Abstract
Introduction
Elderly patients are the most affected and vulnerable to COVID-19 and effective therapeutic interventions are urgently required. We clarified the safety and efficacy of Paxlovid in the treatment of elderly patients with coronavirus disease 2019 (COVID-19).
Methods
Patients aged over 60 years and with mild to moderate COVID-19 were admitted to the Zhongshan Hospital MinHang MeiLong Branch, Fudan University and received either Paxlovid treatment or only conventional therapy, between April 1 and May 31, 2022. Viral shedding time, duration of hospital stay, disease progression, and adverse events were analyzed, and multivariate Cox regression analysis was performed to detect the independent high-risk factors for COVID-19 progression in the patients.
Results
A total of 163 (82 and 81 in the treatment and control groups, respectively) patients had a median age of 82 (71–89) years, and 89.0% had at least one concomitant disease. The duration of hospitalization reduced from 15 to 13 days, and viral shedding time reduced from 20 to 16.5 days after Paxlovid treatment. The differences of these two variables between the groups were significant (p < 0.01). Moreover, no serious adverse events or obvious changes in laboratory test results were observed in patients treated with Paxlovid. One patient (1.2%) treated with Paxlovid experienced rebound 56 days after negative measurement. Multivariate analysis showed that Paxlovid therapy, age, hemoglobin, and nucleic acid Ct values at admission were independent risk factors for hospitalization within 14 days, and the differences were significant (p < 0.01).
Conclusion
The use of Paxlovid in elderly patients may promote recovery from COVID-19 and reduce the viral load without adverse events.
Clinical trial registration
www.ClinicalTrials.gov, ID: ChiCTR2200066990.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-023-00760-x.
Keywords: Elderly patient, COVID-19, SARS-CoV-2, Paxlovid
Key Summary Points
| Why carry out this study? |
| Elderly patients are the most affected and vulnerable to COVID-19 and effective therapeutic interventions are urgently required. |
| We clarified the safety and efficacy of Paxlovid in the treatment of elderly patients with COVID-19. |
| What was learned from the study? |
| The use of Paxlovid in elderly patients may promote recovery from COVID-19 and reduce the viral load without adverse events. |
| Paxlovid might be able to reduce the transition rate to severe COVID-19. |
Introduction
Hundreds of millions of individuals, especially elderly without vaccination or with chronic diseases, have been infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) since the outbreak of COVID-19 in Wuhan, China [1, 2]. According to the World Health Organization, 643,875,406 were infected with COVID-19, including 6,630,082 deaths as of 9 December 2022 [3]. In November 2021, the omicron variant was first identified in Botswana and rapidly became the dominant circulating variant [4]. Numerous studies have focused on the mechanisms of and therapeutic methods for the disease; however, there is still no perfect treatment.
The recent global surge of the B.1.1.529 (omicron) variant of SARS-CoV-2 has further accelerated the COVID-19 pandemic and reignited the concern of society in general [4, 5]. Hospitalization rate, hospitalization length, and death rate of patients infected with omicron variant have declined relative to those infected with other variants, and the symptoms are less severe [6–8]. However, omicron has more than 50 mutations and different amino acids in the spike protein, which poses a large threat to elderly patients with no vaccination and chronic comorbidities owing to the disordered immune system, making treatment difficult for these patients [4, 9, 10]. It has been reported that the omicron variant is resistant to the treatment of convalescent plasma and most neutralizing nonoclonal antibodies [11]. Recently, several oral antiviral drugs have been reported to reduce the frequency of hospitalization, inhibit risk of pneumonia progression, and block transition to a severe stage, representing important alternatives to COVID-19 treatment, even for the omicron variant [12–16]. Remdesivir, molnupiravir, and Paxlovid are the three typical drugs used for antiviral therapy [14–16].
Paxlovid and molnupiravir are the primary, novel, approved antiviral drugs inhibiting the viral replication in host cells [12, 13, 17, 18]. Paxlovid is composed of two agents: nirmatrelvir, which blocks viral replication by targeting the protease of SARS-CoV-2-3CL, and ritonavir, which delays the metabolism of nirmatrelvir by inhibiting cytochrome P4503A and CYP2D [16]. A recent clinical trial indicated that a 5-day dose of Paxlovid can reduce the risk of death and hospitalization risk by 89% compared to that with placebo [12, 19], suggesting the effectiveness of Paxlovid in reducing the transition from mild to severe disease stage. In addition, Paxlovid has shown effectiveness in immunocompromised patients [18]. In kidney transplant recipients infected with SARS-CoV-2, the Paxlovid treatment under a strict protocol was also relatively safe and effective [20]. Another retrospective analysis also demonstrated that Paxlovid could reduce the risk of COVID-19-associated hospitalization [21]. However, these studies were not powered to demonstrate the effect of Paxlovid on the elderly patients, although they were predominantly affected by omicron surge. Therefore, there is a need to investigate the safety and efficacy of Paxlovid in elderly hospitalized patients. Powered to answer the question in the present study, we analyzed the safety, tolerability, and therapeutic effects of Paxlovid administered twice daily for 5 days in the treatment of hospitalized elderly patients with mild or moderate COVID-19 caused by the omicron variant of SARS-CoV-2 in Shanghai.
Methods
Patients and Study Design
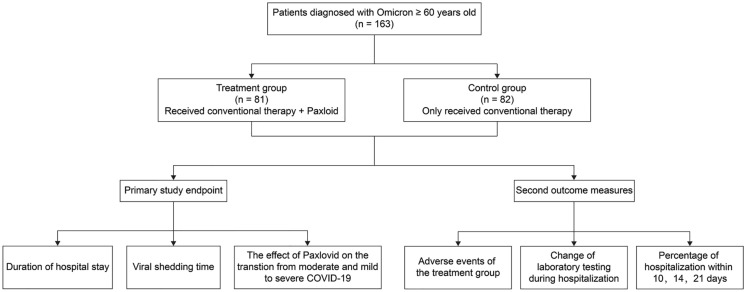
This was a single-center, retrospective study. The medical records of patients aged over 60 years who were diagnosed with mild or moderate COVID-19 caused by omicron variant, according to the Chinese guidelines for diagnosis and treatment of COVID-19, were reviewed from April 1 to May 31, 2022 in the Zhongshan Hospital MinHang MeiLong Branch, Fudan University (Shanghai, China) (Fig. 1). Patients with mild COVID-19 had clinical manifestations related to COVID-19, such as fever and/or respiratory symptoms, but the symptoms were mild, and there was no evidence of pneumonia on imaging. Patients with moderate COVID-19 had clinical manifestations associated with COVID-19 and evidence of pneumonia on imaging. A suspected diagnosis was made on the basis of comprehensive analysis of the patient’s epidemiological history, clinical manifestations, laboratory tests, and other tests, while a positive COVID-19 nucleic acid test is the first criterion to confirm the diagnosis.
Fig. 1.
Flow diagram for study inclusion
According to the diagnosis and treatment protocol for coronavirus pneumonia in China (trial version 9) (Diagnosis and Treatment Protocol for Coronavirus Pneumonia, 2022), conventional therapy included oxygen inhalation as needed (2 L/min for half an hour, three times a day) and Lianhuaqingwen capsules (four capsules administered orally thrice daily for 14 days; Shijiazhuang Yiling Pharmaceutical Co. Ltd., Shijiazhuang, China), while administration of antibacterial agents or methylprednisolone varied depending on disease severity. After a Ct value greater than 35 of SARS-CoV-2 nucleic acid for nucleocapsid protein (N) and open reading frame 1ab (ORF1ab) genes (in two consecutive tests at a time interval of greater than 24 h) in polymerase chain reaction (PCR), patients were discharged or transferred to other hospitals for further treatment depending on their condition.
CT image review and visual severity scores were obtained using previously reported methods [22]. Two non-author radiologists each with 15 years of working experience scored the CT images and were blinded to patients’ clinical statuses. After the initial independent evaluations, the two radiologists discussed any disagreements to reach consensus. Pulmonary disease severity on CT scans was visually assessed. Each scan was assigned to one of the following categories: no ground-glass opacity (GGO) or consolidation, GGO only, consolidation only, or both GGO and consolidation. GGO was defined as an area of haziness with increased attenuation that failed to show any underlying vascular markings [22]. A visual severity score was assigned to each lung lobe using four categories of involved lobes based on GGO and consolidation: 0, none (0% involvement); 1, mild (less than 50% involvement); 2, moderate (50–75% involvement); and 3, severe (greater than 75% involvement) [22]. An overall CT visual severity score was obtained by summing the scores of the five lung lobes (possible range for summed scores, 0–15) [22].
Eligibility Criteria and Trial Treatment
Patients were diagnosed with mild or moderate COVID-19 at admission and received either Paxlovid treatment or only conventional therapy. The treatment group received Paxlovid [300 mg nirmatrelvir (150 mg × 2 tablets) with 100 mg ritonavir (100 mg × 1 tablet) every 12 h for 5 days] and conventional treatment; the control group received conventional treatment only [13]. If the kidneys of the patients had moderate renal damage [30 mL/(min·1.73 m2) ≤ eGFR < 60 mL/ (min·1.73 m2)], the Paxlovid dose was adjusted to 150 mg nirmatrelvir and 100 mg ritonavir twice daily for 5 days. Three patients in the treatment group underwent dose reduction because of impaired renal function.
Exclusion criteria for the study were (1) severe or critical pneumonia at admission, (2) possible allergies to Paxlovid, (3) severe hepatic and renal insufficiency, (4) consuming other antiviral drugs, (5) risk of serious adverse effects from interactions with other drugs, and (6) duration of oral Paxlovid administration of less than 5 days.
Efficacy Assessment of Paxlovid
The primary study endpoint was the duration of hospitalization [from the first day of admission to second time of nucleic acid Ct value being greater than 35 for both ORF1ab and N genes (two consecutive times at an interval of greater than 24 h)], viral shedding time from the first positive test to the second time of nucleic acid Ct value being greater than 35 for both ORF1ab and N genes (two consecutive times at an interval of greater than 24 h) between the treatment and control groups, and the effect of Paxlovid oral administration on the transition from moderate and mild to severe COVID-19. The secondary outcome measures included adverse events in the treatment group, changes in laboratory testing during hospitalization, and percentage of hospitalization within 10, 14, and 21 days (the number of patients discharged within 10, 14, and 21 days accounts for the percentage of the total number of patients in the group). The two groups of patients were followed up for 3 months for SARS-CoV-2 RT-PCR rebound.
Data Extraction
Data extracted from the medical records including age, sex, weight, height, comorbidities, medications, COVID-19 vaccine inoculated (Sinopharm or Sinovac Pharmaceutical Co. Ltd., China), time of detecting nucleic acid abnormality, and latency of omicron were recorded. Clinical data included the patients’ clinical symptoms at admission, baseline Ct values for ORF1ab genes after admission, initial laboratory testing including routine blood tests, blood biochemistry, coagulation profile, C-reactive protein, chest computed tomography (CT) scans, treatment measures (e.g., antibacterial agents, respiratory support, and corticosteroid therapy) during the hospital stay, and other related data.
Compliance with Ethics Guidelines
The study was approved by the Institutional Ethics Board of the Zhongshan Hospital, Fudan University (No. B2022-245R) and was carried out in accordance with the ethical principles for medical research involving human subjects established by the Declaration of Helsinki, protecting the privacy of all participants, as well as the confidentiality of their personal information. This study was registered at www.chictr.org.cn (Identifier: ChiCTR2200066990). Informed consent was obtained from all the patients participating in the study.
Statistical Analysis
Categorical variables are described as frequency rates and percentages, and continuous variables are described using medians and interquartile range (IQR) (25th–75th percentiles). Means for continuous variables were compared using independent group t tests when data were normally distributed. In other instances, the Mann–Whitney U test was used. Proportions of categorical variables were compared using the χ2 test, although Fisher’s exact test was used when data were limited. Potential factors influencing hospitalization within 14 days were analyzed using univariate and multivariate Cox regression models. All statistical tests were two-sided, and p < 0.05 was considered to be statistically significant. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) software version 20.0 (SPSS Inc., Chicago, IL, USA), and statistical mapping was performed using GraphPad Prism 7.0.
Results
Patient Characteristics
Patient characteristics are provided in Table 1. A total of 163 patients meeting the inclusion and exclusion criteria were included in the current study, comprising 81 and 82 in the control and treatment groups, respectively. A total of 36 and 37 cases had moderate disease in the treatment and control groups, respectively. With regard to COVID-19 vaccine inoculation, 129 (79.1%) patients failed to receive COVID-19 vaccine. There were no significant differences between the groups with regards to age, sex, BMI, chronic comorbidities, disease duration prior to admission, severity assessment at admission, or the number of COVID-19 vaccine inoculations (p > 0.05). Moreover, no significant differences were observed between the two groups in terms of laboratory results, nucleic acid Ct values at admission, and CT image visual severity scores (p > 0.05) (Table S1 in the supplementary material).
Table 1.
Baseline characteristics of older patients with COVID-19
| Variables | Total | Treatment group | Control group | p |
|---|---|---|---|---|
| N = 163 | N = 82 | N = 81 | ||
| Age, years | 82 (71–89) | 78 (72–88) | 85 (70–90) | 0.184 |
| Men | 70 (42.9%) | 36 (43.9%) | 34 (42.9%) | 0.804 |
| BMI, kg/m2 | 22.1 (20.8–23.5) | 21.9 (20.3–24.1) | 22.2 (21.0–23.4) | 0.907 |
| Symptoms | ||||
| Fever | 30 (18.4) | 14 (17.1%) | 16 (19.8%) | 0.659 |
| Fatigue | 10 (6.1%) | 5 (6.1%) | 5 (6.2%) | 0.984 |
| Coughing | 79 (48.5%) | 35 (42.7%) | 44 (54.3%) | 0.137 |
| Pharyngalgia | 18 (11.0%) | 10 (12.2%) | 8 (9.9%) | 0.637 |
| Nasal congestion | 14 (8.6%) | 7 (8.5%) | 7 (8.6%) | 0.981 |
| Chronic comorbidity | ||||
| Concomitant diseases | 145 (89.0%) | 72 (87.8%) | 73 (90.1%) | 0.637 |
| Multi-morbidity | 108 (66.3%) | 53 (64.6%) | 55 (67.9%) | 0.659 |
| Hypertension | 88 (54.0%) | 42 (51.2%) | 46 (56.8%) | 0.476 |
| Diabetes | 37 (22.7%) | 16 (19.5%) | 21 (25.9%) | 0.328 |
| Coronary artery disease | 39 (23.9%) | 17 (20.7%) | 22 (27.2%) | 0.336 |
| COPD | 13 (8.0%) | 8 (9.8%) | 5 (6.2%) | 0.398 |
| Cerebrovascular disease | 37 (22.7%) | 19 (23.2%) | 18 (22.2%) | 0.885 |
| Cancer | 18 (11.0%) | 8 (9.8%) | 9 (12.3%) | 0.598 |
| Severity assessment at admission | ||||
| Mild | 90 (55.2%) | 46 (56.1%) | 44 (54.3%) | 0.820 |
| Moderate | 73 (44.8%) | 36 (43.9%) | 37 (45.7%) | |
| Number of COVID-19 vaccine inoculations | ||||
| 0 | 129 (79.1%) | 65 (79.3%) | 64 (79.0%) | 0.965 |
| 1 | 3 (1.8%) | 2 (3.7%) | 1 (1.2%) | |
| 2 | 13 (8.0%) | 5 (6.1%) | 8 (9.9%) | |
| 3 | 17 (10.4%) | 9 (11.0%) | 8 (9.9%) | |
Data for age and BMI are presented as median and interquartile range (IQR). Other data are presented as n (%)
BMI body mass index, COPD chronic obstructive pulmonary disease
Clinical Outcomes
The median time to receive Paxlovid in the treatment group after admission was 2.5 days (IQR 1–4). The median hospitalization duration for all patients was 14 days (IQR 11–17 days). The duration of hospitalization, the primary endpoint, was significantly reduced in the Paxlovid treatment group compared to that in the control group (p = 0.004, Table 2 and Fig. 2a). Survival curves for the duration until discharge in both groups are shown in Fig. 3. Moreover, the viral shedding time from the first positive test to the second time of nucleic acid Ct value being greater than 35 for both ORF1ab and N genes (two consecutive times at an interval of greater than 24 h) was also significantly reduced in the treatment group compared to that in the control group (p = 0.001; Fig. 2b). The percentage of hospital stays within 10 days was significantly higher in the treatment group than that in the control group (30.5% vs. 13.6%, p = 0.009). The percentage of hospital stay within 14 days reached 62.2%, which was significantly higher in the treatment group than that in the control group (p = 0.015, Fig. 2c). The percentage of hospital stay within 21 days tended to be higher in the treatment group than in the control group (97.6% vs. 91.9%; p = 0.099) (Fig. 2d).
Table 2.
Clinical outcomes
| Variables | Total | Treatment group | Control group | p |
|---|---|---|---|---|
| N = 163 | N = 82 | N = 81 | ||
| Duration of hospitalization | 14 (11–17) | 13 (10–16) | 15 (12–18) | 0.004 |
| Viral shedding time from the first positive testing to the second time of nucleic acid Ct value > 35 for ORF1ab and N genes (two consecutive times) | 18 (15–21) | 16.5 (13–20) | 20 (17–22) | 0.000 |
| Percentage of the hospital stay time | ||||
| Day 10 | 36 (22.1%) | 25 (30.5%) | 11 (13.6%) | 0.009 |
| Day 14 | 86 (52.8%) | 51 (62.2%) | 35 (43.2%) | 0.015 |
| Day 21 | 154 (94.5%) | 80 (97.6%) | 74 (91.9%) | 0.099 |
| Antibiotics | 11 (6.7%) | 5 (6.1%) | 6 (7.4%) | 0.739 |
| Use of systemic steroids | 3 (1.8%) | 0 | 3 (3.7%) | 0.080 |
| HFNC | 2 (1.2%) | 1 (1.2%) | 1 (1.2%) | 1 |
| ICU during hospitalization | 5 (3.1%) | 1 (1.2%) | 4 (4.9%) | 0.170 |
| MV | 3 (1.8%) | 0 | 3 (3.7%) | 0.080 |
| Death | 1 (0.6%) | 0 | 1 (1.2%) | 0.314 |
The duration of hospitalization and viral shedding time are presented as the median and interquartile range (IQR). Other data are presented as n (%)
HFNC high-flow nasal cannula, ICU intensive care unit, MV mechanical ventilation
Fig. 2.

The major clinical outcomes of Paxlovid treatment in elderly patients. a Hospitalization time, data are presented as the median and interquartile range (IQR). b Viral shedding time, data are presented as the median and IQR. c Percentage of hospital stay time within 14 days and d percentage of hospital stay time within 21 days are compared between the treatment and control groups
Fig. 3.

Survival curves for the duration until discharge for both groups. HR hazard ratio, CI cumulative index
Furthermore, the effect of Paxlovid oral administration on the transition from moderate and mild to severe COVID-19 was emphatically analyzed. A higher number of patients in the control group tended to require supportive treatment than patients in the treatment group, including high-flow nasal cannula (HFNC), intensive care unit (ICU), and MV (p = 0.08, Table 2). One patient in the treatment group progressed from moderate to severe disease and was transferred to the ICU for HFNC because of pneumonia. Four patients in the control group progressed from moderate to severe disease. Three of the four cases in the control group developed severe pneumonia requiring systemic steroids and MV, and the other required HFNC. All four patients were transferred to the ICU. One of the three aforementioned cases succumbed to acute respiratory distress syndrome, which was secondary to severe lung infection. Therefore, 1 of 82 patients (1.2%) in the treatment group and 4 of 81 (4.9%) in control group had moderate to severe disease, corresponding to a 75.51% relative risk reduction. Patients in both groups who developed severe COVID-19 failed to receive COVID-19 vaccine inoculation.
A total of 148 patients completed 3 months of follow-up, whereas 15 patients were lost to follow-up. Five patients experienced SARS-CoV-2 RT-PCR rebound after discharge (3.1%), including 1 (1.2%) and 4 (4.9%) patients in the treatment and control groups, respectively (p = 0.170, Table 3). None of the patients had completed COVID-19 vaccine inoculations. Patient 1, who had paralysis and spent a long time lying in bed, had a long SARS-CoV-2 RT-PCR rebound time (56 days) after first positive to negative RT-PCR testing. Patient 2 had COPD, diabetes, and pneumonia. Patients 3 and 4 were transferred to ICU for MV during hospitalization due to respiratory failure caused by pulmonary infection. Patient 5 had coronary heart disease and psychiatric illness, but was admitted with lung infection, fever, and high viral load of SARS-CoV-2. The result of SARS-CoV-2 RT-PCR in the patients experiencing rebound quickly turned negative again within 5 days.
Table 3.
SARS-CoV-2 RT-PCR rebound
| First positive to negative RT-PCR | Negative RT-PCR to rebound | Rebound: positive to negative RT-PCR | |
|---|---|---|---|
| Treatment group | |||
| Patient 1 | 12 days | 56 days | 3 days |
| Control group | |||
| Patient 2 | 26 days | 3 days | 2 days |
| Patient 3 | 35 days | 16 days | 5 days |
| Patient 4 | 19 days | 1 days | 2 days |
| Patient 5 | 25 days | 60 days | 1 days |
Safety Evaluation
Paxlovid treatment was well tolerated by elderly patients, and there were no early discontinuations due to adverse effects. Paxlovid treatment was associated with few, mainly low-grade, adverse events (Table 4). Serious adverse events were not observed. The incidence of adverse events during the treatment period was 14.6%.
Table 4.
Adverse events during paxlovid treatment
| Adverse events | Receiving Paxlovid therapy |
|---|---|
| Abdominal pain | 1 (1.21%) |
| Nausea | 1 (1.21%) |
| Vomiting | 1 (1.21%) |
| Diarrhea | 2 (2.44%) |
| Dry mouth | 1 (1.21%) |
| Decreased appetite | 2 (2.44%) |
| Taste perversion | 4 (4.90%) |
| Other | 0 |
| Incidence of drug withdrawal due to adverse events | 0 |
Data are presented as n (%)
Only 31 patients underwent re-examination of laboratory tests in the treatment group, but there were no significant differences in the laboratory parameters between pre-treatment and post-treatment (p > 0.05, Table 5). These parameters included leukocyte count, hemoglobin, lymphocyte count, platelet count, albumin, globulin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase, serum creatinine, glomerular filtration rate (GFR), coagulation function, and C-reactive protein.
Table 5.
Laboratory parameters following therapy
| Variables | Paxlovid therapy (N = 31) | p | |
|---|---|---|---|
| Before therapy | After therapy | ||
| Blood routine | |||
| White blood cell count, × 109/L | 5.6 (3.9–6.4) | 6.2 (5.2–7.4) | 0.190 |
| Hemoglobin (g/L) | 119 (105–128) | 121 (100–129) | 0.658 |
| Lymphocyte count, × 109/L | 1.2 (0.8–1.8) | 1.2 (0.8–1.8) | 0.778 |
| Platelet count, × 109/L | 195 (158–252) | 226 (180–251) | 0.342 |
| Blood biochemistry | |||
| ALT, U/L | 16.8 (10.0–24.9) | 15.0 (12.0–23.6) | 0.678 |
| AST, U/L | 23.0 (17.9–32.0) | 21.0 (16.0–29.0) | 0.176 |
| Albumin, g/L | 37.0 (34.0–39.0) | 36.0 (34.0–39.2) | 0.631 |
| Globulin, g/L | 26.0 (23.7–30.0) | 26.0 (23.5–29.0) | 0.637 |
| Lactate dehydrogenase, U/L | 216 (185–251) | 230 (183–261) | 0.506 |
| Serum creatinine, μmol/L | 83 (68–115) | 83 (64–105) | 0.805 |
| GFR, mL/min | 81.7 (53.4–98.5) | 81.6 (58.3–98.4) | 0.656 |
| C-reactive protein, mg/L | 8.00 (3.90–26.10) | 12.01 (4.00–29.80) | 0.921 |
| Coagulation function | |||
| Prothrombin time, s | 11.8 (11.2–12.7) | 12.0 (11.2–12.9) | 0.938 |
| aPTT, s | 30.0 (27.7–32.8) | 28.5 (26.1–31.0) | 0.163 |
| D-dimer, ng/mL | 0.7 (0.3–1.3) | 0.8 (0.5–1.5) | 0.291 |
Data are presented as median and interquartile range (IQR)
ALT alanine aminotransferase, AST aspartate aminotransferase, GFR glomerular filtration rate, aPTT activated partial thromboplastin clotting time
Multivariate Analysis of Hospitalization Within 14 days
To analyze the independent high-risk factors for COVID-19 progression in elderly patients, stepwise multivariate Cox regression analysis was performed for hospitalization time. Hospitalization days were set as the time variable, and hospitalization days within 14 days (0 = no, 1 = yes) were set as the status. Univariate Cox regression analysis was performed before the stepwise multivariate analysis. Variables that were significant (p < 0.10) in the univariate Cox regression analysis were set as independent variables. The significant variables in the univariate analysis were as follows: Paxlovid therapy, age, serum creatinine, prothrombin time, hemoglobin, GFR, and nucleic acid Ct at admission. Serum creatinine, prothrombin time, and GFR were eliminated to avoid the influence of confounders. Stepwise multivariate Cox regression analysis showed that the model was significant (p < 0.001, Table 6). The significant factors were as follows: Paxlovid therapy (hazard ratio [HR] 1.814, 95% confidence interval [CI] 1.174–2.804), age (HR 0.964, 95% CI 0.943–0.985), hemoglobin level (HR 1.026, 95% CI 1.012–1.040), and nucleic acid Ct at admission (HR 1.114, 95% CI 1.069–1.160). Thus, the results showed that Paxlovid therapy, age, hemoglobin, and nucleic acid Ct at admission were independent factors that determined hospitalization within 14 days after adjusting for other confounding factors.
Table 6.
Risk factors associated with hospitalization within 14 days
| Factors | Partial regression coefficient | Standard error | Wald test | p | HR | 95% CI |
|---|---|---|---|---|---|---|
| Paxlovid therapy | 0.596 | 0.222 | 7.199 | 0.007 | 1.814 | 1.174–2.804 |
| Age | − 0.037 | 0.011 | 10.804 | 0.001 | 0.964 | 0.943–0.985 |
| Hemoglobin | 0.026 | 0.007 | 13.631 | < 0.001 | 1.026 | 1.012–1.040 |
| Nucleic acid Ct at admission | 0.108 | 0.021 | 26.7 | < 0.001 | 1.114 | 1.069–1.160 |
HR hazard ratio, CI confidence interval
Discussion
Elderly patients are the most affected and vulnerable to COVID-19, especially to the omicron variant in Shanghai; therefore, effective therapeutic interventions are urgently required [23]. In the present study, we analyzed the outcomes and safety of Paxlovid in elderly patients aged over 60 years with mild or moderate COVID-19. The hospitalization time and viral shedding time were significantly reduced by Paxlovid treatment for 5 days. Furthermore, we demonstrated the relative safety of Paxlovid for the treatment of elderly patients with multiple chronic diseases.
Symptoms in elderly patients with SARS-CoV-2 infection are often atypical. Patients in the early period of infection display less severe and nonspecific manifestations, lacking enough geriatric care; however, these symptoms progress rapidly during the medium and later periods, leading to a high risk of ICU transfer with MV [24]. To reduce the risk of transition to severe COVID-19 and mortality, the US Food and Drug Administration (FDA) approved the emergency use of Paxlovid for patients with mild and moderate COVID-19, and the drug was urgently introduced into Shanghai in March 2022. Paxlovid is reported to reduce the transition risk by 88% in the EPIC-HR trial and 46% in a real-world study [13]. In contrast, we observed a 75.51% reduction in risk in the present study. Compared to patients’ mean age of 46 and 54 years in these two studies, the mean age of patients in the present study was 80 years. The differences in age distributions, related chronic diseases, vaccination rates, study designs, and SARS-CoV-2 variants may have contributed to the inconsistency between these studies. In a previous study related to the effect of paxlovid on the elderly patients with mean 76.37 years old who were infected with SARS-CoV-2 omicron variants, the nucleic acid shedding time was significantly reduced [25], which is consistent with the result of our research. Meanwhile we also found that the nucleic acid Ct value at admission was low in the elderly patients. High viral load can lead to more severe inflammation and immune response, longer viral shedding time, and longer hospital stay, which may be associated with mental health and severe clinical outcomes, so early intervention and monitoring of nucleic acid Ct value during the SARS-CoV-2 infection were required in the elderly patients [26, 27].
Elderly patients are generally more susceptible to the adverse effects of drug treatment than younger adults. However, the response of elderly patients to Paxlovid therapy remained unclear. The incidence of Paxlovid-related adverse events was higher in the current study (14.6%) than in the EPIC-HR trial (7.8%) [12]. Moreover, patients’ age was the main factor accounting for the differences between the two groups. With advancing age, the self-recovery capacity, immunologic function, biological processes, drug distribution, metabolism, and pharmacodynamic responses of aged organs change drastically compared with younger organs, leading to an increase in adverse events [28, 29]. Furthermore, in the current study, 89.0% of patients had one or more concomitant diseases, and 66.3% had more than two concomitant diseases, increasing their susceptibility to adverse events [30]. In addition, elderly patients who were isolated in specific wards lacked sufficient support from caregivers and geriatric care, and this may have exacerbated the increase in adverse events. However, there were no serious adverse events to cause the discontinuation of Paxlovid. The most frequent adverse events were diarrhea and decreased appetite, followed by dry mouth, vomiting, abdominal pain, and nausea.
As elderly patients have multiple basic disorders, confounders may influence the outcome of Paxlovid therapy. In the current study, we identified Paxlovid therapy, age, hemoglobin levels, and nucleic acid Ct at admission as independent risk factors predicting hospitalization duration within 12 days, further demonstrating the role of Paxlovid in reducing hospitalization duration. In comparison, several other risk factors have been reported, including comorbidity, old age, low lymphocyte count, and high lactate dehydrogenase level in 208 patients enrolled in a previous study [31]. Additionally, several recent studies have demonstrated that immunocompromised condition, oxygen saturation, respiratory rate, lymphocyte count, and CT manifestations are also predictors [18, 32].
Recently, the COVID-19 rebound of symptoms and RT-PCR after Paxlovid treatment has drawn wide public attention [33–37]. In a cohort study of 483 patients with a median age of 63 years old, 0.8% of patients experienced rebound of symptoms after Paxlovid treatment [33]. In the present, we found that one patient (1.2%) in the Paxlovid treatment group experienced SARS-CoV-2 RT-PCR rebound after discharge, which is higher than the reported data. The different characteristics of the enrolled patients might account for the differences of morbidity. Interestingly, the rebound rate of patients (4.9%) in the control group was higher that in the treatment group (1.2%). Actually, it has been reported that it is common for the SARS-CoV-2 to return in patients without Paxlovid treatment [34].
The present study has several limitations. First, the sample size was small as we enrolled only 163 patients and this may have undermined the accuracy of the conclusion. Second, owing to the sudden outbreak of COVID-19 in Shanghai, a case–control study should have been designed. In the future, randomized controlled trials should be performed. Third, the difficulty in entering the isolated wards at any time may have affected the observations of patient behaviors. In addition, further safety follow-up may be required to enhance the quality of the research. The recent reported COVID-19 rebound after Paxlovid treatment has been observed in the present study; however, the present follow-up time was relative short [17].
Conclusion
This real-world study reveals that Paxlovid holds promise in combating the COVID-19 pandemic with adequate safety, especially in vulnerable elderly patients with chronic diseases.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This work was supported by China Primary Health Care Foundation (MTP2022A012), “Technology Innovation Action Plan” of Shanghai Science and Technology Commission (21S11902700), Natural Science Foundation of Shanghai (21ZR1412300), Shanghai “Rising Stars of Medical Talent” Youth Development Program (Youth Medical Talents –Specialist Program, [2020]087), Shanghai Talent Development Fund (2020067), Natural Science Foundation of Fujian (2022J011427). The corresponding author Li-Bo Jiang will be responsible for the journal’s rapid service fee.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Chengzhao Weng, Rongcheng Xie, Shiqin Li, Chao Wang and Libo Jiang collected the epidemiological and clinical data. Chengzhao Weng, Guanjie Han and Libo Jiang conceived and designed the study and analyzed the data. Xiaofeng Wang and Wei Jiang directed and managed the planning. All authors interpreted the data, were involved with drafting or critical revisions of the manuscript, and provided approval of the final manuscript for submission.
Disclosures
Chengzhao Weng, Rongcheng Xie, Guanjie Han, Shiqin Li, Chao Wang, Xiaofeng Wang, Wei Jiang and Libo Jiang have none to declare.
Compliance with Ethics Guidelines
The study was approved by the Institutional Ethics Board of the Zhongshan Hospital, Fudan University (No. B2022-245R) and was carried out in accordance with the ethical principles for medical research involving human subjects established by the Declaration of Helsinki, protecting the privacy of all participants, as well as the confidentiality of their personal information. This study was registered at www.chictr.org.cn (Identifier: ChiCTR2200066990). Informed consent was obtained from all the patients participating in the study.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chengzhao Weng, Rongcheng Xie, and Guanjie Han contributed to this work equally.
Contributor Information
Xiaofeng Wang, Email: wang.xiaofeng@zs-hospital.sh.cn.
Wei Jiang, Email: jiang.wei@zs-hospital.sh.cn.
Libo Jiang, Email: jiang.libo@zs-hospital.sh.cn.
References
- 1.Ross JA, Malone PK, Levy S. The impact of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic on substance use in the United States. Clin Infect Dis. 2022;75(Supplement_1):S81–S5. doi: 10.1093/cid/ciac311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. 2022. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/.
- 4.Vitiello A, Ferrara F, Auti AM, Di Domenico M, Boccellino M. Advances in the Omicron variant development. J Intern Med. 2022;292(1):81–90. doi: 10.1111/joim.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitaker M, Elliott J, Bodinier B, et al. Variant-specific symptoms of COVID-19 in a study of 1,542,510 adults in England. Nat Commun. 2022;13(1):6856. doi: 10.1038/s41467-022-34244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun K, Tempia S, Kleynhans J, et al. SARS-CoV-2 transmission, persistence of immunity, and estimates of Omicron's impact in South African population cohorts. Sci Transl Med. 2022;2022:eabo7081. doi: 10.1126/scitranslmed.abo7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward IL, Bermingham C, Ayoubkhani D, et al. Risk of covid-19 related deaths for SARS-CoV-2 omicron (B.1.1.529) compared with delta (B.1.617.2): retrospective cohort study. BMJ. 2022;378:070695. doi: 10.1136/bmj-2022-070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Modes ME, Directo MP, Melgar M, et al. Clinical characteristics and outcomes among adults hospitalized with laboratory-confirmed SARS-CoV-2 infection during periods of B.1.617.2 (Delta) and B.1.1.529 (Omicron) variant predominance—one hospital, California, July 15-September 23, 2021, and December 21, 2021-January 27, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(6):217–23. doi: 10.15585/mmwr.mm7106e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavda VP, Apostolopoulos V. Omicron variant (B.1.1.529) of SARS-CoV-2: threat for the elderly? Maturitas. 2022;158:78–81. doi: 10.1016/j.maturitas.2022.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ying-Hao P, Yuan-Yuan G, Hai-Dong Z, et al. Clinical characteristics and analysis of risk factors for disease progression of patients with SARS-CoV-2 Omicron variant infection: a retrospective study of 25207 cases in a Fangcang hospital. Front Cell Infect Microbiol. 2022;12:1009894. doi: 10.3389/fcimb.2022.1009894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murakami N, Hayden R, Hills T, et al. Therapeutic advances in COVID-19. Nat Rev Nephrol. 2022 doi: 10.1038/s41581-022-00642-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19. N Engl J Med. 2022;386(15):1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Najjar-Debbiny R, Gronich N, Weber G, et al. Effectiveness of Paxlovid in reducing severe COVID-19 and mortality in high risk patients. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owen DR, Allerton CMN, Anderson AS, et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374(6575):1586–1593. doi: 10.1126/science.abl4784. [DOI] [PubMed] [Google Scholar]
- 16.Sheahan TP, Sims AC, Zhou S, et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 2020 doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Berger NA, Davis PB, Kaelber DC, Volkow ND, Xu R. COVID-19 rebound after Paxlovid and molnupiravir during January-June 2022. medRxiv. 2022 doi: 10.1101/2022.06.21.22276724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun F, Lin Y, Wang X, Gao Y, Ye S. Paxlovid in patients who are immunocompromised and hospitalised with SARS-CoV-2 infection. Lancet Infect Dis. 2022 doi: 10.1016/S1473-3099(22)00430-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahase E. Covid-19: Pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021;375:n2713. doi: 10.1136/bmj.n2713. [DOI] [PubMed] [Google Scholar]
- 20.Devresse A, Sebastien B, De Greef J, et al. Safety, efficacy, and relapse of nirmatrelvir-ritonavir in kidney transplant recipients infected with SARS-CoV-2. Kidney Int Rep. 2022;7(11):2356–2363. doi: 10.1016/j.ekir.2022.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah MM, Joyce B, Plumb ID, et al. Paxlovid associated with decreased hospitalization rate among adults with COVID-19—United States, April-September 2022. MMWR Morb Mortal Wkly Rep. 2022;71(48):1531–7. doi: 10.15585/mmwr.mm7148e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Hu X, Tan W, et al. Multicenter study of temporal changes and prognostic value of a CT visual severity score in hospitalized patients with coronavirus disease (COVID-19) AJR Am J Roentgenol. 2021;217(1):83–92. doi: 10.2214/AJR.20.24044. [DOI] [PubMed] [Google Scholar]
- 23.Soiza RL, Scicluna C, Thomson EC. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50(2):279–283. doi: 10.1093/ageing/afaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauretani F, Ravazzoni G, Roberti MF, et al. Assessment and treatment of older individuals with COVID 19 multi-system disease: clinical and ethical implications. Acta Biomed. 2020;91(2):150–68. doi: 10.23750/abm.v91i2.9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong W, Jiang X, Yang X, et al. The efficacy of paxlovid in elderly patients infected with SARS-CoV-2 omicron variants: results of a non-randomized clinical trial. Front Med (Lausanne) 2022;9:980002. doi: 10.3389/fmed.2022.980002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W, Xiao Q, Fang Z, Lv X, Yao M, Deng M. Correlation analysis between the viral load and the progression of COVID-19. Comput Math Methods Med. 2021;2021:9926249. doi: 10.1155/2021/9926249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20(6):656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jing Y, Diao L, Han L. Adverse events associated with potential drugs for COVID-19: a case study from real-world data. Brief Bioinform. 2021;22(2):1232–1238. doi: 10.1093/bib/bbaa234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei C, Liu Y, Liu Y, et al. Clinical characteristics and manifestations in older patients with COVID-19. BMC Geriatr. 2020;20(1):395. doi: 10.1186/s12877-020-01811-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji D, Zhang D, Xu J, et al. Prediction for progression risk in patients with COVID-19 pneumonia: the CALL score. Clin Infect Dis. 2020;71(6):1393–1399. doi: 10.1093/cid/ciaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranganath N, O'Horo JC, Challener DW, et al. Rebound phenomenon after nirmatrelvir/ritonavir treatment of coronavirus disease-2019 in high-risk persons. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Callaway E. COVID rebound is surprisingly common—even without Paxlovid. Nature. 2022 doi: 10.1038/d41586-022-02121-z. [DOI] [PubMed] [Google Scholar]
- 35.Ganatra S, Dani SS, Ahmad J, et al. Oral nirmatrelvir and ritonavir in non-hospitalized vaccinated patients with covid-19. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Chen X, Xiao W, Zhao D, Feng L. Rapid COVID-19 rebound in a severe COVID-19 patient during 20-day course of Paxlovid. J Infect. 2022 doi: 10.1016/j.jinf.2022.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta K, Strymish J, Stack G, Charness M. Rapid relapse of symptomatic SARS-CoV-2 infection following early suppression with nirmatrelvir/ritonavir. Res Sq. 2022 doi: 10.21203/rs.3.rs-1588371/v1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.