Abstract
Low‐beta (13–23 Hz) event‐related desynchrony (ERD), a neural signature of expressive language, lateralizes from bilateral to left hemisphere in development. In contrast, low‐beta event‐related synchrony (ERS), thought to reflect inhibition, lateralizes from bilateral to the right hemisphere across development. Using whole‐brain directed connectivity analyses, we aimed to characterize hemispheric and regional contributions to expressive language, in childhood. We studied 80 children and adolescents, 4 to less than 19 years of age, performing covert auditory verb generation in magnetoencephalography. Outdegree, indegree, and betweenness centrality were used to differentiate regions acting as drivers, receivers, and bridging hubs, respectively. The number of suprathreshold connections significantly increased with age for delta band (p < .01). Delta outflow was mapped to left inferior frontal gyrus (IFG), while regions of right hemisphere, including right IFG, showed significant inflow. The right parietal cortex showed significant ERS, but without corresponding outdegree or indegree. Betweenness mapped to midline cortical and subcortical structures. Results suggest Broca's area develops a driving role in the language network, while Broca's homologue receives information without necessarily propagating it. Subcortical and midline hubs act as intrahemispheric relays. Findings suggest that Broca's homologue is inhibited during expressive language, in development.
Keywords: brain, development, information flow, language, MEG
Asymmetric information flow underlies language lateralization in children and adults. The left hemisphere increasingly drives the language networks, while the right hemisphere appears to suppress information flow.
1. INTRODUCTION
The left perisylvian cortex supports gross language function in healthy adults (Friederici, 2011; Friederici, 2012; Price, 2000; Price, 2010). However, neuroimaging has shown contributions from outside the canonical language network, including bilateral middle frontal gyrus (MFG), left middle temporal gyrus (MTG), left inferior temporal gyrus (ITG), bilateral precentral gyrus, bilateral angular gyrus, and cerebellum (Binder et al., 1997; Gabrieli et al., 1998; Knecht et al., 2000; Verly et al., 2014). These findings indicate an extended language network beyond classical Broca's and Wernicke's areas, even in healthy adults. Here, we aim to characterize patterns of information flow in language regions that exhibit increased neural engagement (beta desynchrony) and regions showing decreased engagement (beta synchrony, potentially a marker of inhibition), in children performing verb generation (Sharma et al., 2021). This may differentiate critical language drivers from noncritical, which is applicable to presurgical language mapping in pediatric epilepsy. Characterizing the role of tissue and understanding how information is propagated within the distributed network allows us to gain better understanding of the relevant functional neuroanatomy.
Developmental changes in language representation have been studied using functional magnetic resonance imaging (fMRI) and magnetoencephalography (MEG). Using fMRI, studies show that language functions shift from a bilateral and diffuse to left lateralized and focal, in development (Berl et al., 2014; Holland et al., 2001; Holland et al., 2007; Olulade et al., 2020; Pang et al., 2011). MEG research has confirmed this trajectory by tracking the distribution of spectral power changes for language, in development (Kadis et al., 2011; Ressel et al., 2008; Sharma et al., 2021). Specifically, low‐beta (13–23 Hz) event‐related desynchrony (ERD), an index of neural engagement (Crone et al., 1998; Pfurtscheller & Lopes da Silva, 1999; Pfurtscheller & Lopes da Silva, 2017), transitions from bilateral in young children to left lateralized by adolescence or early adulthood.
Recently, we tracked the developmental trajectory for a complementary signal—low‐beta event‐related synchrony (ERS), an index of neural inhibition (Klimesch et al., 2001; Neuper et al., 2006; Pfurtscheller, 1997; Singer, 1999)—in children performing verb generation in MEG. We found that low‐beta ERS becomes increasingly right lateralized through childhood (Sharma et al., 2021). However, the right hemisphere is dominant in only a small minority of healthy adults (Knecht et al., 2000), and sites exhibiting low‐beta ERS (right inferior frontal gyrus (IFG), MFG, and angular gyrus) are usually not considered critical for expressive or receptive language, in that lesions to these regions do not lead to gross language deficits, or aphasias. Rather, lesion studies show that the right hemisphere is important for prosody (Patel et al., 2018; Weintraub et al., 1981). Right hemisphere injury is associated with deficits processing intonation (Ross & Mesulam, 1979), pragmatics (Joanette & Anslado, 1999; Kuperberg et al., 2003; Myers, 2001; Paradis, 1998), discourse maintenance (Brookshire & Nicholas, 1993; Myers, 1999), metaphor comprehension (Bryan, 1994), and synonymous association (Kay et al., 1992). This is consistent with fMRI studies showing increased activation during prosodic discrimination (Vannest et al., 2009). We suggest that these aspects of language may involve higher cognitive processes and possibly a combination of domain‐specific (e.g., semantic) and domain‐general (e.g., attention) functions.
The right hemisphere has been found to support expressive language in people with aphasia after left perisylvian or subcortical stroke; this right lateralization may decrease over the course of recovery (Heiss et al., 1999), or may continue as a compensatory mechanism for when left hemisphere damage is extensive. There is also evidence that inhibitory transcranial magnetic stimulation of the right IFG benefits post‐stroke recovery (Haghighi et al., 2018; Hu et al., 2018; Ren et al., 2019), suggesting functional benefit or support from disengagement of the right hemisphere. Further, the right IFG shows greater engagement in both positron emission tomography and fMRI studies of people with aphasia compared to healthy controls, suggesting possible maladaptive disinhibition of right frontal cortex (Wilson & Schneck, 2021).
Subcortical activity can also be reliably resolved in MEG (Piastra et al., 2020). Importantly, the basal ganglia are densely connected to regions of the IFG, including Broca's area (Alexander et al., 1990). Connectivity between basal ganglia and inferior frontal regions has been associated with procedural processing related to grammar and syntax (Ullman, 2001), as well as processing of morphemes (Ullman, 2006). Orthographic processing and rhyme detection have also been found to co‐activate Broca's area and left putamen (Booth et al., 2007), suggesting that both cortical and subcortical activity are relevant for language despite distinct functions in a whole brain network. There is evidence of a direct pathway from Broca's area to the basal ganglia, thought to be uniquely positioned to participate in expressive language (Jorge et al., 2022).
Here, we examine patterns of information flow during a verb generation task in MEG in a cross‐sectional group of children and adolescents. This group was confirmed to show low‐beta ERD and ERS during VG in Sharma et al. (2021). Phase slope index (PSI; Nolte et al., 2008) was used to assess whole‐brain directional connectivity within canonical frequency bands. PSI is insensitive to volume conduction, requires minimal parameterization, and is inherently interpretable as a test statistic (Nolte et al., 2008). Low‐beta ERD and ERS are associated with expressive language, specifically (Kadis et al., 2011; Sharma et al., 2021). However, significant connectivity has been noted across the spectra (Kadis et al., 2016; Youssofzadeh et al., 2017). By using a whole brain approach, we identify connectivity patterns without imposing assumptions about network composition.
Previous connectivity studies have found brain networks emerge at different rates, with some systems developing before birth (Fransson et al., 2007; Hoff et al., 2013). In contrast, language network development continues into adolescence (Kadis et al., 2016), and centrality appears bilateral in children and left lateralized in adolescents (Youssofzadeh et al., 2017). In children born preterm, network strength in beta and gamma frequencies was found to be related to language performance (Barnes‐Davis et al., 2021).
We predict that degree will increase with age. We hypothesize that regions showing low‐beta ERD will drive activity within the network, as indexed by high outdegree. Regions showing low‐beta ERS are expected to show high indegree as well as low outdegree, suggesting deactivation or task‐related inhibition. Based on previous findings, we also predict that information flow patterns may differ between frequency bands. Finally, we predict that subcortical structures will exhibit high betweenness centrality, suggesting bridge‐like function, reflecting important intermediate positioning within in the distributed language network.
2. METHODS
2.1. Participants
Then, 82 typically developing children (45 females, 37 males), ages 4.03–18.92 years (M = 11.38, SD = 4.52), participated in this study. Two were excluded due to lack of low‐beta ERD in frontal lobes, which suggests a lack of participation in the task. All participants were native English speakers, without history of neurological insult, speech or language impairment, or learning disability (based on parent report). Seventy‐one participants were right‐handed (three left‐handed, six ambidextrous), as determined using the Edinburgh Handedness Inventory (Oldfield, 1971). Data for this project stems from two prior studies with different assessment batteries; 27 participants completed the Peabody Picture Vocabulary Test (Dunn et al., 2015) along with the Expressive Vocabulary Test (Williams, 2007); 53 participants completed the Clinical Evaluation of Language Fundamentals (Semel et al., 2004). Scores on the PPVT and EVT were averaged to estimate overall vocabulary, which serves as a coarse assay for overall language ability; the Core Language Score from the CELF was used to assess general language ability. Performance for the group was within the average range (n = 80; mean = 111.6, SD = 14.47). Informed written consent from a parent and/or legal guardian was obtained for all participants under the age of 18 years; children ages 10 to less than 18 years provided assent, prior to participation. Participants ages 18 years or older provided informed written consent. The study was approved by the Institutional Review Board at Cincinnati Children's Hospital Medical Center (data collection site), and the Research Ethics Board at the Hospital for Sick Children in Toronto (analysis site).
2.2. Verb generation paradigm
Concrete nouns (verb generation trials), or speech‐shaped noise (control trials) were presented auditorily, in MEG. Participants were instructed to think of an action word that corresponds to each noun, as rapidly as possible. Nouns were chosen from normative databases and standardized language assessments consisting of everyday items selected to be familiar to a typically developing 5‐year‐old (e.g., pencil, book, dog, etc.). Verb generation trials required comprehension of each noun stimulus, semantic processing to generate a related verb, and then a covert response. For control trials, participants were asked to attend to speech‐shaped noise stimuli, without responding. Noun and noise stimuli were identical in duration (M = 670 ms, SD = 140 ms). Trials were presented in random order with an interstimulus interval jittered between 4000 and 5000 ms from onset. A total of 72 noun trials and 72 noise trials were presented. A training phase preceded the experimental trials and MEG acquisition, consisting of an overt version of the paradigm to confirm ability for each participant (at least 8/10 items correct), and to promote task compliance during subsequent data acquisition. Following the experiment, participants were asked to overtly recall nouns presented and the corresponding verbs that were generated; in all cases, the experimenter confirmed that participants were able to recall multiple items.
2.3. MEG and anatomical MRI acquisition
MEG was acquired on a whole‐head 275‐channel CTF system (CTF MEG Neuro Innovations Inc., Coquitlam, Canada) at a sampling rate of 1200 Hz. Participants were in supine position with head support from memory foam and/or linens to provide both comfort and stability. MEG data acquisition was limited to sessions <30 min to minimize fatigue and maximize task participation. Stimuli were presented binaurally by an intensity calibrated distal transducer projecting through tubing and ear inserts (Etymotic Research, IL, USA). SPL was set at a comfortable level for each participant. Localization coils were placed onto nasion and preauricular points for continuous monitoring of head position. Head movement was generally low. If participants exceeded 5 mm of movement within the first minute of recording, we aborted the run and relaunched the paradigm and acquisition. If total movement exceeded 5 mm in the final minute of the recording, the data were retained and included, in total, in the current analyses. Following the MEG, the coils were replaced by multimodal radiographic markers for subsequent structural MRI imaging. These fiducial markers facilitated offline co‐registration of MEG and MRI data. Whole‐head 3D T1‐weighted images (1 mm isotropic; flip = 90°, TE = 3.7 ms, TR = 8.1 ms) were acquired at 3.0 T on a Philips Achieva or Ingenia Elition scanner (Philips Medical Systems, International).
2.4. Whole brain connectivity analysis
2.4.1. Preprocessing
MEG data were preprocessed using FieldTrip toolbox in MATLAB (version 2019b; MathWorks Inc., MA, USA). Line noise (60 Hz, plus harmonics at 120 and 180 Hz) was attenuated in the continuous recording using a very sharp discrete Fourier transform filter. Continuous data were then bandpassed from 0.1 to 100 Hz. Ocular artifacts and cardiac rhythms were identified and removed from continuous data using independent component analysis. Verb generation and noise trials were epoched from 0 to 2000 ms poststimulus onset, and demeaned. SQUID jump artifacts were automatically identified; trials containing artifact (range 0–4; mean ± SD = 1.67 ± 1.16) were rejected.
2.4.2. Head modeling and source analysis
Fiducial positions (nasion and preauricular positions) were marked on the skin, prior to scanning in MEG and MRI. In the MEG, head localization coils were placed against each fiducial position, to track head position during recording. Immediately after MEG, the head coils were replaced with radiopaque donut‐shaped markers; these are clearly visible on the MRI, and facilitate precise offline co‐registration. The individual 3D T1‐weighted images were segmented into brain, skull, and scalp compartments using SPM12 routines, and realistic single‐shell (brain) models (Nolte, 2003) were generated in FieldTrip. Source models were developed based on centroids of the 246 parcels (210 cortical, 36 subcortical) from the Brainnetome Atlas (Fan et al., 2016), which were nonlinearly warped to individual space. Parcel centroids serve as network “nodes” in subsequent connectivity for analyses.
2.4.3. Beamforming
Verb generation and noise trials were concatenated for each participant, for development of a common spatial filter in linearly constrained minimum variance (LCMV) beamforming, with 1% Tikhonov regularization of the covariance matrix. Source estimates were normalized by projected noise to compute the neural activity index (NAI). Trial‐wise verb generation data were projected through the common filter to estimate nodal time course. Each subject‐space coordinate was assigned its corresponding MNI coordinate, to permit comparison across subjects.
2.4.4. Directional connectivity analysis
PSI was computed within canonical frequency bands for each node pair across a window of 500–1500 ms poststimulus presentation, corresponding to the time course of ERD and ERS peaks for auditory verb generation (Sharma et al., 2021). Bands were delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (13–29 Hz), and gamma (30–70 Hz). Directionality of information flow between each signal pair is identified using the normalized PSI value (Nolte et al., 2008). Connections were considered significant at a value of |PSI| > 1.96 (Kadis et al., 2016; Nolte et al., 2008). Suprathreshold values were then assigned values of 1 or −1, to indicate directionality of information flux. Only positive suprathrehold values were retained; the directed adjacency matrix contained 0 s (no significant connection) and 1 s (significant directed connection).
2.4.5. Degreeness and betweenness centrality graph metrics
From the thresholded adjacency matrices, indegree and outdegree were calculated for each node. Degree values provided measures of incoming and outgoing information, at each site, for each band. Number of drivers significantly connected to intrahemispheric or interhemispheric receivers were calculated. For visualization, indegree and outdegree were calculated from individual adjacency matrices and averaged across participants, then projected on a template brain. To visualize bridging hubs, betweenness centrality was also calculated from individual adjacency matrices and averaged across participants, then projected onto a template brain.
2.4.6. Graph metric age‐related correlations
Robust correlations (Pearson's r, with outlier assessment/removal using Cook's distance method, at a threshold of 3 SDs) were used to assess relationships between total degree (i.e., the sum of outdegree and indegree) and age for each band. We have adopted a familywise error rate of 5%, with Bonferroni correction for correlation analyses across the five canonical frequency bands; as such, each correlation was evaluated at a conservative, corrected alpha of .01.
2.5. Whole brain low‐beta ERD/ERS for expressive language
To assess the spatial relationship between low‐beta ERD/ERS with information flow (sending vs. receiving), we established whole‐brain maps of low‐beta ERD and ERS for the group. Low‐beta ERD is an established neural signature of expressive language, and low‐beta ERS has been shown to be a complementary signature (Hirata et al., 2004; Kadis et al., 2008, 2011; Ressel et al., 2008; Sharma et al., 2021; Youssofzadeh et al., 2017).
2.5.1. Low‐beta ERD/ERS preprocessing
Continuous MEG data were preprocessed using FieldTrip toolbox in MATLAB (version 2019b; MathWorks Inc.). Line noise (60 Hz, plus harmonics at 120 and 180 Hz) was attenuated in the continuous recording using a very sharp discrete Fourier transform filter. Continuous data were band‐pass filtered from 13–23 Hz then cropped from 700 to 1200 ms poststimulus onset (Youssofzadeh et al., 2017). This time window was selected to focus on the expressive components of the task. Trials containing artifact (range 0–8; mean ± SD = 2.08 ± 1.74) were rejected.
2.5.2. Low‐beta ERD/ERS differential beamforming
A regular 10 mm grid within the MNI152 template brain, was nonlinearly warped to individual MRIs. Verb generation and noise trials were concatenated for each participant to develop a common spatial filter in LCMV beamforming, with 1% Tikhonov regularization of the covariance matrix (Van Veen et al., 1997). Source level time series estimates were normalized by projected noise to compute active and baseline NAIs. Following source estimation, warped grid positions were assigned their corresponding MNI coordinates, which permitted comparison across subjects. T‐statistics were computed between noun and noise conditions at each position; the significance of each t‐statistic was assessed through Monte Carlo permutation where condition labels were randomly shuffled for 5000 permutations; an alpha level of .05 was used (Maris & Oostenveld, 2007). T‐statistics at each location were used to produce spatial maps of low‐beta ERD with negative values and low‐beta ERS with positive values.
2.5.3. Low‐beta ERD/ERS concordance to direction of informational flow
Number of parcels with ≥25% of their voxels exhibiting significant power changes were computed separately for ERD and ERS. Statistical level of association between ERD/ERS and direction of information flow (i.e., outdegree, indegree) was assessed with the chi‐square (χ 2) statistic for frequency bands that show a significant correlation between degree and age (i.e., a developmental trajectory for information flow).
3. RESULTS
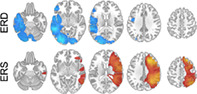
Event‐related spectral changes from Sharma et al. (2021) are presented groupwise in Figure 1. Maxima of low‐beta ERD were found at left IFG, posterior left STG, and left occipital cortex. Left insula, left MTG, left ITG, right occipital cortex, and bilateral cerebellum also showed low‐beta ERD. Maxima of low‐beta ERS was found at right MFG and right angular gyrus. Low‐beta ERS was also found at right IFG, right precentral gyrus, right postcentral gyrus, right STG, and right supramarginal gyrus (see Figure 1).
FIGURE 1.
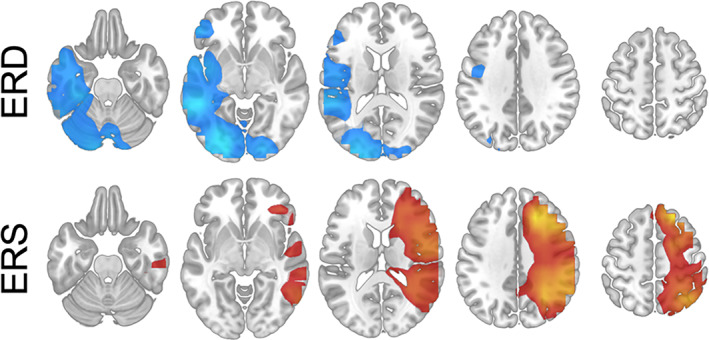
Axial slices (Panel a) showing low‐beta (13–23 Hz) event‐related desynchrony (ERD) in the top row (cool colors), and event‐related synchrony (ERS) in the bottom row (warm colors) in 80 children and adolescents (4–18 years old) performing verb generation.
Age‐related correlations were significant for degree in delta (r = .33, p < .05, corrected). Delta outdegree, indegree, and betweenness centrality showed a bilateral network, with involvement of deep and midline structures (see Figure 2). Statistical level of association between spectral change and degreeness was significant only in the delta band (χ 2 = 13.18, p < .001). Connectivity shows a bilateral perisylvian network with outdegree prominently in left IFG at pars opercularis and pars triangularis, along with left MFG. Indegree was found at Broca's homologue. Remarkably, despite subcortical structures showing information flow, all delta band connections were intrahemispheric. See Table 1 for a complete summary of structures showing delta band degreeness and betweenness.
FIGURE 2.
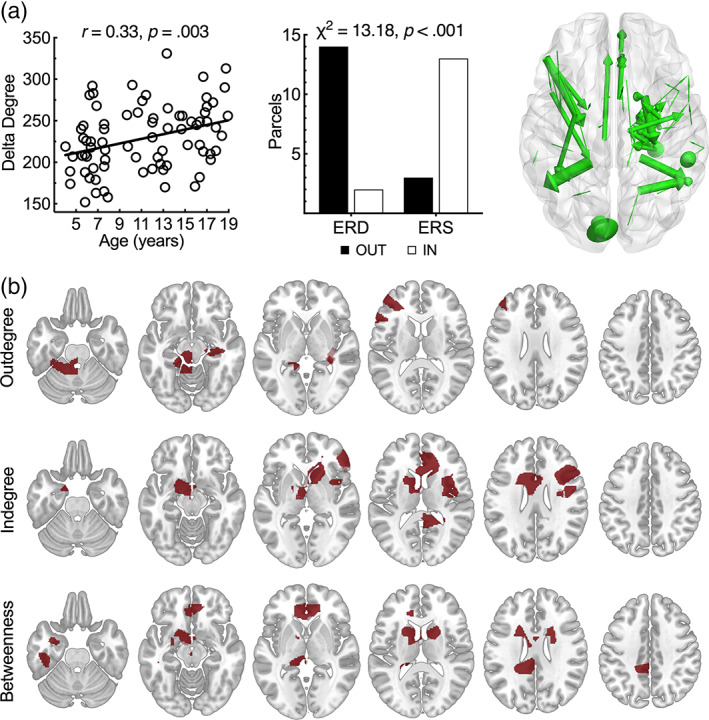
In (a), scatterplot shows significant correlations between age and delta (1–4 Hz) band degree based on normalized phase slope index in 80 children and adolescents performing verb generation. Bar plot (center) displays a significant association between direction of oscillatory changes and direction of information flow. Top 1% of all edges (right) during verb generation is shown across all participants. In (b), axial slices show delta band outdegree (top row) and indegree (middle row). Outdegree and indegree for delta reflect intrahemispheric connections. Axial slices show delta band betweenness centrality based on phase slope index (bottom row).
TABLE 1.
Regions showing delta (1–4 Hz) connectivity. Structures showing suprathreshold degreeness (outdegree/indegree) and betweenness centrality for delta band (1‐4Hz) connectivity.
| Subdivision | Outdegree | Indegree | Betweenness |
|---|---|---|---|
| Cortical |
L IFG (pars triangularis, pars opercularis) L MFG |
R IFG (pars orbitalis, pars triangularis) R MFG |
L Olfactory cortex L Temporal pole |
| Cortical midline | L/R ACC | L/R ACC |
L/R ACC L PCC |
| Subcortical |
L Thalamus R Hippocampus |
L/R Caudate L Thalamus L Globus pallidus |
L Amygdala L/R Caudate L Putamen L Globus pallidus |
Abbreviations: IFG, inferior frontal gyrus; MFG, middle frontal gyrus.
4. DISCUSSION
4.1. Asymmetric information flow underlies language lateralization
Delta band information flow (i.e., degreeness) was correlated to age. This is consistent with findings showing that the number of suprathreshold directed connections for expressive language increases with age (Kadis et al., 2016), though we observed whole‐brain age‐related findings specifically in delta band, here. Delta band showed intrahemispheric connections, only, not interhemispheric.
As predicted, subcortical and midline cortical regions showed betweenness centrality, outdegree, and indegree. Betweenness centrality has previously been found to map to subcortical regions in broadband (Youssofzadeh et al., 2017). Here, we show this effect may be specific to delta band connectivity. Since PSI is insensitive to bidirectionality, outflow and inflow are mutually exclusive per node pair. Therefore, regions with high betweenness act as bridge hubs, relaying information from one region to another.
4.2. Left hemisphere drives expressive language network
Low‐beta ERD and delta outflow show overlap at left IFG at pars triangularis and pars opercularis, left MTG, and left STG. In contrast, low‐beta ERS is associated with intrahemispheric delta band inflow and overlapped at right IFG at pars orbitalis and right MFG. Overlapping low‐beta ERD and delta information outflow in Broca's area suggests neural driving for expressive language. However, the same cannot be said of Broca's homologue. Right IFG and right MFG showed only information inflow and not outflow in delta, while the right parietal cortex did not exhibit significant information flow. Broca's area increasingly drives the distributed language network in development, while its right hemisphere homologue increasingly receives signals without sending. Thus, for expressive language, Broca's area is active and develops a driving role in the language network, its right hemisphere homologue may be idling or inhibited during language processing.
These results also seem consistent with an active inhibitory role for right hemisphere in expressive language that might involve inhibiting information flow, or possibly propagation, at cortex. Since low‐beta ERS was found at both language and nonlanguage sites, the functional role of the right hemisphere in language may involve active inhibition of both language‐specific and domain‐general functions. For example, right supramarginal gyri did not show suprathreshold degreeness but did show low‐beta ERS and betweenness. Right parietal cortex seems to exhibit hubness and low‐beta ERS without information flow, suggesting inhibited information flow. Thus, results here begin to describe a specialized inhibitory role for right hemisphere in expressive language, which seems to involve control over information flow.
4.3. Occipital subnetwork and language
Visual areas of the occipital cortex showed sparse connectivity to the language network but showed low‐beta ERD, implying increased neuronal firing in a visual subnetwork supporting language. We suspect that low‐beta ERD in the occipital cortex might indeed represent visual processing, or possibly visualization, during verb generation (Frings et al., 2006; Horowitz‐Kraus, 2014; Pang et al., 2011; Twait & Horowitz‐Kraus, 2019).
5. CONCLUSIONS
This study fills a gap in the literature by characterizing the relationship between localized measures of neuronal oscillations (ERD/ERS) and intrahemispheric connectivity. In conclusion, language development forms a distributed network, with left hemisphere increasingly driving the network across development. Importantly, inhibitory signaling is concordant with network receivers in Broca's homologue, while signals indexing increased neural firing are concordant to network drivers found in Broca's and Wernicke's areas. Broca's area drives information flow in the language network, while its homologue acts as an inhibitory information sink.
ACKNOWLEDGMENTS
The study was supported by a grant provided to DSK by Research Institute of Cincinnati Children's Hospital Medical Center and National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health (NIH), under award number R21NS106631 (PI: DSK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Data were collected at Cincinnati Children's Hospital and analyzed at Hospital for Sick Children (Toronto) and strictly comply with review board ethical guidelines.
Sharma, V. V. , Vannest, J. , & Kadis, D. S. (2023). Asymmetric information flow in brain networks supporting expressive language in childhood. Human Brain Mapping, 44(3), 1062–1069. 10.1002/hbm.26136
Funding information National Institute of Neurological Disorders and Stroke, Grant/Award Number: R21NS106631; Research Institute of Cincinnati Children's Hospital Medical Center
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.
REFERENCES
- Alexander, G. E. , Crutcher, M. D. , & DeLong, M. R. (1990). Basal ganglia‐thalamocortical circuits: Parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. In Uylings H. B., Van Eden C. G., DeBruin J. P., Corner M. A., & Feenstra M. G. (Eds.), Progress in brain research (Vol. 85, pp. 119–146). Elsevier. [PubMed] [Google Scholar]
- Barnes‐Davis, M. E. , Fujiwara, H. , Drury, G. , Merhar, S. L. , Parikh, N. A. , & Kadis, D. S. (2021). Functional hyperconnectivity during a stories listening task in magnetoencephalography is associated with language gains for children born extremely preterm. Brain Sciences, 11(10), 1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berl, M. M. , Mayo, J. , Parks, E. N. , Rosenberger, L. R. , VanMeter, J. , Ratner, N. B. , Vaidya, C. J. , & Gaillard, W. B. (2014). Regional differences in the developmental trajectory of lateralization of the language network. Human Brain Mapping, 35, 270–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder, J. R. , Frost, J. A. , Hammeke, T. A. , Cox, R. W. , Rao, S. M. , & Prieto, T. (1997). Human brain language areas identified by functional magnetic resonance imaging. Journal of Neuroscience, 17(1), 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth, J. R. , Wood, L. , Lu, D. , Houk, J. C. , & Bitan, T. (2007). The role of the basal ganglia and cerebellum in language processing. Brain Research, 1133(1), 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookshire, R. H. , & Nicholas, L. E. (1993). Discourse comprehension test. Communication Skill Builders. [Google Scholar]
- Bryan, K. L. (1994). The right hemisphere language battery. Whurr. [Google Scholar]
- Crone, N. E. , Miglioretti, D. L. , Gordon, B. , & Lesser, R. P. (1998). Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis: II. Event‐related synchronization in the gamma band. Brain, 121(12), 2301–2315. [DOI] [PubMed] [Google Scholar]
- Dunn, L. M. , Dunn, D. M. , & Lenhard, A. (2015). Peabody picture vocabulary test: PPVT 4. Pearson Assessments. [Google Scholar]
- Fan, L. , Li, H. , Zhuo, J. , Zhang, Y. , Wang, J. , Chen, L. , Yang, Z. , Chu, C. , Xie, S. , Laird, A. R. , Fox, P. T. , Eickhoff, S. B. , Yu, C. , & Jiang, T. (2016). The human Brainnetome atlas: A new brain atlas based on connectional architecture. Cerebral Cortex, 26(8), 3508–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson, P. , Skiöld, B. , Horsch, S. , Nordell, A. , Blennow, M. , Lagercrantz, H. , & Aden, U. (2007). Resting‐state networks in the infant brain. Proceedings of the National Academy of Sciences of the United States of America, 104(39), 15531–15536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici, A. D. (2011). The brain basis of language processing: from structure to function. Physiological Reviews, 91(4), 1357–1392. [DOI] [PubMed] [Google Scholar]
- Friederici, A. D. (2012). The cortical language circuit: From auditory perception to sentence comprehension. Trends in Cognitive Sciences, 16(5), 262–268. [DOI] [PubMed] [Google Scholar]
- Frings, M. , Dimitrova, A. , Schorn, C. F. , Elles, H. G. , Hein‐Kropp, C. , Gizewski, E. R. , Diener, H. C. , & Timmann, D. (2006). Cerebellar involvement in verb generation: An fMRI study. Neuroscience Letters, 409(1), 19–23. [DOI] [PubMed] [Google Scholar]
- Gabrieli, J. D. , Poldrack, R. A. , & Desmond, J. E. (1998). The role of the prefrontal cortex in language and memory. Proceedings of the National Academy of Sciences of the United States of America, 45, 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi, M. , Mazdeh, M. , Ranjbar, N. , & Seifrabie, M. A. (2018). Further evidence of the positive influence of repetitive transcranial magnetic stimulation on speech and language in patients with aphasia after stroke: Results from a double‐blind intervention with sham condition. Neuropsychobiology, 75, 185–192. [DOI] [PubMed] [Google Scholar]
- Heiss, W. D. , Kessler, J. , Thiel, A. , Ghaemi, M. , & Karbe, H. (1999). Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Annals of Neurology, 45(4), 430–438. [DOI] [PubMed] [Google Scholar]
- Hirata, M. , Kato, A. , Taniguchi, M. , Saitoh, Y. , Ninomiya, H. , Ihara, A. , Kishima, H. , Oshino, S. , Baba, T. , Yorifuji, S. , & Yoshimine, T. (2004). Determination of language dominance with synthetic aperture magnetometry: Comparison with the Wada test. NeuroImage, 23, 46–53. [DOI] [PubMed] [Google Scholar]
- Hoff, G. E. , Heuvel, M. P. , Benders, M. J. , Kersbergen, K. J. , & De Vries, L. S. (2013). On development of functional brain connectivity in the young brain. Frontiers in Human Neuroscience, 7, 650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland, S. K. , Plante, E. , Byars, A. W. , Strawsburg, R. H. , Schmithorst, V. J. , & Ball, W. S. (2001). Normal fMRI brain activation patterns in children performing a verb generation task. NeuroImage, 14(4), 837–843. [DOI] [PubMed] [Google Scholar]
- Holland, S. K. , Vannest, J. , Mecoli, M. , Jacola, L. M. , Tillema, J. , Karunanayaka, P. R. , Schmithorst, V. J. , Yuan, W. , Plante, E. , & Byars, W. (2007). Functional MRI of language lateralization during development in children. International Journal of Audiology, 46(9), 533–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz‐Kraus, T. (2014). Pinpointing the deficit in executive functions in adolescents with dyslexia performing the Wisconsin card sorting test: An ERP study. Journal of Learning Disabilities, 47(3), 208–223. [DOI] [PubMed] [Google Scholar]
- Hu, X. , Zhang, R. G. B. , Stone, C. , xu Liu, L. , He, J. , Shan, L. , Yang, L. , Liu, P. , Gao, F. , Yang, Y. , Wu, X. , Ye, C. , & Chen, Y. (2018). Effects of different frequencies of repetitive transcranial magnetic stimulation in stroke patients with non‐fluent aphasia: A randomized, sham‐controlled study. Neurological Research, 40, 459–465. [DOI] [PubMed] [Google Scholar]
- Joanette, Y. , & Anslado, A. I. (1999). Clinical note: Acquired pragmatic impairments and aphasia. Brain and Language, 68, 529–534. [DOI] [PubMed] [Google Scholar]
- Jorge, A. , Lipski, W. J. , Crammond, D. J. , Turner, R. S. , & Richardson, R. M. (2022). Hyperdirect connectivity of opercular speech network to the subthalamic nucleus. Cell Reports, 38, 110477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadis, D. S. , Dimitrijevic, A. , Toro‐Serey, C. A. , Smith, M. L. , & Holland, S. K. (2016). Characterizing information flow within the distributed pediatric expressive language network: A core region mapped through fMRI‐constrained MEG effective connectivity analyses. Brain Connectivity, 6(1), 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadis, D. S. , Pang, E. W. , Mills, T. , Taylor, M. J. , McAndrews, M. P. , & Smith, M. L. (2011). Characterizing the normal developmental trajectory of expressive language lateralization using magnetoencephalography. Journal of the International Neuropsychological Society, 17, 896–904. [DOI] [PubMed] [Google Scholar]
- Kadis, D. S. , Smith, M. L. , Mills, T. , & Pang, E. W. (2008). Expressive language mapping in children using MEG; MEG localization of expressive language cortex in healthy children: Application to paediatric clinical populations. Down Syndrome Quarterly, 10, 5–12. [Google Scholar]
- Kay, J. , Lesser, R. , & Coltheart, M. (1992). Psycholinguistic assessments of language processing in aphasia (PALPA). Erlbaum. [Google Scholar]
- Klimesch, W. , Doppelmayr, M. , Wimmer, H. , Gruber, W. , Röhm, D. , Schwaiger, J. , & Hutzler, F. (2001). Alpha and beta band power changes in normal and dyslexic children. Clinical Neurophysiology, 112(7), 1186–1195. [DOI] [PubMed] [Google Scholar]
- Knecht, S. , Drager, B. , Deppe, M. , Bobe, L. , Lohmann, H. , Flöel, A. , Ringelstein, E.‐B. , & Henningsen, H. (2000). Handedness and hemispheric language dominance in healthy humans. Brain, 123(12), 2512–2518. [DOI] [PubMed] [Google Scholar]
- Kuperberg, G. R. , Sitnikova, T. , Caplan, D. , & Holcomb, P. J. (2003). Electrophysiological distinctions in processing conceptual relationships within simple sentences. Cognitive Brain Research, 17(1), 117–129. [DOI] [PubMed] [Google Scholar]
- Maris, E. , & Oostenveld, R. (2007). Nonparametric statistical testing of EEG‐ and MEG‐data. Journal of Neuroscience Methods, 164(1), 177–190. [DOI] [PubMed] [Google Scholar]
- Myers, P. M. (2001). Towards a definition of RHD syndrome. Aphasiology, 15, 913–918. [Google Scholar]
- Myers, P. S. (1999). Right hemisphere damage: Disorders of communication and cognition. Singular. [Google Scholar]
- Neuper, C. , Wörtz, M. , & Pfurtscheller, G. (2006). ERD/ERS patterns reflecting sensorimotor activation and deactivation. In Neuper C. & Klimesch W. (Eds.), Event‐related dynamics of brain oscillations (pp. 211–259). Elsevier. [DOI] [PubMed] [Google Scholar]
- Nolte, G. (2003). The magnetic lead field theorem in the quasi‐static approximation and its use for magnetoencephalography forward calculation in realistic volume conductors. Physics in Medicine and Biology, 48(22), 3637–3652. [DOI] [PubMed] [Google Scholar]
- Nolte, G. , Ziehe, A. , Nikulin, V. V. , Schlögl, A. , Krämer, N. , Brismar, T. , & Müller, K. R. (2008). Robustly estimating the flow direction of information in complex physical systems. Physical Review Letters, 100, 234101. [DOI] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Olulade, O. A. , Seydell‐Greenwald, A. , Chambers, C. E. , Turkeltaub, P. E. , Dromerick, A. W. , Berl, M. M. , Gaillard, W. D. , & Newport, E. L. (2020). The neural basis of language development: Changes in lateralization over age. Proceedings of the National Academy of Sciences of the United States of America, 117(38), 23477–23483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang, E. W. , Wang, F. , Malone, M. , Kadis, D. S. , & Donner, E. J. (2011). Localization of Broca's area using verb generation tasks in the MEG: Validation against fMRI. Neuroscience Letters, 490(3), 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis, M. (1998). The other side of language: Pragmatic competence. Journal of Neurolinguistics, 11, 1–10. [Google Scholar]
- Patel, S. , Oishi, K. , Wright, A. , Sutherland‐Foggio, H. , Saxena, S. , Sheppard, S. M. , & Hillis, A. E. (2018). Right hemisphere regions critical for expression of emotion through prosody. Frontiers in Neurology, 9, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller, G. (1997). EEG event‐related desynchronization (ERD) and synchronization (ERS). Electroencephalography and Clinical Neurophysiology, 103, 1–26. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller, G. , & Lopes da Silva, F. H. (1999). Event‐related EEG/MEG synchronization and desynchronization: Basic priniciples. Clinical Neurophysiology, 110, 1842–1857. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller, G. , & Lopes da Silva, F. H. (2017). EEG event‐related desynchronization and event‐related synchronization. In Schomer D. L. & Lopes da Silva F. H. (Eds.), Niedermeyer's electroencephalography: Basic priniciples, clinical applications, and related fields (7th ed., pp. 294–316). Wiley‐Blackwell. [Google Scholar]
- Piastra, M. C. , Nüßing, A. , Vorwerk, J. , Clerc, M. , Engwer, C. , & Wolters, C. H. (2020). A comprehensive study on electroencephalography and magnetoencephalography sensitivity to cortical and subcortical sources. Human Brain Mapping, 42, 978–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, C. (2010). The anatomy of language: A review of 100 fMRI studies published in 2009. Annals of the New York Academy Sciences, 1191, 62–88. [DOI] [PubMed] [Google Scholar]
- Price, C. J. (2000). The anatomy of language: Contributions from functional neuroimaging. Journal of Anatomy, 197(3), 335–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, C. , Zhang, G. , Xu, X. , Hao, J. , Fang, H. , Chen, P. , Li, Z. , Ji, Y. , Cai, Q. , & Gao, F. (2019). The effect of rTMS over the different targets on language recovery in stroke patients with global aphasia: A randomized sham‐controlled study. BioMed Research International, 2019, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressel, V. , Wilke, M. , Lidzba, K. , Lutzenberger, W. , & Krageloh‐Mann, I. (2008). Increases in language lateralization in normal children as observed using magnetoencephalography. Brain and Language, 106, 167–176. [DOI] [PubMed] [Google Scholar]
- Ross, E. D. , & Mesulam, M. M. (1979). Dominant language functions of the right hemisphere? Prosody and emotional gesturing. Archives of Neurology, 36(3), 144–148. [DOI] [PubMed] [Google Scholar]
- Semel, E. M. , Wiig, E. H. , & Secord, W. (2004). CELF 4: Clinical evaluation of language fundamentals. Pearson Assessments. [Google Scholar]
- Sharma, V. V. , Vannest, J. , Greiner, H. M. , Fujiwara, H. , Tenney, J. R. , Williamson, B. J. , & Kadis, D. S. (2021). Beta synchrony for expressive language lateralizes to right hemisphere in development. Scientific Reports, 11, 3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, W. (1999). Neuronal synchrony: A versatile code for the definitions of relations. Neuron, 24, 49–65. [DOI] [PubMed] [Google Scholar]
- Twait, E. , & Horowitz‐Kraus, T. (2019). Functional connectivity of cognitive control and visual regions during verb generation is related to improved Reading in children. Brain Connectivity, 9(6), 500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman, M. T. (2001). A neurocognitive perspective on language: The declarative/procedural model. Nature Reviews Neuroscience, 2, 717–726. [DOI] [PubMed] [Google Scholar]
- Ullman, M. T. (2006). Is Broca's area part of a basal ganglia thalamocortical circuit? Cortex, 42, 480–485. [DOI] [PubMed] [Google Scholar]
- Van Veen, B. D. , van Drongelen, W. , Yuchtman, M. , & Suzuki, A. (1997). Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Transactions on Biomedical Engineering, 44(9), 867–880. [DOI] [PubMed] [Google Scholar]
- Vannest, J. , Karunanayaka, P. R. , Schmithorst, V. J. , Szaflarski, J. P. , & Holland, S. K. (2009). Language networks in children: Evidence from functional MRI. American Journal of Roentgenology, 192(5), 1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verly, M. , Verhoeven, J. , Zink, I. , Mantini, D. , Peeters, R. , Deprez, S. , Emsell, L. , Boets, B. , Noens, I. , Steyaert, J. , Lagae, L. , De Cock, P. , Rommel, N. , & Sunaert, S. (2014). Altered functional connectivity of the language network in ASD: Role of classical language areas and cerebellum. NeuroImage: Clinical, 4, 374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub, S. , Mesulam, M. M. , & Kramer, L. (1981). Disturbances in prosody. A right‐hemisphere contribution. Archives of Neurology, 38(12), 742–744. [DOI] [PubMed] [Google Scholar]
- Williams, K. (2007). Expressive vocabulary test (2nd ed.). Pearson Assessments. [Google Scholar]
- Wilson, S. M. , & Schneck, S. M. (2021). Neuroplasticity in post‐stroke aphasia: A systematic review and meta‐analysis of functional imaging studies of reorganization of language processing. Neurobiology of Language, 2(1), 22–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssofzadeh, V. , Williamson, B. J. , & Kadis, D. S. (2017). Mapping critical language sites in children performing verb generation: Whole‐brain connectivity and graph theoretical analysis in MEG. Frontiers in Human Neuroscience, 11(173), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


