Abstract
Macrophage inflammatory protein 1α (MIP-1α)/CCL3 prevents the development of eosinophilic pneumonia (EP) driven by a nonprotective T2-type immunity during infection with a highly virulent strain of Cryptococcus neoformans. The present study evaluated the interaction of MIP-1α with other innate immune system cytokines by comparing the immune responses that followed pulmonary infections with high- (C. neoformans 145A) and low (C. neoformans 52D)-virulence strains. In contrast to what was found for C. neoformans 145A infection, lack of MIP-1α in C. neoformans 52D infection did not cause the development of EP. C. neoformans 52D induced tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and MCP-1 in the lungs of infected wild-type (WT) and MIP-1α knockout (KO) mice by day 7 postinfection. Both WT and MIP-1α KO mice subsequently cleared this infection. Thus, the robust expression of early inflammatory cytokines in C. neoformans 52D-infected mice promoted the development of protective immunity even in the absence of MIP-1α. Alternatively, C. neoformans 145A-infected WT and MIP-1α KO mice had diminished TNF-α, IFN-γ, and macrophage chemoattractant protein 1 (MCP-1) responses, indicating that virulent C. neoformans 145A evaded early innate host defenses. However C. neoformans 145A-infected WT mice had an early induction of MIP-1α and subsequently did not develop EP. In contrast, C. neoformans 145A-infected MIP-1α KO mice developed EP and had increased C. neoformans dissemination into the brain by day 35. We conclude that, in the absence of other innate immune response effector molecules, MIP-1α is crucial to prevent the development of EP and to control C. neoformans dissemination to the brain.
Cryptococcus neoformans is an encapsulated yeast that can cause persistent and often fatal opportunistic infections (3). C. neoformans infection occurs via the respiratory route; in the absence of appropriate clearance mechanisms the infection disseminates from the lung into internal organs, particularly the brain (3). The development of a protective T-cell-mediated immunity is required for clearance of C. neoformans (3).
Clinical isolates of C. neoformans in experimental murine infections can be classified into three categories: low virulent, moderately virulent, and highly virulent. Infection of immunocompetent mice with low or moderately virulent strains of C. neoformans such as 184A, YC-13, and 52D results in clearance (2, 6, 10, 14, 18, 32). This clearance is associated with a prominent inflammatory response in the lung and the development of strong T1-driven specific immunity. Infection of immunocompetent mice with highly virulent C. neoformans strains such as 145A, YC-11, and NU-2 results in a chronic infection (2, 6, 14, 23–25). In these cases the inflammatory response in the lung is reduced or delayed (2, 6, 14, 24). In addition, immune system deviation leading to the development of nonprotective eosinophilic pneumonia (EP) occurs when susceptible mice (C57BL/6, CC chemokine receptor 2 [CCR2] knockout [KO], or gamma interferon [IFN-γ]- and interleukin-12 [IL-12]-neutralized mice) are infected with C. neoformans (12, 13, 31) Thus, persistence of the C. neoformans infection is associated either with minimal immune responses or with the deviation of the immune response leading to EP.
In light of the latter observation, it is not clear why immunocompetent mice infected with highly virulent strains do not develop EP in response to infection (2, 6, 11, 14, 25). However, EP does develop in macrophage inflammatory protein 1α (MIP-1α) KO mice infected with highly virulent C. neoformans (27). MIP-1α KO mice developed a nonprotective T2 pulmonary immune response following infection with highly virulent C. neoformans strain 145A. This infection resulted in (i) EP, (ii) increased pulmonary IL-4 and IL-13 expression, (iii) highly elevated immunoglobulin E levels, (iv) severe lung pathology, and (v) dramatically increased mortality (27). These recent data demonstrate that, just like IFN-γ, CC chemokine MIP-1α plays a role in preventing the development of nonprotective EP.
There is accumulating evidence from other systems that MIP-1α and other chemokines and chemokine receptors can regulate the development and polarization of immune responses. MIP-1α is a classic CC chemokine, more recently designated CCL3 (33). MIP-1α has been shown to (i) promote chemotaxis of Th1, but not Th2, cell lines in vitro (30), (ii) promote differentiation of T-cell receptor transgenic Th0 cells to Th1 cells in vitro (19), and (iii) decrease IL-4 production from cultured Th2-type lymphocytes stimulated with schistosomal egg antigen (20, 26). Anti-MIP-1α antibodies inhibit the development of T1-mediated experimental autoimmune encephalitis (19, 21). Thus, MIP-1α can be classified as a factor that is associated with T1 immune responses and that prevents T2-type responses.
The interaction of MIP-1α with other inflammatory cytokines, such as IFN-γ, tumor necrosis factor alpha (TNF-α), and macrophage chemoattractant protein 1 (MCP-1), in the regulation of immunity to infection has not been studied. Infection by high- and low-virulence strains of C. neoformans provides a useful set of tools to study the interaction of MIP-1α with other inflammatory cytokines in driving T-cell-mediated responses to infection. Our objective was to assess the role of MIP-1α in the presence and absence of inflammatory signals in preventing the development of EP.
MATERIALS AND METHODS
Mice.
Wild type (WT) mice (B6129F2/J; Jackson Laboratories, Bar Harbor, Maine) and MIP-1α KO mice (B6129-Scya3tm1Coo) were used for these studies (5). These mice were housed under specific-pathogen-free conditions in enclosed filter top cages at the University of Michigan Laboratory Animal Facility. Clean food and water were given ad libitum. The mice were handled and maintained using microisolator techniques with daily veterinarian monitoring. The MIP-1α KO mice lack a promoter region, as well as exon 1 and part of exon 2, of the MIP-1α gene (5). Mice were 8 to 16 weeks of age at the time of infection. There were no age-related differences in the responses of these mice to C. neoformans infection.
Cultures of C. neoformans.
Highly virulent C. neoformans strain 145A (ATCC 62070) and low-to-moderately virulent strain 52D (ATCC 24067) were obtained from the American Type Culture Collection (Manassas, Va.) (17). Both strains were isolated originally from cerebrospinal fluid of infected human patients, and both are encapsulated and laccase and urease positive; they have similar rates of in vitro growth. For infection, yeast was grown to stationary phase (at least 72 h) at 36°C in Sabouraud dextrose (SD) broth (1% neopeptone, 2% dextrose; Difco, Detroit, Mich.) on a shaker. The cultures were then washed in nonpyrogenic saline (Travenol, Deerfield, Ill.), counted on a hemocytometer, and diluted to 3.3 ×105 yeast cells/ml in sterile nonpyrogenic saline.
Intratracheal inoculation of C. neoformans.
Mice were anesthetized by intraperitoneal injection of pentobarbital (0.074 mg/g of body weight; Butler, Columbus, Ohio) and restrained on a small board. A small incision was made through the skin over the trachea, and the underlying tissue was separated. A bent 30-gauge needle (Becton Dickinson, Rutherford, N.J.) was attached to a tuberculin syringe (BD & Co., Franklin Lakes, N.J.) filled with the diluted C. neoformans culture. The needle was inserted into the trachea, and 30 μl of inoculum was dispensed into the lungs (104 yeast cells). The skin was closed with cyanoacrylate adhesive. The mice recovered with minimal visible trauma. Aliquots of the inoculum were collected periodically and plated on SD agar to monitor the number of CFU being delivered.
Preparation of lung leukocytes.
The lungs from each mouse were excised, washed in phosphate-buffered saline, minced with scissors, and digested enzymatically at 37°C for 30 min in 15 ml of digestion buffer (RPMI 1640, 5% fetal calf serum, antibiotics, 1 mg of collagenase [Boehringer Mannheim Biochemical, Chicago, Ill.]/ml, 30 μg of DNase [Sigma]/ml)/lung. The cell suspension and tissue fragments were further dispersed by repeated aspiration through the bore of a 10-ml syringe and centrifuged. Erythrocytes in the pellets were lysed by addition of 3 ml of NH4Cl buffer (0.829% NH4Cl, 0.1% KHCO3, 0.0372% Na2-EDTA, pH 7.4) for 3 min, followed by a 10-fold excess of RPMI 1640. Cells were resuspended again, and a second cycle of syringe dispersion and filtration through a sterile 100-μm-pore-size nylon screen (Nitex, Kansas City, Mo.) was performed. The filtrate was centrifuged for 25 min at 1,500 × g in the presence of 20% Percoll (Sigma) to separate leukocytes from cell debris and epithelial cells. Leukocyte pellets were resuspended in 10 ml of complete media and enumerated in a hemocytometer following dilution in trypan blue. Leukocyte recruitment was calculated using the following formula: recruited leukocytes in an infected mouse = total number of leukocytes in the infected mouse − mean number of leukocytes in uninfected mice. Values of leukocyte recovery from uninfected WT and MIP-1α KO mice were (23.26 ± 1.78) × 106 leukocytes (n = 18) and (24.36 ± 1.81) × 106 leukocytes (n = 16), respectively.
Assessment of leukocyte populations.
For the differential count of lung cell suspensions, samples were cytospun (Shandon Cytospin, Pittsburgh, Pa.) onto glass slides and stained by a modification of the Diff-Quik whole-blood stain (VWR Scientific Products). Samples were fixed and prestained for 2 min in a one-step methanol-based Wright-Giemsa stain (Harleco, EM Diagnostics, Gibbstown, N.J.), rinsed in water, and stained using steps 2 and 3 of the Diff-Quik stain procedure. This modification of the Diff-Quik stain procedure improves the resolution of eosinophils from neutrophils in the mouse. A total of 200 to 400 cells were counted for each sample from randomly chosen, high-power microscope fields.
Lung and brain CFU assays.
Aliquots of the lung digest solutions (prior to the first centrifugation) were collected for lung CFU assays. Dissected brains were homogenized in 2 ml of sterile water. Lung and brain suspensions were serially diluted in sterile water. Dilution samples (10 μl each) were plated on SD agar and incubated at room temperature for 48 h. Colony counts were performed and adjusted to reflect the total lung and brain CFU.
Histology.
Lungs were fixed by inflation with 1 ml of 10% neutral buffered formalin, excised en bloc, and immersed in neutral buffered formalin. Brains were carefully excised and immersed in neutral buffered formalin. After paraffin embedding, 5-μm-thick sections of both organs were cut and stained with hematoxylin and eosin. Sections were analyzed with light microscopy.
Detection of cytokine mRNA by RT-PCR.
Whole lungs were removed, homogenized in TRIzol reagent (Gibco BRL, Gaithersburg, Md.), extracted as outlined in the TRIzol protocol, and precipitated with isopropanol. The RNA was washed with 70% ethanol, dissolved in nuclease-free H2O, and quantified by UV spectrophotometry using absorbance at 260 nm. One-step reverse transcriptase PCR (RT-PCR; Access RT-PCR kits; Promega) on equal aliquots of RNA was performed in accordance with the manufacturer's protocol. Three fivefold dilutions of the RNA product (1, 0.2, and 0.04 μg) were used for the RT-PCR to control for possible overamplification of the cDNA in the samples. The oligonucleotide primers for PCRs were as follows: for β-actin, 5′-GTG-GGC-CGC-TCT-AGG-CAC-CA-3′ (sense) and 5′-CTC-AGC-TGT-GGT-GGT-GAA-GC-3′ (antisense); for TNF-α, 5′-AGC-ACA-GAA-AGC-ATG-ATC-CGC-G-3′ (sense) and 5′-GAC-TTT-CTC-CTG-GTA-TGA-GAT-AGC-3′ (antisense); for IFN-γ, 5′-GGC-TGT-TTC-TGG-CTG-TTA-CTG-CCA-CG-3′ (sense) and 5′-GAC-AAT-CTC-TTC-CCC-ACC-CCG-AAT-CAG-3′ (antisense); for MIP-1α, 5′-AAG-GTC-TCC-ACC-ACT-GCC-CTT-G-3′ (sense) and 5′-CTC-AGG-CAT-TCA-GTT-CCA-GGT-C-3′ (antisense).
The number of PCR cycles for β-actin was 25, whereas the cycle number for MIP-1α, IFN-γ, and TNF-α was 35. Annealing temperatures were as follows: 55°C for β-actin, TNF-α, and MIP-1α and 63°C for IFN-γ. RT-PCR products were electrophoresed and visualized by ethidium bromide staining. The sizes of the RT-PCR products were confirmed by comparison with a 100-bp ladder run in parallel on the same gel. RT-PCR products were transferred from the gels onto a Zeta Probe blotting membrane (Bio-Rad, Hercules, Calif.) for approximately 2 h on a vacuum blotter (model 735; Bio-Rad). DNA was cross-linked to the membranes in a UV Stratalinker (model 1800; Stratagene). Specific DNA products were detected via hybridization with labeled internal probes for DNA products of interest: for β-actin, 5′-GGG-ACG-ACA-TGG-AGA-AGA-TCT-GG-3′; for TNF-α, 5′-CCT-GTA-GCC-CAC-GTC-GTA-GC-3′; for IFN-γ, 5′-CAG-CGA-CTC-CTT-TTC-CGC-TT-3′; for MIP-1α, 5′-GTC-AAC-GAT-GAA-TTG-GCG-TGG-AAT-C-3′. The presence of specific DNA products was determined via exposure of the blots on X-ray film.
MCP-1 and IFN-γ detection in BAL fluid.
Bronchoalveolar lavage (BAL) fluid was obtained via a 3-mm-diameter Teflon catheter inserted directly into the exposed trachea of the mouse directly after euthanasia. The catheter was secured with surgical silk, and two 1-ml washes of ice-cold, sterile PBS were instilled into the lungs and withdrawn. BAL fluid was transferred into polyethylene microtubes and frozen until tested. Cytokines in BAL fluid were quantified using a murine enzyme-linked immunosorbent assay (ELISA) kit (Pharmingen). Reactions were performed in 96-well ELISA plates (Costar) in accordance with the manufacturer's instructions. The optical densities (ODs) were read on a microplate reader (Ultra Micro EL 808; Biotek Instruments) at a wavelength of 450 nm. The cytokine content in each well was estimated by interpolation of sample OD values with an appropriate standard by a four-parameter curve-fitting program.
Calculations and statistics.
Data (means ± standard errors [SE]) for each experimental group were derived from at least two independent infections (of compared groups of animals) and analyzed via t test or either one-way or two-way analysis of variance, depending on the number of groups. For individual comparisons of multiple groups, a post hoc test for simple main effects was used to calculate P values. Means with P values <0.05 were considered statistically significant.
RESULTS
Pulmonary growth and clearance of C. neoformans in infections with low- (52D) versus high (145A)-virulence strains.
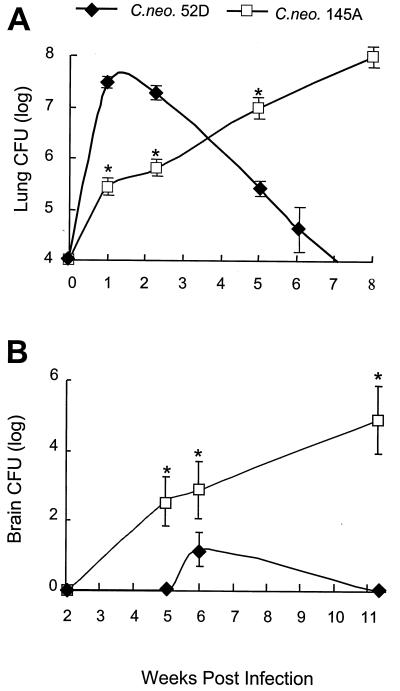
It was important to first determine the dynamics of pulmonary growth of C. neoformans 52D and C. neoformans 145A in B6129F2/J, i.e., WT mice, prior to our studies using MIP-1α KO mice. Rapid growth of C. neoformans 52D within the lung occurred over the first week of infection, resulting in a nearly 1,000-fold increase in lung CFU over the inoculum dose (Fig. 1A). This rapid phase of C. neoformans growth in the lung was followed by a gradual decrease over a period of several weeks and subsequent clearance of C. neoformans 52D from the lungs. In contrast, C. neoformans 145A proliferated in the lung less vigorously, approaching a 30-fold increase over the inoculum during the first week of infection (Fig. 1A). However, it continued to grow progressively during the 7 weeks of infection, approaching 108 CFU at day 70 (10,000-fold increase). Thus, pulmonary infection in B6129F2/J mice with C. neoformans 52D results in the development of a protective response and clearance, while pulmonary infection with C. neoformans 145A is chronic and characterized by progressive pulmonary growth of cryptococci.
FIG. 1.
Comparison of the growth of C. neoformans 52D and 145A in lungs (A) and brains (B). B6129F2/J mice were infected intratracheally with C. neoformans 52D or C. neoformans 145A (104 CFU) and analyzed (n = 5 to 22 per time point per group). Data pooled from separate matched experiments are expressed as the mean CFU per lung or brain ± SE. Asterisk, P < 0.05 in comparison with C. neoformans 52D-infected group.
Pathology of C. neoformans 52D versus C. neoformans 145A infections.
We performed histological examination of lungs 11 to 12 weeks postinfection to compare the long-term pulmonary infections by C. neoformans 52D and C. neoformans 145A. Consistent with the CFU analysis, C. neoformans 52D was no longer present in the lungs by 12 weeks and almost all of the inflammatory response had resolved in the lungs. Lung histology of infected animals resembled lung histology of uninfected animals. Histological features of the repair process—groups of fibroblasts surrounded by modest mononuclear cell infiltration—were noted. At the same time point, lungs of mice infected with C. neoformans 145A contained large numbers of cryptococci, which formed large nodular clusters and masses. These C. neoformans clusters were surrounded by walls of mononuclear cell infiltrates forming diffuse granulomas containing large cryptococci. Thus, while C. neoformans 52D is entirely cleared from lungs by week 12, leaving only minimal pathological changes, C. neoformans 145A progressively grows in the lungs, resulting in chronic inflammation and focal destruction of the lung tissue.
Differential capacity for cerebral dissemination of C. neoformans strains.
The capacity for cerebral dissemination of both strains of C. neoformans was evaluated. C. neoformans 52D was detected only at 6 weeks postinfection in two of eight animals. All remaining WT mice infected with C. neoformans 52D had no C. neoformans in the brain at any time point analyzed (Fig. 1B). Cerebral dissemination began between weeks 2 and 5 in animals infected with C. neoformans 145A. Cryptococci were cultured from the brains of 50% of the mice at 5 weeks. The mean brain CFU, as well as the percentage of mice with positive brain cultures, increased progressively after this time point (Fig. 1B). Thus, in contrast with C. neoformans 52D, C. neoformans 145A rapidly disseminates into the central nervous system (CNS) and establishes a permanent CNS infection.
Pulmonary recruitment of leukocytes.
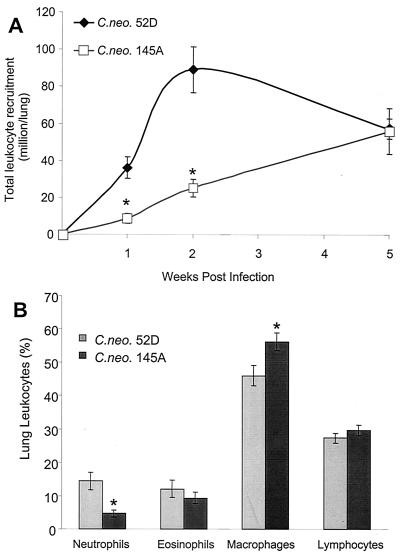
To determine if the differences in clearance were caused by differential host responses to these strains of C. neoformans, we analyzed pulmonary recruitment of leukocytes. Pulmonary leukocyte recruitment in mice infected with C. neoformans 145A was delayed compared with recruitment in mice infected with C. neoformans 52D (Fig. 2A). The numbers of leukocytes in the two groups were similar by day 35 postinfection. Significant clearance of C. neoformans 52D had occurred by this time point, and pulmonary leukocyte numbers were already declining in these mice. Thus, infection with strain 145A produced a much less vigorous and delayed cellular response.
FIG. 2.
Comparison of pulmonary leukocyte recruitment in mice infected with C. neoformans 52D or 145A. Leukocytes were isolated from infected lungs following enzymatic dispersion of whole lungs (see Materials and Methods). (A) Lung leukocyte recruitment at weeks 1, 2, and 5 postinfection was calculated by subtraction of the mean number of leukocytes in uninfected mice from the total number of leukocytes in infected animals. (B) Relative composition of leukocyte subsets in the lungs 2 weeks postinfection. Data (means ± SE) are percentages of subsets in the samples of total lung leukocyte isolates. Asterisk, P < 0.05 in comparison with C. neoformans 52D-infected group (n = 18 uninfected mice and n = 5 to 22 infected mice per time point per group).
Characterization of pulmonary leukocyte subsets.
To determine if qualitative or quantitative differences in pulmonary leukocyte recruitment occurred, we performed analysis of cell subsets recruited into the lungs at 2 weeks postinfection. Leukocyte recruitment in the C. neoformans 145A-infected group was only about 28% of the recruitment in C. neoformans 52D-infected groups; thus recruitment of each individual cell type was significantly higher in the latter. Therefore, we compared the relative numbers of pulmonary leukocytes expressed as percentages. The percentages of eosinophils and lymphocytes were similar in both types of infection. We observed a higher proportion of neutrophils to macrophages in C. neoformans 52D-infected animals than in mice infected with C. neoformans 145A (Fig. 2B), indicating that C. neoformans 52D-infected mice developed a more pronounced acute inflammatory response.
Comparison of the proinflammatory cytokine profiles.
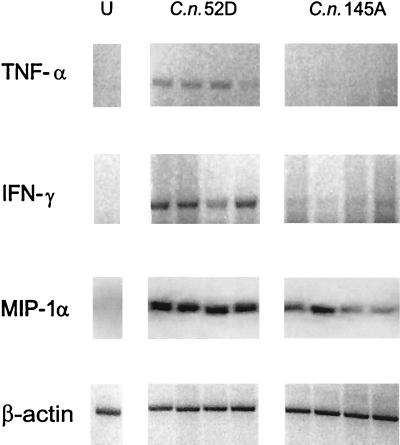
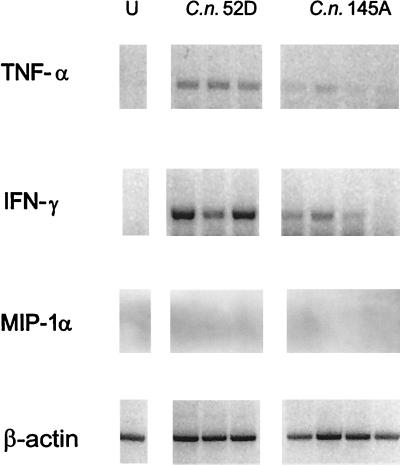
We next analyzed early expression of the proinflammatory cytokines, because leukocyte recruitment was delayed in lungs infected with C. neoformans 145A compared with that in lungs infected with C. neoformans 52D. By day 7, C. neoformans 52D-infected mice demonstrated robust induction of TNF-α, IFN-γ, and MIP-1α in their lungs (Fig 3). In mice infected with C. neoformans 145A, expression of TNF-α was absent in the lungs; the expression of IFN-γ was dramatically reduced (Fig. 3). Interestingly, MIP-1α was induced following infection by both strains of C. neoformans. Thus, mice infected with C. neoformans 52D demonstrated strong induction of TNF-α, IFN-γ, and MIP-1α by day 7 of infection, while mice infected with C. neoformans 145A expressed only MIP-1α.
FIG. 3.
Comparison of pulmonary TNF-α, IFN-γ, and MIP-1α gene expression in WT mice infected intratracheally (C. neoformans 52D or C. neoformans 145A; 104 CFU). Total lung RNA was isolated from the uninfected mice (U) on day 7 postinoculation. Expression of cytokine mRNA was analyzed via RT-PCR. The cytokine PCR products were confirmed by Southern blotting with specific DNA probes.
Comparison of MIP-1α effects on the development of EP in infections with C. neoformans 52D versus C. neoformans 145A.
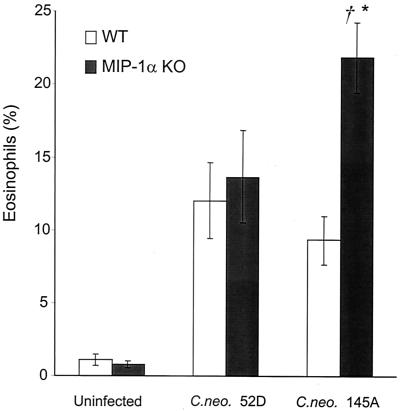
We reported previously that infection of MIP-1α KO mice with C. neoformans 145A caused a switch to a T2-type immune response, resulting in EP, immunoglobulin E production, and subsequent lung pathology. To determine if MIP-1α prevents the development of EP during development of immune responses to C. neoformans 52D, we analyzed eosinophil recruitment in C. neoformans 52D-infected WT and MIP-1α KO mice 2 weeks postinfection. Interestingly, the absence of MIP-1α did not result in increased eosinophil influx into the lungs of MIP-1α KO mice compared with WT mice (Fig 4). However, consistent with our previous report, we observed the development of EP in MIP-1α KO mice infected with C. neoformans 145A (Fig. 4). Thus, deletion of MIP-1α does not result in the development of EP in response to infection with C. neoformans 52D.
FIG. 4.
Effect of MIP-1α gene deletion on eosinophil recruitment into the lungs of mice infected with C. neoformans 52D or 145A (2 weeks postinfection). Leukocytes were isolated from infected lungs of WT and MIP-1α KO mice following enzymatic dispersion of whole lungs. Data (means ± SE) are percentages of eosinophils in cytospun preparations of lung leukocytes (n = 15 to 17 mice per group). Asterisk, P < 0.05, in comparison with 52D-infected group; dagger, P < 0.05, in comparison with WT mice.
Comparison of the proinflammatory cytokine profiles in WT versus MIP-1α KO mice.
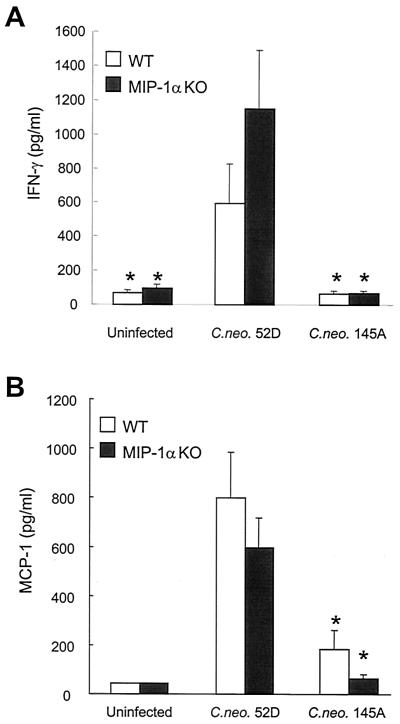
To dissect the molecular mechanism of the differential effects of MIP-1α in the regulation of the immune response to C. neoformans 52D and C. neoformans 145A, we analyzed whether expression of MIP-1α was required for induction of TNF-α, IFN-γ, and MCP-1 at 7 days postinfection. Consistent with the MIP-1α KO genotype, MIP-1α mRNA was undetectable in both types of infection. Just like WT mice (Fig. 3) MIP-1α KO mice infected with C. neoformans 52D demonstrated robust induction of TNF-α and IFN-γ in their lungs (Fig. 5). The level of MCP-1 in BAL fluid was elevated (Fig. 6) in both groups infected with C. neoformans 52D. In contrast, both WT and MIP-1α KO mice infected with C. neoformans 145A demonstrated dramatically reduced expression of TNF-α and IFN-γ compared with C. neoformans 52D-infected mice (Fig. 3 and 5). MCP-1 induction in C. neoformans 145A-infected WT and MIP-1α KO mice was minimal compared to that in animals infected with C. neoformans 52D (Fig. 6). A small increase in MCP-1 level could be detected in C. neoformans 145A-infected WT mice but not in MIP-1α KO mice (Fig. 6). Thus, MIP-1α is not required for the robust early induction of TNF-α, IFN-γ, or MCP-1 in response to C. neoformans 52D but may be required for the modest production of MCP-1 seen in response to C. neoformans 145A.
FIG. 5.
Comparison of pulmonary TNF-α, IFN-γ, and MIP-1α gene expression in MIP-1α KO mice infected intratracheally (C. neoformans 52D or C. neoformans 145A; 104 CFU). Total lung RNA was isolated from the uninfected mice (U) on day 7 postinoculation. Expression of cytokine mRNA was analyzed via RT-PCR. The cytokine PCR products were confirmed by Southern blotting with specific DNA probes.
FIG. 6.
Comparison of IFN-γ (A) and MCP-1 (B) levels in BAL fluid. The cytokines were detected by ELISA in BAL samples from infected (1 week postinfection) and uninfected WT and MIP-1α KO mice. Bars, means ± SE (n = 9 or 10 per group [infected] and 4 per group [uninfected]). Asterisk, P < 0.05 in comparison with C. neoformans 52D-infected group.
Comparison of MIP-1α effects on pulmonary growth of C. neoformans.
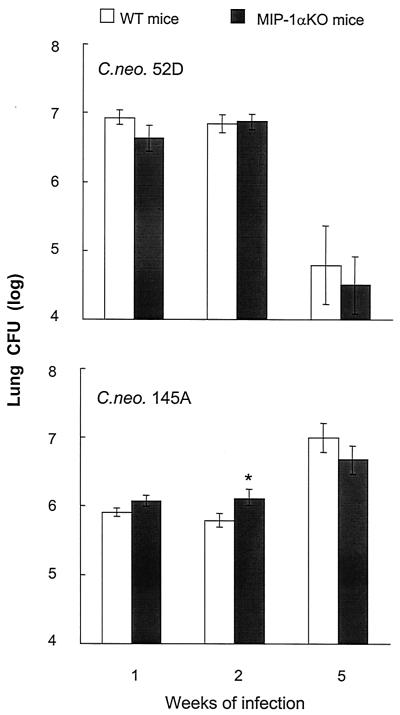
Our next objective was to determine the role of MIP-1α in the control of short- and long-term pulmonary infection with C. neoformans 52D and C. neoformans 145A. In contrast with C. neoformans 145A infection, there were no observed differences in C. neoformans 52D-infected lung CFU between WT and MIP-1α KO mice at 2 weeks postinfection (Fig. 7). Both MIP-1α KO and WT mice cleared the C. neoformans 52D infection. In contrast, neither MIP-1α KO nor WT mice could control growth of C. neoformans 145A in the lungs (Fig. 7). Thus, MIP-1α was not required to control the pulmonary growth of C. neoformans 52D, and the beneficial effect of MIP-1α at week 2 in C. neoformans 145-infected mice was not apparent at 5 weeks postinfection.
FIG. 7.
Effect of MIP-1α gene deletion on pulmonary clearance of C. neoformans in mice infected with C. neoformans 52D (top) and C. neoformans 145A (bottom). Mice were infected intratracheally (C. neoformans 52D or C. neoformans 145A; 104 CFU) and analyzed (n = 5 to 22 per time point per group). Data, pooled from at least two separate matched experiments, are mean CFU per whole lung ± SE. Asterisk, P < 0.05, in comparison with 52D-infected group.
Comparison of MIP-1α effects on control of C. neoformans in the CNS.
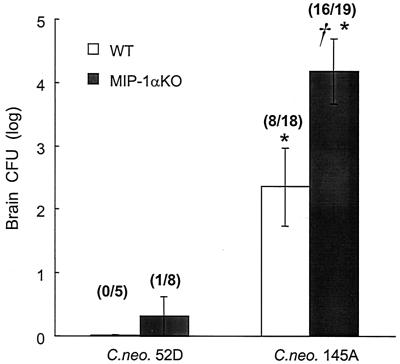
To determine if MIP-1α deletion had an effect on the control of C. neoformans dissemination and growth in the CNS, we analyzed brain CFU at 5 weeks postinfection. One of eight animals in the MIP-1α KO group infected with C. neoformans 52D contained C. neoformans in the CNS; no C. neoformans was detected in the CNS of the remaining 7 MIP-1α KO animals (Fig 8). In contrast, brain dissemination occurred in both WT and MIP-1α KO mice infected with C. neoformans 145A. However, the number of cryptococci was nearly 2 log units greater in MIP-1α KO mice than in WT mice (Fig. 8). Thus, dissemination of the more-virulent C. neoformans 145A is much greater than that of C. neoformans 52D, and MIP-1α is required to control the growth of C. neoformans 145A in the CNS.
FIG. 8.
Effect of MIP-1α gene deletion on cerebral dissemination of C. neoformans in mice infected with C. neoformans 52D and C. neoformans 145A. Mice were infected intratracheally (C. neoformans 52D or C. neoformans 145A; 104 CFU) and analyzed 5 weeks postinfection (n = 5 to 19 per group). Data, pooled from separate matched experiments, are mean CFU per brain ± SE. Asterisk, P < 0.05, in comparison with 52D-infected group; dagger, P < 0.05, in comparison with WT mice.
Comparison of CNS pathology between WT and MIP-1α KO mice infected with C. neoformans145A.
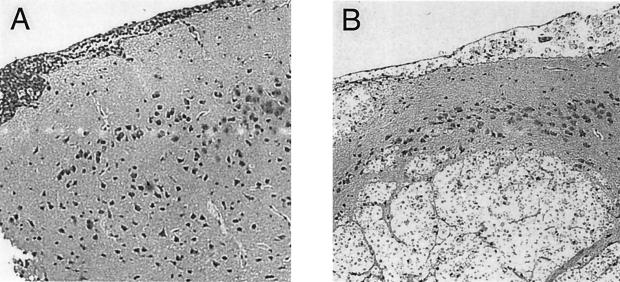
Our final objective was to compare brain pathologies in WT and MIP-1α KO mice infected with C. neoformans 145A. Cryptococcal brain masses were increased in number and size in the brains of MIP-1α KO mice compared with WT mice (Fig. 9). The lesions in the brains of MIP-1α KO mice included the following: (i) profound meningitis (swelling and infiltration of the meninges and numerous cryptococci and mononuclear leukocytes throughout the entire perimeter of brain sections) and (ii) multiple intracerebral lesions (round foci expanding within the brain, containing large cryptococci with no or few inflammatory cells separating them from uninfected brain tissue) (Fig. 9A). In the WT mice, meningitis was far less pronounced; we observed 0 to 2 small lesions/section of the brain, demarcated by the inflammatory cell infiltrate (Fig. 9B). Thus, severe pathology and evidence of uncontrolled growth of C. neoformans were found in the CNS of C. neoformans 145A-infected MIP-1α KO mice.
FIG. 9.
Histology of brain sections 10 weeks post-intratracheal infection with C. neoformans 145A. Magnification, ×33. (A) WT mice. Note the well-organized, local inflammatory cell infiltrate colocalized with cryptococci and the good separation of infected areas of meninges from uninfected brain tissue. (B) MIP-1α KO mice. Note the swelling and infiltration of the meninges and multiple intracerebral lesions, the round foci expanding within the brain with numerous cryptococci with large capsules, no or few inflammatory cells.
DISCUSSION
In this paper, we report that deletion of MIP-1α did not cause development of nonprotective EP in mice infected with moderately virulent strain C. neoformans 52D while infection of MIP-1α KO mice with more-virulent strain C. neoformans 145A resulted in the development of EP. Early induction of inflammatory cytokines TNF-α, IFN-γ, and MCP-1 followed C. neoformans 52D infection but did not occur in C. neoformans 145A-infected animals. The observation that MIP-1α prevents the development of EP following C. neoformans 145A infection, even in the absence of TNF-α, IFN-γ, and MCP-1, documents that MIP-1α is a potent factor regulating the phenotype of the inflammatory response which develops in the lung upon infection with C. neoformans. On the other hand MIP-1α is not required for the development of protective immunity to C. neoformans 52D.
MIP-1α functions as a part of a complex regulatory network. A relationship between TNF-α and MIP-1α induction exists, since neutralization of TNF-α during the first 2 weeks of infection decreases MIP-1α levels in the BAL fluid of mice infected with C. neoformans 52D (14). Thus, we were surprised to observe MIP-1α induction in response to infection by C. neoformans 145A, during which TNF-α induction (RNA [Fig. 3] or protein level [16]) was minimal or absent. The difference between these two strains in the induction signals for MIP-1α likely relates to virulence factor or surface structural differences between these two isolates and is a point of future investigation. The lack of effect of MIP-1α deletion on the immune response to C. neoformans 52D, during which TNF-α, IFN-γ, and MCP-1 are abundant, suggests that cytokines and/or chemokines have overlapping functions with MIP-1α in regulation of the immunity to C. neoformans. Thus, the regulatory function of MIP-1α is particularly important in situations in which other strong proinflammatory molecules are absent.
Differential inflammatory responses were associated with subsequent differences in the clearance of C. neoformans 52D and C. neoformans 145A. The B6129F2/J mice generate a strong inflammatory response and are resistant to C. neoformans 52D infection. Our studies demonstrate that this strain is useful for studying protective immunity, as are BALB/c, C.B-17, CDF1, and CBA/J mice. Information about the response in B6129F2/J mice is important since this strain is utilized often for KO studies. Similar to CBA/J mice, these mice do not generate significant early inflammation during C. neoformans 145A infection and the infection becomes chronic (6, 14). However, MIP-1α is expressed in response to the infection, which prevents the development of a T2 response even in the absence of TNF-α and IFN-γ. Our studies document that the generation of protective immunity in cryptococcal infection is crucially dependent on the early innate immune response. A vigorous early inflammatory response is associated with the development of strong protective immunity and clearance of the infection. On the other hand, a delayed inflammatory response is associated with a “deficient” immune response and chronic infection. Circulating cryptococcal polysaccharide is likely a factor in modulating leukocyte recruitment to the site of infection (8). However, the effect of circulating polysaccharide on leukocyte recruitment is secondary if the appropriate inflammatory signals and chemotactic factors are not generated at the site of infection (such as in a C. neoformans 145A infection). In this situation, leukocytes will not be recruited to the site whether or not there is circulating polysaccharide. Thus, down-regulation or absence of early “danger signals” such as TNF-α is an important factor determining C. neoformans virulence (14, 17, 22). The net result of decreased early danger signals is limited inflammation, altered cytokine production, limited or altered immune responses, and a chronic infection.
The early induction of MCP-1 in C. neoformans 52D infection suggests that MCP-1 may also play a role in early inflammatory cell recruitment. MCP-1 was previously shown to be important molecule during the efferent phase of immune response to C. neoformans (16). The dramatically lower induction of MCP-1 in C. neoformans 145A-infected lungs corresponds to decreased early inflammation in this infection and subsequent problems with clearance of the infection. Kawakami et al. also observed very little or no production of chemokines, including MCP-1, during infection with a highly virulent strain of C. neoformans (24). Moreover, mice deficient in CCR2, the receptor for MCP-1, also have decreased recruitment of mononuclear cells early in C. neoformans 52D infection (31). Thus, down-regulation of early MCP-1 production is consistent with a lack of danger signals induced by C. neoformans 145A.
Meningoencephalitis is the cause of excessive mortality previously reported in C. neoformans 145A-infected MIP-1α KO mice. In spite of the more-severe lung pathology in MIP-1α KO mice (27), no differences in lung CFU between C. neoformans 145A-infected WT and KO mice were noted on day 35. Thus, pulmonary cryptococcal burden itself was not the likely cause of the increased mortality that occurred between weeks 6 and 12 postinfection in MIP-1α KO mice. However, C. neoformans 145A disseminates earlier and in greater numbers in MIP-1α KO mice than in WT mice. Moreover, C. neoformans 145A multiplies in the CNS; CFU values >107 were noted in MIP-1α KO animals, as were multiple cryptococcal mass lesions with proliferating cryptococci (Fig. 9). Neurological abnormalities were present in premoribund animals (paralysis, abnormal gait). Thus, increased mortality in MIP-1α KO mice was predominantly associated with cryptococcal meningoencephalitis rather than pulmonary pathology.
The CNS pathology, survival times, and day 35 lung CFU burdens parallel those from our observations of CCR5 KO mice infected with C. neoformans 145A (15). MIP-1α is a high-affinity ligand of CCR5, and these findings provide further support for our notion that expression of CCR5 and its ligands is crucial for protection of the CNS from C. neoformans infection. The absence of MIP-1α results in pronounced brain dissemination and CNS pathology, showing that the MIP-1α/CCR5 axis is particularly crucial to organ-specific protection of the CNS (15). The importance of the MIP-1α/CCR5 axis may potentially be even greater in human immunodeficiency virus type 1 (HIV-1) patients infected with C. neoformans. Both MIP-1α and HIV gp120 are ligands for CCR5 (28, 29). Thus, CCR5, a receptor via which MIP-1α exerts its protective anticryptococcal effect in the CNS, is also a coreceptor for HIV infection (1, 4, 7, 9). Our studies raise the possibility that dysregulated MIP1α/CCR5 function (potentially caused by HIV or gp120 binding to CCR5) is a significant predisposing factor for C. neoformans infection of the CNS (21).
Microbes persist in mammalian hosts, in part, because they do not elicit innate immune system danger signals. Well-characterized danger signals include TNF-α and IFN-γ, cytokines essential for the development of a protective immune response to C. neoformans. Our data document that MIP-1α also plays a crucial role as an early danger signal in certain microbe-host interactions. MIP-1α alone can prevent the development of a nonprotective EP immune response, particularly in circumstances when a pathogen does not elicit other danger signals.
ACKNOWLEDGMENTS
This work was supported in part by a VA Merit Grant (G.B.T.), a VA REAP grant (G.B.T., M.A.O.), NHLBI R01-HL63670 (G.B.H.), NHLBI R01-HL 65912 (G.B.H.), NHLBI R01-HL51082 (G.B.T.), NHLBI T32-HL07749 (G.B.H., M.A.O.), and the Burroughs-Wellcome Fund (G.B.H.),
We thank Lisa McNeil and Michael Boyd for their assistance in the preliminary phases of this project and Dennis Lindell, Amy Herring, and Raj Pandrangi for their ongoing collaboration in these studies.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Blackstock R, Buchanan K L, Adesina A M, Murphy J W. Differential regulation of immune responses by highly and weakly virulent Cryptococcus neoformans isolates. Infect Immun. 1999;67:3601–3609. doi: 10.1128/iai.67.7.3601-3609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casadevall A, Perfect J R. Cryptococcus neoformans. Vol. 1. Washington, D.C.: ASM Press; 1998. [Google Scholar]
- 4.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 5.Cook D N, Beck M A, Coffman T M, Kirby S L, Sheridan J F, Pragnell I B, Smithies O. Requirement of MIP-1α for an inflammatory response to viral infection. Science. 1995;269:1583–1585. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 6.Curtis J L, Huffnagle G B, Chen G H, Warnock M L, Gyetko M R, McDonald R A, Scott P J, Toews G B. Experimental murine pulmonary cryptococcosis. Differences in pulmonary inflammation and lymphocyte recruitment induced by two encapsulated strains of Cryptococcus neoformans. Lab Investig. 1994;71:113–126. [PubMed] [Google Scholar]
- 7.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 8.Dong Z M, Murphy J W. Mobility of human neutrophils in response to Cryptococcus neoformans cells, culture filtrate antigen, and individual components of the antigen. Infect Immun. 1993;61:5067–5077. doi: 10.1128/iai.61.12.5067-5077.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 10.Dykstra M A, Friedman L, Murphy J W. Capsule size of Cryptococcus neoformans: control and relationship to virulence. Infect Immun. 1977;16:129–135. doi: 10.1128/iai.16.1.129-135.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman D L, Fries B C, Franzot S P, Montella L, Casadevall A. Phenotypic switching in the human pathogenic fungus Cryptococcus neoformans is associated with changes in virulence and pulmonary inflammatory response in rodents. Proc Natl Acad Sci USA. 1998;95:14967–14972. doi: 10.1073/pnas.95.25.14967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoag K A, Lipscomb M F, Izzo A A, Street N E. IL-12 and IFN-γ are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am J Respir Cell Mol Biol. 1997;17:733–739. doi: 10.1165/ajrcmb.17.6.2879. [DOI] [PubMed] [Google Scholar]
- 13.Huffnagle G B, Boyd M B, Street N E, Lipscomb M F. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6) J Immunol. 1998;160:2393–2400. [PubMed] [Google Scholar]
- 14.Huffnagle G B, Chen G H, Curtis J L, McDonald R A, Strieter R M, Toews G B. Down-regulation of the afferent phase of T cell-mediated pulmonary inflammation and immunity by a high melanin-producing strain of Cryptococcus neoformans. J Immunol. 1995;155:3507–3516. [PubMed] [Google Scholar]
- 15.Huffnagle G B, McNeil L K, McDonald R A, Murphy J W, Toews G B, Maeda N, Kuziel W A. Cutting edge: role of C-C chemokine receptor 5 in organ-specific and innate immunity to Cryptococcus neoformans. J Immunol. 1999;163:4642–4646. [PubMed] [Google Scholar]
- 16.Huffnagle G B, Strieter R M, McNeil L K, McDonald R A, Burdick M D, Kunkel S L, Toews G B. Macrophage inflammatory protein-1α (MIP-1α) is required for the efferent phase of pulmonary cell-mediated immunity to a Cryptococcus neoformans infection. J Immunol. 1997;159:318–327. [PubMed] [Google Scholar]
- 17.Huffnagle G B, Toews G B, Burdick M D, Boyd M B, McAllister K S, McDonald R A, Kunkel S L, Strieter R M. Afferent phase production of TNF-α is required for the development of protective T cell immunity to Cryptococcus neoformans. J Immunol. 1996;157:4529–4536. [PubMed] [Google Scholar]
- 18.Huffnagle G B, Yates J L, Lipscomb M F. T-cell-mediated immunity in the lung: a Cryptococcus neoformans pulmonary infection model using SCID and athymic nude mice. Infect Immun. 1991;59:1423–1433. doi: 10.1128/iai.59.4.1423-1433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karpus W J, Kennedy K J. MIP-1α and MCP-1 differentially regulate acute and relapsing autoimmune encephalomyelitis as well as Th1/Th2 lymphocyte differentiation. J Leukoc Biol. 1997;62:681–687. [PubMed] [Google Scholar]
- 20.Karpus W J, Lukacs N W, Kennedy K J, Smith W S, Hurst S D, Barrett T A. Differential CC chemokine-induced enhancement of T helper cell cytokine production. J Immunol. 1997;158:4129–4136. [PubMed] [Google Scholar]
- 21.Karpus W J, Lukacs N W, McRae B L, Strieter R M, Kunkel S L, Miller S D. An important role for the chemokine macrophage inflammatory protein-1α in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. J Immunol. 1995;155:5003–5010. [PubMed] [Google Scholar]
- 22.Kawakami K, Qifeng X, Tohyama M, Qureshi M H, Saito A. Contribution of tumour necrosis factor-α (TNF-α) in host defence mechanism against Cryptococcus neoformans. Clin Exp Immunol. 1996;106:468–474. doi: 10.1046/j.1365-2249.1996.d01-870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawakami K, Qureshi M H, Koguchi Y, Nakajima K, Saito A. Differential effect of Cryptococcus neoformans on the production of IL-12p40 and IL-10 by murine macrophages stimulated with lipopolysaccharide and gamma interferon. FEMS Microbiol Lett. 1999;175:87–94. doi: 10.1111/j.1574-6968.1999.tb13605.x. [DOI] [PubMed] [Google Scholar]
- 24.Kawakami K, Shibuya K, Qureshi M H, Zhang T, Koguchi Y, Tohyama M, Xie Q, Naoe S, Saito A. Chemokine responses and accumulation of inflammatory cells in the lungs of mice infected with highly virulent Cryptococcus neoformans: effects of interleukin-12. FEMS Immunol Med Microbiol. 1999;25:391–402. doi: 10.1111/j.1574-695X.1999.tb01365.x. [DOI] [PubMed] [Google Scholar]
- 25.Kawakami K, Tohyama M, Qifeng X, Saito A. Expression of cytokines and inducible nitric oxide synthase mRNA in the lungs of mice infected with Cryptococcus neoformans: effects of interleukin-12. Infect Immun. 1997;65:1307–1312. doi: 10.1128/iai.65.4.1307-1312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukacs N W, Chensue S W, Karpus W J, Lincoln P, Keefer C, Strieter R M, Kunkel S L. C-C chemokines differentially alter interleukin-4 production from lymphocytes. Am J Pathol. 1997;150:1861–1868. [PMC free article] [PubMed] [Google Scholar]
- 27.Olszewski M A, Huffnagle G B, McDonald R A, Lindell D M, Moore B B, Cook D N, Toews G B. The role of macrophage inflammatory protein-1alpha/CCL3 in regulation of T cell-mediated immunity to Cryptococcus neoformans infection. J Immunol. 2000;165:6429–6436. doi: 10.4049/jimmunol.165.11.6429. [DOI] [PubMed] [Google Scholar]
- 28.Raport C J, Schweickart V L, Chantry D, Eddy R L, Jr, Shows T B, Godiska R, Gray P W. New members of the chemokine receptor gene family. J Leukoc Biol. 1996;59:18–23. doi: 10.1002/jlb.59.1.18. [DOI] [PubMed] [Google Scholar]
- 29.Samson M, Labbe O, Mollereau C, Vassart G, Parmentier M. Molecular cloning and functional expression of a new human CC-chemokine receptor gene. Biochemistry. 1996;35:3362–3367. doi: 10.1021/bi952950g. [DOI] [PubMed] [Google Scholar]
- 30.Siveke J T, Hamann A. T helper 1 and T helper 2 cells respond differentially to chemokines. J Immunol. 1998;160:550–554. [PubMed] [Google Scholar]
- 31.Traynor T R, Kuziel W A, Toews G B, Huffnagle G B. CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. J Immunol. 2000;164:2021–2027. doi: 10.4049/jimmunol.164.4.2021. [DOI] [PubMed] [Google Scholar]
- 32.Xie Q, Kawakami K, Kudeken N, Zhang T, Qureshi M H, Saito A. Different susceptibility of three clinically isolated strains of Cryptococcus neoformans to the fungicidal effects of reactive nitrogen and oxygen intermediates: possible relationships with virulence. Microbiol Immunol. 1997;41:725–731. doi: 10.1111/j.1348-0421.1997.tb01917.x. [DOI] [PubMed] [Google Scholar]
- 33.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]