Abstract
Introduction:
Psychosis is a prominent neuropsychiatric symptom of Parkinson's disease (PD) and is associated with negative outcomes, such as poorer quality of life and greater rate of functional impairment. Early identification of patients with PD at risk of developing psychosis facilitates appropriate management to improve outcomes. However, this phenomenon has not been examined locally. This study aimed to examine the prevalence of PD-associated psychosis in the local setting, identify any associated risk factors, as well as characterise the cognitive trajectory of patients with PD with psychosis.
Methods:
A retrospective cohort of 336 patients with PD, who presented to the National Neuroscience Institute, Singapore, in 2006 and 2007 and attended follow-up visits through to 2013 was analysed. The data analysed included scores from clinician assessments of cognitive function, disease severity and presence of psychotic symptoms, conducted when clinically appropriate during patients’ medical visits. Survival analysis and logistic and linear regression analysis were performed.
Results:
Psychosis was diagnosed in 63 patients with PD, indicating a prevalence of 18.8% for PD-associated psychosis. Incidence of psychosis in PD was calculated to be 40 per 1,000 person-years. No significant association was found between demographic variables and the odds of developing psychosis in PD. Regression analyses found that the presence of psychosis significantly predicted greater cognitive decline and disease severity.
Conclusion:
Psychosis has a significant presence among the PD population in Singapore, possibly serving as an indicator of more rapid cognitive decline and progression of PD severity.
Keywords: Cognitive decline, disease severity, Parkinson's disease, psychosis
INTRODUCTION
Parkinson's disease (PD) is now known as a complex neurodegenerative disorder comprising both motor and non-motor symptomatology. Among the non-motor symptoms, neuropsychiatric symptoms are prominent and add significantly to the burden of disease. Examples of neuropsychiatric symptoms are: cognitive disorders ranging from mild cognitive impairment to dementia; mood disorders, particularly depression and anxiety, which may present as early symptoms of PD and precede motor symptoms; other behavioural disturbances, such as impulse control disorders; apathy; and psychotic symptoms.[1,2]
Psychotic symptoms in PD consist of hallucinations, delusions and minor symptoms, such as sense of presence, visual illusions and passage hallucinations.[3] Early studies tended to attribute the development of such psychotic symptoms in patients with PD to side effects of dopaminergic medications, and this association frequently overshadowed the other important causes of psychosis in PD. Presently, it is known that psychosis can also develop spontaneously or in association with cognitive impairment, on-off fluctuations, mood disturbances, other psychoactive medications and delirium.[3] Numerous studies have shown that risk factors associated with the development of psychotic symptoms in patients with PD include older age, longer duration of disease, cognitive decline, Modified Hoehn and Yahr Staging Scale (H&Y) stage 4 and above, and treatment with a dopamine receptor agonist.[4,5,6,7,8,9] It is important to note that psychotic symptoms may present differently in PD than in schizophrenia or other primary psychiatric disorders, highlighting the need to increase awareness of these symptoms and understand how they present in patients with PD.
Cross-sectional prevalence rates of psychosis in PD have been reported to be in the range of 20%–40%.[10,11] Psychotic symptoms in PD are associated with a range of negative outcomes, including poorer quality of life, greater rate of functional impairment, cognitive and affective dysfunction, caregiver stress and burden, and nursing home placement. Psychotic symptoms can even be severe enough to warrant inpatient psychiatric treatment.[12,13,14] Institutionalisation, in turn, leads to higher mortality rates among those suffering from PD-associated psychosis.[13] The single most important precipitant for placement of patients with PD in a long-term care facility is psychiatric dysfunction, particularly psychosis.[15] In view of these severe consequences, early identification of patients with PD at risk of developing psychosis allows for careful examination of psychotic symptoms and appropriate management to improve outcomes.
Despite the abundance of research that has been conducted globally on this subject matter, to the best of our knowledge, there has not been any local research conducted on the prevalence of psychosis in PD and its associated risk factors. Therefore, it is important to assess its presence in the local setting in order to facilitate appropriate healthcare planning. This study aimed to determine the local prevalence of psychotic symptoms in an outpatient cohort of patients with PD on follow-up with the National Neuroscience Institute (NNI), Singapore, as well as evaluate the risk factors associated with developing psychotic symptoms in PD and assess if they were specifically related to cognitive decline or worsening disease severity over time.
METHODS
We analysed data from a retrospectively identified cohort, using data from the movement disorders database, a database approved by the centralised institutional review board of the Singapore Health Services (SingHealth), which contains information on all patients with PD, who attend medical follow-up at the NNI. Clinical diagnosis for these patients was conducted by movement disorders specialists, adhering to PD diagnostic criteria defined by the National Institute of Neurological Disorders and Stroke (NINDS). We included patients with PD who were entered into the database in 2006 and 2007, reviewing their data available in the database through to 31 December 2013. We excluded those who were diagnosed or entered into the database before 2006 and after 2007. This study period was chosen based on our a priori hypothesis that neuropsychiatric symptoms would emerge within the first five years of PD diagnosis. Patients who were diagnosed with PD by other physicians prior to their first contact with the NNI were entered into the database based on the date of diagnosis they reported. This study received ethical approval from the National Healthcare Group domain specific review boards. This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Demographic and clinical information were recorded during the initial visit and subsequent visits to the movement disorders clinic at the NNI. A movement disorders specialist evaluated patients with PD at baseline and at subsequent follow-up appointments, conducting standardised assessments, when deemed clinically appropriate. Cognition was assessed using the Mini-Mental State Examination (MMSE).[16] Patients with PD were assessed to display cognitive impairment, if they scored below 25 on the MMSE, in accordance with the cut-off score deemed to be locally sensitive for capturing early cognitive impairment among elderly people.[17] Severity of PD was measured based on the duration of illness and H&Y[18] staging (stages 1–5, with higher stage indicating higher disease severity). The longitudinal course of PD symptoms was measured using the Unified Parkinson's Disease Rating Scale (UPDRS).[19]
Information on the presence and severity of hallucinations and delusions was derived from the UPDRS Part I subscore: non-motor aspects of experiences of daily living. The UPDRS thought disorder (UPDRS-TD) item allocates stages 0–4 as follows: 0 = no symptoms; 1 = vivid dreaming; 2 = ‘benign’ hallucinations, with insight retained; 3 = occasional-to-frequent hallucinations or delusions without insight (could interfere with daily activities); and, 4 = persistent hallucinations, delusions or florid psychosis. Psychosis associated with PD was diagnosed if a patient scored 2 or more on UPDRS-TD, or was prescribed antipsychotic medication. These diagnostic criteria were previously used in a study by Forsaa et al.[20]
Demographic characteristics were presented as percentage for proportions of categorical variables and mean ± standard deviation for continuous variables. Patients not assessed with UPDRS were regarded as missing data. Demographic and clinical factors, including age, gender, ethnicity, years of education (continuous), mean duration of follow-up before onset of psychosis, MMSE (score <25, score ≥25), and H&Y staging 1–5, were further compared for patients with PD with and without psychotic symptoms. The Mann-Whitney U test was used for continuous variables, and Chi-square test for categorical variables. Univariate logistic regression analysis was used to analyse the change in MMSE scores and H&Y staging over time for each group.
A multivariate linear regression model was used to analyse the odds of developing psychosis within the cohort, after controlling for age at PD diagnosis, gender, ethnicity and years of education. Missing data was excluded list-wise in the analysis. A survival time analysis was conducted to examine how likely patients with PD, with differing cognitive impairment and disease severity levels, were to develop psychotic symptoms. The observation period was from the date of the patient's initial visit to the endpoint, which was defined as the occurrence of psychosis or the end of the study period, whichever was earlier. All statistical tests were evaluated assuming a level of significance of P < 0.05. Analyses were performed with IBM SPSS Statistics version 23.0 (IBM Corp, Armonk, NY, USA).
RESULTS
This study utilised the data of a total of 336 patients who were diagnosed with PD between January 2006 and December 2007 from the movement disorders database. Demographic characteristics are shown in Table 1. Mean age of patients with PD at diagnosis was 66.15 ± 10.58 years, with 73.2% of patients aged 60 years and above. Of these 336 patients, 199 (59.2%) were men and 288 (85.7%) were ethnically Chinese. Mean education for patients with available data was 7.42 ± 5.22 years. Mean duration of follow-up from diagnosis of PD until the end of our defined time period was 5.14 ± 2.46 years. At diagnosis of PD, only 24 (8.3%) patients presented with H&Y stage 1, with 126 (43.6%) patients presenting with H&Y stage 2.
Table 1.
Basic demographics of patients diagnosed with Parkinson's disease (n=336) during 2006-2007.
| Variable | No. (%) |
|---|---|
| Age at diagnosis (yr)* | 66.15±10.58 |
|
| |
| <60 | 90 (26.8) |
|
| |
| ≥60 | 246 (73.2) |
|
| |
| Gender | |
|
| |
| Men | 199 (59.2) |
|
| |
| Women | 137 (40.8) |
|
| |
| Ethnicity | |
|
| |
| Chinese | 288 (85.7) |
|
| |
| Malay/Indian/other | 48 (14.3) |
|
| |
| Years of education*,† | 7.42±5.22 |
|
| |
| Year of diagnosis | |
|
| |
| 2006 | 181 (53.9) |
|
| |
| 2007 | 155 (46.1) |
|
| |
| Duration of follow-up (yr)*,‡ | 5.14±2.46 |
|
| |
| H&Y staging† | |
|
| |
| Stage 1 | 24 (8.3) |
|
| |
| Stage 2 | 126 (43.6) |
|
| |
| Stage 3 | 42 (14.5) |
|
| |
| Stage 4 | 16 (5.5) |
|
| |
| Stage 5 | 14 (4.8) |
|
| |
| UPDRS Part I non-motor aspects: thought disorder | |
|
| |
| Score ≥2 | 59 (17.6) |
|
| |
| Score <2 | 224 (66.7) |
|
| |
| Missing | 53 (15.8) |
|
| |
| MMSE at first assessment | |
|
| |
| Score <25 | 106 (31.6) |
|
| |
| Score ≥25 | 136 (40.5) |
|
| |
| Missing | 94 (28.0) |
*Data presented as mean±standard deviation. †Missing data not included. ‡From diagnosis to date of last consultation or death. H&Y: Modified Hoehn and Yahr Staging Scale, MMSE: Mini-Mental State Examination, UPDRS: Unified Parkinson’s Disease Rating Scale
In our study, 18.8% (n = 63) of patients were found to exhibit psychotic symptoms during follow-up, 59 of whom were assessed positive using the UPDRS-TD scale and the rest identified through prescription of antipsychotic medication. 53 patients were not assessed using the UPDRS-TD scale during the entire follow-up duration because they did not display any psychotic symptoms and consultants did not deem it clinically essential. The incidence of psychosis was 40 (95% confidence interval [CI] 30–50) per 1,000 person-years, as calculated from the survival distribution curve [Figure 1].
Figure 1.
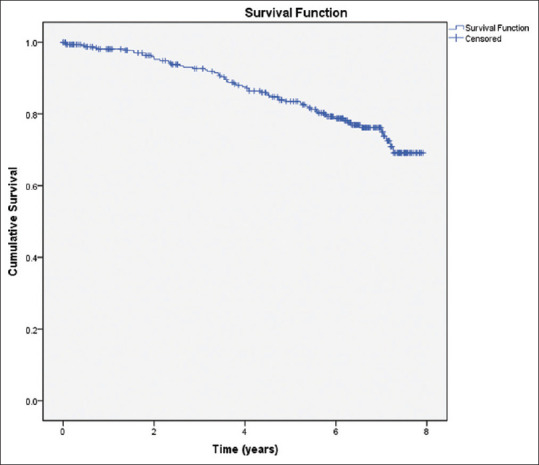
Survival distribution of patients with Parkinson's disease.
Table 2 presents a comparison of patients with PD who developed psychosis with those who did not. No significant differences were found in the mean age of patients at diagnosis of PD (P = 0.982), gender distribution (P = 0.294), racial representation (P = 0.424), and mean years of education (P = 0.617) between patients with psychotic symptoms and those without.
Table 2.
Characteristics of patients with Parkinson's disease (PD) with and without psychotic symptoms.
| Variable | No. (%) | P | |
|---|---|---|---|
|
| |||
| PD with psychotic symptoms (n=63) | PD without psychotic symptoms (n=273) | ||
| Age at PD diagnosis (yr)* | 66.24±9.83 | 66.13±10.77 | 0.982† |
|
| |||
| Gender | |||
|
| |||
| Men | 41 (65.1) | 158 (57.9) | 0.294‡ |
|
| |||
| Women | 22 (34.9) | 115 (42.1) | |
|
| |||
| Ethnicity | |||
|
| |||
| Chinese | 56 (88.9) | 232 (85.0) | 0.424‡ |
|
| |||
| Malay/Indian/other | 7 (11.1) | 41 (15.0) | |
|
| |||
| Years of education* | 7.60±4.45 | 7.37±5.43 | 0.617† |
|
| |||
| Duration of follow-up at first onset of psychosis (yr)* | 3.81±1.95 | ||
|
| |||
| MMSE§ | |||
|
| |||
| MMSE <25 | 22 (34.9) | ||
|
| |||
| MMSE ≥25 | 32 (50.8) | ||
|
| |||
| Missing | 9 (14.3) | ||
|
| |||
| H&Y stage | |||
|
| |||
| Stage 1 | 1 (1.6) | ||
|
| |||
| Stage 2 | 28 (44.4) | ||
|
| |||
| Stage 3 | 7 (11.1) | ||
|
| |||
| Stage 4 | 10 (15.9) | ||
|
| |||
| Stage 5 | 5 (7.9) | ||
|
| |||
| MMSE score per calendar year* | |||
|
| |||
| 2006 | 26.18±3.34 | 25.58±3.16 | <0.001¶ |
|
| |||
| 2007 | 24.74±5.37 | 24.92±4.36 | |
|
| |||
| 2008 | 24.51±4.09 | 24.84±4.29 | |
|
| |||
| 2009 | 22.66±5.76 | 24.17±5.64 | |
|
| |||
| 2010 | 23.83±6.01 | 25.18±3.32 | |
|
| |||
| 2011 | 23.64±4.45 | 24.44±4.18 | |
|
| |||
| 2012 | 24.93±4.92 | 24.83±4.77 | |
|
| |||
| 2013 | 21.70±6.03 | 23.39±4.83 | |
|
| |||
| H&Y staging per calendar year* | |||
|
| |||
| 2006 | 1.97±0.67 | 2.25±0.77 | <0.001¶ |
|
| |||
| 2007 | 2.25±0.76 | 2.18±0.72 | |
|
| |||
| 2008 | 2.27±0.79 | 2.09±0.73 | |
|
| |||
| 2009 | 2.32±0.74 | 2.16±0.74 | |
|
| |||
| 2010 | 2.44±0.87 | 2.20±0.76 | |
|
| |||
| 2011 | 2.54±0.87 | 2.21±0.77 | |
|
| |||
| 2012 | 2.69±1.02 | 2.35±0.91 | |
|
| |||
| 2013 | 3.02±1.13 | 2.55±0.96 | |
*Data presented as mean±standard deviation. †Mann-Whitney U test for continuous variables. ‡Chi-square test for categorical variables. §MMSE score±12 months of onset of psychosis. ¶Univariate regression analysis. H&Y: Modified Hoehn and Yahr Staging Scale, MMSE: Mini-Mental State Examination
Multivariate logistic regression analysis showed that age at PD diagnosis (odds ratio [OR; 95% CI] 1.00 [0.97–1.04], P = 0.821), male gender (OR [95% CI] 1.34 [0.69–2.61], P = 386), Chinese ethnicity (OR [95% CI] 1.28 [0.45–3.64], P = 0.645), and years of education (OR [95% CI] 1.01 [0.94–1.08], P = 0.838), were not significantly associated with increased odds of developing psychotic symptoms in our cohort.
Data also showed that 40.5% (n = 136) of patients had an MMSE score ≥25 at first assessment, and 31.6% (n = 106) had a score <25. Among our patients, 28.0% (n = 94) did not have an MMSE assessment at their initial visit.
We found that, over time, the presence of psychotic symptoms in patients with PD significantly predicted a decrease in mean MMSE scores (F[2,772] = 9.07, P < 0.001, R2 = 0.023). From 2006 to 2013, the mean MMSE scores dropped 4.48 points by the end of the study period for patients with PD with psychotic symptoms when compared to a drop of 2.19 points for those without psychotic symptoms. This is further illustrated in Figure 2, where the decline in mean MMSE score is more marked in the psychotic group over the same duration of follow-up, despite similar scores at the beginning of the study period.
Figure 2.
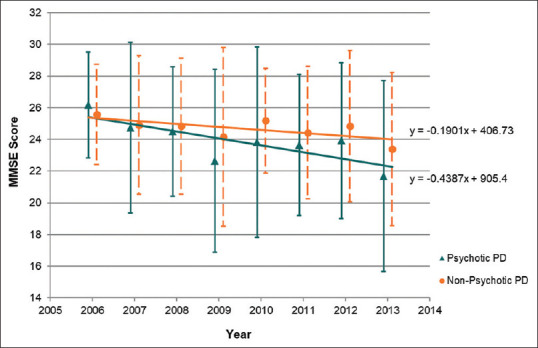
Average Mini-Mental State Examination (MMSE) score of patients with Parkinson's disease (PD) with and without psychotic symptoms from 2006 to 2013.
Similarly, the presence of psychotic symptoms significantly predicted an increase in severity of PD (F[2,3375] = 81.38, P < 0.001, R2 = 0.046). From 2006 to 2013, the mean H&Y staging increased by 1.05 by the end of the study period for patients with PD with psychotic symptoms when compared to an increase of 0.3 among those without psychotic symptoms. As shown in Figure 3, the progression through H&Y staging was more marked in the psychotic group over the same duration of follow-up, despite similar staging at the beginning of follow-up.
Figure 3.
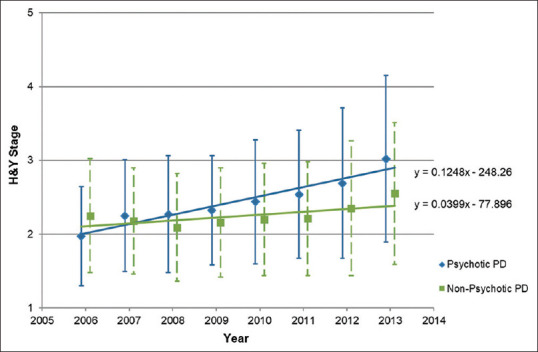
Modified Hoehn and Yahr Staging Scale (H&Y) staging of patients with Parkinson's disease (PD) with and without psychotic symptoms from 2006 to 2013.
In survival time analyses, log rank tests found no significant differences in the survival distributions between patients with H&Y staging 1–3 and 4–5 (χ2 (1) = 0.543, P = 0.461), as well as patients with MMSE scores 0–24 and 25–30 (χ2 (1) = 0.117, P = 0.732).
DISCUSSION
This study allowed us to examine the development and course of PD-associated psychosis and its possible risk factors from the diagnosis of PD to 8 years subsequently, as captured by a movement disorders database of outpatients at a tertiary neuroscience centre. A prevalence of 18.8% for PD-associated psychosis is within range of the findings of several studies, including a community-based study that reported the prevalence of psychosis at 23.6% in a sample of 250 outpatients with PD based on the NINDS/NIMH (National Institute of Mental Health) clinical criteria,[13] as well as another study of 191 patients with PD at two movement disorders centres that found a 21% prevalence of psychotic symptoms.[21] Even so, cross-sectional prevalence rates reported for psychosis in PD vary widely, ranging from 16%–75%.[4,6,9,22,23,24,25,26] This is likely the result of the lack of a uniform definition of psychosis, lack of disease-specific scales to measure psychosis, and the use of scales that are not validated to measure psychotic symptoms.
We were unable to find any significant associations between our hypothesised risk factors (i.e. age at diagnosis of PD, gender, ethnicity and years of education) and the odds of developing psychosis in PD, with age at diagnosis of PD acting as a surrogate marker for age of PD onset, as we could not have definitively known the exact age of onset. This is in contrast to the existing literature, which has reported age of onset and female gender[8,27] as independent risk factors for developing psychosis in PD. Additionally, we wanted to examine whether ethnicity and years of education would be risk factors, given the diversity of ethnicity and education in Singapore. However, multivariate logistic regression analysis did not find them to be significant risk factors. One possible reason that we were unable to find significant associations for these risk factors could be that the study cohort attended medical follow-up within a tertiary setting, which might not be fully representative of the PD population in Singapore. Keeping in mind the paucity of current literature that explores these risk factors in the local context, it would be worthwhile to examine these variables in a larger local cohort in the future.
Our findings showed that the presence of psychotic symptoms significantly predicted more rapid cognitive decline and progression of PD severity after controlling for the effect of time, concurring with the existing literature.[8,21,28,29] While causality cannot be inferred, this highlights the possibility that psychosis is a risk factor for cognitive impairment as well as higher disease severity in PD, which consequently serves to guide and inform clinicians in their treatment of patients. Increasing cognitive impairment and worsening severity of PD may signal a need for vigilance in observing psychotic symptoms and vice versa.
While studies[30,31] have suggested that dopaminergic medications commonly prescribed for patients with PD may have a facilitating role in PD-associated psychosis, this study suffered from a lack of access to medical databases. This meant that we were unable to study the presence and effect of dopamine agonists, as a possible precipitating factor in PD-associated psychosis. Ideally, access to such information would have enabled us to examine the relationship between levodopa dose equivalence and the presence of psychosis in patients with PD. This would have been a worthwhile contribution to our discussion regarding the risk factors associated with psychosis in PD and would be valuable to examine in future studies. Additionally, insufficient information about the prescription of antipsychotic medications prevented a more thorough investigation of cognitive impairment as a possible side effect of their long-term use, as has been reported by some studies that have found increased presence of neurofibrillary tangles (NFTs) associated with the long-term use of such medications,[32,33] as well as cognitive impairment in PD.[34,35]
Owing to its retrospective nature, this study had several limitations. As the movement disorders database was established in 2005, assessment tools thought then to be appropriate may be less relevant to current clinical research in PD-associated psychosis. With regard to assessing psychosis in PD, the single thought disorder item in the UPDRS is not sufficient to explore the variety of psychotic phenomena in PD[36] and does not include any recent updates in the spectrum of PD-associated psychosis, as we understand it.[3] A critique of rating scales used to assess psychosis in PD suggested that multiple scales be used to adequately capture the complete phenomenology of PD-associated psychosis; for example, one could combine the use of the Clinical Global Impression Scale to measure clinical response and change over time with another scale better at cataloguing specific features, such as the Neuropsychiatric Inventory.[36]
To detect the presence of significant changes in MMSE scores and H&Y staging of patients with PD over time, repeated measures analysis of variance would have been the appropriate for our cohort test to use. However, the MMSE and H&Y scale were not administered at fixed time points, resulting in too many missing values in the relevant dataset. Consequently, we conducted multivariate linear regression analyses to determine whether the presence of psychotic symptoms could predict mean MMSE scores and H&Y staging in patients with PD, while controlling for the effect of time.
While the MMSE has been used to assess cognitive impairment in studies that found strong associations between cognitive impairment and psychosis in patients with PD,[36,37] Hoops et al.[38] suggested the use of Montreal Cognitive Assessment over the MMSE, which they found to be a poorer measure of cognitive impairment in patients with PD due to its lack of sensitivity. Additionally, there was a large variability, in our study, in the timing of the assessments that were only conducted when clinically appropriate, leading to significant amounts of missing data. This rendered preferable methods of statistical analysis unviable. Future research would benefit from a prospective cohort study design, with a larger sample size, where patients are monitored over a longer follow-up duration beyond ten years, and assessments are administered according to a fixed schedule to reassess the associated risk factors.
Despite these challenges, this study has demonstrated that psychosis has a significant presence among the PD population in Singapore, shedding light on our local service needs. Knowing the negative implications of psychosis on the quality of life of patients with PD, it would be worthwhile for psychiatrists to collaborate with movement disorders specialists for constructing a standardised screening tool to specifically assess for PD-associated psychosis that is applicable to the local population. Establishing a standardised approach and translating this into clinical guidelines could facilitate the early detection of PD-associated psychosis and its timely intervention, which would be beneficial in the long-term management of patients with PD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mueller C, Rajkumar AP, Wan YM, Velayudhan L, Ffytche D, Chaudhuri KR, et al. Assessment and management of neuropsychiatric symptoms in Parkinson's disease. CNS Drugs. 2018;32:621–35. doi: 10.1007/s40263-018-0540-6. [DOI] [PubMed] [Google Scholar]
- 2.Connolly B, Fox SH. Treatment of cognitive, psychiatric, and affective disorders associated with Parkinson's disease. Neurotherapeutics. 2014;11:78–91. doi: 10.1007/s13311-013-0238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ffytche DH, Creese B, Politis M, Chaudhuri KR, Weintraub D, Ballard C, et al. The psychosis spectrum in Parkinson disease. Nat Rev Neurol. 2017;13:81–95. doi: 10.1038/nrneurol.2016.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fénelon G, Mahieux F, Huon R, Ziégler M. Hallucinations in Parkinson's disease: Prevalence, phenomenology and risk factors. Brain. 2000;123:733–45. doi: 10.1093/brain/123.4.733. [DOI] [PubMed] [Google Scholar]
- 5.Graham C, Ballard C, Saad K. Variables which distinguish patients fulfilling clinical criteria for dementia with Lewy bodies from those with Alzheimer's disease. Int J Geriatr Pscyhiatry. 1997;12:314–8. [PubMed] [Google Scholar]
- 6.Pacchetti C, Manni R, Zangaglia R, Mancini F, Marchioni E, Tassorelli C, et al. Relationship between hallucinations, delusions, and rapid eye movement sleep behavior disorder in Parkinson's disease. Mov Disord. 2005;20:1439–48. doi: 10.1002/mds.20582. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Ramos JR, Ortoll R, Paulson GW. Visual hallucinations associated with Parkinson disease. Arch Neurol. 1996;53:1265–8. doi: 10.1001/archneur.1996.00550120077019. [DOI] [PubMed] [Google Scholar]
- 8.Sawada H, Oeda T, Yamamoto K, Umemura A, Tomita S, Hayashi R, et al. Trigger medications and patient-related risk factors for Parkinson disease psychosis requiring anti-psychotic drugs: A retrospective cohort study. BMC Neurol. 2013;13:145. doi: 10.1186/1471-2377-13-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams DR, Warren JD, Lees AJ. Using the presence of visual hallucinations to differentiate Parkinson's disease from atypical Parkinsonism. J Neurol Neurosurg Psychiatry. 2008;79:652–5. doi: 10.1136/jnnp.2007.124677. [DOI] [PubMed] [Google Scholar]
- 10.Weintraub D, Hurtig HI. Presentation and management of psychosis in Parkinson's disease and dementia with Lewy bodies. Am J Psychiatry. 2007;164:1491–8. doi: 10.1176/appi.ajp.2007.07040715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weintraub D, Chen P, Ignacio RV, Mamikonyan E, Kales HC. Patterns and trends in antipsychotic prescribing for Parkinson disease psychosis. Arch Neurol. 2011;68:899–904. doi: 10.1001/archneurol.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson's disease: A population-based, prospective study. J Am Geriatr Soc. 2000;48:938–42. doi: 10.1111/j.1532-5415.2000.tb06891.x. [DOI] [PubMed] [Google Scholar]
- 13.Goetz CG, Stebbins GT. Mortality and hallucinations in nursing home patients with advanced Parkinson's disease. Neurology. 1995;45:669–71. doi: 10.1212/wnl.45.4.669. [DOI] [PubMed] [Google Scholar]
- 14.Mack J, Rabins P, Anderson K, Goldstein S, Grill S, Hirsch ES, et al. Prevalence of psychotic symptoms in a community-based Parkinson's disease sample. Am J Geriatr Psychiatry. 2012;20:123–32. doi: 10.1097/JGP.0b013e31821f1b41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marsh L. Psychosis in Parkinson's disease. Curr Treat Options Neurol. 2006;6:181–9. doi: 10.1007/s11940-004-0010-y. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Feng L, Chong MS, Lim WS, Ng TP. The Modified Mini-Mental State Examination test: Normative data for Singapore Chinese older adults and its performance in detecting early cognitive impairment. Singapore Med J. 2012;53:458–62. [PubMed] [Google Scholar]
- 18.Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations. Mov Disord. 2004;19:1020–8. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 19.Ramaker C, Marinus J, Stiggelbout AM, Van Hilten BJ. Systematic evaluation of rating scales for impairment and disability in Parkinson's disease. Mov Disord. 2002;17:867–76. doi: 10.1002/mds.10248. [DOI] [PubMed] [Google Scholar]
- 20.Forsaa EB, Larsen JP, Wentzel-Larsen T, Goetz CG, Stebbins GT, Aarsland D, et al. A 12-year population-based study of psychosis in Parkinson disease. Arch Neurol. 2010;67:996–1001. doi: 10.1001/archneurol.2010.166. [DOI] [PubMed] [Google Scholar]
- 21.Lee AH, Weintraub D. Psychosis in Parkinson's disease without dementia: Common and comorbid with other non-motor symptoms. Mov Disord. 2012;27:858–63. doi: 10.1002/mds.25003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holroyd S, Currie L, Wooten GF. Prospective study of hallucinations and delusions in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2001;70:734–8. doi: 10.1136/jnnp.70.6.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marsh L, Williams JR, Rocco M, Grill S, Munro C, Dawson TM. Psychiatric comorbidities in patients with Parkinson disease and psychosis. Neurology. 2004;63:293–300. doi: 10.1212/01.wnl.0000129843.15756.a3. [DOI] [PubMed] [Google Scholar]
- 24.Paleacu D, Schechtman E, Inzelberg R. Association between family history of dementia and hallucinations in Parkinson disease. Neurology. 2005;64:1712–5. doi: 10.1212/01.WNL.0000161872.85903.8E. [DOI] [PubMed] [Google Scholar]
- 25.Papapetropoulos S, Katzen H, Schrag A, Singer C, Scanlon BK, Nation D, et al. A questionnaire-based (UM-PDHQ) study of hallucinations in Parkinson's disease. BMC Neurol. 2008;8:21. doi: 10.1186/1471-2377-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schrag A, Ben-Shlomo Y, Quinn N. How common are complications of Parkinson's disease? J Neurol. 2002;249:419–23. doi: 10.1007/s004150200032. [DOI] [PubMed] [Google Scholar]
- 27.Zhu K, van Hilten JJ, Putter H, Marinus J. Risk factors for hallucinations in Parkinson's disease: Results from a large prospective cohort study. Mov Disord. 2013;28:755–62. doi: 10.1002/mds.25389. [DOI] [PubMed] [Google Scholar]
- 28.Goetz CG, Leurgans S, Pappert EJ, Raman R, Stemer AB. Prospective longitudinal assessment of hallucinations in Parkinson's disease. Neurology. 2001;57:2078–82. doi: 10.1212/wnl.57.11.2078. [DOI] [PubMed] [Google Scholar]
- 29.Merims D, Shabtai H, Korczyn AD, Peretz C, Weizman N, Giladi N. Antiparkinsonian medication is not a risk factor for the development of hallucinations in Parkinson's disease. J Neural Transm (Vienna) 2004;111:1447–53. doi: 10.1007/s00702-004-0209-9. [DOI] [PubMed] [Google Scholar]
- 30.Munhoz RP, Teive HA, Eleftherohorinou H, Coin LJ, Lees AJ, Silveira-Moriyama L. Demographic and motor features associated with the occurrence of neuropsychiatric and sleep complications of Parkinson's disease. J Neurol Neurosurg Psychiatry. 2013;84:883–7. doi: 10.1136/jnnp-2012-304440. [DOI] [PubMed] [Google Scholar]
- 31.Fénelon G, Soulas T, Cleret de Langavant L, Trinkler I, Bachoud-Lévi AC. Feeling of presence in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2011;82:1219–24. doi: 10.1136/jnnp.2010.234799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ballard CG, Perry RH, McKeith IG, Perry EK. Neuroleptics are associated with more severe tangle pathology in dementia with Lewy bodies. Int J Geriatr Psychiatry. 2005;20:872–5. doi: 10.1002/gps.1378. [DOI] [PubMed] [Google Scholar]
- 33.Wisniewski HM, Constantinidis J, Wegiel J, Bobinski M, Tarnawski M. Neurofibrillary pathology in brains of elderly schizophrenics treated with neuroleptics. Alzheimer Dis Assoc Disord. 1994;8:211–27. doi: 10.1097/00002093-199408040-00001. [DOI] [PubMed] [Google Scholar]
- 34.Irwin DJ, Lee VM, Trojanowski JQ. Parkinson's disease dementia: Convergence of a-synuclein, tau and amyloid-b pathologies. Nat Rev Neurosci. 2013;14:626–36. doi: 10.1038/nrn3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jellinger KA. Morphological substrates of Parkinsonism with and without dementia: A retrospective clinico-pathological study. J Neural Transm Suppl. 2007:91–104. doi: 10.1007/978-3-211-73574-9_12. doi: 10.1007/978-3-211-73574-9_12. [DOI] [PubMed] [Google Scholar]
- 36.Morgante L, Colosimo C, Antonini A, Marconi R, Meco G, Pederzoli M, et al. Psychosis associated to Parkinson's disease in the early stages: Relevance of cognitive decline and depression. J Neurol Neurosurg Psychiatry. 2012;83:76–82. doi: 10.1136/jnnp-2011-300043. [DOI] [PubMed] [Google Scholar]
- 37.Ffytche DH, Aarsland D. Psychosis in Parkinson's disease. Int Rev Neurobiol. 2017;133:585–622. doi: 10.1016/bs.irn.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Hoops S, Nazem S, Siderowf AD, Duda JE, Xie SX, Stern MB, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73:1738–45. doi: 10.1212/WNL.0b013e3181c34b47. [DOI] [PMC free article] [PubMed] [Google Scholar]


