Myeloid malignancies, including myelodysplastic syndromes (MDS), polycythemia vera, primary myelofibrosis, and acute myeloid leukemia (AML), have a male predominance.1–4 In addition, male patients with myeloid malignancies have inferior survival compared with female patients.1,5–7 Recently, it has been reported that the response to treatment with hypomethylating agents in MDS patients is different for males and females, suggesting different underlying pathobiology.8 Data from previous clinical cohorts showed different molecular characteristics of myeloid malignancies dependent on sex. For example, in both AML and MDS, it was reported that female patients carry more frequently mutations in DNMT3A and TP53, while mutations in U2AF1 are more frequently observed in males.1,3,9 In addition, ASXL1 mutations are more frequently observed in males with myeloproliferative neoplasms (MPN), unclassifiable MDS/MPN syndrome (MDS/MPN-U), or AML (Suppl. Table S1).3,10 It is currently unknown whether these sex differences in mutational patterns already occur in “pre-leukemic” clonal hematopoiesis (CH) and whether this impacts on the development of hematological malignancies. Here, we investigated sex differences in the prevalence of CH, the mutational spectra of CH and the impact of CH on overall survival (OS) and the development of hematological malignancies in the general population.
This study was performed within the Lifelines Cohort, a multidisciplinary prospective population-based cohort study examining in a unique three-generation design the health and health-related behaviors of 167,729 persons living in the North of the Netherlands. It employs a broad range of investigative procedures in assessing the biomedical, sociodemographic, behavioral, physical, and psychological factors which contribute to the health and disease of the general population, with a special focus on multimorbidity and complex genetics.11 The Lifelines study was performed in accordance with the Declaration of Helsinki and approved by the medical ethical committee of the University Medical Center Groningen. For a selected cohort of participants (n = 4715 individuals ≥60 years), we performed targeted error-corrected next-generation sequencing to acquire data on CH as described earlier.12 Gene mutations were called at variant allele frequency (VAF; Suppl. Table S2, Suppl. Figures S2 and S3) ≥1% and ≥10 mutant reads (referred to as CH). We additionally performed analyses restricting to the VAF cut-off proposed in the definition of CH of indeterminate potential (CHIP; VAF ≥2%).13 Given the interest for CH in the context of peripheral blood cell abnormalities, the cohort was enriched for individuals with blood count abnormalities (Suppl. Figure S1).
Statistical analyses were performed using R version 4.0.2. Two-group nonparametric data including clone size were statistically compared using the Mann-Whitney U test. Odds ratios (ORs), 95% confidence intervals (CIs), and P values for the association between CH, specific mutated genes, or presence of ≥2 mutated genes and sex were derived from logistic regression and corrected for age. Associations between CH or specific mutated genes and sex were additionally corrected for the presence of selected blood count abnormalities and smoking (Suppl. Tables S3 and S4). Development of incident hematological malignancies was retrieved by linkage with the Netherlands Cancer Registry, maintained and hosted by the Netherlands Comprehensive Cancer Organization (IKNL). Cumulative incidence graphs for newly diagnosed hematological malignancies were constructed using the Aalen-Johansen estimator, with death as a competing risk. OS was visualized by Kaplan-Meier graphs. Hazard ratios (HRs) with 95% CIs for death and development of hematological malignancy were reported from Cox proportional hazard regression with age as covariable. All statistical tests were performed two-sided, and P values below 0.05 were considered significant (Suppl. Materials and Methods).
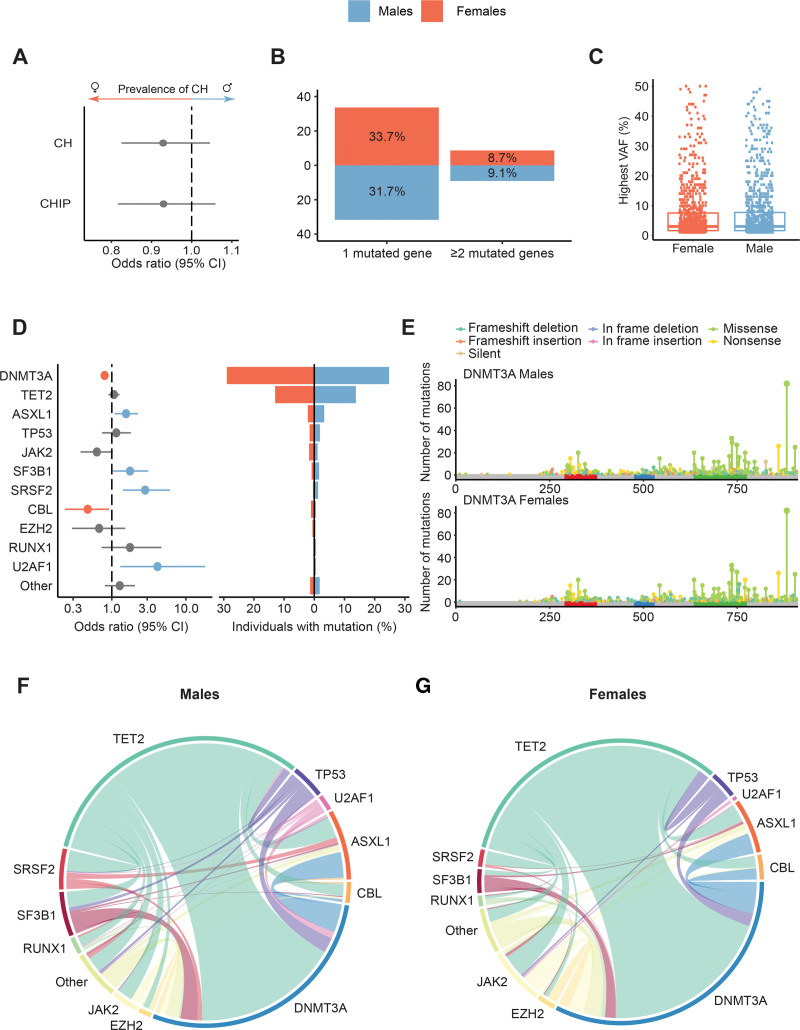
We investigated the prevalence of CH (VAF ≥1%) and CHIP (VAF ≥2%)13 in males and females among 4715 individuals ≥60 years from the population-based Lifelines cohort. This revealed no difference in the prevalence of CH and CHIP for males compared to females (CH 1005/2463 males [40.8%] and 953/2252 females [42.3%]; CHIP 667/2463 males [27.1%] and 638/2252 females [28.3%]; Figure 1A; Suppl. Figure S4). Additionally, we corrected these associations for the presence of blood count abnormalities and smoking, which did not impact on the results (Suppl. Tables S3A and S4A). Moreover, when restricting the analysis to individuals without blood count abnormalities, we also do not find an enrichment of CH (OR 0.90, 95% CI, 0.76-1.06; P = 0.211) nor CHIP (OR 0.92, 95% CI, 0.77-1.11; P = 0.385) in males or females. No difference in the prevalence of multiple mutated genes was observed in males compared to females (P = 0.834; Figure 1B) and the median clone size (VAF) was comparable for males (3.1%) and females (3.0%; P = 0.965; Figure 1C).
Figure 1.
Mutational spectrum of clonal hematopoiesis in males and females. (A) Forest plot indicating the OR and 95% CIs (horizontal lines) for the association between CH/CHIP and male sex, as derived from multivariable logistic regression with correction for age. (B) Proportion of individuals with one mutated gene or ≥2 mutated genes for males (blue) and females (orange) with the percentages indicated in the bar plots. (C) Distribution for the maximum VAF per individual detected in males and females. (D) Forest plot indicating the OR and 95% CIs for the association between recurrently mutated genes and male sex corrected for age. A colored line indicates a significantly higher proportion of the respective gene mutation in males (blue) or females (orange). The pyramid plot displays the proportion of individuals with mutations in most commonly mutated genes for males and females. (E) Lollipop plot indicating the number and type of mutations on each location of the DNMT3A gene for males and females. The red (PWWP), blue (ADD), and green (MTase) colored regions indicate respective protein domains. (F and G) Chord diagrams displaying comutational patterns in males and females. The width of the ribbon corresponds to the relative frequency of the co-occurrence, whereas the length of the arc corresponds to the relative frequency of mutations. Genes with <10 detected mutations in either males or females were grouped in the category other. ADD = ATRX-DNMT3-DNMT3L domain of DNMT3A; CH = clonal hematopoiesis; CHIP = clonal hematopoiesis of indeterminate potential; CI = confidence interval; MTase = catalytic methyltransferase domain of DNMT3A; OR = odds ratio; PWWP = Pro-Trp-Trp-Pro domain of DNMT3A; VAF = variant allele frequency.
Although the overall prevalence of CH was comparable between males and females, the spectrum of mutations showed sex-specific differences. We observed a significant enrichment of mutations in ASXL1 (P = 0.018), SF3B1 (P = 0.043), SRSF2 (P = 0.005), and U2AF1 (P = 0.030) in males compared to females (Figure 1D; Suppl. Figure S7). In contrast, mutations in the most commonly mutated gene DNMT3A (P = 0.001) and CBL (P = 0.031) were significantly enriched in females (Figure 1D; Suppl. Figure S7). This biased mutational spectrum was also observed using the VAF cut-off at ≥2% (i.e, CHIP; Suppl. Figure S5). After additional correction for blood count abnormalities and smoking, the associations for SRSF2, U2AF1, DNMT3A, and CBL were confirmed. For SF3B1, the presence of blood count abnormalities could play a role in the association with male sex (Suppl. Table S3B), and the enrichment of ASXL1 in males could partly be explained by smoking (Suppl. Table S4B). The enrichment of DNMT3A mutations in females could not be explained by a difference in clone size, the type of mutations, or their distribution across the gene coding region (Figure 1E; Suppl. Figure S6). Finally, the comutational patterns for males and females revealed comparable clonal complexity for both sexes (Figure 1F, G). The sex differences in the mutational spectrum of CH were in line with the bias previously reported in myeloid malignancies (Suppl. Table S1).1,3,7,9,10 Apparently, the reported sex differences in the mutational spectrum of myeloid malignancies are already present at a premalignant stage.
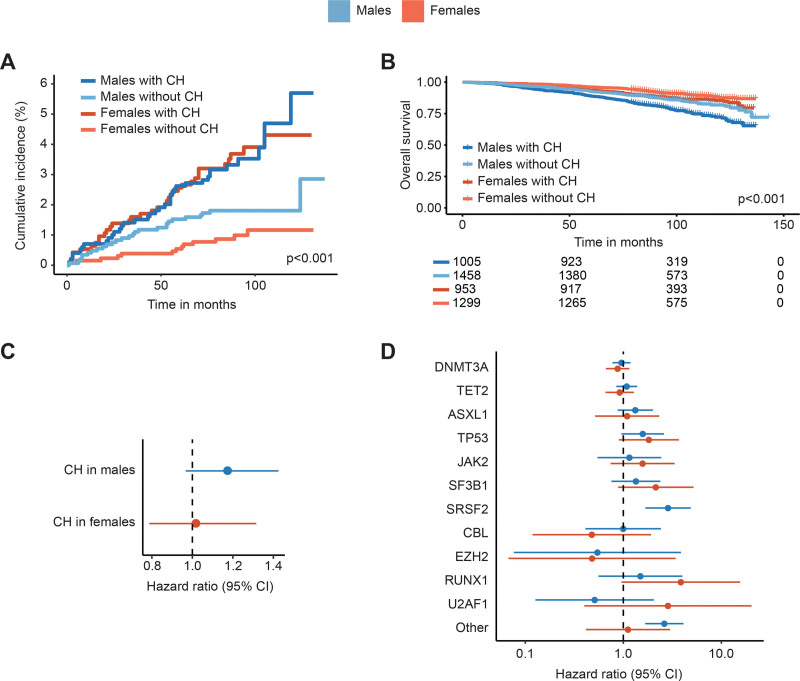
Subsequently we investigated whether CH has sex-specific implications on the development of hematological malignancies and survival. The cumulative incidence of hematological malignancies over 5 years for females with CH was 2.6% (95% CI, 1.5%-3.6%) compared to 0.5% (95% CI, 0.1%-0.9%) for females without CH. For males, the cumulative incidence of hematological malignancies was 2.6% (95% CI, 1.6%-3.6%) versus 1.5% (95% CI, 0.9%-2.2%) over 5 years with and without detectable CH, respectively. Overall, there was a significant increase in risk of incident hematological malignancies when CH was present (males HR 2.22, 95% CI, 1.34-3.67, P = 0.002; females HR 4.18, 95% CI, 2.19-7.96, P < 0.001; Figure 2A). No significant interaction effect between CH and sex was observed, indicating no sex-specific implications of CH on the development of hematological malignancies (P = 0.135).
Figure 2.
Incidence of hematological malignancies and all-cause mortality for males and females. (A) Cumulative incidence of hematological malignancies in males and females, stratified by the presence of CH. (B) Kaplan-Meier curve for OS stratified by sex and the presence of CH. (C) Forest plot showing the risk of all-cause mortality associated with CH in males and females, corrected for age. (D) Forest plot showing the risk of all-cause mortality associated with mutations in specific genes in males and females, corrected for age. The risk of all-cause mortality was not evaluable for SRSF2 mutations in females, as no deaths occurred in this subgroup. Cox proportional hazard regression models included age as covariable. Absence of mutations in the respective gene was used as a reference. Genes with <10 mutations in either males or females were grouped in the category other. CH = clonal hematopoiesis; CI = confidence interval; OS = overall survival.
Male sex associated with inferior OS (Figure 2B). The presence of CH did not significantly affect OS in males and females (males HR 1.17, 95% CI, 0.97-1.42, P = 0.105; females HR 1.02, 95% CI, 0.79-1.32, P = 0.895; Figure 2C). In addition, no significant interaction between sex and CH was observed (P = 0.681), indicating no sex-specific effect of CH on OS. Although hampered by low numbers, our data suggests a different impact of certain mutations on survival for males and females (Figure 2D). Mutations in SRSF2 (P < 0.001) and less commonly mutated genes categorized as “other” (P < 0.001) associated with a significantly higher risk of death in males. The risk of death was not higher for “other” mutated genes in females and not evaluable for SRSF2 mutations in females, as no deaths occurred in this subgroup.
In line with a recent study using UK biobank participants,14 we observed a biased mutational spectrum of CH in males and females, with enrichment of SF3B1, SRSF2, and ASXL1 in males and DNMT3A in females. We additionally observed enrichment of U2AF1 mutations in males and CBL mutations in females, while we could not reproduce the reported enrichment of TP53 and JAK2 mutations in males. Sex-specific differences in mutational patterns for community-based individuals may be the result of different exposures to environmental factors, such as smoking which has been associated with ASXL1 mutations.15 We previously reported on the association between estimated exposure to DNA damaging toxicities (including pesticides) and ASXL1 and spliceosome variants in the oldest old (80+) Lifelines population.12 Also, in that cohort of highly aged individuals, we noticed a higher prevalence of spliceosome mutations in males.12 Although our cohort was enriched for blood count abnormalities, we consider it unlikely that this explains the observed differences in mutational spectrum between males and females, since we corrected for the presence of blood phenotypes in multivariable analyses.
Males have a higher prevalence of myeloid malignancies.1–4 In our study, this male predominance of hematological malignancies could not be explained by the effect of CH, as we observed no sex-specific impact of CH on the development of hematological malignancies and comparable cumulative incidences were observed for males and females with CH. We hypothesize that the male predominance in myeloid malignancies might be due to enrichment of specific mutations in males that increase the risk to develop hematological malignancies, like ASXL1 and spliceosome mutations. Further, the male predominance may partly be explained by a higher proportion of males who develop hematological malignancies without detectable CH. Finally, mutations not included in our panel (such as BCOR, STAG2, ZRSR2) or mosaic chromosomal lesions may be additional underpinnings for this male bias.
In conclusion, sex-specific mutational patterns of myeloid malignancies are already present during the “premalignant” state of CH.
ACKNOWLEDGMENTS
The authors wish to acknowledge the services of the Lifelines Cohort Study, the contributing research centers delivering data to Lifelines, and all the study participants. The authors thank the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for collecting data for the Netherlands Cancer Registry and IKNL staff for scientific advice.
AUTHOR CONTRIBUTIONS
PK, IAvZ, AOdG, and JBS contributed to study design, data collection, analysis and interpretation of the data; MGJMvB, JJS, AGD, and BAvdR were involved in the interpretation of the data; GH and JHJ were principal investigators and involved in the study design, data collection, and interpretation of the results.
DISCLOSURES
The authors have no conflicts of interest to disclose.
SOURCES OF FUNDING
This work was supported by the MDS-RIGHT project, which has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement 634789. The Lifelines Biobank initiative has been made possible by subsidy from the Dutch Ministry of Health, Welfare and Sport; the Dutch Ministry of Economic Affairs; the University Medical Center Groningen; University Groningen; and the Northern Provinces of the Netherlands. AOdG and JHJ were supported by a grant from the Dutch Cancer Society (grant number10813).
Supplementary Material
Footnotes
JHJ and GH have contributed equally to this study.
Supplemental digital content is available for this article.
REFERENCES
- 1.Karantanos T, Jain T, Moliterno AR, et al. Sex-related differences in chronic myeloid neoplasms: from the clinical observation to the underlying biology. Int J Mol Sci. 2021;22:2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karantanos T, Gondek LP, Varadhan R, et al. Gender-related differences in the outcomes and genomic landscape of patients with myelodysplastic syndrome/myeloproliferative neoplasm overlap syndromes. Br J Haematol. 2021;193:1142–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De-Morgan A, Meggendorfer M, Haferlach C, et al. Male predominance in AML is associated with specific preleukemic mutations. Leukemia. 2021;35:867–870. [DOI] [PubMed] [Google Scholar]
- 4.Geyer HL, Kosiorek H, Dueck AC, et al. Associations between gender, disease features and symptom burden in patients with myeloproliferative neoplasms: an analysis by the MPN QOL International Working Group. Haematologica. 2017;102:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nösslinger T, Tüchler H, Germing U, et al. Prognostic impact of age and gender in 897 untreated patients with primary myelodysplastic syndromes. Ann Oncol. 2010;21:120–125. [DOI] [PubMed] [Google Scholar]
- 6.Wang F, Ni J, Wu L, et al. Gender disparity in the survival of patients with primary myelodysplastic syndrome. J Cancer. 2019;10:1325–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karantanos T, Chaturvedi S, Braunstein EM, et al. Sex determines the presentation and outcomes in MPN and is related to sex-specific differences in the mutational burden. Blood Adv. 2020;4:2567–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeZern AE, Zeidan AM, Barnard J, et al. Differential response to hypomethylating agents based on sex: a report on behalf of the MDS Clinical Research Consortium (MDS CRC). Leuk Lymphoma. 2017;58:1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metzeler KH, Herold T, Rothenberg-Thurley M, et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood. 2016;128:686–698. [DOI] [PubMed] [Google Scholar]
- 10.Bose P, Nazha A, Komrokji RS, et al. Mutational landscape of myelodysplastic/myeloproliferative neoplasm-unclassifiable. Blood. 2018;132:2100–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scholtens S, Smidt N, Swertz MA, et al. Cohort profile: life lines, a three-generation cohort study and biobank. Int J Epidemiol. 2015;44:1172–1180. [DOI] [PubMed] [Google Scholar]
- 12.van Zeventer IA, Salzbrunn JB, de Graaf AO, et al. Prevalence, predictors, and outcomes of clonal hematopoiesis in individuals aged ≥80 years. Blood Adv. 2021;5:2115–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kar SP, Quiros PM, Gu M, et al. Genome-wide analyses of 200,453 individuals yield new insights into the causes and consequences of clonal hematopoiesis. Nat Genet. 2022;54:1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawoud AAZ, Tapper WJ, Cross NCP. Clonal myelopoiesis in the UK Biobank cohort: ASXL1 mutations are strongly associated with smoking. Leukemia. 2020;34:2660–2672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.