Dear Sir,
Intrahepatic bile duct adenoma (BDA) (or peribiliary gland hamartoma) is a benign liver tumour arising from intrahepatic duct epithelium. Few cases are reported.[1] An et al. reported that only five in 50,000 autopsies revealed BDA.[2] BDA is considered benign, but malignant transformation to cholangiocarcinoma has been reported.[3] Due to its rarity, it is difficult to distinguish malignancy on radiology and challenging to interpret on histology. Hence, all cases should be reported to supplement the current literature. We report a case of an intrahepatic BDA in a man following a liver resection for suspected hepatocellular carcinoma.
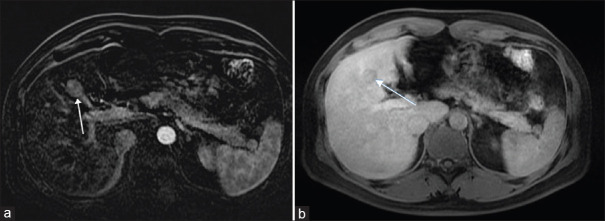
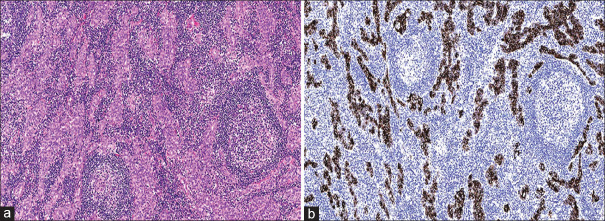
A 49-year-old man who is a hepatitis B carrier with a previous history of laparoscopic cholecystectomy underwent investigation for a right hepatic lesion detected on surveillance ultrasonography. He did not consume excessive alcohol and his body mass index was 25 kg/m2. Serum biochemistry revealed normal full blood count, liver and renal functions and alpha-fetoprotein level. Magnetic resonance (MR) imaging revealed a 2.3-cm arterial-enhancing LI-RADS 4 lesion in segment 4B of the liver [Figure 1]. The lesion demonstrated marked restricted diffusion and increased T2 signal with enhancement in the subtraction sequence and washout appearance in delayed phases. There was no intralesional fat or pseudo capsule. A multi-disciplinary discussion advocated for liver resection. Three-dimensional laparoscopic wedge resection was done; the patient had an uneventful recovery and was discharged home on the third postoperative day. Gross examination of the resected mass showed a circumscribed solid whitish tumour measuring 2.0 cm × 1.7 cm × 1.6 cm. Microscopy revealed a relatively circumscribed nodular proliferation of well-formed ducts lined by cytologically bland cuboidal epithelial cells and accompanied by a prominent lymphoid infiltrate forming reactive follicles with germinal centres [Figure 2a]. Mitotic activity was inconspicuous. The adjacent liver showed no evidence of cirrhosis. On immunohistochemical staining, the ducts were positive for CK19 and CK7, but negative for CK20, chromogranin, synaptophysin and thyroid transcription factor-1 [Figure 2b]. There was a low Ki-67 labelling index (<10%) in the ductal epithelium.
Figure 1.
Magnetic resonance images show (a) the 2.3-cm arterial-enhancing LI-RADS 4 lesion in segment 4B of the liver (arrow) and (b) washout in delayed phase (arrow).
Figure 2.
(a) Photomicrograph of the resected mass shows well-formed ducts lined by cytologically bland cuboidal epithelial cells and accompanying lymphoid infiltrate forming reactive follicles with germinal centres (H&E, ×100). (b) Photomicrograph of the ductal epithelium shows diffuse positivity for CK19 immunohistochemical stain (×100).
Typically, intrahepatic BDAs are detected incidentally following abdominal surgery or associated with alcoholic cirrhosis, biliary obstruction, haemangioma, focal nodular hyperplasia, and hepatitis C-related chronic hepatitis.[1,4] Our patient is a hepatitis B carrier with previous laparoscopic cholecystectomy. Current theories of pathogenesis include a trigger of trauma or inflammation resulting in a reactive focal bile ductular injury.[5] Wei et al. drew the conjecture that BDA represents disorganised peribiliary glands based on their similar secretory gland cell phenotype.[1] They are composed of uniformly small-sized ducts with cuboidal cells containing regular nuclei.
Immunohistochemical stains for CD10, CK19, CK7 and CD56 are usually positive, while AFP and p53 stains usually are negative.[1,5] Cholangiocarcinoma and metastatic adenocarcinoma are distinguished based on cellular atypia and nuclear hyperchromasia. Von Meyenburg complexes are multiple and small, associated with polycystic liver disease and express CD10. Surgical resection is the standard of care to establish histologic diagnosis and rule out malignancy.
In conclusion, intrahepatic BDA is rare and indistinguishable from intrahepatic cholangiocarcinoma. Surgical resection and histology with immunohistochemistry confirms the diagnosis. Due to its rarity, we recommend all cases of BDAs to be reported to generate more awareness of these tumours. This would serve as a beneficial teaching point to recognise this tumour in the examination of post-surgical resection specimens.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wei J, Zhang D, Yang J, Xu C. Intrahepatic bile duct adenoma (peribiliary gland hamartoma): A case report and review of literature. Int J Clin Exp Pathol. 2015;8:5908–13. [PMC free article] [PubMed] [Google Scholar]
- 2.An C, Park S, Choi YJ. Diffusion-weighted MRI in intrahepatic bile duct adenoma arising from the cirrhotic liver. Korean J Radiol. 2013;14:769–75. doi: 10.3348/kjr.2013.14.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasebe T, Sakamoto M, Mukai K, Kawano N, Konishi M, Ryu M, et al. Cholangiocarcinoma arising in bile duct adenoma with focal area of bile duct hamartoma. Virchows Arch. 1995;426:209–13. doi: 10.1007/BF00192644. [DOI] [PubMed] [Google Scholar]
- 4.Allaire GS, Rabin L, Ishak KG, Sesterhenn IA. Bile duct adenoma. A study of 152 cases. Am J Surg Pathol. 1988;12:708–15. doi: 10.1097/00000478-198809000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Bhathal PS, Hughes NR, Goodman ZD. The so-called bile duct adenoma is a peribiliary gland hamartoma. Am J Surg Pathol. 1996;20:858–64. doi: 10.1097/00000478-199607000-00009. [DOI] [PubMed] [Google Scholar]