Abstract
Introduction and hypothesis
Female voiding dysfunction is often due to bladder outlet obstruction (BOO). We investigated pelvic floor muscle training (PFMT) effectiveness in women with functional BOO.
Methods
This is a prospective study recruiting 63 women functionally obstructed, over 18yo, maximum flow rate (Qmax) less than 12 ml/sec, naïve of voiding treatment. Exclusion criteria were anatomical BOO, neurological condition, pelvic intervention, psychiatric or anticholinergic medication, diabetes mellitus and affected upper urinary tract. At baseline, women underwent uroflow, post void residual (PVR) measurement, cystoscopy, cystogram and urodynamic study (UDS) with pelvic electromyography (EMG). Blaivas-Groutz nomogram has been used to define obstruction. After diagnosis, patients underwent six-month PFMT. Re-evaluation was offered four weeks after end of treatment. Data were analyzed with SPSSv22.0.
Results
63 women were recruited and 48 finally included. At baseline, 20 reported 3 urinary tract infections (UTIs) during last year, and 12 had one episode of urine retention. Median Qmax was 7.5 ml/sec and median PVR 110 ml. 40 women were obstructed. 16 (40%) had mild, 16 (40%) moderate and 8 (20%) severe obstruction. All subjects had an overactive pelvic floor on EMG. Obstructed women were re-evaluated. Median Qmax was 8.5 ml/sec, close to baseline (p = 0.16). Median PVR was 65 ml, reduced to baseline (p = 0.02). 33 (82.5%) remained obstructed, 22 (66.67%) with mild, 8 (24.24%) moderate and 3 (9.09%) severe obstruction. 7 (17.5%) were non-obstructed. 4 patients reported one UTI episode with no cases of retention.
Conclusions
A 6 month PFMT reduced UTIs and PVR in women with functional BOO. Additionally, most patients had a de-escalation to milder obstruction.
Keywords: Female lower urinary tract symptoms, Voiding dysfunction, Pelvic floor muscle training, Functional bladder outlet obstruction
What does this study add to the clinical work
| Pelvic floor muscle training is effective for women with dysfunctional voiding, reducing recurrent urinary tract infection and post void residual. |
Introduction
Female voiding dysfunction is nowadays recognized as an important issue in the everyday clinical practice and this could be directly proven by taking a look inside the latest European Association of Urology (EAU) Guidelines, where Female non-neurogenic LUTS are currently discussed in a separate chapter [1]. Definition and classification of LUTS for women are similar to those used for men, using the terminology of the International Continence Society (ICS) [2]. Unsurprisingly, women frequently complain about storage symptoms, but apart from them, urinary hesitancy and low-stream voiding are also reported, sometimes accompanied by an additional feeling of incomplete emptying of the bladder. ICS recognizes this condition as voiding dysfunction (VD), including symptoms and urodynamic investigations characterized by abnormally slow and/or incomplete micturition, based on abnormally slow urine flow rates and/or raised post-void residual (PVR), ideally on repeated measurement to confirm abnormality [3]. Voiding dysfunction is not always a result of bladder outlet obstruction (BOO), as detrusor underactivity (DU), a urodynamic diagnosis, could also lead to VD. A high detrusor pressure followed with a low peak flow rate could be indicative of obstructive voiding in the Abrams-Griffiths nomogram and these urodynamic parameters remain the only way to diagnose BOO instead of DU [1, 4]. Nevertheless, females’ micturition has been separately investigated and more specific voiding patterns and nomograms have been introduced. One of the most commonly used is the Blaivas-Groutz nomogram, which is validated to evaluate the grade of BOO in female patient’s voiding, while Bladder Contractility Index (BCI) has been introduced for the evaluation of the detrusor contractility strength [5, 6]. It should also be highlighted that according to ICS, dysfunctional voiding is a term describing a specific and discrete form of voiding dysfunction and is characterized by an intermittent and/or fluctuating flow rate due to involuntary intermittent contractions of the periurethral striated muscle during voiding in neurologically healthy individuals [3]. BOO could be anatomical when it is due to urethral stricture, prolapse, fibrosis, or previous pelvic surgical interventions, but it also could be functional when it is caused by non-relaxation of the urethra, the bladder neck, or even the whole pelvic floor during the voiding phase [7, 8]. In the cases where the anatomical obstruction can be excluded and the main cause of BOO remains functional, pelvic floor muscle training (PFMT) is one of the suggested treatments [1][9]. The aim of our study is to determine the efficacy of PFMT in women with functional bladder outlet obstruction.
Material and methods
This is a prospective observational study of women with voiding dysfunction due to presumed functional dysfunction. The study has been approved by the local Ethics Committee and was carried out in accordance with the Declaration of Helsinki. All included subjects signed an informed consent.
Women over 18 years old were recruited if they reported hesitancy and poor stream in the last 12 months with or without the feeling of incomplete emptying. Before any evaluation, a midstream urine sample was collected to exclude urinary tract infection (UTI). A medical history and clinical investigation, including gynecological examination, have been documented during first evaluation. Female patients eligible for the study have had an uroflow with a maximum flow rate (Qmax) less than 12 ml/sec. All patients were naive of any other treatment for their voiding problems.
Women with any kind of vaginal prolapse or those with a visible or palpable fibrotic peri-urethral mass have been excluded. Additional exclusion criteria were history of neurologic condition, prior pelvic surgery, malignancy or radiation, psychiatric or anticholinergic medication, and diabetes mellitus. Women with urethral stricture, cystoscopic evidence of an obstructive bladder neck and those with an affected upper urinary tract have been excluded.
Every woman complaining of voiding dysfunction underwent full diagnostic work-up with uroflow, ultrasound, PVR measurement, flexible cystoscopy, cystogram and urodynamic study (UDS). The UDS was equipped with electromyography (EMG) for the documentation of pelvic floor muscle activity and has been performed according to the ICS standards. Blaivas-Groutz nomogram has been used for the identification and classification of obstructive voiding at the UDS [5]. Moreover, the detrusor contractility has been estimated with the Bladder Contractility Index formula (BCI = PdetQmax + 5 Qmax), while the obstruction has been evaluated with the BOO female index formula (BOO = Pdet@Qmax × 2.2 Qmax) [2, 6]. Using EMG findings, pelvic floor activity was classified as normal, overactive or underactive.
Upon functional BOO diagnosis, women have been advised for supervised PFMT protocol for 6 months, executed, and followed up in the same specialized physiotherapy center. The muscle assessment of the pelvic floor has been based on the PERFECT protocol (P: power, E: endurance, R: repetitions, F: fast, E: ability to elevate the posterior vaginal wall during contraction, C: appropriate co-contraction of transverse abdominus, T: co-ordination of contraction prior to coughing) [10]. Re-evaluation with uroflow, PVR measurement, and invasive UDS with pelvic EMG was offered four weeks after the end of PFMT, while the clinical follow-up, including uroflow and PVR, lasted for 6 months after the end of treatment.
The collected data were analyzed with SPSS v22.0 (IBM SPSS Statistics for Macintosh, Version 22.0. Armonk, NY: IBM Corp), using Mann–Whitney statistical approach for non-parametric samples.
The PFMT sessions protocol
At the first visit, patients were examined for the evaluation of their pelvic floor activity. After taking a medical history, they were given the anatomical information of the pelvic area, they were informed about the significant role of PFMT for their treatment and had a definite schedule for the upcoming sessions. They were asked to empty their bladder and then to lie in a supine position and get prepared for a vaginal examination. With a finger inside their vagina, they were asked to contract and relax their pelvic muscles and notice the sensation around the finger. Additionally, a separate evaluation of the whole pelvis was performed, including all pelvic area, hip joints activity, lumbar muscles, and lower extremities. After the first evaluation, patients were advised for a 6-months weekly supervised PFMT program in the physiotherapy center, accompanied with guidance for home practice.
The weekly visits included a re-education course of diaphragmatic breathing and exercises of relaxing pelvic floor muscles, assisted with manual practice, vaginal mollification, and radiofrequencies. The sessions were also expanded to the relaxation of all pelvic soft tissues and muscles fascia decompression. Patients were advised for bladder training, for the right posture and the ideal body position during voiding.
The home practice included sets of exercises for diaphragmatic breathing and pelvic muscles relaxation with vaginal testing of sensation, depending on their ability on vaginal palpation. The diaphragmatic and pelvic home-program required 20 repeats per day in 4 sets of 5 efforts.
Results
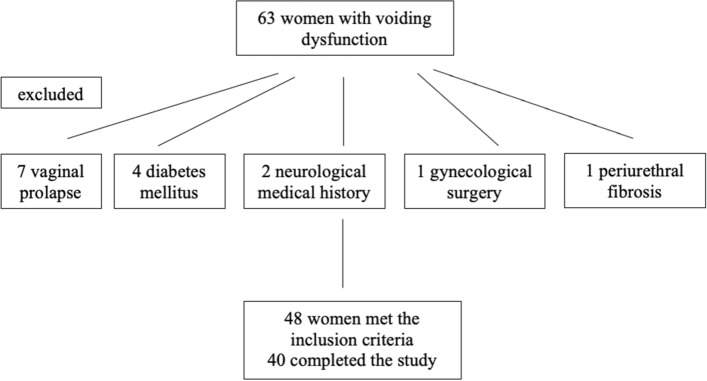
A total of 63 women were recruited in this study and 48 met the inclusion criteria with a median age of 61 years (min–max: 23–72 y.o.). Among the 15 who have been excluded, 7 of them (46.67%) had a vaginal prolapse, 4 (26.67%) had diabetes mellitus, 2 (13.33%) had a neurological medical history, 1 (6.67%) had prior gynecological surgery and 1 (6.67%) had palpable periurethral fibrosis, as a result of an underlying Wegener granulomatosis (Fig. 1).
Fig. 1.
Patients’ enrollment in the study
At baseline, 20 women (41.67%) reported at least 3 UTIs in the last year, and 12 of them (25%) had at least one episode of urine retention at the same time. Moreover, 10 (20.83%) patients were presented with a PVR above 100 ml and they were advised to self-catheterize up to three times daily. The median Qmax was 7.5 ml/sec (min–max: 5.5–12 ml/sec), while the median total flow time has been measured at 26.5 s (min–max: 18–32 s) and the median PVR was 110 ml (min–max: 85–150 ml). The UDS revealed that 40 (83.33%) women were obstructed according to the Blaivas-Groutz nomogram. Among those, 16 (40%) had mild obstruction, 16 (40%) had moderate and 8 (20%) has severe obstruction. Eight (16.7%) women were diagnosed with detrusor underactivity, with a median BCI of 83.5 (min–max: 70–95). All subjects were diagnosed as having overacting pelvic floor based on EMG findings.
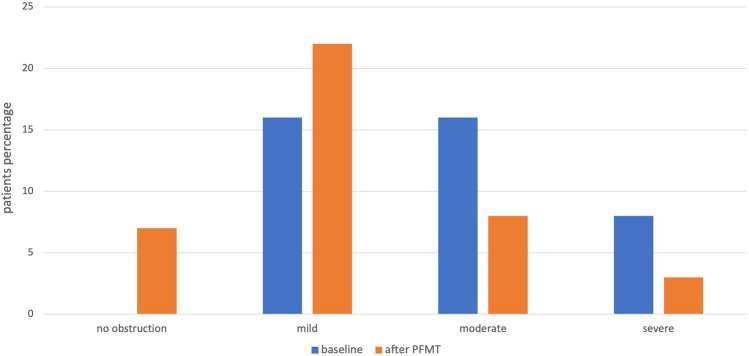
The 40 women found to have a BOO were advised for PFMT program for 6 months. At the re-evaluation 4 weeks after the end of PFMT program, the median Qmax was 8.5 ml/sec (min–max: 6.5-14 ml/sec) and the median voiding time was 18 s (min–max: 15–25 s), with no significant difference with the baseline (p = 0.16 and p = 0.27 respectively). The median PVR has been measured at 65 ml (min–max: 40–75 ml), significantly reduced compared to the baseline evaluation (p = 0.02) (Table 1). More specifically, 4 weeks after PFMT, only 2 (5%) patients needed self-catheterization up to a maximum of twice daily. Considering the results of the UDS re-evaluation after treatment for women with obstruction, 33 (82.5%) women remained obstructive, 22 (66.67%) of them had a mild, 8 (24.24%) a moderate and 3 (9.09%) had severe obstruction. The rest 7 (17.5%) have been diagnosed as non-obstructed, according to their urodynamic findings 4 weeks after PFMT (Fig. 2). Among those women, 6 (85.71%) had a mild obstruction at the baseline and the other 1 (14.29%) had a moderate one. The pelvic EMG remained overactive in the still obstructed patients but became normal in those without any more obstruction.
Table 1.
Variation of the urodynamic parameters before and 4 weeks after PFMT
| Parameters | Baseline (median, min–max) | After PFMT (median, min–max) | p value |
|---|---|---|---|
| Qmax (ml/sec) | 7.5 (5.5–12) | 8.5 (6.5–14) | 0.16 |
| Total flow time (sec) | 26.5 (18–32) | 18 (15–25) | 0.27 |
| PVR (ml) | 110 (85–150) | 65 (40–75) | 0.02 |
PFMT Pelvic Floor Muscle Training, Qmax maximum flow rate, PVR Post Void Residual
Fig. 2.
Obstructed women before and 4 weeks after PFMT
During the 6 months of clinical follow-up for women with BOO, only 4 (10%) patients reported one UTI episode and there were no cases of retention. The median PVR remains at the re-evaluation levels (50 ml, min–max: 40–65 ml), while the median Qmax was 9 ml/sec (min–max: 6.8–14 ml/sec) and the median voiding time was 18.5 s (min–max: 15–23 s), close to the re-evaluation measurements.
Discussion
In our study we evaluated the effectiveness of a 6-month, supervised PFMT program in women with urodynamically proven dysfunctional voiding (DV) and observed a clinically and statistically significant improvement in PVR, reduction of UTI episodes and downgrading of most patients regarding their degree of obstruction according to the Blaivas-Groutz nomogram [5]. Female DV represents a heterogeneous spectrum of urinary difficulties, including both voiding and storage symptoms. At times, it can be complicated by UTIs, or upper tract changes. Due to the non-specific presentation and the similarities to other clinical conditions, the diagnosis may be difficult and frequently involves specialized examinations such as urodynamics [11].
In terms of pathophysiology, it is considered an abnormal voiding behavior, in which increased levator ani or external urethral sphincter (EUS) activity during voiding, disrupts the voiding phase in neurologically intact patients (voiding obstruction) [12]. Restoration of a normal voiding pattern is the main treatment objective in these patients and is achieved through reduction of aberrant muscle activity during voiding phase [13]. Therefore, PFMT is a first-line treatment [1, 13].
Several research teams have proven that muscular, inappropriate hyperactivity is a basic electromyographic finding in patients with VD [14]. Deindl et al., studied 15 women with VD using wire or needle electrodes and reported that women showed either marked activation of both pubococcygeal muscles (part of levator ani muscle group) or EUS during voiding [15]. They also applied a biofeedback-training based on electromyographic activity during micturition and encouraged women to relax their muscles based on EMG recordings [15]. They concluded that women with increased pubococcygeal activity achieved adequate relaxation, in contrast with those showing patterns of EUS hyperactivity, suggesting the potential subcategorization of patients with VD [15].
Involvement of muscular groups in the pathogenesis of VD led to the establishment of PFMT programs for treating patients [16–18]. PFMT consists of education, pelvic floor muscle reinforcement and improvement of posture in order to coordinate muscular compartments more effectively during voiding and achieve a better support for pelvic organs [19]. In our study, the primary goal of using PFMT is muscle relaxation and co-ordination, rather than reinforcement, as functionally obstructive voiding requires a de-escalation of pelvic muscle tone and a synergic activity of pelvic floor. For a PFMT program to be clinically effective it should combine cognitive/behavioral education and physical training [20]. Biofeedback is a type of behavioral therapy which provides information (visual, acoustic, or tactile) about the function of a muscle group in order for the patient to understand how to properly modulate it [13]. Minardi et al., tested two forms of biofeedback in women with DV and recurrent UTIs and realized that significant improvements occurred both regarding UTI prevalence (75–80% reduction) and storage voiding symptoms, regardless the type of biofeedback [13]. Chiang et al., assessed the therapeutic efficacy of biofeedback with PFMT in patients with VD and concluded that the 3-month program led to significant improvements (> 80% of patients) in symptom scores, uroflowmetry parameters and quality of life [21]. Multivariate analysis revealed that recurrent UTIs in the past year were associated with a poor response to PFMT (odds ratio 0.09) [21]. Although this contradicts with previous findings [13], explains partly the non-significant changes of uroflowmetry parameters, since many women reported multiple UTI episodes per year. On the other hand, in a study mainly involved children with non-neurogenic voiding dysfunction, traditional biofeedback proved to be effective only as an adjunctive treatment to PFMT [22]. A randomized controlled study compared biofeedback plus PFMT versus PFMT alone in women with VD and concluded that the former improved voiding symptoms and uroflowmetry parameters compared to latter [23]. It should be, also, important to refer that during pandemic by COVID-19, the need of distant PFMT care had been increased and several programs for women with dysfunction voiding had been based on telehealth. Although, there is a lack of strong evidence, it seems that even telehealth PFMT programs were sufficiently effective [24].
Our findings indicated that high PVR and UTI prevalence improve after a 6 month PFMT even without biofeedback, an evidence which is in line with the literature. An additional finding is that patients with mild forms of functional obstruction according to the Blaivas-Groutz nomogram were more likely to be rendered non-obstructed at the UDS performed four weeks after the end of 6-month training program, compared with those with moderate or severe obstruction. The lack of significant Qmax improvement may potentially be explained by the low baseline Qmax of our sample compared to most published studies reporting higher baseline values. The use of relatively stricter cut-off Qmax at 12 ml/sec instead of 15 ml/sec for inclusion criteria is likely the reason for including more severely affected patients.
Our study suffers from certain limitations. The lack of biofeedback along with PFMT may be a reason for non-significant improvement of Qmax since the behavioral part of these programs is considered crucial to be effective. Although this is not a randomized study, data was collected using a prospective database and a specific protocol in contrast with published studies performed retrospectively. Finally, we did not report metrics from quality-of-life outcomes, but we performed a second urodynamic study at the end of training program to quantify potential improvements which strengthens our results instead of relying only on questionnaires.
In conclusion, PFMT is a first-line treatment option for women with voiding dysfunction due to functional obstruction. In this study we assessed the effect of a 6-month PFMT program on women without previous treatment for their VD and observed an improvement in the prevalence of recurrent UTIs and PVR. At the same time, most patients that remained obstructed had a de-escalation to milder forms of obstruction according to Blaivas-Groutz nomogram four weeks after the end of training program. However, Qmax did not improve significantly to the cohort, potentially due to including more severely affected patients.
Author contributions
TL: manuscript writing, data collection. TI: manuscript writing, data collection, statistical analysis. SV: manuscript reviewing and editing, data collection. PC: physiotherapy execution. SM: project development, data collection, manuscript writing, supervising.
Funding
None of the authors has any conflict of interest or disclosures to declare.
Data availability
Data are available at any time after contacting the corresponding author.
Declarations
Conflict of interest
The authors declares that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Harding CK, Lapitan MC, Arlandis S et al. (2022) EAU Guidelines. Edn. Presented at the EAU Annual Congress Amsterdam March 2022. ISBN 978-94-92671-16-5.
- 2.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the standardisation sub-committee of the international continence society. Neurourol Urodyn. 2002;21:167. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 3.Haylen BT, de Ridder D, Freeman RM, et al. An international urogynecological association (IUGA)/International continence society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4. doi: 10.1002/nau.20798. [DOI] [PubMed] [Google Scholar]
- 4.Lim CS, Abrams P. The Abrams-Griffiths nomogram. World J Urol. 1995;13(1):34–39. doi: 10.1007/BF00182664. [DOI] [PubMed] [Google Scholar]
- 5.Massolt ET, Groen J, Vierhout ME. Application of the Blaivas-Groutz bladder outlet obstruction nomogram in women with urinary incontinence. Neurourol Urodyn. 2005;24(3):237–242. doi: 10.1002/nau.20107. [DOI] [PubMed] [Google Scholar]
- 6.Mytilekas KV, Oeconomou A, Sokolakis I, et al. Defining voiding dysfunction in women: bladder outflow obstruction versus detrusor underactivity. Int Neurourol J. 2021;25(3):244–251. doi: 10.5213/inj.2040342.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel Raheem A, Madersbacher H. Voiding dysfunction in women: how to manage it correctly. Arab J Urol. 2013;11(4):319–330. doi: 10.1016/j.aju.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman DS, Nitti VW. Female bladder outlet obstruction. Curr Urol Rep. 2016;17(4):31. doi: 10.1007/s11934-016-0586-2. [DOI] [PubMed] [Google Scholar]
- 9.Naess I, Bø K. Can maximal voluntary pelvic floor muscle contraction reduce vaginal resting pressure and resting EMG activity? Int Urogynecol J. 2018;29(11):1623–1627. doi: 10.1007/s00192-018-3599-1. [DOI] [PubMed] [Google Scholar]
- 10.Laycock J, Jerwood D. Pelvic floor muscle assessment: the PERFECT scheme. Physiotherapy. 2001;87(12):631–642. doi: 10.1016/S0031-9406(05)61108-X. [DOI] [Google Scholar]
- 11.Pang KH, Campi R, Arlandis S, et al. Diagnostic tests for female bladder outlet obstruction: a systematic review from the European association of urology non-neurogenic female LUTS guidelines panel. Eur Urol Focus. 2021;S2405–4569(21):00231–235. doi: 10.1016/j.euf.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Yagci S, Kibar Y, Akay O, et al. The effect of biofeedback treatment on voiding and urodynamic parameters in children with voiding dysfunction. J Urol. 2005;174(5):1994–1997. doi: 10.1097/01.ju.0000176487.64283.36. [DOI] [PubMed] [Google Scholar]
- 13.Minardi D, d'Anzeo G, Parri G, et al. The role of uroflowmetry biofeedback and biofeedback training of the pelvic floor muscles in the treatment of recurrent urinary tract infections in women with dysfunctional voiding: a randomized controlled prospective study. Urology. 2010;75(6):1299–1304. doi: 10.1016/j.urology.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan WE, Firlit CF, Schoenberg HW. The female urethral syndrome: external sphincter spasm as etiology. J Urol. 1980;124(1):48–49. doi: 10.1016/S0022-5347(17)55287-8. [DOI] [PubMed] [Google Scholar]
- 15.Deindl FM, Vodusek DB, Bischoff C, et al. Dysfunctional voiding in women: which muscles are responsible? Br J Urol. 1998;82(6):814–819. doi: 10.1046/j.1464-410X.1998.00866.x. [DOI] [PubMed] [Google Scholar]
- 16.Artibani W, Cerruto MA. Dysfunctional voiding. Curr Opin Urol. 2014;24(4):330–335. doi: 10.1097/MOU.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 17.Riaz H, Nadeem H, Rathore FA. Recent advances in the pelvic floor assessment and rehabilitation of Women with pelvic floor dysfunction. J Pak Med Assoc. 2022;72(7):1456–1459. doi: 10.47391/JPMA.22-83. [DOI] [PubMed] [Google Scholar]
- 18.Wallace SL, Miller LD, Mishra K. Pelvic floor physical therapy in the treatment of pelvic floor dysfunction in women. Curr Opin Obstet Gynecol. 2019;31(6):485–493. doi: 10.1097/GCO.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 19.Blanchard V, Nyangoh-Timoh K, Fritel X, et al. Importance of a pelvic floor lifestyle program in women with pelvic floor dysfunctions: a pilot study. J Gynecol Obstet Hum Reprod. 2021;50(4):102032. doi: 10.1016/j.jogoh.2020.102032. [DOI] [PubMed] [Google Scholar]
- 20.Frawley HC, Dean SG, Slade SC, et al. Is pelvic-floor muscle training a physical therapy or a behavioral therapy? A call to name and report the physical, cognitive, and behavioral elements. Phys Ther. 2017;97(4):425–437. doi: 10.1093/ptj/pzx006. [DOI] [PubMed] [Google Scholar]
- 21.Chiang CH, Jiang YH, Kuo HC. Therapeutic efficacy of biofeedback pelvic floor muscle exercise in women with dysfunctional voiding. Sci Rep. 2021;11:13757. doi: 10.1038/s41598-021-93283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ladi-Seyedian SS, Sharifi-Rad L, Nabavizadeh B, et al. Traditional biofeedback vs. pelvic floor physical therapy-is one clearly superior? Curr Urol Rep. 2019;20(7):38. doi: 10.1007/s11934-019-0901-9. [DOI] [PubMed] [Google Scholar]
- 23.Sam E, Cinislioglu AE, Yilmazel FK, et al. Is biofeedback-assisted pelvic floor muscle training superior to pelvic floor muscle training alone in the treatment of dysfunctional voiding in women? A prospective randomized study. Int Braz J Urol. 2022;48(3):501–511. doi: 10.1590/s1677-5538.ibju.2021.0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.da Mata KRU, Costa RCM, Carbone ÉDSM, et al. Telehealth in the rehabilitation of female pelvic floor dysfunction: a systematic literature review. Int Urogynecol J. 2021;32(2):249–259. doi: 10.1007/s00192-020-04588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available at any time after contacting the corresponding author.