Abstract
Background
Postoperative pulmonary complications are a source of morbidity after major surgery. In patients at increased risk of postoperative pulmonary complications we sought to assess the association between neuromuscular blocking agent reversal agent and development of postoperative pulmonary complications.
Methods
We conducted a retrospective matched cohort study, a secondary analysis of data collected in the prior STRONGER study. Data were obtained from the Multicenter Perioperative Outcomes Group. Included patients were aged 18 yr and older undergoing non-emergency surgery under general anaesthesia with tracheal intubation with neuromuscular block and reversal, who were predicted to be at elevated risk of postoperative pulmonary complications. This risk was defined as American Society of Anesthesiologists Physical Status 3 or 4 in patients undergoing either intrathoracic or intra-abdominal surgery who were either aged >80 yr or underwent a procedure lasting >2 h. Cohorts were defined by reversal with neostigmine or sugammadex. The primary composite outcome was the occurrence of pneumonia or respiratory failure.
Results
After matching by institution, sex, age (within 5 yr), body mass index, anatomic region of surgery, comorbidities, and neuromuscular blocking agent, 3817 matched pairs remained. The primary postoperative pulmonary complications outcome occurred in 224 neostigmine cases vs 100 sugammadex cases (5.9% vs 2.6%, odds ratio 0.41, P<0.01). After adjustment for unbalanced covariates, the adjusted odds ratio for the association between sugammadex use and the primary outcome was 0.39 (P<0.0001).
Conclusions
In a cohort of patients at increased risk for pulmonary complications compared with neostigmine, use of sugammadex was independently associated with reduced risk of subsequent development of pneumonia or respiratory failure.
Keywords: neostigmine, neuromuscular blocking drug, pneumonia, postoperative pulmonary complications, respiratory failure, reversal of neuromuscular block, sugammadex
Editor's key points.
-
•
A large US electronic health record database was queried to determine the association between use of sugammadex or neostigmine to reverse neuromuscular block with postoperative pulmonary complications in at risk patients.
-
•
In 3817 matched pairs of patients undergoing non-emergent surgery, occurrence of pneumonia or respiratory failure was 2.3-fold greater in patients given neostigmine than sugammadex for reversal of neuromuscular block.
-
•
This multi-institution retrospective cohort study shows that choice of reversal agent might offer an opportunity to improve outcomes in high-risk perioperative care.
Postoperative pulmonary complications (POPCs) affect up to one-third of patients undergoing major noncardiac surgery.1, 2, 3 They prolong length of stay, increase hospital costs, and increase 7-day, 30-day, and long-term mortality.1,2,4 The relationship between patient, procedural, and intraoperative care factors and development of POPCs is well established.5,6 Although current preoperative risk prediction systems allow for identification of high-risk cohorts of patients, there are few options for reducing the risk of POPC.6, 7, 8, 9
Variation in the use of neuromuscular block and its reversal is a modifiable risk factor for POPCs.10, 11, 12, 13, 14, 15 In 2015, sugammadex was introduced to the US market. Sugammadex facilitates rapid recovery from both moderate and deep neuromuscular block, which is unavailable with reversal by acetylcholinesterase inhibitors. Substantial variability in sugammadex use exists.16, 17, 18, 19, 20 Sugammadex is preferentially administered to older patients, and in patients with more comorbidities undergoing more extensive surgery.20 Although sugammadex reduces the incidence of residual neuromuscular block and is associated with decreased risk of development of POPCs in broad patient populations, it is not known if this effect is present in those at increased risk of POPCs.11, 13, 21
The introduction of sugammadex in the USA provides an opportunity to assess whether sugammadex use is associated with reduced risk of POPCs in patients at increased risk based on procedure or patient characteristics. This would represent a potentially modifiable risk factor for POPCs. We hypothesised that in patients at increased risk of POPCs, defined as ASA Physical Status 3 or 4, aged >80 yr undergoing intrathoracic or intra-abdominal surgery, or those who underwent a procedure of >2 h, sugammadex is associated with decreased risk of development of POPCs compared with neostigmine.
Methods
We conducted a retrospective, matched cohort study to assess the association between sugammadex or neostigmine administration and development of POPCs. Data were from the Multicenter Perioperative Outcomes Group (MPOG) electronic health record (EHR) registry.22 A statistical analysis plan specific to this project was developed before data access and was presented to the MPOG Perioperative Clinical Research Committee on January 13, 2020 and finalised on July 27, 2020. Institutional Review Board approval was obtained (HUM00173230, University of Michigan IRB-MED, Ann Arbor, MI, USA, April 6, 2020). The reported work follows the STROBE Reporting Guidelines (Appendix 1).23
Data sources
Data were drawn from the MPOG database. Data are automatically extracted from the source EHR containing all records of anaesthesia care at each of 50 participating institutions across the USA, standardised to a shared lexicon, and transferred to the coordinating centre for analysis after validation.22
The study cohort was derived from the match-eligible population in the previously reported STRONGER study, a retrospective cohort study examining association between choice of reversal agent and development of POPCs.13 The presented increased risk sub-population has not previously been separately described or analysed. Within the match-eligible subjects in the STRONGER cohort, we identified those at increased risk of POPCs to generate the starting population for the present work.
Study design
A matched cohort was constructed using patients who received reversal of neuromuscular block with neostigmine or sugammadex. Approval for marketing of sugammadex in the USA by the Food and Drug Administration was obtained on December 15, 2015. We defined an institution-specific date of introduction based on the first date of documented administration. Patients included in the neostigmine group received neostigmine between January 1, 2014 and the first documented sugammadex administration at that institution. Patients in the sugammadex group were included from 6 months after the first documented use of sugammadex at that institution until August 31, 2018.
Inclusion and exclusion criteria
The inclusion and exclusion criteria were inherited from the STRONGER study, as the starting population was the match-eligible population from this prior study. Patients were eligible for inclusion if: >18 yr of age, underwent general anaesthesia with tracheal intubation, received rocuronium or vecuronium, received reversal with sugammadex (1.8–4.4 mg kg−1 [label dose plus or minus 10%]) or neostigmine, received care at an institution included in the original STRONGER study (requiring participation in MPOG and use of sugammadex for reversal in >10% of cases).13
Patients were excluded if they: were tracheally intubated before arrival in the operating theatre, were transferred directly to the ICU after the procedure, underwent cardiac surgery, underwent lung or liver transplantation surgery, underwent outpatient or emergency surgery, received reversal of neuromuscular block to facilitate neuromonitoring, were ventilated with a median PEEP of ≥10 cm H2O, had a diagnosis of myasthenia gravis or renal failure, received chronic pyridostigmine therapy, received both sugammadex and neostigmine, had missing or incomplete outcome data, or missing or inaccurate intraoperative timestamps.
To arrive at the STIL-STRONGER pre-match population, we retained patients deemed to be at increased risk of POPCs based on the following criteria: ASA physical status 3 or 4, underwent intrathoracic or intra-abdominal surgery, and were either >80 yr of age or underwent a surgical procedure >2 h duration. Additionally, we required a neostigmine dose of 27–77 μg kg−1 (accepted dose plus or minus 10%).
Outcomes
The primary composite outcome was development of a POPC based on the diagnosis of pneumonia or respiratory failure. The secondary outcomes of this study were the component outcomes of pneumonia and respiratory failure alone. We used International Classification of Diseases (ICD) 9 or 10 codes to indicate the presence of pneumonia or respiratory failure. These codes each refer to a specific diagnosis; for example: 481 (ICD-9) or J13 (ICD-10) both refer to pneumococcal pneumonia, or 518.51 (ICD-9) refers to acute respiratory failure after trauma and surgery and J95.821 (ICD-10) refers to acute postprocedural respiratory failure. Pneumonia was defined as the presence of one or more of the following: ICD-9: 481, 482, 482.1, 482.3, 482.4, 482.41, 482.42, 482.82, 482.83, 482.89, 482.9, 486, 483.8, 484.6, 485; ICD-10: J13, J15.0, J15.1, J15.4, J15.20, J15.211, J15.212, J15.5, J15.6, J15.8, J15.9, J18.9, J16.8, B44.0, J18.0. Respiratory failure was defined by the presence of one or more of the following codes ICD-9: 518.51, 518.52, 518.81, 518.82, 518.84; ICD-10: J95.821, J96.00, J95.1, J95.2, J96.00, J96.90, J80, J96.20 (Supplementary Table S1).
Power calculation
Our a priori power calculation was based on a test for two correlated proportions in a matched case-control design implemented in PASS 2019, v19.0.1 software (NCSS Statistical Software, Kaysville, UT UT, USA).24 Based on published risk prediction models considering the anticipated patient population, we assumed the incidence of the primary outcome of 7.5% in the neostigmine group and projected the required sample size based on a range of effect sizes from 20% to 45%.6, 7, 8, 13 With an alpha value of 0.05 and power of 90%, a 35% difference in incidence of primary outcome between the neostigmine and sugammadex groups would require 3571 cases in each group.
Statistical methods
A matched cohort was constructed using a 1:1 match between sugammadex and neostigmine cases. Matching was performed based on institution, sex, age (within 5 yr), ASA physical status, WHO BMI categorisation, anatomic region of surgery, select Elixhauser comorbidities25, 26 (chronic obstructive pulmonary disease, congestive heart failure, paralysis, cardiac arrhythmia, liver disease), and neuromuscular blocking agent (NMBA) used (rocuronium alone vs not-rocuronium alone).
Continuous data were summarised using median and inter-quartile range and categorical data were presented as frequencies and percentages by group. Bivariate unadjusted conditional logistic regression models were used to evaluate differences between patients receiving sugammadex vs neostigmine and the outcomes.
We pre-specified several non-matched variables to assess for difference between the patient groups, including: train-of-four count (TOFc) before reversal, type of general anaesthetic delivered, primary in-room anaesthesia provider, anaesthetic duration, age in years, time from NMBA administration to reversal, time from reversal to extubation, ED95 of the NMBA agents administered, and the presence of an intraoperative blood product transfusion. After matching, to assess imbalance between cohorts, absolute standardised differences (ASD) for pre-specified, potentially confounding, covariates were calculated. Any covariate with an ASD >0.10 was included in multivariable models.
Multivariable conditional logistic regression models were constructed to assess the independent association between reversal agent (sugammadex or neostigmine) and the primary composite outcome of pulmonary complications and secondary component outcomes. Adjusted odds ratios (aORs) with 95% confidence intervals (CIs) were reported for all model covariates. Two-sided hypothesis testing was used. All statistical analyses were performed using SAS® version 9.4 (SAS Institute, Cary, NC, USA). For the primary and secondary outcome models, we calculated the E-value to determine the degree of unmeasured confounding required to change the statistical significance of the outcome presented.27
Sensitivity analyses
This study had several pre-specified sensitivity analyses: (1) Restricting the outcome definition to only include ICD version 9 or 10 (ICD-9/10) codes specific to postsurgical complications, (2) limiting the analysis to cases occurring after the transition to ICD-10 coding (October 2015), (3) restricting the analysis to sugammadex cases occurring within 2 yr of the matched neostigmine case, and (4) examining institution-specific effects by removing each centre from the analysis to ensure that no single institution's data were solely responsible for the presence or absence of an association. We performed two post hoc sensitivity analyses. Firstly, we used a broader ICD-9 and ICD-10 derived definition of POPCs to examine if changes in billing codes impacted the study (Supplementary Table S2). Secondly, we repeated the primary analysis presenting TOFc data without grouping.
Results
Our starting population comprised 119 611 match-eligible cases from the STRONGER study13; 16 042 cases met both the definition of high risk and all other inclusion criteria for the current study (Fig 1). Of these cases, 10 536 received neostigmine and 5506 received sugammadex. After matching, 3817 matched pairs were available (match rate 69.3%). The characteristics of the matched and unmatched patient populations are presented in Table 1. Unmatched covariates were well balanced (Table 2) with ASD >0.1 noted for eight of 40 considered variables.
Fig 1.
Study flow chart describing study population, exclusion criteria, and relationship of this population with the prior STRONGER study. NMB, neuromuscular blocking agent; OR, operating room; ICU, Intensive Care Unit.
Table 1.
Patient and case characteristics used for matching within the matched and unmatched sugammadex cases and neostigmine cases. ∗Age in matched cases refers to the sugammadex patient age, match performed within plus or minus 5 yr. †Because exact matching was used for the parameters presented, the matched characteristics (except age as noted above) do not vary between the patients drawn from neostigmine and sugammadex group and thus a single summary is presented.
| Covariate | Matched cases† |
Unmatched cases |
||||
|---|---|---|---|---|---|---|
| Unmatched sugammadex cases |
Unmatched neostigmine cases |
|||||
|
N=3817 |
N=1689 |
N=6719 |
||||
| N | % | N | % | N | % | |
| Age, yr∗ | ||||||
| 18–40 | 251 | 6.6 | 196 | 11.6 | 597 | 8.9 |
| 41–50 | 408 | 10.7 | 195 | 11.5 | 817 | 12.2 |
| 51–60 | 896 | 23.5 | 341 | 20.2 | 1456 | 21.7 |
| 61–70 | 1251 | 32.8 | 463 | 27.4 | 2099 | 31.2 |
| 71–80 | 773 | 20.3 | 345 | 20.4 | 1150 | 17.1 |
| 81–90+ | 238 | 6.2 | 149 | 8.8 | 600 | 8.9 |
| Sex | ||||||
| Male | 1774 | 46.5 | 799 | 47.3 | 3646 | 54.3 |
| Female | 2043 | 53.5 | 887 | 52.5 | 3066 | 45.6 |
| ASA physical status | ||||||
| 3 | 3784 | 99.1 | 1,528 | 90.5 | 6383 | 95.0 |
| 4 | 33 | 0.9 | 161 | 9.5 | 336 | 5.0 |
| WHO BMI classification | ||||||
| Underweight | 22 | 0.6 | 63 | 3.9 | 160 | 2.6 |
| Normal | 868 | 23.1 | 379 | 23.2 | 1541 | 24.7 |
| Overweight | 1168 | 31.1 | 414 | 25.4 | 1796 | 28.8 |
| Class I | 771 | 20.5 | 360 | 22.0 | 1285 | 20.6 |
| Class II | 429 | 11.4 | 211 | 12.9 | 714 | 11.5 |
| Class III | 501 | 13.3 | 206 | 12.6 | 738 | 11.8 |
| Select Elixhauser comorbidities26 | ||||||
| Cardiac arrhythmias | 453 | 11.9 | 578 | 34.2 | 1279 | 19.0 |
| Chronic pulmonary disease | 579 | 15.2 | 559 | 33.1 | 1621 | 24.1 |
| Congestive heart failure | 50 | 1.3 | 192 | 11.4 | 423 | 6.3 |
| Liver disease | 140 | 3.7 | 232 | 13.7 | 625 | 9.3 |
| Paralysis | 6 | 0.2 | 46 | 2.7 | 65 | 1.0 |
| Body region/type of procedure | ||||||
| Intrathoracic noncardiac | 454 | 11.9 | 432 | 25.6 | 1,390 | 20.7 |
| Abdominal | 3363 | 88.1 | 1257 | 74.4 | 5329 | 79.3 |
| Agent used for neuromuscular block | ||||||
| Rocuronium only | 2849 | 74.6 | 1093 | 64.7 | 5239 | 78.0 |
| Vecuronium or (rocuronium and vecuronium) |
968 |
25.4 |
596 |
35.3 |
1480 |
22.0 |
|
Median |
IQR |
Median |
IQR |
Median |
IQR |
|
| Age, yr | 63 | [54–71] | 63 | [52–72] | 63 | [53–71] |
| BMI, kg m−2 | 29 | [25–35] | 30 | [25–35] | 29 | [25–35] |
IQR, inter-quartile range.
Table 2.
Patient and case characteristics of non-matched covariates after matching sugammadex cases and neostigmine cases. ∗Pre-specified potentially confounding variables with absolute standardised difference >0.1 which were subsequently included in the conditional logistic regression model. †To meet study inclusion criteria, sugammadex dose must have been 1.8–4.4 mg kg−1. ‡To meet study inclusion criteria, neostigmine dose must have been 27–77 μg kg−1. AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus; IQR, inter-quartile range; NMBA, neuromuscular blocking agent.
| Sugammadex cases N=3817 |
Neostigmine cases N=3817 |
Absolute standardised difference | |||
|---|---|---|---|---|---|
| N | N | ||||
| Last train-of-four count documented within 30 min of NMBA reversal∗ | 0.12 | ||||
| Not documented | 774 | 20.2 | 876 | 22.9 | |
| Zero or one twitch | 646 | 16.9 | 316 | 8.3 | |
| Two twitches | 537 | 14.1 | 339 | 8.9 | |
| Three or four twitches or sustained tetany | 1860 | 48.7 | 2286 | 59.9 | |
| General anaesthesia (GA) technique | 0.09 | ||||
| General anaesthetic with volatile agent | 3730 | 97.7 | 3774 | 98.9 | |
| General anaesthetic without volatile or nitrous oxide | 80 | 2.1 | 37 | 1.0 | |
| General anaesthetic with nitrous oxide | 7 | 0.2 | 6 | 0.2 | |
| Other Elixhauser comorbidities | |||||
| AIDS/HIV | 8 | 0.2 | 4 | 0.1 | 0.03 |
| Alcohol abuse∗ | 10 | 0.3 | 48 | 1.3 | 0.12 |
| Blood loss anaemia | 67 | 1.8 | 40 | 1.0 | 0.06 |
| Coagulopathy | 116 | 3.0 | 100 | 2.6 | 0.03 |
| Deficiency anaemia | 151 | 4.0 | 105 | 2.8 | 0.07 |
| Depression | 398 | 10.4 | 500 | 13.1 | 0.08 |
| Diabetes mellitus (complicated) | 53 | 1.4 | 36 | 0.9 | 0.04 |
| Diabetes mellitus (uncomplicated) | 709 | 18.6 | 700 | 18.3 | 0.01 |
| Drug abuse | 62 | 1.6 | 59 | 1.5 | 0.01 |
| Fluid/electrolyte disorders | 414 | 10.8 | 447 | 11.7 | 0.03 |
| Hypertension (complicated)∗ | 61 | 1.6 | 21 | 0.6 | 0.10 |
| Hypertension (uncomplicated) | 2094 | 54.9 | 2112 | 55.3 | 0.01 |
| Hypothyroidism | 442 | 11.6 | 469 | 12.3 | 0.02 |
| Lymphoma | 35 | 0.9 | 44 | 1.2 | 0.02 |
| Metastatic cancer | 736 | 19.3 | 699 | 18.3 | 0.03 |
| Other neurological disorders | 93 | 2.4 | 111 | 2.9 | 0.03 |
| Peptic ulcer disease, excluding bleeding | 46 | 1.2 | 48 | 1.3 | 0.01 |
| Peripheral vascular disorder | 171 | 4.5 | 172 | 4.5 | 0.00 |
| Psychosis | 14 | 0.4 | 26 | 0.7 | 0.04 |
| Pulmonary circulation disorders | 92 | 2.4 | 93 | 2.4 | 0.00 |
| Rheumatoid arthritis collagen vascular diseases | 91 | 2.4 | 89 | 2.3 | 0.00 |
| Solid tumour without metastasis∗ | 2198 | 57.6 | 1607 | 42.1 | 0.31 |
| Valvular disease | 118 | 3.1 | 132 | 3.5 | 0.02 |
| Weight loss | 297 | 7.8 | 329 | 8.6 | 0.03 |
| Primary in-room provider∗ | 0.11 | ||||
| Faculty only | 156 | 4.1 | 101 | 2.6 | |
| Resident/fellow | 2029 | 53.2 | 2199 | 57.6 | |
| Nurse anaesthetist | 1631 | 42.7 | 1516 | 39.7 | |
| Estimated blood loss (ml) | 0.09 | ||||
| 0–500 | 3429 | 89.8 | 3322 | 87.0 | |
| 501–1000 | 260 | 6.8 | 330 | 8.6 | |
| >1000 | 128 | 3.4 | 165 | 4.3 | |
| Surgical duration | 0.02 | ||||
| ≥2 h | 3710 | 97.2 | 3699 | 96.9 | |
| <2 h | 107 | 2.8 | 118 | 3.1 | |
| Sugammadex dosing range† | N/A | ||||
| 1.8–2.2 mg kg−1 | 2231 | 58.4 | N/A | ||
| >2.2 AND <3.6 mg kg−1 | 1108 | 29.0 | |||
| 3.6–4.4 mg kg−1 | 478 | 12.5 | |||
| Neostigmine dosing range‡ | N/A | ||||
| 27–39.9 μg kg−1 | N/A | 1296 | 34.0 | ||
| 40–60 μg kg−1 | 2046 | 53.6 | |||
| 60.1–77 μg kg−1 |
475 |
12.4 |
|||
|
Median |
IQR |
Median |
IQR |
Absoloute Standardised difference |
|
| Surgical duration, h | 3.3 | [2.5–4.5] | 3.3 | [2.5–4.5] | 0.01 |
| Fluid balance, ml kg−1 h−1∗ | 3.1 | [1.8–4.6] | 3.6 | [2.2–5.5] | 0.21 |
| Intraoperative packed red blood cell transfusions (units) | 0 | [0–0] | 0 | [0–0] | 0.02 |
| Intraoperative fresh frozen plasma transfusions (units) | 0 | [0–0] | 0 | [0–0] | 0.01 |
| Oral morphine equivalent, mg kg−1 h−1∗ | 0.23 | [0.16–0.31] | 0.26 | [0.19–0.36] | 0.26 |
| Median ventilator driving pressure | 16 | [13–21] | 17 | [13–22] | 0.06 |
| Age (yr) | 64 | [54–71] | 63 | [54–71] | 0.02 |
| Time from last NMBA dose to first reversal (min) | 60 | [42–88] | 61 | [42–87] | 0.00 |
| Time from first reversal to extubation (min)∗ | 13 | [7–21] | 17 | [11–26] | 0.34 |
| Time from last NMBA to extubation (min) | 75 | [56–105] | 81 | [60–109] | 0.09 |
| ED95 per hour of NMBA∗ | 1.3 | [1.1–1.7] | 1.2 | [1.0–1.6] | 0.12 |
Population description
Within the 3817 matched pairs, the median age was 63 yr (6.2% >80 yr), 88.1% of patients underwent abdominal surgery, 99.1% were ASA physical status 3, 45.2% were obese (BMI > 30 kg m−2), and 74.6% received muscle relaxation with rocuronium only. Within the sugammadex group, 58.4% received a dose of 1.8–2.2 mg kg−1 (consistent with reversal of moderate neuromuscular block) and 12.5% received a dose of 3.6–4.4 mg kg−1 (consistent with reversal of deeper neuromuscular block). Although neostigmine dose selection is based on clinical judgement, 34.0% received a dose between 27 and 40 μg kg−1 and 12.4% received a dose between 60 and 77 μg kg−1. The total administered dose was documented in more than one dose in 71 (1.9%) patients in the sugammadex group and 206 (5.3%) patients in the neostigmine group.
Primary and secondary outcomes
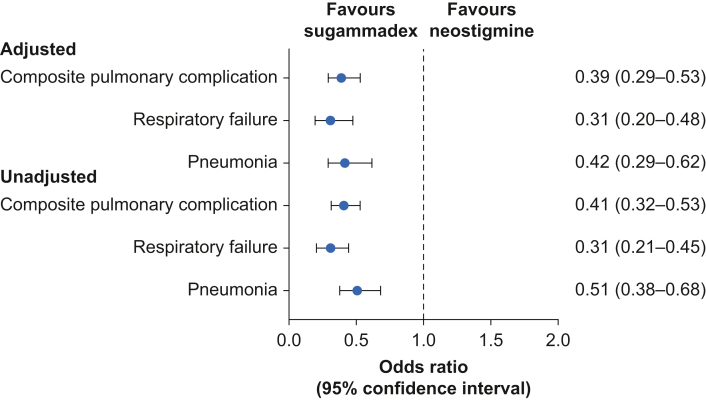
The primary outcome occurred in 100/3817 sugammadex cases and 224/3817 neostigmine cases (2.6% vs 5.9%, unadjusted odds ratio 0.41, P<0.0001, Supplementary Table S4). After adjustment for unbalanced covariates (Fig 2), the aOR for the association between sugammadex use and the primary composite outcome of POPCs was 0.39 (95% CI: 0.29–0.53, P<0.0001, Table 3), and for the secondary outcome of respiratory failure was 0.31 (95% CI: 0.20–0.48, P<0.0001 Supplementary Table S5) and of pneumonia was 0.42 (95% CI: 0.29–0.62, P<0.0001, Supplementary Table S6). The calculated E-value for the primary outcome was 4.57 and for secondary outcomes was 5.91 for pneumonia and 4.19 for respiratory failure.
Fig 2.
Adjusted odds ratio (with confidence intervals) for primary and secondary outcomes.
Table 3.
Adjusted conditional logistic regression assessing composite postoperative pulmonary complications in sugammadex cases vs matched neostigmine cases, N=7588. Calibration: Hosmer-Lemeshow P-value= 0.4299.
| Adjusted odds ratio | 95% lower bound | 95% upper bound | P-value | |
|---|---|---|---|---|
| Reversal category | ||||
| Sugammadex | 0.39 | 0.29 | 0.53 | <0.0001 |
| Neostigmine | 1.00 | Reference | Reference | Reference |
| Last train-of-four count documented within 30 min of reversal | ||||
| Not documented | 1.56 | 0.96 | 2.54 | 0.07 |
| Zero or one twitch | 1.70 | 0.89 | 3.23 | 0.11 |
| Two twitches | 1.38 | 0.70 | 2.70 | 0.35 |
| Three or four twitches or sustained tetany | 1.00 | Reference | Reference | Reference |
| Other Elixhauser comorbidities | ||||
| Alcohol abuse | 0.50 | 0.15 | 1.64 | 0.25 |
| Hypertension (complicated) | 2.27 | 0.65 | 7.98 | 0.20 |
| Solid tumour without metastasis | 0.62 | 0.41 | 0.95 | 0.03 |
| Primary in-room provider | ||||
| Faculty only | 1.00 | Reference | Reference | Reference |
| Resident/fellow | 0.80 | 0.24 | 2.71 | 0.72 |
| Nurse anaesthetist | 0.64 | 0.19 | 2.17 | 0.47 |
| Fluid balance, ml kg−1 h−1 | 1.06 | 1.00 | 1.13 | 0.07 |
| Oral morphine equivalent, mg kg−1 h−1 | 1.05 | 0.26 | 4.21 | 0.94 |
| Time from first reversal to tracheal extubation (5 min interval) | 1.09 | 1.01 | 1.18 | 0.03 |
| ED95 per hour | 0.96 | 0.65 | 1.42 | 0.84 |
Sensitivity analyses
In the preplanned sensitivity analyses, the association remained both when diagnosis codes specific to postsurgical complications were utilised (aOR 0.52 [0.30–0.90], P=0.02, Supplementary Table S7), and when, to limit influence of any secular trend in POPCs, matching was restricted to cases occurring within 24 months (n=4438; aOR 0.42 [95% CI: 0.28–0.63], P<0.001, Supplementary Table S7). However, in the small number of cases where the neostigmine and sugammadex cases both occurred after the coding system change in October 2015, the association was not detected (n=1952; aOR 1.04 [95% CI: 0.50–2.15], P=0.92, Supplementary Table S7). Removal of one institution at a time did not change the primary association (Supplementary Table S8). In the first post hoc sensitivity analysis, the association was present when using a more expansive definition of the primary outcome (aOR 0.60 [95% CI: 0.47–0.77], P<0.001, Supplementary Table S7). In the second, the association also remained when TOFc was analysed without grouping (aOR 0.40 [95% CI: 0.29–0.54], P<0.0001, Supplementary Table S9).
Discussion
In this multi-institution retrospective cohort study of patients at increased risk of POPCs, the use of sugammadex compared with neostigmine for reversal of neuromuscular block was associated with decreased odds of developing a composite of POPCs (respiratory failure or pneumonia). Our study population is commonly encountered during in-patient practice and is at increased risk based on scoring systems. Choice of reversal agent might offer an opportunity to improve outcomes in high-risk perioperative care.
The incidence of pneumonia and respiratory failure in our high-risk population was ∼60% higher than in the previous STRONGER study.13 Although this study focused on more severe pulmonary complications, the similar results suggest that prior findings were not solely attributable to a differential effect within surgical populations. The findings are consistent with observational studies examining the impact of sugammadex use in pulmonary outcomes in a single-centre interrupted-time-series analysis and other studies in patients undergoing laparoscopic gastrectomy and prostatectomy.28, 29, 30, 31 Three small RCTs have examined the impact of sugammadex on pulmonary outcomes: one showed reduction in early desaturation in thoracic surgery patients, a second did not detect a difference in the incidence of POPCs in an older population but was underpowered to detect differences in severe complications, and a third reported a lower incidence of pneumonia, but not a large composite POPC outcome.32, 33, 34 A recent single-centre retrospective cohort study by Li and colleagues35 and colleagues associated a decline in POPC risk with a secular trend coincident with the introduction of sugammadex. However, the study did not use exact matching and lacked key intraoperative data regarding depth of neuromuscular block for the majority of patients. Our study did not seek to elucidate any possible relationship between tracheal intubation itself and pulmonary outcomes,36, 37, 38 as we included only those with both neuromuscular block and tracheal intubation.
Limitations
Despite the inclusion of a staggered, institution-specific transition date from neostigmine to sugammadex use and a sensitivity analysis examining matched pairs occurring within 24 months, which is consistent with the primary analysis, the possibility of an unmeasured secular trend in the incidence of postoperative respiratory failure and pneumonia remains. We deliberately performed a comparison of practice before and after sugammadex became available at each institution to minimise confounding. Prior work has shown that use of sugammadex varies substantially across institutions (2–80%).20 Therefore, we feel that contemporaneous comparisons would have included practice differences that could not be accounted for.
The possibility of other residual unmeasured confounding and unaccounted treatment biases remains. The E-value of 4.57 (the magnitude of the effect of an unmeasured confounder required to alter the significance of the reported association) for the primary outcome argues against this.
Our dataset included TOFc and not quantitatively derived TOF ratio. It is possible that differences may have existed between groups if quantitative neuromuscular monitoring had been used. Additionally, we identified practices that might contribute to incomplete reversal in the neostigmine groups, including apparent use of neostigmine for reversal of deep neuromuscular block or short time intervals between administration of reversal agent and extubation. This, however, reflects documented practice in our US hospital sample. In this study, the intrinsic characteristics of the medication and the manner in which it was used were tested simultaneously, as these may combine to impact outcome. Although this may introduce bias, when compared with a controlled prospective protocol it improves the generalisability of our results because it comments on how these agents are actually used. Our choice of outcome in this study was (1) designed to focus on major complications mechanistically related to neuromuscular block, (2) well defined within administrative data, and (3) aligned where possible with consensus definition.39
In deciding the study population, we attempted to balance a population broad enough to draw generalisable conclusions with one that was sufficiently at risk for the primary outcome, as our study includes patients with or at risk of significant comorbidities (ASA physical status 3, 4 or age >80 yr) presenting for major inpatient surgeries (abdominal or thoracic surgery requiring admission with a duration >2 h). Whereas we restricted the agent to clinically relevant doses of neostigmine and sugammadex, we did not assess if the administered dose was appropriate for the clinical circumstance. Our study population makes up a minority of clinical practice, but is at substantial risk for development of POPCs and is frequently encountered by practitioners working in in-patient settings. We used matching in an attempt to create two similar study groups. Residual differences in important pre-specified variables were adjusted for in our statistical model. Alternative matching methods (including 1:n approaches) might have resulted in a different study population.
As a result of changes in US federal regulations, billing coding changed from ICD-9 to ICD-10 during our study period. We attempted to assess the impact of this in a pre-planned sensitivity analysis, but because of the small number of patients in this subanalysis, it cannot be fully excluded. Billing codes are widely used in observational research but may be subject to inaccuracies when compared with clinician adjudicated outcomes.
Conclusions
In ASA physical status 3 or 4 patients undergoing intrathoracic or intra-abdominal surgery and who were aged >80 yr or underwent a procedure >2 h, reversal of neuromuscular block with sugammadex is independently associated with reduced odds of development of a composite of major POPCs. Decisions about the choice of reversal agent could provide anaesthesiologists an opportunity to impact this outcome.
Authors' contributions
Study design: DAC, MTV, LDB, AJ, GM
Primary interpretation of the analysis, primary drafter of the manuscript: DAC
Final approval of the published version: DAC, SK
Analysis of study data, drafted elements of the manuscript: MTV
Critically revised the manuscript: MTV, LDB, AJ, NS, AG, MS, GM, SK
Approved the final version to be published: MTV, LDB, AJ, NS, AG, MS, GM
Interpretation of results: AJ, NS, AG, MS
Assisted in the analysis: GM
Assisted in the development of the primary interpretation of the results, critically reviewed the manuscript: SK
Acknowledgements
The authors gratefully acknowledge the work of Michelle Romanowski in contributing to the data extraction from the Multicenter Perioperative Outcomes Group database and David Clark and Rachel Hurwitz for administrative support in the development of the manuscript. The authors gratefully acknowledge the valuable contributions to protocol and final manuscript review by the Multicenter Perioperative Outcomes Group Perioperative Clinical Research Committee (non-Author Collaborator Group).
Non-author collaborators
Robert Craft, University of Tennessee, Knoxville, TN, USA
Karen B. Domino, University of Washington, Seattle, WA, USA
Robert E. Freundlich, Vanderbilt University Medical Center, Nashville, TN, USA
Michael R. Mathis, University of Michigan, Ann Arbor, MI, USA
Patrick J. McCormick, Memorial Sloan Kettering Cancer Center, New York, NY, USA
Bhiken I. Naik, University of Virginia, Charlottesville, VA, USA
Joseph Ruiz, MD Anderson Cancer Center, Houston, TX, USA
Robert B. Schonberger, Yale School of Medicine, New Haven, CT, USA
Rebecca A. Schroeder, Duke University Medical Center, Durham, NC, USA
Alvin F. Stewart, University of Arkansas for Medical Sciences, Little Rock, AR, USA
Brad M. Taicher, Duke University Medical Center, Durham, NC, USA
Sarah Tingle, MD, University of Arkansas for Medical Sciences, Little Rock, AR, USA
Brandon Michael Togioka, Oregon Health & Science University, Portland, OR, USA
Richard Urman, Brigham and Women's Hospital, Boston, MA, USA
Shital Vachhani, MD Anderson Cancer Center, Houston, TX, USA
Handling editor: Hugh C Hemmings Jr
Footnotes
This article is accompanied by an editorial: How robust are the STRONGER and STIL-STRONGER studies? by Blobner et al., Br J Anaesth 2023:130:e41–e44, doi: 10.1016/j.bja.2022.08.021
Presented in part at the Association of University Anesthesiologists and International Anesthesia Research Society meetings, held virtually May 13–16, 2021.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2022.04.023.
Contributor Information
Douglas A. Colquhoun, Email: dougcolq@med.umich.edu.
Multicenter Perioperative Outcomes Group (MPOG) Perioperative Clinical Research Committee:
Robert Craft, Karen B. Domino, Robert E. Freundlich, Michael R. Mathis, Patrick J. McCormick, Bhiken I. Naik, Joseph Ruiz, Robert B. Schonberger, Rebecca A. Schroeder, Alvin F. Stewart, Brad M. Taicher, Sarah Tingle, Brandon Michael Togioka, Richard Urman, and Shital Vachhani
Declarations of interest
DAC, NS, GM, SK and MTV declare support from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., (Rahway, NJ, USA) paid to the University of Michigan. LDB is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., which manufactures and distributes sugammadex. LDB declares stock/stock options in Merck & Co. NS and SK declare support paid to the University of Michigan from Apple Inc. (Cupertino, CA, USA) unrelated to the current work. NS declares support paid to the University of Michigan from Edwards Lifesciences (Irvine, CA, USA) unrelated to the current work. AJ declares support paid to the University of Michigan from Becton, Dickinson & Company (Franklin Lakes, NJ, USA), unrelated to the current work.
Funding
Partial funding by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., (Rahway, NJ, USA) to the University of Michigan. Research reported in this publication was supported by US National Institute of General Medical Sciences and National Heart, Lung, and Blood Institute of the National Institutes of Health (Bethesda, MD, USA) under award numbers T32GM103730 (AJ) and K08HL159327 (DAC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Partial funding by a Mentored Research Training Grant from Foundation for Anesthesia Education and Research (FAER) to DAC. Partial funding by departmental and institutional resources at each contributing site.
Partial funding to the Multicenter Perioperative Outcomes Group registry by Blue Cross Blue Shield of Michigan/Blue Care Network as part of the Blue Cross Blue Shield of Michigan/Blue Care Network Value Partnerships program. Although Blue Cross Blue Shield of Michigan/Blue Care Network and Multicenter Perioperative Outcomes Group work collaboratively, the opinions, beliefs and viewpoints expressed by the authors do not necessarily reflect the opinions, beliefs, and viewpoints of Blue Cross Blue Shield of Michigan/Blue Care Network or any of its employees.
The study protocol and statistical analysis plan were reviewed and approved a priori by the Multicenter Perioperative Outcomes Group publications committee, an academic entity independent of funding sources and free from industry and funder involvement.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Fernandez-Bustamante A., Frendl G., Sprung J., et al. Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter study by the perioperative research network investigators. JAMA Surg. 2017;152:157–166. doi: 10.1001/jamasurg.2016.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vegas investigators L.A.S. Epidemiology, practice of ventilation and outcome for patients at increased risk of postoperative pulmonary complications: LAS VEGAS – an observational study in 29 countries. Eur J Anaesthesiol. 2017;34:492–507. doi: 10.1097/EJA.0000000000000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleisher L.A., Linde-Zwirble W.T. Incidence, outcome, and attributable resource use associated with pulmonary and cardiac complications after major small and large bowel procedures. Perioper Med (Lond) 2014;3:7. doi: 10.1186/2047-0525-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khuri S.F., Henderson W.G., DePalma R.G., et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–341. doi: 10.1097/01.sla.0000179621.33268.83. ; discussion 341–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neto A.S., da Costa L.G.V., Hemmes S.N.T., et al. The LAS VEGAS risk score for prediction of postoperative pulmonary complications: an observational study. Eur J Anaesthesiol. 2018;35:691–701. doi: 10.1097/EJA.0000000000000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canet J., Gallart L., Gomar C., et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113:1338–1350. doi: 10.1097/ALN.0b013e3181fc6e0a. [DOI] [PubMed] [Google Scholar]
- 7.Gupta H., Gupta P.K., Schuller D., et al. Development and validation of a risk calculator for predicting postoperative pneumonia. Mayo Clin Proc. 2013;88:1241–1249. doi: 10.1016/j.mayocp.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 8.Gupta H., Gupta P.K., Fang X., et al. Development and validation of a risk calculator predicting postoperative respiratory failure. Chest. 2011;140:1207–1215. doi: 10.1378/chest.11-0466. [DOI] [PubMed] [Google Scholar]
- 9.Arozullah A.M., Khuri S.F., Henderson W.G., Daley J. Participants in the National Veterans Affairs Surgical Quality Improvement Program. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann Intern Med. 2001;135:847–857. doi: 10.7326/0003-4819-135-10-200111200-00005. [DOI] [PubMed] [Google Scholar]
- 10.Kumar G.V., Nair A.P., Murthy H.S., Jalaja K.R., Ramachandra K., Parameshwara G. Residual neuromuscular blockade affects postoperative pulmonary function. Anesthesiology. 2012;117:1234–1244. doi: 10.1097/ALN.0b013e3182715b80. [DOI] [PubMed] [Google Scholar]
- 11.Ledowski T., Falke L., Johnston F., et al. Retrospective investigation of postoperative outcome after reversal of residual neuromuscular blockade: sugammadex, neostigmine or no reversal. Eur J Anaesthesiol. 2014;31:423–429. doi: 10.1097/EJA.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 12.Kirmeier E, Eriksson Li, Lewald H, et al. Post-anaesthesia pulmonary complications after use of muscle relaxants (POPULAR): a multicentre, prospective observational study. Lancet Respir Med. 2019;7 doi: 10.1016/S2213-2600(18)30294-7. 129–40. [DOI] [PubMed] [Google Scholar]
- 13.Kheterpal S., Vaughn M.T., Dubovoy T.Z., et al. Sugammadex versus neostigmine for reversal of neuromuscular blockade and postoperative pulmonary complications (STRONGER): a multicenter matched cohort analysis. Anesthesiology. 2020;132:1371–1381. doi: 10.1097/ALN.0000000000003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grosse-Sundrup M., Henneman J.P., Sandberg W.S., et al. Intermediate acting non-depolarizing neuromuscular blocking agents and risk of postoperative respiratory complications: prospective propensity score matched cohort study. BMJ. 2012;345:e6329. doi: 10.1136/bmj.e6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLean D.J., Diaz-Gil D., Farhan H.N., Ladha K.S., Kurth T., Eikermann M. Dose-dependent association between intermediate-acting neuromuscular-blocking agents and postoperative respiratory complications. Anesthesiology. 2015;122:1201–1213. doi: 10.1097/ALN.0000000000000674. [DOI] [PubMed] [Google Scholar]
- 16.Hurford W.E., Welge J.A., Eckman M.H. Sugammadex versus neostigmine for routine reversal of rocuronium block in adult patients: a cost analysis. J Clin Anesth. 2020;67:110027. doi: 10.1016/j.jclinane.2020.110027. [DOI] [PubMed] [Google Scholar]
- 17.Drzymalski D.M., Schumann R., Massaro F.J., Trzcinka A., Azocar R.J. Effect of a cognitive aid on reducing sugammadex use and associated costs: a time series analysis. Anesthesiology. 2019;131:1036–1045. doi: 10.1097/ALN.0000000000002946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orihara M., Takazawa T., Horiuchi T., et al. Comparison of incidence of anaphylaxis between sugammadex and neostigmine: a retrospective multicentre observational study. Br J Anaesth. 2020;124:154–163. doi: 10.1016/j.bja.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Meistelman C., Donati F. Do we really need sugammadex as an antagonist of muscle relaxants in anesthesia? Curr Opin Anaesthesiol. 2016;29:462–467. doi: 10.1097/ACO.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 20.Dubovoy T.Z., Saager L., Shah N.J., et al. Utilization patterns of perioperative neuromuscular blockade reversal in the United States: a retrospective observational study from the Multicenter Perioperative Outcomes Group. Anesth Analg. 2020;131:1510–1519. doi: 10.1213/ANE.0000000000005080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hristovska A.-M., Duch P., Allingstrup M., Afshari A. Efficacy and safety of sugammadex versus neostigmine in reversing neuromuscular blockade in adults. Cochrane Database Syst Rev. 2017;8:CD012763. doi: 10.1002/14651858.CD012763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colquhoun D.A., Shanks A.M., Kapeles S.R., et al. Considerations for integration of perioperative electronic health records across institutions for research and quality improvement: the approach taken by the Multicenter Perioperative Outcomes Group. Anesth Analg. 2020;130:1133–1146. doi: 10.1213/ANE.0000000000004489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Elm E., Altman D.G., Egger M., et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dupont W.D. Power calculations for matched case-control studies. Biometrics. 1988;44:1157–1168. [PubMed] [Google Scholar]
- 25.Li B., Evans D., Faris P., Dean S., Quan H. Risk adjustment performance of Charlson and Elixhauser comorbidities in ICD-9 and ICD-10 administrative databases. BMC Health Serv Res. 2008;8:12. doi: 10.1186/1472-6963-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 27.VanderWeele T.J., Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 28.Krause M., McWilliams S.K., Bullard K.J., et al. Neostigmine versus sugammadex for reversal of neuromuscular blockade and effects on reintubation for respiratory failure or newly initiated noninvasive ventilation: an interrupted time series design. Anesth Analg. 2020;131:141–151. doi: 10.1213/ANE.0000000000004505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han J., Ryu J.-H., Koo B.-W., Nam S.W., Cho S.-I., Oh A.-Y. Effects of sugammadex on post-operative pulmonary complications in laparoscopic gastrectomy: a retrospective cohort study. J Clin Med. 2020;9:1232. doi: 10.3390/jcm9041232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Min B.-H., Oh T.K., Song I.-A., Jeon Y.-T. Comparison of the effects of sugammadex and neostigmine on hospital stay in robot-assisted laparoscopic prostatectomy: a retrospective study. BMC Anesthesiol. 2020;20:178. doi: 10.1186/s12871-020-01088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu J., Park J.-Y., Lee Y., Hwang J.-H., Kim Y.-K. Sugammadex versus neostigmine on postoperative pulmonary complications after robot-assisted laparoscopic prostatectomy: a propensity score-matched analysis. J Anesth. 2021;35:262–269. doi: 10.1007/s00540-021-02910-2. [DOI] [PubMed] [Google Scholar]
- 32.Moon T.S., Reznik S., Pak T., et al. Sugammadex versus neostigmine for reversal of rocuronium-induced neuromuscular blockade: a randomized, double-blinded study of thoracic surgical patients evaluating hypoxic episodes in the early postoperative period. J Clin Anesth. 2020;64:109804. doi: 10.1016/j.jclinane.2020.109804. [DOI] [PubMed] [Google Scholar]
- 33.Togioka B.M., Yanez D., Aziz M.F., Higgins J.R., Tekkali P., Treggiari M.M. Randomised controlled trial of sugammadex or neostigmine for reversal of neuromuscular block on the incidence of pulmonary complications in older adults undergoing prolonged surgery. Br J Anaesth. 2020;124:553–561. doi: 10.1016/j.bja.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Ledowski T., Szabó-Maák Z., Loh P.S., et al. Reversal of residual neuromuscular block with neostigmine or sugammadex and postoperative pulmonary complications: a prospective, randomised, double-blind trial in high-risk older patients. Br J Anaesth. 2021;127:316–323. doi: 10.1016/j.bja.2021.04.026. [DOI] [PubMed] [Google Scholar]
- 35.Li G., Freundlich R.E., Gupta R.K., et al. Postoperative pulmonary complications’ association with sugammadex versus neostigmine. Anesthesiology. 2021;134:862–873. doi: 10.1097/ALN.0000000000003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Habre W., Disma N., Virag K., et al. Incidence of severe critical events in paediatric anaesthesia (APRICOT): a prospective multicentre observational study in 261 hospitals in Europe. Lancet Respir Med. 2017;5:412–425. doi: 10.1016/S2213-2600(17)30116-9. [DOI] [PubMed] [Google Scholar]
- 37.Hammer M., Santer P., Schaefer M.S., et al. Supraglottic airway device versus tracheal intubation and the risk of emergent postoperative intubation after general anaesthesia in adults: a retrospective cohort study. Br J Anaesth. 2021;126:738–745. doi: 10.1016/j.bja.2020.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blobner M., Hunter J.M. Supraglottic airway, tracheal intubation, and neuromuscular block: will the ménage à trois endure? Br J Anaesth. 2021;127:174–177. doi: 10.1016/j.bja.2021.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Abbott T.E.F., Fowler A.J., Pelosi P., et al. A systematic review and consensus definitions for standardised end-points in perioperative medicine: pulmonary complications. Br J Anaesth. 2018;120:1066–1079. doi: 10.1016/j.bja.2018.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.