INTRODUCTION
The COVID-19 pandemic has posed a major threat to global health. After the first case of infection in Singapore was confirmed on 23 January 2020, local community transmission followed, prompting escalation of disease outbreak control measures. The number of cases spiked dramatically in mid-April 2020 due to rapid formation of infection clusters in migrant worker dormitories; infected migrant workers made up 95% of COVID-19 infections as of January 2021.[1] Kidney transplant recipients (KTRs) who have impaired immunity are susceptible to severe disease, with reported mortality rates of up to 28%–30%.[2,3,4] In this commentary, we shared the challenges we faced in managing two KTRs with COVID-19 in Singapore.
CASE 1
A 43-year-old Chinese Singaporean man who had undergone a living donor kidney transplantation (LDKT) four years earlier presented to our institution. His induction immunosuppression included hydrocortisone and basiliximab, and maintenance immunosuppression included cyclosporine, mycophenolate sodium and prednisolone. The patient experienced only anosmia on 1 April 2020, and presented when he developed fever and cough six days later. Contact tracing revealed that he had visited his mother-in-law who had tested positive for COVID-19 seven days prior. He tested positive for COVID-19 via nasopharyngeal swab using a standard testing protocol.[5] Chest radiography (CXR) was clear. The patient had lymphopenia (0.47 × 109/L) and elevated ferritin levels. He was started on lopinavir/ritonavir (400 mg/100 mg) two tablets twice daily on admission and completed ten days of treatment. His dosage of cyclosporine and mycophenolate sodium were halved on admission.
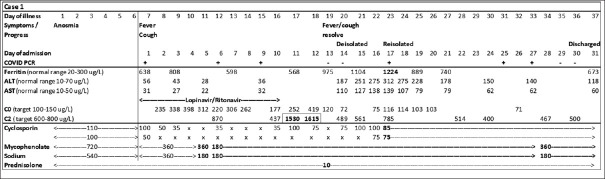
Despite the paucity of guidelines on management of immunosuppression at the time, when making our decision, we took into consideration the high risk of progression to severe disease and subsequent high mortality rate in immunosuppressed patients. His dosage of cyclosporine was reduced further due to its significant drug interaction with lopinavir/ritonavir; titration was based on therapeutic drug monitoring (TDM), targeting trough levels of 100 mcg/L and two-hour post-dose levels of 600–800 mcg/L [Figure 1]. His fever and cough resolved by Day 13, but he developed drug-induced transaminitis with very high cyclosporine levels. Cyclosporine was ceased, then restarted at lower doses after the transaminitis improved. He did not suffer acute kidney injury, and CXR remained clear. On Day 20 of illness, the patient was de-isolated after two negative nasopharyngeal swabs, but he was re-isolated on Day 23 after a repeat swab test performed due to his rising ferritin level was positive (cycle threshold 35). Isolation was imposed, as it was uncertain if the persistent positive polymerase chain reaction (PCR) result was significant for infectivity, and we could not risk transmission to other patients. On Day 37 of illness, he was discharged well but with persistent anosmia after two consecutive negative nasopharyngeal swabs.
Figure 1.
Chart shows the timeline of Case 1's symptoms, clinical progress, laboratory findings and immunosuppression management. The patient was started with lopinavir/ritonavir on admission, resulting in significant drug interaction with cyclosporine, high cyclosporine levels, followed by hepatocellular transaminitis. Cyclosporine and mycophenolate sodium doses were titrated based on drug levels and clinical condition. He was de-isolated on Day 20 of illness after two negative PCR swabs (−), but in view of the rising ferritin trend, a repeat PCR swab was done on Day 23 and found to be positive (+), resulting in re-isolation. Ferritin and transaminase levels eventually improved, and he was discharged on Day 37 of illness after two negative PCR swabs. ×: cyclosporine dose withheld, ALT: alanine aminotransferase, AST: aspartate aminotransferase, C0: cyclosporine trough level, C2: cyclosporine level two hours after dose, PCR: polymerase chain reaction
CASE 2
A 47-year-old Indian national who worked as an electrician in Singapore had undergone LDKT nine years earlier in India. He had been followed up remotely by his transplant physician in India and not by our centre. The patient first presented to our hospital on 27 March 2020 with six days of fever and cough. He reported gradual worsening of allograft function (from baseline creatinine of 100 mmol/L to 280 mmol/L) over the preceding six months, which had yet to be evaluated by his physician in India. Maintenance immunosuppression included mycophenolate sodium, tacrolimus and prednisolone. Admission CXR was clear. COVID-19 infection was excluded after two negative nasopharyngeal swabs. He was treated for community-acquired respiratory tract infection with amoxicillin/clavulanic acid and azithromycin with good response. His creatinine was 523 mmol/L on admission but improved to 392 mmol/L with hydration. He declined evaluation for possible allograft rejection, preferring to defer this to when he returned to his home country, citing financial constraints. He was discharged well four days later while awaiting repatriation.
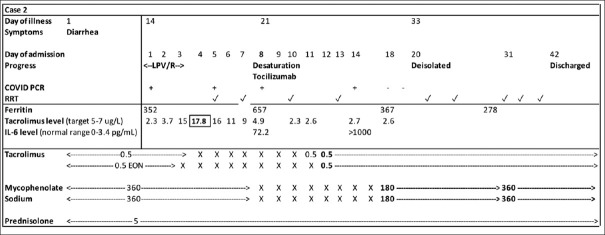
One week post discharge, the patient was transferred to an isolation facility for foreign workers, as co-workers from his dormitory had tested positive for COVID-19. Five weeks later, he was diagnosed with COVID-19 infection on a surveillance swab that was done prior to de-isolation. On admission, he reported a 15-day history of diarrhoea but no fever or respiratory symptoms. Laboratory investigations revealed no lymphopenia and acute-on-chronic kidney dysfunction (creatinine 508 mmol/L). CXR was clear. He had concurrent Clostridium difficile that was treated with oral vancomycin for 14 days. Lopinavir/ritonavir was initiated on admission but was stopped after three days due to persistent vomiting and diarrhoea. Haemodialysis was initiated. The patient deteriorated further, requiring 2 L/min of oxygen supplementation, and computed tomography of the thorax showed severe bilateral consolidation. He was treated with piperacillin/tazobactam for possible concomitant hospital-acquired pneumonia. Due to respiratory deterioration and high tacrolimus levels (peak of 17.8 ng/mL) from drug interaction with lopinavir/ritonavir, his immunosuppression (unchanged on admission, as his mycophenolate dose and tacrolimus levels were already low) was reduced to prednisolone alone [Figure 2]. As his interleukin-6 level was elevated at 72 pg/mL (normal 0–3.4 pg/mL) and he was deteriorating both clinically and radiologically, a single dose of tocilizumab was administered (8 mg/kg).
Figure 2.
Chart shows the timeline of Case 2's symptoms, clinical progress, laboratory findings and immunosuppression management. The patient was started on lopinavir/ritonavir on admission, but it was ceased after three days in view of gastrointestinal symptoms. High tacrolimus trough levels were observed. Clinical deterioration occurred on Day 8 of admission, corresponding to the time point of worsening inflammatory markers. The patient was initiated on haemodialysis due to oliguria and remained dialysis dependent after de-isolation on Day 33 of illness. He was repatriated well on Day 42. ✓: Session of RRT, ×: immunosuppression withheld, EON: every other night, IL-6: interleukin-6, LPV/R: lopinavir/ritonavir, PCR: polymerase chain reaction; RRT: renal replacement therapy
Ten days post tocilizumab, he was weaned off oxygen supplementation but remained dialysis-dependent. Tacrolimus and mycophenolate were gradually re-introduced. After Day 33 of illness, two consecutive COVID-19 nasopharyngeal swabs were negative. He was repatriated to India after a 42-day inpatient stay.
DISCUSSION
The amount of literature on COVID-19 is growing rapidly, but the optimal management for KTRs remains uncertain. What is well reported are atypical presentations[3,6] of KTRs with COVID-19 and their greater susceptibility to complications, critical illness and mortality.[7,8] The biggest concern is the reported high risk of mortality (28%–30%),[2,3,4] reaching 50% mortality in those requiring critical care support.[9] To enable early detection of COVID-19 infection in KTRs and prevent spread, heightened precautions have been implemented since the first reported case in Singapore. An extensive questionnaire screening for symptoms and risk factors in patients and their household contacts is conducted prior to outpatient visits and transplant ward admissions. During the height of the pandemic, outpatient consultations predominantly consisted of teleconsultations, making up 80%–90% of outpatient consultations. Patients with high-risk features had their appointments deferred, managed by teleconsultation, or referred for Emergency Department review if symptomatic. For patients requiring inpatient care, any risk factor that was elicited prompted isolation and full personal protective equipment use until COVID-19 infection was excluded. All KTRs with fever, regardless of the apparent source, were swabbed to allow detection of COVID-19 co-infection with atypical presentations.
We gained valuable insights from managing these two KTRs with COVID-19 infection. Both had atypical presentations with an initial absence of fever or clear respiratory symptoms. Both patients received specific antivirals that were potential options available in Singapore at that time. Notably, lopinavir/ritonavir have since been documented to have unfavourable safety or efficacy profiles and are not currently recommended.[10,11] Another aspect unique to KTRs is management of maintenance immunosuppression. Current guidelines recommend frequent review and adjustment of immunosuppression based on TDM and the patients’ clinical condition. Efforts to prevent rejection are carefully weighed against the risk of COVID-19 disease progression. Although the immunosuppressed state may impair control of viral infection, its discontinuation could exacerbate cytokine-mediated inflammatory responses to the virus.[12] If the disease is severe, guidelines recommend reducing or stopping immunosuppression early, with reduction of anti-proliferative agents followed by calcineurin inhibitors (CNI)[6,13,14] while maintaining the usual steroids. Broad use of high-dose steroids is not recommended for mild disease without pneumonia.[15] Steroids can temper immunopathogenesis,[16] and dexamethasone has been shown to lower 28-day mortality in patients with severe illness requiring respiratory support.[17] This data was not available at the time our second patient was treated, but he received tocilizumab in an attempt to temper the immune response driving the hyperinflammatory response.
Notably, we observed significant drug-drug interactions. Both cases had very high CNI levels upon receiving lopinavir/ritonavir, a potent inhibitor of cytochrome P450 3A-mediated metabolism, prolonging CNI half-life by five- to 20-fold.[18] This has not been observed with remdesivir, which has largely supplanted lopinavir/ritonavir as the main antiviral used for COVID-19 in Singapore and elsewhere.
Transplant centres worldwide have adopted differing management strategies for infected KTRs. Our centre treats COVID-19 in KTRs, as they are at high risk of severe disease with higher mortality rates, using a biphasic approach. Viral replication drives the early course of infection, while an exaggerated inflammatory response drives the later course of infection that results in tissue damage. We use antivirals in the first seven days of infection to reduce viral load, then tocilizumab and potentially other targeted immunotherapies for immunomodulation past seven days of illness to temper the virus-evoked immune response, specifically in patients who are deteriorating clinically due to a hyperinflammatory response. The choice of antivirals has shifted from protease inhibitors to remdesivir, as lopinavir/ritonavir seemed not to demonstrate significant clinical improvement nor reduction in mortality.[10,19] Preliminary studies have shown that remdesivir was superior to a placebo in shortening recovery time.[20] The data is, however, rapidly evolving.
While the effect of tocilizumab in patients infected with COVID-19 remains uncertain, with earlier observational data suggesting some benefit but subsequent randomised control trials demonstrating no clear effects, early findings from the REMAP-CAP trial have led to an interim position statement in the United Kingdom permitting the use of tocilizumab in eligible patients in the intensive care setting.[21] Tocilizumab may yet prove to be useful in patients with severe illness as an emergency intervention.
Specific therapies for KTRs with COVID-19 infection highlight the management challenges in any pandemic. As data on various therapeutics emerges, we have to weigh the risks and benefits using preliminary efficacy and safety data before using any investigational therapeutic. Potential side effects need to be monitored closely. For instance, drug interaction of lopinavir/ritonavir with CNI requires close TDM to avoid acute kidney injury from high CNI levels, which is reversible with reduction of CNI levels to target therapeutic ranges. Case 2 had acute-on-chronic kidney dysfunction largely due to sepsis and intravascular depletion, which was contributed to by transient high tacrolimus levels. It should be emphasised that robust clinical trials that provide quality evidence to guide the use of therapeutic agents must remain the standard of care even during difficult circumstances in a pandemic, with investigational drugs reserved for approved emergency use only.
Apart from the unavailability of proven therapies against COVID-19 infection, uncertainties regarding post-infection immunity and transmission risk remain. Although the World Health Organization recommended a time-based discharge criterion[22] regardless of swab result, this may not be applicable to immunosuppressed patients with a propensity for prolonged viral shedding.[23] Case 1 returned a positive nasopharyngeal swab after two negative swabs, indicating persistent shedding of virus or simply detection of viral particles. As viable virus has not been identified two weeks after symptom onset in other populations, the significance of persistently positive PCR-based tests after symptom resolution remains unclear. At our centre, abundant caution is practised, allowing in-person outpatient visits when a repeat nasopharyngeal swab at three months post illness is negative.
Finally, this pandemic not only revealed deficiencies in healthcare systems worldwide, but also highlighted multiple socio-ethical issues. It has brought much attention to the working and living conditions of the migrant worker population in Singapore, whose healthcare needs hitherto went unnoticed. While their housing conditions have improved over the years, existing conditions do not allow for adequate social distancing in a pandemic.[24] Fortunately, mitigation measures to identify those at highest risk of complications have minimised morbidity and mortality. Case 2, being additionally vulnerable, was particularly challenging as his available past medical history was limited, and disposition after de-isolation was difficult because existing policies prevented his return to his dormitory. Fortunately, the considerable medical costs were covered by special assistance.
In conclusion, we reported the first two cases of COVID-19 infections in KTRs in Singapore and highlighted challenges faced in their management. Specific therapies and immunosuppression were tailored to their condition. Both patients recovered, although one patient developed end-stage kidney disease and required dialysis. Research surrounding KTRs is still evolving. While the search for the optimal therapeutic approach continues, aggressive measures to prevent infection are critical.
REFERENCES
- 1.Ministry of Health, Singapore. COVID-19 situation report. [Last accessed on 04 Jan 2021]. Available from: https://covidsitrep.moh.gov.sg/
- 2.Banerjee D, Popoola J, Shah S, Ster IC, Quan V, Phanish M. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;97:1076–82. doi: 10.1016/j.kint.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akalin E, Azzi Y, Bartash R, Seethamraju H, Parides M, Hemmige V, et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382:2475–7. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Archuleta S, Cross G, Somani J, Lum L, Santosa A, Alagha RA, et al. Responding to COVID-19: How an academic infectious diseases division mobilized in Singapore. BMC Med. 2020;18:179. doi: 10.1186/s12916-020-01641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.López V, Vázquez T, Alonso-Titos J, Cabello M, Alonso A, Beneyto I, et al. Recommendations on management of the SARS-CoV-2 coronavirus pandemic (Covid-19) in kidney transplant patients. Nefrología. 2020;40:265–71. doi: 10.1016/j.nefro.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pereira MR, Mohan S, Cohen DJ, Husain SA, Dube GK, Ratner LE, et al. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20:1800–8. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alberici F, Delbarba E, Manenti C, Econimo L, Valerio F, Pola A, et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97:1083–8. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.British Transplantation Society. Guidance on the management of transplant recipients diagnosed with or suspected of having COVID19. [Last accessed on 04 Jan 2021]. Available from: https://bts.org.uk/wp-content/uploads/2020/07/Clinical-management-of-transplants-and-immunosuppression-updated-9th-July.pdf .
- 10.RECOVERY Collaborative Group. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet. 2020;396:1345–52. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Centre for Infectious Diseases, Singapore. Interim treatment guidelines for COVID-19 (Version 4.0) [Last accessed on 24 Dec 2020]. Available from: https://www.ncid.sg/Documents/Interim%20Treatment%20Guidelines%20for%20COVID-19%20v4%20%2831%20Aug%202020%29-%20final.docx%20for%20upload.pdf .
- 12.Hammami MB, Garibaldi B, Shah P, Liu G, Jain T, Chen PH, et al. Clinical course of COVID-19 in a liver transplant recipient on hemodialysis and response to tocilizumab therapy: A case report. Am J Transplant. 2020;20:2254–9. doi: 10.1111/ajt.15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kronbichler A, Gauckler P, Windpessl M, Il Shin J, Jha V, Rovin BH, et al. COVID-19: Implications for immunosuppression in kidney disease and transplantation. Nat Rev Nephrol. 2020;16:365–7. doi: 10.1038/s41581-020-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maggiore U, Abramowicz D, Crespo M, Mariat C, Mjoen G, Peruzzi L, et al. How should I manage immunosuppression in a kidney transplant patient with COVID-19?An ERA-EDTA DESCARTES expert opinion. Nephrol Dial Transplant. 2020;35:899–904. doi: 10.1093/ndt/gfaa130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–5. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LF. The trinity of COVID-19: Immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–74. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2020;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elens L, Langman LJ, Hesselink DA, Bergan S, Moes DJAR, Molinaro M, et al. Pharmacologic treatment of transplant recipients infected with SARS-CoV-2: Considerations regarding therapeutic drug monitoring and drug-drug interactions. Ther Drug Monit. 2020;42:360–8. doi: 10.1097/FTD.0000000000000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–99. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19--final report. N Engl J Med. 2020;383:1813–26. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wise J. Covid-19: Critically ill patients treated with arthritis drug tocilizumab show improved outcomes, researchers report. BMJ. 2020;371:m4530. doi: 10.1136/bmj.m4530. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Criteria for releasing COVID-19 patients from isolation: Scientific brief 17 June, 2020. [Last accessed on 04 Jan 2021]. Available from: https://www.who.int/news-room/commentaries/detail/criteria-for-releasing-covid-19-patients-from-isolation .
- 23.Zhang M, Zhang J, Shi H, Liu B, Zeng F. Viral shedding prolongation in a kidney transplant patient with COVID-19 pneumonia. Am J Transplant. 2020;20:2626–7. doi: 10.1111/ajt.15996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koh D. Migrant workers and COVID-19. Occup Environ Med. 2020;77:634–6. doi: 10.1136/oemed-2020-106626. [DOI] [PMC free article] [PubMed] [Google Scholar]