Background.
The role of lung transplantation for coronavirus disease 2019 (COVID-19)–related lung failure is evolving as the pandemic persists.
Methods.
From January 2021 to April 2022, 20 patients (median age 62 y; range 31–77) underwent lung transplantation for COVID-related lung failure at our institution. We reviewed their clinical and intraoperative characteristics and early outcomes including postoperative complications.
Results.
Eleven patients (55%) had chronic lung disease when they contracted COVID-19. All 20 patients required hospitalization for antivirus treatment. Median lung allocation score was 74.7 (33.1–94.0). Thirteen patients (65%) underwent single-lung transplants, and 7 patients (35%) underwent double-lung transplants. Concomitant coronary artery bypass graft surgery was performed in 2 (10%) patients because of severe coronary artery disease. Postoperatively, venovenous extracorporeal membrane oxygenation was needed in 3 patients (15%) because of severe primary graft dysfunction; all were eventually weaned. Ten patients (50%) experienced deep venous thrombosis, and 1 eventually developed a major pulmonary embolus. The median intensive care unit stay and hospital stays were 6.5 d (3–44) and 18 d (7–77), respectively. During a median follow-up of 201 d (47–418), we experienced 1 late mortality due to COVID-19–related myocarditis. Among the 13 patients with single-lung transplant, 5 demonstrated improvement in their native lungs.
Conclusions.
Lung transplantation yielded favorable early outcomes in a heterogeneous patient cohort that included older patients, obese patients, and patients with coronary artery disease or preexisting chronic lung disease. Our data also shed light on the transforming role of lung transplantation for the pulmonary sequelae of a complex multisystem COVID-19 disorder.
INTRODUCTION
In response to novel lung pathologies resulting from the coronavirus disease 2019 (COVID-19) pandemic, the United Network for Organ Sharing (UNOS) introduced 2 diagnoses on October 28, 2020‚ that are specific to lung failure caused by COVID-19: COVID-related acute respiratory distress syndrome (CARDS) and post-COVID pulmonary fibrosis (PCPF).1 Since August 1, 2020, 140 lung transplant procedures have been performed for CARDS and 74 for PCPF in North America.2
During the first phase of the pandemic, which comprised all of 2020 and continued into 2021, lung transplantation focused on saving the lives of critically ill patients with CARDS who required extracorporeal membrane oxygenation (ECMO) and prolonged intensive care unit (ICU) management. The outcomes after these lung transplantations were much better than initially anticipated based on the complexities of the patients’ conditions and the surgical challenges, such as extensive intrathoracic adhesions with substantial pleural involvement, hilar lymphadenopathy, increased bleeding and transfusion requirements, and optimal selection and management of intraoperative mechanical circulatory support (MCS) including cardiopulmonary bypass3-7 and encouraged the entire lung transplant community. The younger age of the recipients and their healthier baseline status before contracting COVID-19 infection relative to other potential lung transplant recipients appear to be the primary factors contributing to better outcomes, although the excellence of the surgeons who performed these challenging procedures is well recognized.3,4,6,7
As the pandemic persists, treatment options for COVID-19 continue to evolve, and the role of ECMO as a bridge to recovery or transplantation continues to change. Recent studies demonstrate significantly decreased mortality of patients with CARDS and a decreased need for ECMO to support critically ill patients.8,9 Additionally, an excellent recent review highlights the possible reversibility of the lung injuries associated with PCPF and discusses the optimal timing of lung transplantation.10
The role of lung transplantation in patients with COVID-19–related lung failure appears to be transforming synchronously with the trajectory of the pandemic.11 To better understand these changes, we examined outcomes during the second year of the pandemic at our high-volume transplant center, which is located in a geographical area with high COVID-19 infection rates. Here, we present a case series detailing outcomes of lung transplant recipients in 2021 and 2022 for COVID-19–related end-stage lung disease in a heterogeneous patient population including both CARDS and PCPF, and offer insight into the evolving role of lung transplantation in response to COVID-19.
MATERIALS AND METHODS
Study Design
Patients who underwent lung transplantation for COVID-related lung failure at Temple University Hospital between January 1, 2021‚ and April 12, 2022‚ were enrolled. During the study period, a total of 103 patients including external lung transplant referrals for patients who had a diagnosis of CARDS or PCPF were reviewed by Temple University Lung Transplant Program. Of these, 23 patients (22%) were approved and listed for lung transplantation‚ whereas 20 patients underwent lung transplantation at our center, 1 patient has been a still viable candidate awaiting on the list, and 2 died because of progressive sepsis while waiting for their donor’s lungs. The remaining 80 patients were not approved as candidates for lung transplantation because of multiple different reasons, the most outstanding of which was their physical viability/deconditioning due to prolonged ECMO or ventilator support with deep sedation. We reviewed medical charts and operative records to identify patient characteristics, treatments for COVID-19, surgical procedures including the type of intraoperative MCS, and posttransplant early outcomes. Survival and COVID-19 recurrence after lung transplantation were determined from clinical records. COVID-related lung failure was defined as lung failure caused by COVID-19 infection requiring hospital admission. Acute respiratory distress syndrome was defined according to the Berlin Definition.12 Primary graft dysfunction was defined using the International Society for Heart and Lung Transplantation criteria.13 This study was approved by the Temple University Institutional Review Board (protocol number: 29609). The requirement for the patient’s informed consent was waived because of the minimal risk posed by the study.
COVID-19 Treatment and Indications for Lung Transplant
All the patients received COVID-19 treatment either at an outside institution or at Temple University Hospital. The patients who had completed the COVID-19 treatment at an outside hospital were referred to our hospital for lung transplant evaluation and procedure. The indications we typically use for lung transplantation for COVID-related lung failure are (I) at least 3 wk from the initial onset of COVID-associated symptoms with 2 COVID-negative nasal swab tests postinfection; (II) single-organ failure (lung failure only); (III) an age younger than 80 y; (IV) acceptable physical viability if the patient is on ECMO; and (V) the standard criteria of the Temple University Lung Transplant Program‚ including a BMI of <32. This is our standard cutoff when considering whether BMI may complicate lung transplantation; however, the decision to place a patient on the lung transplant waitlist was made comprehensively based on the preoperative patient medical condition by a multidisciplinary team that included surgeons, pulmonologists, and infectious disease physicians.
Statistical Analysis
Continuous variables are shown as mean ± SD and median (range). Categorical variables are shown as absolute numbers (percentages). All statistical analyses were conducted with JMP Pro 15 software (SAS Institute, Cary, NC).
RESULTS
Study Population
From January 1, 2021, to April 12, 2022, 156 lung transplantations were performed at Temple University Hospital. Of these, 20 (13%) were performed for COVID-related lung failure. Eighteen had PCPF‚ and 2 had CARDS. The median age of these 20 lung transplant recipients was 62 y (range 21–77 y)‚ and 17 (85%) were male (Table 1). Ten patients (50%) had a history of smoking. Eleven patients (55%) had a preexisting chronic lung disease when they contracted COVID-19, 4 of whom (20%) required oxygen support pre–COVID-19 (Table 1). One was experiencing chronic lung allograft dysfunction after a double-lung transplant (DLT) 5.5 y prior. Of note, no patients with prior chronic lung disease had been listed for lung transplantation before they suffered from COVID-19. Five patients (25%) were obese with a BMI >32. Four patients (20%) had concurrent severe coronary artery disease (CAD). Median lung allocation score was 74.7 (range 33.1–94.0) (Table 1).
TABLE 1.
Patient characteristics
| Variablea | n = 20 |
|---|---|
| Recipient characteristics | |
| Age, y | 58 ± 12 |
| 62 (31–77) | |
| Sex | |
| Male | 17 (85%) |
| Female | 3 (15%) |
| Height, cm | 173 ± 9 |
| 174 (155–183) | |
| BMI, kg/m2 | 27.6 ± 4.7 |
| 27.2 (19.1–34.9) | |
| BMI >32 | 5 (25%) |
| Diabetes | 6 (30%) |
| Severe coronary artery disease | 4 (20%) |
| Smoking history | 10 (50%) |
| Pre-COVID lung disease | 11 (55%) |
| Interstitial lung disease | 6 (30%) |
| Chronic obstructive lung disease | 3 (15%) |
| Asthma | 1 (5%) |
| Chronic lung allograft dysfunction | 1 (5%) |
| O2 support pre-COVID | 4 (20%) |
| UNOS diagnosis | |
| PCPF | 18 (90%) |
| CARDS | 2 (10%) |
| Mean pulmonary artery pressure (mmHg) | 22 ± 9 |
| 23 (12–44) | |
| Lung allocation score | 65.7 ± 20.6 |
| 74.7 (33.1–94.0) | |
| Time on waitlist, d | 33 ± 47 |
| 15 (4–219) | |
| Donor characteristics | |
| Age, y | 36 ± 14 |
| 38 (14–61) | |
| Diabetes | 3 (15%) |
| Cigarette use (>20 pack years) | 1 (5%) |
| PaO2/FiO2 ratio | 495 ± 78 |
| 495 (324–603) | |
| Total ischemic time, min | 324 ± 93 |
| 325 (193–590) | |
aContinuous variables are shown as mean ± SD and median (range). Categorical variables are shown as absolute numbers (percentages).
CARDS, COVID-related acute respiratory distress syndrome; PaO2/FiO2, pressure of arterial oxygen to fractional inspired oxygen concentration; PCPF, post-COVID pulmonary fibrosis; UNOS, United Network for Organ Sharing.
Trajectory of COVID-19 Infection Before Lung Transplantation
Table 2 shows a brief summary of the patients’ trajectories following treatment for COVID-19 infection before lung transplantation. Median length of stay after admission for COVID-19 was 47 d (range 2–165 d). Eight (40%) patients were discharged to home, and 12 (60%) patients remained hospitalized until they underwent lung transplantation, including 5 (25%) cared for in the ICU. Among 5 ICU patients, 2 patients were on a ventilator, 2 patients were on a high-flow nasal cannula, and 1 patient was on a bilevel-positive airway pressure machine. A total of 3 (15%) patients‚ including 1 stable non-ICU patient‚ were intubated, and 2 (10%) were placed on ECMO in addition to mechanical ventilation. None of the patients required hemodialysis before lung transplantation. The median times from COVID-19 diagnosis to listing and lung transplantation were 80 d (range 38–332 d) and 107 d (range 42–368 d), respectively.
TABLE 2.
Treatment for COVID-19 infection before lung transplant
| Variablea | n = 20 |
|---|---|
| Remdesivir | 16 (80%) |
| Steroid | 16 (80%) |
| Hospital admission | 20 (100%) |
| Discharged to home | 8 (40%) |
| Remained hospitalized (non-ICU) | 7 (35%) |
| Remained hospitalized (ICU) | 5 (25%) |
| Hospital stay, d | 47 ± 42 |
| 47 (2–165) | |
| Intubation | 3 (15%) |
| ECMO | 2 (10%) |
| Hemodialysis | 0 (0%) |
| O2 requirement postinfection, L/min | 17 ± 20 |
| 6.5 (0–60) | |
| Time from COVID-19 diagnosis to listing, d | 131 ± 102 |
| 80 (38–332) | |
| Time from COVID-19 diagnosis to lung transplant, d | 163 ± 116 |
| 107 (42–368) |
aContinuous variables are shown as mean ± SD and median (range). Categorical variables are shown as absolute numbers (percentages).
COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
Intraoperative Findings
Intraoperative and surgical data are summarized in Table 3. Thirteen (65%) patients underwent a single-lung transplant (SLT)‚ and the remaining 7 (35%) patients underwent a DLT. Concomitant coronary artery bypass graft was performed in 2 (10%) patients because of obstructive lesions‚ including 1 patient who had undergone a prior percutaneous coronary intervention. ECMO was used for intraoperative MCS in 8 patients (40%)‚ and cardiopulmonary bypass was used in 3 (15%). The remaining 9 patients underwent lung transplantation off-pump. The median total units of blood products transfused were 2 units (range 0–18).
TABLE 3.
Intraoperative findings and postoperative outcomes
| Variablea | n = 20 |
|---|---|
| Intraoperative findings | |
| Type of procedure | |
| Single-lung transplant | 13 (65%) |
| Double-lung transplant | 7 (35%) |
| Concomitant coronary artery bypass graft | 2 (10%) |
| Mechanical circulatory support | |
| Off-pump | 9 (45%) |
| ECMO | 8 (40%) |
| Cardiopulmonary bypass | 3 (15%) |
| Transfusion (red blood cell/fresh frozen plasma/platelets), total units | 2 (0–18) |
| Postoperative outcomes | |
| Reexplorations due to bleeding | 0 (0%) |
| Time on ventilator, h | 124 ± 157 |
| 59 (18–566) | |
| New tracheostomy | 0 (0%) |
| Primary graft dysfunction requiring ECMO | 3 (15%) |
| Dialysis | 0 (0%) |
| Deep venous thrombosis | 10 (50%) |
| ICU stay, d | 8.5 ± 8.9 |
| 6.5 (3–44) | |
| Hospital stay, d | 22 ± 16 |
| 18 (7–77) | |
aContinuous variables are shown as mean ± SD and median (range). Categorical variables are shown as absolute numbers (percentages).
ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
Early Outcomes
Early outcomes after lung transplantation are also shown in Table 3. No reexplorations because of bleeding were needed. The median time on a ventilator after lung transplantation was 59 h (range 18–566 h), and none of the patients required tracheostomy after lung transplantation. Peripheral venovenous ECMO was needed in 3 patients (15%) after lung transplantation due to severe primary graft dysfunction, and all were eventually weaned off ECMO. Deep venous thrombosis was found in 10 patients (50%)‚ including 1 recipient who eventually developed a major pulmonary embolus. The median ICU stay after the transplant was 6.5 d (range 3–44 d), and the median hospital stay after the transplant was 18 d (range 7–77 d).
Survival and Notable Posttransplant Changes of the Native Lung
During a median follow-up of 201 d (range 47–418 d), both 30-d and 90-d mortality were 0%. Among 13 SLT recipients, improvement of the native contralateral lung was observed by computed tomography imaging in 5 recipients (Table 4 and Figure 1), whereas all the explanted lungs from these patients exhibited advanced interstitial lung fibrosis‚ as well as extensive acute lung injuries‚ on pathological examination. At the final follow-up in April 2022, 19 patients (95%) were alive; 1 patient had died because of COVID-19–associated myocarditis on posttransplantation day 195 (Table 4). This patient had preexisting nonischemic cardiomyopathy and underwent a single-right lung transplant. His posttransplant recovery was uneventful, and he was discharged without major complications on postoperative day 25. However, when he was readmitted for elective bronchoscopy surveillance, he experienced sudden cardiac arrest with pulseless electrical activity. An institutional root-cause analysis deemed that his cause of death was associated with COVID-19–related myocarditis.
TABLE 4.
Characteristics of each recipient
| Pt. # | Age (y) | Pre-COVID lung disease | Diagnosis | LAS | Major comorbidity | Type of procedure | Intraoperative MCS | Hospital stay (d) | Follow-up (d) | Improvement in native lung (Y/N) | Alive/dead |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | ILD | PCPF | 71.8 | Small chest | DLT | ECMO | 21 | 303 | – | Alive |
| 2 | 54 | COPD | PCPF | 85.8 | None | DLT | CPB | 16 | 418 | – | Alive |
| 3 | 60 | COPD | PCPF | 80.9 | Severe CAD (prior CABG, PCI) | RLT | CPB | 10 | 367 | Y | Alive |
| 4 | 64 | ILD | PCPF | 34.8 | None | LLT | Off-pump | 7 | 353 | Y | Alive |
| 5 | 63 | ILD | PCPF | 40.6 | None | RLT | Off-pump | 13 | 357 | N | Alive |
| 6 | 68 | No | PCPF | 59.3 | Severe CAD (prior PCI) | RLT | ECMO | 16 | 327 | N | Alive |
| 7 | 67 | No | PCPF | 81.3 | Cardiomyopathy | RLT | ECMO | 25 | 195 | Y | Dead |
| 8 | 57 | No | PCPF | 77.6 | Small chest, BMI >32 | RLT | CPB | 34 | 310 | N | Alive |
| 9 | 43 | No | PCPF | 79.8 | None | DLT | ECMO | 12 | 259 | – | Alive |
| 10 | 31 | ILD | CARDS | 89.5 | None | DLT | ECMO | 28 | 235 | – | Alive |
| 11 | 64 | No | PCPF | 33.1 | BMI >32 | RLT | Off-pump | 7 | 200 | N/Aa | Alive |
| 12 | 37 | CLAD | PCPF | 80.5 | Small chest, BMI >32 | Redo DLT | Off-pump | 38 | 202 | – | Alive |
| 13 | 61 | COPD | PCPF | 35.4 | Severe CAD | LLT with CABG x1 | Off-pump | 12 | 185 | N/Aa | Alive |
| 14 | 71 | No | PCPF | 85.2 | None | RLT | ECMO | 10 | 146 | N | Alive |
| 15 | 63 | No | PCPF | 45.0 | BMI >32 | LLT | Off-pump | 39 | 137 | N | Alive |
| 16 | 43 | No | PCPF | 79.2 | None | LLT | ECMO | 14 | 128 | Y | Alive |
| 17 | 77 | ILD | PCPF | 44.0 | Severe CAD (prior PCI) | RLT with CABG x1 | Off-pump | 21 | 47 | N | Alive |
| 18 | 63 | ILD | PCPF | 53.8 | None | LLT | Off-pump | 22 | 53 | Y | Alive |
| 19 | 45 | No | CARDS | 94.0 | BMI >32 | DLT | ECMO | 77 | 172 | – | Alive |
| 20 | 69 | Asthma | PCPF | 62.0 | None | DLT | Off-pump | 19 | 106 | – | Alive |
aFollow-up images were not available.
CABG, coronary artery bypass graft; CAD, coronary artery disease; CARDS, COVID-related acute respiratory distress syndrome; CLAD, chronic lung allograft dysfunction; COPD, chronic obstructive lung disease; CPB, cardiopulmonary bypass; DLT, double-lung transplant; ECMO, extracorporeal membrane oxygenation; ILD, interstitial lung disease; LAS, lung allocation score; LLT, single left-lung transplant; MCS, mechanical circulatory support; N/A, not available; PCI, percutaneous coronary intervention; PCPF, post-COVID pulmonary fibrosis; Pt., patient; RLT, single-right lung transplant.
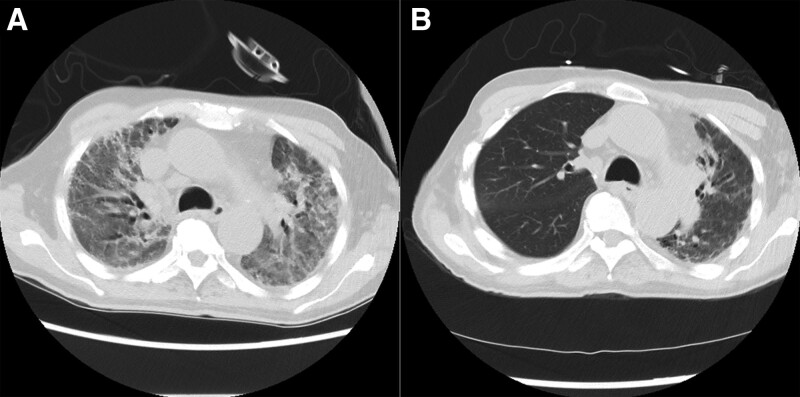
FIGURE 1.
Improvement of the native, contralateral lung in patient 7. A, Diffuse interlobular septal thickening with interstitial reticulation and ground-glass opacities was observed on CT in both lungs before lung transplant. B, CT obtained 6 mo after right SLT demonstrates improvements in the native, left lung. CT, computed tomography; SLT, single-lung transplant.
Recurrent COVID-19 After Lung Transplantation
Three recipients experienced COVID-19 recurrence after lung transplantation on posttransplantation days 149, 257, or 326 (Table 5). All 3 recipients were admitted to Temple University Hospital and were discharged home following the completion of treatment. All were alive at the time of the final follow-up without further episodes of readmission or COVID-19.
TABLE 5.
Summary of recurrent COVID-19 after lung transplantation
| Patient # | Agea | Sex | Type of procedure | Time from lung transplant to repeat COVID (d) | Hospital stay for repeat COVID (d) | Alive/dead |
|---|---|---|---|---|---|---|
| 3 | 60 | Male | SLT | 326 | 6 | Alive |
| 8 | 57 | Female | SLT | 257 | 4 | Alive |
| 12 | 37 | Female | Redo DLT | 149 | 11 | Alive |
aAt lung transplantation.
COVID-19, coronavirus disease 2019; DLT, double-lung transplant; SLT, single-lung transplant.
DISCUSSION
This report of 20 lung transplantations in patients with COVID-19–related lung failure, primarily with PCPF, demonstrates favorable short- and mid-term outcomes that are in line with recent similar studies of lung transplantation for CARDS (Table 6). Our patient cohort included high-risk patients‚ such as older patients, obese patients, and patients with CAD, and more than half of the patients underwent SLT to mitigate their surgical burden and fasten postoperative recovery. Unique posttransplant complications stemming from the complex multisystem nature of COVID-19 were noted in our study, and new findings of post-COVID-19 pulmonary sequelae in the native fibrotic lung after SLT for PCPF are reported that may shed light on the reversibility of the disease‚ as well as the evolving role of lung transplantation for COVID treatment.
TABLE 6.
Literature review of lung transplantation for post-COVID lung failure
| Author | Type of study | Number of recipients | Study period | Preoperative MCS | UNOS disease etiology | Type of transplant | Median hospital stay (d) | Median follow-up (d) | Deaths (n) |
|---|---|---|---|---|---|---|---|---|---|
| Bharat3 | Multicenter | 12 | May 2020–September 2020 | 11 ECMO‚ 1 history of ECMO |
CARDS | DLT | 37 | 80 | 1 |
| Kurihara4 | Single center | 30 | January 2020 - September 2021 | 17 ECMO | CARDS | DLT | 28.5 | 351 | 0 |
| Ko5 | Multicenter | 11 | June 2020–June 2021 | 11 ECMO | CARDS | DLT | 156 | 322 | 1 |
| Lang6 | Multicenter (2) | 19 | January 2020–May 2021 | 19 ECMO | CARDS | DLT | 64 | 134 | 5 |
| Current study | Single center | 20 | January 2021–April 2022 | 2 ECMO | 18 PCPF‚ 2 CARDS |
13 SLT‚ 7 DLT |
18 | 201 | 1 |
CARDS, COVID-related acute respiratory distress syndrome; DLT, double-lung transplant; ECMO, extracorporeal membrane oxygenation; MCS, mechanical circulatory support; PCPF, post-COVID pulmonary fibrosis; SLT, single-lung transplant; UNOS, United Network for Organ Sharing.
An Evolving Role for Lung Transplantation for COVID-19–related Lung Failure: Comorbidities and Surgical Options
Although the central role of lung transplantation as the last resort for patients with end-stage lung diseases holds promise for COVID-19–related lung failure during this enduring pandemic as well, the characteristics of patients with COVID-19–related lung failure, their surgical complexities, optimal timing for the transplant surgery, and their transplant outcomes continue to evolve in parallel with institutional experiences and new treatments for COVID-19‚ including new drugs tested in clinical trials.11,14,15
The potential impact of age-related comorbidities on outcomes after lung transplantation for COVID-19–related lung failure is unique to reports of lung transplantation after the first year of the pandemic, such as this study, because younger patients without comorbidities were well characterized in the first reports of lung transplantation after COVID infection.3,4 Our patient population included several patients over 65 y of age and with outstanding comorbidities, such as morbid obesity and severe CAD requiring concurrent cardiac surgery at the time of transplant. These comorbidities are common among older patients and are associated with a mutual underlying disease mechanism, namely‚ an accelerated aging process.16 Additionally, more than half of the patients had a pre-existing chronic lung disease and thus are thought to be at an increased risk of mortality and morbidity following COVID infection.17,18 Despite these complexities, we observed strong posttransplant outcomes during the follow-up period of the study.
When performing lung transplantation in these high-risk patients during the ongoing pandemic, we think it is important to minimize their surgical burden to potentially reduce postoperative mortality and morbidity and promote faster recovery. To this end, we prioritize SLT as the first surgical option for them unless they have specific pathologies requiring DLT, such as cystic fibrosis and bronchiectasis. In a recently published study, we demonstrated that SLT yields excellent outcomes for patients with severe pulmonary hypertension in whom DLT had been the preferred surgical option for several decades at most transplant centers.19 Of note, the mortality of lung transplant recipients who contract COVID-19 after lung transplantation remains as high as 20% to 40%.11,20 Given the high risks inherent to COVID-19 infection in lung transplant recipients, the option for an SLT with the goal of reducing the patient’s surgical burden should be strongly considered‚ particularly for elderly patients and patients with outstanding comorbidities. Our findings in this series support this approach.
Posttransplant Venous Thromboembolism and Cardiac Manifestations of COVID-19
Venous thromboembolism (VTE) after lung transplantation is common and has been associated with lower survival.21 It is also well known that COVID-19 predisposes patients to VTE because of hypoxia, excessive inflammation, platelet activation, endothelial dysfunction, and stasis.22 Lung transplant recipients for COVID-19–related lung failure are thus quite susceptible to VTE. This was well demonstrated in our patient series in which 50% of the patients exhibited deep venous thrombosis in their extremities during index admission and 10% of them experienced major pulmonary embolism requiring a readmission for intensive anticoagulant therapy despite prophylactic treatment. Based on these experiences, our current lung transplant protocol prioritizes proactive anticoagulation therapy with a therapeutic heparin drip followed by a therapeutic dose of direct oral anticoagulant‚ which is commenced at the time of hospital discharge for all patients with COVID-19.
Other notable late complications, which led to the sole death in this series, were COVID-19–related myocarditis and cardiac arrhythmias. Because of the high frequency of cardiac manifestations in severely ill COVID patients,23 the team caring for sick patients with COVID-19-related lung failure must pay attention to the possibility of COVID-19–related myocarditis and the progress of its unique pathology. Serial Doppler ultrasound tests in extremities and echocardiography‚ which are scheduled at postoperatively 2 wk, 3 and 6 mo following lung transplantation‚ are currently part of the standard protocol for posttransplant follow-up of these patients at our institution.
Renal failure is another well-known consequence of COVID-19;24 however, no patients required hemodialysis following lung transplantation in this series. We speculate that‚ during the lung transplant procedure in particular in patients with severe pulmonary hypertension, intraoperative MCS plays a preemptive role in stabilizing hemodynamics and better preserving organ function, in particular in the kidneys, which is also discussed in our recently published article.19
Optimal Timing to Proceed With Lung Transplantation and Reversible Lung Injuries Associated With COVID-19
Whereas most prior reports detailing lung transplantation for COVID-19–related lung failure advocate close monitoring for 4 to 6 wk after the onset of COVID-19 infection before considering a patient for lung transplantation, essential questions regarding the reversibility of lung injuries associated with COVID-19 infection remain largely unanswered.3,4,7,10 Approximately half of the patients who underwent SLT in our study were noted to have an improvement in their remaining native fibrotic lung postoperatively. All the explanted lungs from these patients exhibited advanced interstitial lung fibrosis‚ as well as extensive acute lung injuries‚ on pathological examination. For the moment, to avoid unnecessary lung transplantation, there would be nothing to do but follow up cautiously to see if the lungs improve even after listing. Correlation of qualitative and quantitative analysis of computed tomography chest images with these pathologies25 is needed to better answer critical questions regarding the reversibility of COVID-19–associated lung injuries and fibrosis. Furthermore, clinical trials of antifibrotic therapy for CARDS and PCPF are currently ongoing.26 These may become groundbreaking for treatment strategies combined with lung transplantation, which is in line with the findings of our study.
LIMITATIONS
The present study has several limitations. First, this study is a retrospective single-center analysis with a small and heterogeneous sample. However, our prior institutional reports on COVID-19 infection demonstrate that the screening, diagnosis, and management of patients with COVID-19, including those who need lung transplantation, have been thoroughly incorporated into protocols that have been followed consistently at our institution over the course of the pandemic.11,27-29 Therefore, we believe that the heterogeneous characteristics are a unique manifestation of the pulmonary sequelae of a complex multisystem disorder. Secondly, currently‚ there are no clear diagnostic criteria or differentiation for the 2 UNOS COVID-19–related codes (CARDS and PCPF). The 2 diagnoses may partially overlap; however, present data clearly showed that our cohort was different from previous reports of lung transplantation for CARDS.3-6 This should become better elucidated with further studies focused on explanted lung pathology and evolving radiographic diagnostic technology. Third, because of the high number of SLTs‚ which is not the current trend, our findings could not be generalized to other institutions. Lastly, long-term survival outcomes are not yet known because of the limited follow-up period since the onset of the COVID-19 pandemic.
CONCLUSION
Our unique experience treating a heterogeneous patient population with COVID-19–related lung failure has yielded favorable transplant outcomes to date. The present series also sheds light on an evolving role for lung transplantation and will help us determine an optimal strategy for managing the pulmonary sequelae of COVID-19, a complex multisystem disorder.
ACKNOWLEDGMENTS
We thank Shannon Wyszomierski for editing a draft of the article.
Footnotes
H.K. participated in study design, data collection, analysis, and writing of the article. C.M. and G.S. participated in data collection. S.I. and R.Y. participated in study design. M.K., Y.P., K.S., A.M., N.M., F.C., G.C., and Y.T. participated in critical revision. N.S. participated in study design, writing of the article, and critical revision.
The authors declare no conflicts of interest.
REFERENCES
- 1.United States Department of Health & Human Services. Organ procurement and transplantation network. OPTN board approves transplant candidate diagnoses associated with COVID-19 related organ failure. Published October 14, 2020. Available at https://optn.transplant.hrsa.gov/news/optn-board-approves-transplant-candidate-diagnoses-associated-with-covid-19-related-organ-failure/. Accessed April 26, 2022.
- 2.Roach A, Chikwe J, Catarino P, et al. Lung transplantation for covid-19-related respiratory failure in the United States. N Engl J Med. 2022;386:1187–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharat A, Machuca TN, Querrey M, et al. Early outcomes after lung transplantation for severe COVID-19: a series of the first consecutive cases from four countries. Lancet Respir Med. 2021;9:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurihara C, Manerikar A, Querrey M, et al. Clinical characteristics and outcomes of patients with COVID-19-associated acute respiratory distress syndrome who underwent lung transplant. JAMA. 2022;327:652–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ko RE, Oh DK, Choi SM, et al. Lung transplantation for severe COVID-19-related ARDS. Ther Adv Respir Dis. 2022;16:7534666221081035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang C, Ritschl V, Augustin F, et al. Clinical relevance of lung transplantation for COVID-19 ARDS: a nationwide study. Eur Respir J. 2022;60:2102404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkins RB, Mehaffey JH, Charles EJ, et al. Lung transplantation for severe post-coronavirus disease 2019 respiratory failure. Transplantation. 2021;105:1381–1387. [DOI] [PubMed] [Google Scholar]
- 8.Yeates EO, Nahmias J, Chinn J, et al. Improved outcomes over time for adult COVID-19 patients with acute respiratory distress syndrome or acute respiratory failure. PLoS One. 2021;16:e0253767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt M, Langouet E, Hajage D, et al. Evolving outcomes of extracorporeal membrane oxygenation support for severe COVID-19 ARDS in Sorbonne hospitals, Paris. Crit Care. 2021;25:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King CS, Mannem H, Kukreja J, et al. Lung transplantation for patients with COVID-19. Chest. 2022;161:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shigemura N, Cordova F, Hayanga AJ, et al. Lung transplantation and coronavirus disease 2019 (COVID-19): a roadmap for the enduring pandemic. J Thorac Dis. 2021;13:6755–6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. [DOI] [PubMed] [Google Scholar]
- 13.Snell GI, Yusen RD, Weill D, et al. Report of the ISHLT working group on primary lung graft dysfunction, part I: definition and grading-A 2016 consensus group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36:1097–1103. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med. 2022;386:1397–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shigemura N, Toyoda Y. Elderly patients with multiple comorbidities: insights from the bedside to the bench and programmatic directions for this new challenge in lung transplantation. Transpl Int. 2020;33:347–355. [DOI] [PubMed] [Google Scholar]
- 17.Drake TM, Docherty AB, Harrison EM, et al. Outcome of hospitalization for COVID-19 in patients with interstitial lung disease. An international multicenter study. Am J Respir Crit Care Med. 2020;202:1656–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higham A, Mathioudakis A, Vestbo J, et al. COVID-19 and COPD: a narrative review of the basic science and clinical outcomes. Eur Respir Rev. 2020;29:200199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sunagawa G, Kehara H, Mangukia C, et al. Single lung transplant remains a viable option for patients with severe secondary pulmonary hypertension. Transplantation. 2022;106:2241–2246. [DOI] [PubMed] [Google Scholar]
- 20.Kamp JC, Hinrichs JB, Fuge J, et al. COVID-19 in lung transplant recipients – risk prediction and outcomes. PLoS One. 2021;16:e0257807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ribeiro Neto ML, Budev M, Culver DA, et al. Venous thromboembolism after adult lung transplantation: a frequent event associated with lower survival. Transplantation. 2018;102:681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiménez D, García-Sanchez A, Rali P, et al. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Chest. 2021;159:1182–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S, Wang J, Yan Y, et al. Clinical characterization and possible pathological mechanism of acute myocardial injury in COVID-19. Front Cardiovasc Med. 2022;9:862571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonnweber T, Sahanic S, Pizzini A, et al. Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur Respir J. 2021;57:2003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen H, Zhang N, Liu Y, et al. The interaction between pulmonary fibrosis and COVID-19 and the application of related anti-fibrotic drugs. Front Pharmacol. 2022;12:805535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandya A, Kaur NA, Sacher D, et al. Ventilatory mechanics in early vs late intubation in a cohort of coronavirus disease 2019 patients with ARDS: a single center’s experience. Chest. 2021;159:653–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chowdhury JM, Patel M, Zheng M, et al. Mobilization and preparation of a large urban academic center during the COVID-19 pandemic. Ann Am Thorac Soc. 2020;17:922–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myers CN, Scott JH, Criner GJ, et al. COVID-19 in lung transplant recipients. Transpl Infect Dis. 2020;22:e13364. [DOI] [PMC free article] [PubMed] [Google Scholar]