244, PMID: , 10.1016/j.envres.2020.109244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Buuren S, Groothuis-Oudshoorn K. 2011. MICE: Multivariate imputation by chained equations in R. J Stat Softw 45(3):1–67, 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 42.Little RJA. 1988. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc 83(404):1198–1202, 10.1080/01621459.1988.10478722. [DOI] [Google Scholar]
- 43.Rubin DB. 1987. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons, i–xxix, 10.1002/9780470316696.fmatter. [DOI] [Google Scholar]
- 44.Cook RD. 1977. Detection of influential observation in linear regression. Technometrics 19(1):15, 10.2307/1268249. [DOI] [Google Scholar]
- 45.Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, et al. . 2004. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med 351(11):1057–1067, PMID: , 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- 46.Guo C, Hoek G, Chang L-Y, Bo Y, Lin C, Huang B, et al. . 2019. Long-term exposure to ambient fine particulate matter (PM2.5) and lung function in children, adolescents, and young adults: a longitudinal cohort study. Environ Health Perspect 127(12):127008, PMID: , 10.1289/EHP5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang BF, Chen YH, Lin YT, Wu XT, Leo Lee Y. 2015. Relationship between exposure to fine particulates and ozone and reduced lung function in children. Environ Res 137:382–390, PMID: , 10.1016/j.envres.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 48.Rice MB, Rifas-Shiman SL, Litonjua AA, Oken E, Gillman MW, Kloog I, et al. . 2016. Lifetime exposure to ambient pollution and lung function in children. Am J Respir Crit Care Med 193(8):881–888, PMID: , 10.1164/rccm.201506-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schultz ES, Hallberg J, Gustafsson PM, Bottai M, Bellander T, Bergström A, et al. . 2016. Early life exposure to traffic-related air pollution and lung function in adolescence assessed with impulse oscillometry. J Allergy Clin Immunol 138(3):930–932.e5, PMID: , 10.1016/j.jaci.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 50.Shao J, Zosky GR, Hall GL, Wheeler AJ, Dharmage S, Melody S, et al. . 2020. Early life exposure to coal mine fire smoke emissions and altered lung function in young children. Respirology 25(2):198–205, PMID: , 10.1111/resp.13617. [DOI] [PubMed] [Google Scholar]
- 51.Lavigne É, Burnett RT, Stieb DM, Evans GJ, Godri Pollitt KJ, Chen H, et al. . 2018. Fine particulate air pollution and adverse birth outcomes: effect modification by regional nonvolatile oxidative potential. Environ Health Perspect 126(7):077012, PMID: , 10.1289/EHP2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saadeh R, Klaunig J. 2014. Child’s development and respiratory system toxicity. J Environ Anal Toxicol 4(5), 10.4172/2161-0525.1000233. [DOI] [Google Scholar]
- 53.Øvrevik J. 2019. Oxidative potential versus biological effects: a review on the relevance of cell-free/abiotic assays as predictors of toxicity from airborne particulate matter. Int J Mol Sci 20(19):4772, PMID: , 10.3390/ijms20194772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Veras MM, de Oliveira Alves N, Fajersztajn L, Saldiva P. 2017. Before the first breath: prenatal exposures to air pollution and lung development. Cell Tissue Res 367(3):445–455, PMID: , 10.1007/s00441-016-2509-4. [DOI] [PubMed] [Google Scholar]
- 55.Abrams JY, Weber RJ, Klein M, Sarnat SE, Chang HH, Strickland MJ, et al. . 2017. Associations between ambient fine particulate oxidative potential and cardiorespiratory emergency department visits. Environ Health Perspect 125(10):107008, PMID: , 10.1289/EHP1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fang T, Verma V, Bates JT, Abrams J, Klein M, Strickland MJ, et al. . 2016. Oxidative potential of ambient water-soluble PM2.5 in the southeastern United States: contrasts in sources and health associations between ascorbic acid (AA) and dithiothreitol (DTT) assays. Atmos Chem Phys 16(6):3865–3879, 10.5194/acp-16-3865-2016. [DOI] [Google Scholar]
- 57.Janssen NAH, Strak M, Yang A, Hellack B, Kelly FJ, Kuhlbusch TAJ, et al. . 2015. Associations between three specific a-cellular measures of the oxidative potential of particulate matter and markers of acute airway and nasal inflammation in healthy volunteers. Occup Environ Med 72(1):49–56, PMID: , 10.1136/oemed-2014-102303. [DOI] [PubMed] [Google Scholar]
- 58.Weichenthal S, Crouse DL, Pinault L, Godri-Pollitt K, Lavigne E, Evans G, et al. . 2016. Oxidative burden of fine particulate air pollution and risk of cause-specific mortality in the Canadian census health and environment cohort (CanCHEC). Environ Res 146:92–99, PMID: , 10.1016/j.envres.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 59.Borlaza LJS, Weber S, Uzu G, Jacob V, Cañete T, Micallef S, et al. . 2021. Disparities in particulate matter (PM10) origins and oxidative potential at a city scale (Grenoble, France) – Part I: Source apportionment at three neighbouring sites. Atmos Chem Phys 21(7):5415–5437, 10.5194/acp-21-5415-2021. [DOI] [Google Scholar]
- 60.Borlaza LJS, Weber S, Jaffrezo JL, Houdier S, Slama R, Rieux C, et al. . Disparities in particulate matter (PM10) origins and oxidative potential at a city-scale (Grenoble, France) – Part II: sources of PM10 oxidative potential using multiple linear regression analysis and the predictive applicability of multilayer perceptron neural network analysis. Atmos Chem Phys 21(12):9719–9739, 10.5194/acp-2021-57. [DOI] [Google Scholar]
- 61.Evangelopoulos D, Katsouyanni K, Keogh RH, Samoli E, Schwartz J, Barratt B, et al. . 2020. PM2.5 and NO2 exposure errors using proxy measures, including derived personal exposure from outdoor sources: a systematic review and meta-analysis. Environ Int 137:105500, PMID: , 10.1016/j.envint.2020.105500. [DOI] [PubMed] [Google Scholar]
- 62.Rao L, Zhang L, Wang X, Xie T, Zhou S, Lu S, et al. . 2020. Oxidative potential induced by ambient particulate matters with acellular assays: a review. Processes 8(11):1410, 10.3390/pr8111410. [DOI] [Google Scholar]
- 63.Fang T, Zeng L, Gao D, Verma V, Stefaniak AB, Weber RJ. 2017. Ambient size distributions and lung deposition of aerosol dithiothreitol-measured oxidative potential: contrast between soluble and insoluble particles. Environ Sci Technol 51(12):6802–6811, PMID: , 10.1021/acs.est.7b01536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Janssen NAH, Yang A, Strak M, Steenhof M, Hellack B, Gerlofs-Nijland ME, et al. . 2014. Oxidative potential of particulate matter collected at sites with different source characteristics. Sci Total Environ 472:572–581, PMID: , 10.1016/j.scitotenv.2013.11.099. [DOI] [PubMed] [Google Scholar]
- 65.Visentin M, Pagnoni A, Sarti E, Pietrogrande MC. 2016. Urban PM2.5 oxidative potential: importance of chemical species and comparison of two spectrophotometric cell-free assays. Environ Pollut 219:72–79, PMID: , 10.1016/j.envpol.2016.09.047. [DOI] [PubMed] [Google Scholar]
- 66.Xiong Q, Yu H, Wang R, Wei J, Verma V. 2017. Rethinking dithiothreitol-based particulate matter oxidative potential: measuring dithiothreitol consumption versus reactive oxygen species generation. Environ Sci Technol 51(11):6507–6514, PMID: , 10.1021/acs.est.7b01272. [DOI] [PubMed] [Google Scholar]
- 67.Ouidir M, Giorgis-Allemand L, Lyon-Caen S, Morelli X, Cracowski C, Pontet S, et al. . 2015. Estimation of exposure to atmospheric pollutants during pregnancy integrating space–time activity and indoor air levels: does it make a difference? Environ Int 84:161–173, PMID: , 10.1016/j.envint.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stapleton A, Casas M, García J, García R, Sunyer J, Guerra S, et al. . 2022. Associations between pre- and postnatal exposure to air pollution and lung health in children and assessment of CC16 as a potential mediator. Environ Res 204(pt A):111900, PMID: , 10.1016/j.envres.2021.111900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.