INTRODUCTION
This article will summarize selected sections of the practice guidance put forth by the American Association for the Study of Liver Diseases in an easy-to-read format for patients and their caregivers. The goal of this summary is to help patients and caregivers better understand liver conditions as they relate to sexual health and pregnancy, but it should not replace expert medical care. The full document is available online at: https://aasldpubs.onlinelibrary.wiley.com/doi/10.1002/hep.31559
See Table 12 for a list of the guidance statements.
TABLE 12.
List of guidance statements
| Section 1. Sexual function and birth control |
| • Sexual dysfunction is common in chronic liver disease, and clinicians should routinely inquire about symptoms. When identified, referral to a specialist may be needed for evaluation and treatment |
| • Clinicians should routinely inquire about sexual activity and pregnancy intentions in all reproductive-aged adolescents and women with chronic liver disease or liver transplant |
| • Estrogen-containing agents should be avoided in patients with decompensated cirrhosis, Budd-Chiari syndrome, hepatocellular adenomas, and in transplant recipients with graft failure |
| • Patients using agents other than long-acting reversible contraceptives are encouraged to combine these with a barrier method given their higher failure rates; this is particularly important for women taking teratogenic medications, such as mycophenolic acid products |
| • All forms of emergency contraception may be used in the setting of chronic liver disease |
| Section 2. Elevated liver tests in pregnancy |
| • Elevation in aminotransferases, bilirubin, or bile acids in pregnancy is abnormal and requires investigation |
| • Abdominal US without contrast is the preferred imaging modality throughout pregnancy. MRI/Magnetic resonance cholangiopancreatography without gadolinium is preferred over CT imaging. Gadolinium is contraindicated in pregnancy |
| • Abdominal CT without contrast is generally safe, but the cumulative ionizing radiation exposure should be as low as possible and <50 mGy |
| • Use of iodinated contrast is recommended only if essential |
| • Breastfeeding is safe after iodinated or gadolinium contrast |
| Section 3. Intrahepatic cholestasis of pregnancy |
| • Women with ICP should be tested for HCV if not previously done |
| • Women with persistent symptoms or elevated liver tests or total bile acids postpartum should be evaluated for other causes of chronic liver diseases, including genetic variants associated with cholestasis |
| • Pruritus presenting in the second or third trimester plus total bile acids >10 µmol/L without other causes of pruritus is diagnostic for ICP. Elevation of AST and ALT are frequent but not required for the diagnosis |
| • Pruritus management is multifaceted and includes UDCA (10–15 mg/kg) as first-line therapy. For refractory symptoms, antihistamines, cholestyramine, rifampin, and SAMe may be considered, although the evidence is scant |
| • UDCA is safe in pregnancy and reduces serum bile acid levels |
| • Vitamin K should be supplemented when prothrombin time (PT) is prolonged. More frequent monitoring of PT may be considered for women on cholestyramine |
| Section 4. Hepatitis B and pregnancy |
| • All pregnant women should be tested for HBsAg. Women newly identified as HBsAg-positive should be linked with a clinician knowledgeable in the long-term management of HBV |
| • HBsAg-positive pregnant women require quantification of HBV DNA in the second trimester, and if maternal HBV DNA is >200,000 IU/mL, antiviral therapy should be started between gestational weeks 28 and 32 to reduce the risk of mother-to-child transmission. Antiviral therapy can be stopped at delivery or up to 3 mo postpartum |
| • HBsAg-positive pregnant women should be informed of the increased risk of mother-to-child transmission with invasive pregnancy procedures, such as amniocentesis |
| • If antiviral therapy is required, tenofovir disoproxil fumarate (Viread) is preferred. There are insufficient safety data to recommend entecavir or tenofovir alafenamide |
| • Monitoring of ALT and HBV DNA for 6 mo postpartum or after cessation of antivirals is recommended |
| • Breastfeeding is not contraindicated, even in women who continue on antiviral therapy |
| Section 5. Hepatitis C and pregnancy |
| • Preconception antiviral therapy should be prioritized for women of childbearing age infected with HCV, to eliminate the risk of mother-to-child transmission during pregnancy |
| • For patients whose hepatitis C therapy includes ribavirin, highly effective contraception is required, and conception should be deferred for at least 6 mo following last ribavirin use |
| • Pregnant women should be tested for HCV during each pregnancy, and those testing positive should be evaluated for antiviral therapy after completion of pregnancy and breastfeeding |
| • There are insufficient safety data on DAAs to recommend their use in pregnancy as a means of reducing mother-to-child transmission |
| • Monitoring for ALT flares in women infected with hepatitis C is not required during pregnancy and postpartum |
| • Women infected with HCV requiring invasive pregnancy procedures, such as amniocentesis, should be informed of the possible risk of mother-to-child transmission |
| • To reduce mother-to-child transmission in women infected with HCV, prolonged rupture of membranes, invasive fetal monitoring, and episiotomies should be avoided |
| • Breastfeeding is not contraindicated in women infected with HCV but is not recommended during antiviral therapy. In addition, caution is needed if skin breakdown occurs with associated bleeding of nipples |
| Section 6. Wilson disease and pregnancy |
| • Preconception counseling should include genetic counseling and discussion of medication safety in pregnancy. Preconception counseling is desirable to optimize pregnancy outcomes |
| • Use of zinc is safe in pregnancy, and delaying conception until the patient is on zinc monotherapy should be encouraged |
| • Chelating agents should be continued during pregnancy because of the high risk of spontaneous abortion if withdrawn. Dose reductions in the second and third trimesters are required |
| • Close monitoring of copper balance is necessary to avoid overchelation during pregnancy. Chelating agents require uptitration to prepregnancy doses after delivery |
| • Breastfeeding is associated with infant risks, as all WD drugs are excreted in breast milk, and there is a risk for copper deficiency in the infant |
| Section 7. Primary biliary cholangitis and pregnancy |
| • UDCA is safe in pregnancy and lactation |
| • Obeticholic acid and fibrate use cannot be recommended in pregnancy or lactation in women with PBC because of a lack of safety data |
| • Clinically significant pruritus typically requires a multifaceted approach with cholestyramine (4–16 g daily, divided dose and separated from other medications by at least 2 hours), rifampin (300–600 mg daily), SAMe (1000–1200 mg daily), and antihistamines considered, although evidence is low |
| Section 8. Primary sclerosing cholangitis and pregnancy |
| • UDCA is safe in pregnancy and lactation, although no data support a therapeutic benefit in PSC |
| • Clinically significant pruritus typically requires a multifaceted approach with cholestyramine (4–16 g daily, divided dose and separated from other medications by at least 2 h), rifampin (300–600 mg daily), SAMe (1000–1200 mg daily), and antihistamines considered, although evidence is low |
| Section 9. Autoimmune hepatitis and pregnancy |
| • Preconception counseling should include discussion of medication safety in pregnancy and potential for disease exacerbation, including decompensation. Delaying conception until liver disease is well controlled on stable doses of immunosuppressants for at least 1 year is suggested |
| • Counseling regarding the potential risks of azathioprine and 6-mercaptopurine is recommended, although these drugs are considered safe in pregnancy and lactation |
| • Prednisone and budesonide are considered low risk in pregnancy and lactation |
| • Mycophenolate products are contraindicated in pregnancy and lactation |
| • Liver test monitoring during each trimester is suggested. More frequent monitoring (every 2–4 wk) is advised for the first 6 mo postpartum |
| • Breastfeeding is not contraindicated |
| Section 10. NAFLD and pregnancy |
| • Preconception counseling should include review of maternal and fetal risks associated with obesity and diabetes and the benefits of optimizing weight and metabolic comorbidities before conception |
| • Optimal gestational weight gain through healthy diet and appropriate exercise should be emphasized |
| • Monitoring of liver tests should be similar to nonpregnant women |
| • Breastfeeding is encouraged, and longer duration of lactation is associated with lower rates of future NAFLD among offspring and reduced maternal metabolic complications, including NAFLD |
| Section 11. Liver masses |
| • Reproductive-aged women with HCAs should be counseled about the possibility of adenoma growth and rupture in pregnancy |
| • US monitoring of HCAs during each trimester of pregnancy and up to 3 mo postpartum is reasonable |
| • Pregnancy in women with HCAs <5 cm in diameter is not contraindicated |
| • For HCAs >5 cm in diameter, prophylactic treatment with embolization or resection should be considered before conception to reduce the risk of rupture during pregnancy |
| • Hepatic hemangiomas, regardless of size, do not require monitoring during pregnancy, but new onset of symptoms should prompt investigation |
| • FNH, regardless of size, does not require monitoring |
| Section 12. Gallstones and pregnancy |
| • US is the imaging modality of choice for suspected gallstone disease in pregnancy |
| • Initial management of gallstone disease in pregnancy is supportive, although ERCP and laparoscopic cholecystectomy can be considered, ideally in the second trimester |
| Section 13. Budd-Chiari syndrome and pregnancy |
| • Doppler US is the imaging modality of choice for diagnosing Budd-Chiari syndrome |
| • Treatment of acute Budd-Chiari syndrome is the same as in nonpregnant patients except for more limited choices of anticoagulation and insufficient safety data to endorse thrombolytic therapy in pregnancy |
| • Low–molecular-weight heparin is the anticoagulant of choice during pregnancy and lactation in women with Budd-Chiari syndrome |
| • Vitamin K antagonists are contraindicated during pregnancy but acceptable during breastfeeding |
| Section 14. Cirrhosis and pregnancy |
| • In women with cirrhosis, prepregnancy endoscopic variceal screening is recommended. Screening in the early second trimester is indicated if not performed within 1 y before conception or if there is ongoing liver injury or decompensation occurs |
| • For pregnant women with small gastroesophageal varices, primary prophylaxis using nonselective beta blockers should be considered |
| • For pregnant women with medium or large gastroesophageal varices, primary prophylaxis using band ligation or nonselective beta blockers is recommended. Propranolol is the preferred nonselective beta blocker |
| • Mode of delivery should be guided only by obstetric indications |
| Section 15. Liver transplant and pregnancy |
| • Mycophenolic acid products are contraindicated in pregnancy and lactation, and contraceptive agents with low failure rates should be used |
| • Pregnancy should be delayed until at least 1 y after transplant and with at least 6 mo of stable graft function |
| • Calcineurin inhibitors, azathioprine/6-mercaptopurine, and corticosteroids are acceptable for use in pregnancy and lactation |
| • mTORs are not recommended in pregnancy or lactation because of a lack of safety data |
| • Calcineurin inhibitors trough levels should be obtained every 2–4 wk during pregnancy, with dosing guided by levels and liver tests |
Abbreviations: ALT, alanine aminotransferase; DAA, direct-acting antiviral; ERCP, endoscopic retrograde cholangiopancreatography; FNH, focal nodular hyperplasia; HCAs, hepatocellular adenomas; mTORs, mammalian target of rapamycin agents; PBC, primary biliary cholangitis; UDCA, ursodeoxycholic acid; US, ultrasound.
Section 1: Sexual function and birth control
Question: Can liver disease affect my sexual health?
Answer: Yes. Liver disease can affect a patient’s sexual health. It can cause erectile dysfunction, vaginal dryness, or pain during sex. This is common with more severe liver disease, such as cirrhosis. These problems can also be caused by some medications used to treat liver diseases. If you experience these problems, tell your health care provider because additional testing and/or treatments may be helpful.
Question: Can I get pregnant if I have liver disease?
Answer: Yes. Most people with liver disease can become pregnant. However, it might be harder to get pregnant. For example, women with cirrhosis may have few or no periods, so getting pregnant might be more difficult (see Cirrhosis and pregnancy).
Question: What are the risks of pregnancy if I have liver disease?
Answer: The risks of pregnancy (to you and your baby) will depend on what kind of liver disease you have and how healthy your liver is. Talk to your health care provider if you are thinking about getting pregnant to learn about these risks. You might need to receive some tests and treatments to lower these risks before you get pregnant. Your provider may also change your medications to ones that are safe in pregnancy. Close follow-up with a high-risk obstetrician may also be needed during your pregnancy.
Question: Is hormonal birth control safe for my liver?
Answer: This depends on what kind of liver disease you have and the severity of your liver disease. There are many types of birth control, including ones that have hormones, that are safe for patients with liver disease. These are shown in Table 1.
TABLE 1.
Contraceptive options in patients with liver disease
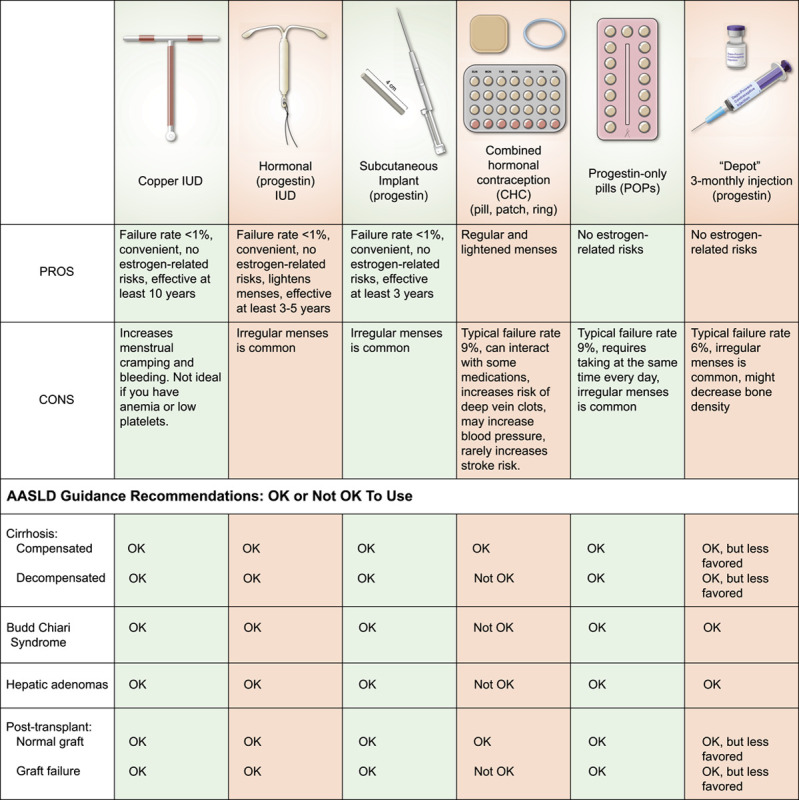
Abbreviation: IUD, intrauterine device.
Birth control options with estrogen and progestin are safe for patients with most types of liver diseases. These are available as a pill, a vaginal ring, or a skin patch. Because of the estrogen, however, these options are NOT safe in some liver conditions. Liver conditions that are not safe with estrogen-containing birth control are Budd-Chiari syndrome (see Budd-Chiari syndrome in pregnancy), decompensated cirrhosis (meaning cirrhosis with complications, such as fluid buildup in the belly or confusion), a liver transplant that is not working well, or hepatic adenomas (see section on Liver Masses).
Birth control options that only have progestin are safe for all liver diseases. Progestin-only options include: (1) the intrauterine device (IUD), (2) the implant (which goes under the skin), (3) the pill form, or (4) an injection. The copper IUD has no hormones and is safe for all liver conditions. IUDs and the implant work best, meaning that <1% of women get pregnant when using these methods. IUDs can be left in place for between 3 and 12 years (depending on which one you choose), so these options are good for women who have a hard time remembering to take medications.
Some medications, such as mycophenolic acid products (ie, CellCept or Myfortic), can harm the baby during pregnancy. These medications increase the risk of birth defects and miscarriages. If you take these medications, you should use 2 forms of birth control to prevent pregnancy, unless you have an IUD or implant because these work very well on their own.
All types of emergency contraception (including ones with estrogen) are safe to use with all types of liver disease, because they are not taken for very long.
If you do not want to get pregnant, talk with your health care provider about which birth control options are safest for your liver and fit best with your lifestyle.
Section 2: Liver tests in pregnancy
Question: When should I be concerned about my liver health in pregnancy?
Answer: You should be concerned about liver health and talk to your health care provider if any of the following is true: (1) you have/had liver disease prior to getting pregnant, (2) your liver tests are abnormal during pregnancy, or (3) you have symptoms including abdominal pain, nausea, vomiting, jaundice (yellowing of your skin or your eyes) or itchiness during pregnancy. While some laboratory changes and symptoms can be normal during pregnancy, your health care provider will help determine if these changes are suggestive of a problem with your liver.
The risks of pregnancy (to you and your baby) will depend on what kind of liver disease you have and how healthy your liver is. Talk to your provider if you are thinking about getting pregnant to learn about these risks. You might need to receive some tests and treatments to lower these risks before you get pregnant and/or during pregnancy. Your health care provider may also change your medications to ones that are safe in pregnancy. Close follow-up with a high-risk obstetrician may also be needed during your pregnancy.
Question: Should I be concerned about high liver tests in pregnancy?
Answer: The liver can be affected by pregnancy. Although some liver tests are expected to increase during pregnancy, others are not. If your liver tests are high, your health care provider may do additional testing to find out why.
Question: Which imaging tests of the liver are safe in pregnancy?
Answer: Ultrasound is safe in pregnancy and is the preferred imaging test to look at the liver. If more testing is needed, MRI without contrast is safe. However, MRI with contrast is not safe in pregnancy. If an abdominal CT scan is needed in pregnancy, it can be done with contrast and with a low amount of radiation. MRI with contrast and a CT scan with contrast are also both safe for women who are breastfeeding. See Table 2 for safety of imaging tests in pregnancy.
TABLE 2.
Abdominal imaging tests
| Imaging | Pregnancy | Breastfeeding |
|---|---|---|
| Ultrasound | OK | OK |
| CT scan without contrast | OK (keep radiation at lowest possible dose) | OK |
| CT with contrast | Perform only if essential | OK |
| MRI without contrast | OK | OK |
| MRI with contrast | Not OK—avoid | OK |
Section 3: Intrahepatic cholestasis of pregnancy
Question: What is intrahepatic cholestasis of pregnancy (ICP)?
Answer: ICP is caused by a problem that prevents the liver from getting rid of bile, leading to a build-up of bile acids in the liver during pregnancy. It usually happens in the third trimester but can occur earlier. ICP typically causes severe itching in the mother (especially the palms of the hands and soles of the feet). ICP can increase the risk of early delivery, breathing problems for the baby, and stillbirth.
Question: Who is at increased risk of developing ICP?
Answer: The risk of developing ICP is increased for certain women, such as those with first-degree relatives (mothers and sisters) who have/had ICP, women carrying twins or triplets, and women with chronic hepatitis C infection. ICP goes away in most women quickly after delivery without any long-term problems.
Question: How do I know if I have ICP?
Answer: Women with ICP experience severe itching, typically on the palms of the hands and soles of the feet. Although a rash is not present, scratch marks may be seen. Typically, the itching starts in the second or third trimester of pregnancy, although rarely it can happen in the first trimester. Jaundice (yellowing of the eyes or the skin) and dark urine can also happen, but are rare. Liver tests can be elevated. A diagnosis of ICP is made by finding elevated bile acid levels in the blood.
Question: How is ICP treated?
Answer: The main treatment for ICP is a medication called ursodeoxycholic acid (or ursodiol). Ursodiol lowers bile acid levels and improves itching. Ursodiol is safe in pregnancy. If itching does not respond to ursodiol, other medications, such as rifampin, antihistamines, cholestyramine, and S-adenosyl-L-methionine, can be used (Table 3).
TABLE 3.
Medications for intrahepatic cholestasis of pregnancy
| Medication | Pregnancy | Breastfeeding | Comments |
|---|---|---|---|
| Ursodeoxycholic acid | OK | OK | |
| Rifampin | OK | OK | |
| Cholestyramine | OK | OK | |
| SAMe | OK | OK | |
| Antihistamines | OK | OK in low doses | Diphenhydramine is preferred |
Note: This information reflects general practice. The specific risks and benefits of these medications should be discussed with your health care provider.
Abbreviation: SAMe, S-adenosyl-L-methionine.
Section 4: Hepatitis B and pregnancy
The risks of pregnancy (to you and your baby) will depend on how healthy your liver is. Talk to your health care provider if you are thinking about getting pregnant to learn about these risks. You might need to receive some tests and treatments to lower these risks before you get pregnant. Your provider may also change your medications to ones that are safe in pregnancy. Close follow-up with a high-risk obstetrician may also be needed during your pregnancy.
Question: Why was I tested for HBV?
Answer: All pregnant women should be tested for hepatitis B since this virus can be passed on from mother to baby. A person can have hepatitis B without symptoms, so doing a blood test is the best way to find out if you have this infection. You may need treatment to lower your chances of passing the infection to your baby. If the test is positive, you should follow up with a health care provider with expertise in hepatitis B.
Question: If I am taking hepatitis B medications, is it safe to become pregnant?
Answer: Yes, but some hepatitis B medications are preferred over others during pregnancy (Table 4). When you learn that you are pregnant, it is important to talk with your health care provider to find out if a change in medication is needed. Tenofovir disoproxil fumarate (Viread) is the preferred medication because it is safe for the baby and works the best to treat hepatitis B during pregnancy.
TABLE 4.
Medications for hepatitis B infection
| Medication | Pregnancy | Breastfeeding | Comments |
|---|---|---|---|
| Lamivudine | OK | OK | Nonpreferred, as less effective than tenofovir DF |
| Tenofovir DF | OK | OK | Preferred treatment |
| Tenofovir AF | Not OK | Not OK | Probably safe but limited data; not preferred |
| Entecavir | Not OK | Not OK | |
| Telbivudine | OK | OK | Nonpreferred, as less effective in treatment of HBV than tenofovir DF |
Note: This information reflects general practice. The specific risks and benefits of these medications should be discussed with your health care provider.
Women with more advanced liver disease (ie, those with cirrhosis) should not stop therapy, because serious liver complications can occur. If hepatitis B treatment is stopped, your virus levels and liver tests should be monitored.
Question: Will having hepatitis B infection affect my pregnancy?
Answer: Hepatitis B infection will not likely affect your pregnancy. However, close monitoring of liver tests is needed as these levels may increase during pregnancy. An increase in the levels of your liver tests can happen if you stop your hepatitis B medications, and can happen after delivery. Usually, an increase in liver tests does not cause problems and gets better without treatment. Sometimes women will need to start hepatitis B medications if liver tests do not get better. So close monitoring of liver tests is needed after delivery.
Question: How can I prevent my baby from getting hepatitis B?
Answer: Your baby should receive the hepatitis B vaccine and hepatitis B immune globulin (HBIG) at delivery. HBIG contains antibodies that immediately protect your baby against the HBV. The vaccine helps the baby make their own antibodies against the HBV for long-term protection. It is important that both HBIG and the vaccine are given within 12 hours of birth to best protect your baby from getting hepatitis B.
Another way to lower the chance of your baby getting hepatitis B is to treat your own hepatitis B infection with medication. If your viral levels are very high, your health care provider may recommend that you start tenofovir disoproxil fumarate (Viread) during the third trimester of pregnancy and continue this medication until delivery. Also, if you need certain procedures during your pregnancy, such as such as an amniocentesis for example, your health care provider should discuss taking hepatitis B medication before the procedure.
Discuss how best to handle your hepatitis B with your health care provider. Your baby should be tested for hepatitis B after they complete their hepatitis B vaccine series.
Question: Can I breastfeed if I have hepatitis B?
Answer: Yes. Breastfeeding is safe even if you have cracked or bleeding nipples since infants are protected by their hepatitis B vaccination at birth. For mothers who are still on hepatitis B medication, breastfeeding is also safe as the amount of medication in the breast milk is very low.
Section 5: Hepatitis C and pregnancy
The risks of pregnancy (to you and your baby) will depend on how healthy your liver is. Talk to your health care provider if you are thinking about getting pregnant to learn about these risks. You might need to receive some tests and treatments to lower these risks before you get pregnant. Your provider may also change your medications to ones that are safe in pregnancy. Close follow-up with a high-risk obstetrician may also be needed during your pregnancy.
Question: Will having hepatitis C infection affect my pregnancy?
Answer: Hepatitis C infection can increase your risk for developing intrahepatic cholestasis of pregnancy (see Section 3 on ICP). Otherwise hepatitis C infection does not usually harm you or your baby during pregnancy. Also, there are no specific tests or treatments that are recommended for hepatitis C during pregnancy. However, there is a small risk (5%) that hepatitis C can be passed on to your baby. This can happen during delivery or during certain procedures, such as amniocentesis (a procedure that removes a small amount of blood from the fetus or from fluid around the fetus). Talk to your health care provider about steps you can take to lower this risk and make a plan to treat your hepatitis C after your pregnancy.
Question: If I test positive for hepatitis C while trying to get pregnant, or during pregnancy, should I undergo treatment?
Answer: Many medications are available that can cure hepatitis C. If you are thinking about getting pregnant, you can be treated before pregnancy, so you do not pass the infection to your baby. Although hepatitis C medications are likely safe in pregnancy, there is not enough information about their safety during pregnancy, so treatment is not usually recommended while you are pregnant. If you have hepatitis C, be sure to discuss a treatment plan with your health care provider after delivery.
Question: How can I prevent my baby from getting hepatitis C?
Answer: Pregnant women with hepatitis C can pass the virus to their baby, but the risk is low (about 5%). To keep this risk low and possibly reduce the risk, you should ask your health care provider about your specific pregnancy. Pregnancy-related procedures such as an amniocentesis can increase this risk. Surgical cuts near the vagina that are sometimes used to help with a difficult delivery can also increase the risk. Finally, it is best to avoid a long period of time between when your water breaks and your delivery, because this can increase the risk of passing hepatitis C to your baby. You should discuss the delivery plan with your obstetrician.
Your child should get a blood test at 18 months of age to check for HCV infection. If your child has HCV infection, they should be seen in a clinic with expertise in treating hepatitis C. Treatments for hepatitis C are available for children as young as 3 years of age.
Question: Can I breastfeed if I have hepatitis C?
Answer: The risk of passing hepatitis C to your newborn from breastfeeding is very low. If you have any bleeding around your nipples, it is best if you stop breastfeeding temporarily. Hepatitis C medications are expected to be found in breast milk and since there is not enough information about the safety of these medications in newborns, it is recommended that you not breastfeed if you are on HCV medications.
Section 6: Wilson disease (WD) and pregnancy
The risks of pregnancy (to you and your baby) will depend on how healthy your liver is. Talk to your health care provider if you are thinking about getting pregnant to learn about these risks. You might need to receive some tests and treatments to lower these risks before you get pregnant. Your provider may also change your medications to ones that are safe in pregnancy. Close follow-up with a high-risk obstetrician may also be needed during your pregnancy.
Question: Does WD affect conception and pregnancy?
Answer: It may be hard for women with WD to get pregnant. You may need to see a fertility expert. Women with WD, especially those who have stopped taking their medications, may also have more miscarriages. Therefore, women with WD should stay on their medications when trying to get pregnant and during pregnancy. There are pros and cons of different WD medications. It is important to discuss with your health care provider what is the best medication for you during pregnancy. Because WD is a genetic disease (passed down from parents), it is important to get counseling on the risk of your children getting WD.
Question: What medications for WD are safe in pregnancy?
Answer: There are 3 medications used to treat WD (Table 5): trientine, D-penicillamine, and zinc. Trientine and D-penicillamine are typically used early in the course of the disease and zinc is often used later in the course of the disease. Zinc does not cause birth defects, and for this reason, delaying pregnancy until you are on zinc therapy is ideal but not required. The decision regarding which drug is best for you during pregnancy should be made in discussion with your health care provider, ideally before you become pregnant.
TABLE 5.
Medications for Wilson disease
| Medication | Pregnancy | Breastfeeding | Comments |
|---|---|---|---|
| D-penicillamine | Discuss with health care provider | Not OK | Birth defects have been reported when used during pregnancy, but discontinuation in pregnancy is associated with negative effects on the mother and pregnancy. If used during breastfeeding, consider monitoring of copper and zinc levels in the baby |
| Trientine | OK | Not OK | Dose adjustments may be needed in pregnancy. If used during breastfeeding, consider monitoring of copper and zinc levels in the baby |
| Zinc | OK | Not OK | If used during breastfeeding, consider monitoring of copper and zinc levels in the baby |
Note: This information reflects general practice. The specific risks and benefits of these medications should be discussed with your health care provider.
Question: How will pregnancy affect my liver health if I have WD?
Answer: Pregnancy will not harm your liver if you have WD, as long as you continue to take your prescribed medications. These medications will help keep your copper levels in the right range. Some WD medications will need to be changed during pregnancy to make sure your baby gets enough copper to develop normally. If you are taking trientine or D-penicillamine, you may need a lower dose during pregnancy. Your health care provider will check your urine copper levels more often during pregnancy and after delivery to make sure you are taking the right dose of your medications.
Question: Can I breastfeed if I have WD?
Answer: Breastfeeding is not generally recommended for women with WD because all medications for WD are found in breast milk. These medications may lower copper levels in the baby. Also, mothers with WD may have low copper levels in their breast milk. Together, these factors put the baby at risk for not having enough copper for normal growth and development. Mothers should discuss the pros and cons of breastfeeding while on WD medications with their health care providers.
Section 7: Primary biliary cholangitis (PBC) and pregnancy
The risks of pregnancy (to you and your baby) will depend on how healthy your liver is. Talk to your health care provider if you are thinking about getting pregnant to learn about these risks. You might need to receive some tests and treatments to lower these risks before you get pregnant. Your provider may also change your medications to ones that are safe in pregnancy. Close follow-up with a high-risk obstetrician (also known as a maternal-fetal medicine specialist) is important during pregnancy.
Question: Does PBC affect pregnancy?
Answer: Unless you have cirrhosis, PBC should not affect your ability to get pregnant. Your risk of early delivery may be increased, but most pregnancies with PBC are otherwise healthy.
Question: Does pregnancy affect my PBC?
Answer: Pregnancy does not usually affect PBC. Liver tests often get better during pregnancy. However, it is possible to have a flare-up of PBC after delivery, meaning an increase in liver tests. In addition, itching might get worse during pregnancy. If you have worsening itching, you should discuss this with your health care provider because other conditions can also cause itching during pregnancy.
Question: Is it safe to continue my PBC medications during pregnancy?
Treatment with ursodeoxycholic acid (ursodiol) is safe to take during pregnancy and breastfeeding. Obeticholic acid or fibrates are not recommended because there is not enough information about their safety in pregnancy. Several medications can be used safely for the treatment of itching during pregnancy and breastfeeding, including cholestyramine, rifampin, and a supplement called S-adenosyl-L-methionine. See Table 6 for safety of medications used in the treatment of PBC during pregnancy and breastfeeding.
TABLE 6.
Medications for primary biliary cholangitis
| Medication | Pregnancy | Breastfeeding | Comments |
|---|---|---|---|
| Ursodeoxycholic acid | OK | OK | |
| Obeticholic acid | Not OK | Not OK | |
| Fenofibrate | Not OK | Not OK | Lacking safety data |
| Rifampin | OK | OK | |
| Cholestyramine | OK | OK | |
| SAMe | OK | OK | |
| Antihistamines | OK | OK in low doses | Diphenhydramine is preferred |
Note: This information reflects general practice. The specific risks and benefits of these medications should be discussed with your health care provider.
Abbreviation: SAMe, S-adenosyl-L-methionine.
Section 8: Primary sclerosing cholangitis (PSC) and pregnancy
The risks of pregnancy (to you and your baby) will depend on how healthy your liver is. Talk to your health care provider if you are thinking about getting pregnant to learn about these risks. You might need to receive some tests and treatments to lower these risks before you get pregnant. Your provider may also change your medications to ones that are safe in pregnancy. Close follow-up with a high-risk obstetrician may also be needed during your pregnancy.
Question: Does PSC affect pregnancy?
Answer: Up to 80% of patients with PSC also have inflammatory bowel disease. Neither PSC nor inflammatory bowel disease affect your ability to get pregnant. However, inflammatory bowel disease activity during pregnancy can increase the risk of miscarriage, stillbirth, early delivery, and low birth weight. Most patients with PSC have healthy pregnancies. PSC increases the risk of early delivery and cesarean births, but does not increase the risk of birth defects or stillbirths.
Question: How will pregnancy affect my PSC?
Answer: Pregnancy does not usually affect PSC. Liver tests are usually stable during pregnancy, although mild flare-ups may occur. Itching may also develop or worsen during pregnancy. If this is new for you, and especially if you have other symptoms, such as pain in the upper right area of your abdomen or a fever, further testing will be necessary. The first test is usually an ultrasound of your liver. Your health care provider will decide if more tests are needed.
Question: Is it safe to continue my PSC medications during pregnancy?
Answer: There are no approved treatments for PSC. However, if you were taking ursodiol before your pregnancy, it is safe to continue throughout pregnancy and breastfeeding (Table 7).
TABLE 7.
Medications for primary sclerosing cholangitis
| Medication | Pregnancy | Breastfeeding | Comments |
|---|---|---|---|
| Ursodeoxycholic acid | OK | OK | |
| Rifampin | OK | OK | |
| Cholestyramine | OK | OK | |
| SAMe | OK | OK | |
| Antihistamines | OK | OK in low doses | Diphenhydramine is preferred |
Note: This information reflects general practice. The specific risks and benefits of these medications should be discussed with your health care provider.
Abbreviation: SAMe, S-adenosyl-L-methionine.
Several medications can be used safely for the treatment of itching during pregnancy and breastfeeding, including cholestyramine, rifampin, and a supplement called S-adenosyl-L-methionine.
Section 9: Autoimmune hepatitis (AIH) and pregnancy
The risks of pregnancy (to you and your baby) will depend on how healthy your liver is. Talk to your health care provider if you are thinking about getting pregnant to learn about these risks. You might need to receive some tests and treatments to lower these risks before you get pregnant. Your provider may also change your medications to ones that are safe in pregnancy. Close follow-up with a high-risk obstetrician (also known as a maternal-fetal medicine specialist) is important during pregnancy.
Question: Does AIH affect pregnancy?
Answer: Unless you have cirrhosis, AIH should not affect your ability to get pregnant. Having AIH increases the risk of certain complications, including risk of early delivery, low birth weight, and fetal loss.
Question: How will pregnancy affect my AIH?
Answer: During pregnancy, liver tests typically improve. A rise in liver tests, or a flare-up of AIH, is more common after delivery, although this can happen at any time. It is very important to prevent these flare-ups of liver tests, as they can cause severe worsening of your liver disease, which can lead to the need for a liver transplant, or even death. This is especially true for patients with cirrhosis. To prevent flare-ups, good control of AIH with medications for at least 1 year before pregnancy is recommended. In addition, you should continue your AIH treatment throughout pregnancy, although the types and doses of medications might be changed by your health care provider. You should have liver tests checked each trimester, and more often in the first 6 months after delivery (Figure 1).
FIGURE 1.
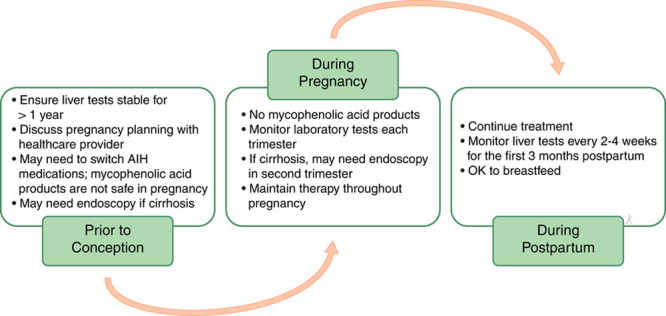
Care of the patient with autoimmune hepatitis (AIH) from preconception through delivery.
Question: Is it safe to continue my AIH medications during pregnancy?
Answer: The following medications are safe for pregnancy and breastfeeding: azathioprine, 6-mercaptopurine, prednisone, and budesonide. There have been some reports of low birth weight and early delivery with azathioprine and 6-mercaptopurine, but that risk is very low compared with the risk of flare-ups during pregnancy. These risks are also not clearly linked to the medications themselves, but, instead, to just having AIH during pregnancy. Less common medications used for AIH include mycophenolic acid products (ie, CellCept or Myfortic), cyclosporine, or tacrolimus. Mycophenolic acid products do increase the risk of birth defects and miscarriage and should be stopped at least 6 weeks prior to getting pregnant. These should not be used while breastfeeding either. Cyclosporine or tacrolimus are considered safe in pregnancy and breastfeeding, as any uncommon risks are outweighed by the importance of keeping AIH under good control. See Table 8 for the safety of AIH medications during pregnancy and breastfeeding.
TABLE 8.
Medications for autoimmune hepatitis
| Medication | Pregnancy | Breastfeeding | Comments |
|---|---|---|---|
| Prednisone | OK | OK | |
| Budesonide | OK | OK | |
| Azathioprine | OK | OK | Associated with low birth weight and preterm birth |
| 6-Mercaptopurine | OK | OK | |
| Mycophenolic acid products | Not OK | Not OK | High risk of miscarriage and birth defects. Stop using 6 wk before conception |
| Cyclosporine | OK | OK | Associated with low birth weight |
| Tacrolimus | OK | OK | Associated with high blood pressure, pre-eclampsia, and preterm birth |
Note: This information reflects general practice. The specific risks and benefits of these medications should be discussed with your health care provider.
Section 10: Non Alcoholic Fatty Liver Disease (NAFLD) and pregnancy
The risks of pregnancy (to you and your baby) will depend on how healthy your liver is. Talk to your health care provider if you are thinking about getting pregnant to learn about these risks. You might need to receive some tests and treatments to lower these risks before you get pregnant. Your provider may also change your medications to ones that are safe in pregnancy. Close follow-up with a high-risk obstetrician may also be needed during your pregnancy.
Question: Are there unique conditions in women that increase risk for NAFLD?
Answer: Yes. A common condition in premenopausal women that increases the risk of NAFLD is called polycystic ovary syndrome (PCOS). PCOS often causes irregular periods and/or excess hair growth in places such as the chest, abdomen, back, or chin. Women with PCOS are often overweight, have high insulin levels or diabetes, and/or high cholesterol. Having PCOS might increase your risk for more severe NAFLD. Your liver specialist may ask about signs of PCOS, such as irregular periods and hair growth, and refer you to a PCOS specialist if they think you may have this condition.
Question: Does having NAFLD affect pregnancy?
Answer: Yes. While most pregnancies in women with NAFLD are healthy, some risks are higher. These include developing gestational diabetes, complications related to high blood pressure (such as pre-eclampsia), and early delivery.
Before and during pregnancy, it is important to make sure metabolic conditions, like high cholesterol, diabetes, and your weight, are under good control. This includes making healthy nutritional choices and getting regular exercise. During pregnancy, it is important not to exceed recommended weight gain goals, based on your weight before pregnancy and how many babies you are carrying (weight gain goals are different if you are carrying a singleton vs. twins, for example). Cesarean deliveries are more common in women with NAFLD, although having NAFLD is not a reason to have a cesarean delivery. You should talk with your obstetrician about the best way for you to deliver your baby.
Question: Is breastfeeding safe with NAFLD?
Answer: Yes. Breastfeeding is safe and encouraged for women with NAFLD because it has many benefits for you and your baby. For example, breastfeeding helps with weight loss after delivery, helps to improve your blood sugar and cholesterol levels, and lowers the risk of your children developing NAFLD in the future.
Section 11: Liver masses
Question: Can I use hormonal birth control if I have a benign liver mass?
Answer: Yes, but the type of hormonal birth control that is safe will depend on what kind of liver mass you have. Common liver masses in women are hemangiomas, hepatocellular adenomas (HCAs), and focal nodular hyperplasia. These are not cancers. All types of hormonal birth control can be used in women with hemangiomas and focal nodular hyperplasia. HCAs can grow in the presence of estrogen so any birth control that contains estrogen should be avoided. These include the combined hormonal contraceptive pill, the vaginal ring, and the skin patch. Progestin-only birth control options can be used in women with HCAs. These options include the IUD, pill form, injection, and an implant (that is placed under the skin) (Table 1).
Question: Do liver masses need treatment or monitoring during pregnancy?
Answer: Hemangiomas and focal nodular hyperplasia do not need to be monitored during pregnancy. HCAs can grow in pregnancy because of high estrogen levels, and large HCAs are at risk of bleeding or rupture. If you have an HCA over 5 cm in size, you may be advised to have it surgically removed or embolized (injecting beads to stop blood flow to the HCA) before or during pregnancy.
Section 12: Gallstones and pregnancy
Question: What are gallstones and why did I get them?
Answer: Gallstones are pieces of solid material that can form in your gallbladder, a small organ in the upper right side of your abdomen. During pregnancy, your gallbladder empties more slowly, which increases the chances of getting gallstones (Figure 2). Gallstones occur in up to 10% of pregnancies but only rarely cause problems.
FIGURE 2.
Gallstones in pregnancy.
Most people with gallstones do not have symptoms, but you may notice right-sided pain after eating or even nausea and vomiting. In addition, the stones can move from your gallbladder into tubes called bile ducts. Sometimes this can cause inflammation of your pancreas (pancreatitis), blockage of your bile ducts (cholangitis), or inflammation of your gallbladder (acute cholecystitis). If your health care provider is concerned about gallstones, an ultrasound is the best first test because there is no risk to you or your baby.
Question: Should my gallstones be treated during pregnancy?
Answer: Most pregnant women with gallstones do not need treatment, which is especially true if you do not have pain. Often, gallstones can be managed with changes in diet alone, such as eating low-fat foods. If problems occur, they can increase your risk of being hospitalized, having earlier delivery, and even losing the baby.
Treatment options that may be considered include surgery to remove your gallbladder (a “cholecystectomy”) or performing a procedure to remove gallstones from the bile ducts (an endoscopic retrograde cholangiopancreatography). These procedures are usually safest during the second trimester. The main risk of endoscopic retrograde cholangiopancreatography during pregnancy is exposure of the baby to radiation, but steps can be taken to reduce this risk.
Section 13: Budd-Chiari syndrome (BCS)
Question: What is BCS?
Answer: BCS occurs when a blood clot forms in the veins that drain blood from the liver (Figure 3). It can cause abdominal pain and fluid to build up in the abdomen.
FIGURE 3.
Budd-Chiari syndrome.
Pregnancy increases the chance of getting blood clots, but pregnancy alone does not typically cause BCS. If you develop BCS during pregnancy, your health care provider should look for other reasons why you developed this condition.
Question: What are treatments options for BCS?
Answer: Treatment of BCS includes medications to thin the blood (anticoagulation). The recommended blood thinner during pregnancy is low–molecular-weight heparin (eg, enoxaparin or Lovenox). Importantly, you should NOT take blood thinners that lowers vitamin K, such as warfarin (Coumadin), because these drugs are not safe during pregnancy. Ask your health care provider which medication is safest during pregnancy and breastfeeding (Table 9).
TABLE 9.
Medications for Budd-Chiari syndrome
| Medication | Pregnancy | Breastfeeding | Comments |
|---|---|---|---|
| Low molecular weight heparin (ie, enoxaparin) | OK | OK | Does not cross the placenta and well-studied in pregnancy. Your health care provider may monitor labs including platelets while taking this medication. This drug does not accumulate in breast milk |
| Direct oral anticoagulants (ie, dabigatran, rivaroxaban, apixaban, edoxaban) | Not OK | Not OK | Lacking safety data |
| Warfarin (ie, coumadin) | Not OK | OK | Can cross placenta and lead to bleeding in the fetus. Does not accumulate in breast milk and can be used during breastfeeding |
Note: This information reflects general practice. The specific risks and benefits of these medications should be discussed with your health care provider.
Other treatment options include procedures to break up the blood clot (thrombolysis), and, rarely, procedures to improve blood flow in the liver, such as placing a shunt in the liver. A liver transplantation may be needed in severe cases.
Section 14: Cirrhosis and pregnancy
The risks of pregnancy (to you and your baby) will depend on how healthy your liver is. Talk to your health care provider if you are thinking about getting pregnant to learn about these risks. You might need to receive some tests and treatments to lower these risks before you get pregnant. Your provider may also change your medications to ones that are safe in pregnancy. Close follow-up with a high-risk obstetrician may also be needed during your pregnancy.
Question: Can I get pregnant if I have cirrhosis?
Answer: Yes. Although cirrhosis might lower your chances of getting pregnant, you can still get pregnant. If you are thinking about getting pregnant, or if you are sexually active and want to avoid a pregnancy, talk with your health care provider.
Question: What are the risks to me or to the baby if I get pregnant?
Answer: Pregnancies in women with cirrhosis have some increased risks. During pregnancy, the amount of water in your body increases, and there are changes in blood pressure and blood flow to the liver that can worsen cirrhosis. There is a higher risk of getting varices (veins in the esophagus or stomach) and a higher risk of bleeding from varices during pregnancy.
Other risks include pre-eclampsia (high blood pressure and protein in the urine) and early delivery. The risk of death for mothers and babies is greater than for women without cirrhosis, although fortunately, this risk is overall low, at <2%. It is very important to tell your health care provider if you are thinking about pregnancy so you can learn about your specific risks based on the condition of your liver. Some tests and treatments done before and during pregnancy might be needed to help lower these risks.
Cesarean deliveries are more common in women with cirrhosis, but cirrhosis is not a reason to have a cesarean delivery. You should talk with your obstetrician about the best way to deliver your baby.
Question: How can I lower my risk for complications during pregnancy?
Answer: The most important step is to tell your health care provider when you are thinking about becoming pregnant. That way, they can discuss your specific risks and perform testing and treatments to lower these risks before you get pregnant. Because pregnancy can worsen varices, you should have an upper endoscopy to look for and treat varices, if you have not had one recently (Figure 4).
FIGURE 4.
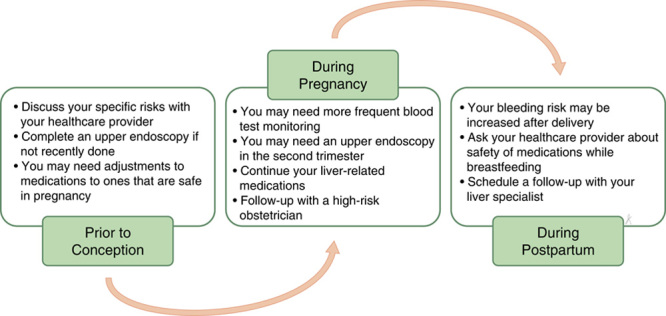
Care of the patient with cirrhosis from preconception through delivery.
Your medications may also need to be changed to ones that are safe for pregnancy. Your health care provider will also check that your liver tests are stable on any new medications before you get pregnant. See Table 10 for the safety of medications used for cirrhosis during pregnancy and breastfeeding.
TABLE 10.
Medications for cirrhosis management
| Medication | Pregnancy | Breastfeeding | Comments |
|---|---|---|---|
| Propranolol | OK | OK | Of these, propranolol is favored for use in pregnancy and breastfeeding |
| Nadolol | OK | OK | |
| Carvedilol | Not OK | Not OK | |
| Fluoroquinolones (ie, ciprofloxacin) | Not OK | OK | — |
Note: This information reflects general practice. The specific risks and benefits of these medications should be discussed with your health care provider.
Close follow-up with a high-risk obstetrician, also known as a maternal-fetal medicine specialist, is important throughout your pregnancy.
Section 15: Liver transplant and pregnancy
The risks of pregnancy (to you and your baby) will depend on how healthy your liver is. Talk to your health care provider if you are thinking about getting pregnant to learn about these risks. You might need to receive some tests and treatments to lower these risks before you get pregnant. Your provider may also change your medications to ones that are safe in pregnancy. Close follow-up with a high-risk obstetrician may also be needed during your pregnancy.
Question: Can I get pregnant after liver transplant?
Answer: Yes, most women can have healthy pregnancies after liver transplant. Regular periods usually return by 1 year after liver transplant, but periods can start within weeks of your transplant surgery. That means that pregnancy can happen very soon after your transplant. There are increased risks for you and the baby if your pregnancy is unplanned or happens within the first year after liver transplant. It is therefore recommended that women wait at least 1 year after liver transplant and have at least 6 months of stable liver tests before getting pregnant. Before that time, it is very important to use contraception if you are sexually active and you want to avoid a pregnancy.
Question: Will pregnancy affect my transplanted liver?
Answer: There is no increased risk of liver failure with pregnancy after liver transplant. However, rejection (or problems with your body attacking the new liver) is more common in pregnancies that happen within the first year after liver transplant.
Question: Does having a liver transplant affect pregnancy?
Answer: There are increased pregnancy risks in women who have a liver transplant. These include high blood pressure and related issues like pre-eclampsia, bleeding after delivery, cesarean deliveries, low birth weight babies, and early delivery. Because of these risks, close follow-up with your transplant provider and a high-risk obstetrician (also known as a maternal-fetal medicine specialist) is important during pregnancy.
Question: Will my transplant medications change during pregnancy?
Answer: Most transplant medications are safe in pregnancy, but some are not (Table 11). Mycophenolic acid products (ie, CellCept or Myfortic) cannot be used in pregnancy because they cause miscarriage and birth defects. These medications should be stopped at least 6 weeks before trying to get pregnant. Your health care provider can replace that medication with ones that are safe in pregnancy, such as azathioprine or 6-mercaptopurine if needed. Steroids, cyclosporine and tacrolimus can be used in pregnancy. Drug levels should be checked every 2–4 weeks during pregnancy. The medications sirolimus and everolimus (known as mammalian target of rapamycin agents) are not recommended in pregnancy. Do not stop taking your transplant medications unless your doctor tells you to stop taking them.
TABLE 11.
Medications in liver transplant recipients
| Medication | Pregnancy | Breastfeeding | Comments |
|---|---|---|---|
| Azathioprine | OK | OK | |
| Cyclosporine | OK | OK | |
| Everolimus | Not OK | Not OK | Associated with low birth weight and preterm birth |
| Mycophenolic acid products | Not OK | Not OK | High risk of miscarriage and birth defects. Stop using 6 wk before conception |
| Prednisone | OK | OK | |
| Sirolimus | Not OK | Not OK | Associated with low birth weight |
| Tacrolimus | OK | OK | Associated with high blood pressure, pre-eclampsia, and preterm birth |
Note: This information reflects general practice. The specific risks and benefits of these medications should be discussed with your health care provider.
Question: Are liver transplant medications safe to take when breastfeeding?
Answer: Some are safe, but some are not (Table 11). The amount of tacrolimus and cyclosporine in breast milk is very low, so these medications can be used while breastfeeding. Azathioprine and prednisone can also be used while breastfeeding, but mycophenolic acid products (ie, CellCept or Myfortic), sirolimus, and everolimus should not.
See Table 12 for a list of the guidance statements.
ACKNOWLEDGMENTS
The authors are grateful for the valuable contributions of the Patient Community Representatives on the AASLD Practice Guideline Committee (PGC), Henry Chang and Jeff McIntyre and the PGC Chairs, Drs Elizabeth C. Verna and George Ioannou.
FUNDING INFORMATION
Funding for the development of this Patient-Friendly Summary of the 2021 AASLD Guidance on Reproductive Health was provided by the American Association for the Study of Liver Diseases.
CONFLICT OF INTEREST
Monika Sarkar is a principal investigator of clinical trials funded by Zydus Pharmaceuticals and GlaxoSmithKline. Norah Terrault receives institutional grant support from Eiger Pharmaceuticals, Gilead Sciences, Roche-Genentech, GlaxoSmithKline, Helio Health and DURECT Corporation, and is a member of the AASLD Governing Board. Cynthia Levy receives institutional grant support from Calliditas, Cara, Cymabay, Escient, Genfit, Gilead, GSK, Intercept, Mirum, Novartis, Pliant, Target RWE and Zydus, and consults for Calliditas, Cara, Cymabay, Escient, Genfit, Gilead, GSK, Escient, Intercept, Ipsen, Mirum, Pliant, and Target RWE. Carla Brady is a member of the AASLD Governing Board. The remaining authors have nothing to report.
Glossary
Cirrhosis–Scarring of the liver caused by long-term liver damage.
Decompensated cirrhosis–Advanced stage of cirrhosis characterized by the progressive loss of liver function.
Hepatic adenoma–Noncancerous liver tumor.
Tubal ligation–A type of surgery involving severing and tying the fallopian tubes that prevents women from getting pregnant.
Vasectomy–A type of surgery that prevents sperm from being expelled in a male’s ejaculate, thereby preventing a man from being able to make a woman pregnant.
Graft–a piece of living tissue that is transplanted surgically.
Teratogenic–Anything that negatively affects the normal development of a baby before it is born.
Anemia–A lower than normal amount of red blood cells or hemoglobin (an oxygen-carrying protein inside red blood cells), leading to low energy, weakness, and other symptoms.
Stroke–Blockage or rupture of a blood vessel supplying the brain; often leads to impaired brain function or death.
Bilirubin–An orange-yellow pigment that is produced as part of the normal process of red blood cell break down.
Aminotransferases–Enzymes that transfer an amino group from one molecule to another; aspartate aminotransferase and alanine aminotransferase are frequently measured to assess the health of the liver.
Magnetic resonance imaging (MRI)–A scan that uses radio waves, a strong magnetic field, and a computer to produce detailed pictures of internal organs
Computed tomography (CT) scan–An imaging technique that uses a computer and x-rays passed through the body at different angles to create a detailed, nearly three-dimensional picture of the body.
Radiation–Energy in the form of particles or waves, such as x-rays and gamma rays. Radiation is often used to help make a diagnosis, as in x-rays, or as a treatment for cancer.
Cholestasis–Any condition in which the flow of bile from the liver stops or slows.
Genetic mutations–Alteration of genetic material.
Gallstones–Hardened deposits of bile that form in the gallbladder.
Jaundice–Yellow staining of skin and fluids due to higher than normal levels of bilirubin in the blood.
Pruritus–Tingling or burning feeling on the any part of the skin that creates an urge to scratch.
Antihistamines–Medications that treat allergies and reduce symptoms such as sneezing and itching by blocking histamine, the substance in the body which causes these symptoms.
Amniocentesis–Medical procedure in which a small amount of amniotic fluid, which contains fetal tissues, is sampled from the amniotic sac surrounding a developing fetus. Used primarily in prenatal diagnosis of chromosomal abnormalities and fetal infections, as well as for sex determination.
Alanine aminotransferase (ALT) flares–Potentially dangerous increase in the level of alanine aminotransferase in the body.
Chelating agents–Chemical compounds that react with metal ions to form a stable, water-soluble complex. Used to treat diseases caused by the excessive amount of specific minerals in the body.
Up-titration–The initiation of therapy at a lower dose, followed by and increase of the dose over time to attain a specific response, or to decrease the risk of adverse effects.
Inflammatory bowel disease (IBD)–A general term for two disorders, ulcerative colitis and Crohn's disease, that cause the intestines to become swollen and inflamed.
Nonalcoholic fatty liver disease (NAFLD)–Fatty build-up in the liver causing the liver to not work as well as it should.
Ultrasound–A painless, noninvasive imaging method that uses high-frequency sound waves.
Transjugular intrahepatic portosystemic shunt (TIPS)–A procedure which connects the vein that brings blood from your gastrointestinal tract and intra-abdominal organs to your liver with the vein that drains blood out of the liver to the heart.
Footnotes
Abbreviations: AIH, autoimmune hepatitis; ALT, alanine aminotransferase; DAA, direct-acting antiviral; ERCP, endoscopic retrograde cholangiopancreatography; FNH, focal nodular hyperplasia; HCA, hepatocellular adenoma; ICP, intrahepatic cholestasis of pregnancy; IUD, intrauterine device; PBC, primary biliary cholangitis; PCOS, polycystic ovary syndrome; PSC, primary sclerosing cholangitis; SAMe, S-adenosyl-L-methionine; UDCA, ursodeoxycholic acid; US, ultrasound; WD, Wilson disease.
Contributor Information
Cynthia Levy, Email: clevy@med.miami.edu.
Carla W. Brady, Email: carla.brady@duke.edu.
Norah Terrault, Email: terrault@usc.edu.
Matthew J. Stotts, Email: matthew.j.stotts@gmail.com.
Monika Sarkar, Email: monika.sarkar@ucsf.edu.


